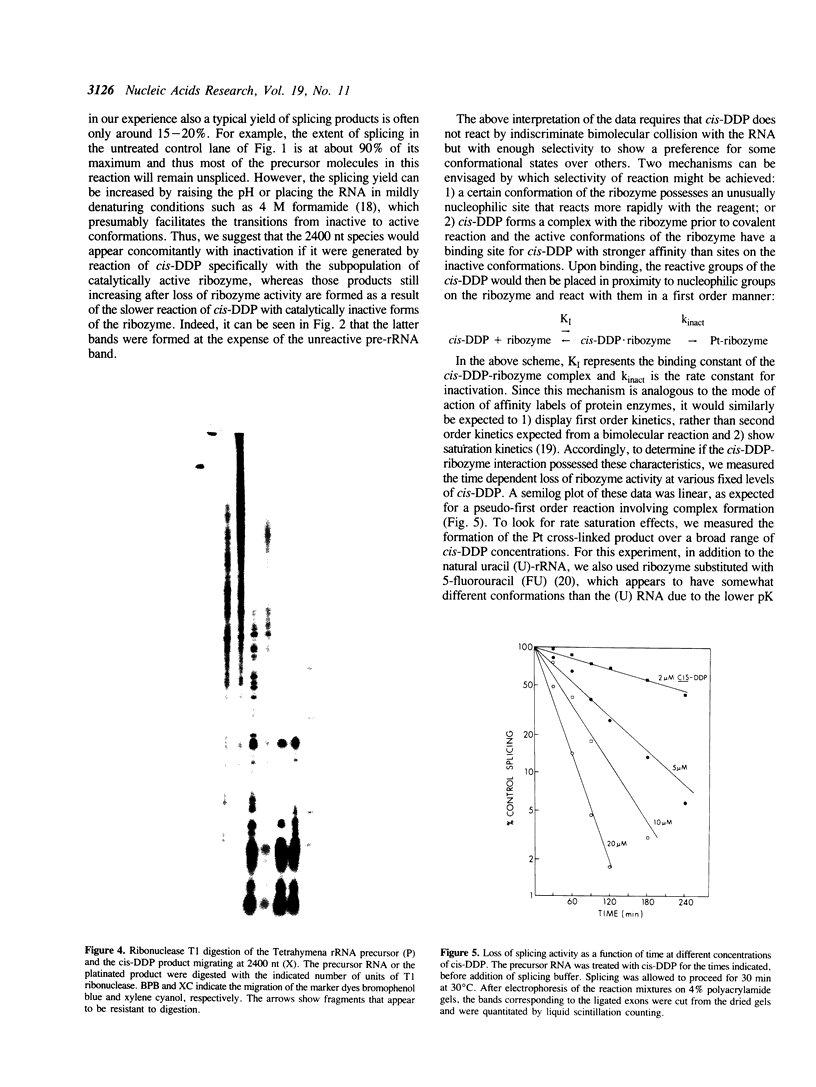
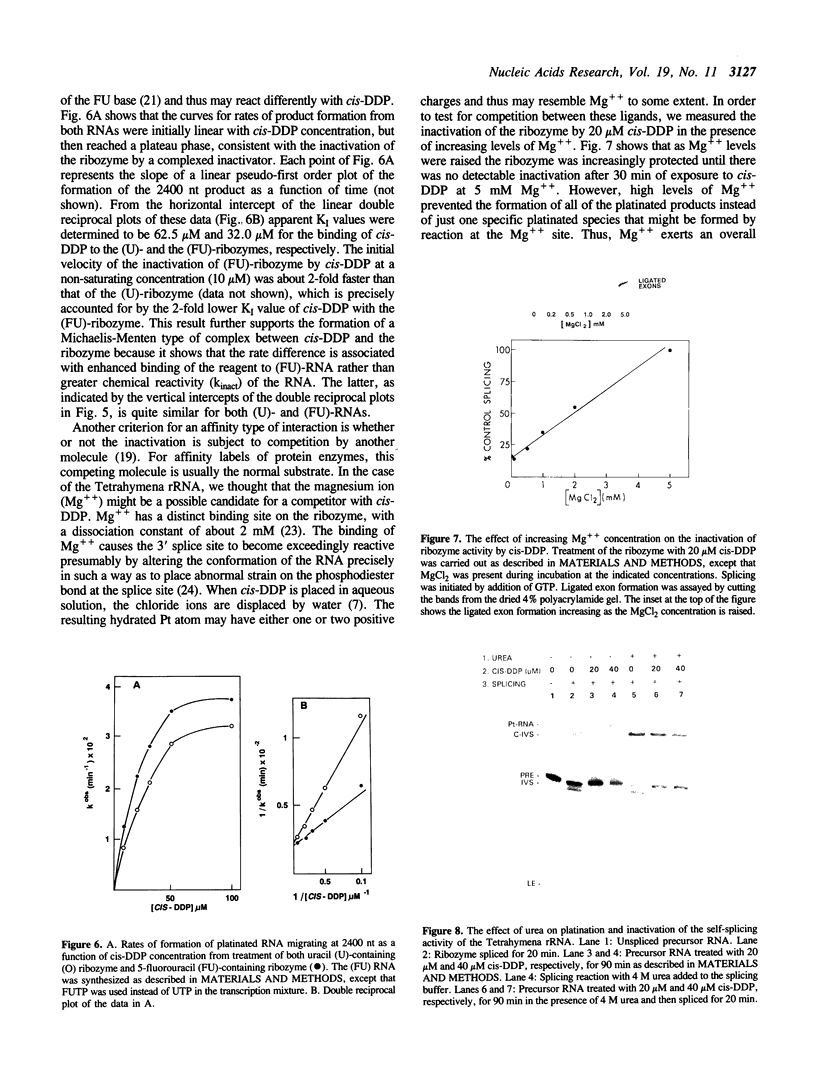
Abstract
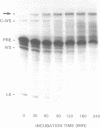
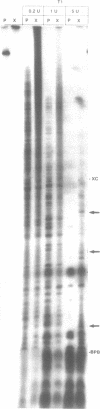
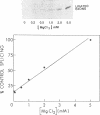
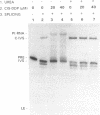
The anti-cancer drug cis-diamminedichloroplatinum (II) (cis-DDP) reacted with Tetrahymena self-splicing rRNA ribozyme, causing loss of self-splicing activity and formation of a number of platinated RNA species. The formation of one distinct platinated product, migrating at an apparent size of 2400 nt, was closely associated with ribozyme inactivation. This platinated RNA was resistant to T1 ribonuclease digestion, suggesting the presence of inter-strand Pt cross-links. The reaction rate of cis-DDP with the ribozyme followed first order kinetics and showed a saturation effect with increasing cis-DDP concentration, characteristic of an affinity-label type of interaction rather than bimolecular collision. The apparent KI for binding of cis-DDP to the ribozyme was 62 microM. Ribozyme treated with urea was not inactivated by cis-DDP, indicating that the native structure of the RNA is required for reaction with cis-DDP. Mg++, which binds to the ribozyme and causes conformational changes in the molecule, protected the ribozyme from inactivation by cis-DDP and also prevented the formation of platinated RNA. These results suggest that binding of cis-DDP to sites formed by certain secondary or tertiary structural elements of the RNA enhance the rate and the specificity of reaction of the reagent with the ribozyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Been M. D., Cech T. R. Selection of circularization sites in a group I IVS RNA requires multiple alignments of an internal template-like sequence. Cell. 1987 Sep 11;50(6):951–961. doi: 10.1016/0092-8674(87)90522-8. [DOI] [PubMed] [Google Scholar]
- Branch A. D., Benenfeld B. J., Paul C. P., Robertson H. D. Analysis of ultraviolet-induced RNA-RNA cross-links: a means for probing RNA structure-function relationships. Methods Enzymol. 1989;180:418–442. doi: 10.1016/0076-6879(89)80115-6. [DOI] [PubMed] [Google Scholar]
- Cech T. R. The chemistry of self-splicing RNA and RNA enzymes. Science. 1987 Jun 19;236(4808):1532–1539. doi: 10.1126/science.2438771. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Zaug A. J., Grabowski P. J. In vitro splicing of the ribosomal RNA precursor of Tetrahymena: involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell. 1981 Dec;27(3 Pt 2):487–496. doi: 10.1016/0092-8674(81)90390-1. [DOI] [PubMed] [Google Scholar]
- Ciccarelli R. B., Solomon M. J., Varshavsky A., Lippard S. J. In vivo effects of cis- and trans-diamminedichloroplatinum(II) on SV40 chromosomes: differential repair, DNA-protein cross-linking, and inhibition of replication. Biochemistry. 1985 Dec 17;24(26):7533–7540. doi: 10.1021/bi00347a005. [DOI] [PubMed] [Google Scholar]
- Danenberg P. V., Shea L. C., Danenberg K. Characterization of the mode of binding of substrates to the active site of Tetrahymena self-splicing RNA using 5-fluorouracil-substituted mini-exons. Biochemistry. 1989 Aug 8;28(16):6779–6785. doi: 10.1021/bi00442a035. [DOI] [PubMed] [Google Scholar]
- Danenberg P. V., Shea L. C., Danenberg K. Effect of 5-fluorouracil substitution on the self-splicing activity of Tetrahymena ribosomal RNA. Cancer Res. 1990 Mar 15;50(6):1757–1763. [PubMed] [Google Scholar]
- Davanloo P., Rosenberg A. H., Dunn J. J., Studier F. W. Cloning and expression of the gene for bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2035–2039. doi: 10.1073/pnas.81.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingman C. W., Fisher M. P., Kakefuda T. Role of molecular conformation in determining the electrophoretic properties of polynucleotides in agarose-acrylamide gels. II. Biochemistry. 1972 Mar 28;11(7):1242–1250. doi: 10.1021/bi00757a020. [DOI] [PubMed] [Google Scholar]
- Harder H. C., Rosenberg B. Inhibitory effects of anti-tumor platinum compounds on DNA, RNA and protein syntheses in mammalian cells in virtro. Int J Cancer. 1970 Sep 15;6(2):207–216. doi: 10.1002/ijc.2910060207. [DOI] [PubMed] [Google Scholar]
- Howle J. A., Gale G. R. Cis-dichlorodiammineplatinum (II). Persistent and selective inhibition of deoxyribonucleic acid synthesis in vivo. Biochem Pharmacol. 1970 Oct;19(10):2757–2762. doi: 10.1016/0006-2952(70)90102-4. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Cech T. R. Three-dimensional model of the active site of the self-splicing rRNA precursor of Tetrahymena. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8788–8792. doi: 10.1073/pnas.84.24.8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehrer P. J., Einhorn L. H. Drugs five years later. Cisplatin. Ann Intern Med. 1984 May;100(5):704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- Pinto A. L., Lippard S. J. Binding of the antitumor drug cis-diamminedichloroplatinum(II) (cisplatin) to DNA. Biochim Biophys Acta. 1985;780(3):167–180. doi: 10.1016/0304-419x(85)90001-0. [DOI] [PubMed] [Google Scholar]
- Rahmouni A., Leng M. Reaction of nucleic acids with cis-diamminedichloroplatinum(II): interstrand cross-links. Biochemistry. 1987 Nov 17;26(23):7229–7234. doi: 10.1021/bi00397a005. [DOI] [PubMed] [Google Scholar]
- Sugimoto N., Kierzek R., Turner D. H. Kinetics for reaction of a circularized intervening sequence with CU, UCU, CUCU, and CUCUCU: mechanistic implications from the dependence on temperature and on oligomer and Mg2+ concentrations. Biochemistry. 1988 Aug 23;27(17):6384–6392. doi: 10.1021/bi00417a029. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Davis P. W., Hardin C. C., Puglisi J. D., Walker G. T., Wyatt J. RNA structure from A to Z. Cold Spring Harb Symp Quant Biol. 1987;52:135–146. doi: 10.1101/sqb.1987.052.01.018. [DOI] [PubMed] [Google Scholar]
- Wu H. N., Lin Y. J., Lin F. P., Makino S., Chang M. F., Lai M. M. Human hepatitis delta virus RNA subfragments contain an autocleavage activity. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1831–1835. doi: 10.1073/pnas.86.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaug A. J., Been M. D., Cech T. R. The Tetrahymena ribozyme acts like an RNA restriction endonuclease. Nature. 1986 Dec 4;324(6096):429–433. doi: 10.1038/324429a0. [DOI] [PubMed] [Google Scholar]
- Zaug A. J., Kent J. R., Cech T. R. A labile phosphodiester bond at the ligation junction in a circular intervening sequence RNA. Science. 1984 May 11;224(4649):574–578. doi: 10.1126/science.6200938. [DOI] [PubMed] [Google Scholar]
- Zaug A. J., Kent J. R., Cech T. R. Reactions of the intervening sequence of the Tetrahymena ribosomal ribonucleic acid precursor: pH dependence of cyclization and site-specific hydrolysis. Biochemistry. 1985 Oct 22;24(22):6211–6218. doi: 10.1021/bi00343a027. [DOI] [PubMed] [Google Scholar]