Abstract
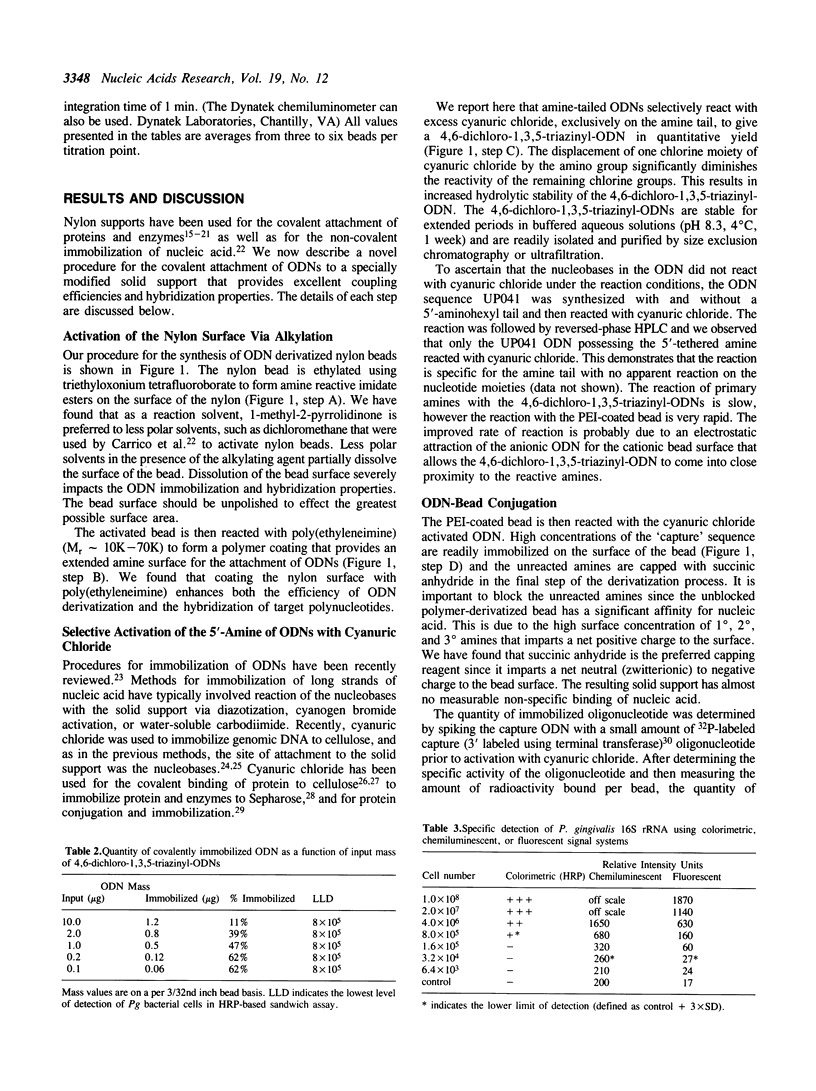
A procedure for immobilization of well-defined quantities of oligodeoxyribonucleotides (ODNs) to a versatile nylon support is described. The solid support, a nylon-6/6 bead, is covalently coated with poly(ethyleneimine) to provide a reactive spacer-arm for attachment of ODNs. 5'-Aminohexyl-tailed ODNs are selectively activated using 2,4,6-trichloro-1,3,5-triazine (cyanuric chloride) and then covalently attached to the bead via the triazine moiety. The modified nylon support has a low level of binding of nonspecific nucleic acid and efficiently captures both RNA and DNA targets.
Full text
PDF

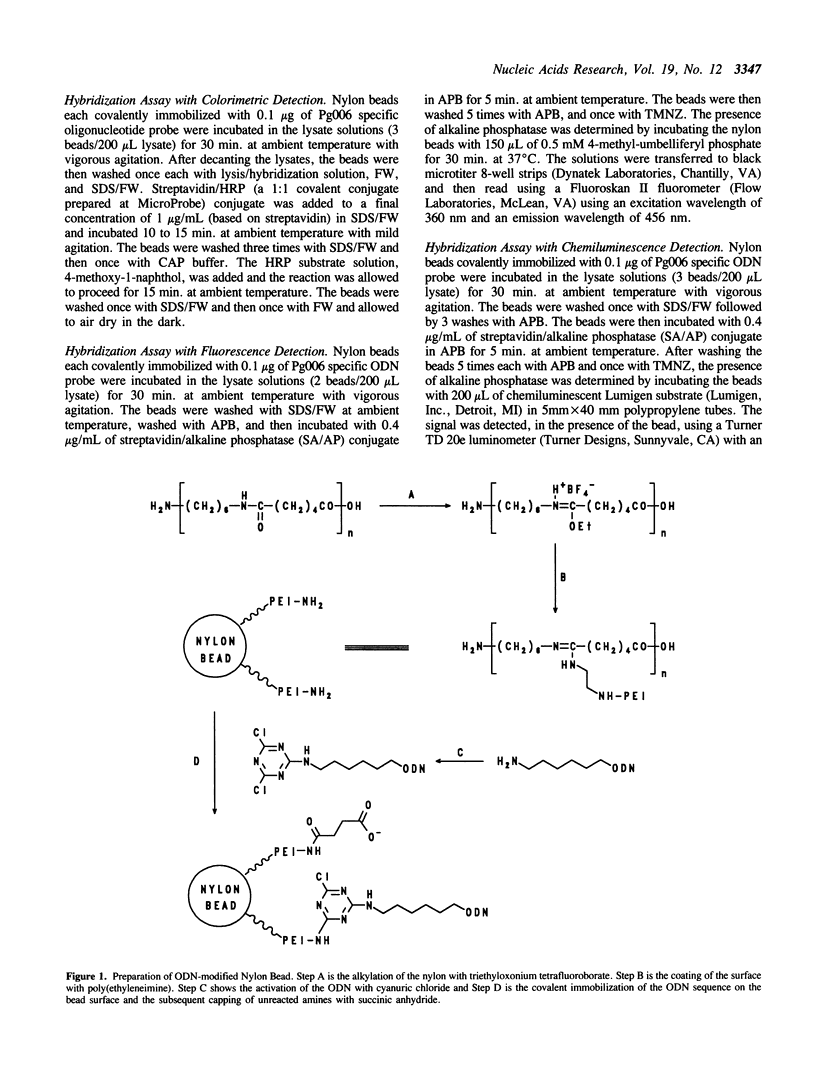


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagioni S., Sisto R., Ferraro A., Caiafa P., Turano C. A new method for the preparation of DNA--cellulose. Anal Biochem. 1978 Sep;89(2):616–619. doi: 10.1016/0003-2697(78)90390-1. [DOI] [PubMed] [Google Scholar]
- Dams E., Hendriks L., Van de Peer Y., Neefs J. M., Smits G., Vandenbempt I., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1988;16 (Suppl):r87–173. doi: 10.1093/nar/16.suppl.r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix K., Watanabe S. M., McArdle S., Lee D. I., Randolph C., Moncla B., Schwartz D. E. Species-specific oligodeoxynucleotide probes for the identification of periodontal bacteria. J Clin Microbiol. 1990 Feb;28(2):319–323. doi: 10.1128/jcm.28.2.319-323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A. R., Hassell J. A. A novel method to map transcripts: evidence for homology between an adenovirus mRNA and discrete multiple regions of the viral genome. Cell. 1977 Sep;12(1):23–36. doi: 10.1016/0092-8674(77)90182-9. [DOI] [PubMed] [Google Scholar]
- Filippusson H., Hornby W. E., McDonald A. The use of immobilised derivatives of urease and urate oxidase in automated analysis. FEBS Lett. 1972 Feb 15;20(3):291–293. doi: 10.1016/0014-5793(72)80089-9. [DOI] [PubMed] [Google Scholar]
- Finlay T. H., Troll V., Levy M., Johnson A. J., Hodgins L. T. New methods for the preparation of biospecific adsorbents and immobilized enzymes utilizing trichloro-s-triazine. Anal Biochem. 1978 Jun 15;87(1):77–90. doi: 10.1016/0003-2697(78)90571-7. [DOI] [PubMed] [Google Scholar]
- Ghosh S. S., Musso G. F. Covalent attachment of oligonucleotides to solid supports. Nucleic Acids Res. 1987 Jul 10;15(13):5353–5372. doi: 10.1093/nar/15.13.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., Kwoh D. Y., Davis G. R. Hybridization properties of immobilized nucleic acids. Nucleic Acids Res. 1987 Jul 10;15(13):5373–5390. doi: 10.1093/nar/15.13.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild J. Conjugates of oligonucleotides and modified oligonucleotides: a review of their synthesis and properties. Bioconjug Chem. 1990 May-Jun;1(3):165–187. doi: 10.1021/bc00003a001. [DOI] [PubMed] [Google Scholar]
- Hemmilä I. Fluoroimmunoassays and immunofluorometric assays. Clin Chem. 1985 Mar;31(3):359–370. [PubMed] [Google Scholar]
- Hornby W. E., Filippusson H., McDonald A. The preparation of glucose oxidase chemically attached to polystyrene and its use in the automated analyses of glucose. FEBS Lett. 1970 Jul 15;9(1):8–10. doi: 10.1016/0014-5793(70)80297-6. [DOI] [PubMed] [Google Scholar]
- Hornby W. E., Inman D. J., McDonald A. The preparation of some immobilised dehydrogenases and their use in automated analysis. FEBS Lett. 1972 Jun 1;23(1):114–116. doi: 10.1016/0014-5793(72)80297-7. [DOI] [PubMed] [Google Scholar]
- Hunger H. D., Coutelle C., Behrendt G., Flachmeier C., Rosenthal A., Speer A., Breter H., Szargan R., Franke P., Stahl J. CCA paper: a new two-dimensional cyanuric chloride-activated matrix for universal application in molecular biology. Anal Biochem. 1986 Aug 1;156(2):286–299. doi: 10.1016/0003-2697(86)90255-1. [DOI] [PubMed] [Google Scholar]
- Inman D. J., Hornby W. E. Preparation of some immobilized linked enzyme systems and their use in the automated determination of disaccharides. Biochem J. 1974 Jan;137(1):25–32. doi: 10.1042/bj1370025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay G., Crook E. M. Coupling of enzymes to cellulose using chloro-s-triazines. Nature. 1967 Nov 4;216(5114):514–515. doi: 10.1038/216514a0. [DOI] [PubMed] [Google Scholar]
- Kremsky J. N., Wooters J. L., Dougherty J. P., Meyers R. E., Collins M., Brown E. L. Immobilization of DNA via oligonucleotides containing an aldehyde or carboxylic acid group at the 5' terminus. Nucleic Acids Res. 1987 Apr 10;15(7):2891–2909. doi: 10.1093/nar/15.7.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J. A., Kricka L. J. Analytical strategies for the use of DNA probes. Anal Biochem. 1988 Feb 15;169(1):1–25. doi: 10.1016/0003-2697(88)90251-5. [DOI] [PubMed] [Google Scholar]
- Means G. E., Feeney R. E. Chemical modifications of proteins: history and applications. Bioconjug Chem. 1990 Jan-Feb;1(1):2–12. doi: 10.1021/bc00001a001. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Ngo T. T., Lenhoff H. M. A sensitive and versatile chromogenic assay for peroxidase and peroxidase-coupled reactions. Anal Biochem. 1980 Jul 1;105(2):389–397. doi: 10.1016/0003-2697(80)90475-3. [DOI] [PubMed] [Google Scholar]
- Ranki M., Palva A., Virtanen M., Laaksonen M., Söderlund H. Sandwich hybridization as a convenient method for the detection of nucleic acids in crude samples. Gene. 1983 Jan-Feb;21(1-2):77–85. doi: 10.1016/0378-1119(83)90149-x. [DOI] [PubMed] [Google Scholar]
- Schaap A. P., Akhavan H., Romano L. J. Chemiluminescent substrates for alkaline phosphatase: application to ultrasensitive enzyme-linked immunoassays and DNA probes. Clin Chem. 1989 Sep;35(9):1863–1864. [PubMed] [Google Scholar]
- Smith N. L., 3rd, Lenhoff H. M. Covalent binding of proteins and glucose-6-phosphate dehydrogenase to cellulosic carriers activated with s-triazine trichloride. Anal Biochem. 1974 Oct;61(2):392–415. doi: 10.1016/0003-2697(74)90406-0. [DOI] [PubMed] [Google Scholar]
- Sundaram P. V., Hinsch W. Single- and coupled-enzyme nylon tube reactors for routine determination of pyruvate and lactate in serum. Clin Chem. 1979 Feb;25(2):285–288. [PubMed] [Google Scholar]
- Sundaram P. V., Igloi M. P., Wassermann R., Hinsch W. Immobilized-enzyme nylon-tube reactor for routine determination of uric acid in serum. Clin Chem. 1978 Oct;24(10):1813–1817. [PubMed] [Google Scholar]
- Sundaram P. V., Igloi M. P., Wassermann R., Hinsch W., Knoke K. J. Immobilized-enzyme nylon-tube reactors for routine determination of urea and citrulline in serum. Clin Chem. 1978 Feb;24(2):234–239. [PubMed] [Google Scholar]


