Abstract
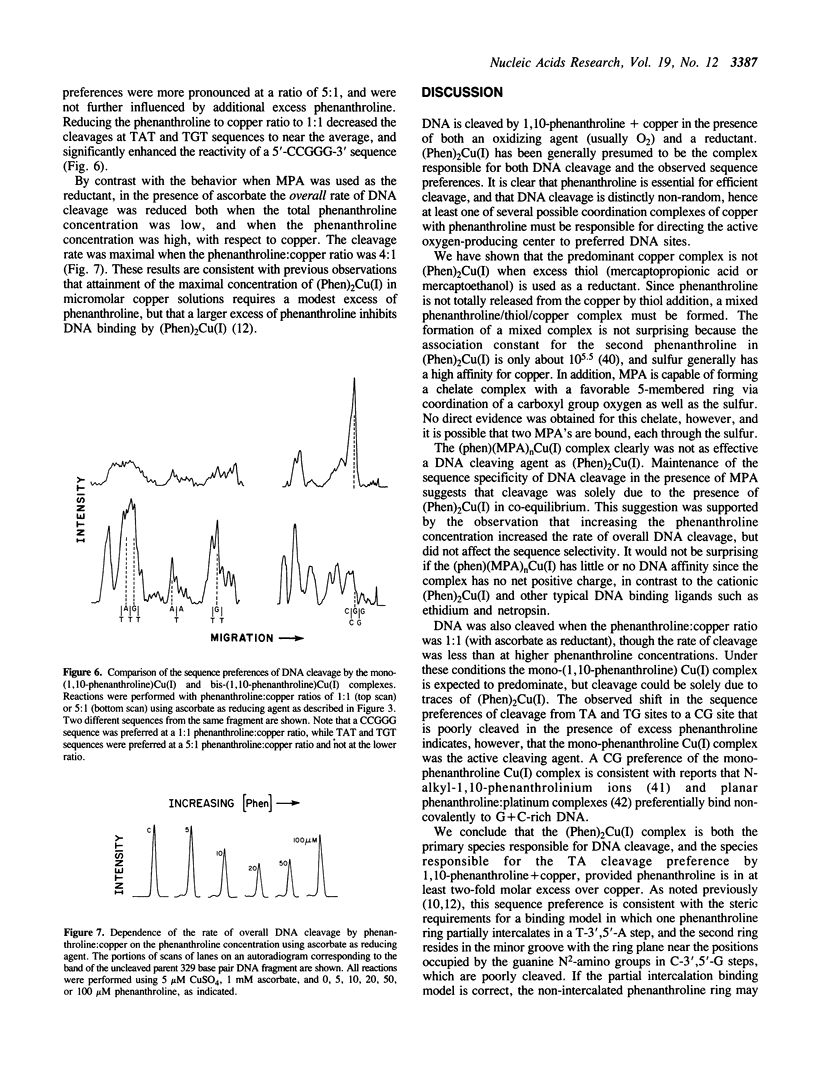
Copper in the presence of excess 1,10-phenanthroline, a reducing agent, and molecular oxygen causes cleavage of DNA with a preference for T-3',5'-A-steps, particularly in TAT triplets. The active molecular species is commonly thought to be the bis-(1,10-phenanthroline)Cu(I) complex, (Phen)2Cu(I), regardless of the reducing agent type. We have found that (Phen)2Cu(I) is not the predominant copper complex when 3-mercaptopropionic acid (MPA) or 2-mercaptoethanol are used as the reducing agents, but (Phen)2Cu(I) predominates when ascorbate is used as the reducing agent. Substitution of ascorbate for thiol significantly enhances the rate of DNA cleavage by 1,10-phenanthroline + copper, without altering the sequence selectivity. We show that (Phen)2Cu(I) is the complex responsible for DNA cleavage, regardless of reducing agent, and that 1,10-phenanthroline and MPA compete for copper coordination sites. DNA cleavage in the presence of ascorbate also occurs under conditions where the mono-(1,10-phenanthroline)Cu(I) complex predominates (1:1 phenanthroline:copper ratio), but preferential cleavage was observed at a CCGG sequence and not at TAT sequences. The second phenanthroline ring of the (Phen)2Cu(I) complex appears essential for determining the T-3',5'-A sequence preferences of phenanthroline + copper when phenanthroline is in excess.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cartwright I. L., Elgin S. C. Analysis of chromatin structure and DNA sequence organization: use of the 1,10-phenanthroline-cuprous complex. Nucleic Acids Res. 1982 Oct 11;10(19):5835–5852. doi: 10.1093/nar/10.19.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. H., Sigman D. S. Chemical conversion of a DNA-binding protein into a site-specific nuclease. Science. 1987 Sep 4;237(4819):1197–1201. doi: 10.1126/science.2820056. [DOI] [PubMed] [Google Scholar]
- Chen C. H., Sigman D. S. Nuclease activity of 1,10-phenanthroline-copper: sequence-specific targeting. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7147–7151. doi: 10.1073/pnas.83.19.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. DNA structural variations in the E. coli tyrT promoter. Cell. 1984 Jun;37(2):491–502. doi: 10.1016/0092-8674(84)90379-9. [DOI] [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Barbier C., Chassignol M., Thuong N. T., Hélène C. Sequence-specific recognition and cleavage of duplex DNA via triple-helix formation by oligonucleotides covalently linked to a phenanthroline-copper chelate. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9702–9706. doi: 10.1073/pnas.86.24.9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Chassignol M., Thuong N. T., Helene C. Sequence-targeted cleavage of single- and double-stranded DNA by oligothymidylates covalently linked to 1,10-phenanthroline. J Biol Chem. 1989 Apr 5;264(10):5891–5898. [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Chassignol M., Thuong N. T., Sun J. S., Hélène C. Periodic cleavage of poly(dA) by oligothymidylates covalently linked to the 1,10-phenanthroline-copper complex. Biochemistry. 1988 Apr 5;27(7):2272–2276. doi: 10.1021/bi00407a004. [DOI] [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Hélène C. Sequence-specific recognition of the major groove of DNA by oligodeoxynucleotides via triple helix formation. Footprinting studies. Nucleic Acids Res. 1988 Dec 23;16(24):11431–11440. doi: 10.1093/nar/16.24.11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay E. J., Scofield R. E., Baxter C. S. Steric effects on the intercalation of aromatic cations to deoxyribonucleic acid. J Am Chem Soc. 1973 Nov 14;95(23):7850–7857. doi: 10.1021/ja00804a047. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. S., Gariépy J., Schoolnik G., Kornberg R. D. Synthetic peptides as nuclear localization signals. Nature. 1986 Aug 14;322(6080):641–644. doi: 10.1038/322641a0. [DOI] [PubMed] [Google Scholar]
- Guo Q., Lu M., Seeman N. C., Kallenbach N. R. Drug binding by branched DNA molecules: analysis by chemical footprinting of intercalation into an immobile junction. Biochemistry. 1990 Jan 16;29(2):570–578. doi: 10.1021/bi00454a034. [DOI] [PubMed] [Google Scholar]
- Guo Q., Seeman N. C., Kallenbach N. R. Site-specific interaction of intercalating drugs with a branched DNA molecule. Biochemistry. 1989 Mar 21;28(6):2355–2359. doi: 10.1021/bi00432a001. [DOI] [PubMed] [Google Scholar]
- Howe-Grant M., Lippard S. J. Binding of platinum(II) intercalation reagents to deoxyribnonucleic acid. Dependence on base-pair composition, nature of the intercalator, and ionic strength. Biochemistry. 1979 Dec 25;18(26):5762–5769. doi: 10.1021/bi00593a003. [DOI] [PubMed] [Google Scholar]
- Jessee B., Gargiulo G., Razvi F., Worcel A. Analogous cleavage of DNA by micrococcal nuclease and a 1-10-phenanthroline-cuprous complex. Nucleic Acids Res. 1982 Oct 11;10(19):5823–5834. doi: 10.1093/nar/10.19.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazakov S. A., Astashkina T. G., Mamaev S. V., Vlassov V. V. Site-specific cleavage of single-stranded DNAs at unique sites by a copper-dependent redox reaction. Nature. 1988 Sep 8;335(6186):186–188. doi: 10.1038/335186a0. [DOI] [PubMed] [Google Scholar]
- Kuwabara M. D., Sigman D. S. Footprinting DNA-protein complexes in situ following gel retardation assays using 1,10-phenanthroline-copper ion: Escherichia coli RNA polymerase-lac promoter complexes. Biochemistry. 1987 Nov 17;26(23):7234–7238. doi: 10.1021/bi00397a006. [DOI] [PubMed] [Google Scholar]
- Kuwabara M., Yoon C., Goyne T., Thederahn T., Sigman D. S. Nuclease activity of 1,10-phenanthroline-copper ion: reaction with CGCGAATTCGCG and its complexes with netropsin and EcoRI. Biochemistry. 1986 Nov 18;25(23):7401–7408. doi: 10.1021/bi00371a023. [DOI] [PubMed] [Google Scholar]
- Marshall L. E., Graham D. R., Reich K. A., Sigman D. S. Cleavage of deoxyribonucleic acid by the 1,10-phenanthroline-cuprous complex. Hydrogen peroxide requirement and primary and secondary structure specificity. Biochemistry. 1981 Jan 20;20(2):244–250. doi: 10.1021/bi00505a003. [DOI] [PubMed] [Google Scholar]
- Pope L. E., Sigman D. S. Secondary structure specificity of the nuclease activity of the 1,10-phenanthroline-copper complex. Proc Natl Acad Sci U S A. 1984 Jan;81(1):3–7. doi: 10.1073/pnas.81.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope L. M., Reich K. A., Graham D. R., Sigman D. S. Products of DNA cleavage by the 1,10-phenanthroline-copper complex. Inhibitors of Escherichia coli DNA polymerase I. J Biol Chem. 1982 Oct 25;257(20):12121–12128. [PubMed] [Google Scholar]
- Sigman D. S. Chemical nucleases. Biochemistry. 1990 Oct 2;29(39):9097–9105. doi: 10.1021/bi00491a001. [DOI] [PubMed] [Google Scholar]
- Sigman D. S., Chen C. H. Chemical nucleases: new reagents in molecular biology. Annu Rev Biochem. 1990;59:207–236. doi: 10.1146/annurev.bi.59.070190.001231. [DOI] [PubMed] [Google Scholar]
- Sigman D. S., Graham D. R., D'Aurora V., Stern A. M. Oxygen-dependent cleavage of DNA by the 1,10-phenanthroline . cuprous complex. Inhibition of Escherichia coli DNA polymerase I. J Biol Chem. 1979 Dec 25;254(24):12269–12272. [PubMed] [Google Scholar]
- Sigman D. S., Spassky A., Rimsky S., Buc H. Conformational analysis of lac promoters using the nuclease activity of 1,10-phenanthroline-copper ion. Biopolymers. 1985 Jan;24(1):183–197. doi: 10.1002/bip.360240115. [DOI] [PubMed] [Google Scholar]
- Spassky A., Sigman D. S. Nuclease activity of 1,10-phenanthroline-copper ion. Conformational analysis and footprinting of the lac operon. Biochemistry. 1985 Dec 31;24(27):8050–8056. doi: 10.1021/bi00348a032. [DOI] [PubMed] [Google Scholar]
- Stockert J. C. An intercalative and minor groove binding model for the DNA cleavage reagent, copper(I) (1,10-phenanthroline)2. J Theor Biol. 1989 Mar 7;137(1):107–111. doi: 10.1016/s0022-5193(89)80152-3. [DOI] [PubMed] [Google Scholar]
- Sun J. S., François J. C., Lavery R., Saison-Behmoaras T., Montenay-Garestier T., Thuong N. T., Hélène C. Sequence-targeted cleavage of nucleic acids by oligo-alpha-thymidylate-phenanthroline conjugates: parallel and antiparallel double helices are formed with DNA and RNA, respectively. Biochemistry. 1988 Aug 9;27(16):6039–6045. doi: 10.1021/bi00416a032. [DOI] [PubMed] [Google Scholar]
- Veal J. M., Rill R. L. Noncovalent DNA binding of bis(1,10-phenanthroline)copper(I) and related compounds. Biochemistry. 1991 Jan 29;30(4):1132–1140. doi: 10.1021/bi00218a035. [DOI] [PubMed] [Google Scholar]
- Veal J. M., Rill R. L. Sequence specificity of DNA cleavage by bis(1,10-phenanthroline)copper(I). Biochemistry. 1988 Mar 22;27(6):1822–1827. doi: 10.1021/bi00406a004. [DOI] [PubMed] [Google Scholar]
- Veal J. M., Rill R. L. Sequence specificity of DNA cleavage by bis(1,10-phenanthroline)copper(I): effects of single base pair transitions on the cleavage of preferred pyrimidine-purine-pyrimidine triplets. Biochemistry. 1989 Apr 18;28(8):3243–3250. doi: 10.1021/bi00434a019. [DOI] [PubMed] [Google Scholar]
- Williams L. D., Thivierge J., Goldberg I. H. Specific binding of o-phenanthroline at a DNA structural lesion. Nucleic Acids Res. 1988 Dec 23;16(24):11607–11615. doi: 10.1093/nar/16.24.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C., Kuwabara M. D., Law R., Wall R., Sigman D. S. Sequence-dependent variability of DNA structure. Influence of flanking sequences and fragment length on digestion by conformationally sensitive nucleases. J Biol Chem. 1988 Jun 15;263(17):8458–8463. [PubMed] [Google Scholar]
- Yoon C., Kuwabara M. D., Spassky A., Sigman D. S. Sequence specificity of the deoxyribonuclease activity of 1,10-phenanthroline-copper ion. Biochemistry. 1990 Feb 27;29(8):2116–2121. doi: 10.1021/bi00460a022. [DOI] [PubMed] [Google Scholar]