Abstract
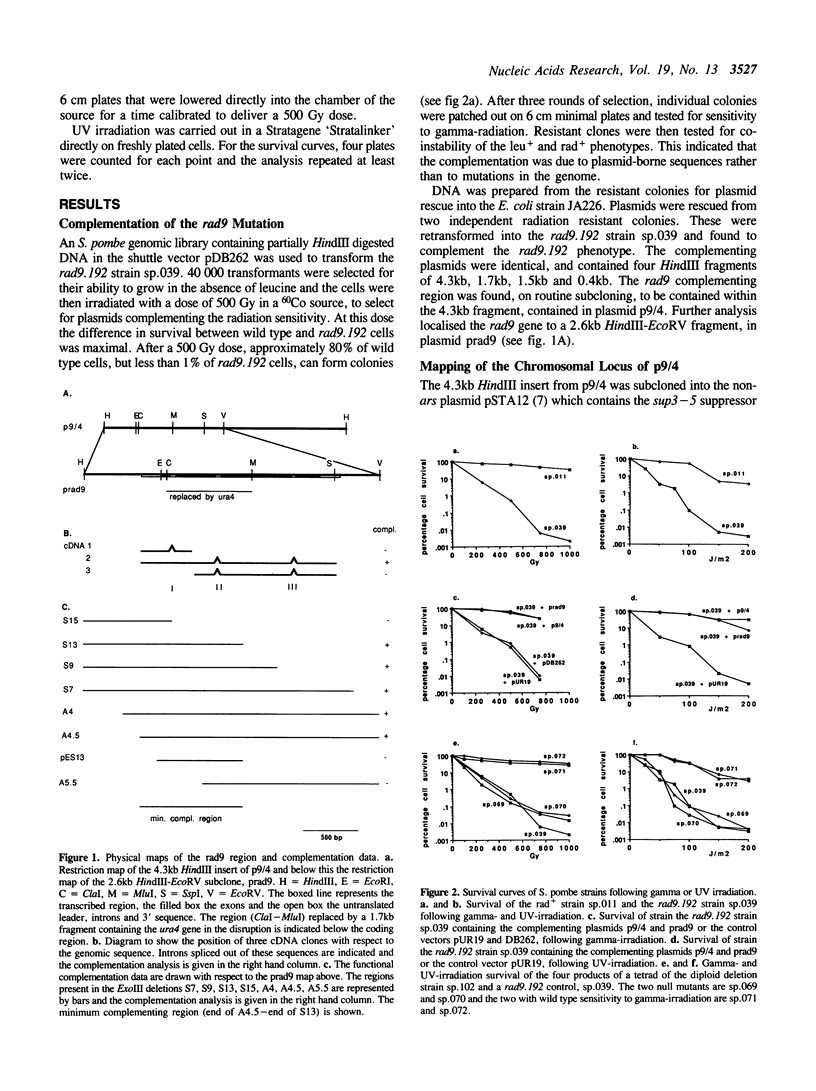
The rad9.192 DNA repair mutant from the fission yeast, Schizosaccharomyces pombe, is sensitive to both UV and ionising radiation. The rad9 gene has been cloned by complementation of the gamma-ray sensitivity of the mutant cell line. A 4.3 kb HindIII fragment was found to confer resistance to both types of radiation. The region of complementation was further localised to a 2.6 kb HindIII-EcoRV fragment, which, by DNA sequence analysis, was found to contain sequences capable of coding for a 427 amino acid protein, if three introns were postulated to remove stop codons. The introns were confirmed by sequence analysis of cDNA clones and PCR products derived from cDNA. The product of transcription is a 1.6 kb mRNA of low abundance. The putative rad9 protein shows no homology to any published sequence. A truncated protein is capable of complementing the radiation sensitivity of the rad9.192 mutant. Deletion of the gene is not lethal and the null allele has a similar phenotype to the rad9.192 mutant.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aves S. J., Durkacz B. W., Carr A., Nurse P. Cloning, sequencing and transcriptional control of the Schizosaccharomyces pombe cdc10 'start' gene. EMBO J. 1985 Feb;4(2):457–463. doi: 10.1002/j.1460-2075.1985.tb03651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battey J., Moulding C., Taub R., Murphy W., Stewart T., Potter H., Lenoir G., Leder P. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983 Oct;34(3):779–787. doi: 10.1016/0092-8674(83)90534-2. [DOI] [PubMed] [Google Scholar]
- Beach D., Piper M., Nurse P. Construction of a Schizosaccharomyces pombe gene bank in a yeast bacterial shuttle vector and its use to isolate genes by complementation. Mol Gen Genet. 1982;187(2):326–329. doi: 10.1007/BF00331138. [DOI] [PubMed] [Google Scholar]
- Durkacz B., Carr A., Nurse P. Transcription of the cdc2 cell cycle control gene of the fission yeast Schizosaccharomyces pombe. EMBO J. 1986 Feb;5(2):369–373. doi: 10.1002/j.1460-2075.1986.tb04221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J. B., Chikashige Y., Smith C. L., Niwa O., Yanagida M., Cantor C. R. Construction of a Not I restriction map of the fission yeast Schizosaccharomyces pombe genome. Nucleic Acids Res. 1989 Apr 11;17(7):2801–2818. doi: 10.1093/nar/17.7.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikes J. D., Becker D. M., Winston F., Guarente L. Striking conservation of TFIID in Schizosaccharomyces pombe and Saccharomyces cerevisiae. Nature. 1990 Jul 19;346(6281):291–294. doi: 10.1038/346291a0. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C. Deoxyribonucleic acid repair in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1988 Mar;52(1):70–102. doi: 10.1128/mr.52.1.70-102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatermann K. B., Hoffmann A., Rosenberg G. H., Käufer N. F. Introduction of functional artificial introns into the naturally intronless ura4 gene of Schizosaccharomyces pombe. Mol Cell Biol. 1989 Apr;9(4):1526–1535. doi: 10.1128/mcb.9.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C., Kohli J., Murray J., Maundrell K. Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement using the ura4 gene as a selectable marker. Mol Gen Genet. 1988 Dec;215(1):81–86. doi: 10.1007/BF00331307. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hindley J., Phear G. A. Sequence of the cell division gene CDC2 from Schizosaccharomyces pombe; patterns of splicing and homology to protein kinases. Gene. 1984 Nov;31(1-3):129–134. doi: 10.1016/0378-1119(84)90203-8. [DOI] [PubMed] [Google Scholar]
- Käufer N. F., Simanis V., Nurse P. Fission yeast Schizosaccharomyces pombe correctly excises a mammalian RNA transcript intervening sequence. Nature. 1985 Nov 7;318(6041):78–80. doi: 10.1038/318078a0. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Lieberman H. B., Riley R., Martel M. Isolation and initial characterization of a Schizosaccharomyces pombe mutant exhibiting temperature-dependent radiation sensitivity due to a mutation in a previously unidentified rad locus. Mol Gen Genet. 1989 Sep;218(3):554–558. doi: 10.1007/BF00332423. [DOI] [PubMed] [Google Scholar]
- Loprieno N., Schüpbach M. On the effect of caffeine on mutation and recombination in Schizosaccharomyces pombe. Mol Gen Genet. 1971;110(4):348–354. doi: 10.1007/BF00438276. [DOI] [PubMed] [Google Scholar]
- Murray J. M., Watts F. Z. Isolation of a Schizosaccharomyces pombe homologue to the rat ribosomal protein, L7. Nucleic Acids Res. 1990 Aug 11;18(15):4590–4590. doi: 10.1093/nar/18.15.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps J., Nasim A., Miller D. R. Recovery, repair, and mutagenesis in Schizosaccharomyces pombe. Adv Genet. 1985;23:1–72. doi: 10.1016/s0065-2660(08)60511-8. [DOI] [PubMed] [Google Scholar]
- Reynolds P., Koken M. H., Hoeijmakers J. H., Prakash S., Prakash L. The rhp6+ gene of Schizosaccharomyces pombe: a structural and functional homolog of the RAD6 gene from the distantly related yeast Saccharomyces cerevisiae. EMBO J. 1990 May;9(5):1423–1430. doi: 10.1002/j.1460-2075.1990.tb08258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P. R., Hall B. D. The primary structure of the alcohol dehydrogenase gene from the fission yeast Schizosaccharomyces pombe. J Biol Chem. 1983 Jan 10;258(1):143–149. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sunnerhagen P., Seaton B. L., Nasim A., Subramani S. Cloning and analysis of a gene involved in DNA repair and recombination, the rad1 gene of Schizosaccharomyces pombe. Mol Cell Biol. 1990 Jul;10(7):3750–3760. doi: 10.1128/mcb.10.7.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. L., Maher V. M., McCormick J. J. Amplification and direct nucleotide sequencing of cDNA from the lysate of low numbers of diploid human cells. Gene. 1989 Nov 30;83(2):347–354. doi: 10.1016/0378-1119(89)90121-2. [DOI] [PubMed] [Google Scholar]