Abstract
Enucleation, the final step in terminal differentiation of mammalian red blood cells, is an essential process in which the nucleus surrounded by the plasma membrane is budded off from the erythroblast to form a reticulocyte. Most molecular events in enucleation remain unclear. Here we show that enucleation requires establishment of cell polarization that is regulated by the microtubule-dependent local activation of phosphoinositide 3-kinase (PI3K). When the nucleus becomes displaced to one side of the cell, actin becomes restricted to the other side, where dynamic cytoplasmic contractions generate pressure that pushes the viscoelastic nucleus through a narrow constriction in the cell surface, forming a bud. The PI3K products PtdIns(3,4)P2 and PtdIns(3,4,5)P3 are highly localized at the cytoplasmic side of the plasma membrane. PI3K inhibition caused impaired cell polarization, leading to a severe delay in enucleation. Depolymerization of microtubules reduced PI3K activity, resulting in impaired cell polarization and enucleation. We propose that enucleation is regulated by microtubules and PI3K signaling in a manner mechanistically similar to directed cell locomotion.
Key words: Erythroblast enucleation, PI3-kinase, Microtubules, Cell polarization
Introduction
In the last step of erythropoiesis, mammalian erythroblasts undergo enucleation, a process that is crucial for the formation of mature functional red blood cells. During enucleation, the erythroblast extrudes its nucleus tightly apposed to the plasma membrane, forming a reticulocyte (Ihle and Gilliland, 2007; Koury et al., 2002; Richmond et al., 2005). Pioneering studies using electron microscopy revealed that at the earliest stage of enucleation the erythroblast nucleus becomes located close to the cell membrane away from the center of the cell (Simpson and Kling, 1967; Skutelsky and Danon, 1967) and that a cytokinetic-like furrow is formed in the region between the extruded nucleus and the incipient reticulocyte (Koury et al., 1989; Skutelsky and Danon, 1967). Actin filaments (F-actin) accumulate in the cytokinetic-like furrow (Ji et al., 2008; Koury et al., 1989) and disruption of F-actin (Ji et al., 2008; Koury et al., 1989; Yoshida et al., 2005) or depletion of mDia2, a regulator of actin polymerization (Ji et al., 2008), blocked enucleation, suggesting that actin-based forces drive nuclear extrusion. However, many questions remain unanswered concerning the process of erythroblast enucleation. In particular, little is known how an asymmetry is established within the erythroblast (i.e. how the nucleus becomes localized to one side of the cell and the cytoplasm to the other), although this polarized state appears to be important for enucleation. Moreover, the detailed organization of actin and microtubules in polarized erythroblasts is unknown.
Phosphoinositide 3-kinase (PI3K) is well known as a central regulator of chemotaxis. In migrating Dictyostelium discoideum, neutrophils and fibroblasts, the PI3K products PtdIns(3,4)P3 and PtdIns(3,4,5)P3 accumulate locally at the leading edge of the surface membrane and control cell polarization (Haugh et al., 2000; Parent et al., 1998; Servant et al., 2000). Although involvement of PI3K in the early stages of Epo (erythropoietin)-regulated differentiation of erythroid progenitors has been established (Ghaffari et al., 2006; Zhao et al., 2006), little is known about its role in the much later steps of enucleation.
We investigated how erythroblasts establish cell polarization and whether this polarization plays a role in expelling the nucleus from the cell. We used a powerful combination of an in vitro cell culture system that mimics normal terminal erythroid proliferation, differentiation and enucleation (Ji et al., 2008), combined with several microscopic imaging techniques. Our results show that proper enucleation requires establishment and maintenance of cell polarization mediated by PI3K in a manner similar to that seen in migrating cells.
Results
Erythroblast enucleation is initiated through establishment of cell polarization, followed by dynamic cytoplasmic contractions
We first wanted to determine when the terminal erythroblast becomes polarized and how the nucleus is extruded from the erythroblast. To this end, we conducted a detailed microscopic analysis of the enucleation process using an in vitro cell culture system employing mouse fetal liver erythroblasts. Enucleation begins ~35 hours after stimulation of erythroid progenitors (Ji et al., 2008). This system employs normal primary erythroid cells and thus does not have the obvious abnormalities associated with virus-infected and/or transformed cell lines. Moreover, the time-course of erythroid differentiation in this system has been well established (Ji et al., 2008), providing us in a time-dependent manner with erythroblasts at different stages of differentiation.
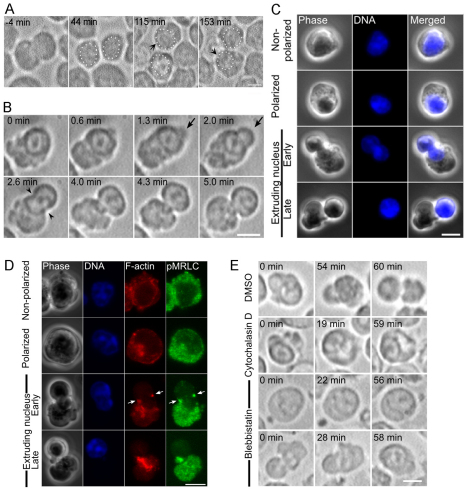
We could follow cultured erythroblast cells after they underwent a final mitotic division (Fig. 1A, −4 minutes) and generated two late erythroblasts in which the nuclei were located at the center of each cell (Fig. 1A, 44 minutes). At ~3 hours after each was generated (210±8.64 minutes, n=8), these late erythroblasts underwent significant polarization, displacing the nucleus to one side of the cell (Fig. 1A, 115 and 153 minutes, arrows). Around 1 hour after cell polarization, enucleation initiated (74.7±8.64 minutes, n=8) and dynamic and random contractions occurred at the side of the cytoplasm opposite to the nucleus (n=16; Fig. 1B and supplementary material Movie 1). Strikingly, a part of the nucleus suddenly protruded from the cell, forming a bleb-like structure from a limited area of the cortex adjacent to the nucleus (3.35±0.64 μm diameter; Fig. 1B, arrows). Then the whole nucleus was quickly squeezed out (5.98±2.73 minutes), in the process undergoing extensive deformations into an hourglass-like shape (supplementary material Movie 1). Fixed late erythroblasts at different stages of enucleation (Fig. 1C) were morphologically similar to live cells (Fig. 1B).
Fig. 1.
Enucleation is initiated through establishment of cell polarization, followed by contractions of asymmetrically localized cytoplasmic actomysoin. (A) Time-lapse images of late erythroblasts undergoing cell polarization. White dots depict the nucleus. Time elapsed in minutes after completion of the final mitotic division of the erythroid progenitor cell is shown. (B) Time-lapse images of late erythroblasts undergoing enucleation. (C) Late erythroblasts were fixed and stained for DNA (blue). The populations of late erythroblasts were classified into non-polarized cells, polarized cells that show nuclear displacement, early phase of nuclear extrusion and late phase of nuclear extrusion. (D) Immunofluorescence of late erythroblasts at different stages of enucleation. Single confocal images of F-actin (red), active myosin II (pMRLC; green) and DNA (blue) are shown. Note that both F-actin and active myosin II become mostly restricted to the opposite side of the cytoplasm after cells become polarized. A small fraction of both F-actin and active myosin (arrows) is found accumulated in the neck region of the bleb-like protrusion. (E) DMSO, cytochalasin D or blebbistatin was applied to cells at 36 hours and then polarized late erythroblasts were identified for time-lapse recording. Scale bars: 5 μm.
Before late erythroblasts became polarized, F-actin formed small particles and patch-like structures distributed throughout the cytoplasm (Fig. 1D, Non-polarized). After cell polarization, F-actin was mostly restricted to the side of the cytoplasm away from the nucleus, and little was localized at the side with the nucleus (Fig. 1D, Polarized). While the nucleus was being expelled, a fraction of F-actin concentrated at the neck region of the bleb (Fig. 1D, Extruding nucleus, F-actin, arrowheads) where it was previously described as the cytokinetic-like furrow or contractile actin ring (Ji et al., 2008; Koury et al., 1989; Skutelsky and Danon, 1967).
Similar to F-actin, after late erythroblasts became polarized small particles of active myosin II (myosin II regulatory light chain phosphorylated at Ser19; pMRLC) (Matsumura et al., 1998) became restricted to the side of the cytoplasm away from the nucleus (Fig. 1D, pMRLC). A fraction of active myosin II also became localized to the neck region of the bleb (Fig. 1D, pMRLC, arrows).
Although previous studies have shown that F-actin is required for enucleation (Ji et al., 2008; Koury et al., 1989; Repasky and Eckert, 1981; Yoshida et al., 2005), it remained unknown whether enucleation is powered by contractions of asymmetrically localized actomyosin. To this end, we disrupted F-actin or inhibited myosin II function specifically in polarized late erythroblasts. We applied specific inhibitors of actin or myosin II to cells at 36 hours of culture, and polarized cells were then identified for time-lapse recording. In polarized cells treated with cytochalasin D, an agent that disrupts F-actin, dynamic contractions were quickly and completely suppressed and enucleation was blocked (Fig. 1E, Cytochalasin D).
Similarly, treatment of polarized cells with blebbistatin, an inhibitor of myosin II activity (Straight et al., 2003), at a concentration (100 μM) that completely inhibits cytokinesis (Mukhina et al., 2007), caused the suppression of cytoplasmic contractions and a failure of enucleation (Fig. 1E, Blebbistatin, top). Strikingly, even in cells already extruding the nucleus, following blebbistatin treatment the nucleus regressed into the cell (Fig. 1E, Blebbistatin, bottom). These results suggest that nuclear extrusion is achieved by pressure generated by contractions of asymmetrically localized actomyosin and thus that the establishment of cell polarization is crucial for enucleation. Our observations also indicate that completion of enucleation probably involves actomyosin-driven constriction of the cortical actin ring at the neck of the bleb-like protrusion.
PI3K activity is required for proper enucleation by regulating the establishment and maintenance of cell polarization
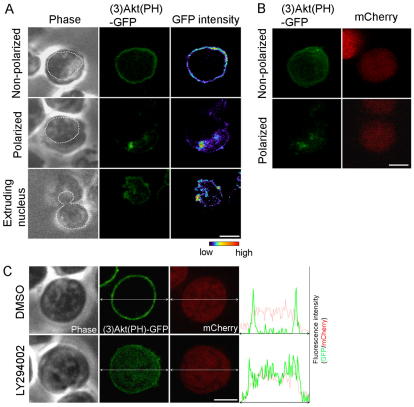
How is cell polarization established and maintained during enucleation? We speculated that PI3K, which regulates cell polarization in migrating cells, might be involved in this process. To test this hypothesis, we first examined the localization of the PI3K products PtdIns(3,4)P2 and PtdIns(3,4,5)P3 by expressing the reporter (3)Akt(PH)–GFP (the trimerized PH domain of Akt tagged with green fluorescent protein) in late erythroblasts (Fig. 2A) (Luo et al., 2005).
Fig. 2.
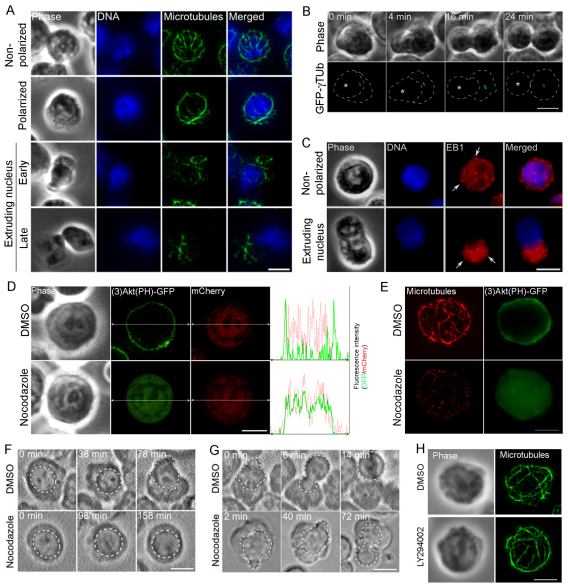
(3)Akt(PH)–GFP is asymmetrically localized in polarized late erythroblasts. (A) Two-dimensional and (B) three-dimensional images of (3)Akt(PH)–GFP in late erythroblasts that are non-polarized, polarized and extruding its nucleus are shown. White dots depict the nucleus. The color bar indicates the intensity of GFP signal from the lowest (black) to highest (red). (C) Localization of (3)Akt(PH)–GFP in cells treated with DMSO or LY294002. Each graph shows the distribution of the GFP (green) and mCherry (red) fluorescence intensity (y axis) in the corresponding distance (x axis; double-headed arrows). Scale bars: 5 μm.
In non-polarized cells, (3)Akt(PH)–GFP was localized throughout the entire plasma membrane (n=11; Fig. 2A, Non-polarized). Strikingly, when cells became polarized, (3)Akt(PH)–GFP became restricted in localization to the segment of plasma membrane facing the cytoplasm (n=8; Fig. 2A, Polarized); this localization pattern remained unchanged during nuclear extrusion (n=16; Fig. 2A, Extruding nucleus). Three-dimensional images of (3)Akt(PH)–GFP and mCherry, used as a cytoplasmic volume marker, showed that the asymmetric distribution of (3)Akt(PH)–GFP in polarized cells was not caused by volume effects in the cytoplasm because mCherry was uniformly distributed throughout the cell (Fig. 2B). In late erythroblasts, (3)Akt(PHR25C)–GFP, a mutant that is unable to bind to PtdIns(3,4)P2 or PtdIns(3,4,5)P3 (Franke et al., 1997), failed to localize to the plasma membrane and was diffusely distributed in the cytoplasm, indicating that (3)Akt(PH)–GFP is a specific reporter for PI3K activity (supplementary material Fig. S1).
Next, we wanted to determine whether PI3K activity is required for cell polarization during enucleation. Because PI3K activity is involved in the early steps of Epo-triggered cell division and differentiation of erythroid progenitor cells (Ghaffari et al., 2006; Zhao et al., 2006), we needed to use a specific inhibitor of PI3K to block its activity only in late erythroblasts – more specifically during enucleation. To this end, we applied LY294002, a well-established specific inhibitor of PI3K, to late erythroblasts expressing (3)Akt(PH)–GFP. Strikingly, after ~1 hour of incubation the membrane association of (3)Akt(PH)–GFP became strongly diminished in 24 cells out of 28 (24/28) treated with LY294002 but not in the DMSO-treated control cells (0/17) (Fig. 2C), indicating that LY294002 effectively inhibits PI3K activity in late erythroblasts.
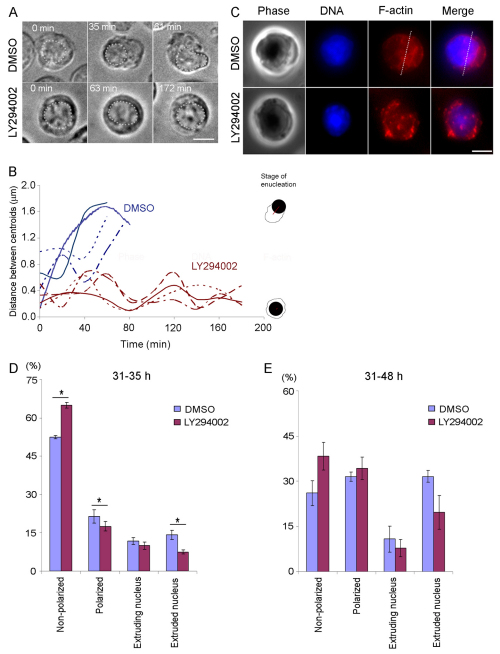
To determine whether PI3K inhibition affects the establishment of cell polarization, we applied LY294002 to cells at 31 hours in culture, where ~80% of late erythroblasts were not yet polarized (see supplementary material Fig. S2). After 1 hour of incubation, non-polarized cells were identified and monitored by time-lapse recording. The majority of control non-polarized cells underwent cell polarization within 3 hours (80%, n=40), whereas 75% of the non-polarized cells treated with LY294002 failed to become polarized during this time period (n=36; Fig. 3A and Fig. 4). The distance between the cellular and nuclear centroids in polarized control cells was 1.59±0.14 μm, whereas the maximum distance between these centroids was 0.62±0.09 μm in LY294002-treated cells that were not able to undergo cell polarization (Fig. 3B), suggesting that the nucleus remained localized in the cell center in LY294002-treated cells. F-actin was found localized throughout the cytoplasm in the LY294002-treated cells that remained non-polarized (n=23; Fig. 3C).
Fig. 3.
PI3K activity is required for establishment of cell polarization during enucleation. (A) Time-lapse images of non-polarized cells treated with DMSO or LY294002. White dots depict the nucleus. (B) Quantification of data obtained with cells similar to those shown in A. The distance between the nuclear and cellular centroids in individual cells treated with DMSO (blue) or LY294002 (red) over time was plotted. Four representative cells from each treatment are shown. (C) Z-projections of F-actin in late erythroblasts treated with DMSO or LY294002. (D,E) Cells treated with DMSO or LY294002 from 31 to 35 hours (D) or from 31 to 48 hours in culture (E) were fixed and stained for F-actin and DNA. We considered an enucleated nucleus as an enucleated cell. More than 100 cells were counted in each experiment. Three independent experiments for each treatment were performed; values are means ± s.e.m. (*P<0.05). Scale bars: 5 μm.
Fig. 4.
Summary of the defects in nuclear displacement and extrusion in cells treated with LY294002 or nocodazole. Live cells were treated with 50 μM LY294002 or 10 μM nocodazole in culture and the percentage of cells showing defects in enucleation was calculated. Control cells were treated with DMSO. Figures in parentheses indicate the number of cells showing the phenotype out of the total number of cells examined.
LY294002- and DMSO-treated (control) cells were also fixed in order to perform quantitative analysis on the progression of enucleation by counting the number of late erythroblasts at each stage of enucleation per total number of late erythroblasts and enucleated cells. We observed only a slight (although statistically significant) decrease in the percentage of polarized cells as well as enucleated cells in cells treated with LY294002 from 31 to 35 hours in culture, compared with control cells (polarized cells, 17.56±1.84% vs 24.48±2.67%; enucleated cells, 7.54±0.68% vs 14.24±1.80%; P<0.05, Fig. 3D). Similar results were obtained when late erythroblasts were treated with another PI3K inhibitor, wortmannin (supplementary material Fig. S3), suggesting that the defects in enucleation caused by LY294002 were indeed due to inhibition of PI3K activity and that PI3K inhibition did not completely block cell polarization. When cells were treated with LY294002 from 31 to 48 hours in culture, the decrease in polarization and enucleation was no longer statistically significant (Fig. 3E). These results suggest that PI3K inhibition caused a severe delay rather than arrest in the establishment of cell polarization.
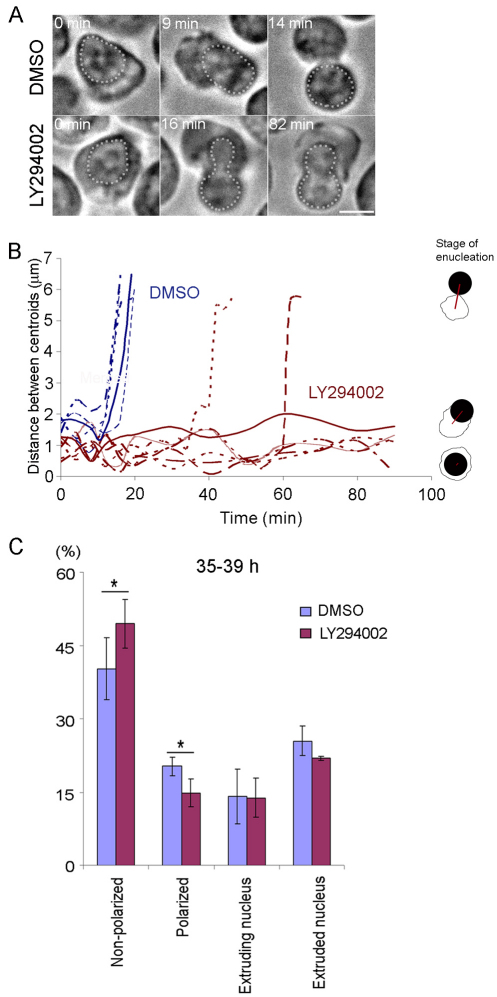
To determine whether PI3K inhibition affects nuclear extrusion, LY294002 was applied to cells at 35 hours in culture and, after 1 hour of incubation, polarized cells were identified and monitored by time-lapse video microscopy. Strikingly, 76% of polarized cells treated with LY294002 exhibited a wide range of delay and/or arrest in nuclear extrusion (35/46), whereas in all control cells enucleation was completed within 25 minutes (46/46; Fig. 5A). Some cells completed nuclear extrusion after a delay (11/46), whereas others moved out of the field of view before the extrusion was completed or did not complete nuclear extrusion during time-lapse imaging (24/46, Fig. 4 and supplementary material Fig. S4).
Fig. 5.
PI3K activity is required for maintenance of cell polarization during enucleation. (A) Time-lapse images of polarized cells treated with DMSO or LY294002. Time 0 indicates the onset of nuclear extrusion. White dots depict the nucleus. (B) Quantification of data obtained with cells similar to those shown in A. The distance between the nuclear and cellular centroids in individual cells treated with DMSO (blue) or LY294002 (red) over time after the onset of nuclear extrusion (time 0) was plotted. Representative cells for each treatment (five for DMSO and seven for LY294002) are shown. (C) Cells treated with DMSO or LY294002 from 35 to 39 hours in culture were fixed and stained for F-actin and DNA. Three independent experiments for each treatment were performed; values are means ± s.e.m. (*P<0.05). Scale bar: 5 μm.
Cells that showed a delay in nuclear extrusion typically exhibited one or both of the following phenotypes: (a) Nuclear extrusion was initiated but the nucleus regressed into the cell before the extrusion was completed; some cells repeated this ‘trial and error’ of nuclear extrusion several times. (b) Nuclear extrusion was initiated but failed to complete, with formation of an hourglass-shaped nucleus (Fig. 5A, supplementary material Movies 2 and 3).
In control cells, nuclear extrusion was completed, with a distance between the cellular and nuclear centroids of 6.11±0.40 μm (Fig. 5B, DMSO), whereas in LY294002-treated cells that showed a delay in nuclear extrusion the distance between these centroids was 1.59±0.32 μm and remained relatively unchanged until extrusion was completed (Fig. 5B, LY294002). Quantitative analysis revealed no significant decrease in the percentage of enucleated cells among cells treated with LY294002 from 35 to 39 hours in culture compared with control cells (Fig. 5C), suggesting that PI3K inhibition caused a delay rather than a complete arrest in nuclear extrusion. These results suggest that PI3K activity is important to maintain cell polarization. Taken together, our observations suggest that PI3K activity is required for proper enucleation by regulating the establishment and maintenance of cell polarization.
Microtubule-dependent local activation of PI3K is required for proper cell polarization during enucleation
Next, we wanted to determine how PI3K activity is regulated during enucleation. In both migrating neutrophils (Xu et al., 2005) and macrophages during phagocytosis (Khandani et al., 2007), localized PI3K activity is dependent on microtubules. Thus, we speculated that microtubules might regulate PI3K activity during enucleation. Before testing this hypothesis, it was important to analyze the microtubule organization during enucleation because this had not been determined, especially in polarized late erythroblasts.
Immunofluorescence showed that, as previously described, before cells became polarized microtubule arrays were radially symmetric, forming a cage-like structure surrounding the nucleus (Fig. 6A) (Koury et al., 1989). Concomitant with cell polarization, microtubules formed an asymmetric array oriented toward the cortex on the side of the cytoplasm opposite to the nucleus (Fig. 6A, Polarized and Extruding nucleus). We also found that, during enucleation, one or two foci of GFP–γ-tubulin were localized together in the cytoplasm in close proximity to the nucleus at the side of the cytoplasm (n=6; Fig. 6B).
Fig. 6.
Enucleation requires microtubule-dependent local activation of PI3K. (A) Microtubule organization in cells prior to and during enucleation. Z-projections of microtubules (green) and DNA (blue) are shown. (B) Time-lapse fluorescence images of cells expressing GFP–γ tubulin (GFP-γTub) during enucleation. Asterisks indicate the position of the nucleus. Dashed lines outline the nucleus and the cell edge. (C) Z-projections of EB1 (red) and DNA (blue) are shown. Typical comet-like structures of EB1 are detected (arrows). (D) Localization of (3)Akt(PH)–GFP in live cells treated with DMSO or nocodazole. Each graph shows the distribution of the GFP (green) and mCherry (red) fluorescence intensity (y axis) in the corresponding distance (x axis; double-headed arrows). (E) Microtubule organization (red) and localization of (3)Akt(PH)–GFP (green) in fixed cells treated with DMSO (top) or nocodazole (bottom). Z-projections of microtubules (red) are shown. (F) Time-lapse images of non-polarized cells treated with DMSO or nocodazole. Dashed lines depict the nucleus. (G) Time-lapse images of polarized cells treated with DMSO or nocodazole. Time 0 indicates the onset of nuclear extrusion. Dashed lines depict the nucleus. (H) Microtubule organization in late erythroblasts treated with DMSO (top) or LY294002 (bottom). Z-projections of microtubules (green) are shown. Scale bars: 5 μm.
Similar localization patterns of endogenous γ-tubulin (n=21) and pericentrin (n=11) were observed in late erythroblasts (supplementary material Fig. S5A,B). Moreover, when late erythroblasts were stained for EB1, a protein that binds to plus-ends of growing microtubules (Mimori-Kiyosue et al., 2000), typical comet-like structures were observed regardless of the stage of enucleation (Fig. 6C, arrows), suggesting that microtubules are dynamic during enucleation. These results suggest that microtubule organization is dramatically rearranged into an asymmetric array with the centrosome when late erythroblasts become polarized.
To determine whether microtubules regulate PI3K activity during enucleation, late erythroblasts expressing (3)Akt (PH)–GFP were treated with nocodazole, a microtubule-depolymerization agent. Strikingly, in nocodazole-treated cells where microtubules were severely disrupted (3)Akt(PH)–GFP failed to associate with the plasma membrane and instead was diffusely localized in the cytoplasm (11/15). By contrast, (3)Akt(PH)–GFP was associated with the membrane in control cells (12/12) (Fig. 6D,E), suggesting that microtubules are important for maintaining PI3K activity.
Nocodazole treatment caused defects in enucleation similar to those seen in cells treated with LY294002. Live-cell analysis showed that when nocodazole was applied to cells at 32 hours in culture only 20% of non-polarized cells were able to undergo polarization within 3 hours (n=20; Fig. 6F, Fig. 4 and supplementary material Fig. S5C). F-actin was localized throughout the cytoplasm in nocodazole-treated cells that remained non-polarized (n=20; supplementary material Fig. S5D). When nocodazole was applied to cells at 36 hours in culture, 63% of polarized cells exhibited a severe delay in nuclear extrusion (n=35; Fig. 6G, Fig. 4, supplementary material Fig. S4, Fig. S5E and Movie 4), with phenotypes similar to those seen in LY294002-treated cells (Fig. 5A).
Quantitative analysis revealed only a slight decrease in the percentage of polarized and enucleated cells among cells treated with nocodazole from 32 to 36 hours in culture, compared with control cells (supplementary material Fig. S5F). It is noteworthy that treatment of erythroblasts with nocodazole from 32 to 48 hours in culture resulted in severe defects in nuclear and cellular morphology (data not shown). The decrease was not significant when cells were treated with nocodazole from 36 to 40 hours in culture (supplementary material Fig. S5G). These results suggest that microtubules are required for proper enucleation by regulating the establishment and maintenance of cell polarization. As detailed below, in large measure this resolves past controversies about whether or not microtubules play an essential role in enucleation (Keerthivasan et al., 2010; Koury et al., 1989; Sonoda et al., 1998). Unlike the crucial role of microtubules in the regulation of PI3K activity, PI3K inhibition caused no drastic defects in microtubule organization in late erythroblasts (Fig. 6H). Taken together, our observations suggest that microtubule-dependent local activation of PI3K is required for proper enucleation.
PI3K regulates cell polarization through promoting the movement of the nucleus but not the MTOC during enucleation
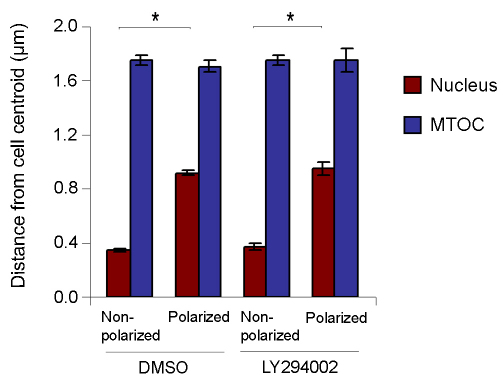
In migrating fibroblasts, reorientation of the microtubule-organizing center (MTOC) is the initial polarization event and occurs by the movement of the nucleus but not the MTOC (Gomes et al., 2005; Schmoranzer et al., 2009). Our live-cell analyses showed that when late erythroblasts became polarized the nucleus moved to one side of the cell (Fig. 3A,B), suggesting that cell polarization might be initiated in a way similar to that seen in migrating cells and that PI3K might regulate this process. To test this, cells treated with LY294002 from 31 to 35 hours in culture were fixed and stained for the nucleus and γ-tubulin (see supplementary material Fig. S6). Then, we measured the distance between the cellular centroid and the MTOC or the nuclear centroid (Fig. 7).
Fig. 7.
PI3K regulates cell polarization through promoting the movement of the nucleus but not the MTOC. Average distance between the centroids of the nucleus (red) or the MTOC (blue) and the cell in late erythroblasts treated with DMSO or LY294002. *P<0.05.
In control cells (Fig. 7, DMSO), the average distance between the MTOC and the cellular centroid in non-polarized cells (1.75±0.06 μm) was similar to that in polarized cells (1.71±0.08 μm), suggesting that the position of the MTOC remains unchanged during cell polarization. By contrast, as observed in live-cell analysis (Fig. 3A,B), the distance between the nuclear and cellular centroids was significantly increased when the cells became polarized (Fig. 7, DMSO). These results indicate that during the early stages of enucleation, MTOC reorientation occurs by the movement of the nucleus but not the MTOC.
In non-polarized cells, LY294002 treatment had no effect on the distances between the MTOC or the nuclear centroid and the cellular centroid (Fig. 7, Non-polarized, LY294002). In the few LY294002-treated cells that managed to become polarized, the distance between the nuclear and the cellular centroids was increased, whereas the position of the MTOC remained unchanged, similar to that seen in control DMSO-treated cells (Fig. 7, Polarized, LY294002). These results suggest that PI3K inhibition causes a delay in nuclear repositioning. Taken together, our observations suggest that PI3K regulates cell polarization through promoting the movement of the nucleus but not the MTOC during enucleation.
Discussion
One of the long-standing questions in erythropoiesis is how the erythroblast extrudes its nucleus to form the reticulocyte that later becomes the mature red blood cell. In particular, little is known about how erythroblasts establish and maintain a polarized state during enucleation. The live-cell analyses we performed in this study have provided a deeper understanding of the mechanisms underlying the regulation of cell polarization and its importance in enucleation.
Early work suggested a mechanism of enucleation similar to that used in cytokinesis of mitotic cells, where local cortical contractions occur between the two daughter cells (Skutelsky and Danon, 1967; Koury et al., 1989). By contrast, our observations demonstrate that nuclear extrusion is driven by contractile forces generated by asymmetrically distributed cytoplasmic actomyosin in late erythroblasts. This finding reiterates the importance of establishment and maintenance of cell polarization during enucleation.
Although previous studies revealed that PI3K was required for early Epo-dependent stages of erythropoiesis (Ghaffari et al., 2006; Zhao et al., 2006), here we demonstrated a second and novel role for PI3K in the establishment and maintenance of cell polarization during enucleation. Moreover, our results also showed that PI3K promotes cell polarization by repositioning of the nucleus during enucleation. In migrating fibroblasts, cell polarization is established by the movement of the nucleus instead of the MTOC (Gomes et al., 2005). Our results indicate that this is also the case in late erythroblasts during enucleation. A possible mechanism that regulates the repositioning of the nucleus by PI3K is discussed below.
The roles of microtubules in enucleation have been controversial (Keerthivasan et al., 2010; Koury et al., 1989; Sonoda et al., 1998). We observed different effects of microtubule disruption agents on enucleation when cells were treated at different stages of erythropoiesis and/or for different time periods of incubation. Imaging and quantitative analysis of live and fixed cells revealed that disruption of microtubules caused a severe delay in enucleation, but did not affect the final extent of enucleation. This indicates that microtubules are required for, but not essential to, enucleation. Currently it is unknown how microtubules regulate PI3K activation during enucleation. Interestingly, biochemical analyses showed that the regulatory subunits of PI3K can bind to tubulin (Inukai et al., 2000; Kapeller et al., 1995), but we do not know whether this occurs in late erythroblasts or whether this affects activation or localization of PI3K.
Interestingly, our results show that cell polarization is initiated through repositioning of the nucleus but not MTOC during enucleation, similar to that seen in migrating fibroblasts (Gomes et al., 2005; Schmoranzer et al., 2009). Nuclear repositioning occurs concomitantly with the rearrangement of the microtubule array that leads to asymmetric distribution of actomyosin and thus the generation of asymmetric force (see below). This force most probably drives the repositioning of the nucleus, somewhat similar to that seen in migrating cells where nuclear movement needs actin flow (Gomes et al., 2005).
In comparison with migrating cells, in late erythroblasts the nucleus moves only a short distance (~1 μm) for the cell to become polarized. Then why do late erythroblasts need a long time (2–3 hours) to establish polarization? This is probably because either rearrangement of the microtubule array or stable association of the nucleus with the plasma membrane, or both, might be a relatively slow event.
Our proposed model for erythroblast enucleation is summarized in Fig. 8. The reorientation from a radial microtubule array to an asymmetric array somehow results in asymmetric activation of PI3K. Asymmetric accumulation of the PI3K products in the plasma membrane then induce asymmetric actin assembly and disassembly by stimulating their effectors, such as α-actinin (Franke et al., 1997), profilin (Lassing and Lindberg, 1985) or gelsolin (Janmey et al., 1987), as well as Rac1 activity (Ji et al., 2008) probably through activation of its upstream regulator (Han et al., 1998; Kunisaki et al., 2006). This asymmetric actin assembly and disassembly and actomyosin contractions promote nuclear displacement as well as nuclear extrusion.
Fig. 8.
Proposed model for PI3K-mediated regulation of erythroblast enucleation. The reorientation from a radial microtubule array to an asymmetric array results in localized activation of PI3K and thereby promotes asymmetric actin assembly. Enucleation is triggered and continued by a pressure generated by contractions of cytoplasmic actomyosin (large arrow) and is probably completed by contractions of concentrated actomyosin at the bleb neck (small arrows).
In cells defective in this microtubule-regulated PI3K-dependent polarization mechanism, enucleation could be achieved by random cytoplasmic contractions alone but in a highly ineffective and slow manner. It is likely that such defects in enucleation cause some proteins to be wrongly sorted to the reticulocytes or the extruded nucleus, leading to the formation of abnormal reticulocytes.
Our results suggest that enucleation is carried out in a way mechanistically similar to directed cell migration. Some migrating cells form a polarized bleb-like protrusion in order to move forward (Charras and Paluch, 2008; Haston and Shields, 1984; Paluch et al., 2006), and the difference between the role of the bleb-like protrusion in cell migration and enucleation is probably influenced by differences in their cytoskeletal organization. The unexpected findings in the present study thus begin to elucidate the regulatory and mechanistic mechanisms underlying mammalian erythroblast enucleation.
Materials and Methods
Cell culture and infection
Pregnant C57 BL/6J mice were purchased from the Genome Institute of Singapore and the Center for Animal Resources (National University of Singapore). The purification and culturing of mouse fetal liver erythroblast precursors (CFU-e; TER119-negative cells) were performed as previously described (Ji et al., 2008; Zhang et al., 2003). For retroviral gene transfer experiments, we used the murine stem cell retroviral vector system (MSCV; Clontech, BD Biosciences). Infection of primary cells with mouse-specific retroviruses encoding fusion proteins of (3)Akt(PH)–GFP (Luo et al., 2005), (3)Akt(PHR25C)–GFP, mCherry, RFP or GFP–γ-tubulin was performed as previously described (Ji et al., 2008). All animal experiments were performed according to the relevant regulatory standards.
Microscopy and image processing
For phase-contrast and bright-field time-lapse live-cell imaging, purified TER119-negative cells grown on fibronectin-coated glass chamber dishes (McKenna and Wang, 1989) were maintained at 37°C in a custom-made incubator built on top of an Axiovert 135 or Axiovert 200M inverted microscope (Carl Zeiss) and viewed with a 100×, Plan Apo NA 1.25 or 100×, NA 1.30, Plan-NEOFLUAR lens. Images were acquired with a video camera (Mintron) or a cooled charge-coupled device camera (CoolSNAPHQ, Roper Scientific) and processed with MetaView (Universal Imaging) or Image-Pro Plus software. Images of cells expressing (3)Akt(PH)–GFP, cells expressing (3)Akt(PH)–GFP and mCherry or RFP, cells expressing (3)Akt(PHR25C)–GFP and cells expressing GFP–γ-tubulin were acquired using an Axiovert 200M inverted microscope (Carl Zeiss) equipped with 100×, NA 1.4 Plan-Apochromat lens, a spinning disk confocal (Yokogawa CSU-21) scan head and Hamamatsu Orca-ER cooled CCD camera. Bright field images (Fig. 1B,D) were uniformly filtered to reduce noise using ImageJ (NIH, Bethesda, MD).
For imaging of F-actin, stacks of images acquired using a LSM 510 Meta confocal microscope system (100×, NA 1.4 Plan-Apochromat lens) were collected at a vertical interval of 0.75 μm and processed with IMARIS (Bitplane, Scientific Solutions).
For imaging of microtubules, Z-stacks of wide-field images were collected at intervals of 0.3 μm, were deconvoluted (for Fig. 3A) using AutoQuant (Media Cybernetics) and processed with a pattern recognition program that detects linear structures as described previously (Wang, 2003). For imaging of EB1 and pericentrin, stacks of wide-field images were collected at vertical intervals of 0.3 μm and projected with a maximum-intensity algorithm using ImageJ. For γ-tubulin, single wide-field images were collected.
Immunofluorescence
Immunofluorescence staining was performed according to the methods previously described (Wheatley and Wang, 1998). We used primary antibodies against rabbit Ser19-phosphorylated myosin light chain II (1:50, Cell Signaling), mouse anti-β-tubulin (1:1000, AbCam), mouse anti-EB1 (1:50, BD Biosciences, Pharmingen), mouse anti-γ-tubulin (1:1000, Sigma); rabbit anti-pericentrin (1:700, Abcam). Secondary antibodies Alexa-Fluor-488-conjugated goat anti-rabbit IgG, Alexa-Fluor-488-conjugated goat anti-mouse IgG and Alexa-Fluor-546-conjugated goat anti-mouse IgG (Molecular Probes), all diluted 1:100. To observe F-actin, Rhodamine–phalloidin (Molecular Probes) was used at 1:300 dilution. Hoechst 33258 (1 μg/ml) was used to locate the nucleus.
Drug treatment
Cytochalasin D (Sigma), blebbistatin (Toronto Research Chemicals), nocodazole (Sigma), LY294002 (Invitrogen) and wortmannin (Sigma) were dissolved in DMSO to make stock solutions of 25 mM, 100 mM, 3.3 mM, 50 mM and 1 mM, respectively, and applied to a culture dish at final concentration of 2 μM, 100 μM, 10 μM, 50 μM and 1 μM, respectively. Note that >10 μM nocodazole was required to completely depolymerize microtubules in late erythroblasts.
Data analysis
To measure the size of the nuclear area, the area of the ellipse corresponding to the shape of the nucleus was calculated using ImageJ. To track the distance between the centroids of the cell and the centroids of the nucleus or the MTOC, each perimeter was drawn and the centroid corresponding to the nucleus and the cell was calculated using ImageJ. The distance between these centroids was calculated.
Supplementary Material
Acknowledgements
We thank TLL Microscopy and Animal Care facilities and Shazmina Rafee for their technical assistance, Koichi Okumura (Cancer Science Institute of Singapore) for helpful suggestions, and G. Wright and J. Brocher for advice on image processing. We thank all members of the Murata-Hori laboratory for discussions and/or critical reading of the manuscript.
Footnotes
Funding
This study was supported by intramural funds from the Temasek Life Sciences Laboratory to M.M.-H. and by the National Institutes of Health [grant number P01 HL 32262] to H.F.L. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.088286/-/DC1
References
- Charras G., Paluch E. (2008). Blebs lead the way: how to migrate without lamellipodia. Nat. Rev. Mol. Cell Biol. 9, 730-736 [DOI] [PubMed] [Google Scholar]
- Franke T. F., Kaplan D. R., Cantley L. C., Toker A. (1997). Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 275, 665-668 [DOI] [PubMed] [Google Scholar]
- Ghaffari S., Kitidis C., Zhao W., Marinkovic D., Fleming M. D., Luo B., Marszalek J., Lodish H. F. (2006). AKT induces erythroid-cell maturation of JAK2-deficient fetal liver progenitor cells and is required for Epo regulation of erythroid-cell differentiation. Blood 107, 1888-1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes E. R., Jani S., Gundersen G. G. (2005). Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell 121, 451-463 [DOI] [PubMed] [Google Scholar]
- Han J., Luby-Phelps K., Das B., Shu X., Xia Y., Mosteller R. D., Krishna U. M., Falck J. R., White M. A., Broek D. (1998). Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science 279, 558-560 [DOI] [PubMed] [Google Scholar]
- Haston W. S., Shields J. M. (1984). Contraction waves in lymphocyte locomotion. J. Cell Sci. 68, 227-241 [DOI] [PubMed] [Google Scholar]
- Haugh J. M., Codazzi F., Teruel M., Meyer T. (2000). Spatial sensing in fibroblasts mediated by 3′ phosphoinositides. J. Cell Biol. 151, 1269-1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Gilliland D. G. (2007). Jak2: normal function and role in hematopoietic disorders. Curr. Opin. Genet. Dev. 17, 8-14 [DOI] [PubMed] [Google Scholar]
- Inukai K., Funaki M., Nawano M., Katagiri H., Ogihara T., Anai M., Onishi Y., Sakoda H., Ono H., Fukushima Y., et al. (2000). The N-terminal 34 residues of the 55 kDa regulatory subunits of phosphoinositide 3-kinase interact with tubulin. Biochem. J. 346, 483-489 [PMC free article] [PubMed] [Google Scholar]
- Janmey P. A., Iida K., Yin H. L., Stossel T. P. (1987). Polyphosphoinositide micelles and polyphosphoinositide-containing vesicles dissociate endogenous gelsolin-actin complexes and promote actin assembly from the fast-growing end of actin filaments blocked by gelsolin. J. Biol. Chem. 262, 12228-12236 [PubMed] [Google Scholar]
- Ji P., Jayapal S. R., Lodish H. F. (2008). Enucleation of cultured mouse fetal erythroblasts requires Rac GTPases and mDia2. Nat. Cell Biol. 10, 314-321 [DOI] [PubMed] [Google Scholar]
- Kapeller R., Toker A., Cantley L. C., Carpenter C. L. (1995). Phosphoinositide 3-kinase binds constitutively to alpha/beta-tubulin and binds to gamma-tubulin in response to insulin. J. Biol. Chem. 270, 25985-25991 [DOI] [PubMed] [Google Scholar]
- Keerthivasan G., Small S., Liu H., Wickrema A., Crispino J. D. (2010). Vesicle trafficking plays a novel role in erythroblast enucleation. Blood 116, 3331-3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandani A., Eng E., Jongstra-Bilen J., Schreiber A. D., Douda D., Samavarchi-Tehrani P., Harrison R. E. (2007). Microtubules regulate PI-3K activity and recruitment to the phagocytic cup during Fcgamma receptor-mediated phagocytosis in nonelicited macrophages. J. Leukoc. Biol. 82, 417-428 [DOI] [PubMed] [Google Scholar]
- Koury M. J., Sawyer S. T., Brandt S. J. (2002). New insights into erythropoiesis. Curr. Opin. Hematol. 9, 93-100 [DOI] [PubMed] [Google Scholar]
- Koury S. T., Koury M. J., Bondurant M. C. (1989). Cytoskeletal distribution and function during the maturation and enucleation of mammalian erythroblasts. J. Cell Biol. 109, 3005-3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisaki Y., Nishikimi A., Tanaka Y., Takii R., Noda M., Inayoshi A., Watanabe K., Sanematsu F., Sasazuki T., Sasaki T., et al. (2006). DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J. Cell Biol. 174, 647-652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. (1985). Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature 314, 472-474 [DOI] [PubMed] [Google Scholar]
- Luo J., Field S. J., Lee J. Y., Engelman J. A., Cantley L. C. (2005). The p85 regulatory subunit of phosphoinositide 3-kinase down-regulates IRS-1 signaling via the formation of a sequestration complex. J. Cell Biol. 170, 455-464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F., Ono S., Yamakita Y., Totsukawa G., Yamashiro S. (1998). Specific localization of serine 19 phosphorylated myosin II during cell locomotion and mitosis of cultured cells. J. Cell Biol. 140, 119-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna N. M., Wang Y. L. (1989). Culturing cells on the microscope stage. Methods Cell Biol. 29, 195-205 [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y., Shiina N., Tsukita S. (2000). The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr. Biol. 10, 865-868 [DOI] [PubMed] [Google Scholar]
- Mukhina S., Wang Y. L., Murata-Hori M. (2007). Alpha-actinin is required for tightly regulated remodeling of the actin cortical network during cytokinesis. Dev. Cell 13, 554-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluch E., Sykes C., Prost J., Bornens M. (2006). Dynamic modes of the cortical actomyosin gel during cell locomotion and division. Trends Cell Biol. 16, 5-10 [DOI] [PubMed] [Google Scholar]
- Parent C. A., Blacklock B. J., Froehlich W. M., Murphy D. B., Devreotes P. N. (1998). G protein signaling events are activated at the leading edge of chemotactic cells. Cell 95, 81-91 [DOI] [PubMed] [Google Scholar]
- Repasky E. A., Eckert B. S. (1981). The effect of cytochalasin B on the enucleation of erythroid cells in vitro. Cell Tissue Res. 221, 85-91 [DOI] [PubMed] [Google Scholar]
- Richmond T. D., Chohan M., Barber D. L. (2005). Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 15, 146-155 [DOI] [PubMed] [Google Scholar]
- Schmoranzer J., Fawcett J. P., Segura M., Tan S., Vallee R. B., Pawson T., Gundersen G. G. (2009). Par3 and dynein associate to regulate local microtubule dynamics and centrosome orientation during migration. Curr. Biol. 19, 1065-1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servant G., Weiner O. D., Herzmark P., Balla T., Sedat J. W., Bourne H. R. (2000). Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287, 1037-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson C. F., Kling J. M. (1967). The mechanism of denucleation in circulating erythroblasts. J. Cell Biol. 35, 237-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skutelsky E., Danon D. (1967). An electron microscopic study of nuclear elimination from the late erythroblast. J. Cell Biol. 33, 625-635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda Y., Sasaki K., Suda M., Itano C., Iwatsuki H. (1998). Effects of colchicine on the enucleation of erythroid cells and macrophages in the liver of mouse embryos: ultrastructural and three-dimensional studies. Anat. Rec. 251, 290-296 [DOI] [PubMed] [Google Scholar]
- Straight A. F., Cheung A., Limouze J., Chen I., Westwood N. J., Sellers J. R., Mitchison T. J. (2003). Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 299, 1743-1747 [DOI] [PubMed] [Google Scholar]
- Wang Y. L. (2003). Computational restoration of fluorescence images: noise reduction, deconvolution, and pattern recognition. Methods Cell Biol. 72, 337-348 [DOI] [PubMed] [Google Scholar]
- Wheatley S. P., Wang Y. L. (1998). Indirect immunofluorescence microscopy in cultured cells. Methods Cell Biol. 57, 313-332 [DOI] [PubMed] [Google Scholar]
- Xu J., Wang F., Van Keymeulen A., Rentel M., Bourne H. R. (2005). Neutrophil microtubules suppress polarity and enhance directional migration. Proc. Natl. Acad. Sci. USA 102, 6884-6889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Kawane K., Koike M., Mori Y., Uchiyama Y., Nagata S. (2005). Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature 437, 754-758 [DOI] [PubMed] [Google Scholar]
- Zhang J., Socolovsky M., Gross A. W., Lodish H. F. (2003). Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood 102, 3938-3946 [DOI] [PubMed] [Google Scholar]
- Zhao W., Kitidis C., Fleming M. D., Lodish H. F., Ghaffari S. (2006). Erythropoietin stimulates phosphorylation and activation of GATA-1 via the PI3-kinase/AKT signaling pathway. Blood 107, 907-915 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.