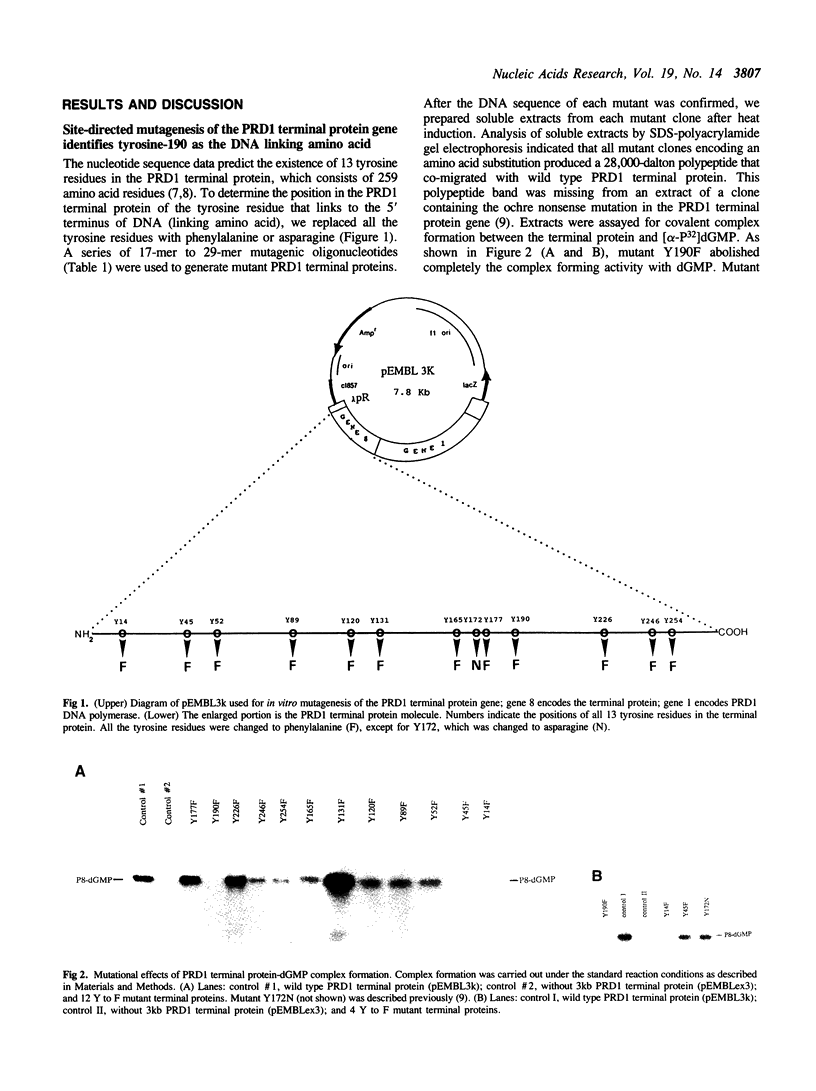
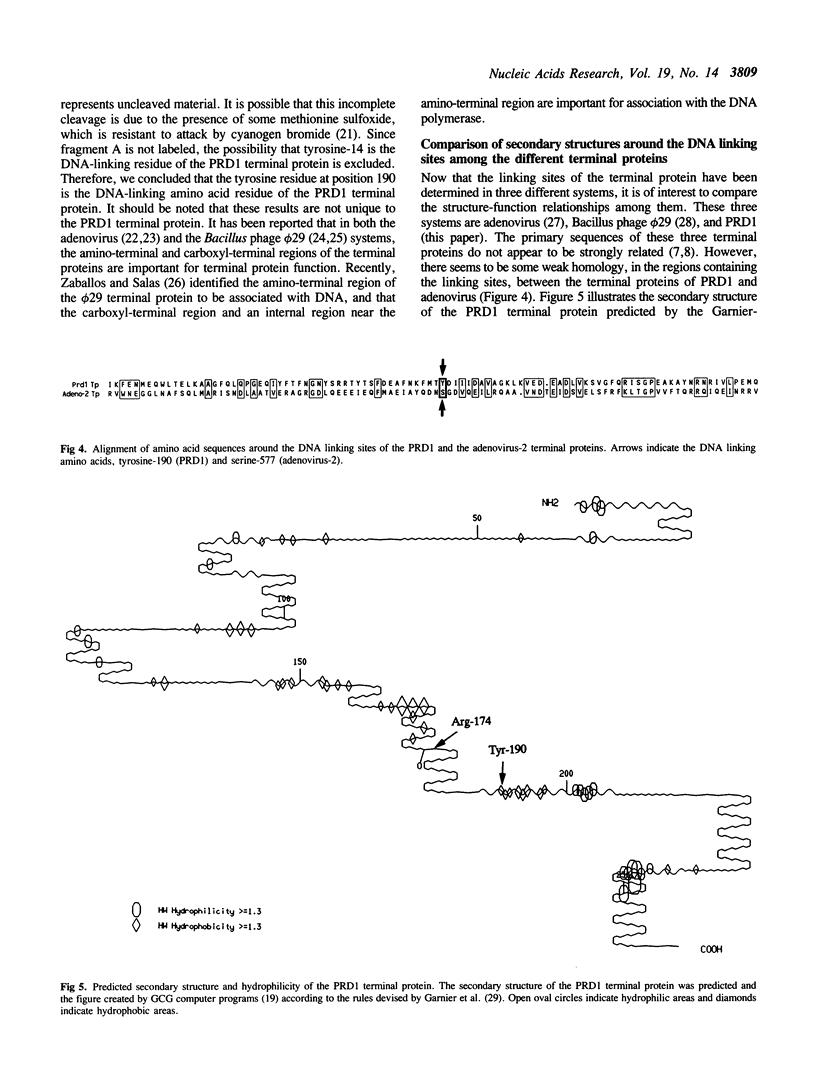
Abstract
DNA replication of PRD1, a lipid-containing phage, is initiated by a protein-priming mechanism. The terminal protein encoded by gene 8 acts as a protein primer in DNA synthesis by forming an initiation complex with the 5'-terminal nucleotide, dGMP. The linkage between the terminal protein and the 5' terminal nucleotide is a tyrosylphosphodiester bond. The PRD1 terminal protein contains 13 tyrosine residues in a total of 259 amino acids. By site-directed mutagenesis of cloned PRD1 gene 8, we replaced 12 of the 13 tyrosine residues in the terminal protein with phenylalanine and the other tyrosine residue with asparagine. Functional analysis of these mutant terminal proteins suggested that tyrosine-190 is the linking amino acid that forms a covalent bond with dGMP. Cyanogen bromide cleavage studies also implicated tyrosine-190 as the DNA-linking amino acid residue of the PRD1 terminal protein. Our results further show that tyrosine residues at both the amino-terminal and the carboxyl-terminal regions are important for the initiation complex forming activity. Predicted secondary structures for the regions around the DNA linking amino acid residues were compared in three terminal proteins (phi 29, adenovirus-2, and PRD1). While the linking amino acids serine-232 (phi 29) and serine-577 (adenovirus-2) are found in beta-turns in hydrophilic regions, the linking tyrosine-190 of the PRD1 terminal protein is found in a beta-sheet in a hydrophobic region.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamford D. H., Mindich L. Characterization of the DNA-protein complex at the termini of the bacteriophage PRD1 genome. J Virol. 1984 May;50(2):309–315. doi: 10.1128/jvi.50.2.309-315.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Desiderio S. V., Kelly T. J., Jr Structure of the linkage between adenovirus DNA and the 55,000 molecular weight terminal protein. J Mol Biol. 1981 Jan 15;145(2):319–337. doi: 10.1016/0022-2836(81)90208-4. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimuth P. I., Ginsberg H. S. Codon insertion mutants of the adenovirus terminal protein. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7816–7820. doi: 10.1073/pnas.83.20.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García P., Hermoso J. M., García J. A., García E., López R., Salas M. Formation of a covalent complex between the terminal protein of pneumococcal bacteriophage Cp-1 and 5'-dAMP. J Virol. 1986 Apr;58(1):31–35. doi: 10.1128/jvi.58.1.31-35.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hermoso J. M., Méndez E., Soriano F., Salas M. Location of the serine residue involved in the linkage between the terminal protein and the DNA of phage phi 29. Nucleic Acids Res. 1985 Nov 11;13(21):7715–7728. doi: 10.1093/nar/13.21.7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoso J. M., Salas M. Protein p3 is linked to the DNA of phage phi 29 through a phosphoester bond between serine and 5'-dAMP. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6425–6428. doi: 10.1073/pnas.77.11.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J. C., Jung G. H., Leavitt M. C., Ito J. Primary structure of the DNA terminal protein of bacteriophage PRD1. Nucleic Acids Res. 1987 Nov 11;15(21):8999–9009. doi: 10.1093/nar/15.21.8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J. C., Yoo S. K., Ito J. An essential arginine residue for initiation of protein-primed DNA replication. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8665–8669. doi: 10.1073/pnas.87.21.8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Mindich L., McGraw T. Molecular cloning of bacteriophage PRD1 genomic fragments. Mol Gen Genet. 1983;190(2):233–236. doi: 10.1007/BF00330645. [DOI] [PubMed] [Google Scholar]
- Pettit S. C., Horwitz M. S., Engler J. A. Mutations of the precursor to the terminal protein of adenovirus serotypes 2 and 5. J Virol. 1989 Dec;63(12):5244–5250. doi: 10.1128/jvi.63.12.5244-5250.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Savilahti H., Bamford D. H. The complete nucleotide sequence of the left very early region of Escherichia coli bacteriophage PRD1 coding for the terminal protein and the DNA polymerase. Gene. 1987;57(1):121–130. doi: 10.1016/0378-1119(87)90183-1. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Smart J. E., Stillman B. W. Adenovirus terminal protein precursor. Partial amino acid sequence and the site of covalent linkage to virus DNA. J Biol Chem. 1982 Nov 25;257(22):13499–13506. [PubMed] [Google Scholar]
- Sollazzo M., Frank R., Cesareni G. High-level expression of RNAs and proteins: the use of oligonucleotides for the precise fusion of coding-to-regulatory sequences. Gene. 1985;37(1-3):199–206. doi: 10.1016/0378-1119(85)90273-2. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Watabe K., Shin M., Ito J. Protein-primed initiation of phage phi 29 DNA replication. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4248–4252. doi: 10.1073/pnas.80.14.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Zaballos A., Mellado R. P., Salas M. Initiation of phage phi 29 DNA replication by mutants with deletions at the amino end of the terminal protein. Gene. 1988;63(1):113–121. doi: 10.1016/0378-1119(88)90550-1. [DOI] [PubMed] [Google Scholar]
- Zaballos A., Salas M. Functional domains in the bacteriophage phi 29 terminal protein for interaction with the phi 29 DNA polymerase and with DNA. Nucleic Acids Res. 1989 Dec 25;17(24):10353–10366. doi: 10.1093/nar/17.24.10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaballos A., Salas M., Mellado R. P. Initiation of phage phi 29 DNA replication by mutants with deletions at the carboxyl end of the terminal protein. Gene. 1986;43(1-2):103–110. doi: 10.1016/0378-1119(86)90013-2. [DOI] [PubMed] [Google Scholar]