Background: c-Abl and DGKα are important enzymes for many cellular processes. However, substrates of c-Abl and the regulation mechanism of DGKα are not fully understood.
Results: c-Abl directly phosphorylates DGKα at Tyr-218.
Conclusion: The c-Abl phosphorylation regulates the serum-induced nuclear export of DGKα.
Significance: The c-Abl phosphorylation contributes to the spatio-temporal regulation of DGKα.
Keywords: Cell Cycle, Lipid Metabolism, Nuclear Transport, Phosphorylation, Tyrosine Protein Kinase (Tyrosine Kinase), Diacylglycerol Kinase
Abstract
c-Abl is a tyrosine kinase involved in many cellular processes, including cell cycle control and proliferation. However, little is known about its substrates. Here, we show that c-Abl directly phosphorylates diacylglycerol kinase α (DGKα), an important regulator of many cellular events through its conversion of diacylglycerol to phosphatidic acid. We found that DGKα was transported from the cytoplasm to the nucleus in response to serum starvation, and serum restoration induced the nuclear export of the enzyme to the cytoplasm. This serum-induced export involves two tyrosine kinases, c-Src and c-Abl. The latter, c-Abl, is activated by c-Src, phosphorylates DGKα, and shuttles between the nucleus and the cytoplasm in a direction opposite to that of DGKα in response to serum restoration. Moreover, an in vitro phosphorylation assay using purified mutants of DGKα identified Tyr-218 as a site of phosphorylation by c-Abl. We confirmed these results for endogenous DGKα using an antibody specific for phospho-Tyr-218, and this phosphorylation was necessary for the serum-induced export of DGKα. These results demonstrate that the nucleo-cytoplasmic shuttling of DGKα is orchestrated by tyrosine phosphorylation by the Src-activated tyrosine kinase c-Abl and that this phosphorylation is important for regulating the function of cytoplasmic and/or nuclear DGKα.
Introduction
Cellular activities are controlled by various substances, including lipids. Lipids are the principal components not only of the cytoplasmic membrane but also of the membrane structure of organelles, including the mitochondria, nucleus, endoplasmic reticulum, and Golgi apparatus. In addition, lipids have important roles in signal transduction. The cytoplasmic membrane contains phosphatidylinositol 4,5-bisphosphate, which is hydrolyzed to diacylglycerol (DG)3 and inositol 1,4,5-trisphosphate by phospholipase Cβ upon stimulation. DG activates protein kinase C (PKC) in cooperation with Ca2+ release induced by inositol 1,4,5-trisphosphate. Thus, DG is an important lipid messenger in signal transduction.
Diacylglycerol kinase (DGK) phosphorylates DG to produce phosphatidic acid (PA) (1), resulting in an attenuation of PKC activity. PA is also an activator for many other kinases, including phosphatidylinositol 4-phosphate 5-kinase (2, 3), protein kinase Cζ (4), and the mammalian target of rapamycin (5). Therefore, DGK is thought to be one of the key enzymes in lipid signal transduction. Mammalian DGKs have 10 subtypes and are categorized into five groups, depending on their primary structure (6). All DGKs, except DGKθ, have two cysteine-rich regions (C1A and C1B domains) in the regulatory domain at the N terminus and a catalytic domain at the C terminus. DGKθ has three C1 domains, and catalytic domains of type II DGKs are separated. In addition to these domains, they have different structures, depending on their groups. Type I DGKs, DGKα, -β, and -γ, have an EF-hand motif and a recoverin homology (RVH) domain. Type II DGKs, DGKδ, -η, and -κ, have a pleckstrin homology domain instead of an EF-hand and RVH domain at the N terminus. DGKδ and -η have a sterile α motif domain at the C terminus. DGKκ has an EPAP repeat at the N terminus and a PDZ binding domain at the C terminus. DGKϵ is a Type III DGK, and it contains only a C1 domain. Type IV DGKs, DGKζ and -ι, have a myristoylated alanine-rich protein kinase C substrate phosphorylation site-like region at the N terminus and four ankyrin repeats at the C terminus. Finally, DGKθ, which is a Type V DGK, has a proline- and glycine-rich domain and a pleckstrin homology domain with an overlapping Ras association domain. DGKs are thought to be involved in development, differentiation, construction of neural networks, and immunity (7). However, subtype-specific functions and regulatory mechanisms remain poorly characterized.
Some DGKs show translocation to the plasma membrane, where DG is produced, in response to various stimulations (8–10). However, lipid metabolism also exists in the nucleus, and the production of DG and PA in the nucleus has been reported (11–13). Moreover, it was reported that DGKζ, -θ, and -γ were localized in the nucleus (14–16). These findings suggest that DGKs are important for nuclear DG/PA metabolism.
In a series of experiments aimed at elucidating the subtype-specific functions of DGKs, we found that DGKα shuttles between the cytoplasm and the nucleus. However, DGKα does not have a conserved nuclear localization signal or a nuclear export signal. Therefore, we tried to clarify the mechanism of DGKα nucleo-cytoplasmic shuttling and found that the nuclear export of the enzyme is regulated by phosphorylation at Tyr-218 by C-Abl.
EXPERIMENTAL PROCEDURES
Materials
NIH3T3 cells and COS-7 cells were purchased from the RIKEN Cell Bank. DDT1-MF2 (DTT) cells were obtained from B. B. Fredholm (Karolinska Institute, Stockholm, Sweden). The plasmids encoding either wild-type Src or a kinase-negative Src C-terminally fused to a FLAG tag were obtained from Dr. Fukami (Kobe University, Kobe, Japan). The plasmids encoding either wild-type c-Abl or a kinase negative c-Abl were kindly provided by Dr. Tanaka (Hokkaido University, Sapporo, Japan). The anti-GFP antibodies were made in house, and the anti-DGKα antibodies were described previously (18). We obtained mouse anti-lamin A/C antibody, mouse anti-Src antibody, and mouse anti-Tyr(P) antibody (4G10) from Upstate Biotechnology, Inc. (Lake Placid, NY), rabbit anti-c-Abl antibody from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), mouse anti-Lck from BD Transduction Laboratories (San Jose, CA), rabbit anti-Tyr(P)-412-c-Abl antibody from IMGENEX (San Diego, CA), rabbit anti-FLAG antibody from Affinity Bioreagents (Golden, CO), and rabbit anti-MBP antibody from DIATHEVA (Viale Piceno, Fano, Italy) as primary antibodies. We purchased peroxidase-labeled goat anti-rabbit and mouse IgG from Jackson ImmunoResearch Laboratories (West Grove, PA) and peroxidase-labeled goat anti-guinea pig IgG from Chemicon International (Temecula, CA) as secondary antibodies. DGKα siRNA from Santa Cruz Biotechnology, Inc. was donated by Dr. Yamashita and Dr. Akifusa (Kyusyu University, Fukuoka, Japan) (19).
Methods
Cell Culture
COS-7 and NIH3T3 cells were cultured in Dulbecco's modified Eagle's medium (Nacalitesque, Kyoto, Japan). All cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2. All media contained 25 mm glucose, and they were all buffered with 44 mm NaHCO3 and supplemented with 10% fetal bovine serum, penicillin (100 units/ml), and streptomycin (100 μg/ml) (Invitrogen). The fetal bovine serum used was not heat-inactivated.
Construction of Plasmids Encoding Wild-type or Mutant DGKα Fused to GFP
The constructs encoding DGKα N-terminally fused to GFP (GFP-DGKα) were described previously (8). To construct the plasmid encoding DGKα with the substitution of Phe for Tyr-218 (Y218F), mutagenesis was carried out as described by the manufacturer's protocol using a QuikChange multisite-directed mutagenesis kit (Stratagene, La Jolla, CA). The primers for Y218F were 5′-AGACCCAAGAGGTTCCCCAGGCCTGTCTTCTGCAACCTGTGCGAGTCG-3′ and 5′-CGACTCGCACAGGTTGCAGAAGACAGGCCTGGGGAACCTCTTGGGTCTC-3′. The mutation was confirmed by verifying its sequence.
Construction of Plasmids Encoding c-Abl Fused to GFP or mDsRed
cDNA fragments of either wild-type or kinase-negative c-Abl with a HindIII site at the N terminus and a KpnI site at the C terminus were produced by PCR using mouse c-Abl cDNA as the template. The PCR products were first subcloned into a pUC118 vector (Takara, Shiga, Japan). After digestion with HindIII and KpnI, the cDNA encoding each fragment was subcloned into the HindIII and KpnI site in the pEGFP-C1 vector or the pDsRed-Monomer-C1 vector (Clontech). The primers used were 5′-TTAAGCTTCGGCCACCATGGGGCAGCCTGGA-3′ and 5′-TTGGTACCGTCCTCCGGACAATGTCGCT-3′.
Lipofection
NIH3T3 cells (7.5 × 104 cells/dish) grown on a glass bottom dish (MatTek Corp., Ashland, MA) were transfected using 3 μl of FuGENETM6 Transfection reagent (Roche Applied Science) and 1 μg of DNA according to the manufacturer's protocol. Transfected cells were cultured at 37 °C for about 24 h before use.
Confocal Laser Scanning Microscopy Analysis
Cy3 and GFP fluorescence was observed under a confocal laser scanning fluorescent microscope (Carl Zeiss, Jena, Germany). The GFP fluorescence was monitored at 488 nm using an argon laser for excitation with a 515-nm-long pass barrier filter. Cy3 fluorescence was monitored at 543 nm using a HeNe 1 laser for excitation with a 560–615-nm band pass filter.
Quantitation of the subcellular distribution of the GFP fusion proteins was defined as described below. The cells in which the ratio of the GFP fluorescence intensity of the cytoplasm and the nucleus was under 0.5 were defined as “only in the cytoplasm,” and the cells in which the ratio was 0.5–0.7 were defined as “abundantly in the cytoplasm.” If the ratio was over 0.7, the cells were defined as “equally in the cytoplasm and the nucleus.”
Fractionation
Fractionation was performed as described previously (20). NIH3T3 or DDT cells were harvested and resuspended in cytoplasmic lysis buffer containing 10 mm HEPES, pH 7.9, 10 mm KCl, 0.1 mm EDTA, 0.1 mm EGTA, 1 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mm sodium orthovanadate. Cells were incubated on ice for 15 min. Nonidet P-40 was added to a final concentration of 0.4%. Cells were vortexed vigorously for 10 s and then centrifuged at 18,000 × g for 30 s. The supernatant was kept as the cytoplasmic fraction. The pellet was washed twice in lysis buffer containing 0.2% Nonidet P-40 and resuspended in nuclear lysis buffer (20 mm HEPES, pH 7.9, 0.4 m NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm dithiothreitol, and 1 mm phenylmethylsulfonyl fluoride). The tube was vigorously rotated at 4 °C for 15 min and centrifuged for 5 min at 18,000 × g. The supernatant was designated as the nuclear fraction. Nuclear fractions were subjected to immunoblotting.
Immunoprecipitation
The lysates from COS-7 cells transfected with wild-type or kinase-negative GFP-c-Abl were incubated with rotation with an anti-GFP antibody immobilized on protein A-Sepharose beads in homogenizing buffer without Triton-X 100 at 4 °C for 2 h. After incubation, the protein A-Sepharose beads were washed with c-Abl kinase buffer three times.
Immunoblotting
Plasmids (∼32 μg) were electroporated into COS-7 cells using a gene pulser (Bio-Rad, 975 microfarads, 220 mV) or transfected into NIH3T3 cells using the FuGENETM6 transfection reagent. After being cultured, the cells were harvested and centrifuged at 5,500 × g for 3 min. The cells were resuspended in homogenizing buffer (250 mm sucrose, 10 mm EGTA, 2 mm EDTA, 50 mm Tris-HCl, 200 μg/ml leupeptin, 1 mm phenylmethylsulfonyl fluoride, 1% Triton X-100, pH 7.4, 1 mm NaF, 1 mm sodium vanadate) and sonicated (UD-210 TOMY, Tokyo, Japan; output 3, 15 s, two times).
For immunoblotting, the samples were subjected to 7.5% SDS-PAGE, followed by blotting onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA). Nonspecific binding sites were blocked by incubation with 5% skim milk in 0.01 m PBS containing 0.03% Triton X-100 (PBS-T) at 4 °C overnight. The membrane was incubated with primary antibody for 1 h at room temperature. After washing with PBS-T, the membrane was incubated with secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) for 30 min. After three rinses with PBS-T, the immunoreactive bands were visualized using a chemiluminescence detection kit (ECL, Amersham Biosciences).
Production of Anti-Tyr(P)-218 DGKα Antibody
For the preparation of the anti-phospho-Tyr-218 of DGKα antibody, an oligopeptide corresponding to amino acids 213–223 of pig DGKα containing phospho-Tyr-218 (FPRPVpYVNLCE) was used as an antigen. The synthetic peptide was conjugated to keyhole limpet hemocyanin, and 20 μg of keyhole limpet hemocyanin-conjugated antigen was emulsified and injected into a 10-week-old female guinea pig. Booster injections (20 μg each) were given at 2-week intervals. Three days after the sixth boost, serum was collected and purified with an affinity column using the antigen peptide.
Construction of Plasmids Encoding DGKα Fragments Fused to MBP
cDNA fragments of DGKα fragments with an EcoRI site at the N terminus and a SalI site at the C terminus were produced by PCR using pig DGKα cDNA as the template. The PCR products were first subcloned into a pUC118 vector (TAKARA, Shiga, Japan). After digestion with EcoRI and SalI, the cDNA encoding each fragment was subcloned into the EcoRI and SalI sites in the pMAL-C2 vector (New England BioLabs, Ipswich, MA). The primers for the DGKα regulatory domain (bp 1–1116) were 5′-TTGAATTCATGTCCAAGGAGAGGGGG-3′ and 5′-TTGTCGACTCAGTTAGAAACGGGGTC-3′. The primers for RDΔhinge (318 aa) (bp 1–954), for RVH-EF-C1 domains (252 aa) (bp 1–756), for RVH-EF-C1a (204 aa) (bp 1–612), and for RVH-EF hand (112 aa) (bp 1–336) were 5′-TTGTCGACTCAACACTCATGGCCCATGGC-3′, 5′-TTGTCGACTCAAACACAGGGCAGGGCCTTCAT-3′, 5′-TTGTCGACTCACTGCCCATTGTCCTTCAA-3′, and 5′-TTGTCGACTCAGTCTTCTGGCCGGCCGCC-3′, respectively.
Purification of DGKα Fragments
DH5α cells were transformed with expression plasmids for MBP fusion proteins. MBP fusion proteins were expressed and purified according to the manufacturer's protocols (New England Biolabs). Expression of MBP fusion proteins was induced with 0.3 mm IPTG at 25 °C for 16 h. The induced cells were harvested and sonicated in column buffer (20 mm Tris-HCl, pH 7.4, 1 mm EDTA, 1 mm DDT, 200 mm NaCl, 1% Triton X-100, 20 μg/ml leupeptin, 1 mm PMSF). After centrifugation at 10,000 × g for 30 min, MBP fusion proteins were applied to amyrose resin and incubated overnight at 4 °C. After washing with column buffer, to cut MBP, 10× factor Xa cleavage buffer and factor Xa were added and incubated overnight at 4 °C. Eluate was dialyzed against PBS(−).
c-Abl Kinase Assay
Purified proteins were incubated in c-Abl kinase buffer (50 mm HEPES, pH 7.4, 10 mm MgCl2, 10 mm MnCl2, 2 mm DDT, 0.1 mm sodium vanadate) with the precipitants obtained from the immunoprecipitation using an anti-GFP antibody and [γ-32P]ATP (MP Biomedicals, Solon, OH) for 15 min at 30 °C. Reaction products were subjected to SDS-PAGE and analyzed using BSA2500 (Fujifilm, Tokyo, Japan).
Effect of siRNA c-Abl on Serum-induced Export of DGKα
To specifically detect the cells transfected with c-Abl siRNA, we employed thymidine dimer methods described previously (21). Briefly, 30 μg of siRNA was irradiated with 2.0 J/cm2 UV. The irradiated siRNA (300 nm) and 1 μg of the plasmid encoding GFP-DGKα were simultaneously transfected into 4 × 104 NIH3T3 cells. Twenty-four hours after the transfection, serum was depleted for 24 h, and then serum was added and incubated for 3 h. Before and after serum starvation and 3 h after serum restoration, the cells were fixed. After immunostaining with anti-thymidine dimer (Kyowa Medics), the localization of GFP-DGKα in the thymidine-dimer positive cells was categorized into three groups and plotted.
RESULTS
Nucleo-cytoplasmic Shuttling of DGKα
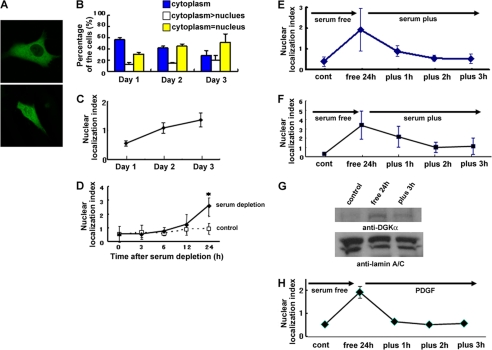
When GFP-DGKα was transfected into NIH3T3 cells, it localized mainly in the cytoplasm but not in the nucleus (Fig. 1A, top). However, the percentage of the cells expressing GFP-DGKα both in the cytoplasm and in the nucleus gradually increased over a several-day course of culturing (Fig. 1A, bottom). At day 1 (24 h after transfection), the percentage of cells expressing GFP-DGKα only in the cytoplasm was 56% (Fig. 1B), which decreased to 28% at Day 3 (72 h after transfection). In contrast, the percentage of cells expressing GFP-DGKα equally in the cytoplasm and the nucleus increased from 31 to 52% within 3 days (Fig. 1B). To quantify the nuclear localization of the enzyme, the ratio of the cells expressing GFP-DGKα equally in the cytoplasm and in the nucleus to those cells expressing the protein only in the cytoplasm was defined as the nuclear localization index, which is plotted in Fig. 1C. The index increased from 0.55 at day 1 to 1.36 at day 3, indicating that GFP-DGKα was transported from the cytoplasm to the nucleus in a time-dependent manner. The fact that this nuclear transportation took 2–3 days suggests the lack or shortage of some factor(s) from the medium required for triggering nuclear import. Therefore, we determined the effect of serum depletion on nuclear import. Serum starvation accelerated the rate of the nuclear transportation; significant nuclear transportation was detected within 24 h after serum depletion (Fig. 1D).
FIGURE 1.
DGKα shows nucleo-cytoplasmic shuttling in response to serum starvation and restoration. A, a typical fluorescent image of cells expressing GFP-DGKα only in the cytoplasm (top) and both in the cytoplasm and in the nucleus (bottom). B, time-dependent nuclear import of GFP-DGKα in NIH3T3 cells. At 24 (day 1), 48 (day 2), and 72 h (day 3) after transfection, cells were fixed using 4% PFA and observed under a laser scanning microscope. Hundreds of cells expressing GFP-DGKα were categorized into three groups: “cytoplasm” (blue column), the cells expressing GFP-DGKα only in the cytoplasm; “cytoplasm > nucleus” (unshaded column), the cells expressing GFP-DGKα more abundantly in the cytoplasm than in the nucleus; and “cytoplasm = nucleus” (yellow column), the cells expressing GFP-DGKα equally in both the cytoplasm and the nucleus. The graph shows the average ± S.E. (error bars) of the percentage of the cells in each group from three independent experiments. C, this graph shows the nuclear localization index (the ratio of the number of cells expressing GFP-DGKα equally in both the cytoplasm and the nucleus to that expressing GFP-DGKα only in the cytoplasm) at day 1, day 2, and day 3. D, serum depletion-induced import of DGKα. The transfected cells with GFP-DGKα were exposed to serum depletion and observed 0, 3, 6, 12, and 24 h after serum depletion. *, p < 0.05 by t test. E and F, serum-dependent nucleo-cytoplasmic shuttling of DGKα in NIH3T3 (E) and DDT cells (F). The transfected cells were exposed to 24-h serum starvation before the medium was changed to one containing serum. The transfected cells were fixed at 1, 2, and 3 h after serum restoration and categorized into three groups as described in A. G, nucleo-cytoplasmic shuttling of endogenous DGKα. NIH3T3 cells were harvested before (control) and after 24 h of serum starvation (free 24 h) and at 3 h after serum restoration (plus 3 h). Fractionation of NIH3T3 cells was carried out as described under “Experimental Procedures,” followed by SDS-PAGE and immunoblotting using anti-DGKα and anti-lamin A/C antibodies to represent equal loading and accurate fractionation. H, PDGF-induced export of DGKα. Serum starvation was done as described in E and F. PDGF (100 ng/ml) was added in the serum-free medium.
Interestingly, the subsequent restoration of serum induced the export of DGKα from the nucleus to the cytoplasm. The nuclear localization index increased from 0.40 to 1.93 during 24 h of serum starvation but reversed to 0.53 within 3 h of serum addition (Fig. 1E), indicating that DGKα shuttles between the cytoplasm and nucleus, depending on the culture conditions. The serum-dependent shuttling of DGKα was also detected in DDT cells derived from Syrian hamster leiomyosarcoma (Fig. 1F), indicating that this phenomenon is not unique to NIH3T3 cells. Furthermore, biochemical fractionation followed by immunoblotting revealed that endogenous DGKα also shuttles between the cytoplasm and the nucleus in a serum-dependent manner (Fig. 1G).
The Tyrosine Kinases c-Src and c-Abl Are Involved in Nuclear Export of DGKα
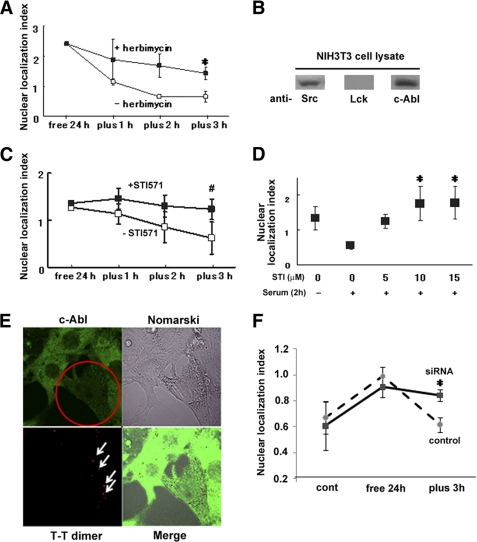
To identify the factor(s) involved in the serum-induced nuclear export of DGKα, we tested several growth factors instead of serum. Among them, platelet-derived growth factor (PDGF) similarly induced the nuclear export (Fig. 1H), suggesting the involvement of tyrosine kinases in this process. Previous reports showed that tyrosine phosphorylation by Src family protein-tyrosine kinases is required for the recruitment of DGKα to the membrane (12). Therefore, we investigated the effect of a tyrosine kinase inhibitor, herbimycin, on the serum-induced nuclear export of DGKα. The herbimycin treatment significantly inhibited the movement of DGKα from the nucleus to the cytoplasm (Fig. 2A). Next, we checked for the presence of Lck and/or c-Src in the NIH3T3 cells because it has been reported that c-Src and Lck directly phosphorylate DGKα (9, 10). The cells endogenously express c-Src but not Lck (Fig. 2B), suggesting that c-Src is a possible candidate. However, GFP-tagged c-Src mainly localized in the cytoplasm under all of the conditions we tested (data not shown), suggesting that the serum-induced nuclear export of DGKα involves an additional factor, which is regulated by c-Src and phosphorylates DGKα in the nucleus.
FIGURE 2.
Tyrosine kinase, c-Src, and c-Abl are involved in the serum-induced export of GFP-DGKα. A, inhibitory effect of herbimycin. After serum starvation for 24 h, cells expressing GFP-DGKα were incubated in the medium containing serum with 1 μm herbimycin for the appropriate time periods. *, p < 0.05. n = 100 × 3. B, immunoblotting with anti-c-Src, Lck, and c-Abl. Whole cell lysates from NIH3T3 cells were subjected to analysis by immunoblotting using anti-c-Src, Lck, and c-Abl antibodies, respectively. C, inhibitory effect of a specific inhibitor for c-Abl. Using STI571 at 10 μm similar experiments were carried out as described in A. n = 100 × 3. #, p < 0.1. D, dose-dependent effect of STI571 on the nuclear export of DGKα. The same experiments were performed as described in C using different concentrations of STI571, and the nuclear localization index at 3 h after serum restoration was plotted against the concentration of STI571. *, p < 0.05. n = 100 × 3. E, down-regulation of endogenous c-Abl by the siRNA treatment. Twenty-four hours after transfection of 300 nm irradiated siRNA for c-Abl, NIH3T3 cells were fixed and stained with anti-c-Abl (green) and T-T dimer antibodies (red). The arrows indicate the transfected siRNA. The amount of endogenous c-Abl was reduced only in the T-T-positive cells (indicated by a circle). F, inhibitory effect of siRNA for c-Abl. The plasmid encoding GFP-DGKα was transfected with either a thymidine-dimer control or the irradiated c-Abl siRNA. Before and after 24-h serum starvation and 3 h after serum restoration, the cells were fixed. After immunostaining with anti-thymidine dimer, the localization of GFP-DGKα in the T-T dimer-positive cells was categorized into three groups and plotted as described in the legend to Fig. 1, A and E. *, p < 0.05. n = 100 × 3. Error bars, S.E.
c-Abl tyrosine kinase was an attractive candidate as an additional factor regulating the nuclear export of DGKα because it is activated by Src (21, 22) and exhibits nucleo-cytoplasmic shuttling (23). Therefore, we checked if NIH3T3 cells endogenously express c-Abl, and we determined the effects of a specific c-Abl inhibitor, STI571, on the serum-induced nuclear exportation of DGKα. c-Abl is endogenously expressed in NIH3T3 cells (Fig. 2B), and STI571 inhibited the nuclear export of GFP-DGKα in a dose-dependent manner (Fig. 2, C and D), suggesting the involvement of c-Abl. To confirm it, we used the irradiated siRNA of c-Abl, as described under “Methods.” First we checked whether endogenous c-Abl was down-regulated by the irradiated siRNA treatment. Fig. 2E indicates that endogenous c-Abl was down-regulated in the cells transfected with siRNA, which was recognized by anti-T-T dimer antibody. We then investigated the localization of GFP-DGKα in the T-T-positive cells. As shown in Fig. 2F, the serum-induced nuclear exportation of DGKα was significantly inhibited by the c-Abl siRNA treatment, confirming the importance of the enzyme for the exportation.
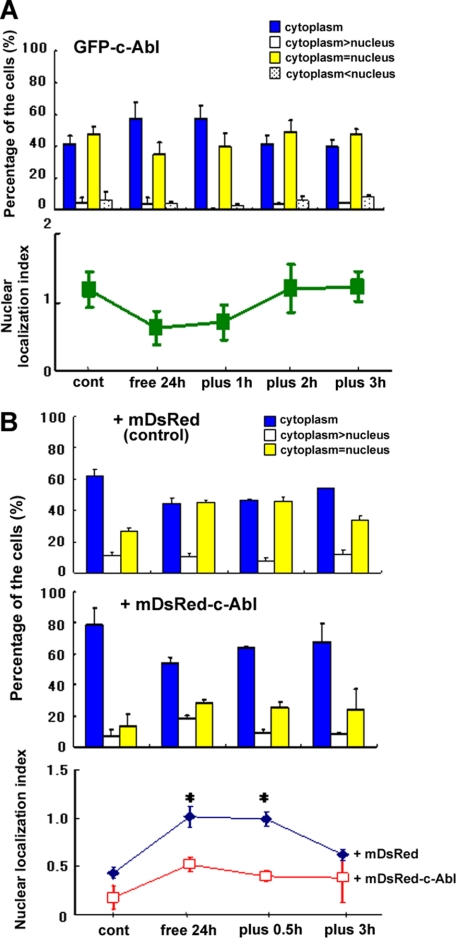
Next, we investigated the shuttling of c-Abl in response to serum. GFP-c-Abl showed nucleo-cytoplasmic shuttling in response to serum, but the direction was opposite that of DGKα; GFP-c-Abl was exported from the nucleus to the cytoplasm by serum depletion, whereas it was imported to the nucleus by serum restoration (Fig. 3A), fitting with our hypothesis that c-Abl directly regulates the serum-induced nuclear export of DGKα. Furthermore, co-expression of mDsRed-tagged c-Abl enhanced the cytoplasmic localization of GFP-DGKα before serum depletion (Fig. 3B); co-expression of c-Abl increased the percentage of cells expressing GFP-DGKα only in the cytoplasm from 60 to 80% and decreased that of the cells both in the cytoplasm and the nucleus from 30 to 15%. In addition, co-expression of c-Abl inhibited the serum depletion-induced nuclear import of DGKα; the nuclear index was lower than 1 in the presence of c-Abl (Fig. 3B, bottom). Together, these results suggest that growth factors, including PDGF, in the serum activate c-Src and that activated c-Src phosphorylates c-Abl, resulting in the activation and nuclear import of c-Abl, and finally, c-Abl phosphorylates DGKα in the nucleus to export DGKα.
FIGURE 3.
c-Abl shows opposite nucleo-cytoplasmic shuttling in response to serum and inhibits the serum-induced nuclear import of DGKα. A, nucleo-cytoplasmic shuttling of c-Abl. GFP-c-Abl was transfected into NIH3T3 cells. Serum starvation, serum restoration, fixation, and observation were performed as described in the legend to Fig. 1. The localization of c-Abl was categorized into three groups as described above. The nuclear localization index is shown in the lower panel. n = 100 × 3. B, effect of co-expression of c-Abl on the localization of DGKα. GFP-DGKα was transfected into NIH3T3 cells with mDsRed-tagged c-Abl (+mDsRed-c-Abl) or mDsRed alone (+mDsRed). The lower panel shows the nuclear localization index of GFP-DGKα in the presence (red squares) or absence (blue diamonds) of c-Abl at each time point. *, p < 0.05. n = 100 × 3. Error bars, S.E.
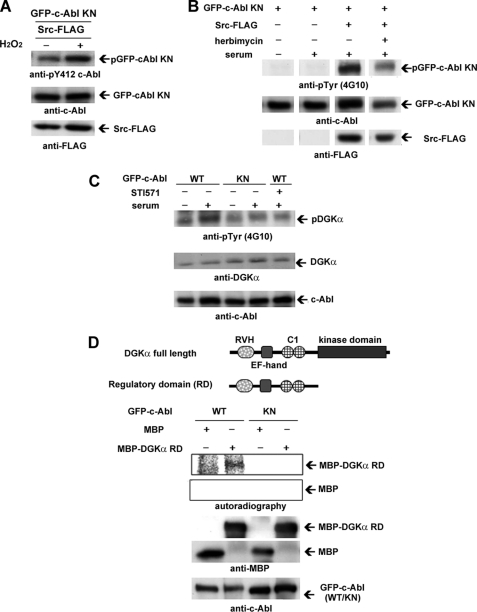
To confirm this hypothesis, we tried to detect the c-Src-dependent activation of c-Abl using an anti-phospho-Tyr-412-c-Abl antibody, because the phosphorylation of Tyr-412 is a marker for the activation of c-Abl (21, 22). In these experiments, we employed a kinase-negative c-Abl (c-Abl KN) to avoid autophosphorylation. When co-transfected with Src-FLAG, the tyrosine phosphorylation at 412 on GFP-c-Abl KN was enhanced by H2O2 stimulation, which is an activator of c-Src (Fig. 4A). Serum restoration induced the tyrosine phosphorylation of GFP-c-Abl KN only when Src-FLAG was co-expressed (Fig. 4B) and tyrosine phosphorylation was inhibited by herbimycin (Fig. 4B), indicating that serum-activated c-Src phosphorylates c-Abl in NIH3T3 cells. Next, we investigated whether c-Abl phosphorylates DGKα in NIH3T3 cells under serum restoration. Serum treatment on the NIH3T3 cells overexpressing c-Abl enhanced tyrosine phosphorylation of endogenous DGKα, but the enhancement was not detected in the case of the KN c-Abl (Fig. 4C). Importantly, the serum-induced enhancement of tyrosine phosphorylation was inhibited by STI571 (Fig. 4C), indicating that DGKα is tyrosine-phosphorylated by c-Abl, which is activated in a serum-dependent manner.
FIGURE 4.
c-Src activated in a serum-dependent manner phosphorylates c-Abl, resulting in the subsequent phosphorylation of DGKα. A, H2O2-induced activation of c-Abl by c-Src. GFP-c-Abl kinase-negative (KN) and FLAG-tagged c-Src (Src-FLAG) were co-transfected into COS-7 cells as described under “Experimental Procedures.” After a 30-min incubation with 3 mm H2O2, the transfected cells were harvested and homogenized. The lysates were subjected to SDS-PAGE, followed by immunoblotting using anti-Tyr(P)-412-c-Abl, anti-c-Abl, and anti-FLAG antibodies. B, serum-induced activation of c-Abl by c-Src. GFP-c-Abl KN and c-Src-FLAG (Src WT) or kinase-negative FLAG-Src (Src KN) were co-transfected into NIH3T3 cells by lipofection. After serum starvation for 24 h, the cells were preincubated with herbimycin for 15 min, followed by incubation with fresh medium containing serum with 1 μm herbimycin for 30 min. The homogenate of the cells was subjected to immunoblotting analysis using anti-Tyr(P) (4G10 antibody), anti-c-Abl, and anti-FLAG antibody. Images cut out from one gel are put together because the order was different. C, tyrosine phosphorylation of endogenous DGKα serum-activated c-Abl. After 24 h of serum starvation, NIH3T3 cells expressing GFP-c-Abl were preincubated with 5 μm STI571 for 15 min, followed by further treatment with serum and 5 μm STI571 for 30 min. The lysates were subjected to immunoblotting analysis using anti-Tyr(P), anti-DGKα, and anti-c-Abl antibodies. D, direct phosphorylation of DGKα by c-Abl. An in vitro c-Abl kinase assay was carried out using purified MBP-DGKα regulatory domain and immunoprecipitated GFP-c-Abl or GFP-c-Abl KN. Purified MBP was used as a control. The reaction products were subjected to immunoblotting analysis using anti-MBP and c-Abl antibodies, and radioactive signals were detected by BSA2500. Schematic representations of DGKα regulatory domains are shown at the top. Error bars, S.E.
To see the direct phosphorylation of DGKα by c-Abl, an in vitro kinase assay was performed. Incubation of purified MBP fused to the regulatory domain (RD) of DGKα with WT GFP-c-Abl, which was immunoprecipitated from COS-7 cell lysates by an anti-GFP antibody, caused the phosphorylation of MBP-DGKαRD (Fig. 4D). In contrast, GFP-c-Abl KN did not phosphorylate the MPB-RD. These results demonstrate that c-Abl directly phosphorylates the RD of DGKα. Together, these results support the hypothesis that the c-Src/c-Abl cascade is involved in the serum-induced nuclear export of DGKα.
Tyr-218 of DGKα Is a Phosphorylation Site for c-Abl
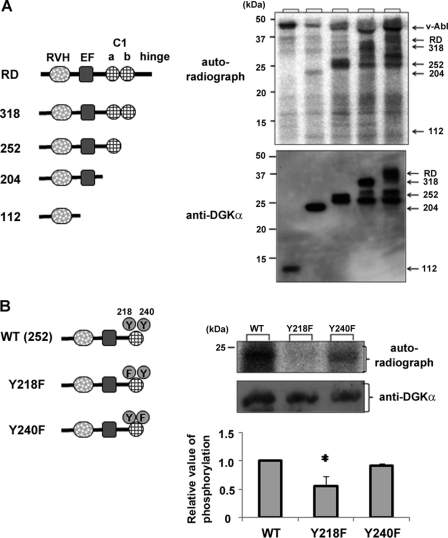
To identify the phosphorylation site in DGKα, we produced a series of mutated RD domains fused with MBP. After cutting out MBP with factor Xa (Novagen), we performed an in vitro phosphorylation assay using the purified proteins and v-Abl (New England BioLabs), which has a catalytic region homologous to c-Abl.
Again, direct phosphorylation of RD by v-Abl was confirmed (Fig. 5A). Even when the hinge region and C1b were truncated, the strong phosphorylation signals were still detected (see 252 in Fig. 5A). However, deletion of the C1a domain resulted in a significant reduction of phosphorylation (Fig. 5A), indicating that there is, at least, a phosphorylation site in the C1a domain. Because there are two tyrosines in the C1a region (Tyr-218 and -240), we produced mutants in which Tyr-218 and -240 were substituted with Phe and performed an in vitro phosphorylation assay. Y240F, but not Y218F, was phosphorylated by v-Abl (Fig. 5B), indicating that v-Abl directly phosphorylates Tyr-218 of DGKα in vitro. Fig. 5 also shows that there is an additional phosphorylation site in the EF-hand domain because 204 showed a signal that disappeared when the EF-hand was further deleted. However, we could not identify the phosphorylation sites in the EF-hand (data not shown).
FIGURE 5.
Tyr-218 is a phosphorylation site for v-Abl. A, the C1a domain is phosphorylated by v-Abl in vitro. MBP-tagged regulatory domain of DGKα (RD), RD lacking hinge region (318), RVH-EF-hand-C1a domain (252), RVH-EF-hand (204), and RVH region (112) were purified. After cutting out MBP, the respective constructs were used as substrates. The upper panel shows autoradiography and the lower panel shows Western blot using anti-DGKα. B, in vitro phosphorylation assay using RVH-EF-C1a containing a point mutation. Similarly, RVH-EF-C1a containing a point mutation was purified and used for an in vitro phosphorylation assay using v-Abl. Average intensities of the autoradiographs in the three independent experiments are plotted in the lower graph. *, significant difference between WT and Tyr-218 (p < 0.05). Error bars, S.E.
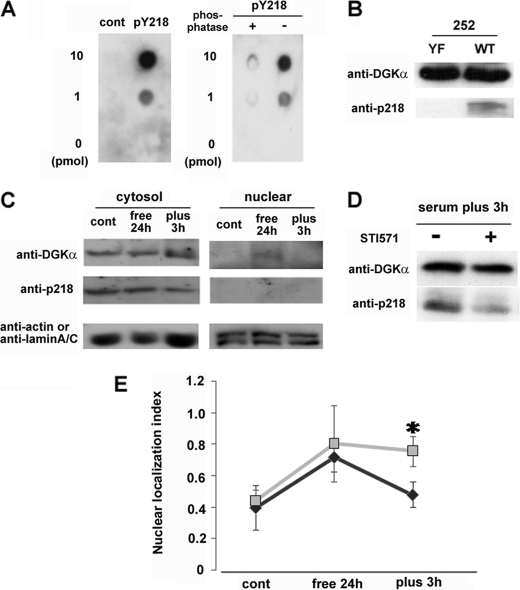
To confirm that endogenous DGKα is phosphorylated by c-Abl in vivo, we produced an antibody specific for phospho-Tyr-218. The antibody recognized the phosphopeptide, including Tyr(P)-218 (pY218), but not the control phosphopeptide (CQKYMEpYSTKKV) or Tyr(P)-218 after treatment with λ-phosphatase (Fig. 6A). To further confirm the specificity of the antibody, peptide 252 (WT) and its YF mutant at 218 (YF) were employed. The peptides were phosphorylated by v-Abl in vitro and applied to SDS-PAGE followed by Western blotting. The Tyr(P)-218 antibody recognized the phosphorylated WT 252 but not the YF mutant (Fig. 6B). Next, we performed Western blotting using this antibody, to investigate whether endogenous DGKα is really phosphorylated at Tyr-218 and where the phosphorylation occurs. Fig. 6C shows that cytosolic DGKα was phosphorylated at Tyr-218, indicating that Tyr-218 is phosphorylated in vivo. However, we did not detect phospho-Tyr-218 DGKα in the nuclear fraction. Moreover, to confirm that the phosphorylation at 218 was dependent on c-Abl, we tested the effect of STI571 on the phosphorylation. The Tyr-218 phosphorylation was inhibited by STI571 (Fig. 6D).
FIGURE 6.
Tyr-218 is phosphorylated in vivo and necessary for the serum-induced nuclear export of DGKα. A, specificity of anti-phospho-Tyr-218 of DGKα. The indicated amount of the antigen peptide (FPRPVpYVNLCE), control peptide corresponding to a different part of DGKα (CQKYMEpYSTKKV), or dephosphorylated antigen peptide was dotted on the PVDF membrane. Immunostaining was performed using purified anti-phospho-Tyr-218 antibody (500× dilution). B, detection of phospho-Tyr-218 in vitro. The peptide 252 with (YF) or without (WT) the point mutation at Tyr-218 described in the legend to Fig. 5B was phosphorylated by v-Abl in vitro and then applied to SDS-PAGE followed by Western blotting using the Tyr(P)-218 antibody. The WT 252 peptide, but not the YF mutant, was detected by the antibody, indicating that the antibody specifically recognized the phospho-Tyr-218. C, detection of phospho-Tyr-218 in vivo. Before and after serum starvation and after 3 h of serum restoration, NIH3T3 cells were harvested and fractionated as described under “Experimental Procedures.” The cytosolic fraction (50 μg) and nuclear fraction (75 μg) were subjected to SDS-PAGE, followed by immunostaining using anti-DGKα, Tyr(P)-218, actin, and lamin A/C antibodies. D, effect of STI571 on the serum-induced phosphorylation of endogenous DGKα at 218. After 24 h of serum starvation, NIH-3T3 cells were cultured with (+) or without (−) 20 μm STI571 in the normal medium for 3 h. The cells were harvested, and total lysate (100 μg) was subjected to SDS-PAGE, followed by immunostaining using anti-DGKα and Tyr(P)-218 antibodies. E, effect of Tyr-218 mutation on the serum-induced nuclear export of DGKα. Cells expressing GFP-DGKα or the Y218F mutant were exposed to 24-h serum starvation, and the medium was then changed to one containing serum. The transfected cells were fixed before and after serum depletion and 3 h after serum restoration and categorized into three groups as described in the legend to Fig. 1A. *, p < 0.05. n = 100 × 3. Error bars, S.E.
Phosphorylation of Tyr-218 Is Involved in Serum-induced Nuclear Export of DGKα
Finally, to confirm that phosphorylation of Tyr-218 is necessary for the serum-induced export of DGKα, we checked the shuttling of the GFP-Y218F mutant, which is a GFP fusion of the full-length DGKα with a Phe substitution for Tyr-218. The GFP-Y218F mutant showed nuclear import similar to WT upon serum depletion, but it could not be exported from the nucleus in response to the addition of serum (Fig. 6E), indicating that phosphorylation of Tyr-218 is necessary for the serum-induced export.
DISCUSSION
This is the first report of the nucleo-cytoplasmic shuttling of DGKα under serum starvation and restoration (Fig. 1). The serum-induced export of DGKα is regulated by the sequential activation of c-Src and c-Abl. This is supported by the following results: herbimycin, STI571, and siRNA knockdown of c-Abl inhibited the serum-induced export (Fig. 2); c-Abl was phosphorylated by c-Src in response to serum (Fig. 4B); DGKα was phosphorylated by c-Abl in vivo and in vitro (Fig. 4, C and D); c-Abl showed the same serum-dependent nucleo-cytoplasmic shuttling but in the direction opposite that of DGKα (Fig. 3A); and co-expression of c-Abl prevented the serum-induced nuclear import of DGKα (Fig. 3B). In addition, Tyr-218 was identified as a phosphorylation site for c-Abl (Figs. 5 and 6), and this phosphorylation is important for the serum-induced export of DGKα (Fig. 6E).
These results suggest that DGKα is an important substrate of c-Abl. The tyrosine kinase was originally identified as the cellular homolog of the v-Abl oncogene product of the Abelson murine leukemia virus (24, 25). Subsequently, c-Abl was shown to be involved in human leukemia as a result of chromosomal translocation events that fuse the bcr and c-abl genes, producing the Bcr-Abl chimeric oncogene (26, 27). It has been reported that the normal c-Abl protein has a role related to the cell cycle, but little is known about its physiological roles and its substrates (28). Together with our results, these facts suggest an important correlation between the shuttling of DGKα and the function of c-Abl. However, discerning the physiological function of DGKα is beyond the scope of this paper. One possible speculation is that cytoplasmic DGKα positively regulates cell proliferation, and it may be pooled in the nucleus under non-proliferative conditions, because serum depletion induced the nuclear transportation of DGKα, and serum restoration caused its nuclear export. This fits with the facts that the hyperproliferative cell line K562 possesses a form of Bcr/Abl with constitutive activity, and that a DGK inhibitor prevents the proliferation of K562 cells (data not shown). Alternatively, to keep cells non-proliferative, nuclear DGKα may cause cells to arrest in G1, similar to DGKζ (29), or keep the cells in the G0 phase. Indeed, a stable line of KN DGKα displayed an abnormal cell cycle (data not shown), and the DGK inhibitor prevented the G0 to G1 transition in NIH3T3 cells following release from serum starvation (data not shown). This hypothesis fits the previous report that c-Abl is involved in S phase entry (30) and that nuclear levels of DG and PA fluctuate during the cell cycle (11–13, 31). Furthermore, DGKα may participate in nuclear membrane lipid metabolism because the nuclear membrane obviously contains many kinds of lipids, and DGKα is translocated to the nuclear matrix (32). In speculating about the physiological functions of nuclear DGKα, we have to be careful because treatment with DGK inhibitors and overexpression of KN DGKα affect both cytosolic and nuclear DGK activities, and cytosolic and nuclear DGKα may have opposing functions.
How can the phosphorylation at Tyr-218 regulate the export of DGKα? The phosphorylation at this site may inhibit the nuclear localization signal function of the C1 domain because Tyr-218 is in the C1 domain, because we previously reported that the C1 domain of DGKγ has an important role in nuclear transport (17). Binding to importin-α, a carrier protein that transports some proteins to the nucleus (33–35), or to an as yet unidentified carrier protein may be regulated by this phosphorylation. Alternatively, the phosphorylation may regulate the interaction between the C1 domain and the N-terminal region of DGKα, because the N-terminal region, including the RVH domain and the EF-hand motif, functions as a nuclear export signal (data not shown); the RD was localized in the cytoplasm, although it contains the C1 domain. Another possibility is that the phosphorylation affects the lipid-binding ability of the C1 domain because it is a well known lipid-binding domain, and other proteins, including PKC, c-Raf and Chimaerin, also have the C1 domain (36). To reveal the precise mechanism, further research is needed.
Needless to say, there may be additional phosphorylation sites recognized by c-Abl. For example, there seems to be, at least, a c-Abl phosphorylation site in the EF-hand. Furthermore, there may be more phosphorylation sites in catalytic domains because we did not address them in this study. In addition, it has been reported that Tyr-334 is phosphorylated by c-Src and Lyn (9, 10, 37). Interestingly, Tyr-334 phosphorylation is involved in the plasma membrane accumulation of DGKα that occurs in response to several stimulations (9, 10, 37), whereas Tyr-218 phosphorylation is necessary for the serum-induced export of DGKα from the nucleus to the cytoplasm. These results indicate that phosphorylation of different tyrosine residues leads to different directions of translocation. Such a mechanism could be important for DGKα activity. These results suggest that DGKα can change its binding partner, depending on the combination of multiple phosphorylation events, with different translocation outcomes.
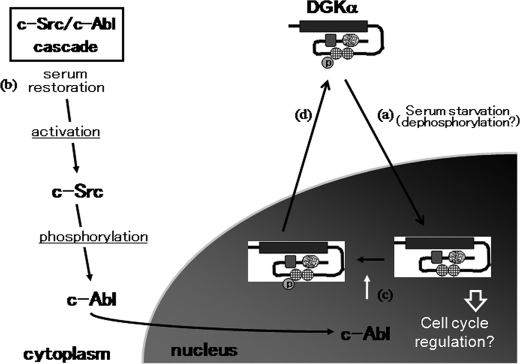
Finally, our model for DGKα nucleo-cytoplasmic shuttling is shown in Fig. 7. DGKα is localized in the cytoplasm under normal conditions, where the C1 domain, the putative nuclear transportation signal, is blocked by the N-terminal region. Under serum starvation, DGKα is transported to the nucleus because the C1 domain gets exposed. The trigger for such a conformational change is unknown, but dephosphorylation may be a key step. Serum restoration induces the sequential activation of c-Src and c-Abl. The activated c-Abl is transported to the nucleus and phosphorylates DGKα at residue 218, although we did not succeed in detecting phospho-Tyr-218 in the nucleus. This may be because once that tyrosine is phosphorylated, DGKα is immediately exported to the cytoplasm. However, our results cannot completely rule out the possibility that c-Abl phosphorylates DGKα in cytoplasm. In conclusion, the well designed nucleo-cytoplasmic shutting system could contribute to the function of DGKα in the nucleus and/or the cytoplasm.
FIGURE 7.
Proposed model of DGKα nucleo-cytoplasmic shuttling. The C1 domain, the putative nuclear transportation signal, is blocked by the N-terminal region under normal conditions. Under serum starvation, DGKα is transported to the nucleus because the C1 domain becomes exposed (a). The trigger for the conformational change is unknown, but dephosphorylation may be a key step. This nuclear transportation is independent of DGKα activity. Serum restoration induces the sequential activation of c-Src and c-Abl (b). The activated c-Abl is transported to the nucleus and phosphorylates DGKα at residue 218 (c). The tyrosine phosphorylation triggers the nuclear export of DGKα, probably by inducing a conformational change regulated by the balance of the kinase domain and the N-terminal region as a nuclear export signal (d).
This work was supported in part by a Grant-in-aid for Scientific Research (C) and the Global Center of Excellence Program of the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
- DG
- diacylglycerol kinase
- DGK
- diacylglycerol kinase
- PA
- phosphatidic acid
- RVH
- recoverin homology
- MBP
- maltose-binding protein
- aa
- amino acids
- KN
- kinase-negative
- RD
- regulatory domain.
REFERENCES
- 1. Hodgkin M. N., Pettitt T. R., Martin A., Michell R. H., Pemberton A. J., Wakelam M. J. (1998) Diacylglycerols and phosphatidates. Which molecular species are intracellular messengers? Trends Biochem. Sci. 23, 200–204 [DOI] [PubMed] [Google Scholar]
- 2. Jones D. R., Sanjuan M. A., Mérida I. (2000) Type Iα phosphatidylinositol 4-phosphate 5-kinase is a putative target for increased intracellular phosphatidic acid. FEBS Lett. 476, 160–165 [DOI] [PubMed] [Google Scholar]
- 3. Luo B., Prescott S. M., Topham M. K. (2004) Diacylglycerol kinase ζ regulates phosphatidylinositol 4-phosphate 5-kinase Iα by a novel mechanism. Cell. Signal. 16, 891–897 [DOI] [PubMed] [Google Scholar]
- 4. Limatola C., Schaap D., Moolenaar W. H., van Blitterswijk W. J. (1994) Phosphatidic acid activation of protein kinase C-ζ overexpressed in COS cells. Comparison with other protein kinase C isotypes and other acidic lipids. Biochem. J. 304, 1001–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fang Y., Vilella-Bach M., Bachmann R., Flanigan A., Chen J. (2001) Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 294, 1942–1945 [DOI] [PubMed] [Google Scholar]
- 6. Sakane F., Imai S., Kai M., Yasuda S., Kanoh H. (2007) Diacylglycerol kinases. Why so many of them? Biochim. Biophys. Acta 1771, 793–806 [DOI] [PubMed] [Google Scholar]
- 7. Zha Y., Marks R., Ho A. W., Peterson A. C., Janardhan S., Brown I., Praveen K., Stang S., Stone J. C., Gajewski T. F. (2006) T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-α. Nat. Immunol. 7, 1166–1173 [DOI] [PubMed] [Google Scholar]
- 8. Shirai Y., Segawa S., Kuriyama M., Goto K., Sakai N., Saito N. (2000) Subtype-specific translocation of diacylglycerol kinase α and γ and its correlation with protein kinase C. J. Biol. Chem. 275, 24760–24766 [DOI] [PubMed] [Google Scholar]
- 9. Baldanzi G., Cutrupi S., Chianale F., Gnocchi V., Rainero E., Porporato P., Filigheddu N., van Blitterswijk W. J., Parolini O., Bussolino F., Sinigaglia F., Graziani A. (2008) Diacylglycerol kinase-α phosphorylation by Src on Y335 is required for activation, membrane recruitment, and Hgf-induced cell motility. Oncogene 27, 942–956 [DOI] [PubMed] [Google Scholar]
- 10. Merino E., Sanjuán M. A., Moraga I., Ciprés A., Mérida I. (2007) Role of the diacylglycerol kinase α-conserved domains in membrane targeting in intact T cells. J. Biol. Chem. 282, 35396–35404 [DOI] [PubMed] [Google Scholar]
- 11. Martelli A. M., Tabellini G., Bortul R., Manzoli L., Bareggi R., Baldini G., Grill V., Zweyer M., Narducci P., Cocco L. (2000) Enhanced nuclear diacylglycerol kinase activity in response to a mitogenic stimulation of quiescent Swiss 3T3 cells with insulin-like growth factor I. Cancer Res. 60, 815–821 [PubMed] [Google Scholar]
- 12. Irvine R. (2000) Nuclear lipid signaling. Sci. STKE 2000, re1. [DOI] [PubMed] [Google Scholar]
- 13. Martelli A. M., Falà F., Faenza I., Billi A. M., Cappellini A., Manzoli L., Cocco L. (2004) Metabolism and signaling activities of nuclear lipids. Cell Mol. Life Sci. 61, 1143–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goto K., Kondo H. (1996) A 104-kDa diacylglycerol kinase containing ankyrin-like repeats localizes in the cell nucleus. Proc. Natl. Acad. Sci. U.S.A. 93, 11196–11201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bregoli L., Tu-Sekine B., Raben D. M. (2002) DGK and nuclear signaling nuclear diacylglycerol kinases in IIC9 cells. Adv. Enzyme Regul. 42, 213–226 [DOI] [PubMed] [Google Scholar]
- 16. Tabellini G., Bortul R., Santi S., Riccio M., Baldini G., Cappellini A., Billi A. M., Berezney R., Ruggeri A., Cocco L., Martelli A. M. (2003) Diacylglycerol kinase-θ is localized in the speckle domains of the nucleus. Exp. Cell Res. 287, 143–154 [DOI] [PubMed] [Google Scholar]
- 17. Matsubara T., Shirai Y., Miyasaka K., Murakami T., Yamaguchi Y., Ueyama T., Kai M., Sakane F., Kanoh H., Hashimoto T., Kamada S., Kikkawa U., Saito N. (2006) Nuclear transportation of diacylglycerol kinase γ and its possible function in the nucleus. J. Biol. Chem. 281, 6152–6164 [DOI] [PubMed] [Google Scholar]
- 18. Goto K., Watanabe M., Kondo H., Yuasa H., Sakane F., Kanoh H. (1992) Gene cloning, sequence, expression, and in situ localization of 80-kDa diacylglycerol kinase specific to oligodendrocyte of rat brain. Mol. Brain Res. 16, 75–87 [DOI] [PubMed] [Google Scholar]
- 19. Kamio N., Akifusa S., Yamashita Y. (2011) Diacylglycerol kinase α regulates globular adiponectin-induced reactive oxygen species. Free Radic. Res. 45, 336–341 [DOI] [PubMed] [Google Scholar]
- 20. Irie N., Sakai N., Ueyama T., Kajimoto T., Shirai Y., Saito N. (2002) Subtype- and species-specific knockdown of PKC using short interfering RNA. Biochem. Biophys. Res. Commun. 298, 738–743 [DOI] [PubMed] [Google Scholar]
- 21. Plattner R., Kadlec L., DeMali K. A., Kazlauskas A., Pendergast A. M. (1999) c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 13, 2400–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brasher B. B., Van Etten R. A. (2000) c-Abl has high intrinsic tyrosine kinase activity that is stimulated by mutation of the Src homology 3 domain and by autophosphorylation at two distinct regulatory tyrosines. J. Biol. Chem. 275, 35631–35637 [DOI] [PubMed] [Google Scholar]
- 23. Taagepera S., McDonald D., Loeb J. E., Whitaker L. L., McElroy A. K., Wang J. Y., Hope T. J. (1998) Nuclear-cytoplasmic shuttling of C-ABL tyrosine kinase. Proc. Natl. Acad. Sci. U.S.A. 95, 7457–7462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goff S. P., Gilboa E., Witte O. N., Baltimore D. (1980) Structure of the Abelson murine leukemia virus genome and the homologous cellular gene. Studies with cloned viral DNA. Cell 22, 777–785 [DOI] [PubMed] [Google Scholar]
- 25. Wang J. Y., Ledley F., Goff S., Lee R., Groner Y., Baltimore D. (1984) The mouse c-abl locus. Molecular cloning and characterization. Cell 36, 349–356 [DOI] [PubMed] [Google Scholar]
- 26. Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. (1984) Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell 36, 93–99 [DOI] [PubMed] [Google Scholar]
- 27. Melo J. V. (1996) The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype. Blood 88, 2375–2384 [PubMed] [Google Scholar]
- 28. Pendergast A. M. (2002) The Abl family kinases. Mechanisms of regulation and signaling. Adv. Cancer Res. 85, 51–100 [DOI] [PubMed] [Google Scholar]
- 29. Topham M. K., Bunting M., Zimmerman G. A., McIntyre T. M., Blackshear P. J., Prescott S. M. (1998) Protein kinase C regulates the nuclear localization of diacylglycerol kinase-ζ. Nature 394, 697–700 [DOI] [PubMed] [Google Scholar]
- 30. Rosti V., Bergamaschi G., Lucotti C., Danova M., Carlo-Stella C., Locatelli F., Tonon L., Mazzini G., Cazzola M. (1995) Oligodeoxynucleotides antisense to c-abl specifically inhibit entry into S-phase of CD34+ hematopoietic cells and their differentiation to granulocyte-macrophage progenitors. Blood 86, 3387–3393 [PubMed] [Google Scholar]
- 31. Deacon E. M., Pettitt T. R., Webb P., Cross T., Chahal H., Wakelam M. J., Lord J. M. (2002) Generation of diacylglycerol molecular species through the cell cycle. A role for 1-stearoyl, 2-arachidonyl glycerol in the activation of nuclear protein kinase C-βII at G2/M. J. Cell Sci. 115, 983–989 [DOI] [PubMed] [Google Scholar]
- 32. Wada I., Kai M., Imai S., Sakane F., Kanoh H. (1996) Translocation of diacylglycerol kinase α to the nuclear matrix of rat thymocytes and peripheral T-lymphocytes. FEBS Lett. 393, 48–52 [DOI] [PubMed] [Google Scholar]
- 33. Goldfarb D. S., Corbett A. H., Mason D. A., Harreman M. T., Adam S. A. (2004) Importin α. A multipurpose nuclear transport receptor. Trends Cell Biol. 14, 505–514 [DOI] [PubMed] [Google Scholar]
- 34. Cingolani G., Petosa C., Weis K., Müller C. W. (1999) Structure of importin-β bound to the IBB domain of importin-α. Nature 399, 221–229 [DOI] [PubMed] [Google Scholar]
- 35. Pemberton L. F., Paschal B. M. (2005) Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic 6, 187–198 [DOI] [PubMed] [Google Scholar]
- 36. Colón-González F., Kazanietz M. G. (2006) C1 domains exposed. From diacylglycerol binding to protein-protein interactions. Biochim. Biophys. Acta 1761, 827–837 [DOI] [PubMed] [Google Scholar]
- 37. Fukunaga-Takenaka R., Shirai Y., Yagi K., Adachi N., Sakai N., Merino E., Merida I., Saito N. (2005) Importance of chroman ring and tyrosine phosphorylation in the subtype-specific translocation and activation of diacylglycerol kinase α by d-α-tocopherol. Genes Cells 10, 311–319 [DOI] [PubMed] [Google Scholar]