SUMMARY
Inner ear hair cell differentiation requires Atoh1 function, while Eya1, Six1 and Sox2 are coexpressed in sensory progenitors and mutations in these genes cause sensorineural hearing loss. However, how these genes are linked functionally and the transcriptional networks controlling hair cell induction remain unclear. Here, we show that Eya1/Six1 are necessary for hair cell development and their coexpression in mouse cochlear explants is sufficient to induce hair cell fate in the nonsensory epithelium expressing low level Sox2 by activating not only Atoh1-dependent but also -independent pathways and that both pathways induce Pou4f3 to promote hair cell differentiation. Sox2 cooperates with Eya1/Six1 to synergistically activate Atoh1 transcription via direct binding to the conserved Sox- and Six-binding sites in Atoh1 enhancers and these proteins physically interact. Our findings demonstrate that direct and cooperative interactions between the Sox2, Six1 and Eya1 proteins coordinate Atoh1 expression to specify hair cell fate.
INTRODUCTION
The organ of Corti necessary for hearing is composed of sensory hair cells and nonsensory supporting cells; both are derived from common precursors within the prosensory domain that is distinctively marked by the expression of p27Kip1 and the SOXB1-HMG box transcription factor Sox2 at embryonic day 13.5 (E13.5) to E14.5 in mice (Chen and Segil, 1999; Fekete, 2000; Kiernan et al., 2005; Ruben, 1967). Recent genetic studies have shown that Sox2 is required for specifying the precursors (Kiernan et al., 2005), while the basic helix-loop helix (bHLH) transcription factor Atoh1 (also known as Math1) is essential for the differentiation of precursors into hair cells but not for their initial specification (Bermingham et al., 1999; Chen et al., 2002; Kiernan et al., 2005). Overexpression of Atoh1 in cochlear nonesensory epithelium induces new hair cells (Izumikawa et al., 2005; Zheng and Gao, 2000). The 1.4 kb Atoh1 enhancer, located ~3.4 kb 3′ of the Atoh1 coding sequence and containing two conserved elements, can direct expression of reporter transgenes to the inner ear hair cells (Chow et al., 2006; Helms et al., 2000). However, the relevance and sufficiency of these conserved elements in modulating Atoh1 activity are not established.
The transcription coactivator and phosphatase Eya1 and its cofactor homeodomain protein Six1 play essential roles in sensory organ development (Xu et al., 1999; Zheng et al., 2003; Zou et al., 2004). They are coexpressed with Sox2 in ventral otocyst, which elongates to form the cochlear duct, but their expression gradually becomes restricted to the differentiating hair cells, where Atoh1 is expressed (Zheng et al., 2003; Zou et al., 2008). Although Eya1 physically interacts with Six1 and Sox2 (Buller et al., 2001; Zou et al., 2008), how these transcription factors are linked functionally during hair cell fate induction and whether they directly activate Atoh1 transcription remain unclear.
Haploinsufficiency for EYA1 or SIX1 causes Branchio-Oto-Renal (BOR) or Branchio-Oto (BO) syndrome (Abdelhak et al., 1997a; Abdelhak et al., 1997b; Ruf et al., 2004), which are characterized by combinations of craniofacial defects, hearing loss and with or without kidney anomalies. Approximately 93% of BOR/BO patients show hearing loss, accounting for as many as 2% of profoundly deaf children (Abdelhak et al., 1997b). Inactivation of Eya1 or Six1 in mice results in early arrest of otic development at the otocyst stage (Xu et al., 1999; Zheng et al., 2003). Such early phenotype in their null mutants precluded evaluation of the specific roles of Eya1 or Six1 in sensory cell development.
In this study, we identify a gradient of Six1 expression in cells within the organ of Corti, which parallels the normal process of hair cell differentiation, but its onset of expression occurs slightly earlier than that of Atoh1. This suggests that Six1 may serve as a critical positive inducer for Atoh1 activation. We demonstrate that Eya1/Six1 act cooperatively with Sox2 to induce hair cell fate in cochlear nonsensory epithelium by activating Atoh1 transcription via direct binding to the Atoh1 enhancers. Our results provide functional and molecular linkages between these genes responsible for hair cell induction.
RESULTS
Six1 shows a gradient of expression that parallels normal process of hair cell differentiation
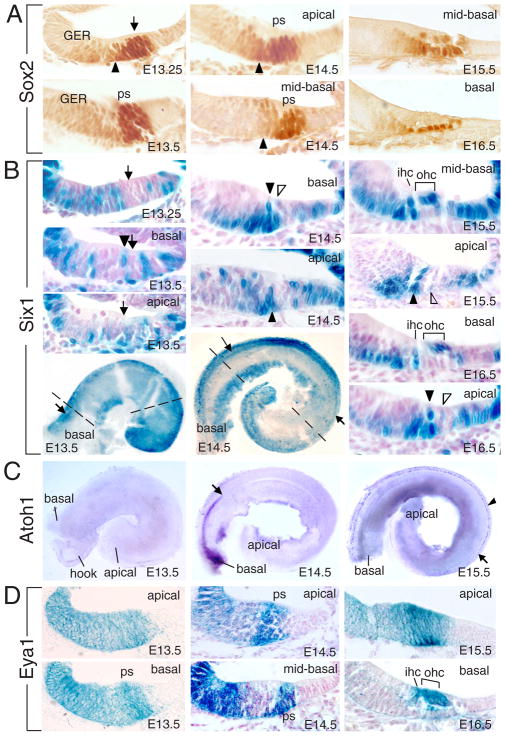
The ventral otocyst elongates and begins to coil ~E12 to reach a full 1.5 turns of cochlear duct by E17.5. Cells that give rise to the entire organ of Corti exit the cell cycle from apex towards base of the cochlear duct between E12 to E14 (Chen et al., 2002; Lee et al., 2006). In the nascent organ of Corti, the expression of Atoh1 begins in the base of the cochlea between E13.5 and E14.5, and spreads to the apex at ~E17.5 (Chen et al., 2002). How such developmental patterns of Atoh1 expression are achieved is unknown. To test how Sox2, Eya1 and Six1 might act to induce Atoh1 activation, we comparatively studied the spatiotemporal expression patterns of Sox2, Eya1 and Six1 in relation to the pattern of Atoh1 in the cochlea. Sox2, Eya1 and Six1 are coexpressed in the otic placode from as early as E8.5, the ventral portion of the otocyst, and the entire cochlear duct at E11.5 (Zheng et al., 2003; Zou et al., 2008). Between E12.5–13.5 when the progenitors within the prosensory domain begin to exit the cell cycle, high level of Sox2 expression becomes restricted to the postmitotic progenitors (Figure 1A). Later when the progenitors begin differentiating into supporting cells or hair cells, Sox2 expression is downregulated in nascent hair cells from E15.5 and disappears in more mature hair cells (Figure 1A). In contrast, Six1 expression disappears in the postmitotic progenitors within the prosensory domain between E12.5–13.5 (Figure 1B). From E13.5, Six1 expression was only observed in a single column of cells at the medial border of the prosensory domain near the basal cochlea duct where inner hair cells will appear, but not in the apical or medial regions (Figure 1B). At this time, Atoh1 expression has not begun (Figure 1C). By E14.5, Six1 expression domain extended towards the apex but its expression was not observed in the apical end. Sections revealed two columns of Six1+cells near the base where the inner and innermost outer hair cells will appear, suggesting that the gradient of Six1 expression proceeds medially to laterally. Within the discrete column of cells, Six1 expression initiates from base where supporting cells reside towards apical region where hair cells form. In a more apical turn of the cochlea, Six1 expression in the second column of cells has also initiated (Figure 1B). In contrast, in situ hybridization (ISH) of cochleae from Six1lacZ/+ littermate controls revealed that Atoh1 expression was only detectable in the basal region (Figure 1C). From E15.5, the sensory epithelium in the base of the cochlea has matured into a bilayered structure and Six1 is expressed in the differentiating inner and outer hair cells (Figure 1B). In less mature E15.5–16.5 sensory epithelium of the apical end where Atoh1 expression has not been induced (Figure 1C), the same pattern of Six1+ cells seen a day earlier in the base are now visible (Figure 1B). This temporal gradient of Six1 expression is similar to the pattern of Atoh1 but appears to occur before the onset of Atoh1 expression.
Figure 1. Expression of Sox2, Six1 and Eya1 in the organ of Corti.
(A) Cochlear sections at E13.25–E16.5 stained with anti-Sox2 antibody. Arrowheads point to border cells within the GER. Arrow points to the prosensory domain (ps). (B) Whole-mount and sections of X-gal stained Six1lacZ/+ cochlea from E13.25–E16.5. Arrows point to primordial organ of Corti. Arrowhead points to Six1+ cells in the medial border of the prosensory domain where inner hair cells (ihc) develop near the basal cochlea duct. Open arrowheads point to second column of Six1+ cells where innermost outer hair cells (ohc) appear. Dashed lines point to the planes of sections shown for E13.5 and E14.5. (C) Atoh1 ISH of cochleae from Six1lacZ/+ littermate controls as used in panel B. The apical limit of Atoh1 expression at E14.5 and in medial region at E15.5 (arrow) or in lateral region at E15.5 (arrowhead) are indicated. (D) Sections of X-gal stained Eya1lacZ/+ cochlea at E13.5–E16.5.
In contrast, Eya1 expression was observed in a broad group of cells throughout the thickness of the epithelium including the site of the future organ of Corti at E12.5–13.5 (Figure 1D), overlapping with Sox2, but later becomes abundantly distributed in differentiating hair cells (Figure 1D), which express Six1 and Atoh1.
In addition to the sensory epithelium, all three genes show differential expression in nonsensory epithelium with Eya1 and Sox2 in the greater epithelial ridge (GER) and Six1 in both the GER and LER (lesser epithelial ridge). While the expression patterns of these genes suggest that they may play essential roles in regulating differentiation of the organ of Corti, the pattern of Six1 expression in the organ of Corti suggests that it may serve as a critical positive regulator for stimulating Atoh1 activation near the basal cochlear duct.
Coexpression of Eya1/Six1 is sufficient to induce ectopic hair cells in cochlear GER cells
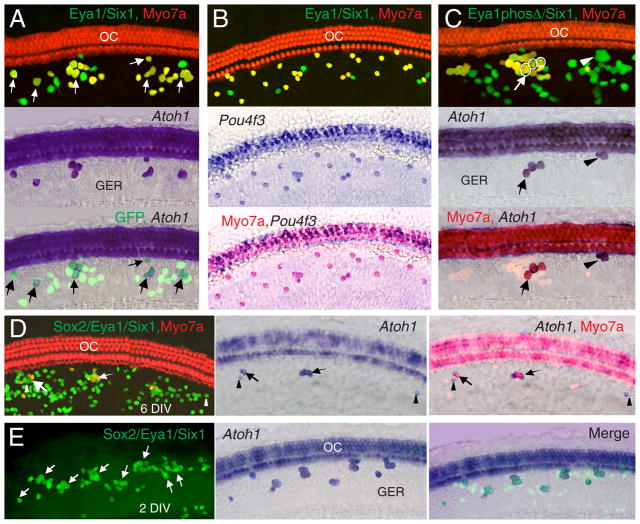
We next tested whether Eya1 and Six1 alone or combined are sufficient to induce hair cell development by electroporating E13.5–14.0 cochleae with Eya1.GFP and Six1.GFP plasmids to induce their ectopic expression in GER cells. After 6 DIV (days in vitro), we analyzed the development of hair cells by examining the expression of hair cell-specific marker Myosin 7a (Myo7a) (Hasson et al., 1995). Multiple transfected cells in the GER were observed (Figure 2), but only very few transfected with either Eya1.GFP or Six1.GFP were Myo7a+ (Figure 2A,B), while none transfected with a control.GFP vector were Myo7a+ (Tables 1, S1). In contrast, ~89% of GFP+ cells transfected with both Eya1/Six1 were Myo7a+ (Figure 2C; Table 1, S1), indicating that the combined action of Eya1/Six1 is necessary for inducing hair cells in the nonsensory epithelium.
Figure 2. Coexpression of Eya1/Six1 induces ectopic hair cells in GER cells.
E13.5 cochlea electroporated with Eya1.GFP (A), Six1.GFP (B) or both (C) stained with anti-Myo7a antibody after 6 DIV. Transfected cells are identified as GFP+ (green) and hair cells are identified as Myo7a+ (red). oc, organ of Corti. Arrows point to Myo7a+GFP+ cells.
Table 1.
Induction of endogenous Atoh1 expression and hair cell differentiation by Eya1, Six1 and Sox2
| Constructs | GFP+/Explants 6 DIV |
Atoh1+ Myo7a 6 DIV |
Atoh1+ Myo7a− 6 DIV |
Atoh1− Myo7a 6 DIV |
%/% (Atoh1+:GFP+/Myo7a+:GFP+) | %/% (Atoh1+:Myo7a+/Atoh1−:Myo7a+) |
%(Atoh1+:GFP+) (Explants 2, 3 or 4 DIV |
%(Pou4f3+: GFP+) (Explants 2, 3, 4 or 6 DIV |
|---|---|---|---|---|---|---|---|---|
| Eya1 | 325/3 | 22 | 2* | 1 | 7.4±0.6/7.1±0.5 | 95.7±0.9/4.3±0.3 | 7.3±0.8(9:124)(3), 2 DIV | |
| Six1 | 271/3 | 15 | 2* | 3 | 6.3±0.7/6.6±0.6 | 83.3±1.0/16.7±0.9 | 6.2±0.2(11:178)(3), 2 DIV | |
| Eya1/Six1** | 441/6 | 152 | 0 | 240 | 34.5±1.2/88.9±0.5 | 38.8±0.7/61.2±0.5 | 33.6±1.0(80:238)(3), 2 DIV | 88.2±1.1(284:322) (2), 6 DIV |
| Eya1/Six4 | 266/4 | 15 | 1* | 23 | 6.0±0.2/14.3±0.3 | 39.5±1.0/60.5±0.7 | 6.2±0.9(28:449)(5), 2 DIV | |
| Eya4/Six1 | 438/5 | 51 | 32* | 206 | 19.0±0.9/58.7±1.1 | 19.8±0.7/80.2±0.3 | 19.0±0.9(98:517)(5), 2 DIV | |
| Eya1phosΔ/Six1 | 351/4 | 11 | 5* | 72 | 4.6±1.1/23.7±0.8 | 13.3±0.7/86.7±0.5 | 4.9± 1.1(22:451)(5), 2 DIV | |
| Sox2 | 498/5 | 0 | 6* | 0 | 1.2±0.3/0 | 0/0 | 3.1±0.3(4:129)(3), 2 DIV | |
| Sox2/Eya1 | 520/7 | 1 | 2* | 3 | 0.5±0.8/0.7±0.8 | 25.0±0.8/75.0±0.8 | 9.3±0.9(26:280)(3), 2 DIV | |
| Sox2/Six1 | 336/4 | 0 | 0 | 1 | 0/0.3±0.7 | 0/100 | 7.5±1.2(20:268)(3), 2 DIV | |
| Sox2/Eya1/Six1 | 389/4 | 15 | 6* | 23 | 5.4±0.7/9.8±0.8 | 39.5±1.6/60.5±2.1 | 39.2±0.9(133:339)(4), 2 DIV | |
| 29.9±0.7(88:290)(4), 3 DIV | 2.0±0.8(7:290)(4), 3 DIV | |||||||
| 30.0±0.4(78:259)(4), 4 DIV | 2.3±0.6(6:259)(4), 4 DIV | |||||||
| Sox2/Atoh1 | 234/3 | 0 | 1 | 0 | 0/0*** | 0/0*** | 95.6±4.5(214:220)(3), 2 DIV | 16.7±2.4(38:220)(3), 2 DIV |
| 66±1.0(40:167)(2), 3 DIV | 7.2±0.1(12:167)(2), 3 DIV | |||||||
| 29.2±0.9(31:181)(2), 4 DIV | 2.7±0.5(5:181)(2), 4 DIV | |||||||
| Atoh1 | 311/4 | 309 | 0 | 0 | 99.4±0.6/99.4±0.6 | 100/0 | 99.1± 0.9(205:207)(3), 2 DIV | 17.0±2.5(36:207)(3), 2 DIV |
| 100(150:150)(2), 3 DIV | 48.7±0.1(73:150)(2), 3 DIV | |||||||
| 100(148:148)(2), 4 DIV | 78.9±1.1(117:148)(2), 4 DIV | |||||||
| Control | 288/3 | 0 | 0 | 0 | 0 | 0 | 0(0:221)(3), 3 DIV | 0(0:221)(3), 3 DIV |
Transfected cells in the GER of E13.5–14.0 explants were identified as GFP+ cells. Explants after 2–6 DIV were processed for in situ hybridization for detecting Atoh1 expression and immunohistochemistry for detecting Pou4f3 or Myo7a expression.
Note that some of the Atoh1+ cells were negative for Myo7a, indicating that they failed to differentiate into hair cells.
Coexpression of Eya1/Six1 promoted Atoh1 expression and hair cell induction. Among the ectopic Myo7a+ cells, ~38.8% were Atoh1+, but ~61.2% were Atoh1−.
When Sox2 was coexpressed with Atoh1, it antagonized the effect of Atoh1 to induce hair cells. Although Pou4f3 expression was initiated normally at 2 DIV when compared with those induced by Atoh1 alone, its expression was decreased after 2 DIV, in contrast to that induced by Atoh1 alone. In the absence of hair cell differentiation, Atoh1 expression eventually disappeared. Atoh1+, Pou4f3+ or Myo7a+ or GFP+ cells were counted from each explant and the ratios of Atoh1+ vs. GFP+, Myo7a+ vs. GFP+ or Pou4f3+ vs. GFP+ from each explant were quantified and values are expressed as means±standard deviations. Significance was determined by comparing each of the sample groups using StatView’s t-test. P-values were between P<0.0001 and P=0.0379.
To determine the specificity of hair cell induction mediated by Eya1/Six1, we tested other Eya or Six gene family members. Although mutations in the human SIX5 gene also cause BOR/BO syndrome (Hoskins et al., 2007), coexpression of Six5/Eya1 did not show obvious synergism in inducing hair cells, similar to Eya1/Six2 or Eya2/Six1 (Figure S1; Table S1). In contrast, coexpression of Eya1/Six4 induced ~14% while Eya4/Six1 induced ~59% Myo7a+ cells (Figure S1; Table S1), suggesting potential redundancies between these Eya and Six genes during hair cell induction. This is consistent with previous observations that Six4 and Eya4 are expressed in otic placode (Borsani et al., 1999; Streit, 2002) and that Eya4 has a role in hair cell development (Wayne et al., 2001). Together, these results demonstrate that the cooperative interaction of Eya1/Six1 acts most efficiently to activate a specific downstream regulatory program controlling hair cell induction.
EYA1 or SIX1 mutations identified from human patients and deletion of distinct biochemical activities of Eya1 abolish their ability to induce hair cells
Five missense mutations that cause single amino acid substitution in the Eya domain of Eya1 (E330K, G393S, S454P, L472R and R514G) have been identified from human patients (Figure S2), and we previously reported that only R514G mutation did not appear to obviously affect the Eya1-Six1 interaction in yeast-two hybrid and GST pulldown assays (Buller et al., 2001). To directly investigate the effect of these mutations in hair cell induction, each of these mutant constructs was cotransfected with Six1. Coexpression of Eya1R514G/Six1 significantly reduced the number of Myo7a+ cells (Figure S2; Table S1), while the remaining mutants abolished the ability of Eya1 to induce hair cells (Figure S2; Table S1). Similarly, no or very few hair cells were observed when Eya1 was expressed with Six1 mutant constructs, including R110W substitution within the Six-specific domain, Y129C substitution and delE133 (glutamic acid deletion) within the homeodomain isolated from BOR/BO patients (Ruf et al., 2004) (Figure S2; Table S1). This in vivo functional assessment of human mutations in hair cell induction directly provides a basis for hearing loss that occurs in human patients.
Eya1 possesses a N-terminal transactivation domain (Xu et al., 1997a) and a conserved C-terminal Eya domain (Xu et al., 1997b) with intrinsic tyrosine phosphatase activity (Tootle et al., 2003), and interacts with Six1. Deletion of the N- or C-terminus or a point mutation that causes a single amino acid substitution of aspartic acid to asparagine at amino acid residue 327 (Eya1D327N) within the Eya domain, which disrupts the tyrosine phosphatase activity (Tootle et al., 2003), abolished the ability of Eya1 to induce hair cells, as coexpression of each of these Eya1 mutants with Six1 only induced a few Myo7a+ cells, similar to that observed with Six1 alone (Figure S2; Table S1). Thus, these results demonstrate the requirement of the distinct biochemical activities of Eya1 in promoting hair cell development.
Induction of hair cell fate by Eya1-Six1 is mediated via not only Atoh1-dependent but also – independent pathways
Atoh1 is essential for the differentiation of hair cells from sensory precursors (Bermingham et al., 1999; Chen et al., 2002). Therefore, we examined whether Eya1/Six1 act upstream of and in the same pathway as Atoh1 to activate the hair cell developmental program. Surprisingly, coexpression of Eya1/Six1 resulted in two types of ectopic hair cells: Atoh1+ (~39% of Myo7a+ cells) and Atoh1− cells (~61% of Myo7a+ cells) (Figure 3A; Table 1). Coexpression of Eya1/Six4 or Eya4/Six1 also resulted in Atoh1+ and Atoh1− cells; however, among the ectopic hair cells, the number of Atoh1− cells was largely increased (~80% of Myo7a+ cells) and ~39% of Atoh1+ cells failed to differentiate into hair cells (Figure S3; Table 1). Atoh1+Myo7a− cells were also observed when Eya1 and Six1 alone or both Eya1/Six4 were expressed but not with Eya1/Six1 at 6 DIV (Table 1). Thus, in addition to activating Atoh1 expression, the combined action of Eya1/Six1 appears to be required for hair cell differentiation.
Figure 3. Coexpression of Eya1/Six1 induces Atoh1-dependent and – independent pathways, and Sox2 acts cooperatively with Eya1/Six1 to ectopically activate endogenous Atoh1 gene.
Explants transfected with the indicated constructs stained with Atoh1 or Pou4f3 probe, anti-Myo7a antibody (red) or anti-GFP antibody (green). Arrows point to Atoh1+Myo7a+ or Atoh1+ and arrowheads point to Atoh1+Myo7a− cells.
To characterize the identity of the Atoh1-independent pathway activated by Eya1/Six1, we examined the expression of the POU domain transcription factor Pou4f3, which is important for inner ear hair cell development and may act downstream of Atoh1 and participate in the maintenance of Atoh1 expression (Hertzano et al., 2004). ISH or immunohistochemistry revealed that all Myo7a+ cells induced by Eya1/Six1 were Pou4f3+ (Figure 3B; Tables 1, S2). Thus, Pou4f3 appears to be the common downstream factor that is activated by both pathways.
To rule out the possibility that Atoh1−Myo7a+ cells induced by Eya1/Six1 was not due to downregulation of Atoh1 expression in those cells, we examined the expression of Atoh1 from 1.5–6 DIV and found that it reached ~43% by 4 DIV when Myo7a became expressed, then decreased to 34–35% by 5 DIV when Myo7a reached a peak (Table S2). Atoh1 expression reached a peak (~47% at 88 hours) before Myo7a, and although Pou4f3 expression appeared later than Atoh1, the number of Atoh1+Pou4f3+ cells also reached a peak (~46.3% at 88 hours), and both declined thereafter (Table S2). In contrast, the induction of Atoh1−Pou4f3+ cells rapidly increased after 88 hours and reached a peak of ~54.9% by 5 DIV (Table S2). Since we observed a few Atoh1+Myo7a− cells at 5 DIV but not at 6 DIV, the reduction of Atoh1+ or Atoh1+Pou4f3+ cells between 3–5 DIV might reflect the eventual disappearance of Atoh1 and Pou4f3 expression in cells that fail to differentiate into Myo7a+ cells.
Further confirmation that Eya1/Six1 promotes hair cell development via Atoh1-independent pathway was achieved through knockdown of endogenous Atoh1 using Atoh1 shRNA (shAtoh1). Consistent with previous observation (Zheng and Gao, 2000), ectopic expression of Atoh1 in GER cells induced ~99.6% of Myo7a+ cells (Table S2) which was completely blocked by coexpression of shAtoh1 (Table S2). Coexpression of shAtoh1 with Eya1/Six1 specifically blocked the production of Atoh1+Myo7a+ cells but not Atoh1−Myo7a+ cells (Table S2). Together, these results demonstrate that Eya1/Six1 are sufficient to transactivate not only Atoh1-dependent but also -independent transcriptional cascades to promote hair cell development.
Phosphorylation of two conserved MAPK sites modulates Eya1 activity in hair cell differentiation
The Drosophila Eya has been suggested to be a substrate for mitogen-activated protein kinase (MAPK), which acts downstream in the receptor tyrosine kinase (RTK) signaling pathway (Hsiao et al., 2001), and the two phosphorylation sites (proline-X-serine/threonine-P) located ~80 residues upstream of the Eya domain are conserved in mammalian Eya gene products (Figure S2). To investigate if phosphorylation of the Eya1 protein is essential for hair cell induction, we mutagenized the MAPK phosphorylation consensus sites by substituting threonine to alanine at both positions (T248A and T284A, the resulting mutant was named as Eya1phosΔ) and confirmed that this mutant blocked phosphorylation of Eya1 by MAPK p38α (data not shown). Coexpression of Eya1phosΔ with Six1 severely diminished the efficiency of hair cell induction, and ~31% of ectopic Atoh1+ cells were Myo7a− after 6 DIV (Figure 3C; Table 1). A large reduction in the number of Atoh1+ cells was similarly observed even after 2 DIV (Table 1). Together, these results indicate that the MAPK-mediated phosphorylation of Eya1 is essential not only for inducing Atoh1 activation but also for regulating hair cell differentiation. Thus, Eya1 may link the extracellular RTK/Ras/MAPK signaling cascade to hair cell development.
Sox2 antagonizes the ability of Eya1/Six1 to induce hair cell differentiation but acts cooperatively with Eya1/Six1 to transactivate Atoh1 expression
As Sox2 is coexpressed with Eya1/Six1 in prosensory progenitors before the onset of Atoh1 expression and in nascent hair cells, we tested if Sox2 may have a synergistic effect with Eya1/Six1 in inducing hair cell fate but not differentiation by cotransfecting Sox2 with Eya1 and/or Six1 into the GER. Coexpression of three factors together only induced a few Myo7a+ cells (Figure 3D; Table 1), similar to that observed with Eya1 or Six1 alone. Sox2 alone failed to induce Myo7a+ cells, while Sox2/Eya1 or Sox2/Six1 only induced a few (Table 1). Thus, Sox2 appears to exert antagonistic effects on Eya1-Six1 in promoting hair cell differentiation.
A recent report has shown that Sox2 antagonizes the ability of Atoh1 to induce hair cell fate in vitro (Dabdoub et al., 2008). Thus, our observation raises two possibilities. First, Sox2 may repress the activity of Atoh1 or its downstream factors such as Pou4f3 to inhibit hair cell differentiation. Second, the marked reduction of Myo7a+ cells might be caused by an antagonistic effect between Sox2 and Eya1/Six1 in activating Atoh1 to induce hair cell fate. ISH revealed that Atoh1+ cells were present in explants transfected with Sox2 alone, Sox2/Eya1 or Sox2/Eya1/Six1 after 6 DIV (Figures 3D, S3), but the number was largely reduced (Table 1). Similarly, Atoh1+Myo7a− and Atoh1−Myo7a+ cells were observed (Figure 3D; Table 1). The reduction of Atoh1+ cells might reflect decreased activation o0r disappearance of Atoh1 mRNA in the absence of hair cell differentiation. To investigate this, explants were processed for ISH after 2 DIV for detecting Atoh1 expression. Surprisingly, Atoh1+ cells were increased from ~34% to ~39% when Sox2 was coexpressed with Eya1/Six1 (Figure 3E; Table 1). When Sox2 was expressed alone, ~3% of transfected cells were also Atoh1+, while combination of Sox2/Eya1 or Sox2/Six1 slightly increased the number of Atoh1+ cells induced (Table 1). Thus, Sox2 appears to act synergistically with Eya1/Six1 to induce Atoh1 activation.
To investigate whether Sox2 antagonizes the activity of Atoh1 or other factors downstream of Atoh1 such as Pou4f3 to inhibit hair cell differentiation, we performed immunostaining for Pou4f3 and found that ~30% of the cells transfected with three factors after 3 DIV were still Atoh1+ (Table 1). However, the majority of Atoh1+ cells were Pou4f3− and very few Pou4f3+Atoh1+ cells were detected (Table 1). Coexpression of Sox2/Atoh1 showed normal induction of Pou4f3 expression at 2 DIV (~16.7%), similar to those induced by Atoh1 alone (~17%). However, in contrast to the rapid increase in Pou4f3 expression induced by Atoh1 after 2 DIV, not only was Pou4f3 expression significantly reduced when Sox2 was coexpressed with Atoh1, but also the level of Atoh1 expression failed to be maintained (Table 1). Thus, as Sox2 does not appear to antagonize the activity of Atoh1 to initiate Pou4f3 activation, it may inhibit hair cell differentiation by directly antagonizing Pou4f3 or other factors required for maintaining Atoh1 expression.
Sox2/Eya1/Six1 cooperatively regulate Atoh1 enhancers
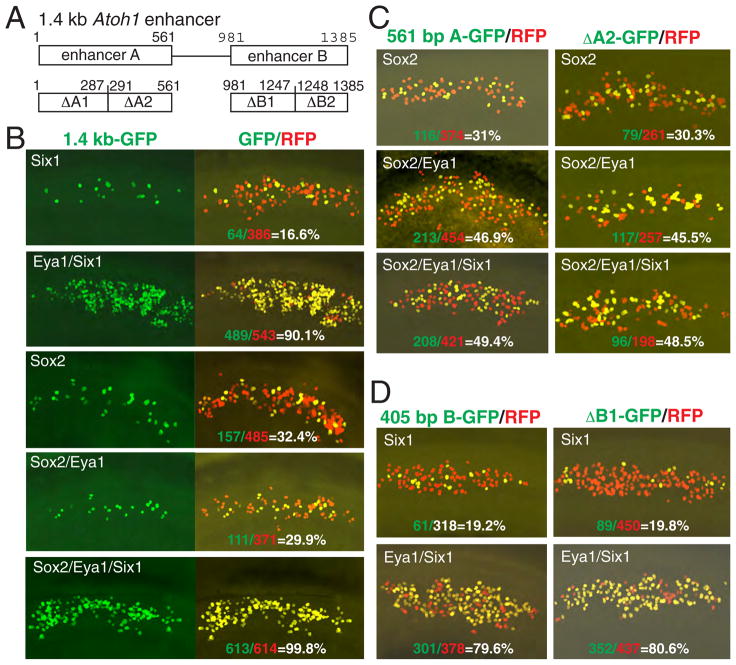
To determine whether the synergistic activation of Atoh1 expression by Sox2, Eya1 and Six1 reflects direct coregulation, we characterized the 1.4 kb Atoh1 enhancer. A 1.4 kb Atoh1 enhancer reporter construct driving GFP (Figure 4A) was electroporated into the GER together with a red fluorescent protein (RFP) vector, which was used to identify transfected cells, and analyzed after 24–36 hours. Transfection of the reporter alone or together with Eya1 resulted in no or very few GFP+ cells (Table S3). In contrast, cotransfection of the reporter together with Six1 or Sox2 induced the reporter transcription (16.6% or 32.4% of total RFP+ cells) (Figure 4B; Table S3), indicating that the 1.4 kb enhancer might contain elements that bind with Six1 or Sox2 protein. Coexpression of Eya1/Sox2 slightly reduced the reporter activity (from 32.4% to 29.9%). However, consistent with our earlier observation that Eya1 is a transcriptional coactivator (Xu et al., 1997a), a dramatic increase in GFP+ cells was observed when Six1 was coexpressed with Eya1 (~90.1%) (Figure 4B), suggesting that Six1 effectively synergizes with Eya1 to induce the reporter activity. Coexpression of Six1/Sox2 also increased the number of GFP+ cells (~52%) (Table S3), indicating that Sox2/Six1 act cooperatively to regulate the reporter expression. Notably, however, combination of all three factors resulted in a robust activation of the reporter in almost all transfected cells (~100%) (Figure 4B; Table S3), demonstrating a synergistic enhancement of transcription from the reporter. Together, these observations demonstrate a requirement of cooperative activities between Sox2, Eya1 and Six1 in activating Atoh1 transcription and that the 1.4 kb enhancer is sufficient to mediate such cooperativity.
Figure 4. Synergistic actions of Sox2/Eya1/Six1 on transcription via Atoh1 enhancers.
(A) Schematic of Atoh1 enhancers driving GFP reporter under control of β-globin minimal promoter. (B) Explants electroporated with the 1.4 kb Atoh1-GFP and different combinations of Sox2, Eya1 and Six1. A control plasmid expressing RFP was cotransfected to identify transfected cells. (C) The 561 bp enhancer A or 271 bp ΔA2 shows similar responsiveness to Sox2-mediated transcription. (D) The 405 bp enhancer B or 267 bp ΔB1 shows similar responsiveness to Six1-mediated transcription.
Our observations suggest that multiple elements may be required for effective coresponsiveness of the enhancer to Sox2, Eya1 and Six1. The 1.4 kb enhancer sequence contains two highly conserved elements (enhancer A and B, Figure 4A) and each can drive a lacZ transgene expression in the inner ear (Helms et al., 2000). To investigate their requirement and cooperativity in mediating Sox2- and Six1-dependent transcription, we made two smaller reporter constructs (561 bp for enhancer A and 405 bp for enhancer B) and tested each by electroporation. The 561 bp Atoh1-GFP reporter responded very weakly to Eya1, Six1 or Six1/Eya1 (<5%), but its responsiveness to Sox2 (~31%) was comparable to the 1.4 kb enhancer (~32.4%) (Figure 4B,C; Table S3), indicating this region as essential for Sox2-mediated transcriptional activation. However, unlike the 1.4 kb enhancer, a noticeable enhancement of the 561 bp Atoh1-GFP reporter activity was observed when Sox2/Eya1 were coexpressed (46.9%), and combination of Sox2/Eya1/Six1 resulted in further increase of the reporter activity (~49.4%) (Figure 4C), indicating a synergistic interaction between these factors. In contrast, the 405 bp Atoh1-GFP failed to respond to Sox2 but its responsiveness to Six1-mediated transcription was almost as active as the 1.4 kb Atoh1-GFP reporter (19.2% vs 16.6%) (Figure 4B,D; Table S3). Coexpression of Six1/Eya1 markedly increased the reporter activity (~80.9%), but Sox2/Eya1/Six1 did not lead to further enhancement of the reporter activity (Table S3), demonstrating that the 405 bp enhancer contains Six1-responsive element.
To define more precisely the Sox2- and Six1-responsive elements, we made four smaller reporter constructs containing subregions of the enhancers (ΔA1, ΔA2, ΔB1 and ΔB2) (Figure 4A). The 287 bp ΔA1-GFP or the 138 bp of ΔB2-GFP reporter failed to respond to Sox2 or Six1 (Table S3), whereas the 271 bp ΔA2-GFP or 267 bp ΔB1-GFP reporter responded to Sox2- or Six1-mediated transcription respectively and their activities were comparable to the 561 bp or 405 bp enhancer (Figure 4C,D; Table S3). This deletion analysis identifies the 271 bp ΔA2 and 267 bp ΔB1 regions as essential elements for mediating the Sox2- and Six1-dependent transcriptional activation respectively.
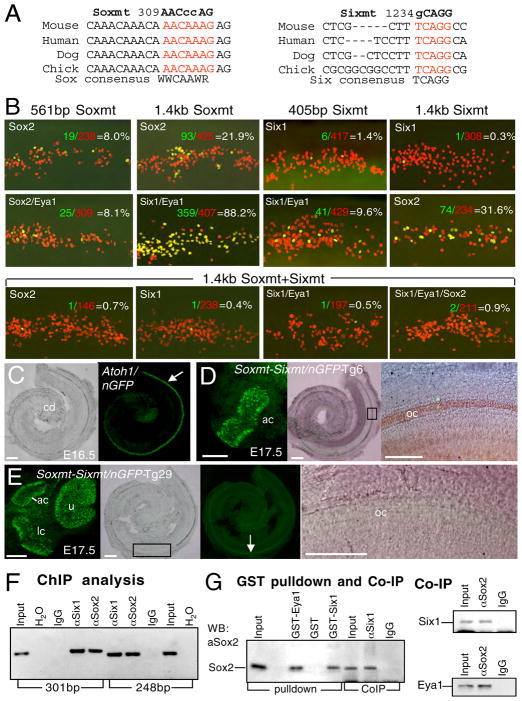
We next searched for potential DNA binding sites for Sox2 and Six1 in those regions and found a highly conserved AACAAAG (309–315) motif in the 271 bp ΔA2 sequence (Figures 5A, S4), perfectly matching the SOX binding site consensus sequence (A/T)(A/T)CAA(A/T)(G/A/T), while two additional, less conserved Sox-binding sites were also present (Figure S4). A 100% conserved binding consensus site TCAGG (1234–1238) for Six proteins (Spitz et al., 1998; Yu et al., 2006) was found within the 267 bp ΔB1 sequence (Figure 5A). To map the Sox2- and Six1-responsive sequences, we first evaluated the conserved sequences by introducing point mutations into the Sox- (AACAAAG to AACccAG) and Six- (TCAGG to gCAGG) binding sites to generate 561 bp Soxmt-GFP and 405 bp Sixmt-GFP reporter constructs, respectively (Figure 5A). Expression of Sox2 alone or together with Eya1 only weakly drove transcription from the Soxmt-GFP (~8%) (Figure 5B; Table S3), while point mutation of the Six-binding site almost abolished the reporter responsiveness to Six1 or Six1/Eya1 (Figure 5B; Table S3). We mutagenized another Sox-binding site (ATTTGTT to ATggGTT, named as Soxmt2) (Figure S4) and tested its involvement in mediating Sox2-dependent transcription. The Soxmt2-GFP reporter showed similar responsiveness as the wild-type reporter (Table S3), but when both sites were mutated, the responsiveness of the reporter to Sox2-mediated transcription was completely abolished (Table S3). Thus, this nonconserved Sox-binding site appears to mediate weak responsiveness of the reporter to Sox2 only when the conserved Sox-binding site is mutated.
Figure 5. Interactions between Eya1/Six1/Sox2 and Atoh1 in inducing hair cell specification.
(A) Multiple DNA sequence alignment of vertebrate Atoh1 enhancer A containing the conserved Sox-binding site (red) and enhancer B containing the conserved Six-binding site (red). Point mutations of the Sox- or Six-binding site (Soxmt or Sixmt) are listed. (B) Responsiveness of different Soxmt- or Sixmt-GFP reporters to Sox2- or Six1-mediated transcription in explants. (C) Bright- and dark-field images of cochlea duct (cd) from 1.4 kb Atoh1-GFP transgenic embryo at E16.5 expressing GFP in the hair cells. (D) Anterior crista (ac) and cochlea from 1.4 kb Atoh1Soxmt+Sixmt-GFP transgenic embryo at E17.5. Darkfield image showing GFP+ cells in anterior crista. Merged bright- and dark-field image showing very few GFP+ cells in the organ of Corti (oc). Right panel is a higher magnification of boxed area. (E) Dissected utricle (u), anterior (ac), lateral crista (lc) and cochlea from 1.4 kb Atoh1Soxmt+Sixmt-GFP transgenic embryo at E17.5. Darkfield image showing GFP expression in vestibular hair cells. Bright- and dark-field images showing no GFP+ cells in the organ of Corti. Arrow indicates autofluorescence. Right panel is a higher magnification of boxed area. Scale bars: 100μm. (F) ChIP analysis. Anti-Sox2 or –Six1 antibody is used for IP. Normal rabbit or goat IgG for ChIP and water for PCR were used as negative control. (G) GST pulldown assay showing in vitro translated Sox2 can be pulled-down by GST-Eya1D or –Six1 fusion protein and co-IP assays demonstrating physical interaction between Sox2/Eya1/Six1.
Sequence examination also revealed a conserved binding site for the Drosophila So protein (C/TGATA) present adjacent to the Sox-binding site (Figure S4). Point mutation of this site (TGATA to TtgTg, named as Somt) completely abolished the weak responsiveness to Six1 and weak synergism between Six1 and Sox2/Eya1 in activating enhancer A-GFP reporter, and additionally reduced the weak activation of the Soxmt-GFP reporters mediated by Sox2 (Table S3), suggesting that Six1 may bind to the So-binding site to cooperate with Sox2 in regulating Atoh1 activation.
When the Soxmt and Sixmt mutations were introduced into the 1.4 kb Atoh1-GFP reporter, the Sixmt-GFP failed to respond to Six1-mediated transcription but showed normal responsiveness to Sox2-mediated transcription (Figure 5B; Table S3), while the 1.4 kb Soxmt-GFP showed normal responsiveness to Six1- or Six1/Eya1-mediated transcription but reduced responsiveness to Sox2-mediated transcription (21.9%, compare with 32.4% of wild-type) (Figure 5B; Table S3), which was repressed when Eya1 and/or Six1 was coexpressed and became similar to that stimulated by Eya1 and/or Six1 (Table S3). Similar observation was obtained when Soxmt2 was added, while Soxmt2-GFP alone showed similar responsiveness as the wild-type reporter. These results further suggest that when the conserved Sox-binding site is mutated, the other Sox-binding sites become responsive to Sox2-dependent transcription, but do not appear to mediate cooperative activation by coexpression of Eya1 and/or Six1. Remarkably, however, when both Soxmt and Sixmt were present, the reporter totally failed to respond to either Sox2- or Six1-mediated transcription (Figure 5B; Table S3). Furthermore, when this mutant Atoh1-GFP transgene was assayed in vivo via transient transgenic analysis, no or very few GFP+ cells were observed in the organ of Corti (Figures 5C–E, S4), whereas strong expression was observed in the vestibular hair cells (Figures 5D,E; Table S4).
Electrophoretic mobility shift assays (EMSAs) confirmed that the Sox2 and Six1 proteins bound to these sites and the protein-DNA binding was totally abolished by the mutated binding sites (Figure S4). Together, these results indicate that the Sox2- or Six1-dependent transactivation of the Atoh1-GFP reporters requires direct binding of Sox2 or Six1 protein to the conserved Sox- or Six-binding site respectively, and such binding is necessary for activating Atoh1 transcription in vivo.
Sox2 and Six1 bind Atoh1 enhancers in vivo and the three Sox2/Six1/Eya1 proteins physically interact
To investigate whether endogenous Sox2 and Six1 proteins bind to the conserved Sox- and Six-binding sites in the progenitors within the prosensory domain, we performed chromatin immunoprecipitation (ChIP) assays (Figure 5F). Genomic fragments bound by the Sox2 or Six1 protein were immunoprecipitated with specific antibodies and analyzed by PCR with primer sets designed to amplify a 301 bp containing the Sox-binding site or a 248 bp region containing the Six-binding site respectively. As a result, both enhancer regions were immunoprecipitated by either the anti-Sox2 or -Six1 antibody but not by normal rabbit or goat IgG (Figure 5F) or when using primers that amplify a part of Atoh1 exon (data not shown). These experiments demonstrate specific binding of the Sox2 and Six1 proteins to the conserved regions of the Atoh1 enhancer in vivo.
The above observation also indicates that Six1 and Sox2 are physically associated in vivo, so we tested for a direct interaction between these proteins. GST-Six1 or –Eya1 (Eya domain only) was able to pulldown in vitro translated Sox2 protein, indicating that Sox2 directly interacts with Eya1 or Six1 (Figure 5G). Coimmunoprecipitation (co-IP) assays using proteins isolated from E13.0–E13.5 cochleae and anti-Six1 or –Sox2 antibody revealed that these proteins were coimmunoprecipitated (Figure 5G). Collectively, these results demonstrate that Six1, Eya1 and Sox2 interact directly with each other in vitro and in vivo.
Endogenous Sox2 and normal Eya1 or Six1 activity are required for Atoh1 activation
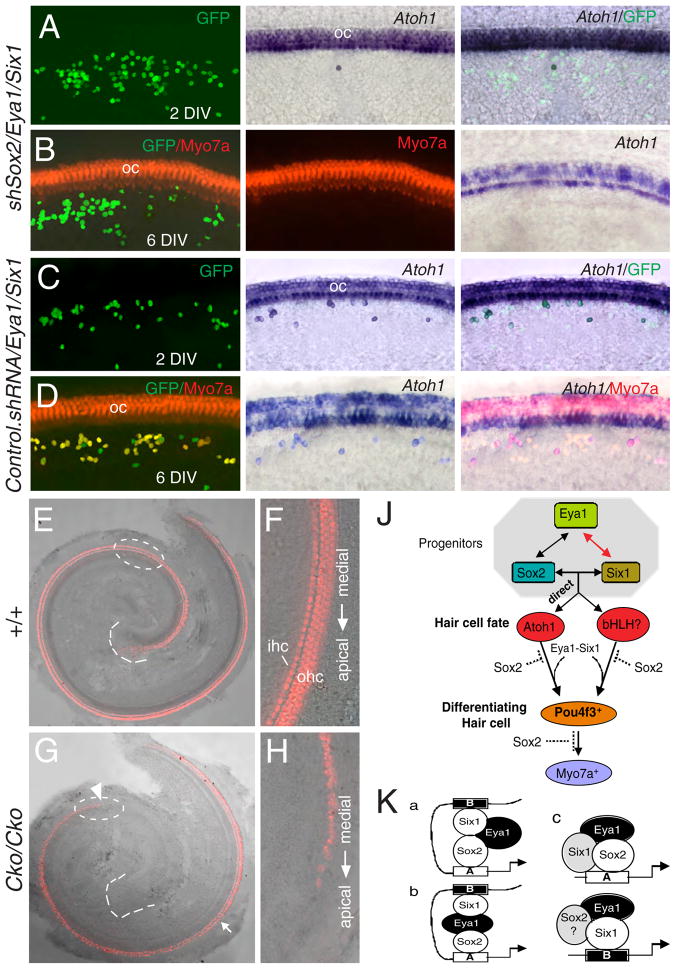
Although Eya1 acts as a transcriptional coactivator, expression of Eya1 alone in the GER induces a few Atoh1+ and Myo7a+ cells (Table 1), suggesting that endogenous Sox2 or Six1 activity in the GER could collaborate with exogenous Eya1 to activate Atoh1 transcription and hair cell differentiation. To confirm that endogenous Sox2 activity is necessary for activating Atoh1 expression, we coexpressed Sox2 shRNA (shSox2) together with Eya1/Six1 and found that knocking down endogenous Sox2 activity blocked not only endogenous Atoh1 activation but also the 1.4 kb Atoh1-GFP reporter activation (Figure 6A–D; Tables 2, S5).
Figure 6. Coexpression of shSox2 with Eya1/Six1 and temporal and cell-specific deletion of Eya1 blocks hair cell induction.
(A–D) Explants transfected with the indicated constructs stained with Atoh1 probe, anti-Myo7a (red) or –GFP (green) antibody after 2 or 6 DIV. (E) Merged image of bright- and dark-field wild-type cochlea at E18.5 stained with anti-Myo7a (red). (F) Higher magnification of circled area in E showing Myo7a+ inner (ihc) and outer hair (ohc) cells. (G) Merged image of bright- and dark-field Eya1Cko/Cko cochlea at E18.5 stained with anti-Myo7a. Arrow points to irregular organization of hair cells, especially outer hair cells from medial-to-apical direction (from arrow-to-arrowhead). (H) Higher magnification of circled area in G showing irregular Myo7a+ cells in the mutant organ of Corti. (J) Molecular relationships among the key transcription factors for hair cell differentiation. This study demonstrates that a direct interaction between Eya1/Six1/Sox2 proteins coordinately regulates Atoh1 expression, and that Pou4f3 is a common downstream factor of the Atoh1-dependent and –independent pathways. Dashed lines indicate that Sox2 may repress Pou4f3 or downstream factors of Pou4f3. (K) Possible mechanisms for Atoh1 activation by Sox2/Eya1/Six1. Eya1/Six1 in collaboration with Sox2 activity in prosensory progenitors can induce Atoh1 activation via direct binding to the Sox- and Six-binding sites within enhancer A and B respectively. These three factors may directly interact (model a) or Eya1 may bridge Six1 and Sox2 (model b). These three factors may also form an active complex to regulate Atoh1 activation via enhancer A (model c), while Eya1/Six1 efficiently upregulate Atoh1 via enhancer B. Question mark indicates that the involvement of the factor is unclear.
Table 2.
Endogenous Sox2 activity in the GER is necessary for Eya1/Six1 to induce hair cells and dosage-dependent response of endogenous Atoh1 activation to Sox2 in combination with Eya1/Six1
| DNA constructs | Total Atoh1+ cells | Atoh1+ Myo7a+ cells | Atoh1+ Myo7a− cells | Atoh1− Myo7a+ cells | Total Myo7a+ cells | GFP+ cells/Total explants | %/% (Atoh1+:GFP+/Myo7a+:GFP+) |
|---|---|---|---|---|---|---|---|
| shSox2/Eya1/Six1, 2 DIV | 1 | – | - | - | - | 549/7 | 0.2±0.5/- |
| shSox2/Eya1/Six1, 6 DIV | 0 | 0 | 0 | 0 | 0 | 291/3 | 0/0 |
| shSox2/Eya1, 2 DIV | 0 | – | - | - | 0 | 365/4 | 0/− |
| Control.shRNA/Eya1/Six1, 2 DIV | 110 | – | - | - | - | 324/4 | 34.2±0.7/− |
| Control.shRNA/Eya1/Six1, 6 DIV | 106 | 106 | 0 | 170 | 276 | 310/4 | 34.2±0.3/89.0±0.3 |
| Control.shRNA/Six1/Sox2, 2 DIV | 16 | - | - | - | - | 214/3 | 7.5±0.2/− |
| Control.shRNA/Six1/Sox2, 6 DIV | 0 | 0 | 0 | 1 | 1 | 312/4 | 0/0.3±0.5 |
| Control.shRNA/Eya1/Sox2, 2 DIV | 21 | - | - | - | - | 232/3 | 9.1±0.2/− |
| Control.shRNA/Eya1/Sox2, 6 DIV | 2 | 1 | 1 | 2 | 3 | 342/4 | 0.6±0.3/0.9±0.2 |
| Control.shRNA/Sox2, 2 DIV | 7 | - | - | - | - | 228/3 | 3.1±0.5/− |
| Control.shRNA/Sox2, 6 DIV | 4 | 0 | 4 | 0 | 0 | 373/4 | 1.1±0.4/0 |
| Eya1(1.0×)/Six1(1.0x)/Sox2(1.0x), 2 DIV | 126 | - | - | - | - | 319/4 | 39.5±0.3/− |
| shSox2/Eya1(1.0×)/Six1(1.0x)/Sox2(1.0x), 2 DIV | 0 | - | - | - | - | 150/2 | 0/− |
| shSox2/Eya1(1.0×)/Six1(1.0x)/Sox2(1.5x), 2 DIV | 14 | - | - | - | - | 152/2 | 9.2±0.7/−* |
| shSox2/Eya1(1.0×)/Six1(1.0x)/Sox2(2.0x), 2 DIV | 31 | - | - | - | - | 163/2 | 19.0±0.2/−* |
| shSox2/Eya1(1.0×)/Six1(1.0x)/Sox2(2.5x), 2 DIV | 58 | - | - | - | - | 172/2 | 33.7±1.0/−* |
| shSox2/Eya1(1.5×)/Six1(1.5x)/Sox2(1.0x), 2 DIV | 22 | - | - | - | - | 162/2 | 13.7±0.6/−* |
| shSox2/Eya1(1.5×)/Six1(1.5x)/Sox2(1.5x), 2 DIV | 31 | - | - | - | - | 154/2 | 20.2±0.6/−* |
| shSox2/Eya1(1.5×)/Six1(1.5x)/Sox2(2.0x), 2 DIV | 49 | - | - | - | - | 146/2 | 33.6±0.3/−* |
| shSox2/Eya1(1.5×)/Six1(1.5x)/Sox2(2.5x), 2 DIV | 64 | - | - | - | - | 161/2 | 39.1±1.2/−* |
Transfected cells in the GER of E13.5–14.0 explants were identified as GFP+ cells. Explants after 2 or 6 DIV were processed for Atoh1 in situ hybridization only or Myo7a antibody staining. The initial dose of each plasmid at 2 μg/μl for electroporation was designated as 1× (all unspecified samples were 1×).
The levels of Atoh1 expression in response to different doses of Eya1, Six1 and Sox2 were examined in the presence of shSox2 in order to maximize the cooperativity of Sox2 with Eya1/Six1. The ratio of Atoh1+ vs. total GFP+ cells was calculated for each explant, and values were expressed as means±standard deviations. Significance was determined for each of the sample groups using StatView’s t-test. P-values were between P<0.0001 and P=0.0397.
Since only ~5% increase of endogenous Atoh1 and ~10% increase of 1.4 kb Atoh1-GFP reporter transcription was induced when Sox2 was coexpressed with Eya1/Six1, the presence of endogenous Sox2 in the GER, or high levels of exogenous Eya1/Six1, which would have interacted too strongly, may limit the effect of exogenous Sox2. To further confirm the requirement of Sox2 and maximize its cooperativity with Eya1/Six1, we titrated the dose of Sox2 in combination with Eya1/Six1 in the presence of shSox2 in activating the transcription of endogenous Atoh1 as well as the Atoh1-GFP reporters. A much wider range of a dose-dependent and larger increase in the expression levels of endogenous Atoh1 and the Atoh1-GFP reporters induced by Sox2 were observed (Tables 2, S5). These results clearly demonstrate the requirement and cooperativity of Sox2 in activating Atoh1 transcription in combination with Eya1/Six1.
Since EYA1 or SIX1 mutations block hair cell induction after 6 DIV (Figure S2; Table S1), we further investigated if the mutations would affect the initial induction of Atoh1 activation. Atoh1 ISH of explants after 2 DIV clearly show that all Eya1 or Six1 mutations completely or severely blocked the initial induction of Atoh1 (Table S1), indicating that normal Eya1 or Six1 activity is required for inducing ectopic activation of Atoh1 in the GER.
Temporal and cell-specific deletion establishes that Eya1 is necessary for hair cell development in vivo
To directly address the requirement of Eya1 or Six1 in hair cell development in vivo, we used an inducible system to delete these genes in a temporally controlled fashion during hair cell development. If these genes are required for hair cell development, deletion of either one of them at ~E13.5 would block hair cell development in the apex but not in the base of the cochlea where hair cell development has already initiated. Conditional mice with a floxed allele of Eya1 gene (Eya1flox) (see supplemental information) were intercrossed with transgenic animals expressing a tamoxifen regulated Cre recombinase (CreER-T2) under control of the 1.4 kb Atoh1 enhancer (Atoh1CreER-T2) (Machold and Fishell, 2005). The efficiency of Atoh1CreER-T2 recombinase-mediated recombination in the hair cell population was determined by crossing with the Rosa26-based reporter R26REYFP (Srinivas et al., 2001). As Atoh1 induction occurs between E13.5–E14.5 and as shown previously that the CreER fusion protein translocates to the cell nucleus within 6 hours of tamoxifen administration and that peak marking occurs over a subsequent period of 12–24 hours (Danielian et al., 1998; Zervas et al., 2004), tamoxifen treatment was administered at a single dose or two doses between E12.75–E14.5 to transiently activate the CreER-T2, and cochleae were analyzed at E18.5. Quantification analysis indicated that a single dose of tamoxifen at E12.75 resulted in YFP expression in 43.2±1.8% of Myo7a+ hair cells (n=4) (39.8±1.3% for inner, 44.3±3% for outer), and that addition of a second dose 24 hours later increased YFP+ cells to 95.6±1.1% of Myo7a+ cells (n=4) (91.1±2.6% for inner, 97.1±1% for outer) (Figure S5). No YFP+ cells were observed in the absence of tamoxifen treatment (Figure S5). Based on this, we chose to give tamoxifen on two consecutive days (E12.75 and E13.75) to transiently and conditionally delete Eya1 in the developing organ of Corti of Eya1flox/flox;Atoh1CreER-T2 embryos. The mutant cochlea reached a full 1.5 turns, and four rows of hair cells were present in the basal turn, but showed a pattern of severity that parallels the normal process of hair cell differentiation, with no hair cells in the apical turn, and outer rows more affected than the inner row in the medial turn (Figure 6G, compare with 6E). The hair cells that initially formed in the basal and medial turns also revealed abnormal morphology (Figure 6H, compare with 6F): these eventually disappeared when cultured in vitro (Figure S5). The observed phenotypes were fully penetrant in all mutants analyzed (n=6) and consistent with the alteration of Atoh1 expression, which was reduced in the basal turn, markedly reduced in the medial turn, especially in the outer hair cells, but was undetectable in the apical turn, as labeled by Atoh1 (n=6, Figure S6). We also confirmed that Eya1 expression was absent in the developing hair cells in the mutant cochleae (n=4) (Figure S6). Similar observation was obtained with Six1flox mice (data not shown). These observations establish that Eya1 or Six1 is necessary for hair cell development and Atoh1 expression in vivo.
Discussion
In this study, we began to probe the molecular mechanism of the transcriptional regulation of the hair cell differentiation factor Atoh1. Our results indicate that three key transcription factors Sox2, Eya1 and Six1 cooperatively interact to activate Atoh1, and this cooperativity could be mediated by direct protein-protein interaction. Thus, we propose a model in which all three proteins, Sox2, Eya1 and Six1, create a specific conformation as well as DNA bending when associated in a multiprotein complex that interacts with the transcriptional machinery more efficiently than any of the individual factors (Figure 6K). Such interactions among these key factors may act to organize the developmental program of cochlear sensory cells in the mammalian inner ear.
How do we explain why Atoh1 induction does not initiate in all prosensory cells at once in which Eya1/Sox2 are coexpressed? Based on its expression pattern between E13–E15, Six1 may serve as a critical positive inducer for Atoh1 activation near the base of the cochlea. Similar to the pattern of Atoh1 expression, Six1 expression initiates in discrete columns of cells that span the entire depth of the sensory epithelium in a basal-to-apical direction, as well as in a medial-to-lateral direction, but its onset appears to occur before Atoh1 expression in the base of the cochlea. Thus, Six1 may stimulate the initial activation of Atoh1 in cooperation with Sox2/Eya1 to initiate hair cell differentiation within the organ of Corti.
Our data suggest that Eya1 may bridge Six1 and Sox2 (Figure 6K), as Eya1 is a transcriptional coactivator and these three factors directly interact. These proteins may undergo protein-interaction-dependent and binding sequence-dependent conformational changes, and form a compact and active complex only after such conformational changes, which is then capable of transcriptional activation. In the absence of Six1, Eya1/Sox2 may not lead to the formation of an active complex, which explains why coexpression of Eya1/Sox2 resulted in no synergy of the reporter activity when both enhancer A and B were present. This also explains why in the primordial organ of Corti, Atoh1 activation is not initiated in all prosensory precursors at the same time.
In addition to the sensory epithelium, Eya1/Six1 are expressed in nonsensory epithelial cells surrounding the prosensory domain and low level of Sox2 exists in GER cells but Atoh1 is not. How do we explain this and why Atoh1 is only induced in GER cells when Eya1/Six1 are overexpressed? Since lateral inhibition by Notch-signaling is well known to be involved in restricting Atoh1 expression, Eya1/Six1/Sox2 may interact with Notch-signaling and Hes proteins (Hayashi et al., 2008; Tateya et al.) to inhibit Atoh1 expression in those nonsensory cells. However, as high levels of Eya1/Six1 act as positive regulators for inducing Atoh1 expression in the sensory cells, when they are overexpressed in GER cells, these transcription factors are clearly able to induce ectopic Atoh1 activation. It is also possible that low level of Sox2/Eya1/Six1 may be necessary to keep GER cells in a multipotent state and maintain their competence to be induced into either neurons or hair cells. Overexpression of Eya1/Six1 in GER cells may cause the cells to undergo a certain level of epigenetic reprogramming and shift to a Sox2-Eya1-Six1 code in order to release cells from nonsensory into hair cell mode.
The conclusion that Sox2 is required for directly activating Atoh1 transcription is supported by the findings that mutation of two nucleotides critical for Sox2 binding to the Atoh1 enhancer compromised the activity of the enhancer and abolished its responsiveness to Sox2-mediated transcription. Once Atoh1 is activated, both Eya1/Six1 but not Sox2 may act together to upregulate and maintain high levels of Atoh1 expression via direct binding to the Six-binding site to mediate hair cell differentiation. Consistent with this, Eya1/Six1 but not Sox2 are coexpressed with Atoh1 in differentiating hair cells and synergise to activate Atoh1en hancer B.
Our results establish the relevance and sufficiency of two conserved enhancers of Atoh1 in directing its expression in sensory hair cells. Considering the requirement of Sox2 in activating Atoh1 transcription discussed above, enhancer A is likely to only mediate the initial activation of Atoh1 transcription, but enhancer B may play additional roles in modulating the levels of Atoh1 expression in order to promote hair cell differentiation program. Interestingly, in the absence of enhancer B, Sox2/Eya1 synergistically enhanced reporter activity driven by enhancer A, while Sox2/Six1 or Six1/Eya1 also weakly synergized. However, when all three factors were present, the reporter activity was greatly enhanced, further suggesting that Sox2-mediated transcriptional activation of Atoh1 via enhancer A may also require Eya1/Six1 in vivo. It is known that Sox proteins generally require the cooperation of partner factors that bind DNA in the vicinity of the Sox site and it only exerts gene regulatory control in combination with a partner (Kondoh and Kamachi). Mutagenisis analysis of the So-binding site adjacent to the Sox-binding site demonstrated that Six1 may bind to this site to cooperate with Sox2 in regulating Atoh1 activation. The reduction of reporter activity from ~90% to ~80% induced by Eya1/Six1 when enhancer A was deleted further suggests that enhancer A may mediate weak responsiveness to Six1-dependent transcriptional activation. However, such weak responsiveness appears to be Sox2-DNA-binding dependent, since when the Sox-binding site was mutated, expression of Six1 or Six1/Eya1 failed to show any effect on the reporter transcription. Thus, the Sox2/Eya1/Six1 complex may be necessary to create a Sox2-dependent and binding sequence-dependent conformational changes to form an active complex capable of Atoh1 transcriptional activation, but only at ~50% of the reporter activity driven by the 1.4 kb enhancer. This result also explains why the lacZ transgene driven by enhancer A could direct expression in hair cells at a reduced level (Helms et al., 2000).
In contrast to enhancer A, the reporter driven by enhancer B predominantly responds to Six1-mediated transcriptional activation and can be largely synergized with Eya1. Addition of Sox2 with Eya1/Six1 did not significantly enhance the reporter activity. Thus, based on the distinct responsiveness of the Atoh1-GFP reporters to different combinations of Sox2, Eya1 and Six1 and abundant coexpression of Eya1/Six1 and Atoh1 in the differentiating hair cells, enhancer B on its own is likely to be essential for the maintenance of Atoh1 expression to promote hair cell differentiation.
It should be noted that besides the Sox- and Six-binding sites, binding sequences for many other transcription factors, including Atoh1 itself, Gfi1, Myc, Foxd and Zic proteins, which are all expressed in the organ of Corti, are found in the 1.4 kb Atoh1 enhancer. Previous studies have shown that Atoh1 can bind to an essential E-box site located in enhancer B to autoregulate Atoh1 expression (Helms et al., 2000) and that Gfi1 may be required for maintenance of Atoh1 expression in the differentiating hair cells (Hertzano et al., 2004). Thus, these factors are likely to bind to their binding sites located in the Atoh1 enhancer to regulate the Atoh1-GFP expression in vivo. This explains why some sporadic GFP+ cells throughout the cochlear duct in two transgenic lines were seen when the 1.4 kb Soxmt+Sixmt mutant enhancer was assayed in vivo. In fact, a few GFP+ cells (~1% of those driven by the wild-type enhancer) were also seen when assayed in explants with Sox2, Eya1 and Six1. Nonetheless, our analysis clearly demonstrates the functional significance of the Sox- and Six-binding sites in regulating Atoh1 expression in vivo.
Strikingly, however, in addition to Atoh1-dependent hair cell differentiation pathway, Eya1-Six1 appears to function upstream of a pathway, independent of Atoh1 but upstream of Pou4f3, which appears to be more efficient than Atoh1 in inducing a hair cell fate. Since Sox2 also antagonizes the Atoh1-independent pathway, such a pathway is likely to be controlled by a bHLH transcription factor. Although we currently cannot determine whether this pathway is also synergistically activated by Sox2/Eya1/Six1, as coexpression of Eya1/Six1/Sox2 similarly induces Atoh1+Myo7a+ and Atoh1−Myo7a+ cells (Table 1), similar cooperative mechanism is likely to operate in activating this pathway. If so, robust activation of Atoh1-dependent and –independent pathways may be similarly coregulated by Sox2/Eya1/Six1. We are in the process of performing microarray analysis to identify this novel pathway. Although it is currently unclear whether this pathway operates in normal hair cell development, the observation that some Myo7a+ cells still exist in the Atoh1 mutant cochlea at postnatal stages in which Atoh1 was deleted by Pax2-Cre (Pan et al.) suggests that these Myo7a+ cells may be induced by other factors. Future studies are required to determine whether Atoh1-independent pathway plays a role in normal hair cell development.
In conclusion, our studies reveal molecular linkages among the genes responsible for hair cell induction and differentiation. These findings of hair cell induction and a robust combinatorial regulatory mechanism may not only lead to the development of a new therapeutic strategy but also allow reprogramming of new hair cells for restoring hearing.
EXPERIMENTAL PROCEDURES
DNA constructs
3xFlag-Eya1-IRES-GFP/pCDNA3 and His-Six1-IRES-GFP was obtained by inserting the IRES-GFP cassette into the 3xFlag-Eya1/pcDNA3 or His-Six1/pcDNA3.
Cochlear explant cultures and electroporation
Cochlear isolated from E13.5–14.0 embryos were cultured as previously described (Dabdoub et al., 2003). GER cells were electroporated as previously described (Jones et al., 2006).
In situ hybridization (ISH)and Immunohistochemistry
ISH was performed using dig-labeled Atoh1 or Pou4f3 riboprobe, followed by immunostaining to detect GFP, Myo7a, Pou4f3 or Atoh1.
Primary antibodies: rabbit anti-Sox2 (Chemicon), -Myo7A and -Myo6 (Proteus), -GFP (Novus), -Pou4f3 (Aviva) and mouse anti-Atoh1 (Hybridoma). Cy3-, fluorescein- and HRP-conjugated secondary antibodies were used.
Cell counts and statistical analysis
Inner and outer Myo7a+ hair cells, YFP+ or GFP+ cells were counted separately in the basal, medial and apical turn of the entire cochlea. For explants, cell count and ratios of Atoh1+, Myo7a+, Pou4f3+ vs. GFP+ from each cochlea were quantified and values are expressed as means±standard deviations. Significance was determined by comparing each of the sample groups using StatView’s t-test.
ChIP, Co-IP, GST pulldown and EMSA assays
For ChIP analysis, E13.0–13.5 cochleae were dissected, fixed, homogenized and processed for chromatin isolation as described (Negre et al., 2006). Rabbit anti-Sox2 and goat anti-Six1 (Santa Cruz) antibodies were used for IP. Input DNAs were diluted 10-fold and used as positive controls. See Supplemental Information for primers.
Co-IP assays were performed as described (Nie et al.). Protein extracts were isolated from E13.0–13.5 cochleae and rabbit anti-Sox2, goat anti–Six1 or rabbit anti-Eya1 (Zou et al., 2008) antibody was used for IP or Western blot.
For GST pulldown, in vitro translated Sox2 protein and GST-Eya1D or Six1 were used.
EMSAs was performed as described (Ruf et al., 2004) with 32P-dCTP-labeled DNA probes of either 3×Sox- or 3×Six-binding site or mutant sites and lysates from 293 cells transfected with Sox2 or Six1.
Drug treatment
3 mg tamoxifen (Sigma)/35 g mouse in corn oil was administered by oral gavage to the pregnant mouse. Embryos were collected at E18.5, fixed and stained for Myo7a, Atoh1 or Eya1.
Supplementary Material
Highlights.
Direct and cooperative interactions of Sox2/Six1/Eya1 coordinate Atoh1 activation
Sox2 and Six1 directly bind to Atoh1 enhancers
Sox2 antagonizes factors downstream of Atoh1 to inhibit hair cell differentiation
Eya1/Six1 are necessary for Atoh1 expression and hair cell development in vivo
Acknowledgments
This work was supported by NIH RO1 DC005824 and DC005824-S1(P -X. Xu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Levi-Acobas F, Cruaud C, Le Merrer M, Mathieu M, et al. Clustering of mutations responsible for branchio-oto-renal (BOR) syndrome in the eyes absent homologous region (eyaHR) of EYA1. Hum Mol Genet. 1997a;6:2247–2255. doi: 10.1093/hmg/6.13.2247. [DOI] [PubMed] [Google Scholar]
- Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Weil D, Cruaud C, Sahly I, Leibovici M, et al. A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat Genet. 1997b;15:157–164. doi: 10.1038/ng0297-157. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Borsani G, DeGrandi A, Ballabio A, Bulfone A, Bernard L, Banfi S, Gattuso C, Mariani M, Dixon M, Donnai D, et al. EYA4, a novel vertebrate gene related to Drosophila eyes absent. Hum Mol Genet. 1999;8:11–23. doi: 10.1093/hmg/8.1.11. [DOI] [PubMed] [Google Scholar]
- Buller C, Xu X, Marquis V, Schwanke R, Xu PX. Molecular effects of Eya1 domain mutations causing organ defects in BOR syndrome. Hum Mol Genet. 2001;10:2775–2781. doi: 10.1093/hmg/10.24.2775. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chow LM, Tian Y, Weber T, Corbett M, Zuo J, Baker SJ. Inducible Cre recombinase activity in mouse cerebellar granule cell precursors and inner ear hair cells. Dev Dyn. 2006;235:2991–2998. doi: 10.1002/dvdy.20948. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Donohue MJ, Brennan A, Wolf V, Montcouquiol M, Sassoon DA, Hseih JC, Rubin JS, Salinas PC, Kelley MW. Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development. 2003;130:2375–2384. doi: 10.1242/dev.00448. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci U S A. 2008;105:18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Fekete DM. Making sense of making hair cells. Trends Neurosci. 2000;23:386. doi: 10.1016/s0166-2236(00)01668-4. [DOI] [PubMed] [Google Scholar]
- Hasson T, Heintzelman MB, Santos-Sacchi J, Corey DP, Mooseker MS. Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proc Natl Acad Sci U S A. 1995;92:9815–9819. doi: 10.1073/pnas.92.21.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Kokubo H, Hartman BH, Ray CA, Reh TA, Bermingham-McDonogh O. Hesr1 and Hesr2 may act as early effectors of Notch signaling in the developing cochlea. Dev Biol. 2008;316:87–99. doi: 10.1016/j.ydbio.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms AW, Abney AL, Ben-Arie N, Zoghbi HY, Johnson JE. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development. 2000;127:1185–1196. doi: 10.1242/dev.127.6.1185. [DOI] [PubMed] [Google Scholar]
- Hertzano R, Montcouquiol M, Rashi-Elkeles S, Elkon R, Yucel R, Frankel WN, Rechavi G, Moroy T, Friedman TB, Kelley MW, et al. Transcription profiling of inner ears from Pou4f3(ddl/ddl) identifies Gfi1 as a target of the Pou4f3 deafness gene. Hum Mol Genet. 2004;13:2143–2153. doi: 10.1093/hmg/ddh218. [DOI] [PubMed] [Google Scholar]
- Hoskins BE, Cramer CH, Silvius D, Zou D, Raymond RM, Orten DJ, Kimberling WJ, Smith RJ, Weil D, Petit C, et al. Transcription factor SIX5 is mutated in patients with branchio-oto-renal syndrome. Am J Hum Genet. 2007;80:800–804. doi: 10.1086/513322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao FC, Williams A, Davies EL, Rebay I. Eyes absent mediates cross-talk between retinal determination genes and the receptor tyrosine kinase signaling pathway. Dev Cell. 2001;1:51–61. doi: 10.1016/s1534-5807(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Jones JM, Montcouquiol M, Dabdoub A, Woods C, Kelley MW. Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during the development of the organ of Corti. J Neurosci. 2006;26:550–558. doi: 10.1523/JNEUROSCI.3859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kondoh H, Kamachi Y. SOX-partner code for cell specification: Regulatory target selection and underlying molecular mechanisms. Int J Biochem Cell Biol. 42:391–399. doi: 10.1016/j.biocel.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Lee YS, Liu F, Segil N. A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development. 2006;133:2817–2826. doi: 10.1242/dev.02453. [DOI] [PubMed] [Google Scholar]
- Machold R, Fishell G. Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron. 2005;48:17–24. doi: 10.1016/j.neuron.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Negre N, Lavrov S, Hennetin J, Bellis M, Cavalli G. Mapping the distribution of chromatin proteins by ChIP on chip. Methods Enzymol. 2006;410:316–341. doi: 10.1016/S0076-6879(06)10015-4. [DOI] [PubMed] [Google Scholar]
- Nie X, Sun J, Gordon RE, Cai CL, Xu PX. SIX1 acts synergistically with TBX18 in mediating ureteral smooth muscle formation. Development. doi: 10.1242/dev.045757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. Conditional deletion of Atoh1 using Pax2-Cre results in viable mice without differentiated cochlear hair cells that have lost most of the organ of Corti. Hear Res. 275:66–80. doi: 10.1016/j.heares.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;(Suppl 220):221–244. [PubMed] [Google Scholar]
- Ruf RG, Xu PX, Silvius D, Otto EA, Beekmann F, Muerb UT, Kumar S, Neuhaus TJ, Kemper MJ, Raymond RM, Jr, et al. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci U S A. 2004;101:8090–8095. doi: 10.1073/pnas.0308475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz F, Demignon J, Porteu A, Kahn A, Concordet JP, Daegelen D, Maire P. Expression of myogenin during embryogenesis is controlled by Six/sine oculis homeoproteins through a conserved MEF3 binding site. Proc Natl Acad Sci U S A. 1998;95:14220–14225. doi: 10.1073/pnas.95.24.14220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A. Extensive cell movements accompany formation of the otic placode. Dev Biol. 2002;249:237–254. doi: 10.1006/dbio.2002.0739. [DOI] [PubMed] [Google Scholar]
- Tateya T, Imayoshi I, Tateya I, Ito J, Kageyama R. Cooperative functions of Hes/Hey genes in auditory hair cell and supporting cell development. Dev Biol. 352:329–340. doi: 10.1016/j.ydbio.2011.01.038. [DOI] [PubMed] [Google Scholar]
- Tootle TL, Silver SJ, Davies EL, Newman V, Latek RR, Mills IA, Selengut JD, Parlikar BE, Rebay I. The transcription factor Eyes absent is a protein tyrosine phosphatase. Nature. 2003;426:299–302. doi: 10.1038/nature02097. [DOI] [PubMed] [Google Scholar]
- Wayne S, Robertson NG, DeClau F, Chen N, Verhoeven K, Prasad S, Tranebjarg L, Morton CC, Ryan AF, Van Camp G, et al. Mutations in the transcriptional activator EYA4 cause late-onset deafness at the DFNA10 locus. Hum Mol Genet. 2001;10:195–200. doi: 10.1093/hmg/10.3.195. [DOI] [PubMed] [Google Scholar]
- Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet. 1999;23:113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- Xu PX, Cheng J, Epstein JA, Maas RL. Mouse Eya genes are expressed during limb tendon development and encode a transcriptional activation function. Proc Natl Acad Sci U S A. 1997a;94:11974–11979. doi: 10.1073/pnas.94.22.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PX, Woo I, Her H, Beier DR, Maas RL. Mouse Eya homologues of the Drosophila eyes absent gene require Pax6 for expression in lens and nasal placode. Development. 1997b;124:219–231. doi: 10.1242/dev.124.1.219. [DOI] [PubMed] [Google Scholar]
- Yu Y, Davicioni E, Triche TJ, Merlino G. The homeoprotein six1 transcriptionally activates multiple protumorigenic genes but requires ezrin to promote metastasis. Cancer Res. 2006;66:1982–1989. doi: 10.1158/0008-5472.CAN-05-2360. [DOI] [PubMed] [Google Scholar]
- Zervas M, Millet S, Ahn S, Joyner AL. Cell behaviors and genetic lineages of the mesencephalon and rhombomere 1. Neuron. 2004;43:345–357. doi: 10.1016/j.neuron.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zheng W, Huang L, Wei ZB, Silvius D, Tang B, Xu PX. The role of Six1 in mammalian auditory system development. Development. 2003;130:3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Erickson C, Kim EH, Jin D, Fritzsch B, Xu PX. Eya1 gene dosage critically affects the development of sensory epithelia in the mammalian inner ear. Hum Mol Genet. 2008;17:3340–3356. doi: 10.1093/hmg/ddn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Silvius D, Fritzsch B, Xu PX. Eya1 and Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes. Development. 2004;131:5561–5572. doi: 10.1242/dev.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.