Abstract
Human papillomavirus (HPV)-related cancers are a major worldwide public health concern. Virtually all cervical cancer is HPV-related, with 70% caused by HPV16 and -18. Variable proportions of certain non-cervical cancers (e.g., anal, vulvar, oropharyngeal) are HPV-related; over 90% of the HPV-related ones are related to HPV16, -18. The HPV-related cancers are dominated by cervical cancer in the developing world, where cervical cancer screening is limited. In this setting, widespread uptake of current HPV vaccines by adolescent girls could reduce this cancer's incidence and mortality by approximately two-thirds, with cost-effective screening programs of adult women having the potential to reduce mortality more rapidly. In the industrialized world, non-cervical HPV-related cancers, especially oropharyngeal, are rapidly increasing, and now rival the incidence of cervical cancer, whose rates continue to decline thanks to established cervical screening programs. Therefore, reducing HPV-associated non-cervical cancers with HPV vaccination has greater importance in the industrialized world, especially since there are no approved screening programs for these cancers. Preventing the substantial number of non-cervical HPV cancers in men will require either “herd” immunity through high vaccination rates in females or male vaccination. Current HPV vaccination can complement cervical screening in protecting against cervical cancer and may permit the safe reduction of screening intensity in industrialized countries. Second-generation HPV vaccines (active against a broader array of cervical cancer–related HPV types) could prevent an even higher proportion of cervical precancer and cancer and might permit further reductions in screening intensity.
The Burden of HPV-associated Cancer in the Developing versus Industrialized World
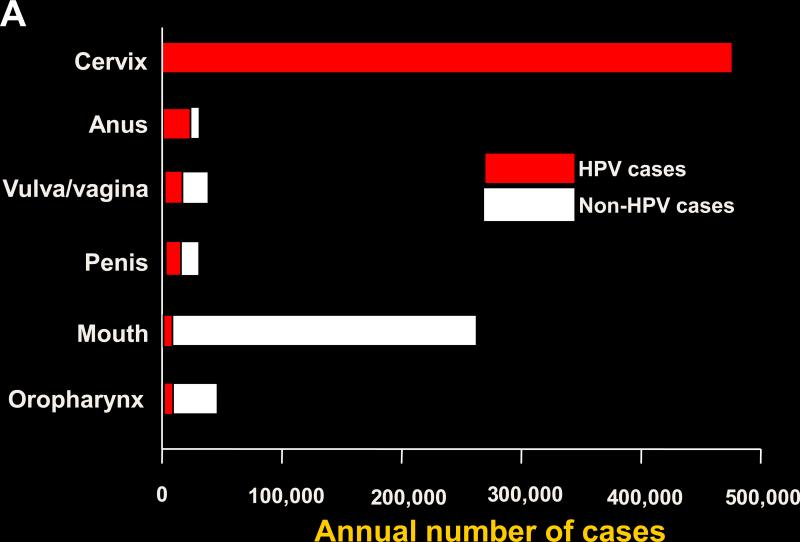
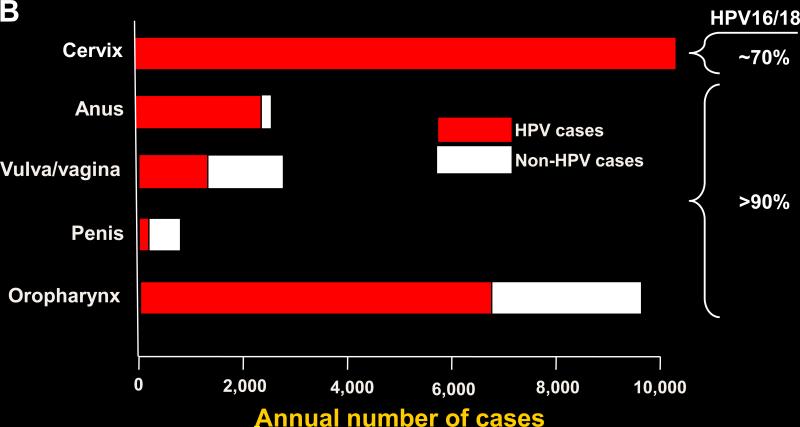
Human papillomavirus (HPV) infection causes virtually all cases of cervical cancer and a variable proportion of certain non-cervical malignancies including vulvar, vaginal, penile, anal, and oropharyngeal cancer (1). Cervical cancer dominates the worldwide HPV-associated cancers (Fig. 1). The worldwide incidence profile is similar to that of the HPV-associated cancers in the developing world, as approximately 80% of the worldwide cervical cancers occur in the nations in the developing world, where this cancer is frequently the most common cause of cancer-related deaths in women. However, the developing and industrialized world have substantially different patterns of HPV-associated cervical and non-cervical cancers (Table 1). The high cervical cancer incidence in the developing world results mainly from a lack of resources devoted to widespread effective cervical cancer screening programs. By contrast, screening programs in industrialized countries have substantially reduced the incidence and mortality from cervical cancer (2). Because of the reduction in cervical cancer, combined with a rising incidence in HPV-related anal and especially oropharyngeal cancer, which increased more than four-fold 1988-2004, the incidence of HPV-associated non-cervical cancers may be similar to that of cervical cancer in industrialized countries such as the U.S. (Fig. 2; refs. (3, 4). Furthermore, more than one-quarter of the HPV-associated cancers in the U.S. occur in males, largely because most patients with oropharyngeal cancer are male as well as almost one-half of anal cancer patients. The proportion of non-cervical HPV-associated cancers is much lower in the developing world. Thus, HPV-associated malignancies in the developing world are dominated by cervical cancer, implying that the vast majority of HPV infections that lead to cancer in this setting occur in women. HPV-associated malignancies in the U.S., however, are not dominated by cancer at a single anatomic site, and a substantial minority occur in males.
Figure 1.
A, worldwide incidence and distribution of HPV-associated cancers (data from ref. 1). Red, HPV-positive cancer; white, HPV-negative cancer. B, incidence and distribution of HPV-associated cancers in the U.S. (data from refs. 3 and 4). The approximate percentage of HPV-associated cancer attributable to HPV16 and -18 is also shown. Red, HPV-positive cancer; white, HPV-negative cancer.
Table 1.
HPV-related disease and roles for screening and vaccination in the developing versus industrialized world
| Existing intervention | Potential for impact | ||||
|---|---|---|---|---|---|
| Disease burdena | Screening | HPV vaccine | Screening | HPV vaccine | |
| Developing world | |||||
| Cervical | High | Limited | Limited | High | Highb |
| Non-cervicalc | |||||
| Oropharyngeal | Low | None | Limitedg | None | Lowd |
| Other | Low | None | Limited | None | Lowd |
| Non-cervical (male) | |||||
| Oropharyngeal | Low | None | Limitedg | None | Lowd |
| Other | Low | None | Limited | None | Lowd |
| Industrialized world | |||||
| Cervical | High | Extensive | Extensivee | High | High |
| Non-cervicalc | |||||
| Oropharyngeal | High | None | Extensiveg | Nonef | Highg |
| Other | Moderate | Limited | Extensive | Unknownh | Moderatei |
| Non-cervical (male) | |||||
| Oropharyngeal | High | None | Limitedf,g | Nonef | Highg |
| Other | Moderate | Limited | Limitedf | Unknownh | Moderatei |
Disease burden relative to cervical cancer in developing world and in industrialized world. The incidence of cervical cancer is substantially higher in developing world.
Currently being developed by the GAVI Alliance and other potential donors.
Includes males and females.
Although the vaccine may be effective against these diseases, their currently low prevalence would limit the potential population-wide impact.
The extent of vaccine uptake in the U.S. lags behind other industrialized countries. It was therefore recommended for males by the U.S. CDC Advisory Committee on Immunization Practices in October 2011. For countries with high HPV vaccine uptake among females, herd immunity from female vaccination should protect most males from HPV-associated disease.
No established screening for oropharyngeal HPV or its related neoplasia is currently available.
Although the vaccine should be highly effective against the HPV types associated with this disease, there are no vaccine data on oral/oropharyngeal infection or disease.
No established screening for anal cancer, but is under consideration for high-risk populations.
Although the vaccine should be highly effective against the HPV types associated with these diseases, the “moderate” designation refers to the relative population-wide impact of reducing the relevant diseases.
The Current HPV Vaccines
Persistent infection by HPV16 and HPV18 accounts for about 70% of cervical cancer worldwide, with relatively small region-specific differences (5); about ten other HPV types contribute to the remaining 30% (6). More than 90% of the HPV-associated non-cervical cancers are attributable to HPV16 and -18, with HPV16 accounting for the vast majority. The two FDA-approved HPV vaccines are manufactured by Merck (Gardasil) and GlaxoSmithKline (Cervarix). They are non-infectious subunit vaccines produced by expressing the viral L1 major capsid protein in yeast (for the Merck vaccine) or insect cells (for the GSK vaccine). The L1 protein has the ability to efficiently self-assemble into virus-like particles (VLPs), which are highly immunogenic (7). GSK's vaccine is bivalent, being composed of VLPs from HPV16 and -18 (8). Merck's vaccine is a quadrivalent, being composed of VLPs from HPV6, -11, -16, and -18 (HPV6 and -11 account for close to 90% of genital warts; ref. (9).
The large-scale international phase III clinical trials of HPV vaccines were focused on women because of the high global burden of cervical cancer. Each vaccine induced high protection against persistent incident infection and premalignant anogenital disease associated with HPV16 and -18 (and against HPV6 and -11–associated genital warts for the Merck vaccine; refs. (8, 9). The vaccines also induced some cross-protection against other HPV types, with the GSK vaccine apparently more protective in this regard than was the Merck vaccine (10, 11). Smaller trials of the Merck vaccine in males have demonstrated protection against genital warts and premalignant anal neoplasia (12, 13). In the U.S. and many other countries, the GSK vaccine is approved for females aged 10–25 years, and the Merck vaccine is approved for females and males aged 9–26 years. It is uncertain whether trials to evaluate these vaccines’ efficacy against oropharyngeal cancer will ever by conducted, largely because this cancer, unlike other HPV-associated cancers, is not associated with a recognized premalignant lesion for which to develop a screen (14).
Reducing Cervical Cancer in the Developing World: The Non-overlapping Roles of Vaccination and Screening
Regional differences in the distribution of HPV-associated cancer imply that approaches to reduce the burden of HPV-associated disease may vary to some degree with the setting. In regions with a high incidence of cervical cancer, reducing this cancer is the primary goal. The Global Alliance for Vaccines and Immunization (GAVI Alliance), which strongly supports widespread implementation of vaccines to the developing world, recently decided to add HPV vaccines to its list of vaccines subsidized in the poorest countries (http://www.gavialliance.org/library/news/press-releases/2011/gavi-takes-first-steps-to-introduce-vaccines-against-cervical-cancer-and-rubella/), thanks in part to tiered pricing by the manufacturers that drastically reduces the cost of each dose. It has, therefore, now becomes feasible to seriously consider widespread sustained vaccination of populations where the vaccine may have the greatest public health impact. To be most cost-effective, the vaccine will target mainly adolescent girls who have not yet become sexually active. They are the preferred group for vaccination because the HPV vaccines prevent new infections, many of these infections occur soon after initiation of sexual activity, and the vaccines are ineffective against pre-existing infections (15, 16).
Although the Merck and GSK vaccines have been licensed to be given in three doses over a six-month period, it could be worthwhile to consider a two-dose regimen in some settings, as already implemented in some parts of Canada and Mexico (vaccine doses are given at zero and six months; if necessary, a third dose will be given at 60 months). Compared with three doses, two doses are easier to administer and less expensive. The rationale for this approach is substantial. The large phase III vaccine trials were conducted in sexually active young women who were 16–23 years old at the time of vaccination, whereas the main target population for the vaccine is young adolescents who are not yet sexually active. The vaccines have been found to be more immunogenic in 10–15-year-old adolescents than in the young women, with peak antibody titers being approximately two times higher in the young adolescents (17, 18). Furthermore, two vaccine doses separated by six months in young adolescents resulted in sustained antibody titers that were comparable to those resulting from three doses in the young women (19). Although the National Cancer Institute (NCI) clinical trial of the GSK vaccine in Costa Rica indicated that vaccine efficacy, as measured by persistent HPV16 and -18 infection over the four-year period of the trial, was as strong for one or two doses as for the planned three doses, the longer-term duration of protection is unknown (20). Given that the immune response may be less robust in some developing world settings than others, it will be important to test the antibody titers induced by the vaccine as it is implemented in the developing world.
What about females who are too old for cost-effective population-wide vaccination? In this regard, cervical cancer has some unusual aspects. First, most vaccines are developed to protect against disease that develops soon after infection. However, although the members of the target population for the HPV vaccine likely to be exposed to HPV infection soon after vaccination, cervical cancer does not usually develop until at least 20 years after infection (6). Therefore, a population-wide impact on this disease would not be expected until 20–30 years after implementation of an HPV vaccination program. Second, population-wide screening is a proven public health measure for preventing cervical cancer in infected women. In contrast, most other viral-induced diseases for which there are effective vaccines do not have public health interventions that can substantially reduce the risk of a serious outcome in infected patients. Although screening does not prevent infection, it has the advantage over vaccination of being able to reduce cervical cancer incidence and mortality after a much shorter interval. For example, in rural India, an HPV-based test used as a once in a lifetime screen in women 30–59 years old resulted in a sustained reduction in cervical cancer mortality within 5 years (21). Therefore, cervical cancer screening could be used in developing regions for adult women who are too old for vaccination.
To the extent that the public health goal is to reduce cervical cancer, screening is as valid an approach as vaccination, with cost-effectiveness, logistical concerns, and side effects guiding recommendations for both modalities. Traditionally, it has been thought that effective screening programs are too expensive for widespread implementation in low-resource settings. However, the recent development and validation of inexpensive HPV-based testing may make it possible for such programs to be cost-effective in low-resource settings (22). If this happens, implementation of a program of HPV vaccination for young women who are not yet sexually active could be combined with screening of adult women once to three times in a lifetime, until the vaccinated women become old enough for screening to be phased out (or possibly retained if it remained cost-effective to continue screening them; refs. (23, 24). In sum, screening and vaccination would be a non-overlapping dual approach that could be a cost-effective way to target this disease, with screening having an impact on cervical cancer until the beneficial effects of adolescent vaccination are achieved.
Implementation of vaccination and screening will require considerable effort and commitment, as there is neither an adolescent vaccine platform in most developing countries nor established screening for adults. Some countries lack other pre-requisites for such programs. For example, the lack of a cancer registry would make it difficult to determine the effectiveness of these interventions. Therefore, implementation of these programs may require the development of additional infrastructure and capacity. However, such development might also be beneficial for other aspects of medical care.
Reducing HPV-associated Cancer in the Industrialized World
In places that already have effective cervical cancer screening programs, the main goal of vaccination is to have an impact on the various precancers and cancers attributable to HPV infection, including cervical precancer and cancer (15). Because screening is used to identify and treat higher-grade cervical dysplasia, the vaccination benefit of reducing these dysplasias and their associated ablative therapy has a clinical impact much sooner than does a reduction in cervical cancer.
The ability of the HPV vaccines to protect against the HPV-associated non-cervical cancers is a benefit not mimicked by screening, as there are no approved screening programs for these cancers. Regarding cervical cancer, however, cervical cancer screening can identify and treat many of the lesions that would be prevented by vaccination. In this sense, there is some overlap of the roles of cervical cancer screening and HPV vaccination in preventing cervical neoplasia when the same woman is vaccinated and subsequently screened, as occurs in the industrialized world. A non-overlapping benefit of screening, however, is that it should be able to detect most cervical precancers and cancers not prevented by the current vaccines. In addition, there are two reasons why vaccine should be more effective than screening by cytology in preventing cervical adenocarcinoma. First, cytologic screening is substantially less sensitive in detecting adenocarcinoma and its precursors compared with detecting squamous cell carcinoma and its precursors, which has contributed to adenocarcinoma accounting for a progressively larger proportion of cervical cancer in industrialized countries (25). (It should be noted, however, that screening by HPV testing can identify lesions that give rise to both types of tumors and their precursors with high sensitivity; ref. (26).) Second, about 80% of cervical adenocarcinoma is attributable to HPV16 and -18 infection, compared with about 70% for squamous cell carcinoma (27), which implies that the vaccines should be more effective in preventing adenocarcinoma than squamous cell carcinoma (28). Therefore, there is a strong case for vaccination and screening each making important non-overlapping contributions to the prevention of cervical cancer, in addition to the overlapping disease that either can prevent. A possible additional benefit of vaccination is that it may be safe to reduce the screening intensity of vaccinated women (15).
Since HPV infection is sexually transmitted, modeling suggests that high vaccination rates of one gender should confer herd immunity for both genders (29). Consistent with this prediction, within two years of vaccine implementation in Australia, where about two-thirds of young women had been fully vaccinated with the Merck vaccine, there was almost a 60% reduction in new cases of genital warts and a more modest, but also significant, 28% reduction in genital warts among young males who have sex with women, but not among young males who have sex with men (30). Vaccination rates have been substantially lower in the U.S. than in Australia. At the end of 2010, 49% of U.S. girls 13–17 years old had received at least one dose, and 32% had received all three doses, which may be attributable to several factors (31) (32).
The U.S. vaccination rate is not expected to result in substantial herd immunity, and modeling based on these conditions suggests that male vaccination can contribute to protection in women, although less efficiently than would be achieved by vaccinating the same number of additional females (29). Other factors that favor male vaccination in this setting include its ability to directly reduce HPV-associated cancer risk for the vaccinees, and the greater likelihood of male vaccination to reduce HPV-associated cancer in men who have sex with men, who are less likely to benefit from female vaccination. For these reasons, the Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP), which makes federal recommendations for vaccines, recently upgraded its recommendation for male vaccination to “routine,” the same level as for female vaccination (http://www.cdc.gov/media/releases/2011/t1025_hpv_12yroldvaccine.html). The CDC is making strong efforts to encourage increased vaccine uptake in both genders.
A Glimpse of the Future: Potential Utility of Second-generation Vaccines
Limitations of the current HPV vaccines include the need for multiple parenteral doses, the lack of protection against some HPV types that cause cervical cancer, and a relatively high cost. The opportunity to overcome one or more of these limitations provides a rationale for developing candidate second-generation vaccines. Low-cost second-generation vaccines that could induce long-term protective immunity with fewer doses would be especially attractive for the developing world. One attractive approach for developing such a vaccine is to express the L1 capsid protein in an existing vaccine, as has been reported for Salmonella and measles vaccines (33, 34). Vaccines with activity against a broader range of the HPV types that cause cervical cancer could increase their effectiveness throughout the world, and Merck has indicated that a nonavalent (nine HPV targets) VLP vaccine is currently in clinical trials (35). If successful, such a vaccine might reduce the frequency of potentially oncogenic infections to a degree that would permit a drastic reduction in cervical cancer screening, which accounts for most of the cost of HPV-associated disease in developed countries (36).
Summary
In the developing world, HPV-associated malignancies are dominated by cervical cancer, and widespread uptake of the current HPV vaccines has the potential to reduce the incidence and mortality from this disease by at least two-thirds. A reduction in mortality could be achieved more rapidly if cost-effective cervical cancer screening programs for adult women were implemented along with vaccination of young adolescent girls. Although they currently have only a limited presence in developing countries, cervical screening plus HPV vaccination could have non-overlapping benefits in reducing cervical cancer. In the industrialized world, the number of non-cervical HPV-associated cancers rivals that of cervical cancers, thanks to established cervical cancer screening programs as well as increases in the incidence of noncervical cancers. Therefore, reducing the non-cervical cancers associated with HPV has greater importance in the industrialized world, and HPV vaccination is a major modality for achieving this goal since there are no approved screening programs for non-cervical HPV-associated cancers. A substantial minority of non-cervical cancers occur in men; protecting them requires either the development of herd immunity through high vaccination rates of females, or male vaccination if there is relatively low vaccine uptake in females, as is occurring in the U.S. For cervical cancer, HPV vaccination and screening for the same women can promote the highest level of protection, and vaccination may have the potential to permit the safe reduction of screening intensity of vaccinated women. Second-generation HPV vaccines with a lower cost and needing fewer doses could be especially useful for developing countries, while those with activity against a broader array of the HPV types that cause cervical cancer could prevent an even higher proportion of cervical precancer and cancer and might also permit further reductions in screening intensity in industrialized countries.
Acknowledgments
We thank Mark Schiffman and Maureen Johnson for helpful suggestions. This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and Center for Cancer Research.
Footnotes
Note: The views expressed in this article are those of the authors and do not necessarily represent the views of, and should not be attributed to, the National Cancer Institute.
Disclosure of Potential Conflicts of Interest
The authors are named inventors on U.S. government–owned HPV vaccine patents that are licensed to GSK and Merck, and are entitled to limited royalties as specified by federal law.
References
- 1.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118(12):3030–44. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 2.Gustafsson L, Ponten J, Zack M, Adami HO. International incidence rates of invasive cervical cancer after introduction of cytological screening. Cancer Causes Control. 1997;8(5):755–63. doi: 10.1023/a:1018435522475. [DOI] [PubMed] [Google Scholar]
- 3.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113(10 Suppl):3036–46. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111(2):278–85. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 6.Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103(5):368–83. doi: 10.1093/jnci/djq562. PMCID: 3046952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992;89(24):12180–4. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2011 doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 9.Munoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102(5):325–39. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- 10.Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J Infect Dis. 2009;199(7):926–35. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler CM, Castellsague X, Garland SM, Szarewski A, Paavonen J, Naud P, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2011 doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- 12.Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Jr., Penny ME, Aranda C, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011;364(5):401–11. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Jr., Aranda C, Jessen H, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576–85. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 14.Fakhry C, Rosenthal BT, Clark DP, Gillison ML. Associations between oral HPV16 infection and cytopathology: evaluation of an oropharyngeal “pap-test equivalent” in high-risk populations. Cancer Prev Res (Phila) 2011;4(9):1378–84. doi: 10.1158/1940-6207.CAPR-11-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowy DR, Solomon D, Hildesheim A, Schiller JT, Schiffman M. Human papillomavirus infection and the primary and secondary prevention of cervical cancer. Cancer. 2008;113(7 Suppl):1980–93. doi: 10.1002/cncr.23704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiller JT, Lowy DR. Vaccines to prevent infections by oncoviruses. Annu Rev Microbiol. 2010;64:23–41. doi: 10.1146/annurev.micro.112408.134019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reisinger KS, Block SL, Lazcano-Ponce E, Samakoses R, Esser MT, Erick J, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J. 2007;26(3):201–9. doi: 10.1097/01.inf.0000253970.29190.5a. [DOI] [PubMed] [Google Scholar]
- 18.Petaja T, Pedersen C, Poder A, Strauss G, Catteau G, Thomas F, et al. Long-term persistence of systemic and mucosal immune response to HPV-16/18 AS04-adjuvanted vaccine in preteen/adolescent girls and young women. Int J Cancer. 2010 doi: 10.1002/ijc.25887. [DOI] [PubMed] [Google Scholar]
- 19.Krajden M, Cook D, Yu A, Chow R, Mei W, McNeil S, et al. Human papillomavirus 16 (HPV 16) and HPV 18 antibody responses measured by pseudovirus neutralization and competitive Luminex assays in a two- versus three-dose HPV vaccine trial. Clin Vaccine Immunol. 2011;18(3):418–23. doi: 10.1128/CVI.00489-10. PMCID: 3067374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103(19):1444–51. doi: 10.1093/jnci/djr319. PMCID: 3186781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360(14):1385–94. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 22.Qiao YL, Sellors JW, Eder PS, Bao YP, Lim JM, Zhao FH, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9(10):929–36. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]
- 23.Levin CE, Sellors J, Shi JF, Ma L, Qiao YL, Ortendahl J, et al. Cost-effectiveness analysis of cervical cancer prevention based on a rapid human papillomavirus screening test in a high-risk region of China. Int J Cancer. 2010;127(6):1404–11. doi: 10.1002/ijc.25150. [DOI] [PubMed] [Google Scholar]
- 24.Campos NG, Kim JJ, Castle PE, Ortendahl JD, O'Shea M, Diaz M, et al. Health and economic impact of HPV 16/18 vaccination and cervical cancer screening in Eastern Africa. Int J Cancer. 2011 doi: 10.1002/ijc.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nieminen P, Kallio M, Hakama M. The effect of mass screening on incidence and mortality of squamous and adenocarcinoma of cervix uteri. Obstet Gynecol. 1995;85(6):1017–21. doi: 10.1016/0029-7844(95)00063-W. [DOI] [PubMed] [Google Scholar]
- 26.Katki HA, Kinney WK, Fetterman B, Lorey T, Poitras NE, Cheung L, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12(7):663–72. doi: 10.1016/S1470-2045(11)70145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 28.Ault KA, Joura EA, Kjaer SK, Iversen OE, Wheeler CM, Perez G, et al. Adenocarcinoma in situ and associated human papillomavirus type distribution observed in two clinical trials of a quadrivalent human papillomavirus vaccine. Int J Cancer. 2011;128(6):1344–53. doi: 10.1002/ijc.25723. [DOI] [PubMed] [Google Scholar]
- 29.Brisson M, van de Velde N, Franco EL, Drolet M, Boily MC. Incremental impact of adding boys to current human papillomavirus vaccination programs: role of herd immunity. J Infect Dis. 2011;204(3):372–6. doi: 10.1093/infdis/jir285. [DOI] [PubMed] [Google Scholar]
- 30.Donovan B, Franklin N, Guy R, Grulich AE, Regan DG, Ali H, et al. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect Dis. 2011;11(1):39–44. doi: 10.1016/S1473-3099(10)70225-5. [DOI] [PubMed] [Google Scholar]
- 31.Colgrove J, Abiola S, Mello MM. HPV vaccination mandates--lawmaking amid political and scientific controversy. N Engl J Med. 2010;363(8):785–91. doi: 10.1056/NEJMsr1003547. [DOI] [PubMed] [Google Scholar]
- 32.National and state vaccination coverage among adolescents aged 13 through 17 years--United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(33):1117–23. [PubMed] [Google Scholar]
- 33.Fraillery D, Baud D, Pang SY, Schiller J, Bobst M, Zosso N, et al. Salmonella enterica serovar Typhi Ty21a expressing human papillomavirus type 16 L1 as a potential live vaccine against cervical cancer and typhoid fever. Clin Vaccine Immunol. 2007;14(10):1285–95. doi: 10.1128/CVI.00164-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantarella G, Liniger M, Zuniga A, Schiller JT, Billeter M, Naim HY, et al. Recombinant measles virus-HPV vaccine candidates for prevention of cervical carcinoma. Vaccine. 2009;27(25-26):3385–90. doi: 10.1016/j.vaccine.2009.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peres J. For cancers caused by HPV, two vaccines were just the beginning. J Natl Cancer Inst. 2011;103(5):360–2. doi: 10.1093/jnci/djr053. [DOI] [PubMed] [Google Scholar]
- 36.Chesson HW, Ekwueme DU, Saraiya M, Lowy DR, Markowitz LE. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. manuscript submitted. [DOI] [PMC free article] [PubMed]