Abstract
Aim
Humans exhibit genetic polymorphism in NAT2 resulting in rapid, intermediate and slow acetylator phenotypes. Over 65 NAT2 variants possessing one or more SNPs in the 870-bp NAT2 coding region have been reported. The seven most frequent SNPs are rs1801279 (191G>A), rs1041983 (282C>T), rs1801280 (341T>C), rs1799929 (481C>T), rs1799930 (590G>A), rs1208 (803A>G) and rs1799931 (857G>A). The majority of studies investigate the NAT2 genotype assay for three SNPs: 481C>T, 590G>A and 857G>A. A tag-SNP (rs1495741) recently identified in a genome-wide association study has also been proposed as a biomarker for the NAT2 phenotype.
Materials & methods
Sulfamethazine N-acetyltransferase catalytic activities were measured in cryopreserved human hepatocytes from a convenience sample of individuals in the USA with an ethnic frequency similar to the 2010 US population census. These activities were segregated by the tag-SNP rs1495741 and each of the seven SNPs described above. We assessed the accuracy of the tag-SNP and various two-, three-, four- and seven-SNP genotyping panels for their ability to accurately infer NAT2 phenotype.
Results
The accuracy of the various NAT2 SNP genotype panels to infer NAT2 phenotype were as follows: seven-SNP: 98.4%; tag-SNP: 77.7%; two-SNP: 96.1%; three-SNP: 92.2%; and four-SNP: 98.4%.
Conclusion
A NAT2 four-SNP genotype panel of rs1801279 (191G>A), rs1801280 (341T>C), rs1799930 (590G>A) and rs1799931 (857G>A) infers NAT2 acetylator phenotype with high accuracy, and is recommended over the tag-, two-, three- and (for economy of scale) the seven-SNP genotyping panels, particularly in populations of non-European ancestry.
Keywords: acetylator genotype, acetylator phenotype, cryopreserved, human hepatocyte, NAT2, SNP
The N-acetylation polymorphism was discovered over 50 years ago when individual variability in isoniazid neurotoxicity was attributed to genetic variability in N-acetylation capacity [1]. In addition to isoniazid, many aromatic amine drugs, such as sulfamethazine (SMZ), are subject to the acetylation polymorphism, thus affecting therapeutic efficacy and toxicity for many therapeutic drugs [2,3]. NAT2 is encoded by a 870-bp gene (NAT2) and genetic polymorphisms in NAT2 is common in the human population [3–6]. NAT2 genetic polymorphism modifies both the efficacy and toxicity of numerous arylamine and hydrazine drugs [2,3,6–8] and increases risk towards several arylamine carcinogen-related cancers [3,9,10].
The reference NAT2*4 allele and over 65 NAT2 allelic variants have been identified in human populations [101]. The NAT2 variant alleles possess one or a combination of SNPs in the 870 bp NAT2 coding region. As recently reviewed [11,12], most NAT2 variant alleles include one or more of the following seven most frequent SNPs: rs1801279 (191G>A), rs1041983 (282C>T), rs1801280 (341T>C), rs1799929 (481C>T), rs1799930 (590G>A), rs1208 (803A>G) and rs1799931 (857G>A). The majority of studies investigating the relationship of NAT2 genotype with drug therapy and/or disease risk use assays that detect only three SNPs (481C>T, 590G>A and 857G>A) to infer NAT2 acetylation status. Although several NAT2 SNPs are in linkage disequilibrium, assessment of only these three SNPs results in the misclassification of some NAT2 alleles [9,13].
The detection of 11 SNPs (seven SNPs listed above plus 111T>C, 434A>C, 759C>T and 845A>C) as previously described [14] has been proposed as the gold standard approach for NAT2 allele identification [13]. However, following comparisons of NAT2 genotyping results using both the 11-SNP and seven-SNP panels, the seven-SNP assay was sufficient for NAT2 genotyping in Caucasian and African–American populations [13].
Recently, a new tag-SNP rs1495741, at location 18317161, approximately 14.5 kb 3′ of the NAT2 coding region was identified in an ongoing bladder cancer genome-wide association study [15]. Agreement between the seven-SNP NAT2 inferred phenotype and the tag-SNP, rs1495741, was assessed in over 2000 subjects from the Spanish Bladder Cancer Study and association of rs1495741 genotypes with N-acetyltransferase catalytic activity was assessed in cryopreserved hepatocytes from over 150 individuals of European background [16]. Estimates for the association between NAT2 slow phenotype and bladder cancer risk and its interaction with cigarette smoking were comparable for the seven-SNP inferred NAT2 phenotype and rs1495741 [15]. In addition, rs1495741 genotypes were strongly related to N-acetyltransferase activity measured in hepatocytes derived from over 150 individuals of European descent [16].
In the current study, we investigated associations of SMZ N-acetyltransferase catalytic activity in cryopreserved human hepatocytes with the tag-SNP rs1495741 and each of the seven SNPs from the coding region described previously. We assessed the accuracy of the tag-SNP and various two-, three-, four- and seven-SNP genotyping panels to accurately infer NAT2 phenotype. Based on these results, we offer recommendations for NAT2 genotype and inferred phenotype determinations.
Materials & methods
Source & ethnicity of cryopreserved human hepatocytes
Over 250 cryopreserved human hepatocyte samples were collected for public sale and received from Celsis in vitro Technologies (MD, USA). They were not collected for this study. Upon receipt, hepatocytes were stored in liquid nitrogen until use. The subject identifier or any information regarding the source of the hepatocytes was not provided. Information on gender, age, race, cause of death, substance abuse, serology and other enzyme activities for the hepatocytes was accessed at [102]. Ethnicity was available for over 99% of the samples. The ethnic frequency in our convenience sampling from 256 individuals was 79.3% Caucasian, 10.55% African–American, 7.03% Hispanic, 1.17% Asian, 1.17% Polynesian and 0.78% unknown. The convenience sample was obtained from individuals in the USA and the ethnic frequency is similar to the 2010 US population census [103].
DNA isolation & SNP determinations
Upon removal from the liquid nitrogen, the hepatocytes were thawed and centrifuged as described previously [17]. Genomic DNA was isolated from pelleted cells prepared as described above using the QIAamp® DNA Mini Kit (Qiagen, MD, USA) according to manufacturer’s instructions. SNPs in the NAT2 coding region and their corresponding alleles and haplotypes were determined with the seven-SNP panel assay as previously described [18]. Briefly, SNP-specific PCR primers and fluorogenic probes were designed using Primer Express™ (Applied Biosystems, CA, USA). The fluorogenic probes are labeled with a reporter dye (either carboxyfluorescein or VIC®, Applied Biosystems) and are specific for one of the two possible bases identified at the seven SNPs rs1801279 (191G>A), rs1041983 (282C>T), rs1801280 (341T>C), rs1799929 (481C>T), rs1799930 (590G>A), rs1208 (803A>G) and rs1799931 (857G>A) in the NAT2 coding region.
The presence of the rs1495741 SNP was determined using a predeveloped TaqMan® SNP Genotyping Assays, Assay ID C_86841101–0 (Applied Biosystems) according to manufacturer’s instructions. Briefly the 20× primer and probe mix supplied (Assay ID C_8684110_10) was used at a 1× concentration with a half volume of 2× TaqMan Universal PCR Master Mix and 12.0 ng of genomic sample per well. Assays were designed and optimized to work with TaqMan® Universal PCR Master Mix (with or without AmpErase® UNG) using the same thermal cycling conditions, which were 60°C for 30 s, 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The PCR amplification and end point reading were performed on StepOnePlus™ real-time instrument (Applied Biosystems).
Controls (no DNA template) were run to ensure that there was no amplification of contaminating DNA.
NAT2 genotype & inferred phenotype
Determination of the seven NAT2 coding region SNPs described above, define 34 of the reported human NAT2 alleles or haplotypes (Table 1). NAT2 alleles possessing additional SNPs in the NAT2 coding region are quite rare [101]. Individuals possessing two of the NAT2 alleles associated with rapid acetylation activity (NAT2*4, NAT2*11, NAT2*12 and NAT2*13 clusters) were classified as rapid acetylators; individuals possessing one of these alleles and one allele associated with slow acetylation (NAT2*5, NAT2*6, NAT2*7 and NAT2*14 clusters) were classified as intermediate acetylators, and those individuals that possessed two slow acetylation alleles were classified as slow acetylators as previously described [18].
Table 1.
Common NAT2 single nucleotide pairs and their corresponding alleles/haplotypes.
| NAT2 allele (haplotype) | Nucleotide change(s) and rs identifiers | Amino acid change(s) |
|---|---|---|
| NAT2*4 | Reference | Reference |
|
| ||
| NAT2*5A | 341T>C (rs1801280)† | I114T† |
| 481C>T (rs1799929) | L161L (synonymous) | |
|
| ||
| NAT2*5B | 341T>C (rs1801280)† | I114T† |
| 481C>T (rs1799929) | L161L (synonymous) | |
| 803A>G (rs1208) | K268R | |
|
| ||
| NAT2*5C | 341T>C (rs1801280)† | I114T† |
| 803A>G (rs1208) | K268R | |
|
| ||
| NAT2*5D | 341T>C (rs1801280)† | I114T† |
|
| ||
| NAT2*5E | 341T>C (rs1801280)† | I114T† |
| 590G>A (rs1799930) | R197Q | |
|
| ||
| NAT2*5G | 282C>T (rs1041983) | Y94Y (synonymous) |
| 341T>C (rs1801280)† | I114T† | |
| 481C>T (rs1799929) | L161L (synonymous) | |
| 803A>G (rs1208) | K268R | |
|
| ||
| NAT2*5J | 282C>T (rs1041983) | Y94Y (synonymous) |
| 341T>C (rs1801280)† | I114T† | |
| 590G>A (rs1799930) | R197Q | |
|
| ||
| NAT2*5K | 282C>T (rs1041983) | Y94Y (synonymous) |
| 341T>C (rs1801280)† | I114T† | |
|
| ||
| NAT2*6A | 282C>T (rs1041983) | Y94Y (synonymous) |
| 590G>A (rs1799930)† | R197Q† | |
|
| ||
| NAT2*6B | 590G>A (rs1799930)† | R197Q† |
|
| ||
| NAT2*6C | 282C>T (rs1041983) | Y94Y (synonymous) |
| 590G>A (rs1799930)† | R197Q† | |
| 803A>G (rs1208) | K268R | |
|
| ||
| NAT2*6E | 481C>T (rs1799929) | L161L (synonymous) |
| 590G>A (rs1799930)† | R197Q† | |
|
| ||
| NAT2*6F | 590G>A (rs1799930)† | R197Q† |
| 803A>G (rs1208) | K268R | |
|
| ||
| NAT2*6J | 282C>T (rs1041983) | Y94Y (synonymous) |
| 590G>A (rs1799930)† | R197Q† | |
| 857G>A (rs1799931) | G286E | |
|
| ||
| NAT2*6N | 282C>T (rs1041983) | Y94Y (synonymous) |
| 481C>T (rs1799929) | L161L (synonymous) | |
| 590G>A (rs1799930)† | R197Q† | |
|
| ||
| NAT2*7A | 857G>A (rs1799931)† | G286E† |
|
| ||
| NAT2*7B | 282C>T (rs1041983) | Y94Y (synonymous) |
| 857G>A (rs1799931)† | G286E† | |
|
| ||
| NAT2*7C | 282C>T (rs1041983) | Y94Y (synonymous) |
| 803A>G (rs1208) | K268R | |
| 857G>A (rs1799931)† | G286E† | |
|
| ||
| NAT2*7D | 191G>A (rs1801279) | R64Q |
| 282C>T (rs1041983) | Y94Y (synonymous) | |
| 857G>A (rs1799931)† | G286E† | |
|
| ||
| NAT2*7E | 282C>T (rs1041983) | Y94Y (synonymous) |
| 481C>T (rs1799929) | L161L (synonymous) | |
| 857G>A (rs1799931)† | G286E† | |
|
| ||
| NAT2*11A | 481C>T (rs1799929)† | L161L (synonymous)† |
|
| ||
| NAT2*12A | 803A>G (rs1208)† | K268R† |
|
| ||
| NAT2*12B | 282C>T (rs1041983) | Y94Y (synonymous) |
| 803A>G (rs1208)† | K268R† | |
|
| ||
| NAT2*12C | 481C>T (rs1799929) | L161L (synonymous) |
| 803A>G (rs1208)† | K268R† | |
|
| ||
| NAT2*13A | 282C>T (rs1041983)† | Y94Y (synonymous)† |
|
| ||
| NAT2*14A | 191G>A (rs1801279)† | R64Q† |
|
| ||
| NAT2*14B | 191G>A (rs1801279)† | R64Q† |
| 282C>T (rs1041983) | Y94Y (synonymous) | |
|
| ||
| NAT2*14C | 191G>A (rs1801279)† | R64Q† |
| 341T>C (rs1801280) | I114T | |
| 481C>T (rs1799929) | L161L (synonymous) | |
| 803A>G (rs1208) | K268R | |
|
| ||
| NAT2*14D | 191G>A (rs1801279)† | R64Q† |
| 282C>T (rs1041983) | Y94Y (synonymous) | |
| 590G>A (rs1799930) | R197Q | |
|
| ||
| NAT2*14E | 191G>A (rs1801279)† | R64Q† |
| 803A>G (rs1208) | K268R | |
|
| ||
| NAT2*14F | 191G>A (rs1801279)† | R64Q† |
| 341T>C (rs1801280) | I114T | |
| 803A>G (rs1208) | K268R | |
|
| ||
| NAT2*14G | 191G>A (rs1801279)† | R64Q† |
| 282C>T (rs1041983) | Y94Y (synonymous) | |
| 803A>G (rs1208) | K268R | |
|
| ||
| NAT2*14I | 191G>A (rs1801279)† | R64Q† |
| 481C>T (rs1799929) | L161L (synonymous) | |
| 803A>G (rs1208) | K268R | |
Signature SNP for each haplotype cluster.
Data from [101].
The two-SNP inferred NAT2 acetylator phenotype was determined by analysis of two SNPs: rs1041983 (282C>T) and rs1801280 (341T>C). The three-SNP inferred NAT2 acetylator phenotype was determined by analysis of three SNPs: rs1799929 (481C>T), rs1799930 (590G>A) and rs1799931 (857G>A). The four-SNP inferred NAT2 acetylator phenotype was determined by an assay of four SNPs: rs1801279 (191G>A), rs1801280 (341T>C), rs1799930 (590G>A) and rs1799931 (857G>A). For each of the two-, three- and four-SNP panel assays, samples homozygous common for all SNPs were classified as rapid acetylator phenotype, samples heterozygous for any one of the SNPs were classified as intermediate acetylator phenotype and samples homozygous variant for one or more SNPs or heterozygous for two or more SNPs were classified as slow acetylator phenotype.
NAT2 catalytic activity
Upon removal from the liquid nitrogen, the hepatocytes were thawed and centrifuged as described previously [17]. Hepatocytes were lysed in 20 mM sodium phosphate (pH 7.4), 1 mM EDTA, 1 mM dithiothreitol, 100 μM phenylmethanesulfonyl fluoride, 1 μg/ml aprotinin and 1 μM pepstatin A by three rounds of freeze (−70°C)/thawing (37°C). Lysates were centrifuged at 15,000 × g for 20 min at 4°C and the resulting supernatant assayed for protein and enzymatic activity as described later.
SMZ N-acetyltransferase assays were conducted as previously described [20]. Briefly, reactions containing the supernatant of hepatocyte lysate (<2 mg/ml protein), SMZ (300 μM) and acetyl coenzyme A (1 mM) were incubated at 37°C. Reactions were terminated at 10 min by the addition of 1/10 volume 1 M acetic acid. The reaction tubes were centrifuged to precipitate the protein. The reaction tubes were centrifuged to precipitate the protein and supernatant was injected onto a Lichrospher® 100 RP-18 (5 mM) reversed phase column. Samples were eluted using a Beckman System Gold® HPLC system equipped with a model 126 programmable solvent module pump and a model 166 programmable UV-visible detector, and the absorbance was measured at 260 nm. The minimum level of detection of N-acetyl-SMZ was 5 pmol. Briefly, the substrates and acetylated products were separated with a mobile-phase of 20 mM sodium perchlorate (pH 2.5; solvent A) and acetonitrile (solvent B). A gradient of solvent B from 9 to 29%, over 5 min at a flow rate of 2 ml/min was used to separate SMZ and N-acetyl-SMZ. Under these conditions SMZ and N-acetyl-SMZ were eluted at 5.2 and 8.4 min, respectively.
Data analysis
Differences in NAT2 catalytic activity among SNPs or haplotypes were analyzed for significance by one-way analysis of variance and Tukey post hoc tests using GraphPad Prism® Software (Graphpad Software, CA, USA). NAT2 acetylator genotype/phenotype misclassification was estimated by visual inspection of the data.
Results
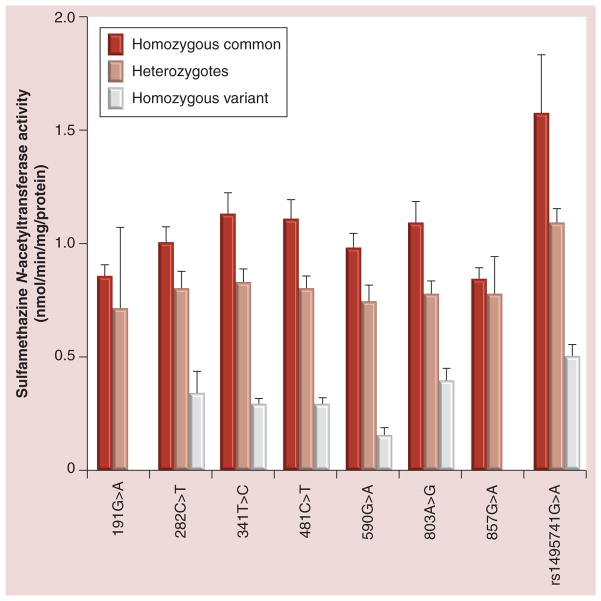
SMZ N-acetyltransferase catalytic activities in cryopreserved human hepatocytes were analyzed for each individual NAT2 SNP. Many of the SNPs (282C>T, 341T>C, 481T>C, 590G>A, 803G>A and rs1495741G>A) differed significantly (p < 0.0001) in SMZ N-acetyltransferase activities among homozygous common, heterozygote and homozygous variant samples following one way analysis of variance (Figure 1).
Figure 1. Sulfamethazine N-acetyltransferase catalytic activities (mean ± standard error of the mean) in cryopreserved human hepatocytes are plotted on the ordinate versus each individual NAT2 SNP on the abscissa.
The white bar is not shown for 191G>A and 857G>A because no homozygous variant samples were available. Sulfamethazine N-acetyltransferase activities for 282C>T, 341T>C, 481T>C, 590G>A, 803G>A and rs1495741G>A all differed significantly (p < 0.0001) among homozygous common, heterozygote and homozygous variant following one-way analysis of variance. For each of these SNPs, homozygous common was significantly higher than heterozygote (p < 0.05), which was significantly higher than homozygous variant samples (p < 0.05) following one-way analysis of variance and Tukey post hoc tests. Homozygous common was not significantly different than the heterozygote for 191G>A (p = 0.6522) or 857G>A (p = 0.7185).
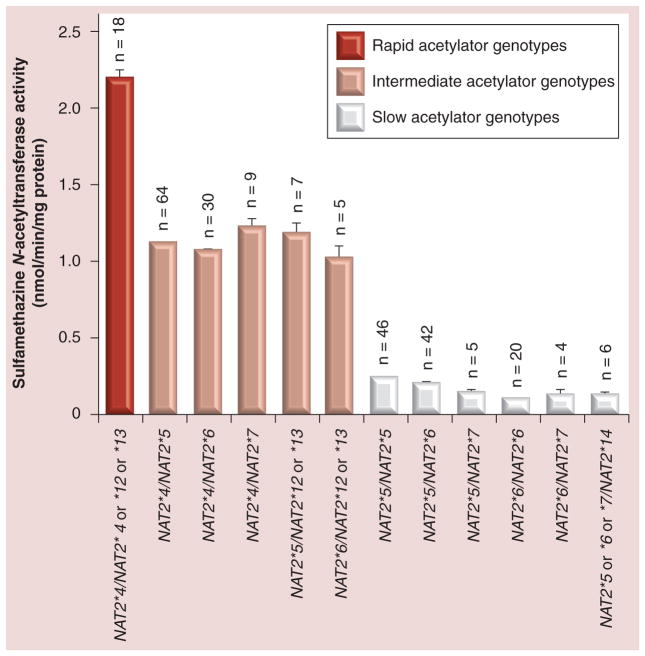
SMZ N-acetyltransferase catalytic activities were segregated by individual NAT2 SNPs and no individual SNP, including rs1495741 yielded a nonoverlapping trimodal distribution (Figure 2) of rapid, intermediate and slow acetylator phenotypes. However, when SMZ N-acetyltransferase activities were segregated by NAT2 genotype (Figure 3), a clear trimodal and nonoverlapping distribution of rapid, intermediate and slow acetylator phenotypes was observed and differences among NAT2 acetylator phenotypes within an NAT2 genotype group were trivial.
Figure 2. Scatter plots of sulfamethazine N-acetyltransferase catalytic activities in cryopreserved human hepatocytes are plotted on the ordinate versus each NAT2 single nucleotide pair on the abscissa.
Each homozygous common sample is illustrated with an open square, each heterozygote is illustrated with an open circle, and each homozygous variant is illustrated with an open triangle. Mean activities are shown by the horizontal lines. Statistical analysis of these data is shown in the legend to Figure 1.
Figure 3. Sulfamethazine N-acetyltransferase catalytic activities (mean ± standard error of the mean) in cryopreserved human hepatocytes are plotted on the ordinate versus NAT2 genotype(s) on the abscissa.
The number of samples in each NAT2 acetylator genotype group is shown and related NAT2 genotypes were combined when sample sizes were less than 4.
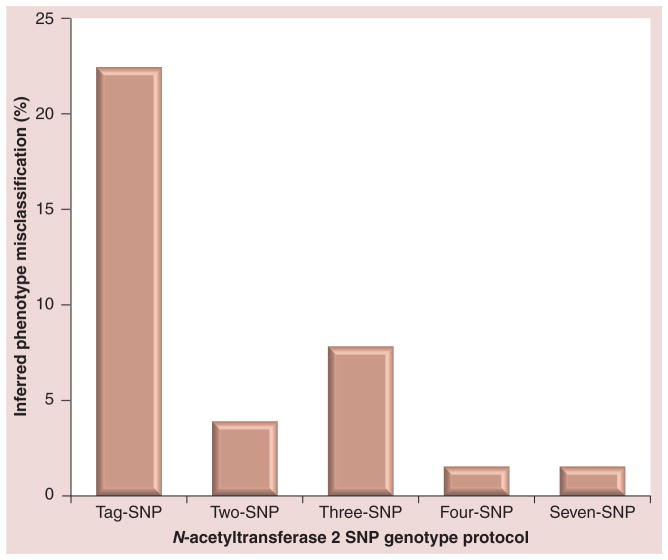
In the absence of a gold-standard reference, determinations of specificity and selectivity cannot be calculated with certainty. However, based on visual inspection of the data, NAT2 acetylator genotype/phenotype misclassification can be estimated (Figure 4). Use of the rs1495741 genotype alone resulted in an inferred phenotype accuracy of 199/256 or 77.7% of the hepatocytes sampled in this study. The misclassification included 32 rapid acetylators, 22 intermediate acetylators and only three slow acetylators. Thus, the rs1495741 SNP misclassified rapid and intermediate acetylators to a much greater extent than it did slow acetylators. Use of the historical three-SNP rs1799929 (481C>T), rs1799930 (590G>A) and rs1799931 (857G>A) genotyping protocol resulted in an inferred phenotype accuracy of 236/256 or 92.2% of the hepatocytes sampled this study. Misclassification was almost entirely intermediate acetylators (19/20; 95%). A two-SNP rs1041983 (282C>T) and rs1801280 (341T>C) genotyping protocol resulted in an inferred phenotype accuracy of 246/256 or 96.1% of the hepatocytes sampled in this study. Misclassification with the two-SNP genotype protocol was equally divided between slow (five) and intermediate (five) acetylators. A four-SNP panel of rs1801279 (191G>A), rs1801280 (341T>C), rs1799930 (590G>A) and rs1799931 (857G>A) resulted in an inferred NAT2 acetylator phenotype accuracy of 252/256 or 98.4% of the hepatocytes sampled this study. The misclassification included no rapid acetylators, three intermediate acetylators and one slow acetylator. The seven-SNP assay resulted in an identical inferred NAT2 acetylator phenotype accuracy determined by the four-SNP assay. The extent of acetylator phenotype misclassification with these NAT2 genotyping protocols is illustrated in Figure 5.
Figure 4. Scatter plots of SMZ N-acetyltransferase catalytic activities plotted on the abscissa versus inferred acetylator phenotype as determined by various NAT2 genotyping protocols.
Each NAT2 rapid acetylator sample is illustrated with an open square, each NAT2 intermediate acetylator sample is illustrated with an open circle, and each NAT2 slow acetylator sample is illustrated with an open triangle. Mean activities are shown by the vertical lines. Since the sulfamethazine N-acetyltransferase catalytic activity distribution was not explicitly trimodal in our population of samples, the identification of misclassified inferred NAT2 phenotypes was estimated by visual inspection of the data (illustrated by the inserted boxes).
Figure 5.
Misclassification (percentage) of inferred acetylator phenotype as determined with various NAT2 SNPs genotyping protocols.
Discussion
N-acetylation capacities determined in vivo have primarily discriminated between rapid versus slow acetylator phenotypes. Although less commonly reported, trimodal (rapid, intermediate and slow acetylator phenotype) distributions of N-acetylation capacity in vivo have been reported in humans following administrations of SMZ [21,22], isoniazid [23–26], sulfasalazine [27] and caffeine [28–29].
Hepatic N-acetylation capacity in vitro often segregates into rapid, intermediate, and slow acetylator phenotypes as reported in humans [17,23], mice [30], Syrian hamsters [31,32] and rats [33,34]. NAT2 is highly expressed in cryopreserved liver hepatocytes [17] and SMZ is a highly selective substrate for NAT2 versus NAT1 [35,36], facilitating comparisons of NAT2 phenotype accuracy inferred from different NAT2 SNP genotype panels.
In our study, we assessed the relative accuracy of various NAT2 SNP genotyping panels to accurately distinguish among rapid, intermediate and slow acetylator phenotypes. Previous studies include calculations of sensitivity and specificity for various assays. We chose not to analyze our data in this manner because:
There is no ‘gold’ standard for definitive assessment of rapid, intermediate and slow acetylator phenotypes
The calculations of sensitivity and specificity are dependent upon both the method used to determine phenotype and upon the ethnic diversity of the sample population
Thus, our genotyping ‘accuracy’ comparisons are based upon the phenotype method chosen (SMZ N-acetyltransferase catalytic activities in cryopreserved human hepatocytes) and the ethnic composition of our sample set.
SMZ N-acetyltransferase catalytic activities in cryopreserved human hepatocytes were segregated by individual NAT2 SNPs and no individual SNP yielded a nonoverlapping trimodal distribution. The effects of each SNP on NAT2 activity was consistent with the effects that have been shown following recombinant expression of human NAT2 alleles in bacteria [37–39], yeast [40,41] and mammalian cells [4,42,43].
Use of the rs1495741 genotype alone resulted in an inferred phenotype accuracy of 77.7% of the hepatocytes sampled in this study. The rs1495741 is located within a large linkage disequilibrium block including each of the seven SNPs determined our study. As reported by Garcia-Closas et al., linkage disequilibrium of the seven NAT2 SNPs to the rs1495741 SNP was highest for 341T>C (pairwise r2 = 0.24), 481T>C (pairwise r2 = 0 0.23) and 803A>G (pairwise r2 = 0.21) [16]. These three SNPs define the NAT2*5B haplotype (Table 1), which is the most common slow acetylator haplotype in European populations. By contrast, linkage disequilibrium of the 857G>A SNP, common in Asian populations was dramatically less (pairwise r2 = 0.01), suggesting that the rs1495741 SNP alone would be much less accurate in the inference of NAT2 phenotype in populations of Asian ancestry. The three-SNP panel and the rs1495741 SNP should be more accurate to infer NAT2 phenotype if restricted to populations of European ancestry.
Subsequent to the submission of our manuscript, a recent study proposed that a two-SNP (282C>T, 341T>C) assay was more accurate than the tag-SNP and nearly as accurate as the seven-SNP assay for discriminating rapid versus slow acetylator NAT2 phenotype as determined in vivo with caffeine [44]. We determined the accuracy of this two-SNP assay for discriminating rapid, intermediate or slow acetylator phenotypes as measured by SMZ NAT2 catalytic activity in cryopreserved human hepatocytes and also found it to be more accurate than both the tag-SNP and the historical three-SNP assay.
However, we found that a four-SNP panel rs1801279 (191G>A), rs1801280 (341T>C), rs1799930 (590G>A) and rs1799931 (857G>A) was still more accurate for inferring SMZ N-acetyltransferase activity phenotypes in cryopreserved human hepatocytes. This four-SNP assay measures the common SNPs that directly confer reductions in NAT2 catalytic activity. Unlike the two-SNP assay, it does not misclassify NAT2*13A alleles as slow acetylators. NAT2*13A allele frequency is substantially more common among populations of African than European ancestry [45–47], indicating this misclassification takes on greater significance in non-European populations.
Determination of acetylator genotype has been shown to improve efficacy and reduce toxicity in tuberculosis patients treated with isoniazid [48–50]. Indeed, NAT2 genotyping may be superior to therapeutic drug monitoring for isoniazid dose individualization in tuberculosis therapy as specific and distinct dosing recommendations have been proposed for rapid, intermediate and slow acetylator genotypes [26] The NAT2 tag-SNP rs1495741, is strongly associated with urinary bladder cancer [15], plasma triglyceride levels and coronary artery disease [51] in recent genome-wide association studies. Although DNA sequencing of the entire coding exon would provide the most comprehensive haplotype and genotype information, our results suggest that use of the four-SNP panel will infer NAT2 acetylator phenotype with high accuracy, and is recommended over the tag-, two-, three- and (for economy of scale) the seven-SNP genotyping panels, particularly in populations that include individuals of non-European ancestry.
Executive summary.
Background
The metabolism of isoniazid and many aromatic amine drugs such as sulfamethazine (SMZ) are subject to the NAT2 polymorphism, thus affecting therapeutic efficacy and toxicity for many therapeutic drugs.
NAT2 is highly expressed in cryopreserved liver hepatocytes and SMZ is a highly selective substrate for NAT2, facilitating comparisons of NAT2 phenotype accuracy inferred from different NAT2 SNP genotype panels.
Materials & methods
SMZ N-acetyltransferase catalytic activities were measured in cryopreserved human hepatocytes from a convenience sample of individuals in the USA with ethnic frequency similar to the 2010 US population census.
SMZ N-acetyltransferase catalytic activities were segregated by the tag-SNP rs1495741 and each of the seven most common SNPs in the NAT2 coding region.
Results
282C>T, 341T>C, 481T>C, 590G>A, 803G>A and rs1495741G>A differed significantly (p < 0.0001) in SMZ N-acetyltransferase activity among homozygous common, heterozygote and homozygous variant genotypes, but no SNP yielded a nonoverlapping trimodal distribution.
The functional effect of each SNP on NAT2 activity in cryopreserved human hepatocytes was consistent with the effects that have been shown following recombinant expression of human NAT2 alleles in bacteria, yeast and mammalian cells.
The accuracy of the various NAT2 SNP genotype panels to infer NAT2 phenotype was as follows: tag-SNP: 77.7%; two-SNP: 96.1%; three-SNP: 92.2%; four-SNP: 98.4%; and seven-SNP: 98.4%.
Conclusion
A NAT2 four-SNP panel of rs1801279 (191G>A), rs1801280 (341T>C), rs1799930 (590G>A) and rs1799931 (857G>A) infers NAT2 acetylator phenotype with high accuracy, and is recommended over the tag-SNP, two-SNP, three-SNP (and for economy of scale the seven-SNP) genotyping panels, particularly in populations of non-European ancestry.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
This research project was supported by National Institute of Health grants R01-CA034627 and P30-ES014443. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Evans DA, Manley KA, McKusick VA. Genetic control of isoniazid metabolism in man. Br Med J. 1960;2:485–491. doi: 10.1136/bmj.2.5197.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber WW, Hein DW. N-acetylation pharmacogenetics. Pharmacol Rev. 1985;37:25–79. [PubMed] [Google Scholar]
- 3.Cascorbi I, Brockmoller J, Mrozikiewicz PM, Muller A, Roots I. Arylamine N-acetyltransferase activity in man. Drug Metab Rev. 1999;31:489–502. doi: 10.1081/dmr-100101932. [DOI] [PubMed] [Google Scholar]
- 4▪.Blum M, Demierre A, Grant DM, Heim M, Meyer UA. Molecular mechanism of slow acetylation of drugs and carcinogens in humans. Proc Natl Acad Sci USA. 1991;88:5237–5241. doi: 10.1073/pnas.88.12.5237. Described the initial report into the functional effects of three common NAT2 SNPs and formed the basis for the historical three-SNP assay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vatsis KP, Martell KJ, Weber WW. Diverse point mutations in the human gene for polymorphic N-acetyltransferase. Proc Natl Acad Sci USA. 1991;88:6333–6337. doi: 10.1073/pnas.88.14.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boukouvala S, Fakis G. Arylamine N-acetyltransferases: what we learn from genes and genomes. Drug Metab Rev. 2005;37:511–564. doi: 10.1080/03602530500251204. [DOI] [PubMed] [Google Scholar]
- 7.Grant DM, Goodfellow GH, Sugamori K, Durette K. Pharmacogenetics of the human arylamine N-acetyltransferases. Pharmacology. 2000;61:204–211. doi: 10.1159/000028402. [DOI] [PubMed] [Google Scholar]
- 8.Sim E, Lack N, Wang CJ, et al. Arylamine N-acetyltransferases: structural and functional implications of polymorphisms. Toxicology. 2008;254:170–183. doi: 10.1016/j.tox.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 9▪.Hein DW, Doll MA, Fretland AJ, et al. Molecular genetics and epidemiology of the NAT1 and NAT2 acetylation polymorphisms. Cancer Epidemiol Biomarkers Prev. 2000;9:29–42. One of the first comprehensive reviews on the role of NAT2 polymorphisms in cancer risk. The paper also discusses possible genotyping misclassification with the historical three-SNP genotype assay. [PubMed] [Google Scholar]
- 10.Agundez JA. Polymorphisms of human N-acetyltransferases and cancer risk. Curr Drug Metab. 2008;9:520–531. doi: 10.2174/138920008784892083. [DOI] [PubMed] [Google Scholar]
- 11.Walraven JM, Zang Y, Trent JO, Hein DW. Structure/function evaluations of single nucleotide polymorphisms in human N-acetyltransferase 2. Curr Drug Metab. 2008;9:471–486. doi: 10.2174/138920008784892065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪.Hein DW. N-acetyltransferase SNPs: emerging concepts serve as a paradigm for understanding complexities of personalized medicine. Expert Opin Drug Metab Toxicol. 2009;5:353–366. doi: 10.1517/17425250902877698. Summarizes the functional effects of NAT2 SNPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13▪.Deitz AC, Rothman N, Rebbeck TR, et al. Impact of misclassification in genotype-exposure interaction studies: example of N-acetyltransferase 2 (NAT2), smoking, and bladder cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1543–1546. Reports NAT2 genotyping misclassification with the historical three-SNP assay and the consequences for gene–environmental interaction analyses. [PubMed] [Google Scholar]
- 14.Doll MA, Fretland AJ, Deitz AC, Hein DW. Determination of human NAT2 acetylator genotype by restriction fragment-length polymorphism and allele-specific amplification. Anal Biochem. 1995;231:413–420. doi: 10.1006/abio.1995.9978. [DOI] [PubMed] [Google Scholar]
- 15▪▪.Rothman N, Garcia-Closas M, Chatterjee N, et al. A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nat Genet. 2010;42:978–984. doi: 10.1038/ng.687. Describes the genome-wide association study that identified a new tag-SNP for slow acetylation and increased risk for urinary bladder cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16▪▪.Garcia-Closas M, Hein DW, Silverman D, et al. A single nucleotide polymorphism tags variation in the arylamine N-acetyltransferase 2 phenotype in populations of European background. Pharmacogenet Genomics. 2011;21:231–236. doi: 10.1097/FPC.0b013e32833e1b54. Reported associations between the newly identified tag-SNP and arylamine N-acetyltransferase activities in human hepatocytes from a population of individuals of European ancestry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doll MA, Zang Y, Moeller T, Hein DW. Codominant expression of N-acetylation and O-acetylation activities catalyzed by N-acetyltransferase 2 in human hepatocytes. J Pharmacol Exp Ther. 2010;334:540–544. doi: 10.1124/jpet.110.168567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doll MA, Hein DW. Comprehensive human NAT2 genotype method using single nucleotide polymorphism-specific polymerase chain reaction primers and fluorogenic probes. Anal Biochem. 2001;288:106–108. doi: 10.1006/abio.2000.4892. [DOI] [PubMed] [Google Scholar]
- 19.Hein DW. N-acetyltransferase 2 genetic polymorphism: effects of carcinogen and haplotype on urinary bladder cancer risk. Oncogene. 2006;25:1649–1658. doi: 10.1038/sj.onc.1209374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leff MA, Epstein PN, Doll MA, et al. Prostate-specific human N-acetyltransferase 2 (NAT2) expression in the mouse. J Pharmacol Exp Ther. 1999;290:182–187. [PubMed] [Google Scholar]
- 21.Chapron DJ, Kramer PA, Mercik SA. Kinetic discrimination of three sulfamethazine acetylation phenotypes. Clin Pharmacol Ther. 1980;27:104–113. doi: 10.1038/clpt.1980.16. [DOI] [PubMed] [Google Scholar]
- 22.Chen B, Zhang WX, Cai WM. The influence of various genotypes on the metabolic activity of NAT2 in a Chinese population. Eur J Clin Pharmacol. 2006;62:355–359. doi: 10.1007/s00228-006-0110-6. [DOI] [PubMed] [Google Scholar]
- 23.Deguchi T, Mashimo M, Suzuki T. Correlation between acetylator phenotypes and genotypes of polymorphic arylamine N-acetyltransferase in human liver. J Biol Chem. 1990;265:12757–12760. [PubMed] [Google Scholar]
- 24.Parkin DP, Vandenplas S, Botha FJ, et al. Trimodality of isoniazid elimination: phenotype and genotype in patients with tuberculosis. Am J Respir Crit Care Med. 1997;155:1717–1722. doi: 10.1164/ajrccm.155.5.9154882. [DOI] [PubMed] [Google Scholar]
- 25.Smith CA, Wadelius M, Gough AC, Harrison DJ, Wolf CR, Rane A. A simplified assay for the arylamine N-acetyltransferase 2 polymorphism validated by phenotyping with isoniazid. J Med Genet. 1997;34:758–760. doi: 10.1136/jmg.34.9.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26▪.Kinzig-Schippers M, Tomalik-Scharte D, Jetter A, et al. Should we use N-acetyltransferase type 2 genotyping to personalize isoniazid doses? Antimicrob Agents Chemother. 2005;49:1733–1738. doi: 10.1128/AAC.49.5.1733-1738.2005. Describes the importance of NAT2 genotyping in tuberculosis therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma JJ, Liu CG, Li JH, Cao XM, Sun SL, Yao X. Effects of NAT2 polymorphism on SASP pharmacokinetics in Chinese population. Clin Chim Acta. 2009;407:30–35. doi: 10.1016/j.cca.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 28.Grant DM, Tang BK, Kalow W. A simple test for acetylator phenotype using caffeine. Br J Clin Pharmacol. 1984;17:459–464. doi: 10.1111/j.1365-2125.1984.tb02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cascorbi I, Drakoulis N, Brockmoller J, Maurer A, Sperling K, Roots I. Arylamine N-acetyltransferase (NAT2) mutations and their allelic linkage in unrelated Caucasian individuals: correlation with phenotypic activity. Am J Hum Genet. 1995;57:581–592. [PMC free article] [PubMed] [Google Scholar]
- 30.Hein DW, Trinidad A, Yerokun T, Ferguson RJ, Kirlin WG, Weber WW. Genetic control of acetyl coenzyme A-dependent arylamine N-acetyltransferase, hydrazine N-acetyltransferase, and N-hydroxy-arylamine O-acetyltransferase enzymes in C57BL/6J, A/J, AC57F1, and the rapid and slow acetylator A.B6 and B6. A congenic inbred mouse. Drug Metab Dispos. 1988;16:341–347. [PubMed] [Google Scholar]
- 31.Hein DW, Rustan TD, Bucher KD, Miller LS. Polymorphic and monomorphic expression of arylamine carcinogen N-acetyltransferase isozymes in tumor target organ cytosols of Syrian-hamsters congenic at the polymorphic acetyltransferase locus. J Pharmacol Exp Ther. 1991;259:699–704. [PubMed] [Google Scholar]
- 32.Hein DW, Doll MA, Rustan TD, Gray K, Ferguson RJ, Feng Y. Construction of Syrian hamster lines congenic at the polymorphic acetyltransferase locus (NAT2): acetylator genotype-dependent N- and O-acetylation of arylamine carcinogens. Toxicol Appl Pharmacol. 1994;124:16–24. doi: 10.1006/taap.1994.1003. [DOI] [PubMed] [Google Scholar]
- 33.Hein DW, Rustan TD, Bucher KD, Martin WJ, Furman EJ. Acetylator phenotype-dependent and -independent expression of arylamine N-acetyltransferase isozymes in rapid and slow acetylator inbred rat liver. Drug Metab Dispos. 1991;19:933–937. [PubMed] [Google Scholar]
- 34.Hein DW, Bendaly J, Neale JR, Doll MA. Systemic functional expression of N-acetyltransferase polymorphism in the F344 Nat2 congenic rat. Drug Metab Dispos. 2008;36:2452–2459. doi: 10.1124/dmd.108.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grant DM, Blum M, Beer M, Meyer UA. Monomorphic and polymorphic human arylamine N-acetyltransferases: a comparison of liver isozymes and expressed products of two cloned genes. Mol Pharmacol. 1991;39:184–191. [PubMed] [Google Scholar]
- 36.Hein DW, Doll MA, Rustan TD, et al. Metabolic activation and deactivation of arylamine carcinogens by recombinant human NAT1 and polymorphic NAT2 acetyltransferases. Carcinogenesis. 1993;14:1633–1638. doi: 10.1093/carcin/14.8.1633. [DOI] [PubMed] [Google Scholar]
- 37.Hein DW, Ferguson RJ, Doll MA, Rustan TD, Gray K. Molecular genetics of human polymorphic N-acetyltransferase: enzymatic analysis of 15 recombinant wild-type, mutant, and chimeric NAT2 allozymes. Hum Mol Genet. 1994;3:729–734. doi: 10.1093/hmg/3.5.729. [DOI] [PubMed] [Google Scholar]
- 38.Hein DW, Doll MA, Rustan TD, Ferguson RJ. Metabolic activation of N-hydroxyarylamines and N-hydroxyarylamides by 16 recombinant human NAT2 allozymes: effects of 7 specific NAT2 nucleic acid substitutions. Cancer Res. 1995;55:3531–3536. [PubMed] [Google Scholar]
- 39.Grant DM, Hughes NC, Janezic SA, et al. Human acetyltransferase polymorphisms. Mutat Res. 1997;376:61–70. doi: 10.1016/s0027-5107(97)00026-2. [DOI] [PubMed] [Google Scholar]
- 40.Fretland AJ, Leff MA, Doll MA, Hein DW. Functional characterization of human N-acetyltransferase 2 (NAT2) single nucleotide polymorphisms. Pharmacogenetics. 2001;11:207–215. doi: 10.1097/00008571-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Hein DW, Fretland AJ, Doll MA. Effects of single nucleotide polymorphisms in human N-acetyltransferase 2 on metabolic activation (O-acetylation) of heterocyclic amine carcinogens. Int J Cancer. 2006;119:1208–1211. doi: 10.1002/ijc.21957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zang Y, Zhao S, Doll MA, States JC, Hein DW. The T341C (Ile114Thr) polymorphism of N-acetyltransferase 2 yields slow acetylator phenotype by enhanced protein degradation. Pharmacogenetics. 2004;14:717–723. doi: 10.1097/00008571-200411000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Zang Y, Doll MA, Zhao S, States JC, Hein DW. Functional characterization of single-nucleotide polymorphisms and haplotypes of human N-acetyltransferase 2. Carcinogenesis. 2007;28:1665–1671. doi: 10.1093/carcin/bgm085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selinski S, Blaszkewicz M, Lehmann ML, et al. Genotyping NAT2 with only two SNPs (rs1041983 and rs1801280) outperforms the tagging SNP rs1495741 and is equivalent to the conventional 7-SNP NAT2 genotype. Pharmacogenet Genomics. 2011;21(10):673–678. doi: 10.1097/FPC.0b013e3283493a23. [DOI] [PubMed] [Google Scholar]
- 45.Loktionov A, Moore W, Spencer SP, et al. Differences in N-acetylation genotypes between Caucasians and black South Africans: implications for cancer prevention. Cancer Detect Prev. 2002;26:15–22. doi: 10.1016/s0361-090x(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 46.Sabbagh A, Langaney A, Darlu P, Gerard N, Krishnamoorthy R, Poloni ES. Worldwide distribution of NAT2 diversity: implications for NAT2 evolutionary history. BMC Genet. 2008;9:21. doi: 10.1186/1471-2156-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sabbagh A, Darlu P, Crouau-Roy B, Poloni ES. Arylamine N-acetyltransferase 2 (NAT2) genetic diversity and traditional subsistence: a worldwide population survey. PLoS ONE. 2011;6:e18507. doi: 10.1371/journal.pone.0018507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohno M, Yamaguchi I, Yamamoto I, et al. Slow N-acetyltransferase 2 genotype affects the incidence of isoniazid and rifampicin-induced hepatotoxicity. Int J Tuberc Lung Dis. 2000;4:256–261. [PubMed] [Google Scholar]
- 49.Hiratsuka M, Kishikawa Y, Takekuma Y, et al. Genotyping of the N-acetyltransferase 2 polymorphism in the prediction of adverse drug reactions to isoniazid in Japanese patients. Drug Metab Pharmacokinet. 2002;17:357–362. doi: 10.2133/dmpk.17.357. [DOI] [PubMed] [Google Scholar]
- 50.Cho HJ, Koh WJ, Ryu YJ, et al. Genetic polymorphisms of NAT2 and CYP2E1 associated with antituberculosis drug-induced hepatotoxicity in Korean patients with pulmonary tuberculosis. Tuberculosis. 2007;87:551–556. doi: 10.1016/j.tube.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 51.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:7071–7073. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101▪▪.Department of pharmacology and toxicology. University of Louisville; KY, USA: www.n-acetyltransferasenomenclature.louisville.edu Official website for consensus human arylamine N-acetyltransferase gene nomenclature. [Google Scholar]
- 102.Celsis Characterization tables. www.celsis.com/ivt/characterization-tables/
- 103.US Census Burea. Overview of race and Hispanic origin. 2010 www.census.gov/prod/cen2010/briefs/c2010br-02.pdf.