Abstract
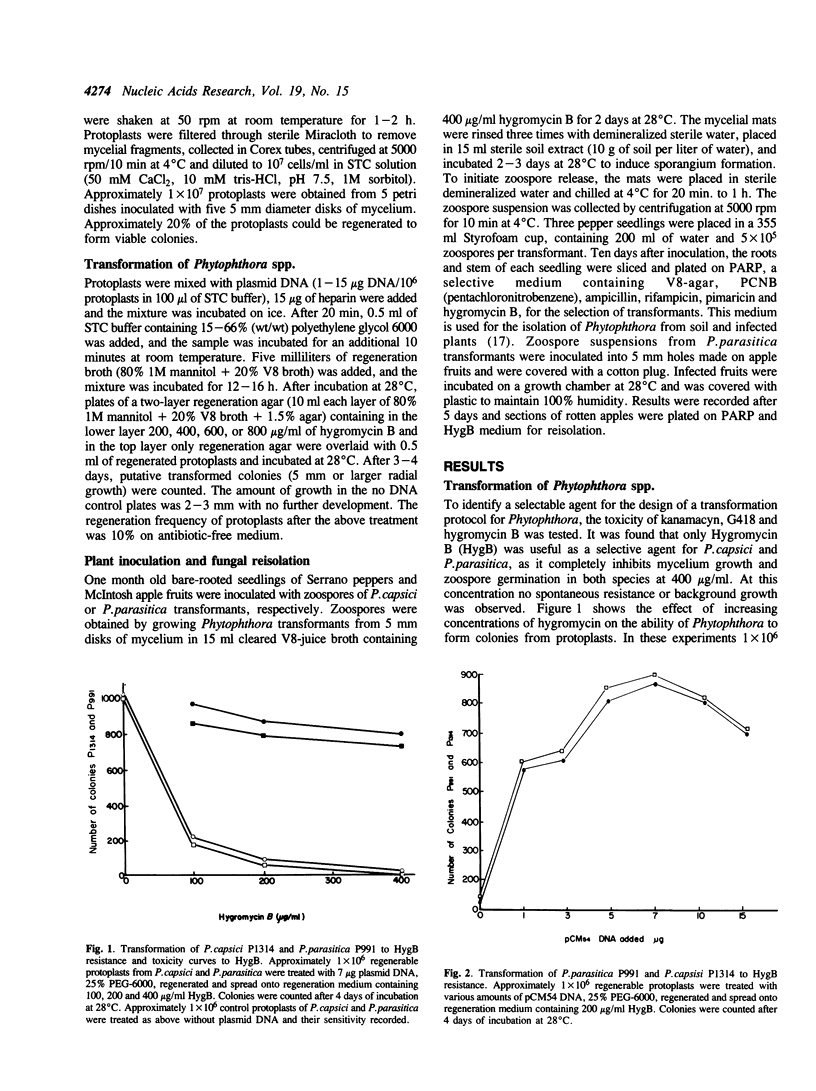
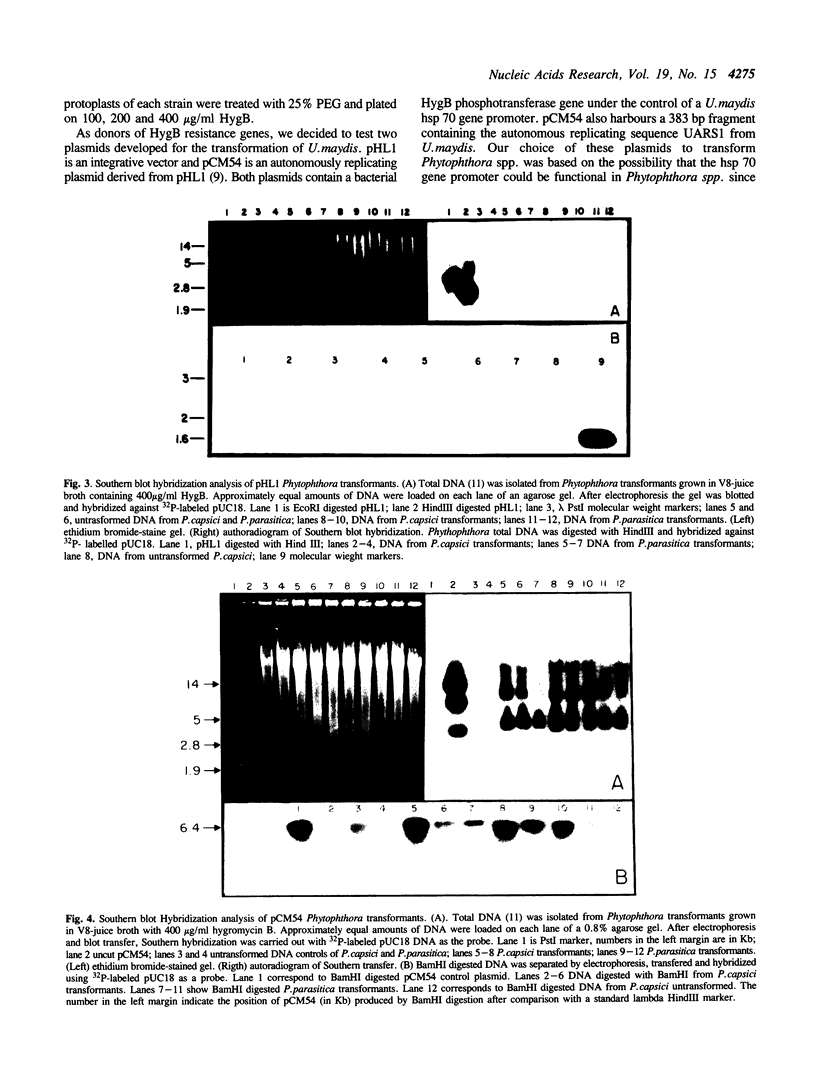
Phytophthora capsici and P.parasitica were transformed to hygromycin B resistance using plasmids pCM54 and pHL1, which contain the bacterial hygromycin B phosphotransferase gene (hph) fused to promoter elements of the Ustilago maydis heat shock hsp70 gene. Enzymes Driselase and Novozyme 234 were used to generate protoplasts which were then transformed following exposure to plasmid DNA and polyethylene glycol 6000. Transformation frequencies of over 500 transformants per micrograms of DNA per 1 x 10(6) protoplasts were obtained. Plasmid pCM54 appears to be transmitted in Phytophthora spp. as an extra-chromosomal element through replication, as shown by Southern blot hybridization and by the loss of plasmid methylation. In addition, transformed strains retained their capacity of infecting Serrano pepper seedlings and Mc. Intosh apple fruits, the host plants for P.capsici and P.parasitica, respectively.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hsiao C. L., Carbon J. Characterization of a yeast replication origin (ars2) and construction of stable minichromosomes containing cloned yeast centromere DNA (CEN3). Gene. 1981 Nov;15(2-3):157–166. doi: 10.1016/0378-1119(81)90125-6. [DOI] [PubMed] [Google Scholar]
- Hynes M. J., Corrick C. M., King J. A. Isolation of genomic clones containing the amdS gene of Aspergillus nidulans and their use in the analysis of structural and regulatory mutations. Mol Cell Biol. 1983 Aug;3(8):1430–1439. doi: 10.1128/mcb.3.8.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malardier L., Daboussi M. J., Julien J., Roussel F., Scazzocchio C., Brygoo Y. Cloning of the nitrate reductase gene (niaD) of Aspergillus nidulans and its use for transformation of Fusarium oxysporum. Gene. 1989 May 15;78(1):147–156. doi: 10.1016/0378-1119(89)90322-3. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Szostak J. W. Pedigree analysis of plasmid segregation in yeast. Cell. 1983 Oct;34(3):961–970. doi: 10.1016/0092-8674(83)90553-6. [DOI] [PubMed] [Google Scholar]
- Parsons K. A., Chumley F. G., Valent B. Genetic transformation of the fungal pathogen responsible for rice blast disease. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4161–4165. doi: 10.1073/pnas.84.12.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R. J., Yoder O. C. Selectable genes for transformation of the fungal plant pathogen Glomerella cingulata f. sp. phaseoli (Colletotrichum lindemuthianum). Gene. 1987;54(1):73–81. doi: 10.1016/0378-1119(87)90349-0. [DOI] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukuda T., Carleton S., Fotheringham S., Holloman W. K. Isolation and characterization of an autonomously replicating sequence from Ustilago maydis. Mol Cell Biol. 1988 Sep;8(9):3703–3709. doi: 10.1128/mcb.8.9.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Holden D. W., Leong S. A. Gene transfer system for the phytopathogenic fungus Ustilago maydis. Proc Natl Acad Sci U S A. 1988 Feb;85(3):865–869. doi: 10.1073/pnas.85.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]