Abstract
Short-term elevations in ambient fine particulate matter (PM2.5) may increase resting systolic (SBP) and diastolic (DBP) blood pressure, but whether PM2.5 alters hemodynamic responses to orthostatic challenge has not been studied in detail. We repeatedly measured SBP and DBP during supine rest and 1 and 3 minutes after standing among 747 elderly (aged 78.3 ± 5.3 years, mean ± SD) participants from the MOBILIZE Boston Study. We used linear mixed models to assess the association between change in SBP (ΔSBP=standing SBP − supine SBP) and DBP (ΔDBP) upon standing and mean PM2.5 levels over the preceding 1 to 28 days, adjusting for meteorological covariates, temporal trends, and medical history. We observed a 1.4 (95% confidence interval (CI): 0.0, 2.8; p=0.046) mmHg higher ΔSBP and a 0.7 (95% CI: 0.0, 1.4; p=0.053) mmHg higher ΔDBP at 1 minute of standing per interquartile range increase (3.8 μg/m3) in mean PM2.5 levels in the past 7 days. ΔSBP and ΔDBP measured 3 minutes after standing were not associated with PM2.5. Resting DBP (but not SBP or pulse pressure) was positively associated with PM2.5 at longer averaging periods. Responses were more strongly associated with black carbon than sulfate levels. These associations did not differ significantly according to hypertension status, obesity, diabetes, or gender. These results suggest that ambient particles can increase resting DBP and exaggerate blood pressure responses to postural changes in elderly people. Increased vasoreactivity during posture change may be responsible, in part, for the adverse effect of ambient particles on cardiovascular health.
Keywords: air pollution, environment, blood pressure, elderly, orthostatic, baroreflex, autonomic nervous system
Short-term changes in ambient levels of fine particulate matter (PM2.5) have been associated with increased risk of acute cardiovascular events.1 These effects are likely mediated, at least in part, by a shift in autonomic nervous system balance towards relative sympathetic dominance.2–4 Several,5–10 but not all,11–17 epidemiologic studies suggest that short-term variation in PM2.5 levels may be associated with higher resting blood pressure (BP).
Active standing from supine rest is associated with a shift in blood volume from the upper body to the abdomen and lower extremities and a transient drop in both systolic (SBP) and diastolic (DBP) blood pressure. In healthy adults, the compensatory withdrawal of parasympathetic activity and subsequent activation of the sympathetic and renin-angiotensin systems helps preserve cardiac output and end-organ perfusion during active postural changes. Aging and diseases of the central or peripheral autonomic nervous system can impair these reflexes and exacerbate the drop in BP. Orthostatic hypotension – defined as a reduction in SBP of ≥20 mmHg or a reduction in DBP of ≥10 mmHg within 3 minutes of standing18 – is common in the elderly, is an important risk factor for falls,19 and has been associated with increased risk of acute cardiovascular events.20, 21 On the other hand, patients with essential hypertension, diabetes, and specific autonomic disorders can exhibit higher BP after standing.22 This orthostatic hypertension, presumably due to excessive sympathoexcitation, has been associated with increased risk of stroke and end-organ damage.23, 24
Given its documented effects on autonomic nervous system function and resting BP, PM2.5 may also affect the dynamic regulation of BP in response to orthostatic challenge, but this hypothesis has only been evaluated in one prior study.25 Evaluating the effects of PM2.5 on postural BP changes may provide novel insights into the role of PM2.5 in the regulation of dynamic BP control mechanisms. Accordingly, we assessed the association between ambient PM2.5 levels and dynamic BP responses to postural changes in a cohort of community-dwelling seniors.
Materials and Methods
Study Design
We evaluated the association between short-term changes in ambient PM2.5 levels and dynamic BP responses to orthostatic challenge within the context of the MOBILIZE Boston Study (MBS), a prospective, community-based cohort study of healthy aging.26 Between 2005 and 2008, we recruited 747 non-institutionalized men and women aged 70 years or older, able to communicate in English, residing within 5 miles (8.0 km) of the study clinic, and able to walk 20 feet (6.1 m) without personal assistance. Individuals not planning to reside in the study area for at least 2 years and those with terminal diseases, severe vision or hearing impairment, or cognitive impairment defined as a Mini-Mental State Examination score <18 were excluded. Upon enrollment, subjects participated in an in-home interview followed within 4 weeks by a clinic examination. A second assessment consisting of an in-home interview and clinic examination was performed a median of 16.5 months after the baseline assessment. All subjects provided written informed consent upon enrollment. This analysis was approved by the Institutional Review Boards at Hebrew SeniorLife and Brown University.
BP Measurements
During the clinic examination, trained staff using a standardized protocol measured BP while minimizing potential sources of error. Following a 5-min period of supine rest, SBP and DBP were measured by auscultation in the dominant arm using an aneroid sphygmomanometer on two occasions separated by 2 minutes and averaged. Participants were then asked to stand and, with the arm supported at heart level, SBP and DBP were measured 1 and 3 minutes after both feet touched the floor. The brachial pulse was documented at each time point and used as a measure of heart rate. Measurements were obtained at least 2 hours after breakfast or lunch. Data from both the baseline and first follow-up visits were used, resulting in a total of 1362 subject observations, including two repeated measurements for most (82%) subjects.
Participants were classified as normotensive if BP was <140/90mmHg and there was no history of hypertension or receiving antihypertensive medications; controlled hypertensive if BP was <140/90 mmHg and there was a history of hypertension or receiving antihypertensives; and uncontrolled hypertensive if BP was ≥140/90mmHg. A blood sample was collected during the clinic visit and participants were classified as having diabetes mellitus if they reported a past diagnosis of diabetes, they reported using any diabetes medications, hemoglobin A1c levels ≥7%, or random glucose measurement ≥200 mg/dl. Participants with LDL ≥130 mg/dl or total cholesterol ≥200 mg/dl, or reporting using any lipid-lowering medications were classified as having hyperlipidemia. Height and weight were measured during the clinic visit according to a standard protocol and body mass index calculated. Smoking history was obtained during the home visit.
Air pollution and Meteorological data
Ambient levels of PM2.5, black carbon (a marker of traffic pollution), and SO42− (generally a marker of regional pollution from coal-fired power plants) were measured continuously at the Boston/Harvard ambient monitoring station and daily averages (9am-9am) calculated, as previously described.27 Our monitoring station is located <10 km from the study clinic site and <20 km from the residential address of any study participant. We obtained hourly meteorological data from the National Weather Service station at Boston’s Logan Airport.
Statistical Methods
We calculated pulse pressure as SBP minus DBP. The change in SBP, DBP, and HR at 1 and 3 minutes of standing (denoted ΔSBP, ΔDBP, and ΔHR, respectively) were calculated as the standing values minus the supine values. We used linear mixed models with a random subject intercept to evaluate the association between each outcome and PM2.5 levels while accounting for the within-subject correlation. In all analyses, we controlled for age (natural cubic spline with 3 degrees of freedom), sex, race (white versus other), smoking status (never, former, current), hypertension status (normotension, controlled hypertension, uncontrolled hypertension), diabetes, body mass index (natural cubic spline with 3 degrees of freedom), visit number, day of week, ambient and dew point temperature (natural cubic splines with 3 degrees of freedom each), season (sine and cosine of calendar day) and long-term temporal trends (calendar day as a linear continuous variable). Adjusting for treatment with antihypertensive medication instead of hypertension status did not materially alter the results. We modeled PM2.5 as a continuous variable and the assumption of a linear exposure-response relationship was confirmed by standard techniques. Results are expressed as a change in each outcome per interquartile range increase in PM2.5.
Previous studies have reported that resting BP is associated with PM2.5 levels averaged over 2 hours to 28 days prior to BP measurement. Accordingly, in separate models we considered pollutant levels averaged over the 1, 3, 5, 7, 14, 21 and 28 days prior to BP measurement. We repeated the main analyses considering in separate models black carbon and sulfate levels instead of PM2.5. We evaluated whether the observed associations with PM2.5 differed according to hypertension, a history of diabetes, presence of obesity (BMI ≥ 30 vs < 30), or gender by adding interaction terms to the model. Analyses were performed using SAS (v9.2; SAS Institute Inc., Cary, NC) and R statistical software (R v2.10). A two-sided p value of <0.05 was considered statistically significant.
Results
MOBILIZE Boston Study participants were elderly, and predominantly white and female (Table 1). At baseline, more than half of participants had controlled hypertension and an additional 25% had uncontrolled hypertension. Postural changes resulted in transient changes in SBP, DBP and HR, denoted ΔSBP, ΔDBP, and ΔHR, respectively (Table S1, please see the online Data Supplement at http://hyper.ahajournals.org.). As expected, ΔSBP and ΔDBP exhibited considerable variability across individuals. The prevalence of clinical orthostatic hypotension, defined as a drop in SBP of ≥20 mmHg or a drop in DBP of ≥10 mmHg within 3 minutes of standing, was 12.6%.
Table 1.
Baseline characteristics of 747 participants aged ≥70 years from the MOBILIZE Boston Study, 2005–2009.
| Characteristic | Mean ± SD or n (%) |
|---|---|
| Age, Mean ± SD | 78.3 ± 5.3 |
| Female, n (%) | 471 (63.1) |
| White, n (%) | 578 (77.4) |
| Hypertension, n (%) | |
| Normotension | 151 (20.2) |
| Controlled hypertension | 398 (53.3) |
| Uncontrolled hypertension | 190 (25.4) |
| Diabetes Mellitus, n (%) | 151 (20.2) |
| Hyperlipidemia, n (%) | 354 (47.3) |
| History of Stroke, n (%) | 74 (9.9) |
| History of Coronary Artery Disease, n (%) | 197 (26.4) |
| Smoking History, n (%) | |
| Never | 331 (44.3) |
| Former | 381 (51.0) |
| Current | 34 (4.6) |
| Body mass index, Mean ± SD | 27.3 ± 5.1 |
| Antihypertensive Medication | |
| ACE Inhibitor or ARB | 278 (37.2) |
| Thiazide Diuretic | 200 (26.8) |
| Calcium Channel Blocker | 177 (23.7) |
| Beta Blocker | 342 (45.8) |
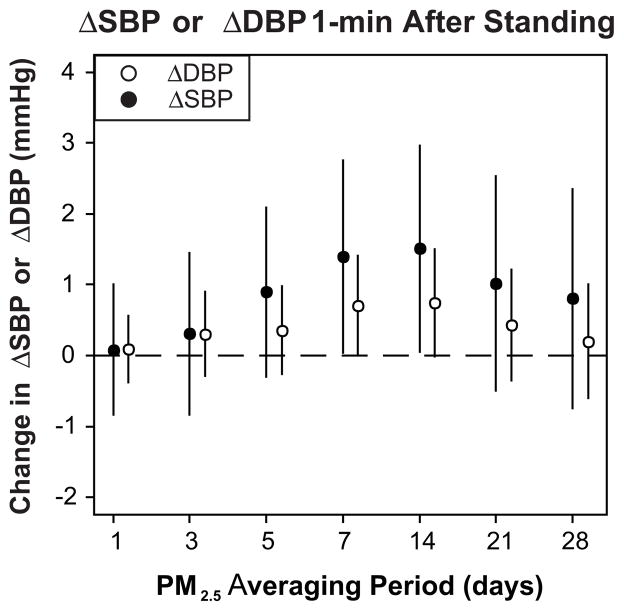
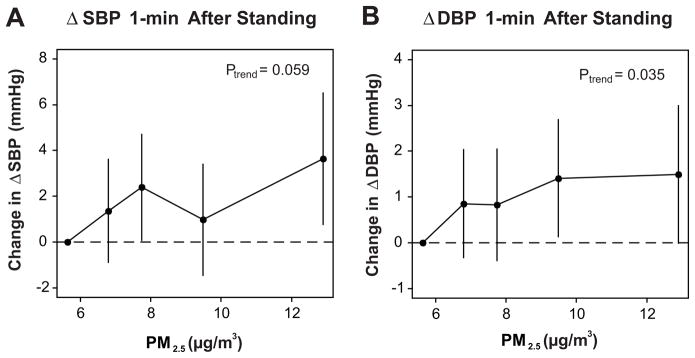
Daily levels of PM2.5 varied throughout the study period (8.6 ± 4.9 μg/m3; mean ± SD). ΔSBP and ΔDBP 1 minute after standing were associated with mean PM2.5 levels averaged over the past 7 and 14 days (Fig. 1). For example, we observed a 1.4 (95% confidence interval (CI): 0.0, 2.8; p=0.046) mmHg higher ΔSBP and a 0.7 (0.0, 1.4; p=0.053) mmHg higher ΔDBP per interquartile range increase in mean PM2.5 levels in the past 7 days (3.8 μg/m3), implying a larger increase in BP upon standing. These associations were approximately linear, although a flattening of the dose-effect relationship at higher levels of PM2.5 cannot be excluded (Fig. 2). Additional control for supine SBP or DBP did not materially alter these results. PM2.5 levels 7 days prior to the clinic visit were also associated with a lower ΔHR (−0.5 [95% CI: −1.1, 0.1; p=0.14] per interquartile range increase in PM2.5), implying a smaller increase in HR upon standing. ΔSBP and ΔDBP 1 minute after standing were more strongly associated with black carbon (a marker of traffic pollution) than with sulfate (generally a marker of regional pollution from coal-fired power plants), but neither reached statistical significance (Table S2, please see http://hyper.ahajournals.org). ΔSBP and ΔDBP measured 3 minutes after standing were not associated with PM2.5 (Table S3, please see http://hyper.ahajournals.org), black carbon, or sulfate levels (data not shown).
Figure 1.
Change (95% confidence intervals) in ΔSBP and ΔDBP 1 minute after standing associated with an interquartile range increase in PM2.5 over different averaging periods. ΔSBP and ΔDBP represent the change in systolic and diastolic blood pressure 1 minute after standing from a supine rest calculated as standing value minus supine value, respectively. In all models we controlled for age, sex, race, smoking, hypertension, diabetes, body mass index, visit number, season, day of week, ambient temperature, dew point temperature, and time.
Figure 2.
Dose-response relationship between quintiles of mean PM2.5 levels 7 days prior to assessment and change in ΔSBP (A.) and ΔDBP (B.) 1 minute after standing, controlling for potential confounders as in Figure 1. Solid circles denote the magnitude of the association at quintiles of exposure and the vertical lines denote 95% confidence intervals. The x-axis shows the median PM2.5 levels (μg/m3) in each quintile.
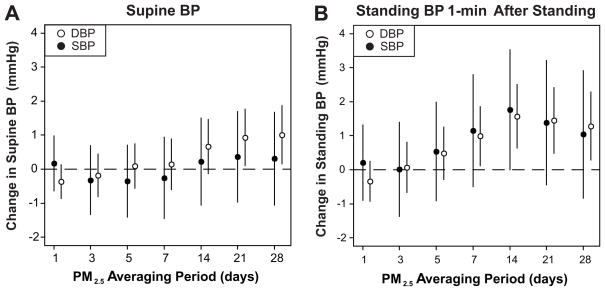
Supine DBP was positively associated with mean PM2.5 levels in the past 21 to 28 days (Fig. 3A). Supine SBP, pulse pressure, and heart rate were not associated with PM2.5 levels. Standing DBP at 1-minute was positively associated with mean PM2.5 levels 7 to 28 days prior to the clinic visit with the strongest association observed with PM2.5 levels over the past 14 days (Fig. 3B). This pattern of results was similar, but less pronounced, for DBP 3-min after standing, and SBP 1- and 3-minutes after standing. Supine DBP was positively associated with 28-day mean levels of both black carbon and sulfates, although the association was somewhat stronger for black carbon (Table S2, please see http://hyper.ahajournals.org).
Figure 3.
Change in SBP and DBP during supine rest (A.) and 1 minute after standing (B.) associated with an interquartile range increase in PM2.5 levels over different averaging periods controlling for potential confounders as in Figure 1.
We found no statistical evidence that the association between PM2.5 and ΔSBP and ΔDBP 1 minute after standing differed according to hypertension status, antihypertensive medication use, and participant characteristics, although there was some suggestion that the association between PM2.5 and ΔSBP was attenuated among participants using calcium channel blockers (Table S4, please see http://hyper.ahajournals.org). Similarly, we found no statistical evidence that the association between PM2.5 and supine SBP or DBP differed according to hypertension status or participant characteristics, although again there was some suggestion that specific anti-hypertensive medications might attenuate the association (Table S5, please see http://hyper.ahajournals.org).
Discussion
In this population-based cohort of community-dwelling elderly residents we found that PM2.5 levels were associated with larger (ie: more hypertensive) values of ΔSBP and ΔDBP measured 1 minute after standing from supine rest. These associations were approximately linear, driven by PM2.5-related increases in standing BP, and accompanied by PM2.5-related changes in ΔHR that were marginally statistically significant. We did not observe statistical evidence that these associations differed by patient characteristics, although some responses may have been attenuated by specific antihypertensive medications.
Within the context of the all-male Normative Aging Study (NAS),25 we previously found a 0.7 mmHg (95% CI: −0.2, 1.5) change in ΔSBP per 10 μg/m3 increase in mean PM2.5 over the past 48 hours, but no association for ΔDBP. For comparison, the results from the current study scaled to a 10 μg/m3 increase in PM2.5 would be 3.6 mmHg (95% CI: 0.1, 7.2) change in ΔSBP and a 1.8 mmHg (95% CI: 0.0, 3.7) change in ΔDBP. However, the results of these two studies are not directly comparable because the NAS measured BP responses 30 seconds after standing from a seated position while we measured BP responses 1 and 3 minutes after standing from a supine position. The physiologic mechanisms – and therefore the effects of external stimuli – may differ depending on the timing of ΔSBP and ΔDBP measurements.28 However, the fact that both studies showed similar effects on orthostatic BP regulation within one minute of standing and we found no effect after 3 minutes of standing suggests that PM2.5 may have a more pronounced effect on rapid autonomic control mechanisms, such as parasympathetic withdrawal, rather than the slower sympathetic or renin-angiotensin activation.
ΔSBP and ΔDBP 1 minute after standing were most strongly associated with mean PM2.5 levels over the past 7 to 14 days, with the association weakening at longer averaging times (Fig. 1). This pattern of results can be explained by the observation that although PM2.5 was associated with both supine and standing BP, the strongest associations were seen in relation to different PM2.5 averaging periods (Fig. 3). For example, although the association between standing DBP and PM2.5 was similar in magnitude considering PM2.5 averaged over the prior 14 and 28 days, 28-day PM2.5 was associated with higher supine DBP while 14-day PM2.5 was not.
That we did not find evidence of an association between supine BP and mean PM2.5 levels over the past 5 or 7 days is in contrast to some previous studies.7, 10, 11 However, we did find that mean PM2.5 levels in the past 28 days were associated with increased supine DBP (but not supine SBP or pulse pressure). The magnitude of this association (3.4 mmHg [95% CI: 0.5, 6.4] per 10 μg/m3 increase in PM2.5) is similar to that observed in prior studies.6 If causal and sustained, an increase in DBP of this magnitude in an elderly population would be expected to be associated with ~20% increase in risk of death from stroke or ischemic heart disease.29 Indeed, because nearly everyone is exposed, the public health burden of ambient particulate matter on cardiovascular events can be substantial even in comparison to less common exposures associated with much higher relative risks.30
The mechanisms by which PM2.5 may influence BP are incompletely understood. In healthy adults, the initial drop in BP associated with active standing results in activation of compensatory responses including increased peripheral vascular resistance. Sun et al.31 found that 9 weeks of PM2.5 pre-exposure potentiated hypertension in response to angiotensin II infusion in Sprague-Dawley rats, increased the vasoconstrictor response of aortic rings to phenylephrine, and attenuated the response of aortic rings to the endothelium-dependent vasodilator acetylcholine, suggesting that longer PM2.5 exposures can augment vascular responses to pressor stimuli. Our finding that PM2.5 levels over the past week or two were associated with increased ΔSBP and ΔDBP is consistent with this hypothesis.
Hypertension blunts the vagally-driven baroreflex change in heart rate, but not the sympathetically-mediated vascular responses.32 Thus, our finding that PM2.5 tended to be associated with lower ΔHR might be expected assuming that longer term PM2.5 levels are indeed associated with higher blood pressure, but stands in contrast to a prior study which found that PM2.5 exposure increased cardiovagal baroreflex sensitivity in dogs.33 Additional studies assessing the relationship between pollutants and the cardiovagal baroreflex in humans and studies evaluating how baroreflex-mediated alterations in sympathetic outflow are modified by PM2.5 exposure are needed to further elucidate these mechanisms.
PM2.5 represents a heterogeneous mixture of constituents derived from multiple sources and subjected to complex atmospheric reactions. Identifying the source(s) or constituents of PM2.5 most responsible for the observed effects is of great public health and regulatory interest. A detailed source apportionment analysis of Boston-area PM2.5 is currently underway to specifically address this question. In the meantime, we note that the observed responses were more strongly associated with black carbon than with sulfate levels, suggesting that traffic pollution may be particularly important in terms of the effects on systemic hemodynamics, in accordance with prior studies in other geographic areas.9, 34
Our study has some limitations. First, the use of air quality measures from a single monitoring site may lead to misclassification of exposure, potentially increasing the width of our confidence intervals, but not otherwise biasing our health effect estimates in either direction.35 Second, since the effects of PM2.5 likely vary depending on pollution sources, particle constituents, and population characteristics, our results are not necessarily generalizable to other geographic locations or study populations. Third, we did not have data on the sodium or hydration status of participants. Although these factors are known to affect orthostatic BP changes, they are unlikely to confound our health effect estimates. Fourth, we measured supine BP twice and standing BP once at each time point. Additional BP determinations would likely have reduced the width of our confidence intervals, but not otherwise alter our health effect estimates.
On the other hand, important strengths of our study include detailed assessment of BP responses in a large, prospective cohort of community-dwelling elderly evaluated repeatedly. Because MOBILIZE Boston Study participants are representative of seniors in the Boston area in terms of age, sex, race and ethnicity,26 our results are broadly relevant to elderly individuals rather than a selected patient population.
In conclusion, the results of this study suggest that exposure to elevated levels of PM2.5 can exaggerate blood pressure responses to postural changes in community-dwelling elderly people. The observed effects are consistent with animal studies showing that several weeks of exposure to PM2.5 increases the sensitivity of vascular endothelium to pressor stimuli, although additional human mechanistic studies are needed to elucidate the role of altered baroreflex responses. Increases in blood pressure and vasoreactivity during posture change may be responsible, in part, for the adverse effect of ambient particles on cardiovascular events.
Perspectives
A recent scientific statement from the American Heart Association concluded that PM2.5 is a modifiable risk factor contributing to cardiovascular morbidity and mortality. The putative mechanisms of the acute effects of PM2.5 include sympathetic activation/parasympathetic withdrawal leading to hemostatic and hemodynamic changes that are presumed to increase the risk of cardiovascular events. The current study adds to the existing knowledge by demonstrating that in elderly adults PM2.5 exposure may also affect the dynamic, beat-to-beat regulation of blood pressure and heart rate, either through altered vascular responses to pressor stimuli or modulation of baroreflex responses. Studies directly evaluating vascular and baroreflex function in human subjects are needed to verify these results and further clarify the effects of PM2.5 on dynamic blood pressure regulation.
Supplementary Material
Acknowledgments
The authors are grateful to the MOBILIZE Boston Study research team and participants for their time, effort, and dedication.
Sources of Funding
The project described was supported by grants AG004390, AG25037, ES015774, ES009825, and ES000002 from the National Institutes of Aging (NIA) and Environmental Health Sciences (NIEHS), NIH, and grants R832416 and RD83479801 from the US Environmental Protection Agency (US EPA). Dr. Wilker was supported by grant T32-HL007374 from the National Heart, Lung, and Blood Institute. Dr. Lipsitz holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the sponsoring institutions. Further, the sponsoring institutions do not endorse the purchase of any commercial products or services mentioned in this publication.
Footnotes
Disclosures
One or more co-authors are currently receiving, or have received in the past, grant support or honoraria from the Health Effects Institute, Boston, MA and/or the Electric Power Research Institute (EPRI), Palo Alto, CA.
References
- 1.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, Allen G, Verrier M, Cherry R, Verrier R. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- 3.Liao D, Duan Y, Whitsel EA, Zheng ZJ, Heiss G, Chinchilli VM, Lin HM. Association of higher levels of ambient criteria pollutants with impaired cardiac autonomic control: A population-based study. American journal of epidemiology. 2004;159:768–777. doi: 10.1093/aje/kwh109. [DOI] [PubMed] [Google Scholar]
- 4.Pope CA, 3rd, Hansen ML, Long RW, Nielsen KR, Eatough NL, Wilson WE, Eatough DJ. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 2004;112:339–345. doi: 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanobetti A, Canner MJ, Stone PH, Schwartz J, Sher D, Eagan-Bengston E, Gates KA, Hartley LH, Suh H, Gold DR. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110:2184–2189. doi: 10.1161/01.CIR.0000143831.33243.D8. [DOI] [PubMed] [Google Scholar]
- 6.Auchincloss AH, Roux AV, Dvonch JT, Brown PL, Barr RG, Daviglus ML, Goff DC, Kaufman JD, O’Neill MS. Associations between recent exposure to ambient fine particulate matter and blood pressure in the multi-ethnic study of atherosclerosis (mesa) Environ Health Perspect. 2008;116:486–491. doi: 10.1289/ehp.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Ruddy T, Dalipaj M, Poon R, Szyszkowicz M, You H, Dales RE, Wheeler AJ. Effects of indoor, outdoor, and personal exposure to particulate air pollution on cardiovascular physiology and systemic mediators in seniors. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine. 2009;51:1088–1098. doi: 10.1097/JOM.0b013e3181b35144. [DOI] [PubMed] [Google Scholar]
- 8.Dvonch JT, Kannan S, Schulz AJ, Keeler GJ, Mentz G, House J, Benjamin A, Max P, Bard RL, Brook RD. Acute effects of ambient particulate matter on blood pressure: Differential effects across urban communities. Hypertension. 2009;53:853–859. doi: 10.1161/HYPERTENSIONAHA.108.123877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delfino RJ, Tjoa T, Gillen DL, Staimer N, Polidori A, Arhami M, Jamner L, Sioutas C, Longhurst J. Traffic-related air pollution and blood pressure in elderly subjects with coronary artery disease. Epidemiology. 2010;21:396–404. doi: 10.1097/EDE.0b013e3181d5e19b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brook RD, Bard RL, Burnett RT, Shin HH, Vette A, Croghan C, Phillips M, Rodes C, Thornburg J, Williams R. Differences in blood pressure and vascular responses associated with ambient fine particulate matter exposures measured at the personal versus community level. Occup Environ Med. 2011;68:224–230. doi: 10.1136/oem.2009.053991. [DOI] [PubMed] [Google Scholar]
- 11.Brauer M, Ebelt ST, Fisher TV, Brumm J, Petkau AJ, Vedal S. Exposure of chronic obstructive pulmonary disease patients to particles: Respiratory and cardiovascular health effects. Journal of exposure analysis and environmental epidemiology. 2001;11:490–500. doi: 10.1038/sj.jea.7500195. [DOI] [PubMed] [Google Scholar]
- 12.Ibald-Mulli A, Timonen KL, Peters A, Heinrich J, Wolke G, Lanki T, Buzorius G, Kreyling WG, De Hartog J, Hoek G, Ten Brink HM, Pekkanen J. Effects of particulate air pollution on blood pressure and heart rate in subjects with cardiovascular disease: A multicenter approach. Environ Health Perspect. 2004;112:369–377. doi: 10.1289/ehp.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mar TF, Koenig JQ, Jansen K, Sullivan J, Kaufman J, Trenga CA, Siahpush SH, Liu LJ, Neas L. Fine particulate air pollution and cardiorespiratory effects in the elderly. Epidemiology. 2005;16:681–687. doi: 10.1097/01.ede.0000173037.83211.d6. [DOI] [PubMed] [Google Scholar]
- 14.Jansen KL, Larson TV, Koenig JQ, Mar TF, Fields C, Stewart J, Lippmann M. Associations between health effects and particulate matter and black carbon in subjects with respiratory disease. Environ Health Perspect. 2005;113:1741–1746. doi: 10.1289/ehp.8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebelt ST, Wilson WE, Brauer M. Exposure to ambient and nonambient components of particulate matter: A comparison of health effects. Epidemiology. 2005;16:396–405. doi: 10.1097/01.ede.0000158918.57071.3e. [DOI] [PubMed] [Google Scholar]
- 16.Madsen C, Nafstad P. Associations between environmental exposure and blood pressure among participants in the oslo health study (hubro) European journal of epidemiology. 2006 doi: 10.1007/s10654-006-9025-x. [DOI] [PubMed] [Google Scholar]
- 17.Linn WS, Gong H, Jr, Clark KW, Anderson KR. Day-to-day particulate exposures and health changes in los angeles area residents with severe lung disease. Journal of the Air & Waste Management Association (1995) 1999;49:108–115. doi: 10.1080/10473289.1999.10463890. [DOI] [PubMed] [Google Scholar]
- 18.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz IJ, Schondorf R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci. 2011;161:46–48. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Gangavati A, Hajjar I, Quach L, Jones RN, Kiely DK, Gagnon P, Lipsitz LA. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: The maintenance of balance, independent living, intellect, and zest in the elderly of boston study. Journal of the American Geriatrics Society. 2011;59:383–389. doi: 10.1111/j.1532-5415.2011.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose KM, Tyroler HA, Nardo CJ, Arnett DK, Light KC, Rosamond W, Sharrett AR, Szklo M. Orthostatic hypotension and the incidence of coronary heart disease: The atherosclerosis risk in communities study. Am J Hypertens. 2000;13:571–578. doi: 10.1016/s0895-7061(99)00257-5. [DOI] [PubMed] [Google Scholar]
- 21.Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (the malmo preventive project) Eur Heart J. 2010;31:85–91. doi: 10.1093/eurheartj/ehp329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson D. Orthostatic hypertension: The last hemodynamic frontier. Hypertension. 2011;57:158–159. doi: 10.1161/HYPERTENSIONAHA.110.163485. [DOI] [PubMed] [Google Scholar]
- 23.Yatsuya H, Folsom AR, Alonso A, Gottesman RF, Rose KM. Postural changes in blood pressure and incidence of ischemic stroke subtypes: The aric study. Hypertension. 2011;57:167–173. doi: 10.1161/HYPERTENSIONAHA.110.161844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsubayashi K, Okumiya K, Wada T, Osaki Y, Fujisawa M, Doi Y, Ozawa T. Postural dysregulation in systolic blood pressure is associated with worsened scoring on neurobehavioral function tests and leukoaraiosis in the older elderly living in a community. Stroke; a journal of cerebral circulation. 1997;28:2169–2173. doi: 10.1161/01.str.28.11.2169. [DOI] [PubMed] [Google Scholar]
- 25.Wilker E, Mittleman MA, Litonjua AA, Poon A, Baccarelli A, Suh H, Wright RO, Sparrow D, Vokonas P, Schwartz J. Postural changes in blood pressure associated with interactions between candidate genes for chronic respiratory diseases and exposure to particulate matter. Environ Health Perspect. 2009;117:935–940. doi: 10.1289/ehp.0800279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leveille SG, Kiel DP, Jones RN, Roman A, Hannan MT, Sorond FA, Kang HG, Samelson EJ, Gagnon M, Freeman M, Lipsitz LA. The mobilize boston study: Design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC geriatrics. 2008;8:16. doi: 10.1186/1471-2318-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dockery DW, Luttmann-Gibson H, Rich DQ, Link MS, Mittleman MA, Gold DR, Koutrakis P, Schwartz JD, Verrier RL. Association of air pollution with increased incidence of ventricular tachyarrhythmias recorded by implanted cardioverter defibrillators. Environ Health Perspect. 2005;113:670–674. doi: 10.1289/ehp.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: Review of a forgotten condition. Clin Sci (Lond) 2007;112:157–165. doi: 10.1042/CS20060091. [DOI] [PubMed] [Google Scholar]
- 29.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 30.Nawrot TS, Perez L, Kunzli N, Munters E, Nemery B. Public health importance of triggers of myocardial infarction: A comparative risk assessment. Lancet. 2011;377:732–740. doi: 10.1016/S0140-6736(10)62296-9. [DOI] [PubMed] [Google Scholar]
- 31.Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R, Hwang JS, Zweier JL, Chen LC, Rajagopalan S. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of rho/rock. Arterioscler Thromb Vasc Biol. 2008;28:1760–1766. doi: 10.1161/ATVBAHA.108.166967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grassi G, Trevano FQ, Seravalle G, Scopelliti F, Mancia G. Baroreflex function in hypertension: Consequences for antihypertensive therapy. Prog Cardiovasc Dis. 2006;48:407–415. doi: 10.1016/j.pcad.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Bartoli CR, Wellenius GA, Diaz EA, Lawrence J, Coull BA, Akiyama I, Lee LM, Okabe K, Verrier RL, Godleski JJ. Mechanisms of inhaled fine particulate air pollution-induced arterial blood pressure changes. Environ Health Perspect. 2009;117:361–366. doi: 10.1289/ehp.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mordukhovich I, Wilker E, Suh H, Wright R, Sparrow D, Vokonas PS, Schwartz J. Black carbon exposure, oxidative stress genes, and blood pressure in a repeated-measures study. Environ Health Perspect. 2009;117:1767–1772. doi: 10.1289/ehp.0900591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, Cohen A. Exposure measurement error in time-series studies of air pollution: Concepts and consequences. Environ Health Perspect. 2000;108:419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.