Abstract
Alzheimer's disease (AD) is the most common type of dementia worldwide. Hippocampal atrophy and ventricular enlargement have been associated with AD but also with normal aging. We analyzed 1.5T brain MRI data from 46 cognitively normal elderly (NC), 33 mild cognitive impairment (MCI) and 43 AD subjects. Hippocampal and ventricular analyses were conducted with two novel semi-automated segmentation approaches followed by the radial distance mapping technique. Multiple linear regression was used to assess effects of age and diagnosis on hippocampal and ventricular volumes and radial distance. Additional 3D map correction for multiple comparisons was conducted with permutation testing. As expected, most significant hippocampal atrophy and ventricular enlargement were seen in the AD vs. NC comparison. MCI subjects showed intermediate levels of hippocampal atrophy and ventricular enlargement. Significant effects of age on hippocampal volume and radial distance were seen in the pooled sample as well as in the NC and AD groups considered separately. Age-associated differences were detected in all hippocampal subfields and the frontal and body/occipital horn portions of the lateral ventricles. Aging affects both the hippocampus and lateral ventricles independent of AD pathology and should be included as covariate in all structural hippocampal and ventricular analyses when possible.
Keywords: Alzheimer's disease (AD), mild cognitive impairment (MCI), aging, hippocampal atrophy, lateral ventricle enlargement
INTRODUCTION
Alzheimer's disease (AD) is the most common type of dementia worldwide, and currently affects 5.5 million people in the U.S. alone1. The recent estimate of the direct and indirect healthcare cost incurred worldwide by those affected by AD was $156 billion annually2. Mortality rates are declining among the elderly, and AD is more prevalent at older ages. As baby-boomer generation ages, we will soon be confronted with an enormous increase in the incidence and prevalence of individuals affected by AD.
Accelerated hippocampal atrophy is a consistent finding in the AD and mild cognitive impairment (MCI) stage. Jack et al. estimated that annual atrophy occurs as a result of normal aging at a mean rate of 1.6 –1.7 % in elderly controls. The rates in the MCI and AD stages are higher − 2.8 % in stable MCI, 3.7 % in MCI transitioning to AD (MCI progressors) and 3.5 – 4.0 % in AD3, 4. AD pathology accumulates for years and maybe even decades before AD is typically diagnosed and sensitive imaging biomarker techniques may be able to pick up signs of neurodegeneration pre-symptomatically. Using the radial distance mapping technique5 we have already demonstrated that hippocampal atrophy is an early diagnostic biomarker and can predict which MCI subjects would progress to AD during 3-year follow-up.6 We also have found that hippocampal atrophy is a promising biomarker for pre-symptomatic disease as it can be detected in cognitively normal elderly up to 3 years prior to MCI and 6 years prior to AD diagnosis7.
The exact localization of aging effects on hippocampal structure has been long disputed. Some groups report isolated involvement of the CA3/dentate gyrus and CA1 subregions8. Others claim that CA1 and subiculum9, or subiculum and dentate gyrus10 or subiculum alone are preferentially involved11. These seemingly conflicting reports may be due to the facts that the hippocampal subregions are fairly hard to identify reliably while at the same time both the effect and sample sizes in these studies are usually small. Given the only partial replication of subregional aging effects on the hippocampus in these reports, it seemed also plausible that all subfields are subject to structural changes with increasing age – a hypothesis that we were able to address with our hippocampal surface mapping technique.
Ventriculomegaly is commonly observed in most neurodegenerative disorders and results from passive enlargement of the lateral, third and fourth ventricles following brain parenchymal shrinkage. Significant ventricular enlargement has been associated with AD12–15, but it is a rather nonspecific finding. Nevertheless ventriculomegaly has a large effect size in AD and is a strong biomarker of disease progression. AD subjects demonstrate significantly greater rates of ventriculomegaly compared to NC and MCI5, 12, 13, 16–19. NC and MCI subjects who experience cognitive decline also show greater ventricular enlargement over time relative to their cognitively stable counterparts12, 14, 15. Ventricular enlargement is strongly correlated with decline in cognitive performance5, 16, 20 as well as with cerebrospinal fluid16 and pathologic markers of AD20. As less labor-intensive automated segmentation methods for lateral ventricle segmentation have been developed, the lateral ventricles are increasingly being studied as promising AD biomarkers12, 15, 17, 19, 21, 22. Ventricular changes over time are also very strongly associated with aging both in cognitively normal and diseased populations13, 18, 20, 23. Our group recently demonstrated that ventricular enlargement performs better in discriminating cognitively normal elderly from MCI as opposed to discriminating MCI from AD subjects suggesting that lateral ventricular measures may be an early and potentially pre-symptomatic disease biomarker24. Finally another important observation in respect to ventricular changes over time is its very strong association with aging both in cognitively normal and diseased populations13, 18, 20, 23.
In this study we aimed to assess the independent effects of age and diagnosis on the hippocampus and the lateral ventricles. Our goals were to establish any unique ageing effect on the hippocampal subfields and ventricular subregions and compare the ageing pattern of hippocampal and ventricular involvement to that in AD.
METHODS
Subjects
Our study cohort consisted of 46 cognitively normal elderly controls (NC), 33 MCI (30 amnestic and 3 nonamnestic subtype) and 43 AD subjects. All subjects were participants in the University of California Los Angeles (UCLA) AD Research Center's database and provided informed consent according to the Declaration of Helsinki and the restrictions and the policies of the UCLA Institutional Review Board. The diagnostic work-up consisted of physician interview, general and neurological examination and detailed neuropsychological evaluation consisting of the following tests: Mini-Mental State Examination (MMSE)25, the Wechsler Adult Intelligence Scale – 3rd edition (WAIS-3: 8 subsets)26, the Boston Naming Test27, the Controlled Oral Word Association Test (FAS and Animals)28, the Wechsler Memory Scale – 3rd edition (WMSIII: Logical Memory and Visual Reproduction)26, the Rey-Osterrieth Complex Figure test – copy and delayed recall (ROCFT)29, the Stroop Test30, Trailmaking A and B31, and the Wisconsin Card Sorting Test (WCST)32. Diagnostic impressions were reached by consensus among neurologists, psychiatrists and neuropsychologists, and were based on the National Institute of Neurologic and Communicative Disorders and Stroke and the AD and Related Disorders Association (NINCDS-ADRDA) criteria for AD33 and Petersen criteria for MCI34. The latter required cognitive decline of at least 1.5 SD below the age- and education-adjusted neuropsychological norms on at least one neuropsychological test, in the context of generally preserved intellectual function and activities of daily living. NC subjects scored within expectation on all cognitive tests. Additional inclusion criteria were age 55–90 years, no evidence of concurrent general medical condition of sufficient severity to impact cognition, no history of significant drug or alcohol abuse, no concurrent psychiatric or other neurological illness and a Mini-Mental State Evaluation (MMSE) score above 18 for the mild AD group.
Imaging data acquisition and preprocessing
Imaging data were collected on a 1.5 T Signa MRI scanner (Milwaukee, Wisconsin) with the following protocol: 3D spoiled gradient echo, gapless coronal acquisition, TR 28 msec, TE 6 msec, FOV 220 mm, 256 ×192 matrix, slice thickness 1.5 mm, voxel size 0.9×0.9×1.5 mm (non-isotropic). We used the Minctracc algorithm35 to rotate and globally scale all MRI scans to match the International Consortium for Brain mapping (ICBM) ICBM53 average brain imaging template36 using a 9-parameter linear transformation (3 translations, 3 rotations and 3 scales) and a regularized tricubic B-spline approach for image intensity inhomogeneity correction37. The post-processed images had a reconstructed isotropic voxel dimension of 1×1×1 mm. The ICBM53 (obtainable on request from the authors) was chosen over the ICBM152 and the ICBM305 templates as it has better contrast and more sharply defined cortical and subcortical structural definition, which may be helpful for improved cross-subject image registration.
Hippocampal tracing
Hippocampal traces included the hippocampus proper, dentate gyrus and subiculum and were traced on gapless coronal slices by two experienced raters blinded to subjects' age, gender, and diagnosis following a detailed, well-established protocol38, 39. We used Crohnbach's alpha coefficient (a measure of intercorrelation, i.e. internal consistency among test items40) to determine inter- and intra-rater variability followed by one – or two-tailed t-test as appropriate to test for statistically significant mean differences in hippocampal volume between the two raters and in intra-rater test-retest conditions (LGA, intra-rater reliability Cronbach's alpha=0.987, one-sample two-tailed t-test p=0.24 and AEG, inter-rater reliability Cronbach's alpha=0.975, one-sample two-tailed t-test p=0.3; LGA vs. AEG inter-rater reliability Cronbach's alpha=0.9, one-sample two-tailed t-test p=0.37). The reliability for hippocampal tracing is determined on a single data set after each tracer receives extensive training with feedback on several tracing datasets different from the reliability dataset. When boundaries were ambiguous, a standard neuroanatomical atlas was consulted41. Volumetric data were extracted for subsequent statistical analyses. We used t-tests to assess any systematic biases between the raters; a high Cronbach's alpha is not sufficient to assess inter-rater consistency, as it can be high if differences between two independent raters are in a consistent direction (i.e. one constantly underestimates or overestimates volumes relative to the other).
Ventricular tracing and extraction
Ventricular extraction followed a semi-automated ventricular segmentation approach17. Briefly, an experienced human rater (AEG, intra-rater reliability Cronbach's Alpha =0.995) traced the lateral ventricles of four subjects in three partitions per hemisphere – superior horn, temporal horn and ventricular body/occipital horn. These traces were converted into 3D parametric ventricular mesh models, termed atlases. Next each of the four atlases was fluidly registered to each unsegmented study image17. The resulting four ventricular segmentations per study subject were averaged resulting in one final 3D ventricular model for each study participant. Averaging four automated segmentations for each subject minimizes as much as possible automated labeling errors and most accurately captures individual anatomy.
Radial distance mapping approach
The hippocampal and ventricular contours were next split into top and bottom components and redigitized to normalize the spatial frequency of the surface points within and across brain slices. We stretched a regular parametric grid (100×150 surface points) over each surface, resampling and redigitizing the surface, to achieve spatial uniformity for precise point-wise comparison between subjects5. To create a measure of `radial distance' we derived first the medial curve (centroid) of the structure of interest and then calculated the distance from this curve to each surface point. The resulting hippocampal or ventricular “thickness” metric (in mm) is sensitive to local atrophy, or expansion - in the case of the lateral ventricle.
Statistical analyses
We conducted between-group comparisons of demographic characteristics and neuropsychological scores using analysis of variance (ANOVA) statistics for continuous (age, education and cognitive scores) and chi-squared tests for categorical variables (gender). All subsequent analyses were adjusted accordingly for demographic variables showing significant between-group differences.
Two types of imaging data – volumetric and 3D radial distance maps – were used for statistical analyses. For our main analyses, we used multiple regression with volume or radial distance as the dependent variables, diagnosis or age as the predictor variables and demographic variables showing statistically significant between-group differences as covariates. For 3D image analyses, independent point-wise regressions were performed and p-values assigned at each surface point. Type I error correction was conducted by statistically permuting the predictor variable - in this case, clinical diagnosis - and iteratively testing the global significance of the maps in each permuted experiment using the stringent significance threshold of p<0.01. We assessed the proportion of the anatomical surface exceeding this threshold, and compared it with what would have been obtained by chance in random re-assignments of covariates to the subjects. We applied 100,000 permutations to each comparison and derived a single global corrected p-value.
RESULTS
Demographic comparisons
The means of the demographic and cognitive variables for each diagnostic group are provided in Table 1. There were significant age, education and sex differences between the groups - AD subjects were oldest, least educated and had a greater proportion of women than the other groups, while the NC were youngest, most educated and had a greater proportion of men. The MCI group was intermediate with respect to all these demographic factors. All statistical analyses were corrected for age, sex and education. Table 2 lists the mean and standard deviations of the neuropsychologic test results for each diagnostic group.
Table 1.
Demographic and MMSE data
| Variable | Hippocampal analysis | Ventricular analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| NC N=46 | MCI N=33 | AD N=43 | ANOVA/Chi-Square p-value | NC N=44 | MCI N=32 | AD N=43 | ANOVA/Chi-Square p-value | |
| Age, yr | 66.4 (7.8) | 73.1 (6.0) | 75.7 (7.6) | <0.0001 | 66.3 (8.0) | 72.8 (5.9) | 75.7 (7.6) | <0.0001 |
| Gender, M:F | 25:21 | 22:11 | 16:27 | 0.035 | 24:20 | 21:11 | 16:27 | 0.044 |
| Education, yr | 17.2 (2.6) | 16.3 (2.6) | 14.6 (2.7) | <0.0001 | 17 (2.5) | 16.4 (2.6) | 14.6 (2.7) | <0.0001 |
| MMSE | 29.5 (0.6) | 27.8 (2.3) | 22.2 (4.9) | <0.0001 | 29.5 (0.6) | 27.8 (2.3) | 22.2 (4.9) | <0.0001 |
NC – cognitively normal elderly, MCI – mild cognitive impairment, AD- Alzheimer's disease, yr – years, M-male gender, F-female gender, MMSE – Mini-Mental State Examination score, ANOVA – analysis of variance
Table 2.
Neuropsychologic test performance
| Neuropsychologic test | NC Mean (SD) | MCI Mean (SD) | AD Mean (SD) |
|---|---|---|---|
| WAIS-III Digit Span Forward | 6.9 (1.2)*,## | 6.1 (1.3)^ | 5.9 (1.2)^^ |
| WAIS-III Digit Span Backwards | 5.1 (1.2)## | 4.9 (1.1)# | 4.1 (1.0)*,^^ |
| WAIS-III Digit Symbol | 65.7 (13.8)**,## | 51.7 (13.3)##,^^ | 37.8 (13.6)**,^^ |
| WAIS-III Block Design | 12.6 (2.4)## | 11.0 (3.2)## | 7.9 (3.0)**,^^ |
| Boston Naming Test | 57.1 (2.6)## | 53.8 (4.1)## | 39.9 (13.6)**,^^ |
| Phonemic fluency (FAS) | 47.8 (12.7)*,## | 37.6 (12.4)#,^ | 28.7 (12.9)*,^^ |
| Animal fluency | 22.5 (5.1)**## | 17.3 (4.3)##,^^ | 9.4 (4 1)**,## |
| WMS -III Logical Memory 1 story 1 | 45.6 (10.0)**,## | 30.2 (12.9)##,^^ | 15.6 (8.8)**,^^ |
| WMS -III Visual Reproduction I | 81.4 (11.8)**,## | 58.4 (17.4)##,^^ | 38.5 (15.2)**,^^ |
| WMS -III Visual Reproduction II | 59.6 (19.0)**,## | 19.9 (21.3)##,^^ | 38.5 (15.2)**,^^ |
| ROCF Copy | 32.8 (3.0)## | 31.1 (4.3)## | 22.9 (8.7)**,^^ |
| ROCF Recall | 17.8 (5.7)^^,## | 9.7 (7.0)##,^^ | 2.9 (2.9)**,^^ |
| Trails A | 30.2 (9.1)## | 42.8 (16.2)## | 70.0 (38.0)**,^^ |
| Trails B | 61.4 (17.1)**,## | 108.6 (50.6)##,^^ | 188.1 (80.8)**,^^ |
| Stroop A | 59.4 (10.2)*,## | 71.2 (13.5)##,^^ | 88.1 (22.8)**,^^ |
| Stroop B | 44.8 (6.6)## | 50.4 (13.0) | 56.2 (11.9)^^ |
| Stroop C | 115.5 (19.6)**,## | 149.6 (41.2)##,^^ | 206.4 (57.3)**,^^ |
p<0.001
p<0.05 relative to NC
p<0.001
p<0.05 relative to MCI
p<0.001
p<0.05 relative to AD
Hippocampal analyses
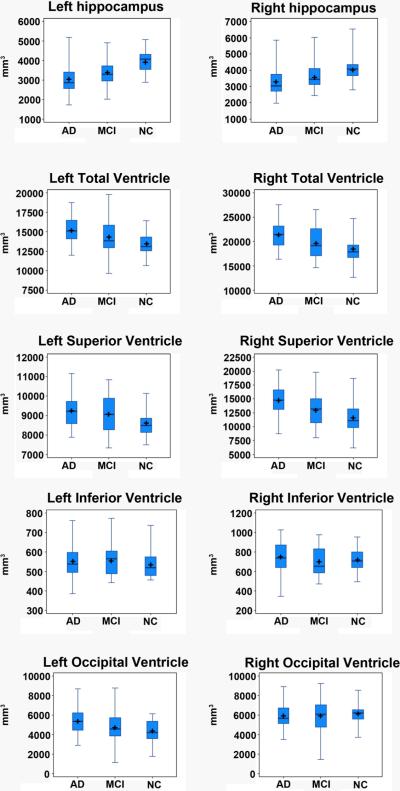
The results of the age-, sex- and education-corrected hippocampal volumetric analyses are presented in the top portion of Table 3. All hippocampal volumes listed in the table are adjusted for the effects of age, sex and educational level. The group-specific univariate statistics for the age-, sex- and education-adjusted hippocampal volumes can be seen in Figure 1. AD subjects had significantly smaller left and right hippocampal volumes when compared to NC (left p<0.0001, right p=0.001). When compared to MCI AD subjects showed trend volumetric differences on the left only (left p=0.088; right p=0.32). MCI subjects had significantly smaller hippocampal volumes bilaterally relative to NC (left p<0.0001; right p=0.034). After excluding the three nonamnestic subjects MCI still had significantly smaller hippocampal volumes relative to NC (left p<0.0001, right p=0.024) while the trend for volumetric different with AD on the left was no longer present (p>0.05).
Table 3.
Volumetric analyses. The age and diagnosis effects were obtained in a multiple linear regression model with hippocampal or ventricular volumes as the outcome and age, gender, education and diagnosis as predictor variables.
| Group 1 vs. Group 2 | Side | Group 1, Adjusted* volume, mm3 | Group 2 Adjusted* volume, mm3 | Age effect | Diagnosis effect | ||
|---|---|---|---|---|---|---|---|
| t | p-value | t | p-value | ||||
| Hippocampi | |||||||
|
| |||||||
| NC vs. MCI | Left | 3947 (787) | 3387 (787) | −1.84 | 0.07 | −3.16 | <0.0001 |
|
| |||||||
| Right | 4027 (713) | 3597 (757) | −0.59 | 0.6 | −2.66 | 0.034 | |
|
| |||||||
| MCI vs. AD | Left | 3387 (787) | 3022 (698) | −2.85 | 0.006 | −2.97 | 0.088 |
|
| |||||||
| Right | 3597 (757) | 3291 (798) | −1.82 | 0.07 | −1.55 | 0.13 | |
|
| |||||||
| NC vs. AD | Left | 3947 (787) | 3022 (698) | −2.42 | 0.018 | −6.05 | <0.0001 |
|
| |||||||
| Right | 4027 (713) | 3291 (798) | −2.37 | 0.02 | −3.36 | 0.001 | |
|
| |||||||
| Ventricles | |||||||
|
| |||||||
| NC vs. MCI | Left ventricle | 13481 (1327) | 14339 (2227) | 2.9 | 0.004 | 0.66 | 0.5 |
| Left frontal horn | 8608 (633) | 9090 (884) | 1.13 | 0.3 | 2.10 | 0.039 | |
| Left temporal horn | 535 (64) | 553 (82) | 2.06 | 0.043 | 0.16 | 0.9 | |
| Left body/occipital horn | 4338 (1102) | 4695 (1675) | 3.11 | 0.003 | −0.35 | 0.7 | |
|
|
|||||||
| Right ventricle | 18411 (2599) | 19705 (3016) | 2.71 | 0.008 | 1.0 | 0.32 | |
| Right frontal horn | 11555 (2770) | 13053 (2898) | 3.28 | 0.002 | 1.07 | 0.3 | |
| Right temporal horn | 717 (121) | 702 (143) | −0.31 | 0.8 | −0.03 | 1 | |
| Right body/occipital horn | 6139 (921) | 5950 (1664) | −1.1 | 0.3 | −0.12 | 0.9 | |
|
| |||||||
| MCI vs. AD | Left ventricle | 14339 (2227) | 15172 (1545) | 1.63 | 0.11 | 1.47 | 0.14 |
| Left frontal horn | 9090 (884) | 9256 (797) | 1.13 | 0.3 | 0.14 | 0.9 | |
| Left temporal horn | 553 (82) | 552 (74) | 0.04 | 1 | 0.19 | 0.9 | |
| Left body/occipital horn | 4695 (1675) | 5363 (1202) | 1.28 | 0.2 | 1.86 | 0.07 | |
|
|
|||||||
| Right ventricle | 19705 (3016) | 21371 (2780) | 1.88 | 0.07 | 1.75 | 0.021 | |
| Right frontal horn | 13053 (2898) | 14711 (2786) | 2.59 | 0.012 | 1.92 | 0.031 | |
| Right temporal horn | 702 (143) | 748 (151) | 1.22 | 0.2 | −0.4 | 0.7 | |
| Right body/occipital horn | 5950 (1664) | 5913 (1209) | −1.32 | 0.2 | −0.13 | 0.9 | |
|
| |||||||
| NC vs. AD | Left ventricle | 13481 (1327) | 15172 (1545) | 3.69 | 0.004 | 2.81 | <0.0001 |
| Left frontal horn | 8608 (633) | 9256 (797) | 1.72 | 0.09 | 2.02 | 0.047 | |
| Left temporal horn | 535 (64) | 552 (74) | 0.54 | 0.6 | 0.58 | 0.6 | |
| Left body/occipital horn | 4338 (1102) | 5363 (1202) | 3.42 | 0.001 | 2.14 | 0.036 | |
|
|
|||||||
| Right ventricle | 18411 (2599) | 21371 (2780) | 3.29 | 0.001 | 3.39 | 0.001 | |
| Right frontal horn | 11555 (2770) | 14711 (2786) | 3.28 | 0.002 | 3.12 | 0.002 | |
| Right temporal horn | 717 (121) | 748 (151) | 0.66 | 0.5 | 0.23 | 0.8 | |
| Right body/occipital horn | 6139 (921) | 5913 (1209) | −1.76 | 0.08 | 0.53 | 0.6 | |
all volumes are adjusted for age, sex and education
NC – cognitively normal elderly, MCI – mild cognitive impairment, AD- Alzheimer's disease, mm3- cubic millimeters
Figure 1.
Box plot of the age-, gender- and education-normalized hippocampal and ventricular volumes in NC, MCI and AD. The central box displays the data between the upper and lower quartiles with the line representing the median and the cross representing the mean. The “whiskers” display the range for the upper and lower quartiles, resp. The feet of the whiskers represent the full range of each variable.
The effects of age on the hippocampus within each diagnostic group after correcting for gender and education can be seen in Table 3.
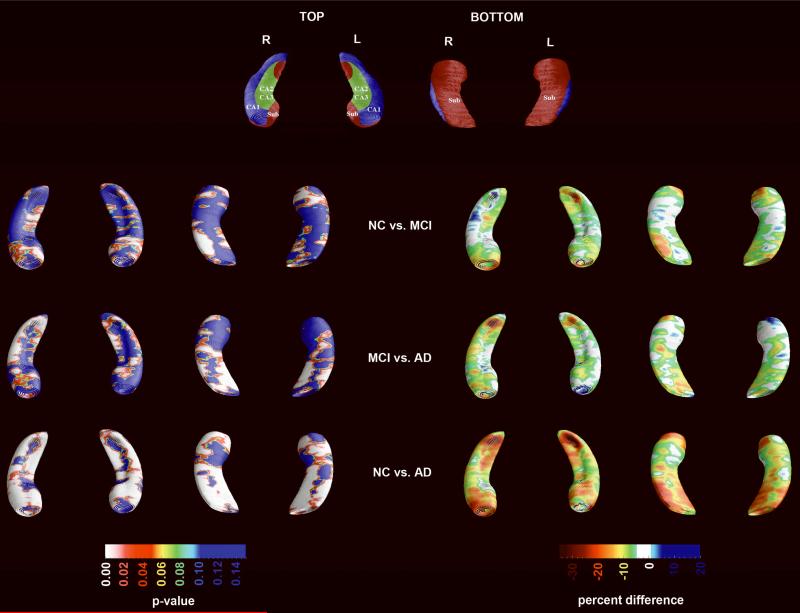
The results of the 3D hippocampal radial distance analyses are presented in Figure 2. AD subjects showed highly significant atrophy in all hippocampal subfields relative to NC (left pcorrected <0.0001; right pcorrected <0.0001) and MCI (left pcorrected =0.0123; right pcorrected =0.0001). MCI subjects showed bilaterally significant atrophy relative to NC (left pcorrected =0.002; right pcorrected =0.0014). Quantitatively, the absolute differences in average radial distance, for each group, ranged between 10–40% in the AD vs. NC, 10–25% in the MCI vs NC and 5–30% in the MCI vs. AD comparisons (Figure 2, right panel). After excluding the three nonamnestic MCI subjects the between group differences remained significant for both comparisons (NC vs. amnestic MCI left pcorrected =0.028, right pcorrected =0.0021; AD vs. amnestic MCI left pcorrected =0.026, right pcorrected =0.0001).
Figure 2.
3D hippocampal between-group statistical (left) and quantitative (right) comparisons. The statistical maps are corrected for age, sex and education.
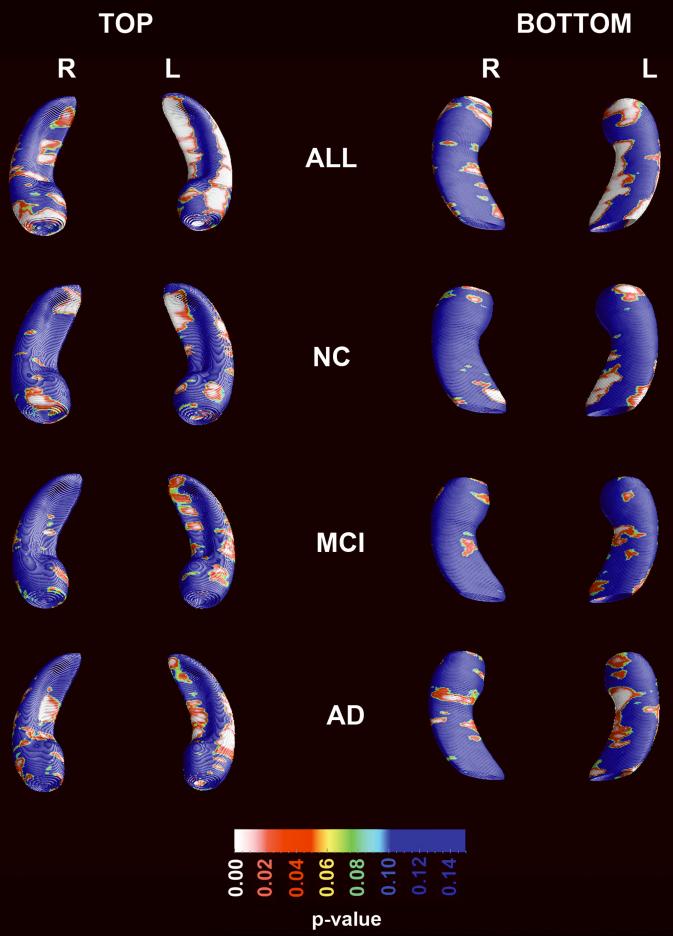
Figure 3 shows the effect of age on hippocampal radial distance while controlling for diagnosis, sex and educational level, in the pooled sample. The permutation-corrected statistical significance in the pooled sample was pcorrected =0.0001 on the left and pcorrected =0.1 on the right. Within diagnostic categories significant age effects were also seen on the left in NC (left pcorrected =0.0024; right pcorrected =0.08) and AD subjects (left pcorrected =0.014; right pcorrected =0.1) but not in MCI (left pcorrected=0.065, right pcorrected=0.44).
Figure 3.
Effect of age on hippocampal radial distance while controlling for diagnosis, sex and education in the pooled sample and in each diagnostic group while controlling for gender and education.
Ventricular analyses
The results of the age-, sex- and education-corrected ventricular volumetric analyses are presented in the bottom portion of Table 3. All ventricular volumes listed in the table are adjusted for age, sex and educational level. AD subjects had significantly larger left and right lateral ventricular volumes when compared to NC subjects (p<0.0001 on the left and p=0.001 on the right). These differences were largely driven by differences in the frontal horns bilaterally, but differences in the left body/occipital horn were also significant. Relative to NC, MCI subjects showed significantly larger left frontal horn volumes (p=0.039). After excluding the three nonamnestic MCI subjects the significance for the left frontal horn became p=0.059, however now the total left ventricular volumes reached significance (left p=0.036, right p=0.071). The MCI subjects had significantly larger right frontal horn (p=0.031) and total right ventricular volume (p=0.021) relative to AD subjects. After excluding the three nonamnestic MCI subjects significant differences persisted for the right total ventricular volume (p=0.043) and showed a trend for the right frontal horn (p=0.069).
3D ventricular maps are presented in Figure 4. AD subjects showed significantly enlarged frontal (left pcorrected <0.022; right pcorrected <0.013) and body/occipital horn (left pcorrected <0.035; right pcorrected <0.017) relative to the NC group. Relative to MCI, AD subjects showed trend-level significant enlargement of the left body/occipital horn (pcorrected =0.088) as well as the right temporal horn (pcorrected =0.065). After excluding the three nonamnestic MCI subjects the trend persisted for the right temporal horn only (pcorrected=0.09). MCI subjects showed trend-level significant enlargement of the right frontal horn relative to NC (pcorrected =0.098). The trend disappeared after excluding the three nonamnestic MCI subjects. Quantitatively, the absolute differences in mean ventricular radial distance, between groups, ranged between 5–30% in AD vs. NC, 5–20% in the MCI vs. NC and 3–13% in the MCI vs. AD comparisons (Figure 4, right panel).
Figure 4.
3D ventricular between-group statistical (left) and quantitative (right) comparisons. The statistical maps are corrected for age, sex and education.
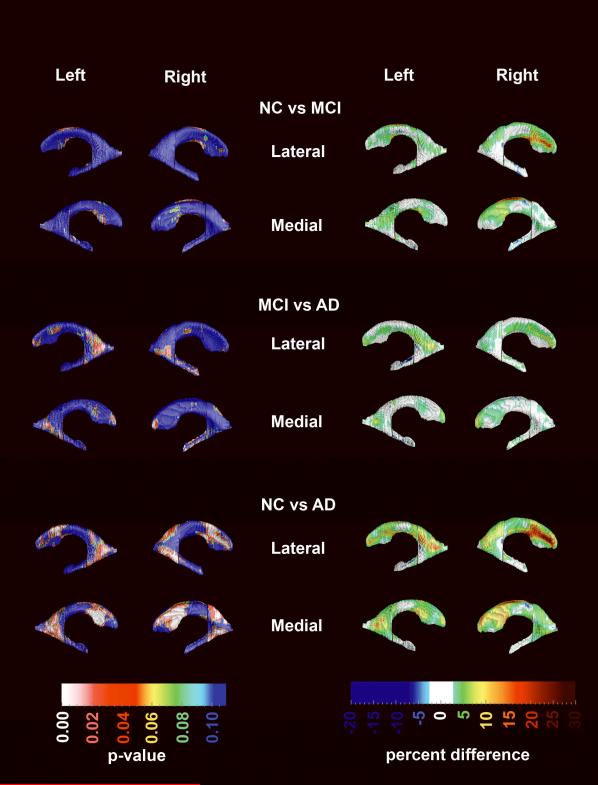
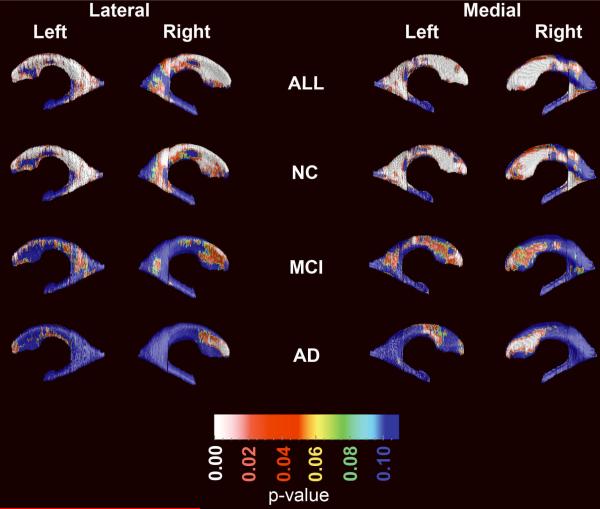
Figure 5 shows the effect of age on ventricular radial distance in the pooled sample while controlling for diagnosis, gender and education (top row) and each diagnostic category while controlling for gender and education (rows 2–4). Permutation-corrected statistically significant aging effects in the pooled sample were seen for the frontal horn (left pcorrected =0.0001, right pcorrected =0.0007) and for the body/occipital horn portions (left pcorrected =0.0012, right pcorrected =0.036) of the lateral ventricles. A trend level effect was seen for the left temporal horn (pcorrected =0.09). After excluding the three nonamnestic MCI subjects these effects persisted – frontal horn left pcorrected = 0.0001, right pcorrected =0.0009; occipital horn left pcorrected =0.0017, right pcorrected =0.059, left temporal horn pcorrected =0.065. Within diagnostic categories, the age effects survived stringent permutation-correction for multiple comparisons in NC and AD only. Among NC, significant effects were seen in the frontal horns (left pcorrected =0.00085; right pcorrected =0.0025) as well as the body/occipital horn (left pcorrected =0.0002; right pcorrected =0.013). The temporal horn in NC showed a trend for age-related expansion (left pcorrected =0.05, right pcorrected =0.09). Among AD subjects, the permutation-corrected significant age effects were detected in the right frontal horn only (left pcorrected =0.092; right pcorrected =0.02). In MCI, the permutation-corrected significance for the frontal horns reached trend-level on the left (left pcorrected =0.067) but became nonsignificant after excluding the three nonamnestic MCI subjects.
Figure 5.
Effect of age on hippocampal radial distance while controlling for diagnosis, gender and education in the pooled sample and in each diagnostic group while controlling for gender and education.
DISCUSSION
AD, the most common neurodegenerative disorder, results in profound cognitive and functional deterioration, and ultimately death. AD pathology is triggered by the aberrant processing and deposition of two proteins – Aß (beta-amyloid) and tau - leading to neuronal dysfunction and neuronal death. The cumulative loss of neurons and their connections results in macroscopic changes such as cortical and hippocampal atrophy and ventricular enlargement. Brain atrophy is a common feature of virtually all neurodegenerative disorders, and each disorder has a unique pattern of involvement. The classic AD pattern consists of early prominent involvement of the entorhinal cortex and the hippocampus, followed by significant atrophy of the posterior (i.e., temporal, parietal and occipital) and later on the frontal association cortices. Sulcal dilation and ventriculomegaly reflecting both gray and white matter loss are prominent and easily seen on conventional diagnostic images. While indeed AD is considered a primary gray matter disorder, there are significant secondary changes in the white matter in subjects with AD. Most likely white matter loss is due to secondary loss of the axons following neuronal death. We could further support this argument by the findings reported in42. The authors of this paper report a gray/white matter ratio of 1.7 (corresponding to approximately 63% gray and 37% white matter) in healthy elderly (mean age 71 y, range 65–76 y) and a gray/white matter ratio of 1.9 (corresponding to approximately 66% gray and 34% white matter) in AD subjects (mean age 70 y, range 61–75 y).
Normal aging is also associated with hippocampal atrophy9 and ventricular dilation13, 23. However, unlike in AD, these changes are modest and their rate of progression over time is relatively slow. Both AD and normal aging result in similar changes, so separating these two states is frequently challenging. Here we have investigated effects of age and AD independent of each other.
Despite the cross-sectional nature of this work the pervasive AD effect on the hippocampal formation is clearly demonstrable. We found significant between-group differences between NC and AD in all hippocampal subfields. Statistically significant differences between MCI and NC were largely restricted to the hippocampal head and the subiculum bilaterally as well as the CA2–3 area on the right. The structural differences between MCI and AD were most pronounced in the CA1 subfield but were also seen in the CA2–3 areas bilaterally.
The effects of full-blown AD on the lateral ventricles localized not only to the posterior portions (body/occipital horn) but also to the frontal horns. This predominantly posterior ventricular enlargement in MCI agrees with the known early posterior predominant disease effect, which later spreads to the frontal horns. Whether involvement of the frontal horns coincides or predates the conversion to full-blown AD syndrome and hence marks the transition to functional impairment remains to be proven.
Previous studies report that aging affects only selected hippocampal subfields, but no two studies have agreed on the exact regions involved. Some report involvement of the CA3/dentate gyrus and CA18, others of CA1 and subiculum9, the subiculum and the dentate gyrus10 or the subiculum alone11. The disagreement between these studies likely reflects MR acquisition and methodological differences, including limited statistical power and relatively small effect sizes. Our results suggest an age effect on all these subfields and confirm all of these previous observations. Our finding of trend-level effect in the MCI group in the context of significant effects in the NC and AD groups may be due to relative lack of power due to the smaller size of our MCI group.
In agreement with prior reports14, 43, we found a significant effect of age on the shape and size of the lateral ventricles. This effect is most pronounced in the frontal horns perhaps as the result of structural changes in one or more tissue classes in the frontal lobes44–47. These data are also in keeping with the pattern of cognitive decline seen in the elderly, including decline in working memory, information processing speed and retrieval of previously learned information48. We found the strongest ventricular age effect in our youngest group – the NC group, and weaker age effects in our older groups – the MCI and AD groups. This may seem counterintuitive, but may be attributable to several factors. One could speculate that either the effect of age on ventricular volume/radial distance is nonlinear with steeper slopes in the younger as opposed to older elderly subjects as was recently demonstrated49. Further evidence is the data reported by Sowell et al.50, 51 where the investigators plotted trajectories of cortical atrophy over the lifespan, and some age effects slowed down. Another possible reason for this in a longitudinal study, is cohort attrition due to mortality or inability to remain in the study when atrophy has exceeded a certain level. This might lead to a censoring of data available to understand the age effects. Yet here we only work with cross-sectional data. It may also be that once the brain and ventricular volumes are affected by Alzheimer's pathology the strength of the association between age and ventricular volume or radial distance weakens. The only way to find out whether any of these two speculations are true would be to study the effect of aging longitudinally in sample of cognitively normal elderly who later in life have succumbed to AD.
Several strengths and limitations of this work should be recognized. The strengths of this work lie in the thorough evaluation of this relatively large subject pool and the advanced imaging software used. Additionally, we carefully modeled the effects of age and by doing so we build upon previous reports of associations between age and change in hippocampal and ventricular volume over time. Our 3D results show aging effects in all hippocampal subfields. We also demonstrated a fronto-parietal pattern of age effects on the lateral ventricles. Given these findings, we conclude that aging accounts for some of the variability of hippocampal and lateral ventricular structural measures and thus should be included as covariate in all structural hippocampal and ventricular analyses when possible. The major weakness of this study is its cross-sectional design. Subject follow-up and the use of longitudinal datasets such as those from the Alzheimer's Disease Neuroimaging Initiative are planned. The second limitation is the fact that original imaging data collection was conducted on 1.5Tesla MR scanner. It would certainly be valuable to collect high resolution hippocampal scans, such as T2-weighted scans at 3 or 7 Tesla52–55 however this type of scan is not routinely aquired in large scale database projects such as the UCLA imaging database or ADNI56, where the whole brain needs to be assessed and scan time is limited. Finally, we corrected for potentially confounding variables such as age, sex, and education using a general linear model, but it may be that age and education effects are nonlinear in nature.
Acknowledgements
The analyses reported in this manuscript were funded by NIA K23 AG026803 (jointly sponsored by NIA, AFAR, The John A. Hartford Foundation, The Atlantic Philanthropies, The Starr Foundation and an anonymous donor), NIA P50 AG16570, NIMH R01 MH071940, NCRR P41 RR013642 and NIH U54 RR021813. PT is also supported by EB008432, EB008281, EB007813, HD050735 and AG036535.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Archives of Neurology. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Wimo A, Jonsson L, Winblad B. An estimate of the worldwide prevalence and direct costs of dementia in 2003. Dement Geriatr Cogn Disord. 2006;21:175–181. doi: 10.1159/000090733. [DOI] [PubMed] [Google Scholar]
- 3.Jack CR, Jr., Petersen RC, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack CR, Jr., Petersen RC, Xu Y, et al. Rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurology. 1998;51:993–999. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson PM, Hayashi KM, De Zubicaray GI, et al. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22:1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 6.Apostolova LG, Dutton RA, Dinov ID, et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006;63:693–699. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- 7.Apostolova LG, Mosconi L, Thompson PM, et al. Subregional hippocampal atrophy predicts Alzheimer's dementia in the cognitively normal. Neurobiol Aging. 2010;31:1077–1088. doi: 10.1016/j.neurobiolaging.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller SG, Weiner MW. Selective effect of age, Apo e4, and Alzheimer's disease on hippocampal subfields. Hippocampus. 2009;19:558–564. doi: 10.1002/hipo.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frisoni GB, Ganzola R, Canu E, et al. Mapping local hippocampal changes in Alzheimer's disease and normal ageing with MRI at 3 Tesla. Brain. 2008;131:3266–3276. doi: 10.1093/brain/awn280. [DOI] [PubMed] [Google Scholar]
- 10.Small SA, Tsai WY, DeLaPaz R, et al. Imaging hippocampal function across the human life span: is memory decline normal or not? Ann Neurol. 2002;51:290–295. doi: 10.1002/ana.10105. [DOI] [PubMed] [Google Scholar]
- 11.Chetelat G, Fouquet M, Kalpouzos G, et al. Three-dimensional surface mapping of hippocampal atrophy progression from MCI to AD and over normal aging as assessed using voxel-based morphometry. Neuropsychologia. 2008;46:1721–1731. doi: 10.1016/j.neuropsychologia.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 12.Nestor SM, Rupsingh R, Borrie M, et al. Ventricular enlargement as a possible measure of Alzheimer's disease progression validated using the Alzheimer's disease neuroimaging initiative database. Brain. 2008;131:2443–2454. doi: 10.1093/brain/awn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmichael OT, Kuller LH, Lopez OL, et al. Ventricular volume and dementia progression in the Cardiovascular Health Study. Neurobiol Aging. 2007;28:389–397. doi: 10.1016/j.neurobiolaging.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmichael OT, Kuller LH, Lopez OL, et al. Cerebral ventricular changes associated with transitions between normal cognitive function, mild cognitive impairment, and dementia. Alzheimer Dis Assoc Disord. 2007;21:14–24. doi: 10.1097/WAD.0b013e318032d2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jack CR, Jr., Shiung MM, Gunter JL, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou Y, Lepore N, Avedissian C, et al. Mapping correlations between ventricular expansion and CSF amyloid and tau biomarkers in 240 subjects with Alzheimer's disease, mild cognitive impairment and elderly controls. Neuroimage. 2009;46:394–410. doi: 10.1016/j.neuroimage.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou YY, Lepore N, de Zubicaray GI, et al. Automated ventricular mapping with multi-atlas fluid image alignment reveals genetic effects in Alzheimer's disease. Neuroimage. 2008;40:615–630. doi: 10.1016/j.neuroimage.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Driscoll I, Davatzikos C, An Y, et al. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72:1906–1913. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou YY, Lepore N, Saharan P, et al. Ventricular maps in 804 ADNI subjects: correlations with CSF biomarkers and clinical decline. Neurobiol Aging. 2010;31:1386–1400. doi: 10.1016/j.neurobiolaging.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Josephs KA, Whitwell JL, Ahmed Z, et al. Beta-amyloid burden is not associated with rates of brain atrophy. Ann Neurol. 2008;63:204–212. doi: 10.1002/ana.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chou Y, Lepore N, Zubicaray GI, et al. Automated 3D mapping and shape analysis of the lateral ventricles via fluid registration of multiple surface-based atlases. 4th IEEE International Symposium on Biomedical Imaging: From Nano to Macro.2007. pp. 1288–1291. [Google Scholar]
- 22.Ferrarini L, Palm WM, Olofsen H, et al. Ventricular shape biomarkers for Alzheimer's disease in clinical MR images. Magn Reson Med. 2008;59:260–267. doi: 10.1002/mrm.21471. [DOI] [PubMed] [Google Scholar]
- 23.Jack CR, Jr., Weigand SD, Shiung MM, et al. Atrophy rates accelerate in amnestic mild cognitive impairment. Neurology. 2008;70:1740–1752. doi: 10.1212/01.wnl.0000281688.77598.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou Y, Lepore N, Avedissian C, et al. Mapping correlations between ventricular expansion and CSF amyloid and tau biomarkers in 240 subjects with Alzheimer's disease, mild cognitive impairment and elderly controls. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2009.02.015. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cogntive state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler Memory Scale - Revised. The Psycholocical Corporation; San Antonio, TX: 1987. [Google Scholar]
- 27.Kaplan EF, Goodgalss H, Weintraub S. The Boston Naming Test. Lea&Febiger; Philadelphia: 1983. [Google Scholar]
- 28.Benton AL, Hamsher K. Multilingual apahasia examination. AJA Associates; Iowa City, Iowa: 1989. [Google Scholar]
- 29.Osterrieth PA. Le test de copie d'une figure complexe. Archieves de Psychologie. 1944;30:206–356. [Google Scholar]
- 30.Stroop JR. Studies in interference of serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- 31.Kelland DZ, Lewis R, Gurevitch D. Evaluation of the Repeatable Cognitive-Perceptual-Motor battery: reliability, validity and sensitivity to diazepam. Journal of Clinical & Experimental Neuropsychology. 1992;14:65. abstract. [Google Scholar]
- 32.Berg L. A simple objective treatment for measuring flexibility in thinking. Journal of General Psychology. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- 33.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disese: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 34.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 35.Collins DL, Neelin P, Peters TM, et al. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 36.Mazziotta J, Toga A, Evans A, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos Trans R Soc Lond B Biol Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shattuck DW, Sandor-Leahy SR, Schaper KA, et al. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 38.Narr KL, Thompson PM, Sharma T, et al. Three-dimensional mapping of temporo-limbic regions and the lateral ventricles in schizophrenia: gender effects. Biol Psychiatr. 2001;50:84–97. doi: 10.1016/s0006-3223(00)01120-3. [DOI] [PubMed] [Google Scholar]
- 39.Pantel J, O'Leary DS, Cretsinger K, et al. A new method for the in vivo volumetric measurement of the human hippocampus with high neuroanatomical accuracy. Hippocampus. 2000;10:752–758. doi: 10.1002/1098-1063(2000)10:6<752::AID-HIPO1012>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 40.Crohnbach LJ. Coefficient alpha and the internal structure of tests. Psychometrica. 1951;16:297–334. [Google Scholar]
- 41.Duvernoy H. An atlas of applied anatomy. J.F. Bergmann Verlag; Munich: 1988. The human hippocampus. [Google Scholar]
- 42.Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch Neurol. 1999;56:338–344. doi: 10.1001/archneur.56.3.338. [DOI] [PubMed] [Google Scholar]
- 43.Carmichael OT, Kuller LH, Lopez OL, et al. Acceleration of cerebral ventricular expansion in the Cardiovascular Health Study. Neurobiol Aging. 2007;28:1316–1321. doi: 10.1016/j.neurobiolaging.2006.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Resnick SM, Pham DL, Kraut MA, et al. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikram MA, Vrooman HA, Vernooij MW, et al. Brain tissue volumes in the general elderly population. The Rotterdam Scan Study. Neurobiol Aging. 2008;29:882–890. doi: 10.1016/j.neurobiolaging.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Ge Y, Grossman RI, Babb JS, et al. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR Am J Neuroradiol. 2002;23:1327–1333. [PMC free article] [PubMed] [Google Scholar]
- 48.Raz N. Decline and compensation in aging brain and cognition: promises and constraints. Preface. Neuropsychol Rev. 2009;19:411–414. doi: 10.1007/s11065-009-9122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walhovd KB, Westlye LT, Amlien I, et al. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sowell ER, Peterson BS, Kan E, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sowell ER, Peterson BS, Thompson PM, et al. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 52.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 53.Mueller SG, Stables L, Du AT, et al. Measurement of hippocampal subfields and age-related changes with high resolution MRI at 4T. Neurobiol Aging. 2007;28:719–726. doi: 10.1016/j.neurobiolaging.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeineh MM, Engel SA, Thompson PM, et al. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]
- 55.Augustinack JC, van der Kouwe AJ, Blackwell ML, et al. Detection of entorhinal layer II using 7Tesla [corrected] magnetic resonance imaging. Ann Neurol. 2005;57:489–494. doi: 10.1002/ana.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jack CR, Jr., Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]