The optimal selection of patients and timing of surgery and selection of chemotherapy for colorectal cancer patients with potentially resectable liver metastases are discussed by addressing a series of questions. Regional therapies are also discussed.
Keywords: Colorectal neoplasms, Liver neoplasms, Metastasis, Chemotherapy, Surgery
After completing this course, the reader will be able to:
Among patients with liver metastases from colorectal cancer, determine which would benefit from liver resection, the timing for surgery, and an appropriate perioperative chemotherapy regimen.
Determine which patients are candidates for perioperative chemotherapy and the appropriate timing of chemotherapy, and describe the relevant toxicities and their impact on morbidity and mortality.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Colorectal cancer is a very common malignancy and frequently manifests with liver metastases, often without other systemic disease. Margin-negative (R0) resection of limited metastatic disease, in conjunction with systemic antineoplastic agents, is the primary treatment strategy, leading to long survival times for appropriately selected patients. There is debate over whether the primary tumor and secondaries should be removed at the same time or in a staged manner. Chemotherapy is effective in converting some unresectable liver metastases into resectable disease, with a correspondingly better survival outcome. However, the ideal chemotherapy with or without biological agents and when it should be administered in the course of treatment are uncertain. The role of neoadjuvant chemotherapy in initially resectable liver metastases is controversial. Local delivery of chemotherapy, with and without surgery, can lead to longer disease-free survival times, but it is not routinely used with curative intent. This review focuses on methods to maximize the disease-free survival interval using chemotherapy, surgery, and local methods.
Introduction
Colorectal cancer (CRC) is the second most common cancer in women and the third most common cancer in men worldwide, with an estimated 1.24 million cases worldwide in 2008 [1]. It is the third most common cancer in Australia, after nonmelanoma skin cancers and prostate cancer, with 14,234 cases diagnosed in 2007 [2]. It is the second most common cancer in men and women, after prostate and breast cancer, respectively [3]. Fifteen to twenty percent of patients have liver metastases at diagnosis of CRC [4], and 60% of patients who develop metastatic disease will develop liver metastases [5]. Cytotoxic chemotherapy is effective, and the role of biologic agents continues to expand.
Resection of liver-limited metastases from CRC has become the standard of care [6]. However, the place of chemotherapy in the perioperative period remains unclear. Up to 23% of patients with metastatic CRC (mCRC) are deemed initially resectable, and with modern cytotoxic chemotherapy an additional 12% can be made resectable [7]. Liver resection in appropriately selected patients can result in a 5-year survival rate of 30% and a 10-year survival rate of 17%–25%, which is widely regarded as a cure [8, 9]. For liver metastases to be resectable, the procedure must be technically feasible, the liver remnant volume must be >20%, and removal of extrahepatic metastases, if present, must be achievable [10]. A patient is considered potentially resectable if it appears reasonable that resectability may be achieved with the use of neoadjuvant systemic or local therapies. If the metastases are deemed unresectable, outcomes are substantially worse, with a 5-year overall survival (OS) rate for patients with synchronous mCRC of 11% in the U.S., and a 10-year survival rate close to zero [4].
This review discusses the optimal selection of patients and timing of surgery and selection of chemotherapy for CRC patients with potentially resectable liver metastases by addressing a series of questions. Regional therapies are also discussed.
Treatment of mCRC
Over the past decade, the standard first-line therapy for mCRC has progressed from 5-fluorouracil and leucovorin (5-FU–LV) alone to the addition of oxaliplatin or irinotecan, and now bevacizumab. In a randomized controlled trial (RCT) comparing 5-FU–LV with 5-FU–LV plus oxaliplatin (FOLFOX) in 420 patients, a longer progression-free survival (PFS) interval, 9.0 months versus 6.3 months, was obtained with the addition of oxaliplatin (p = .0001) [11]. In that study, 3.3% of the 5-FU–LV-treated patients and 6.7% of those receiving FOLFOX had secondary removal of metastases. Saltz et al. [12] compared 5-FU–LV with 5-FU–LV plus irinotecan (IFL), and with irinotecan alone. There was a significant benefit with irinotecan combined with 5-FU–LV over irinotecan alone or 5-FU–LV alone, in terms of the PFS interval (median, 7.0 months versus 4.3 months; p = .004), response rate (39% versus 21%; p < .001), and OS times (14.8 months versus 12.6 months; p = .04). Douillard et al. [13] had similar findings. In another study, comparing FOLFOX with 5-FU–LV plus irinotecan (FOLFIRI), FOLFOX had a therapeutic benefit equivalent to that of FOLFIRI (PFS time, 8.0 months versus 8.5 months; p = .26), with less nausea, vomiting, diarrhea, and febrile neutropenia but at a cost of a higher rate of neuropathy [14]. Metastasectomy was possible in 22% and 9% of patients after using FOLFOX and FOLFIRI, respectively (p = .02).
More recently, biological agents such as bevacizumab and cetuximab are being used in the metastatic setting. In 2004, a German study of 813 patients with mCRC showed that, in the first-line setting, the addition of bevacizumab to IFL resulted in significant additional benefit in terms of the OS duration (20.3 months versus 15.6 months), PFS interval (10.6 months versus 6.2 months), and response rate (44.8% versus 6.2%) [15]. The use of the newer infusional FOLFIRI regimen with bevacizumab is at least equally efficacious [16, 17]. Saltz et al. [18] showed, in 2008, that bevacizumab with FOLFOX4 or capecitabine plus oxaliplatin (XELOX), compared with FOLFOX4 or XELOX alone, gave a PFS advantage of 9.4 months versus 8.0 months (p = 0.0023), but without any difference in the response rate (47% versus 49%; p = .31) or OS time (21.3 months versus 19.9 months; p = .77). That study recruited 1,401 patients who were eligible for first-line chemotherapy for mCRC. Of those patients, 8.4% in the bevacizumab arm and 6.0% in the comparator arm underwent an attempt at curative metastasectomy. The Cetuximab Combined With Irinotecan in First-Line Therapy for Metastatic Colorectal Cancer (CRYSTAL) study, published first in 2009, was a first-line treatment study using cetuximab and chemotherapy that included 1,198 patients with mCRC [19]. Cetuximab added to FOLFIRI resulted in a longer PFS interval (8.9 months versus 8.0 months) and higher response rate (46.9% versus 38.7%), but no difference in the OS time. The R0 resection rate for metastases was 4.8% in the cetuximab-containing arm versus 1.7% with FOLFIRI alone (p = .0002). Subgroup analysis for KRAS mutation status showed that the benefit was limited to those expressing wild-type (wt) KRAS only (PFS interval, 9.9 months versus 8.7 months). There were more patients with wt KRAS in the FOLFIRI arm than in the cetuximab–FOLFIRI arm (66.9% versus 62.1%). The rate of surgery in those with wt KRAS was not reported. In the Oxaliplatin and Cetuximab in First-Line Treatment of mCRC (OPUS) study in patients with wt KRAS, cetuximab added to FOLFOX, compared with FOLFOX alone, led to a higher response rate (57% versus 34%; p = .0027) and longer PFS time (8.3 months versus 7.2 months; p = .0064), but the survival duration was not significantly longer [20]. The addition of cetuximab also led to a higher rate of surgery for metastatic lesions (12% versus 3%; p = .0242), albeit with small numbers. Given these findings, it can be inferred that a proportion of patients with initially unresectable disease could be rendered resectable by chemotherapy. In the studies described above, it was not reported how many patients were potentially resectable, and therefore the true ability of these regimens as conversion therapy could not be assessed.
Is Chemotherapy Beneficial for Patients with Initially Resectable Liver Metastases?
The role of antitumor therapy prior to liver resection remains unclear in patients whose liver metastases are considered to be resectable at the time of diagnosis. The rationale for giving neoadjuvant chemotherapy is to eradicate micrometastases that may progress while the patient is recovering from surgery, to evaluate the chemoresponsiveness of the tumor, and to shrink the known metastases to a more readily resectable size [21]. As a comparison, neoadjuvant chemotherapy can be given to patients with locally advanced breast cancer, and it has been shown that the disease-free survival (DFS) and OS outcomes are equivalent to those achieved in initially operable tumors, with better locoregional control and a higher lumpectomy rate [22].
The idea that chemotherapy could act as an aid to selecting which patients will benefit from neoadjuvant chemotherapy came from a French study showing that those who progressed on chemotherapy had a much lower 5-year survival probability than those who had a response or stable disease (8% versus 37% and 30%, respectively; p < .0001) [23]. The population studied had highly advanced disease, including extrahepatic metastases, and additional ablational techniques were used. The same group also reported on the 5-year survival rate of complete pathological responders compared with patients without a complete pathological response (76% versus 45%; p = .004) [24]. Allen et al. [25] also reported that 17 patients who progressed on neoadjuvant chemotherapy had a 5-year survival rate of 38%, compared with 85% of 29 similar patients who did not progress. It can be inferred that, if a patient progresses on neoadjuvant chemotherapy but is still resectable, then the rate of survival if surgery is offered is still much better than the 0%–10% 5-year survival rate if surgery is withheld. Contrary to the above findings, a German series of 160 patients who had liver resection after chemotherapy showed no difference in the 5-year survival rate (34% versus 36%) [26].
The European Organization for Research and Treatment of Cancer (EORTC) 40983 study attempted to solve the dilemma of whether or not there is a benefit to neoadjuvant chemotherapy in patients with initially resectable liver-limited mCRC. It randomized 364 patients to upfront surgery or six cycles of neoadjuvant FOLFOX4, followed by surgery and then another six cycles of FOLFOX4 [27]. The difference in the median PFS time was not statistically significant, at 11.7 months for the surgery alone arm and 18.7 months for the chemotherapy plus surgery arm (hazard ratio [HR], 0.79; p = .058). Eleven patients in both arms were deemed ineligible after randomization, mainly as a result of having more advanced disease than originally suspected. Analysis of all eligible patients showed a higher 3-year PFS rate (36.2%) in the chemotherapy plus surgery arm than in the surgery alone arm (28.1%) (HR, 0.77; p = .041). When patients who were unresectable were excluded from the analysis (17% in both arms), those who completed surgery had a significantly higher 3-year PFS rate, 33.2% versus 42.4% (HR, 0.73; p = .025). Seventy-nine percent of those allocated to chemotherapy completed the planned six preoperative cycles, and 44% completed the planned six postoperative cycles, suggesting that this regimen is not well tolerated, at least after liver resection. Chemotherapy led to a higher postoperative complication rate (25% versus 16%; p = .04), but all complications were reversible. Seven percent of the patients randomized to neoadjuvant chemotherapy were rendered unresectable because of progressive disease while on treatment. That study indicated a benefit for perioperative chemotherapy, but it is still uncertain whether the benefit seen relates to the neoadjuvant or adjuvant component of the chemotherapy.
Better patient selection will allow patients to receive the greatest benefit from neoadjuvant chemotherapy. A retrospective review of data from the LiverMetSurvey International Registry by Adam et al. [28] analyzed 1,417 patients with initially resectable, metachronous, single lesion, liver-limited mCRC who had either upfront surgery or neoadjuvant chemotherapy and then surgery. The OS rate in both the surgery only group and the surgery plus chemotherapy group was 60% (p = .57). In those with tumors >5 cm, postoperative chemotherapy resulted in a higher 5-year OS rate than in those who did not receive postoperative chemotherapy (58% versus 33%; p < .01). In those with a lesion <5 cm, neoadjuvant chemotherapy did not affect the survival outcome.
A study titled “A Trial in the Timing of Surgery and Adjuvant Chemotherapy for Hepatic Metastases from Colorectal Cancer (ATTACHE), developed by the Australasian Gastrointestinal Trials Group (AGITG), is currently recruiting and will address this issue of timing of chemotherapy in relation to surgery. It is a RCT comparing EORTC-style preoperative and adjuvant chemotherapy with adjuvant chemotherapy alone using oxaliplatin and 5-FU or capecitabine in patients with initially operable liver metastases from CRC. A small nonrandomized comparison of neoadjuvant or adjuvant chemotherapy in patients with initially resectable liver metastases did not find an OS or DFS difference between the strategies [29].
Can Chemotherapy Reliably Convert Unresectable Metastases to Resectable?
For the last two decades, some broad-based trials in mCRC have included patients with liver metastases that became resectable with curative intent after a period of chemotherapy, and around 6%–9% of these were resected [11, 14]. In this setting of potentially resectable liver metastases, tumor response is critical to allow surgery to occur [30]. De Gramont et al. [11] confirmed that the addition of oxaliplatin to 5-FU–LV was beneficial, resulting in a 50.7% response rate, versus 22.3% using 5-FU–LV alone, with maximum response at 9 weeks and 12 weeks, respectively. In a randomized comparison, FOLFOX6 and FOLFIRI were equivalent in the metastatic setting, with response rates of 54% and 56% and median times to response of 1.8 months and 2.1 months [14]. These data suggest that over half of all patients with potentially resectable disease could be rendered resectable by pretreatment with irinotecan- or oxaliplatin-based chemotherapy.
In studies of patients specifically selected with the aim of curative resection, when the initial disease is borderline or unresectable, the resection rates after chemotherapy are actually much higher than in unselected patient groups. Adam et al. [7] published a case series of 1,104 patients with initially unresectable liver metastases who were given chemotherapy, in which 12.5% were able to be resected. In another French study, by Bismuth et al. [31], including 330 patients, neoadjuvant FOLFOX allowed a 16% resection rate, with 36% of those resected surviving disease free after a median of 42 months of follow-up. Chemotherapy was given for a median of 8 months preoperatively and for 6 months postoperatively. In a similar Italian study in 40 patients treated with preoperative FOLFIRI for 3–6 months followed by 3 months of postoperative FOLFIRI, 13 (32.5%) became resectable and the median DFS duration was 14 months [32]. A 70.4% response rate was achieved in a more recent study using 5-FU–LV, oxaliplatin, and irinotecan (FOLFOXIRI) in this population of patients with potentially resectable metastases to the liver, lung, peritoneum, and lymph nodes [33]. Nineteen percent of patients were resected and the 5-year DFS rate was 29%, at a cost of more toxicity. In addition, the four studies described above have shown that repeat hepatic resection for recurrent liver metastases is feasible and effective [7, 31–33].
The value of the addition of biological agents to chemotherapy in the setting of resectable or potentially resectable liver metastases remains uncertain. Results with biological agents have been variable in the limited literature available, comprising mostly phase I–II data. First-line bevacizumab plus oxaliplatin and 5-FU leads to a longer PFS interval in mCRC patients than with oxaliplatin and 5-FU plus placebo, but it does not influence the response rate or OS time [18]. In that study population, who were not selected with the aim of resecting metastases, 8.4% of patients in the bevacizumab-containing arm became resectable, versus 6.1% of patients in the placebo arm (p-value not reported). The number who actually had an R0 resection was not available at the time of the report. In a phase II study of 58 patients selected as potentially resectable and given neoadjuvant capecitabine and bevacizumab, the response rate was 73.2%, with only 5.4% progressing during the six preoperative cycles [34]. Metastasectomy was performed on 92% of these high-risk patients. In that study, bevacizumab was withheld 5 weeks before and 5 weeks after surgery, and there were low rates of adverse effects, as listed in Table 1. Six percent of patients required blood transfusions. Collectively, these studies demonstrated that bevacizumab is feasible in the perioperative setting, with no material impact on surgical complication rates, and it might possibly contribute to better outcomes. However, efficacy needs to be tested formally in a RCT.
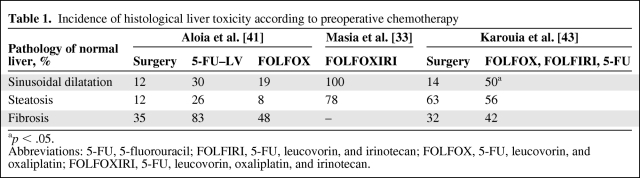
Table 1.
Incidence of histological liver toxicity according to preoperative chemotherapy
ap < .05.
Abbreviations: 5-FU, 5-fluorouracil; FOLFIRI, 5-FU, leucovorin, and irinotecan; FOLFOX, 5-FU, leucovorin, and oxaliplatin; FOLFOXIRI, 5-FU, leucovorin, oxaliplatin, and irinotecan.
Cetuximab has been studied in the setting of resectable or potentially resectable liver metastases. Van Cutsem et al. [19] added cetuximab to FOLFIRI, which allowed metastasectomy in 7.0% of patients, versus 3.7% of patients in the FOLFIRI alone arm, with a low R0 resection rate (4.8% versus 1.7% of the total population). However, these patients had various manifestations of mCRC and were not selected as potential liver resection candidates. A randomized phase II comparison of cetuximab with either FOLFOX6 or FOLFIRI in 114 patients with potentially resectable liver-limited mCRC showed that downstaging and subsequent R0 resection were possible in 38% and 30% of patients, respectively [35]. A higher response rate was noted when the patients were retrospectively stratified by KRAS mutational status, and resectability in the wt KRAS group increased from 32% at baseline to 60% after chemotherapy. Toxicity from chemotherapy included skin reactions, neutropenia, and neuropathy; one patient died as a result of a pulmonary embolus and one died as a result of disease progression and diarrhea. The operative complications were not substantially different. Again, that study indicates that preoperative cetuximab is feasible and might contribute to greater resectability in the wt KRAS subgroup, but further confirmatory studies are required before a definitive conclusion can be reached.
Will Neoadjuvant Treatment Increase Surgical Complications?
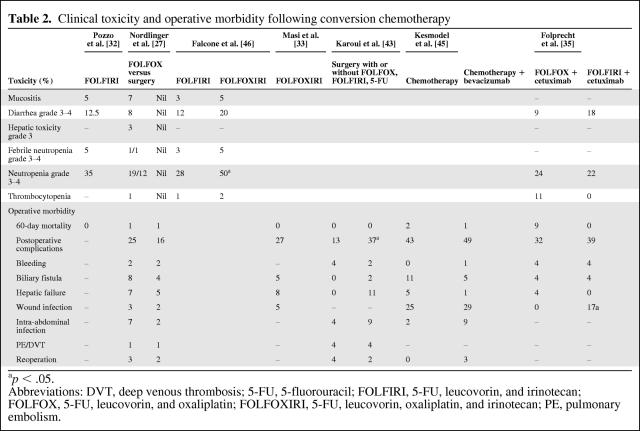
The safety of preoperative chemotherapy is of concern to liver surgeons, because perioperative mortality and morbidity have the potential to be exaggerated by drug toxicity. There are reports that both irinotecan- and oxaliplatin-based regimens damage the liver and affect morbidity (Tables 1 and 2) [27, 32, 33, 35, 45, 46]. Whether or not judiciously administered chemotherapy affects long-term outcomes is still the subject of debate and clinical investigation.
Table 2.
Clinical toxicity and operative morbidity following conversion chemotherapy
ap < .05.
Abbreviations: DVT, deep venous thrombosis; 5-FU, 5-fluorouracil; FOLFIRI, 5-FU, leucovorin, and irinotecan; FOLFOX, 5-FU, leucovorin, and oxaliplatin; FOLFOXIRI, 5-FU, leucovorin, oxaliplatin, and irinotecan; PE, pulmonary embolism.
The form of steatosis associated with irinotecan is categorized as nonalcoholic steatohepatitis (NASH), and the subsequent accumulation of lipids in the liver causes fibrosis [36]. Although the precursor to NASH, fatty liver, is most often benign, inflammation associated with NASH leads to cirrhosis in 9%–20% of cases, of whom 40%–60% develop liver failure or hepatoma within 5–7 years. Considering that fatty liver is the most common form of chronic liver disease in the western world, its presence has implications for the agents being given. FOLFIRI has been implicated in steatohepatitis in 4%–28% of patients, and in one study, those with steatohepatitis had a higher 90-day mortality rate (14.7% versus 1.6%) [37, 38]. A 2010 review analyzed 334 consecutive surgical specimens and showed a 33% rate of steatosis, but the only independent risk factor identified was a body mass index >30 kg/m2, and the 50 patients who were given neoadjuvant irinotecan did not have a significantly higher rate of steatosis or steatohepatitis [39]. NASH is more likely with longer durations and higher total doses of irinotecan, as shown in Figure 1, so clinicians should exercise caution in its use in the perioperative setting.
Figure 1.
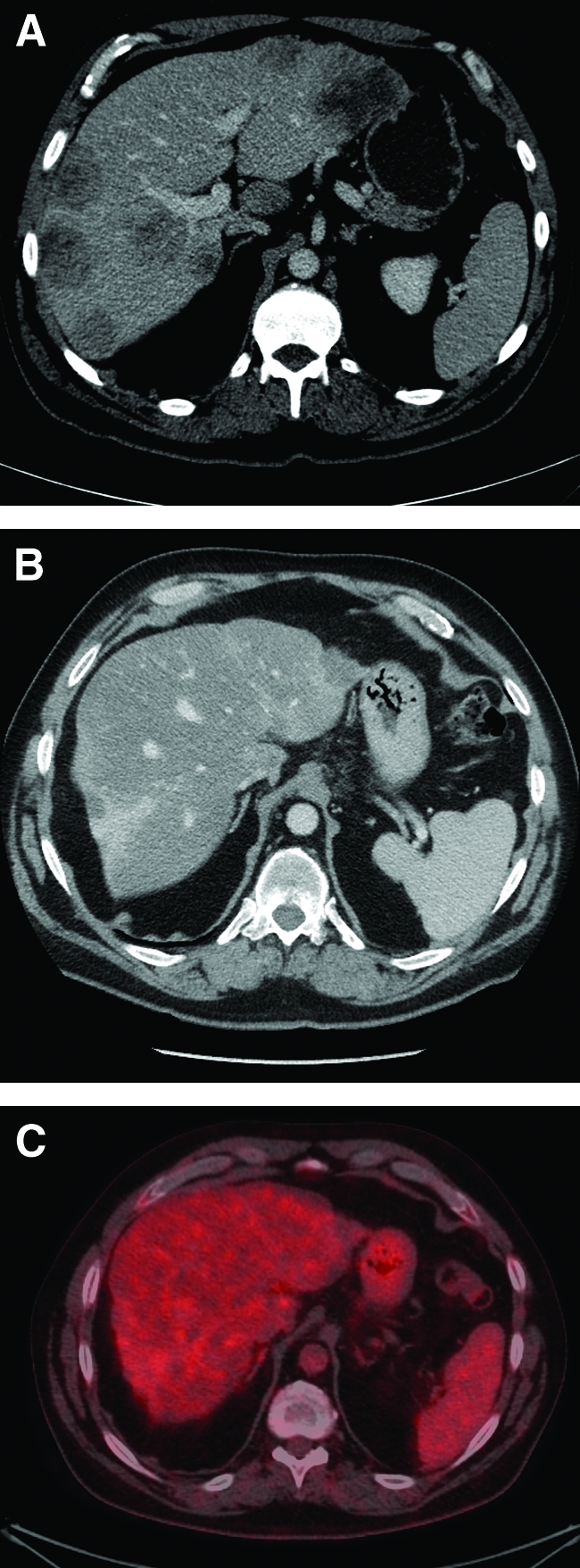
Liver metastases before and after combination chemotherapy. (A): Pretreatment portal venous phase computed tomography (CT) scan showing extensive bilobar liver metastases. (B): Post-treatment portal venous phase CT scan showing fibrosteatotic replacement of metastases and nodular, contracted, steatotic liver, consistent with chemotherapy-associated steatohepatitis. Patient had received 12 months of capecitabine, irinotecan, and bevacizumab. (C): Fused CT–18F-fluorodeoxyglucose positron emission tomography scan after 12 months of chemotherapy showing complete response of liver metastases.
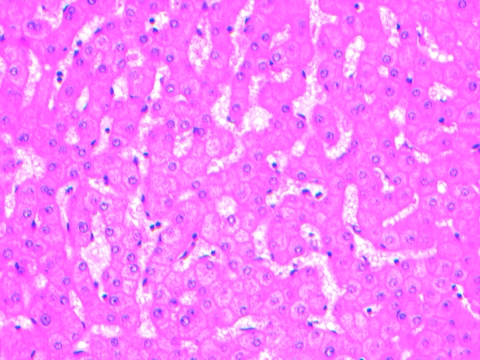
Oxaliplatin was shown to cause sinusoidal dilatation (Fig. 2), perisinusoidal fibrosis, and fibrotic venular occlusion in a histopathology series by Rubbia-Brandt et al. [40]. In that report, 78% of patients had sinusoidal dilatation, but no correlation with morbidity or mortality was reported. There is conflicting evidence about whether or not FOLFOX influences the rates of bleeding, wound infection, or perioperative death [27]. A 2006 series by Vauthey et al. [38] examined biopsies after FOLFOX, showing that 19% had sinusoidal dilatation, but no greater surgical morbidity or mortality was seen. Aloia et al. [41] found that, in patients treated with FOLFOX, 19% had sinusoidal dilatation, which was associated with greater morbidity but did not affect mortality. In the EORTC 40983 study, the surgical complications with significant differences between preoperative FOLFOX chemotherapy and surgery alone were overall morbidity (25% versus 16%), biliary fistulae (8% versus 4%), hepatic failure (7% versus 5%), and intra-abdominal infection (7% versus 2%), but the postoperative mortality rate was not higher [27]. A more recent case series of 196 patients correlated liver histology with long-term outcomes and found that those treated with oxaliplatin, with a tumor size >5 cm, and with an elevated alkaline phosphatase or γ-glutamyl-transferase level were more likely to develop grade 2 or 3 sinusoidal dilatation and had a significantly shorter relapse-free survival interval (HR, 2.05; p = .005) and a higher rate of intrahepatic recurrence (66.7% versus 30.5%; p = .003) [42]. In that study, NASH and fibrosis were not associated with a worse survival outcome.
Figure 2.
Sinusoidal dilatation in liver parenchyma of a patient who had received eight cycles of neoadjuvant 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) prior to liver resection. This specimen was taken from tissue unaffected by metastases. Hematoxylin and eosin stain. Image courtesy of Dr. Anna Price, Department of Anatomical Pathology, John Hunter Hospital, NSW, Australia.
A higher response rate can be achieved by combining oxaliplatin, irinotecan, and 5-FU–LV, but at a cost of greater toxicity. A review of liver histology adjacent to metastases after neoadjuvant FOLFOXIRI showed that 100% of specimens had sinusoidal dilatation (grade 1, 52%; grade 2, 48%), 78% had steatosis (grade 3, 5%), and 5% had steatohepatitis [33]. There were no deaths in the 3 months after surgery. Up to six cycles (3 months) of chemotherapy has been shown to be safe, but a longer duration of chemotherapy results in a greater surgical morbidity rate [43]. That analysis suggests that short preoperative regimens using all three drugs are feasible and might be a way forward in significantly downstaging liver metastases before resection.
The changes caused by neoadjuvant chemotherapy can persist for up to 4 months; however, the surgical risk appears highest in the month after the last dose. A case series of 750 consecutive hepatic resections demonstrated that, if surgery is performed within 4 weeks of chemotherapy, there is an 11% complication rate, compared with a 5.5% rate at 5–8 weeks and a 2.6% rate at 9–12 weeks postoperatively (p = .0009) [44].
Bevacizumab can cause bleeding, delayed wound healing, and bowel perforation, but if it is withheld for 4–6 weeks before and after surgery, complication rates are not greater [45]. Cetuximab has not been shown to cause greater surgical morbidity and mortality when combined with chemotherapy before liver resection [11, 19].
Who Should Have Their Liver Resected?
An expert consensus statement of criteria for technical resectability proposed that a minimum of 20% of the initial liver volume should remain after surgery (or greater if there is pre-existing liver disease), formed of at least two contiguous segments with adequate biliary drainage and vascular inflow and outflow [10]. Several scores have been used in an attempt to prospectively evaluate and select patients who are most likely to benefit from liver resection. An evaluation of the scores proposed by Nordlinger, Fong, Iwatsuki and the Mayo Clinic did not reveal one that was clearly better, showing prediction of the 5-year survival rate to be slightly better than chance [47]. An additional predictive score, the Basingstoke Index, describes six preoperative criteria that stratify the risk for recurrence and cancer-specific mortality [5]. The predictors of poor survival were: more than three hepatic metastases, a node-positive primary, a poorly differentiated primary, extrahepatic disease, tumor diameter ≥5 cm, and a carcinoembryonic antigen (CEA) level >60 ng/mL. Patients in the worst prognostic category had a median cancer-specific survival time of 0.7 years, compared with those in the best category, who had a median cancer-specific survival time of 7.4 years. These clinical risk scores are not predictive prior to neoadjuvant chemotherapy but have been found to be predictive after neoadjuvant therapy has been given, prior to liver resection being performed [48].
The appropriate selection of patients for liver resection is critical if the outcomes seen in clinical trials are to be reproduced in the general clinical setting. The OncoSurge model is a computer program protocol for resectability that was developed using a validated method to assess 252 cases being considered for hepatic resection [49]. Upfront resection was appropriate in those who had four or fewer metastases in an otherwise normal liver, unilobar involvement, adequately radiologically defined margins, and the absence of portal adenopathy. Surgery after neoadjuvant chemotherapy was suggested if there was a response in those with more than four lesions and those with portal adenopathy. Absolute contraindications are liver failure, unresectable extrahepatic disease, >70% liver involvement, and being surgically unfit. Factors that did not influence the treatment strategy were age, primary tumor stage, timing of metastasis detection, past blood transfusion, liver resection type, preresection CEA level, and previous hepatectomy. The OncoSurge model was validated against the recommendations made for 98 patients at a British hepatobiliary referral center multidisciplinary meeting, showing concordance in 93 of the 98 cases (κ = 0.850) [50]. It is a useful tool for hepatobiliary surgeons to facilitate management decisions, particularly when there is uncertainty about the risk–benefit ratio of liver resection.
The small number of patients (4%) in whom liver lesions disappear with preoperative chemotherapy represents a management challenge for surgeons (Fig. 1). In those who manifest a complete radiologic response, resection of the affected liver segments should still be performed because there is a poor correlation with pathologic response, with the vast majority of patients having viable tumor still present [51]. A randomized trial of 150 patients with mCRC who were being considered for liver resection showed that combined 18F-fluorodeoxyglucose positron emission tomography (PET) and computed tomography (CT) led to fewer futile laparotomies than with CT alone (28% versus 45% futile laparotomies; p = .042) [52]. A futile laparotomy was defined as subtotal tumor clearance, having revealed benign disease, or a DFS period <6 months. A systematic review of studies comparing CT with PET–CT for detecting liver metastases from CRC found that PET–CT had a higher accuracy for the detection of extrahepatic and hepatic metastases and for predicting local recurrence [53]. An observational study of 100 patients who had PET–CT staging prior to liver resection demonstrated a 5-year OS rate of 58%, which is superior to comparable historical survival data [54]. Of the cohort studied, 52 patients had synchronous metastases, 63 patients had single lesions, lesions were unilateral in 78 patients, lesions were <5 cm in diameter in 60 patients, margins were ≥1 cm in 52 patients, and 75 patients had a major resection (more than three segments). None of the studies described above involved patients who were given neoadjuvant chemotherapy. PET imaging has less sensitivity if performed within 4 weeks of the last dose of neoadjuvant chemotherapy, which limits its ability to assess response.
Second and third resection of recurrent liver metastases is possible in a subset of patients, leading to a 39% 5-year survival rate in those with hepatic only recurrence [55]. In this small subgroup, R0 resection and an interval >1 year between hepatectomies were independent predictors of survival [56].
The Role of Adjuvant Chemotherapy After Liver Resection
Given that there is established evidence for adjuvant treatment of node-positive CRC, it could be assumed that adjuvant therapy after liver resection is mandatory, but this has not been proven to be the case. The Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colorectal Cancer (MOSAIC) trial showed that the 5-year DFS rate using FOLFOX was superior to that of 5-FU–LV (66.4% versus 58.9%; p = .005) in patients with resected stage III CRC [57]. Irinotecan plus 5-FU–LV has not been shown to be superior to 5-FU–LV in the adjuvant setting [58]. The overall mortality rate is 40% higher if there is a delay ≥2 months before adjuvant chemotherapy is started [59]. In one study, after resection of CRC liver metastases, adjuvant 5-FU–LV resulted in a greater 4-year DFS rate, 42% versus 32% (p = .28), than with surgery alone, but did not affect the OS outcome [60]. A systematic review and meta-analysis of four studies of perioperative chemotherapy around the time of liver resection found no OS benefit (HR, 0.74; 95% confidence interval [CI], 0.53–1.04) but did find a recurrence-free survival benefit of 25% (HR, 0.75; 95% CI, 0.62–0.91) [61]. The OS results did not include the larger EORTC study, which may change the findings. The studies were of the older 5-FU regimen, except for the EORTC study, which used FOLFOX4. A comparison of FOLFIRI and 5-FU–LV showed no DFS or OS benefit and additional toxicity with the irinotecan-containing regimen [62]. A retrospective analysis of FOLFOX or FOLFIRI versus 5-FU–LV for metachronous disease showed a benefit to the pooled results of the former, but the majority of patients did not have previous oxaliplatin- or irinotecan-containing regimens [63]. Bevacizumab added to modified (m)FOLFOX6 and cetuximab added to mFOLFOX6 in patients with wt KRAS tumors have not been shown to have benefit in the adjuvant setting after resection of stage II or stage III CRC [64, 65]. Therefore, the addition of either of these biological agents to chemotherapy after liver resection cannot be supported until there are high quality data available. The upcoming AGITG ATTACHE study is anticipated to determine if neoadjuvant therapy adds a PFS benefit to adjuvant therapy alone.
The Role of Local Treatments for Liver Metastases
Local ablative methods may be employed in the management of liver metastases from mCRC, if resection is not considered optimal, or if curative resection is not feasible. The options include radiofrequency ablation (RFA), portal vein embolization (PVE), hepatic arterial infusional (HAI) chemotherapy, yttrium-90 embolization, cryotherapy, and microwave therapy. The results for RFA are variable, with 5-year survival rates of 14%–55% and local recurrence rates 3.6%–60%, with low morbidity and mortality [66]. Resection produces a better survival outcome than resection plus RFA or RFA alone [67]. Selective PVE involves obstructing the right or left portal vein to cause atrophy of the half of the liver supplied by that vein and hypertrophy of the liver remnant [68]. This technique can allow surgery in those who would otherwise not have been surgical candidates because of inadequate hepatic reserve. A two-stage hepatectomy can be performed if the liver metastases cannot be removed in one procedure, whereby metastases within the future liver remnant are resected and after 2–3 months a major hepatectomy of the lobe with the remaining metastases is performed [69].
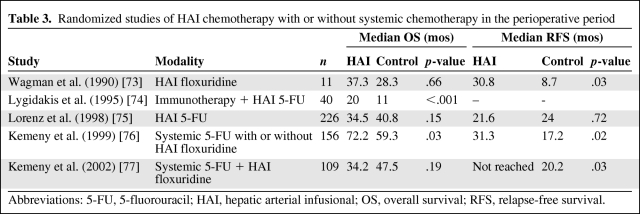
HAI chemotherapy is delivered by a surgically implanted catheter into the segment of the liver affected by metastases. It delivers the dose directly to the tumor on the premise that macrometastases (>0.5 cm) derive >80% of their blood supply from the hepatic artery, in contrast to hepatocytes, which are supplied by the portal circulation [70]. Table 3 shows results from randomized studies of HAI chemotherapy [73–77]. A systematic review of seven studies involving 592 patients receiving HAI chemotherapy in the adjuvant setting did not find any OS benefit (HR, 1.089; 95% CI, 0.887–1.334) [71]. Radiation can be delivered to metastases via infusion of yttrium-90 microspheres (SIR-Spheres) into the hepatic artery, which lodge in the hepatic microcirculation, but this is mainly used in the palliative setting [72].
Table 3.
Randomized studies of HAI chemotherapy with or without systemic chemotherapy in the perioperative period
Abbreviations: 5-FU, 5-fluorouracil; HAI, hepatic arterial infusional; OS, overall survival; RFS, relapse-free survival.
What Is the Optimal Timing of Liver Resection?
In those who present with synchronous primary and metastases, controversy exists over which patients should have simultaneous versus staged surgery. Martin et al. [78] consider resection of the primary lesion and liver metastases at the same time as being preferable if adjuvant chemotherapy is planned, to minimize the delay before starting systemic treatment. In that retrospective series comparing simultaneous with staged surgery, there was no obvious difference in surgical outcomes except for length of stay. The liver lesion size and number and the proportion of major hepatectomies (32% versus 33%) were similar, and the length of stay was 10 days for simultaneous versus 18 days for staged procedures (p = .001). Contrary to this view, a retrospective review of 135 simultaneous and 475 staged procedures showed that the mortality rate was similar if a minor hepatectomy was performed (1% for simultaneous versus 0.5% for staged), but there was a higher mortality rate in those who had a major hepatectomy performed at the same time as colectomy (8.3% for simultaneous versus 1.4% for staged) [79]. A systematic review of 16 controlled trials showed that the 5-year survival rates in patients with staged and synchronous resections are similar; however, the groups are not directly comparable and there are no randomized data [80]. Synchronous surgery has traditionally been limited to right-sided primaries and those with less extensive hepatic disease, but Capussotti et al. [81] challenged this view with a report of safe and effective major hepatectomies for left-sided and rectal tumors. In that study of 79 patients, they found that the overall morbidity rate was higher (56.3% versus 32.6%; p = .0369) and hospital stay was longer (20.5 days versus 13.9 days; p = .00001) in the delayed surgery group when both procedures were considered together. The rates of blood and plasma transfusions were higher in patients with synchronous resections—41.9% versus 16.7% (p = .0131) and 54.8% versus 31.3% (p = .0370), respectively. Patients who have larger or more numerous liver lesions, comorbidities, and left-sided colonic tumors are more likely to have a staged resection; however, with the above data there is a trend toward extending the criteria for simultaneous surgery [80].
There is evidence that recurrence rates are worse, and possibly similar to those seen with observation alone, if there is a delay >8 weeks in starting adjuvant chemotherapy after resection of a colonic primary without metastases [82]. This suggests that a simultaneous procedure may be preferential in those who are planned to have adjuvant chemotherapy, because a staged procedure would cause a substantial delay in starting chemotherapy. Alternatively, these patients may benefit from neoadjuvant chemotherapy, although there are no data to support this approach. Some advocate a delay in liver resection to allow the biological behavior of the tumor to become evident [83]. No difference in survival outcome was seen in a retrospective review of 73 patients who were either operated on immediately or given a period of observation, although that study was not likely to have detected modest-sized effects [84]. A high proportion of patients considered eligible for liver resection (especially those patients with poor prognostic features) are likely to have occult metastases at the time of initial evaluation. If, in the waiting period, new liver lesions develop outside the proposed resection margins or other metastatic sites develop, then upfront surgery is unlikely to result in a survival benefit.
If neoadjuvant chemotherapy is given, the intention is to provide maximal shrinkage of liver lesions to facilitate surgery as well as to control micrometastatic disease. Ideally, the duration of treatment would be long enough to maximize response but short enough to minimize toxicity and surgical morbidity. In a study by de Gramont et al. [11], the median time to response with FOLFOX was 9 weeks. Time to maximal response is seldom reported in large, randomized trials. A study of 35 patients on neoadjuvant FOLFOX and bevacizumab found that the maximal response occurred at 2–4 months, with no significant incremental response at 4–6 months [85]. Three months of chemotherapy has been shown to be safe, as described above, and so 9–16 weeks of treatment seems ideal.
It is less clear what should be done if adjuvant therapy has been given after resection of a stage III primary colonic tumor and hepatic metastases develop at a later date. If chemotherapy is required, the choice of regimen is based on expert opinion—whether to retreat with the same drugs or to switch to an alternative on the assumption that the initial agents did not provide sufficient disease control. There are no studies reported or under way that address this question. A disease-free interval <6 months normally suggests resistance and that a second-line regimen should be considered. When isolated liver metastases recur after partial hepatectomy, second and third liver resections are reported as safe and continue to result in superior 5-year OS rates, with survival rates of 34% and 32%, respectively [86, 87]. Adam et al. [87] reported on a prospective database of 883 hepatectomies, of which 615 were first, 199 (32%) were second, 60 (30% of those who had a second resection) were third, and nine (15% of those who had a third resection) were fourth resections of liver metastases, showing a favorable survival outcome compared with those who did not have repeat resections. This approach is aggressive, and in many centers a very small minority of patients would be offered so many repeat procedures.
Conclusion
For patients who present with mCRC confined to the liver, resection of the primary tumor and the metastases should be performed in a simultaneous procedure if feasible. Those who have bilobar metastases, significant comorbidities, a rectal primary, more than three metastases, a technically difficult colonic resection, or are treated in centers where there is no access to a hepatobiliary unit should be considered for a staged resection. In those with high-risk features, neoadjuvant chemotherapy provides an opportunity to gather information on the biological activity of the tumor and its chemoresponsiveness, in addition to improving resectability. The aim is to avoid a futile liver resection; however, there is evidence that, despite progression on chemotherapy in initially resectable disease, the 5-year survival rates are respectable. Local treatments such as RFA and HAI chemotherapy are inferior to R0 hepatectomy, but when used judiciously they prolong survival in patients with unresectable disease. PVE can cause hypertrophy of future liver remnant to allow sufficient hepatic reserve after resection.
Perioperative chemotherapy results in longer DFS and OS times. It is not clear whether it is the neoadjuvant or the adjuvant component that provides the benefit. There is likely to be a subgroup of patients who benefit from having neoadjuvant chemotherapy, but it is not recommended for all patients with initially resectable disease. It is a reasonable approach to give neoadjuvant and adjuvant FOLFOX with or without bevacizumab to high-risk patients with initially resectable disease. These include those with multiple lesions and lesions >5 cm. Enthusiasm for giving all resectable patients neoadjuvant chemotherapy is tempered by liver toxicity, greater morbidity and possibly mortality, and the risk that a patient may progress and become unresectable in the intervening period.
In patients who are initially unresectable, there are effective options for conversion into surgical candidates. FOLFOX plus bevacizumab is effective and has a favorable side-effect profile, whereas irinotecan carries a higher risk for hepatic toxicity. Cetuximab and FOLFIRI or FOLFOX can be considered for patients with wt KRAS tumors as conversion therapy; however, irinotecan causes a higher risk for steatohepatitis with an attendant slightly higher surgical complication rate. A maximum of six cycles of chemotherapy is recommended. The role of purely adjuvant therapy after initial resection of liver metastases, whether synchronous or metachronous, is not well defined. Adjuvant FOLFOX is recommended on the basis of data in patients with stage III CRC, and studies such as ATTACHE will clarify the role of chemotherapy in this subgroup of patients. Cetuximab and bevacizumab currently do not have evidence to support their use after liver resection. In patients who have had adjuvant chemotherapy and experience a hepatic recurrence that is resected, it is not clear if there is a benefit to adjuvant therapy, let alone which agents to use. FOLFOX or XELOX or FOLFIRI or XELIRI for six cycles is recommended. Depending on the initial response and disease free interval, a regimen may be reused or an alternative strategy may be pursued. What is known is that liver resection provides the only reasonable possibility for cure of mCRC and that the rate of resection can be increased by judicious use of cytotoxic and targeted agents.
Studies are under way to investigate the role of neoadjuvant and adjuvant cetuximab and bevacizumab, the combination of chemotherapy and HAI chemotherapy in the adjuvant setting, whether or not neoadjuvant and adjuvant chemotherapy is better than adjuvant chemotherapy alone, and the role of bevacizumab and chemotherapy then surgery and RFA.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Nicholas Zdenkowski, Stephen Ackland
Collection and/or assembly of data: Nicholas Zdenkowski, Stephen Ackland
Data analysis and interpretation: Nicholas Zdenkowski, Stanley Chen, Stephen Ackland, Andre van der Westhuizen
Manuscript writing: Nicholas Zdenkowski, Stanley Chen, Stephen Ackland
Final approval of manuscript: Nicholas Zdenkowski, Stephen Ackland, Andre van der Westhuizen
References
- 1.Ferlay J, Shin HR, Bray F, et al. Lyon, France: International Agency for Research on Cancer; [accessed June 26, 2011]. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Available at http://globocan.iarc/fr. [Google Scholar]
- 2.Australian Institute of Health and Welfare (AIHW) & Australasian Association of Cancer Registries. Cancer in Australia: An Overview, 2010. Cancer Series No. 60. Cat. No. CAN 56. Canberra: AIHW:1–228. [Google Scholar]
- 3.Cancer in Australia: A Snapshot, 2004–2005. Australian Bureau of Statistics. 2006. [accessed January 18, 2011]. Available at http://www.abs.gov.au.
- 4.Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007, based on November 2009 SEER data submission, posted to the SEER Web site 2010. Bethesda, MD: National Cancer Institute. [accessed June 6, 2010]; Available at http://seer.cancer.gov/csr/1975_2007/ [Google Scholar]
- 5.Rees M, Tekkis PP, Welsh FK, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network (NCCN) NCCN Guidelines, Colorectal Cancer, Version 2 2010. [accessed June 21, 2010]. Available at http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- 7.Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: A model to predict long-term survival. Ann Surg. 2004;240:644–657. doi: 10.1097/01.sla.0000141198.92114.f6. discussion 657–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-Year Survival After Resection of Colorectal Liver Metastases Defines Cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 9.Simmonds PC, Primrose JN, Colquitt JL, et al. Surgical resection of hepatic metastases from colorectal cancer: A systematic review of published studies. Br J Cancer. 2006;94:982–999. doi: 10.1038/sj.bjc.6603033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charnsangavej C, Clary B, Fong Y, et al. Selection of patients for resection of hepatic colorectal metastases: Expert consensus statement. Ann Surg Oncol. 2006;13:1261–1268. doi: 10.1245/s10434-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 11.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 12.Saltz LB, Cox JV, Banke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 13.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 14.Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 15.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 16.Sobrero A, Ackland S, Clarke S, et al. Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer. The Oncologist. 2009;77:113–119. doi: 10.1159/000229787. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs CS, Marshall J, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-Line treatment of metastatic colorectal cancer: Updated results from the BICC-C study. J Clin Oncol. 2008;26:689–690. doi: 10.1200/JCO.2007.15.5390. [DOI] [PubMed] [Google Scholar]
- 18.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 19.Van Cutsem E, Köhne C, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 20.Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: The OPUS study. Ann Oncol. 2011;22:1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 21.Kemeny N. Presurgical chemotherapy in patients being considered for liver resection. The Oncologist. 2007;12:825–839. doi: 10.1634/theoncologist.12-7-825. [DOI] [PubMed] [Google Scholar]
- 22.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 23.Adam R, Pascal G, Castaing D, et al. Tumor progression while on chemotherapy: A contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1061. doi: 10.1097/01.sla.0000145964.08365.01. discussion 1061–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adam R, Wicherts DA, de Haas RJ, et al. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: Myth or reality? J Clin Oncol. 2008;26:1635–1641. doi: 10.1200/JCO.2007.13.7471. [DOI] [PubMed] [Google Scholar]
- 25.Allen PJ, Kemeny N, Jarnagin W, et al. Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J Gastrointest Surg. 2003;7:109–115. doi: 10.1016/S1091-255X(02)00121-X. discussion 116–117. [DOI] [PubMed] [Google Scholar]
- 26.Neumann UP, Thelen A, Röcken C, et al. Nonresponse to pre-operative chemotherapy does not preclude long-term survival after liver resection in patients with colorectal liver metastases. Surgery. 2009;146:52–59. doi: 10.1016/j.surg.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adam R, Bhangui P, Poston G, et al. Is perioperative chemotherapy useful for solitary, metachronous, colorectal liver metastases? Ann Surg. 2010;252:774–787. doi: 10.1097/SLA.0b013e3181fcf3e3. [DOI] [PubMed] [Google Scholar]
- 29.Lubezky N, Geva R, Shmueli E, et al. Is there a survival benefit to neoadjuvant versus adjuvant chemotherapy, combined with surgery for resectable liver metastases? World J Surg. 2009;33:1028–1034. doi: 10.1007/s00268-009-9945-1. [DOI] [PubMed] [Google Scholar]
- 30.Folprecht G, Grothey A, Alberts S, et al. Neoadjuvant treatment of unresectable colorectal liver metastases: Correlation between tumour response and resection rates. Ann Oncol. 2005;16:1311–1319. doi: 10.1093/annonc/mdi246. [DOI] [PubMed] [Google Scholar]
- 31.Bismuth H, Adam R, Lévi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–520. doi: 10.1097/00000658-199610000-00009. discussion 520–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pozzo C, Basso M, Cassano A, et al. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004;15:933–939. doi: 10.1093/annonc/mdh217. [DOI] [PubMed] [Google Scholar]
- 33.Masi G, Loupakis F, Pollina L, et al. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg. 2009;249:420–425. doi: 10.1097/SLA.0b013e31819a0486. [DOI] [PubMed] [Google Scholar]
- 34.Gruenberger B, Tamandl D, Schueller J, et al. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol. 2008;26:1830–1835. doi: 10.1200/JCO.2007.13.7679. [DOI] [PubMed] [Google Scholar]
- 35.Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 36.Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11:1–16. doi: 10.1016/j.cld.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Cleary JM, Tanabe KT, Lauwers GY, et al. Hepatic toxicities associated with the use of preoperative systemic therapy in patients with metastatic colorectal adenocarcinoma to the liver. The Oncologist. 2009;14:1095–1105. doi: 10.1634/theoncologist.2009-0152. [DOI] [PubMed] [Google Scholar]
- 38.Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 39.Ryan P, Nanji S, Pollett A, et al. Chemotherapy-induced liver injury in metastatic colorectal cancer: Semiquantitative histologic analysis of 334 resected liver specimens shows that vascular injury but not steatohepatitis is associated with preoperative chemotherapy. Am J Surg Pathol. 2010;34:784–791. doi: 10.1097/PAS.0b013e3181dc242c. [DOI] [PubMed] [Google Scholar]
- 40.Rubbia-Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 41.Aloia T, Sebagh M, Plasse M, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal liver metastases. J Clin Oncol. 2006;24:4983–4990. doi: 10.1200/JCO.2006.05.8156. [DOI] [PubMed] [Google Scholar]
- 42.Tamandl D, Klinger M, Eipeldauer S, et al. Sinusoidal obstruction syndrome impairs long-term outcome of colorectal liver metastases treated with resection after neoadjuvant chemotherapy. Ann Surg Oncol. 2011;18:421–430. doi: 10.1245/s10434-010-1317-4. [DOI] [PubMed] [Google Scholar]
- 43.Karoui M, Penna C, Amin-Hashem M, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1–7. doi: 10.1097/01.sla.0000193603.26265.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welsh FKS, Tilney HS, Tekkis PP, et al. Safe liver resection following chemotherapy for colorectal metastases is a matter of timing. Br J Cancer. 2007;96:1037–1042. doi: 10.1038/sj.bjc.6603670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kesmodel SB, Ellis LM, Lin E, et al. Preoperative bevacizumab does not significantly increase postoperative complication rates in patients undergoing hepatic surgery for colorectal cancer liver metastases. J Clin Oncol. 2008;26:5254–5260. doi: 10.1200/JCO.2008.17.7857. [DOI] [PubMed] [Google Scholar]
- 46.Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: The Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 47.Zakaria S, Donohue JH, Que FG, et al. Hepatic resection for colorectal metastases: Value for risk scoring systems? Ann Surg. 2007;246:183–191. doi: 10.1097/SLA.0b013e3180603039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ayez N, Lalmahomed ZS, van der Pool AE, et al. Is the clinical risk score for patients with colorectal liver metastases still useable in the era of effective neoadjuvant chemotherapy? Ann Surg Oncol. 2011;18:2757–2763. doi: 10.1245/s10434-011-1819-8. DOI 10.1245/s1434–011-1819–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poston GJ, Adam R, Alberts S, et al. OncoSurge: A strategy for improving resectability with curative intent in metastatic colorectal cancer. J Clin Oncol. 2005;23:7125–7134. doi: 10.1200/JCO.2005.08.722. [DOI] [PubMed] [Google Scholar]
- 50.O'Reilly DA, Chaudhari M, Ballal M. The OncoSurge strategy for the management of colorectal liver metastases—an external validation study. Eur J Surg Oncol. 2008;34:538–540. doi: 10.1016/j.ejso.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka K, Takakura H, Takeda K, et al. Importance of complete pathologic response to prehepatectomy chemotherapy in treating colorectal cancer metastases. Ann Surg. 2009;250:935–942. doi: 10.1097/sla.0b013e3181b0c6e4. [DOI] [PubMed] [Google Scholar]
- 52.Ruers TJM, Wiering B, van der Sijp JRM, et al. Improved selection of patients for hepatic surgery of colorectal liver metastases with 18F-FDG PET: A randomized study. J Nucl Med. 2009;50:1036–1041. doi: 10.2967/jnumed.109.063040. [DOI] [PubMed] [Google Scholar]
- 53.Patel S, McCall M, Ohinmaa A, et al. Positron emission tomography/computed tomographic scans compared to computed tomographic scans for detecting colorectal liver metastases: A systematic review. Ann Surg. 2011;253:666–671. doi: 10.1097/SLA.0b013e31821110c9. [DOI] [PubMed] [Google Scholar]
- 54.Fernandez FG, Drebin JA, Linehan DC, et al. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET) Ann Surg. 2004;240:438–447. doi: 10.1097/01.sla.0000138076.72547.b1. discussion 447–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mise Y, Imamura H, Hashimoto T, et al. Cohort study of the survival benefit of resection for recurrent hepatic and/or pulmonary metastases after primary hepatectomy for colorectal metastases. Ann Surg. 2010;251:902–909. doi: 10.1097/SLA.0b013e3181c9868a. [DOI] [PubMed] [Google Scholar]
- 56.Adam R, Bismuth H, Castaing D, et al. Repeat hepatectomy for colorectal liver metastases. Ann Surg. 1997;225:51–60. doi: 10.1097/00000658-199701000-00006. discussion 60–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Gramont A, Boni C, Navarro M, et al. Oxaliplatin/5FU/LV in adjuvant colon cancer: Updated efficacy results of the MOSAIC trial, including survival, with a median follow-up of six years. J Clin Oncol. 2007;25(18 suppl) Abstract 4007. [Google Scholar]
- 58.Van Cutsem E, Labianca R, Bodoky G, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol. 2009;27:3117–3125. doi: 10.1200/JCO.2008.21.6663. [DOI] [PubMed] [Google Scholar]
- 59.Hershman D, Hall MJ, Wang X, et al. Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer. Cancer. 2006;107:2581–2588. doi: 10.1002/cncr.22316. [DOI] [PubMed] [Google Scholar]
- 60.Portier G, Elias D, Bouche O, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976–4982. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 61.Weiser M, Sauerland S, Arnold D, et al. Peri-operative chemotherapy for the treatment of resectable liver metastases from colorectal cancer: A systematic review and meta-analysis of randomized trials. BMC Cancer. 2010;10:309–322. doi: 10.1186/1471-2407-10-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ychou M, Hohenberger W, Thezenas S, et al. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann Oncol. 2009;20:1964–1970. doi: 10.1093/annonc/mdp236. [DOI] [PubMed] [Google Scholar]
- 63.Liu JH, Hsieh YY, Chen WS, et al. Adjuvant oxaliplatin- or irinotecan-containing chemotherapy improves overall survival following resection of metachronous colorectal liver metastases. Int J Colorectal Dis. 2010;25:1243–1249. doi: 10.1007/s00384-010-0996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allegra CJ, Yothers G, O'Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: Results of the NSABP protocol C-08. J Clin Oncol. 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alberts SR, Sargent DJ, Smyrk TC, et al. Adjuvant mFOLFOX6 with or without cetuxiumab in KRAS wild-type patients with resected stage III colon cancer: Results from NCCTG Intergroup Phase III Trial N0147. J Clin Oncol. 2010;28(18 suppl) Abstract CRA3507. [Google Scholar]
- 66.Wong SL, Mangu PB, Choti MA, et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28:493–508. doi: 10.1200/JCO.2009.23.4450. [DOI] [PubMed] [Google Scholar]
- 67.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. discussion 825–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abdalla EK, Barnett CC, Doherty D, et al. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolisation. Arch Surg. 2002;137:675–680. doi: 10.1001/archsurg.137.6.675. discussion 680–681. [DOI] [PubMed] [Google Scholar]
- 69.Chun YS, Vauthey JN, Ribero D, et al. Systemic chemotherapy and two-stage hepatectomy for extensive bilateral colorectal liver metastases: Perioperative safety and survival. J Gastrointest Surg. 2007;11:1498–1504. doi: 10.1007/s11605-007-0272-2. discussion 1504–1505. [DOI] [PubMed] [Google Scholar]
- 70.Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–977. [PMC free article] [PubMed] [Google Scholar]
- 71.Nelson R, Freels S. Hepatic artery adjuvant chemotherapy for patients having resection or ablation of colorectal cancer metastatic to the liver. Cochrane Database Syst Rev. 2006;(4):CD003770. doi: 10.1002/14651858.CD003770.pub3. DOI: 10.1002/14651858.CD003770.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cosimelli M, Golfieri R, Cagol PP, et al. Italian Society of Locoregional Therapies in Oncology (SITILO). Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer. 2010;103:324–331. doi: 10.1038/sj.bjc.6605770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wagman LD, Kemeny MM, Leong L, et al. A prospective, randomized evaluation of the treatment of colorectal cancer metastatic to the liver. J Clin Oncol. 1990;8:1885–1893. doi: 10.1200/JCO.1990.8.11.1885. [DOI] [PubMed] [Google Scholar]
- 74.Lygidakis NJ, Ziras N, Parissis J. Resection versus resection combined with adjuvant pre- and post-operative chemotherapy—immunotherapy for metastatic colorectal liver cancer. A new look at an old problem. Hepatogastroenterology. 1995;42:155–161. [PubMed] [Google Scholar]
- 75.Lorenz M, Ml̈ler HH, Schramm H, et al. Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer. German Cooperative on Liver Metastases (Arbeitsgruppe Lebermetastasen) Ann Surg. 1998;228:756–762. doi: 10.1097/00000658-199812000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kemeny N, Huang Y, Cohen AM. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–2048. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 77.Kemeny MM, Adak S, Gray B, et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: Surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy—an intergroup study. J Clin Oncol. 2002;20:1499–1505. doi: 10.1200/JCO.2002.20.6.1499. [DOI] [PubMed] [Google Scholar]
- 78.Martin RC, 2nd, Augenstein V, Reuter NP, et al. Simultaneous versus staged resection for synchronous colorectal cancer liver metastases. J Am Coll Surg. 2009;208:842–850. doi: 10.1016/j.jamcollsurg.2009.01.031. discussion 850–852. [DOI] [PubMed] [Google Scholar]
- 79.Reddy SK, Pawlik TM, Zorzi D, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: A multi-institutional analysis. Ann Surg Oncol. 2007;14:3481–3491. doi: 10.1245/s10434-007-9522-5. [DOI] [PubMed] [Google Scholar]
- 80.Hillings JG, Wille-Jrgensen P. Staged or simultaneous resection of synchronous liver metastases from colorectal cancer—a systematic review. Colorectal Dis. 2009;11:3–10. doi: 10.1111/j.1463-1318.2008.01625.x. [DOI] [PubMed] [Google Scholar]
- 81.Capussotti L, Ferrero A, Viganò L, et al. Major liver resections synchronous with colorectal surgery. Ann Surg Oncol. 2007;14:195–201. doi: 10.1245/s10434-006-9055-3. [DOI] [PubMed] [Google Scholar]
- 82.Glimelius B, Dahl O, Cedermark B, et al. Adjuvant chemotherapy in colorectal cancer: A joint analysis of randomised trials by the Nordic Gastrointestinal Tumour Adjuvant Therapy Group. Acta Oncol. 2005;44:904–912. doi: 10.1080/02841860500355900. [DOI] [PubMed] [Google Scholar]
- 83.Yoshidome H, Kimura F, Shimizu H, et al. Interval period tumor progression: Does delayed hepatectomy detect occult metastases in synchronous colorectal liver metastases? J Gastrointest Surg. 2008;12:1391–1398. doi: 10.1007/s11605-008-0540-9. [DOI] [PubMed] [Google Scholar]
- 84.Lambert LA, Colacchio TA, Barth RJ., Jr Interval hepatic resection of colorectal metastases improves patient selection. Arch Surg. 2000;135:473–479. doi: 10.1001/archsurg.135.4.473. discussion 479–480. [DOI] [PubMed] [Google Scholar]
- 85.White RR, Schwartz LH, Munoz JA, et al. Assessing the optimal duration of chemotherapy in patients with colorectal liver metastases. J Surg Oncol. 2008;97:601–604. doi: 10.1002/jso.21042. [DOI] [PubMed] [Google Scholar]
- 86.Petrowsky H, Gonen M, Jarnagin W, et al. Second liver resections are safe and effective treatment for recurrent hepatic metastases from colorectal cancer: A bi-institutional analysis. Ann Surg. 2002;235:863–871. doi: 10.1097/00000658-200206000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adam R, Pascal G, Azoulay D, et al. Liver resection for colorectal metastases: The third hepatectomy. Ann Surg. 2003;238:871–883. doi: 10.1097/01.sla.0000098112.04758.4e. discussion 883–884. [DOI] [PMC free article] [PubMed] [Google Scholar]