Abstract
We used a model of combined bone marrow and heart transplantation, in which tolerance and stable chimerism is induced after conditioning with fractionated irradiation of the lymphoid tissues and anti–T-cell antibodies. Graft acceptance and chimerism required host CD4+CD25+ Treg production of IL-10 that was in-turn enhanced by host invariant natural killer (NK) T-cell production of IL-4. Up-regulation of PD-1 on host Tregs, CD4+CD25− conventional T (Tcon) cells, and CD8+ T cells was also enhanced by NKT cell production of IL-4. Up-regulated PD-1 expression on Tregs was linked to IL-10 secretion, on CD8+ T cells was linked to Tim-3 expression, and on CD4+ Tcon cells was associated with reduced IFNγ secretion. Changes in the expression of PD-1 were induced by the conditioning regimen, and declined after bone marrow transplantation. In conclusion, NKT cells in this model promoted changes in expression of negative costimulatory receptors and anti-inflammatory cytokines by Tregs and other T-cell subsets in an IL-4–dependent manner that resulted in tolerance to the bone marrow and organ grafts.
Introduction
The role of CD4+CD25+ Treg cells and natural killer (NK) T cells in the induction of immune tolerance and acceptance of organ and/or bone marrow transplantations has been studied extensively.1–8 In the case of Treg cells, both naturally occurring (nTregs) and induced (iTregs) cells can promote tolerance.5,6 Whereas the naturally occurring Treg cells suppress immune responses to a broad spectrum of alloantigens in vitro and in vivo, the induced Treg cells have been shown to suppress responses to specific alloantigens that are the targets of tolerance.5,6 The effector mechanisms of immune suppression involve both cell contact interactions with conventional T (Tcon) cells that initiate responses to alloantigens, and the secretion of cytokines such as IL-10 that reduce inflammatory responses and Tcon cell immunity.9,10 The suppression of Tcon cell immunity by Treg cells has been shown to involve the interactions between the programmed death-1 (PD-1) T-cell activation molecule, and the ligand, PDL-1.11 Blocking of this interaction abrogates tolerance to cardiac allografts induced by CTLA-4-Ig treatment.12 Up-regulation of PD-1 on Treg cells has been associated with increased IL-10 production.13,14
NKT cells differ from Tcon-cell subsets because the NKT cell T-cell receptor (TCR) recognizes glycolipids associated with the nonpolymorphic antigen presenting molecule, CD1d, whereas the TCR of the Tcon-cell subsets recognizes peptides associated with the highly polymorphic antigen presenting molecules, MHC classes I and II.15,16 There are 2 major subtypes of NKT cells, type I and type II, that express either an invariant TCRα chain that contains a segment encoded by the Jα18 gene, or a noninvariant TCRα chain, respectively.17,18 Jα18−/− mice are deficient in the type I with the invariant TCR; whereas CD1d−/− mice are deficient in both types as well as the CD1d TCR ligand.17 NKT cells have regulatory functions that promote transplantation tolerance and suppress GVHD after bone marrow transplantation.7,8,19,20 Both in vitro and in vivo model systems showed that NKT cells can interact with Treg cells and change Treg cell function.21–23
Previous studies of bone marrow transplantation that used hosts conditioned with fractionated irradiation of the lymphoid tissues (total lymphoid irradiation; TLI) and anti–T-cell antibodies showed that prevention of GVHD by the conditioning regimen required the presence of both host NKT cells and donor Treg cells.23 The NKT cells interacted with the Treg cells, and NKT cell production of IL-4–enhanced Treg cell expansion and secretion of IL-10 that prevented GVHD.23 Similarly, the same conditioning regimen promoted tolerance to combined organ and bone marrow transplants, and hosts became stable mixed chimeras with long-term acceptance of donor but not third-party heart grafts.8,24 Tolerance required the presence of both host NKT cells and host Treg cells, because pretransplantation depletion of Treg cells in WT (WT) hosts or the deficiency of NKT cells in CD1d−/− hosts prevented engraftment of the organ and bone marrow transplants.24 However, interactions between the T-cell subsets were not studied.24
The goal of the current study was to determine whether the host NKT cells, Treg cells and Tcon cells can interact, and the mechanisms of interactions that can facilitate tolerance in the above model of combined organ and bone marrow transplantation. We found that tolerance was dependent on Treg production of IL-10 that was in turn dependent on NKT cell production of IL-4. The bias of Tregs toward IL-10 and of CD4+ Tcon cells away from IFNγ was associated with up-regulation of PD-1. In addition, CD8+ T cells developed the PD-1hi Tim-3+ “exhausted phenotype.”25–27
Methods
Mice
Adult 8- to 10-week-old male WT and IL-10−/− BALB/c (H-2Kd), IL-4−/− BALB/c (H-2Kd) mice, and WT C57BL/6 (H-2Kb) mice were obtained from The Jackson Laboratory. C57BL/6 neonates were purchased from the Charles River Laboratories. CD1d−/− BALB/c mice,28 and Ja18−/− BALB/c mice29 were bred in the Department of Comparative Medicine, Stanford University, and all mice were maintained in the department according to institutional guidelines approved by the National Institutes of Health (NIH).
Cardiac transplantation and monitoring for graft survival
Neonatal C57BL/6 heart grafts were transplanted into a pouch in the ear pinna of BALB/c hosts on day 0 according to the procedure described by Trager et al.30 Heart grafts were monitored daily for visible contractions, and survival was based on the time interval after transplantation when contractions stopped.
Bone marrow transplantation and TLI
The bone marrow harvesting and transplantation procedure was performed as previously described.8 TLI was delivered to the lymph nodes above and below the diaphragm, thymus, and spleen with lead shielding of the skull, limbs, pelvis, and tail using a 250Kv X-ray machine.31 Ten doses of 240 cGy each were administered starting on the day of heart transplantation using 5 doses per week.24 The last dose of TLI was administered to BALB/c mice 24 hours before the allogeneic bone marrow cell infusions on day 15 after heart transplantation.
Rabbit antithymocyte serum and anti-CD25 monoclonal antibody treatment
Rabbit antithymocyte serum (ATS) was purchased from Accurate Chemical and Scientific. BALB/c recipients were injected intraperitoneally with 0.05 mL of ATS in 0.5 mL saline on days 0, 2, 6, 8, and 10 after heart transplantation. Depletion of CD4+CD25+ Tregs was accomplished by a single intraperitoneal injection of anti-CD25 (clone PC61.5) monoclonal antibody (mAb) purchased from eBioscience, and kinetics of changes in the absolute numbers were previously described.24,32
Immunoflourescent staining reagents and monoclonal antibodies
Anti-CD4 (RM4-5)–APC, anti-CD25 (PC65.1)–PE, anti-CD25 (PC65.1)–APC, anti–H-2Kb (AF6-88.5)–FITC, anti-TCRβ(H57-597)–FITC, anti-TCRβ(H57-597)–PE, anti-TCRβ (H57-597)–APC, anti-Mac1 (M1/70)–PE, anti-Gr1 (RB6-8C5)–PE, anti-B220 (RA3-6B2)–PE, anti-CD1d (Ly-38, 1B1)–PE, anti-Ki67–FITC, anti-Ki67–PE, anti-PD1 (J43)–FITC, and anti-CD16/32(2.4G2) mAbs were purchased from BD Pharmingen. Anti–Tim-3 (B8.2C12) was purchased from BioLegend. Anti-CD25 (clone PC61.5) and anti-Foxp3 (FJK-16s) mAbs (eBioscience) were used for combination staining of surface CD25 and intracellular Foxp3 expression. For intracellular Foxp3 and Ki67 staining, cells were first stained with ethidium monoazide (EMA), a dead cell exclusion dye before fixation and permeabilization as per the manufacturer's protocol (eBioscience, BD Biosciences). Phycoerythrin conjugated CD1d-tetramers were obtained from the NIH Tetramer Facility.
Immunoflourescent staining and chimerism analysis
Single-cell suspensions of blood or spleen cells were stained for surface markers with appropriate mAbs at 4°C in staining buffer containing propidium iodide and Fc receptor blocking antibodies as previously described.24 Analysis of chimerism in the blood was performed by 1 color staining of total white blood cells with the anti–H-2Kb mAb. Staining procedures for CD4+CD25+Foxp3+ T cells were a modification of those previously reported24 using eBioscience regulatory T-cell staining kit. Samples were acquired and analyzed with an LSRII flow cytometer (BD Biosciences), and FlowJo Version 8.2 software (TreeStar).
Purification and adoptive transfer of CD4+CD25+ (Treg) and iNKT cells
For Treg isolation, spleen cells were stained with anti-CD25–PE, incubated with anti-PE microbeads (Miltenyi Biotec), and then enriched using LS Columns (Militenyi Biotec). Purified cells were then stained with anti-CD4–APC and anti-TCRβ–FITC. Splenic cells were sorted as TCRβ+CD4+CD25+ T cell on an Aria flow cytometer (BD Biosciences). Sorted cells were ≥ 98% pure by postsort analysis. Sorted Treg cells (1 × 106/mouse) were injected intravenously into BALB/c hosts depleted with anti-CD25 mAb. Invariant NKT (iNKT) cells were isolated as previously described.23 Briefly splenocytes from WT BALB/c mice were stained with glycolipid loaded PE-conjugated CD1d tetramers (NIH Tetramer Facility), and PE-conjugated α-Galcer loaded CD1d dimer-mouse IgG fusion protein (BD Biosciences). iNKT cells were enriched subsequently by incubating with anti-PE microbeads (Miltenyi Biotech), and passing through a magnetic column (Miltenyi Biotech). The cells were then stained with anti-TCRβ–FITC and sorted by FACS Aria II (BD Biosciences). The purity of sorted iNKT cells (TCRβ+ CD1d-tet+) was more than 97%. Sorted iNKT cells (0.4 × 106/mouse) from WT or IL-4−/− mice were injected intravenously into Ja18−/− BALB/c hosts.
In vitro culture and cytokine analysis
In vitro polyclonal activation, and cytokine analyses of sorted CD4+CD25+ and CD4+CD25− T-cell subpopulations were performed using phorbol myristate acetate (PMA) and ionomycin as previously reported.24 Single-cell suspensions were isolated from spleen of BALB/c hosts on day19 after bone marrow transplantation. Cells were stained with anti-CD90 (Thy1.2) microbeads and enriched using LS columns (Miltenyi Biotec). Enriched cells were stained using anti-TCRβ, anti–H-2Kb (host-type), anti-CD4, and anti-CD25 mAbs and sorted on an Aria flow cytometer (BD Biosciences). Sorted host type H-2Kb−CD4+CD25+ (Treg) and H-2Kb−CD4+CD25− (Tcon) T cells were plated at 0.5 × 105 cells/well, and cultured in complete RPMI 1640 media (Mediatech) in vitro, with or without PMA and ionomycin at 37°C in 5% CO2. After 48 hours of culture, cell culture supernatants were incubated with microbeads and analyzed on a luminex reader (Millipore) for IL-4, IL-10, and IFNγ secretion.
Statistical analysis
Statistical analyses of differences in heart graft survival between different groups of mice were performed using the log-rank test of Kaplan-Meier plots at day 100. Comparisons of the fraction of mice in different groups that were chimeras were performed by χ2 analysis. Comparison of mean percentages of absolute numbers of T cells, and subsets in the spleen and cytokine analysis of different groups of mice were made using 2-tailed Student t test of independent means.
Results
Tolerance is dependent on Tregs, their expression of IL-10, and NKT cells
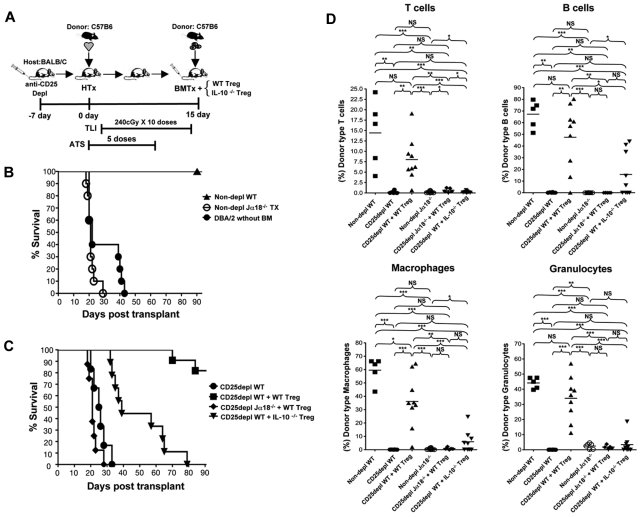
The experimental scheme shown in Figure 1A has been previously used24 to induce tolerance after the transplantation of WT C57BL/6 (H-2Kb) heart grafts on day 0 to WT BALB/c (H-2Kd) recipients conditioned with 10 doses of TLI (240cGy each) and 5 doses of anti-thymocyte serum (ATS) after transplantation. The recipients were injected with 50 × 106 bone marrow cells from the C57BL/6 donor strain immediately after the completion of conditioning 15 days after heart transplantation. In previous experiments, the Tregs were depleted from the recipients by a single injection of anti-CD25 mAb on day −7.24 The Treg percentage among CD4+ T cells in the spleen was reduced to less than 1% for at least 14 days after the completion of conditioning, and caused uniform graft rejection.24 In the current study, some groups of Treg depleted recipients were given an injection of purified Tregs from WT, or IL-10−/− BALB/c mice at the time of the bone marrow transplantation (Figure 1A) to determine whether graft acceptance can be restored. Representative 2 color flow cytometric profiles of the purified and added back Tregs from WT mice are shown in supplemental Figure 1A (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The purity of the injected Tregs was > 99% CD4+CD25+ cells. At least 90% of the latter cells were FoxP3+ (supplemental Figure 1A).
Figure 1.
Tolerance and chimerism is dependent on Tregs, their expression of IL-10, and NKT cells. (A) Experimental scheme: WT or Jα18−/− BALB/c host mice were given C57BL/6 neonatal heart transplants on day 0. ATS was injected intraperitoneally on days 0, 2, 6, 8, and 10. TLI was given over 14 days at 10 doses of 240 cGy each. Some hosts were given a single dose of anti-CD25 mAb intraperitoneally on day −7 to deplete Treg cells. On day 15, 50 × 106 C57BL/6 bone marrow cells were injected intravenously without, or with CD4+CD25+ T cells sorted from WT, or IL-10−/− BALB/c mice. (B) Heart graft survival in nondepleted TLI/ATS WT, or nondepleted Jα18−/− BALB/c mice given C57BL/6 heart transplants and bone marrow cell infusion (nondepl WT, n = 9 mice), (nondepl Jα18−/−, n = 10 mice), or nondepleted BALB/c hosts given DBA/2 heart transplants without bone marrow cell infusion (DBA/2 without BM, n = 10 mice). Nondepl WT versus nondepl Jα18−/−, (P < .01) by log-rank Mantel-Cox test. (C) Heart graft survival in Treg depleted and TLI/ATS conditioned WT hosts given heart transplants and bone marrow cell infusion (CD25 depl WT, n = 12 mice), Treg depleted and TLI/ATS conditioned WT hosts given heart transplants and bone marrow cell infusion with add-back of sorted 1 × 106 CD4+CD25+ T cells from WT BALB/c mice (CD25 depl WT + WT Treg, n = 11 mice), Treg depleted TLI/ATS Ja18−/− BALB/c mice given heart transplants and bone marrow cell infusion (CD25 depl Ja18−/− + WT Treg, n = 8) with add-back of sorted 1 × 106 CD4+CD25+ T cells from WT BALB/c mice, and Treg depleted and TLI/ATS conditioned WT hosts given heart transplants and bone marrow cell infusion with add-back of sorted 1 × 106 CD4+CD25+ T cells from IL-10−/− BALB/c mice (CD25 depl WT + IL-10−/− Treg, n = 9 mice). CD25 depl WT versus CD25 depl + WT Treg (P < .001); CD25 depl WT + WT Treg versus CD25 depl Jα18−/− + WT Treg (P < .001), CD25 depl WT + WT Treg versus + IL-10−/− Treg (P < .001) by log-rank Mantel-Cox test. (D) Mean percentages of donor type cells on day 28 after marrow transplantation in nondepleted WT BALB/c mice given heart and marrow transplants (nondepl WT, n = 8 mice), CD25 depleted WT hosts given transplants (CD25 depl WT, n = 6 mice), CD25 depleted WT hosts given transplants and sorted 1 × 106 CD4+CD25+ T cells from WT BALB/c mice (CD25 depl WT + WT Treg, n = 9 mice), nondepleted TLI/ATS Ja18−/− BALB/c mice given transplants (nondepl Ja18−/−, n = 10 mice), CD25 depleted TLI/ATS Ja18−/− BALB/c mice given transplants with add-back of sorted 1 × 106 CD4+CD25+ T cells from WT BALB/c mice (CD25 Ja18−/− + WT Treg, n = 7 mice), and CD25 depleted WT hosts given transplants and sorted 1 × 106 CD4+CD25+ T cells from IL-10−/− BALB/c mice (CD25 depl WT + IL-10−/− Treg, n = 9 mice). Comparison of means (Student 2-tailed t test) gave P values shown on the panels; NS > .05, *P < .05, **P < .01, ***P < .001. T cells-TCR+; B cells-B220+; Macrophages- Mac1+; Granulocytes-Gr1+ cells.
Figure 1B shows the survival of C57BL/6 heart grafts in the BALB/c recipients during a 100-day observation period. All WT recipients that were conditioned and given combined bone marrow and heart transplants without Treg depletion maintained their grafts for at least 90 days. Previous studies showed that the C57BL/6 bone marrow cells must be injected to achieve acceptance of the C57BL/6 heart grafts.8 Figure 1B also shows that even DBA/2 heart grafts that are MHC matched with BALB/c hosts (H-2Kd) are uniformly rejected in the absence of a bone marrow cell infusion. When a combination of C57BL/6 heart and bone marrow cells were injected into conditioned Jα18−/− BALB/c recipients that are deficient in invariant NKT cells, the heart grafts were all rejected by day 30 (Figure 1B). All WT BALB/c recipients depleted of Tregs with the single injection of anti-CD25 mAb rejected their grafts by 35 days (P = .01; depleted vs undepleted recipients; Figure 1C). Long-term acceptance of the heart grafts was restored in approximately 80% of the anti-CD25 mAb treated recipients by injecting 1 × 106 purified CD4+CD25+ T cells obtained from untreated WT BALB/c mice at the time of the marrow transplantation (P = .01; with vs without add back).
Because full in vivo suppression of alloimmunity or autoimmunity by Tregs has been shown to be dependent on their secretion of IL-10,10,33 the add-back experiments were repeated using purified Tregs from IL-10−/− instead of WT BALB/c mice. As shown in Figure 1C, the injection of the IL-10−/− Tregs failed to restore long-term acceptance of the heart grafts, and all were rejected by approximately day 80 (P = .05; IL-10−/− vs WT). Although add-back of WT Treg cells to WT hosts depleted of Tregs restored graft acceptance, the add-back failed to restore acceptance in the invariant NKT cell–deficient Jα18−/− hosts (Figure 1C).
Chimerism is dependent on Tregs, their expression of IL-10, and on NKT cells
Recipients in the groups shown in Figures 1B and C were monitored not only for heart graft survival, but also for the development of chimerism at days 28 and 100 after bone marrow transplantation. Figure 1D shows chimerism analyses of samples of peripheral blood mononuclear cells obtained from recipients at day 28, which were stained for the donor type marker (H-2Kb) versus lineage markers for T cells, B cells, macrophages, and granulocytes (TCR, B220, Mac-1, and Gr-1, respectively). Samples from WT recipients without Treg depletion showed that there was mixed chimerism in all lineages in the range of 24% to 79% donor type cells, and T cells showed the lowest levels. When Treg depleted WT recipients were examined, then donor type cells accounted for less than 1% of all lineages, and there was no evidence of chimerism. The injection of the Tregs from WT BALB/c mice restored chimerism in all lineages in the depleted WT recipients (Figure 1D). Injection of IL-10−/− Tregs failed to restore chimerism in T cells, or more than 10% among macrophages, granulocytes, and B cells in the majority of recipients (Figure 1D). Substitution of Jα18−/− hosts for WT hosts resulted in failure to restore chimerism with the add-back of WT Tregs (Figure 1D). In addition, Jα18−/− hosts without depletion failed to develop chimerism (Figure 1D). Thus, the patterns of heart graft acceptance and those of bone marrow graft acceptance were similar. When the percentage of donor type cells in the different lineages was examined at day 100 for WT mice without depletion, then the patterns were similar to that of day 28 (supplemental Figure 1B). This indicated that the mixed chimerism established in the recipients was stable.
Up-regulation of IL-10 and PD-1 by Treg cells after heart and bone marrow transplantation is enhanced by NKT cells in an IL-4–dependent manner
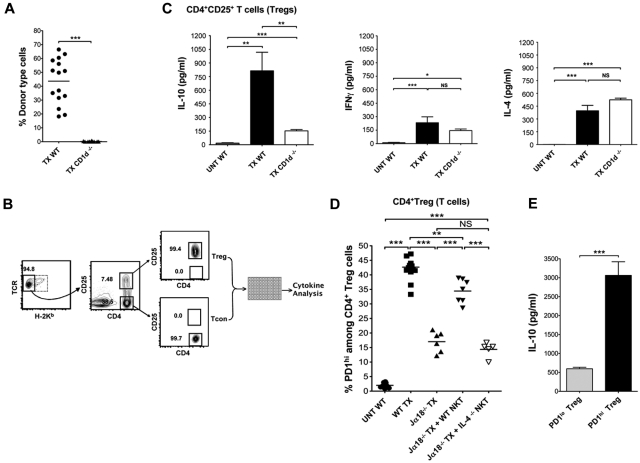
In further experiments, the cytokine secretion patterns of Tregs from the WT transplant recipients were compared with Tregs from a second type of NKT cell deficient (CD1d−/−) recipient to determine whether the deficiency in host NKT cells affected the secretion patterns induced by in vitro activation. Figure 2A shows that the CD1d−/− hosts uniformly failed to develop chimerism compared with WT hosts after bone marrow and heart transplantation. Sorted host type H-2Kb−CD4+CD25+ Tregs cells were obtained from the spleen of heart and marrow transplant recipients 19 days after infusion of marrow cells. Secretion of IL-4, IFNγ, and IL-10 were assayed after stimulation of cells in vitro with PMA and ionomycin as shown in the scheme in Figure 2B. Day 19 was chosen because that was the earliest time point after transplantation with sufficient cell yields to perform the analyses.
Figure 2.
Up-regulation of IL-10 and PD-1 by Treg cells after heart, and bone marrow transplantation is enhanced by NKT cells in an IL-4–dependent manner. (A) Mean percentages of donor type cells at day 19 after marrow transplantation in WT BALB/c mice given heart and bone marrow transplants (TX WT, n = 27 mice), and in CD1d−/− BALB/c mice given transplants (TX CD1d−/−, n = 20 mice). (B) Experimental scheme: representative FACS pattern of gated recipient type H-2Kb−CD4+CD25+ and H-2Kb− CD4+CD25− T cells among CD4+ splenocytes before sorting (left and middle panels) or after sorting (right panel), obtained 19 days after marrow transplantation. Sorted cells were cultured with PMA and ionomycin, and cytokine concentrations in culture supernatants were assessed by luminex beads. (C) Mean (± SEM) IL-10 (left panel), IL-4 (right panel), and IFNγ (middle panel) concentrations in supernatants from sorted CD4+CD25+ T cells, after 48 hours of culture with PMA/ Ionomycin from untreated WT BALB/c mice (UNT WT, n = 15 mice), WT BALB/c mice given heart and bone marrow transplants (TX WT, n = 27 mice) and CD1d−/− mice given heart and bone marrow transplants (TX CD1d−/−, n = 20 mice). Data are combined from 3 or 4 independent experiments with 2 to 3 wells in each experiment. NS, not significant, P > .05; *P < .05, **P < .01, ***P < .001 (Student 2-tailed t test of independent means). Culture supernatants from Treg cells without PMA/ionomycin had less than 20 pg/mL of IFNγ, IL-4, or IL-10. (D) Percentages (horizontal lines show means) of PD-1hi cells among gated CD4+CD25+ T cells from spleen in untreated WT BALB/c mice (UNT WT, n = 8 mice); WT BALB/c mice given heart and bone marrow transplants (WT TX, n = 13 mice), Ja18−/− BALB/c mice given transplants (Ja18−/− TX, n = 6 mice); Ja18−/− BALB/c mice given transplants with add-back of sorted 0.4 × 106 NKT cells from WT BALB/c mice (Ja18−/− TX + WT NKT, n = 7 mice), and Ja18−/− BALB/c mice given transplants with add-back of sorted 0.4 × 106 NKT cells from IL-4−/− BALB/c mice (Ja18−/− TX + IL-4−/− NKT, n = 5 mice). Comparison of means (Student 2-tailed t test) gave P values shown on the panels; NS > 0.05, *P < .05, **P < .01, ***P < .001. (E) Mean IL-10 concentrations of supernatants of sorted host PD-1lo and PD-1hi Treg cells obtained from the spleen of WT heart and bone marrow transplants recipients on day 19 after marrow transplantation (WT TX, n = 8). There were 8 replicate cultures from 2 experiments used for stimulation of cells with PMA and ionomycin. Unstimulated cultures had less than 20 pg/mL IL-10.
Figure 2C shows the mean concentrations of the cytokines in the supernatants of the stimulated Treg cell cultures from WT and CD1d−/− transplant recipients as well as the concentrations from untreated WT BALB/c mice. Treg cells obtained from transplant recipients had significantly increased concentrations of all 3 cytokines compared with cells from the untreated mice (Figure 2C). Whereas the levels of IL-4 and IFNγ were not significantly different from the WT or CD1d−/− transplant recipients, the IL-10 levels were significantly higher in the WT versus the CD1d−/− recipients. The results show that the transplantation procedure markedly increases IL-10 secretion of Treg cells, but the lack of host CD1d expression significantly attenuates the increased secretion.
The expression of the negative costimulatory receptor, PD-1, was also studied on the Treg cells in untreated WT BALB/c mice and in transplant recipients, because interactions between PD-1 and the ligand, PDL-1, have been shown to play an important role in the promotion of transplantation tolerance.11,12 Figure 2D shows that the gated CD4+CD25+ Treg cells in untreated mice contained less than 5% PD-1hi cells, whereas in WT transplanted mice the mean percentage of PD-1hi cells was approximately 42%. Representative flow cytometric profiles for PD-1 staining with thresholds for PD-1hi and PD-1lo cells are shown in Figure 3.
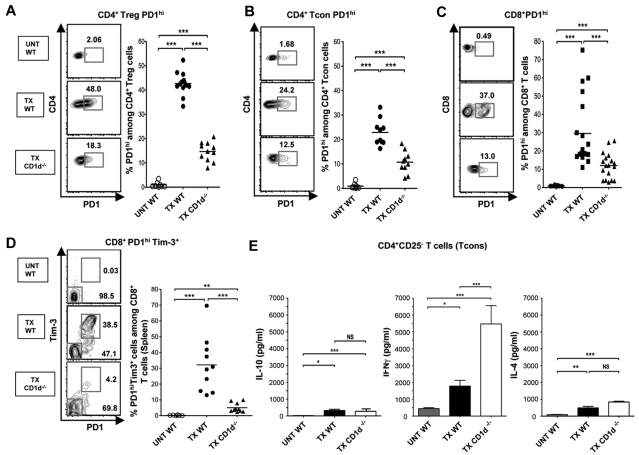
Figure 3.
NKT cells regulate PD-1 and IFNγ expression of CD4+ Tcon cells and PD-1 and Tim-3 of CD8+ T cells. (A-C) Representative FACS patterns of CD4 versus PD-1 on gated Treg (A), CD4 versus PD-1 on gated CD4+ Tcons (B), or CD8 versus PD-1 on gated CD8+ T cells (C), and percentages of PD-1hi cells among subsets from untreated WT (UNT WT, n = 8) or from WT or CD1d−/− transplant recipients at day 19 after marrow transplantation (TX WT, n = 12; TX CD1d−/−, n = 18). (D) Representative FACS patterns, and percentages of PD-1hiTim-3+ cells among gated CD8+ T cells from untreated WT (UNT WT, n = 6) or from WT or CD1d−/− transplant recipients at day 19 after marrow transplantation (TX WT, n = 10; TX CD1d−/−, n = 11). Comparison of means (Student 2-tailed t test) gave P values shown on the panels; NS > 0.05, *P < .05, **P < .01, ***P < .001. (E) Mean (± SEM) IL-10 (left panel), IL-4 (right panel), and IFNγ (middle panel) concentrations in supernatants of sorted CD4+CD25− Tcon cells, after 48 hours of culture with PMA/ Ionomycin from untreated WT BALB/c mice (UNT WT, n = 15 mice), WT BALB/c mice given transplants (TX WT, n = 27 mice), and CD1d−/− BALB/c mice given transplants (TX CD1d−/−, n = 20 mice). Data are combined from 3 or 4 independent experiments with 2 to 3 wells in each experiment. NS, not significant, P > .05; * P < .05, ** P < .01, *** P < .001 (Student 2-tailed t test of independent means). Culture supernatants from Treg cells without PMA/ionomycin had less than 20 pg/mL of IFNγ, IL-4, or IL-10.
Figure 2D also shows that NKT cell deficiency of Ja18−/− transplant recipients resulted in significantly lower percentages of PD-1hi cells among the Tregs, and that the percentages were significantly increased by the add-back of purified WT NKT cells but not by purified IL-4−/− NKT cells. Thus, the NKT cells regulated the levels of expression of PD-1 on Treg cells in an IL-4–dependent manner. Interestingly, the PD-1hi Treg cells from WT transplanted mice produced high levels of IL-10, whereas the PD-1lo Treg cells produced markedly lower levels of IL-10 after in vitro activation (Figure 2E). Thus, the NKT cells regulated the linked expression of IL-10 and PD-1 on Treg cells in an IL-4–dependent manner.
NKT cells regulate PD-1 and IFNγ expression of CD4+ Tcon cells and PD-1 and Tim-3 expression of CD8+ T cells
In further studies, we examined the expression of PD-1 and Tim-3 on CD4+CD25− Tcon and CD8+ T cells. Figure 3A through C shows that the WT transplant recipients had significantly increased percentages of PD-1hi cells compared with untreated mice not only in Treg cells, but also in host type (H-2Kb−) CD4+ Tcon cells and in CD8+ T cells. Representative examples of flow cytometric profiles are shown. As in the case of Treg cells, deficiency of NKT cells in CD1d−/− recipients resulted in a significant decrease in the percentage of PD-1hi cells in the CD4+ Tcon and CD8+ T cells as well (Figure 3A-C). Because increased expression of PD-1 among CD8+ T cells has been associated with increased expression of Tim-3 in mice with chronic viral infections and tumors,25–27 we stained CD8+ T cells for both markers as shown in Figure 3D. Approximately 80% of the PD-1hi cells were Tim-3+, and a mean of approximately 30% of CD8+ T cells were PD-1hi Tim-3+ in WT transplant hosts compared with less than 2% in untreated WT mice. The percentage of PD-1hi Tim-3+ cells among CD8+ T cells was significantly reduced in NKT cell deficient recipients (Figure 3D). Tim-3 expression was near background among CD4+ Tcon cells in all groups of mice (data not shown).
To study the importance of NKT cell deficiency on cytokine secretion pattern of host type CD4+ Tcon cells in the transplanted mice, the protocol outlined in Figure 2B was used. Purified H-2Kb− CD4+ Tcon cells were harvested from the spleen on day 19 after transplantation of marrow cells in WT or CD1d−/− recipients. The purified cells were stimulated in vitro and cytokine concentrations in the supernatants were measured. There was a significant increase in IL-10, IL-4, and IFNγ secretion when untreated WT mice were compared with transplanted WT mice (Figure 3E). The deficiency in NKT cells did not result in a significant change in IL-4 or IL-10 compared with WT transplanted mice. In contrast, NKT cell deficiency markedly increased the IFNγ secretion in transplanted mice (Figure 3E). Thus, the presence of host NKT cells promoted the up-regulation of PD-1 and a bias away from IFNγ in the CD4+ Tcon cells in transplanted mice.
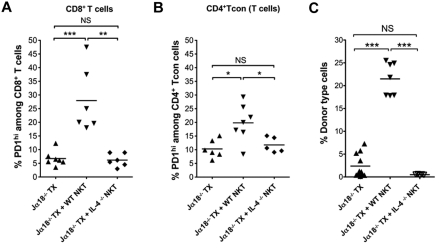
In further studies, add-back experiments were performed to determine whether NKT cell secretion of IL-4 played an important role in regulating PD-1 expression on host CD4+ Tcon and CD8+ T cells as was the case with host Treg cells. Accordingly, the percentages of PD-1hi cells were compared in Jα18−/− transplanted mice with or without the add-back of WT or IL-4−/− NKT cells. As shown in Figure 4A and B, WT NKT cells injected into Jα18−/− transplanted mice significantly increased the percentage of PD-1hi cells among CD8+ T cells and CD4+ Tcon cells, respectively. However, when IL-4−/− NKT cells were injected, no significant increase was observed compared with the Jα18−/− transplanted mice without a cell injection. The results are similar to that observed with Treg cells, and show that the up-regulation of PD-1 on all host T-cell subsets in transplanted mice is dependent on host NKT cells and their secretion of IL-4.
Figure 4.
Chimerism and up-regulation of PD-1 on CD4+ Tcon and CD8+ T cells are enhanced by NKT cells in an IL-4–dependent manner. (A-B) Percentage of PD-1hi cells among gated CD8+ (A) or CD4+CD25− Tcon cells (B) T cells from spleen in Ja18−/− BALB/c mice given transplants (Ja18−/− TX, n = 6 mice); Ja18−/− BALB/c mice given transplants with add-back of sorted 0.4 × 106 NKT cells from WT BALB/c mice (Ja18−/− TX + WT NKT, n = 7 mice), and Ja18−/− BALB/c mice given transplants with add-back of sorted 0.4 × 106 NKT cells from IL-4−/− BALB/c mice (Ja18−/− TX + IL-4−/− NKT, n = 5 mice). (C) Mean percentages of donor type cells in splenic mononuclear cell gate from Ja18−/− BALB/c mice given transplants (Ja18−/− TX, n = 9 mice); Ja18−/− BALB/c mice given transplants with add-back of sorted 0.4 × 106 NKT cells from WT BALB/c mice (Ja18−/− TX + WT NKT, n = 7 mice); and Ja18−/− BALB/c mice given transplants with add-back of sorted 0.4 × 106 NKT cells from IL-4−/− BALB/c mice (Ja18−/− TX + IL-4−/− NKT, n = 5 mice). Comparison of means (Student 2-tailed t test) gave P values shown on the panels; NS > .05, *P < .05, **P < .01, ***P < .001.
To determine whether NKT cell secretion of IL-4 also had impact on C57BL/6 bone marrow graft acceptance, the hosts observed in Figure 4A and B were assayed for chimerism in the spleen. Chimerism was assayed by staining for donor type (H-2Kb+) cells in the splenic mononuclear cell gate. As shown in Figure 4C, the mean percentage of donor type cells in the Jα18−/− transplanted mice were approximately 2%. The add-back of WT NKT cells significantly increased chimerism to approximately 22%. However, the add-back of IL-4−/− NKT cells resulted in no significant change. In summary, not only is the Tim-3 and/ or PD-1 receptor up-regulation dependent on NKT cell secretion of IL-4, but also the acceptance of bone marrow transplantations and development of robust chimerism.
Up-regulation of PD-1 and Tim-3 is induced by TLI and ATS conditioning and declines after transplantation of bone marrow
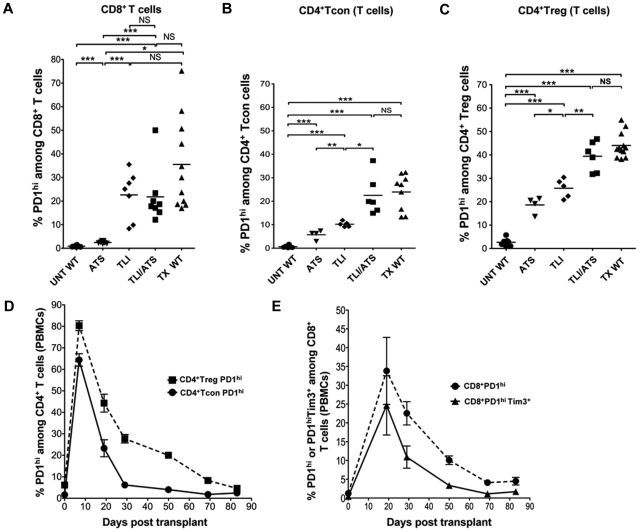
In further experiments, TLI and/ or ATS conditioning alone was compared with conditioning combined with heart and bone marrow transplantation on the up-regulation of PD-1 on T cells. Figure 5A through C compares the percentages of PD-1hi cells among host type CD8+, CD4+CD25−, and CD4+CD25+ T cells, respectively in groups of mice that were untreated, ATS treated, TLI treated, TLI+ATS treated, and TLI+ATS treated and transplanted. In all instances, splenic T cells were harvested 34 days after the first dose of TLI and / or ATS to maintain the time of analysis the same as in Figures 3 and 4. For CD8+ T cells, the ATS treatment alone increased the mean percentage of PD-1hi cells to approximately 5% compared with the mean of 1% in the untreated mice. TLI treatment alone or combined with ATS increased the percentage to approximately 22%. Although combined conditioning and transplantation increased the mean to approximately 35%, the difference between TLI+ATS conditioning with or without transplantation was not significant.
Figure 5.
Up-regulation of PD-1 and Tim-3 is induced by TLI and ATS conditioning and declines after transplantation of bone marrow. (A-C) Percentages of PD-1hi cells among gated CD8+ (A), CD4+CD25− (B), or CD4+CD25+ (C) T cells from the spleen are shown in untreated WT BALB/c mice (UNT WT, n = 8), ATS conditioned WT BALB/c mice (ATS, n = 7), TLI conditioned WT BALB/c mice (TLI, n = 7), ATS + TLI conditioned WT BALB/c mice (ATS + TLI, n = 8), and ATS + TLI conditioned WT BALB/c mice given transplants (WT TX, n = 11). (D) Mean percentages (± SEM) of PD1hi cells among CD4+CD25+ T cells (filled square; n = 10), or CD4+CD25− T cells (filled circle; n = 10) in PBMCs at different time points after transplantation in WT BALB/c hosts. (E) Mean percentages (± SEM) of PD1hi among CD8+ T cells (filled circle; n = 10), or PD1hiTim-3+ (filled triangle; n = 10) among gated CD8+ T cells in PBMCs at different time points after transplantation in WT BALB/c hosts.
A similar pattern of up-regulation of PD-1 on CD4+CD25− and CD4+CD25+ T cells was observed such that the mean percentage of PD-1hi cells increased from approximately 1% to approximately 23% and 40%, respectively after treatment with TLI+ATS alone or combined with transplantation (Figure 5B-C). However, in this case the up-regulation was significantly increased after TLI+ATS versus either TLI or ATS alone. The addition of transplantation to TLI+ATS did not make a significant difference. In summary, the TLI+ATS conditioning regimen markedly increased PD-1 up-regulation on all 3 T-cell subsets, and no significant increment was induced by transplantation procedures.
Figure 5D shows the kinetics of PD-1 up-regulation on host type CD4+CD25− and CD4+CD25+ T cells in the blood after TLI+ATS conditioning combined with transplantation. Mean percentages of PD-1hi cells showed a peak of 65% to 80% at completion of conditioning, and a rapid decline by day 30, and a slower decline to baseline by day 85. Similar kinetics of PD-1 and Tim-3 up-regulation was observed on CD8+ T cells in the blood (Figure 5E). However, the peak mean percentages of PD-1+ cells or PD-1+ Tim-3+ cells were delayed until day 20, and peak percentages were less (25%-35%) than the CD4+ T cells. A rapid decline to day 50 was followed by a slow decline to baseline at day 85.
Discussion
Previous studies showed that conditioning of hosts with TLI and ATS induced tolerance to combined organ and marrow transplants, and host NKT and Treg cells were required.8,24 In the current study, we demonstrate that tolerance, and chimerism are restored by transferring Treg cells from WT BALB/c mice to the Treg depleted WT BALB/c recipients of heart and bone marrow transplants. The Treg restoration of tolerance and chimerism was dependent on their ability to produce IL-10, because IL-10−/− Tregs were ineffective. The Treg requirement for IL-10 for promotion of tolerance is consistent with the IL-10 requirement for donor type Treg cells to fully prevent GVHD by donor type conventional T cells and to fully suppress immune responses in other model systems.10,33
Further studies showed that NKT cells regulate the IL-10 production of the Treg cells in transplanted mice, because the marked increase in IL-10 secretion in vitro by purified host Tregs after transplantation was lost in NKT cell deficient mice. The increase in IL-10 secretion by Tregs was linked to the marked up-regulation of PD-1 after transplantation, and only PD-1hi Tregs secreted high levels of IL-10 after in vitro activation. The increased expression of PD-1 was dependent on NKT cell secretion of IL-4, because PD-1 expression was reduced in NKT cell deficient mice, and restored by WT but not IL-4−/− NKT cells.
Studies of donor type Treg suppression of GVHD in TLI and ATS conditioned hosts showed that donor Treg secretion of IL-10 was regulated by host type NKT cell secretion of IL-4.8,24 The IL-4 facilitated Treg cell proliferation and polarization toward an IL-10 bias.10,33 In another model of tolerance to combined bone marrow and skin transplantations that used conditioning with a nondepleting anti-CD8 mAb and dimethylmyeleran, tolerance and chimerism were abrogated when recipients were treated with a combination of anti–IL-4 anti–IL-10 mAbs.23 The results of the latter study show that these Th2 cytokines suppressed rejection of grafts by host CD8+ T cells are consistent with the findings of the current study.
Host NKT cells not only regulated the expression of PD-1 on host Treg cells, but also on host CD4+ Tcon cells and CD8+ T cells. After transplantation, the percentage of PD-1hi cells rose among the latter T cell subsets, and in the case of CD8+ T cells there was a rise in PD-1hi Tim-3+ cells. The latter “exhausted” phenotype has been associated with decreased proliferation and decreased secretion of IFNγ in mice with chronic viral infections or with acute myelogenous leukemia.25–27 As is the case of Tregs, the increase in PD-1 on CD4+ Tcon and CD8+ T cells was significantly reduced in NKT cell–deficient compared with WT recipients, and the reduction could be restored by add-back of WT NKT cells but not IL-4−/− NKT cells. Thus, PD-1 up-regulation in all T-cell subsets was dependent on IL-4 secretion by NKT cells. In addition, bone marrow transplantation acceptance was dependent on NKT cell secretion of IL-4.
Additional experiments showed that the increased expression of PD-1 on Tregs, CD4+ Tcon and CD8+ T cells was observed after conditioning of WT BALB/c mice with TLI and ATS without transplantation of heart or bone marrow grafts. This is consistent with previous reports that TLI induces a Th2 bias in NKT cells.34 Thus, the altered host immune milieu promotes the acceptance of the bone marrow transplants, that in turn results in the development of stable mixed chimerism. The mechanisms by which mixed chimerism promote tolerance have been studied exhaustively, and include clonal deletion.35,36 As in other models, PD-1 expression has been shown to suppress conventional T cell alloreactivity, and provide a costimulatory signal that enhances IL-10 secretion of regulatory T cells.11,12,37–39
Although our model of tolerance induction requires the injection of donor bone marrow cells that leads to chimerism and clonal deletion,8 mixed chimerism itself is not sufficient to induce tolerance to the heart grafts. When TLI was used for posttransplantation conditioning instead of TLI and ATS, then mixed chimerism was achieved uniformly, but 7 of 8 heart grafts were rejected before day 60.8 Therefore, it is probable that immune regulation in addition to clonal deletion associated with chimerism are required to induce tolerance to organ grafts in this model. The findings in the current study can be extrapolated to other mouse strains and to other species. Tolerance after 10 doses of TLI and 5 doses of ATS was also induced when mouse donors were BALB/c and recipients were C57BL/6 (the reverse of the current study),8 or when vascularized heart graft and bone marrow donors were ACI rats, and TLI and ATS conditioned recipients were Lewis rats.40 More importantly, the findings could be extrapolated to humans, and tolerance could be induced in approximately 70% of recipients given combined kidney and hematopoietic cell transplantations from HLA-matched donors after conditioning with 10 doses of TLI and 5 doses of ATG.41,42 The rationale for using completely posttransplantation conditioning was the ability to extend the protocol in humans to transplants from deceased donors, because conditioning cannot be scheduled in advance. In conclusion, interactions between NKT and Treg cells are required to promote tolerance in this system, and the regimen has been translated to the successful induction of tolerance in humans.
Supplementary Material
Acknowledgments
The authors thank Glenna Letsinger for assistance in the submission of the paper, and Piyanka Chandra and Bill Robinson for their assistance with data analysis using the luminex machine. They also thank Dr P. Savage (Brigham Young University, UT) for providing the α-GalCer, and the NIH Tetramer Facility (Rockville, MD) for providing the CD1d-tetramer.
This work was supported by NIH research grants RO1-AI-037683, PO1-HL-075462, and PO1-CA-49605.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.H. designed and performed research, contributed vital analytical methods, collected, analyzed and interpreted data, and wrote the paper; X.T. purified NKT cells and helped perform adoptive transfer experiments; S.D. purified Treg cells and helped perform adoptive transfer experiments; R.G.N. helped design experiments and performed research; and S.S. provided overall research supervision and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for R.G.N. is Lung Transplant Program, University of Alberta, Edmonton, AB.
Correspondence: Samuel Strober, MD, Division of Immunology & Rheumatology/Medicine, Stanford University School of Medicine, CCSR Bldg, Rm 2215-C, 269 Campus Dr, Stanford, CA 94305-5166; e-mail: sstrober@stanford.edu.
References
- 1.Wood KJ, Ushigome H, Karim M, Bushell A, Hori S, Sakaguchi S. Regulatory cells in transplantation. Novartis Found Symp. 2003;252:177–188. discussion 88-93, 203-210. [PubMed] [Google Scholar]
- 2.Kang SM, Tang Q, Bluestone JA. CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects. Am J Transplant. 2007;7(6):1457–1463. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 3.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med. 2002;195(12):1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graca L, Daley S, Fairchild PJ, Cobbold SP, Waldmann H. Co-receptor and co-stimulation blockade for mixed chimerism and tolerance without myelosuppressive conditioning. BMC Immunol. 2006;7:9. doi: 10.1186/1471-2172-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 6.Schiopu A, Wood KJ. Regulatory T cells: hypes and limitations. Curr Opin Organ Transplant. 2008;13(4):333–338. doi: 10.1097/MOT.0b013e3283061137. [DOI] [PubMed] [Google Scholar]
- 7.Seino KI, Fukao K, Muramoto K, et al. Requirement for natural killer T (NKT) cells in the induction of allograft tolerance. Proc Natl Acad Sci U S A. 2001;98(5):2577–2581. doi: 10.1073/pnas.041608298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higuchi M, Zeng D, Shizuru J, et al. Immune tolerance to combined organ and bone marrow transplants after fractionated lymphoid irradiation involves regulatory NK T cells and clonal deletion. J Immunol. 2002;169(10):5564–5570. doi: 10.4049/jimmunol.169.10.5564. [DOI] [PubMed] [Google Scholar]
- 9.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192(9):1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196(3):389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandner SE, Clarkson MR, Salama AD, et al. Role of the programmed death-1 pathway in regulation of alloimmune responses in vivo. J Immunol. 2005;174(6):3408–3415. doi: 10.4049/jimmunol.174.6.3408. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka K, Albin MJ, Yuan X, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007;179(8):5204–5210. doi: 10.4049/jimmunol.179.8.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aramaki O, Shirasugi N, Takayama T, et al. Programmed death-1-programmed death-L1 interaction is essential for induction of regulatory cells by intratracheal delivery of alloantigen. Transplantation. 2004;77(1):6–12. doi: 10.1097/01.TP.0000108637.65091.4B. [DOI] [PubMed] [Google Scholar]
- 14.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 15.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 16.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 17.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 18.Terabe M, Swann J, Ambrosino E, et al. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202(12):1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng D, Lewis D, Dejbakhsh-Jones S, et al. Bone marrow NK1.1(-) and NK1.1(+) T cells reciprocally regulate acute graft versus host disease. J Exp Med. 1999;189(7):1073–1081. doi: 10.1084/jem.189.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto D, Asakura S, Miyake S, et al. Stimulation of host NKT cells by synthetic glycolipid regulates acute graft-versus-host disease by inducing Th2 polarization of donor T cells. J Immunol. 2005;174(1):551–556. doi: 10.4049/jimmunol.174.1.551. [DOI] [PubMed] [Google Scholar]
- 21.Liu R, La Cava A, Bai XF, et al. Cooperation of invariant NKT cells and CD4+CD25+ T regulatory cells in the prevention of autoimmune myasthenia. J Immunol. 2005;175(12):7898–7904. doi: 10.4049/jimmunol.175.12.7898. [DOI] [PubMed] [Google Scholar]
- 22.Jiang S, Game DS, Davies D, Lombardi G, Lechler RI. Activated CD1d-restricted natural killer T cells secrete IL-2: innate help for CD4+CD25+ regulatory T cells? Eur J Immunol. 2005;35(4):1193–1200. doi: 10.1002/eji.200425899. [DOI] [PubMed] [Google Scholar]
- 23.Pillai AB, George TI, Dutt S, Strober S. Host natural killer T cells induce an interleukin-4-dependent expansion of donor CD4+CD25+Foxp3+ T regulatory cells that protects against graft-versus-host disease. Blood. 2009;113(18):4458–4467. doi: 10.1182/blood-2008-06-165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nador RG, Hongo D, Baker J, Yao Z, Strober S. The changed balance of regulatory and naive T cells promotes tolerance after TLI and anti-T-cell antibody conditioning. Am J Transplant. 2010;10(2):262–272. doi: 10.1111/j.1600-6143.2009.02942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Q, Munger ME, Highfill SL, et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood. 2010;116(14):2484–2493. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wherry EJ, Ha SJ, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27(4):670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275(5302):977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 29.Cui J, Shin T, Kawano T, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278(5343):1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 30.Trager DK, Banks BA, Rosenbaum GE, et al. Cardiac allograft prolongation in mice treated with combined posttransplantation total-lymphoid irradiation and anti-L3T4 antibody therapy. Transplantation. 1989;47(4):587–591. doi: 10.1097/00007890-198904000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Slavin S, Strober S, Fuks Z, Kaplan HS. Induction of specific tissue transplantation tolerance using fractionated total lymphoid irradiation in adult mice: long-term survival of allogeneic bone marrow and skin grafts. J Exp Med. 1977;146(1):34–48. doi: 10.1084/jem.146.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schenk S, Kish DD, He C, et al. Alloreactive T cell responses and acute rejection of single class II MHC-disparate heart allografts are under strict regulation by CD4+ CD25+ T cells. J Immunol. 2005;174(6):3741–3748. doi: 10.4049/jimmunol.174.6.3741. [DOI] [PubMed] [Google Scholar]
- 33.Uhlig HH, Coombes J, Mottet C, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177(9):5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lan F, Zeng D, Higuchi M, Huie P, Higgins JP, Strober S. Predominance of NK1.1+TCR alpha beta+ or DX5+TCR alpha beta+ T cells in mice conditioned with fractionated lymphoid irradiation protects against graft-versus-host disease: “natural suppressor” cells. J Immunol. 2001;167(4):2087–2096. doi: 10.4049/jimmunol.167.4.2087. [DOI] [PubMed] [Google Scholar]
- 35.Sykes M. Mechanisms of tolerance induced via mixed chimerism. Front Biosci. 2007;12:2922–2934. doi: 10.2741/2282. [DOI] [PubMed] [Google Scholar]
- 36.Strober S, Spitzer TR, Lowsky R, Sykes M. Translational studies in hematopoietic cell transplantation: Treatment of hematologic malignancies as a stepping stone to tolerance induction. Semin Immunol. 2011;23(4):273–281. doi: 10.1016/j.smim.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dai H, Wan N, Zhang S, Moore Y, Wan F, Dai Z. Cutting edge: Programmed Death-1 defines CD8+ CD122+ T cells as regulatory versus memory T cells. J Immunol. 2010;185(2):803–807. doi: 10.4049/jimmunol.1000661. [DOI] [PubMed] [Google Scholar]
- 38.Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao W, Demirci G, Strom TB, Li XC. Stimulating PD-1-negative signals concurrent with blocking CD154 co-stimulation induces long-term islet allograft survival. Transplantation. 2003;76(6):994–999. doi: 10.1097/01.TP.0000085010.39567.FB. [DOI] [PubMed] [Google Scholar]
- 40.Lan F, Hayamizu K, Strober S. Cyclosporine facilitates chimeric and inhibits nonchimeric tolerance after posttransplant total lymphoid irradiation. Transplantation. 2000;69(4):649–655. doi: 10.1097/00007890-200002270-00029. [DOI] [PubMed] [Google Scholar]
- 41.Scandling JD, Busque S, Shizuru JA, Engleman EG, Strober S. Induced immune tolerance for kidney transplantation. N Engl J Med. 2011;365(14):1359–1360. doi: 10.1056/NEJMc1107841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and withdrawal of immunosuppressive drugs in patients given kidney and hematopoietic cell transplants. Am J Transplant. doi: 10.1111/j.1600-6143.2012.03992.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.