Abstract
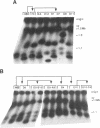
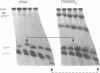
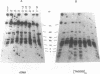
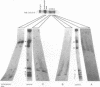
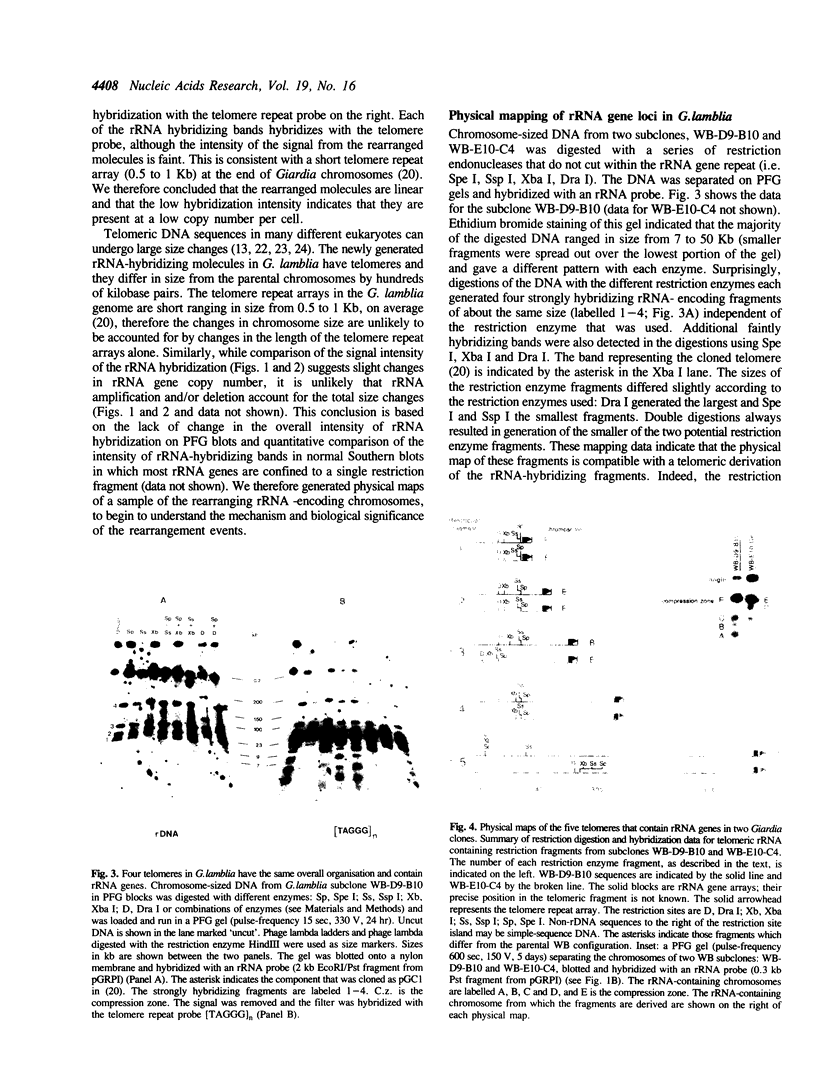
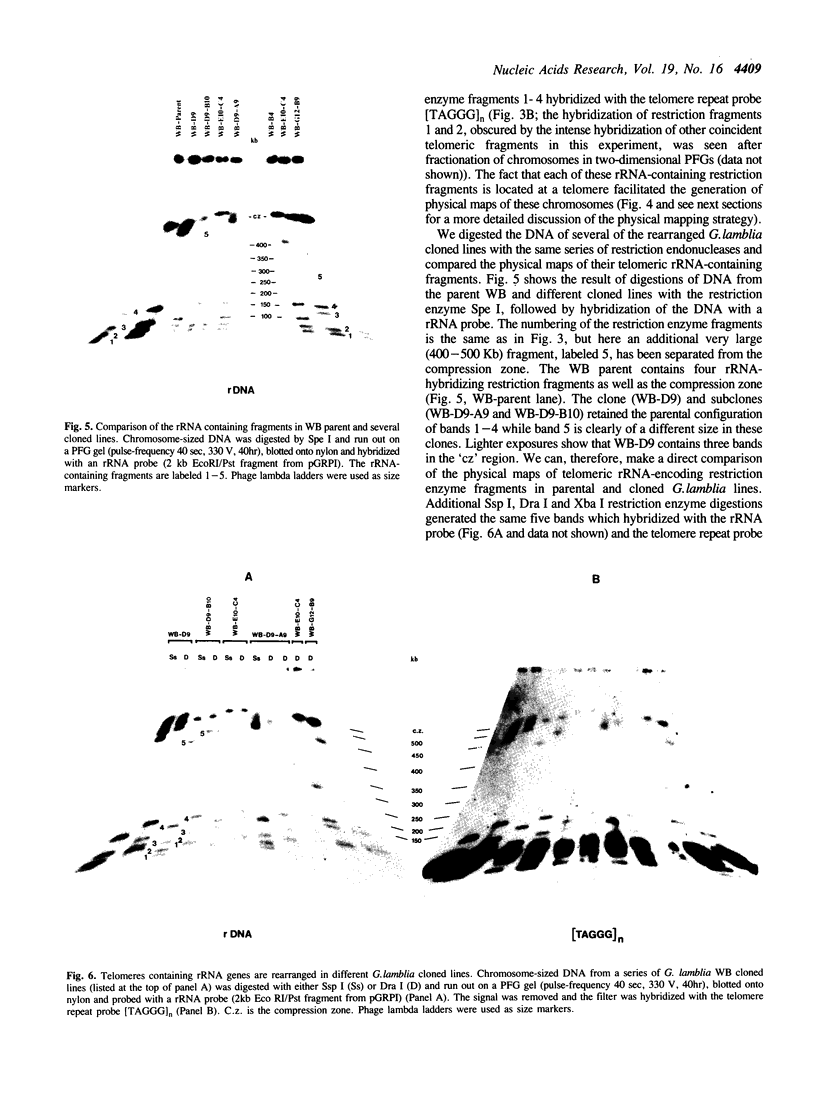
The ribosomal RNA (rRNA) genes in Giardia lamblia are present as short tandem arrays of a 5.6 Kb repeat unit on at least six telomeres. Four of these telomeres have the same overall organisation comprising a domain ranging in size from 25 to 300 Kb, delineated chromosome internally by a conserved island of restriction enzyme sites. Cloned lines of G. lamblia derived from the WB strain contain polymorphic subsets of chromosomes encoding rRNA genes. However, changes in the size of the rRNA telomere domains of these polymorphic chromosomes alone cannot account for the total size changes in the chromosomes. The rearrangement events are very frequent: 60% of subcloned lines had discrete rearranged karyotypes that differed from each other, suggesting either an estimated rearrangement rate that may be as high as 3% per division or a cloning-induced rearrangement event. The extreme plasticity of the genome has obvious implications for the maintenance of a functional genome and the control of gene expression in Giardia.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam R. D., Aggarwal A., Lal A. A., de La Cruz V. F., McCutchan T., Nash T. E. Antigenic variation of a cysteine-rich protein in Giardia lamblia. J Exp Med. 1988 Jan 1;167(1):109–118. doi: 10.1084/jem.167.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam R. D., Nash T. E., Wellems T. E. The Giardia lamblia trophozoite contains sets of closely related chromosomes. Nucleic Acids Res. 1988 May 25;16(10):4555–4567. doi: 10.1093/nar/16.10.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum K. F., Berens R. L., Jones R. H., Marr J. J. A new method for cloning Giardia lamblia, with a discussion of the statistical considerations of limiting dilution. J Parasitol. 1988 Apr;74(2):267–269. [PubMed] [Google Scholar]
- Bernards A., Michels P. A., Lincke C. R., Borst P. Growth of chromosome ends in multiplying trypanosomes. Nature. 1983 Jun 16;303(5918):592–597. doi: 10.1038/303592a0. [DOI] [PubMed] [Google Scholar]
- Boothroyd J. C., Wang A., Campbell D. A., Wang C. C. An unusually compact ribosomal DNA repeat in the protozoan Giardia lamblia. Nucleic Acids Res. 1987 May 26;15(10):4065–4084. doi: 10.1093/nar/15.10.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Greaves D. R. Programmed gene rearrangements altering gene expression. Science. 1987 Feb 6;235(4789):658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- Cappai R., van Schravendijk M. R., Anders R. F., Peterson M. G., Thomas L. M., Cowman A. F., Kemp D. J. Expression of the RESA gene in Plasmodium falciparum isolate FCR3 is prevented by a subtelomeric deletion. Mol Cell Biol. 1989 Aug;9(8):3584–3587. doi: 10.1128/mcb.9.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran L. M., Thompson J. K., Walliker D., Kemp D. J. Homologous recombination within subtelomeric repeat sequences generates chromosome size polymorphisms in P. falciparum. Cell. 1988 Jun 3;53(5):807–813. doi: 10.1016/0092-8674(88)90097-9. [DOI] [PubMed] [Google Scholar]
- Edlind T. D., Chakraborty P. R. Unusual ribosomal RNA of the intestinal parasite Giardia lamblia. Nucleic Acids Res. 1987 Oct 12;15(19):7889–7901. doi: 10.1093/nar/15.19.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel A., Yen T. J., Schwartz D. C., Smith C. L., Boeke J. D., Sollner-Webb B., Cleveland D. W. A rapidly rearranging retrotransposon within the miniexon gene locus of Crithidia fasciculata. Mol Cell Biol. 1990 Feb;10(2):615–624. doi: 10.1128/mcb.10.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin F. D., Diamond L. S. Entamoeba histolytica and Giardia lamblia: effects of cysteine and oxygen tension on trophozoite attachment to glass and survival in culture media. Exp Parasitol. 1981 Aug;52(1):9–17. doi: 10.1016/0014-4894(81)90055-2. [DOI] [PubMed] [Google Scholar]
- Gottesdiener K., Garciá-Anoveros J., Lee M. G., Van der Ploeg L. H. Chromosome organization of the protozoan Trypanosoma brucei. Mol Cell Biol. 1990 Nov;10(11):6079–6083. doi: 10.1128/mcb.10.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz M., Korman S. H., Shapiro M., Har-Even U., Tamir I., Strauss N., Deckelbaum R. J. Asymptomatic giardiasis in children. Pediatr Infect Dis J. 1989 Nov;8(11):773–779. doi: 10.1097/00006454-198911000-00009. [DOI] [PubMed] [Google Scholar]
- Kabnick K. S., Peattie D. A. In situ analyses reveal that the two nuclei of Giardia lamblia are equivalent. J Cell Sci. 1990 Mar;95(Pt 3):353–360. doi: 10.1242/jcs.95.3.353. [DOI] [PubMed] [Google Scholar]
- Keister D. B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77(4):487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Kim R. A., Wang J. C. A subthreshold level of DNA topoisomerases leads to the excision of yeast rDNA as extrachromosomal rings. Cell. 1989 Jun 16;57(6):975–985. doi: 10.1016/0092-8674(89)90336-x. [DOI] [PubMed] [Google Scholar]
- Kirk-Mason K. E., Turner M. J., Chakraborty P. R. Cloning and sequence of beta tubulin cDNA from Giardia lamblia. Nucleic Acids Res. 1988 Mar 25;16(6):2733–2733. doi: 10.1093/nar/16.6.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash T. E., Aggarwal A., Adam R. D., Conrad J. T., Merritt J. W., Jr Antigenic variation in Giardia lamblia. J Immunol. 1988 Jul 15;141(2):636–641. [PubMed] [Google Scholar]
- Nash T. E., Herrington D. A., Levine M. M., Conrad J. T., Merritt J. W., Jr Antigenic variation of Giardia lamblia in experimental human infections. J Immunol. 1990 Jun 1;144(11):4362–4369. [PubMed] [Google Scholar]
- Pologe L. G., Ravetch J. V. A chromosomal rearrangement in a P. falciparum histidine-rich protein gene is associated with the knobless phenotype. 1986 Jul 31-Aug 6Nature. 322(6078):474–477. doi: 10.1038/322474a0. [DOI] [PubMed] [Google Scholar]
- Ponzi M., Janse C. J., Dore E., Scotti R., Pace T., Reterink T. J., van der Berg F. M., Mons B. Generation of chromosome size polymorphism during in vivo mitotic multiplication of Plasmodium berghei involves both loss and addition of subtelomeric repeat sequences. Mol Biochem Parasitol. 1990 Jun;41(1):73–82. doi: 10.1016/0166-6851(90)90098-7. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H., Elwood H. J., Alonso R. A., Peattie D. A. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989 Jan 6;243(4887):75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Szostak J. W., Wu R. Unequal crossing over in the ribosomal DNA of Saccharomyces cerevisiae. Nature. 1980 Apr 3;284(5755):426–430. doi: 10.1038/284426a0. [DOI] [PubMed] [Google Scholar]
- Upcroft J. A., Boreham P. F., Upcroft P. Geographic variation in Giardia karyotypes. Int J Parasitol. 1989 Aug;19(5):519–527. doi: 10.1016/0020-7519(89)90082-9. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Schwartz D. C., Cantor C. R., Borst P. Antigenic variation in Trypanosoma brucei analyzed by electrophoretic separation of chromosome-sized DNA molecules. Cell. 1984 May;37(1):77–84. doi: 10.1016/0092-8674(84)90302-7. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Smith C. L., Polvere R. I., Gottesdiener K. M. Improved separation of chromosome-sized DNA from Trypanosoma brucei, stock 427-60. Nucleic Acids Res. 1989 Apr 25;17(8):3217–3227. doi: 10.1093/nar/17.8.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie A. O., Higgs D. R., Rack K. A., Buckle V. J., Spurr N. K., Fischel-Ghodsian N., Ceccherini I., Brown W. R., Harris P. C. Stable length polymorphism of up to 260 kb at the tip of the short arm of human chromosome 16. Cell. 1991 Feb 8;64(3):595–606. doi: 10.1016/0092-8674(91)90243-r. [DOI] [PubMed] [Google Scholar]