Abstract
Background and Aims
The family Balsaminaceae is essentially herbaceous, except for some woodier species that can be described as ‘woody’ herbs or small shrubs. The family is nested within the so-called balsaminoid clade of Ericales, including the exclusively woody families Tetrameristaceae and Marcgraviaceae, which is sister to the remaining families of the predominantly woody order. A molecular phylogeny of Balsaminaceae is compared with wood anatomical observations to find out whether the woodier species are derived from herbaceous taxa (i.e. secondary woodiness), or whether woodiness in the family represents the ancestral state for the order (i.e. primary woodiness).
Methods
Wood anatomical observations of 68 Impatiens species and Hydrocera triflora, of which 47 are included in a multigene phylogeny, are carried out using light and scanning electron microscopy and compared with the molecular phylogenetic insights.
Key Results
There is much continuous variation in wood development between the Impatiens species studied, making the distinction between herbaceousness and woodiness difficult. However, the most woody species, unambiguously considered as truly woody shrubs, all display paedomorphic wood features pointing to secondary woodiness. This hypothesis is further supported by the molecular phylogeny, demonstrating that these most woody species are derived from herbaceous (or less woody) species in at least five independent clades. Wood formation in H. triflora is mostly confined to the ribs of the stems and shows paedomorphic wood features as well, suggesting that the common ancestor of Balsaminaceae was probably herbaceous.
Conclusions
The terms ‘herbaceousness’ and ‘woodiness’ are notoriously difficult to use in Balsaminaceae. However, anatomical observations and molecular sequence data show that the woodier species are derived from less woody or clearly herbaceous species, demonstrating that secondary woodiness has evolved in parallel.
Keywords: Balsaminaceae, herbaceousness, Hydrocera, Impatiens, insular woodiness, light microscopy, primary woodiness, secondary woodiness, wood anatomy
INTRODUCTION
Balsaminaceae are a family of horticulturally important herbs that are characterized by an enormous floral diversity (Yuan et al., 2004; Janssens et al., 2006, 2009). Although the family exceeds 1000 species, only two genera are recognized: Impatiens and Hydrocera. The species-rich genus Impatiens is primarily distributed in the highlands and mountains of the Paleotropics, yet a few species also occur in temperate Eurasia and North America (Yuan et al., 2004; Janssens et al., 2009). In contrast, the genus Hydrocera contains only one species, Hydrocera triflora (Fig. 1A), and is confined to the lowlands of Indo-Malaysia (Grey-Wilson, 1980b). Large differences in habitat can be observed between both genera: Impatiens is nearly always associated with an often humid environment as it often grows along small rivers (Fig. 1B) or in the spray zone of waterfalls – although some species grow on physiologically dry limestone outcrops (Fig. 1C) – while Hydrocera is a semi-aquatic plant, thriving in stagnant water of pools and ditches (Grey-Wilson, 1980a). The high species diversity in Impatiens is reflected by the hypervariable floral morphology, of which the spurred sepal and the lateral petals show an extreme variability (Yuan et al., 2004; Caris et al., 2006; Janssens et al., 2008). Despite the enormous floral variation, the vegetative morphology of Impatiens is well preserved, always having glandular toothed leaves and often a fleshy semi-succulent stem (Fig. 1B).
Fig. 1.
Examples of the variation in growth forms of (A) Hydrocera and (B–D) Impatiens. (A) Hydrocera triflora, overview of the top part of the flowering stem, 4–5 ribbed (insert). (B) Impatiens glandulifera, relatively thick but herbaceous, semi-succulent stem with a limited amount of wood development, growing near the river Dijle, Leuven (Belgium). (C) Impatiens mirabilis (bottom left), very thick herbaceous, succulent stem consisting of abundant parenchyma tissue and a negligible amount of wood, growing on a limestone outcrop in Pulau Langgun (Malaysia); I. mirabilis is deciduous in the dry season (photo credit: Dr Max van Balgooy). (D) Impatiens niamniamensis, woody stems, growing in a tropical montane greenhouse of the Botanical Garden of Ulm (Germany).
The majority of the (sub-)tropical balsams are considered to be annual, especially the species growing in wet microhabitats without dry periods (Grey-Wilson, 1980a). However, a considerable number of species are perennial and have specific root adaptations, such as, for example, tubers in I. tuberosa (Madagascar) and I. mirabilis (Thailand), which are needed to survive the (usually short) dry season (Perrier de la Bathie, 1948; Grey-Wilson, 1980a; Newman, 2008). Also epiphytic or semi-epiphytic species are considered to be perennials that are often adapted to short periods of water shortage due to the formation of tubers (Grey-Wilson, 1980a; Cheek and Fischer, 1999; Janssens et al., 2010).
A small number of species in Africa, South India and Madagascar have robust shoots that initially thicken and gradually become woody with age (Fig. 1D), sometimes almost becoming shrubby (Hooker and Thomson, 1859; Grey-Wilson, 1980a). Interestingly, Grey-Wilson (1980a) suggested that a woody habit is probably not related to any specific habitat type, but independently originated throughout the genus. We want to investigate this hypothesis, and assess (1) whether the woody species in Impatiens have originated from herbaceous relatives (secondary woodiness) or resemble the woody ancestral state for the Ericales order (primary woodiness); and investigate (2) whether these habit shifts have happened several times within the genus. Three independent strategies can be applied to investigate whether herbaceous lineages have evolved into secondarily woody species. A first strategy is to trace evolutionary shifts towards secondary woodiness using a robust, species-dense molecular phylogenetic framework. Most of these secondarily woody lineages are found on islands and are therefore also referred to as insular woody lineages (e.g. Böhle et al., 1996; Francisco-Ortega et al., 2002; Lee et al., 2005). A second option is to make woody mutants from herbaceous wild types (Groover, 2005; Melzer et al., 2008; Lens et al., 2012). If molecular data are insufficient or even unavailable, which is still the case in many groups, a third source of evidence is to look for so-called paedomorphic features in the wood anatomy of the species under study (Carlquist, 1962, 1974, 1992, 2009; Koek-Noorman, 1976; Lens et al., 2005a, b, 2007, 2009; Dulin and Kirchoff, 2010). Paedomorphic or juvenile wood features resemble characters of the primary xylem that are protracted into the more mature secondary xylem (=wood) of secondarily woody species. Examples are the continuous decrease of vessel element length from the pith towards the cambium, the presence of wide gaping or gash-like intervessel pits resembling helical or reticulate tracheids in the primary xylem, and the absence of rays and/or the presence of rays with mainly square to upright ray cells. As stressed in Lens et al. (2009, 2012) and Dulin and Kirchoff (2010), scientists should make use of independent strategies to obtain sound conclusions on habit shifts towards secondary/insular woodiness, because studying merely wood anatomical observations or molecular data separately may lead to misinterpretation of the origin of woodiness within a particular group.
Stem anatomical observations in Balsaminaceae are extremely scarce. As far as we know, there are only two papers that describe the wood anatomy of Impatiens: Gerard (1917) includes a very brief description of only one species, and Lens et al. (2005b) provide a more detailed description of only two species. To rectify this lack of information, we have studied the stem anatomy of 68 Impatiens species from all major clades of the present molecular phylogeny (Yuan et al., 2004; Janssens et al., 2006, 2009) and Hydrocera triflora, and compared the anatomical observations with an improved phylogeny.
The objectives of this study are to present an overview of the stem anatomical variation in Balsaminaceae, and to investigate the origin of woodiness based on the anatomical observations in combination with an up-to-date molecular phylogeny. However, above all, this study wants to find a way to distinguish between herbaceousness and woodiness in a group that shows a continuous variation in wood development.
MATERIALS AND METHODS
Material
Stem samples from 69 Balsaminaceae species were collected from the living collection of the National Botanic Gardens of Belgium (BR), and the spirit and herbarium collection of the Netherlands Centre for Biodiversity Naturalis-section NHN (NCB Naturalis, L) (Appendix). Our sampling covers all major Balsaminaceae clades following the latest molecular phylogeny of Janssens et al. (2009). To increase the number of species for which molecular data are also available from earlier studies, we sequenced additional chloroplast atpB-rbcL and nuclear ImpDEF1 and ImpDEF2 sequences for the following species: Impatiens eriosperma, I. grandis, I. kilimanjari × pseudoviola, I. stuhlmannii and I. repens (GenBank accession nos HE617195–HE617200).
Since wood development – if present at all – is limited in many Balsaminaceae, we investigated only stem samples at the base of a mature plant during flowering. After sampling, we immediately stored the stems in 70 % alcohol to prevent the stems from drying out. This is important, because the majority of Balsaminaceae species have stems containing much parenchyma tissue that would otherwise completely shrink due to the drying process. Consequently, most of our samples had to be sampled from living collections grown in botanical gardens or from available spirit collections, and only a few samples of the woodiest species in the NCB Naturalis herbarium collection were added to our sampling (Appendix).
Wood anatomical descriptions and microtechnique
Stems of Balsaminaceae are typically soft because of the high ratio of parenchymatous vs. lignified tissues. Therefore, the standardized way of wood sectioning following Lens et al. (2005b) could only be applied for the most woody herbarium species. All the other species were embedded in LR White resin (hard grade, London Resin, UK) and sectioned with a rotary microscope according to the protocol described in Hamann et al. (2011). The LR White sections were stained with either toluidine blue or Etzolds dye (a mixture of 10 mg of fuchsin, 40 of mg safranin and 150 of mg astra blue dissolved in 100 mL of water, added with 2 mL of acetic acid). Transverse sections and longitudinal sections were made for the most woody species, while the other species were represented by transverse sections only. For length-on-age curves, measurements for vessel elements were made using radial sections from the pith towards the cambium, and added with maceration slides taken from various distances between pith and cambium. The wood anatomical terminology follows the ‘IAWA list of microscopic features for hardwood identification’ (IAWA Committee, 1989).
Molecular analysis
DNA was extracted using a modified cetyltrimethylammonium bromide (CTAB) protocol (Janssens et al., 2006). Primers used for amplification and sequencing of the chloroplast atpB-rbcL spacer and the nuclear ImpDEF1 and ImpDEF2 are obtained from Janssens et al. (2006, 2007). Amplified DNA was sequenced by the Macrogen sequencing facility (Macrogen, Seoul, South Korea). Sequences obtained in this study were submitted to GenBank (see the Materials and methods). Alignment of the nuclear and chloroplast sequences was conducted with MUSCLE under default parameters (Edgar, 2004) as implemented in the software program Geneious v.4·7·5 (Biomatters Ltd, Auckland) and subsequently fine-tuned by hand. Chloroplast atpB-rbcL and nuclear ImpDEF1/ImpDEF2 data matrices were analysed separately and combined using the probabilistic maximum likelihood (ML) method. Maximum likelihood analyses were performed using the RaxML search algorithm (Stamatakis et al., 2005) under the GTRGAMMA approximation of rate heterogeneity for each gene (Stamatakis, 2006). Five hundred bootstrap trees were inferred using the RaxML Rapid bootstrap algorithm (ML-BS) to provide support values for the best-scoring ML tree. A partition homogeneity test, as implemented in PAUP*4·0b10a (Swofford, 2002), was used to appraise whether the data sets provide different signals in the combined analyses.
Character mapping
It is known that most herbaceous species produce a limited amount of wood (Dulin and Kirchoff, 2010; Schweingruber et al., 2011; Lens et al., 2012), but how much wood does a species need to produce in order to be considered woody? All the species studied that do not form a wood cylinder are treated here as herbaceous (Table 1). In order to distinguish between ‘herbaceousness’ and ‘woodiness’ amongst the species that do form a wood cylinder at least at the base of their stems, we carefully measured the ratio of the double thickness of the wood cylinder over the entire stem diameter. Since the stems and wood cylinders are usually not perfectly cylindrical, we measured both stems and wood cylinders multiple times for each stem (eight measurements to calculate mean double wood cylinder thickness, four measurements to calculate mean stem diameter), and divided the mean values to obtain a habit quotient. The quotient for each species can be considered as a proxy for the continuously varying wood development we are interested in. Subsequently, this continuous character was treated as an ordered multistate character with 26 different character states using the gap weighting method of Thiele (1993), and implemented by the open-source software program MorphoCode (Schols et al., 2004). Consequently, besides species without a wood cylinder which are considered herbaceous, this method allows three other classes amongst the species with a complete wood cylinder to be chosen: ‘woody’ herbs (character states 1–9), slightly woody species (character states 12–14) and truly woody species (character states 17–26; see Supplementary Data Fig. S1 for a distribution of the character states among the species). An alternative option is to have only two classes, i.e. herbaceous species (all the species without a wood cylinder and species having character states 1–9) and woody species (character states 12–26; Table 1; Supplementary Data Fig. S1). Nevertheless, plotting the two- or four-character state features onto our up-to-date phylogenetic tree leads to the same conclusions with respect the origin of woodiness (Fig. 7). Parsimony character optimization was carried out using MacClade 4·05 (Maddison and Maddison, 2002) under the ACCTRAN algorithm.
Table 1.
Overview of selected stem anatomical characters within Balsaminaceae
| Taxon | Outer layer, meristimatic activity | No. of cell layers collenchyma, meristimatic activity | No. of cell layers cortex, meristimatic activity | No. of cell layers wood InterF vs. intraF region | Wood distributed as cylinder or as individual islands | Thickness double wood cylinder (mm) | Average diameter entire stem (mm) | Ratio thickness double wood cylinder/average stem diameter | Character state after gap weighting (n = 26) for species with wood cylinder | Four-character states | Two-character states |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrocera triflora | Epidermis, + | 2–3, + | Aerenchyma 10, ± | 0–7 vs. 18–45 | Island | – | 15·1 | – | – | Herbaceous | Herbaceous |
| Impatiens amplexicaulis | Periderm, + | ? | ? | 12–36 | Cylinder | 1·49 | 4·15 | 0·36 | 14 | Slightly woody | Woody |
| I. arguta | Epidermis, – | 3–4, – | 2–4, – | 10–15 vs. 12–20 | Cylinder | 0·56 | 3·85 | 0·15 | 5 | Woody herb | Herbaceous |
| I. aurea | Initiating periderm, + | 1–2, – | 1–2, – | 19–24 vs. 33–40 | Cylinder | 1·33 | 6·4 | 0·21 | 8 | Woody herb | Herbaceous |
| I. auricoma | Initiating periderm, + | 15–18, + | 12–17, + | 0 vs. 12–23 | Island | – | 18·1 | – | – | Herbaceous | Herbaceous |
| I. balfourii | Periderm, + | ? | ? | 50–100 | Cylinder | 2·73 | 5·28 | 0·52 | 21 | Truly woody | Woody |
| I. balsamina | Epidermis, + | 3–4, + | 4–6, + | 6–8 vs. 14–30 | Cylinder | 0·3 | 19 | 0·02 | 1 | Woody herb | Herbaceous |
| I. bicaudata | Initiating periderm, + | 20–25, + | 12–15, + | 0 vs. 40–45 | Island | – | 30 | – | – | Herbaceous | Herbaceous |
| I. biflora | Periderm, + | 1–3, – | 8–12, – | 24–67 | Cylinder | 1·56 | 5·16 | 0·3 | 12 | Slightly woody | Woody |
| I. burtonii ssp. burtonii | Epidermis, ± | 2–4, – | 5–8, ± | 0 vs. 2–3 | Island | – | 3 | – | – | Herbaceous | Herbaceous |
| I. campanulata | Epidermis, – | 5–7, + | 5–7, + | 18–20 vs. 25–35 | Cylinder | 1 | 7·9 | 0·13 | 5 | Woody herb | Herbaceous |
| I. capensis | Periderm, + | 1–2, – | 2–3, – | 20–25 vs. 25–30 | Cylinder | 1·34 | 6·3 | 0·21 | 8 | Woody herb | Herbaceous |
| I. catati | Epidermis, – | 2–7, – | 2–4, + | 0 vs. 2–3 | Island | – | 3·1 | – | – | Herbaceous | Herbaceous |
| I. cecili | Epidermis, – | 4–6, ± | 4–7, + | 40–45 vs. 40–55 | Cylinder | 2·01 | 5·8 | 0·35 | 14 | Slightly woody | Woody |
| I. clavigera | Initiating periderm, + | 6–8, – | 6–10, – | 0 vs. 6–8 | Island | – | 5·2 | – | – | Herbaceous | Herbaceous |
| I. curvipes | Epidermis, ± | 3–5, ± | 3–5, ± | 0 vs. 1–2 | Island | – | 2·8 | – | – | Herbaceous | Herbaceous |
| I. dewildeana | Periderm, + | ? | ? | 52–68 vs. 60–88 | Cylinder | 2·77 | 5·73 | 0·48 | 20 | Truly woody | Woody |
| I. edgeworthii | Epidermis, – | 3–5, – | 2–4, – | 0–3 vs. 12–20 | Island | – | 3·4 | – | – | Herbaceous | Herbaceous |
| I. eriosperma | Epidermis, – | 2–5, ± | 3–5, + | 0 vs. 2–3 | Island | – | 2·2 | – | – | Herbaceous | Herbaceous |
| I. eubotrya | Periderm, + | ? | ? | 37–58 vs. 56–69 | Cylinder | 1·74 | 5·1 | 0·34 | 14 | Slightly woody | Woody |
| I. flaccida | Initiating periderm, + | 5–8, + | 3–5, + | 60–80 | Cylinder | 2·89 | 6·78 | 0·43 | 17 | Truly woody | Woody |
| I. gesneroidea | Periderm, + | ? | ? | 47–68 | Cylinder | 3·45 | 6·14 | 0·56 | 23 | Truly woody | Woody |
| I. glandulifera | Epidermis, + | 6–11, ± | 4–9, ± | 17–20 vs. 30–35 | Cylinder | 0·5 | 27 | 0·018 | 1 | Herbaceous | Herbaceous |
| I. glandulosa | Epidermis, + | 8–10, – | 5–12, + | 0 vs. 1–2 | Island | – | 17 | – | – | Herbaceous | Herbaceous |
| I. grandis | Epidermis, – | 6–7, ± | 4–7, ± | 0 vs. 3–10 | Island | – | 10 | – | – | Herbaceous | Herbaceous |
| I. griffithii | Periderm, + | 1–2, – | 2–3, – | 10–14 vs. 15–29 | Cylinder | 0·62 | 3·35 | 0·19 | 7 | Woody herb | Herbaceous |
| I. havilandii | Periderm, + | ? | ? | 53–58 vs. 50–67 | Cylinder | 2·78 | 4·42 | 0·63 | 26 | Truly woody | Woody |
| I. hawkeri | Epidermis, + | 4–5, ± | 3–5, ± | 18–22 | Cylinder | 0·6 | 5 | 0·12 | 4 | Woody herb | Herbaceous |
| I. henslowiana | Epidermis, – | 4–6, + | 3–7, ± | 1–5 vs. 5–10 | Cylinder | 0·15 | 3·51 | 0·12 | 1 | Woody herb | Herbaceous |
| I. hians var. hians | Epidermis, + | 6–9, – | 3–7, ± | 12–20 vs. 25–35 | Cylinder | 0·6 | 8·2 | 0·07 | 3 | Woody herb | Herbaceous |
| I. hochstetteri ssp. hochstetteri | Epidermis, – | 9–11, – | 10–13, + | 0 vs. 8–18 | Island | – | 16 | – | – | Herbaceous | Herbaceous |
| I. inaperta | Epidermis, – | 2–3, – | 4–5, – | 0 vs. 1–2 | Island | – | 1·2 | – | – | Herbaceous | Herbaceous |
| I. irvingii | Epidermis, – | 4–8, + | 6–11, + | 18–23 vs. 30–40 | Cylinder | 1 | 7·5 | 0·13 | 5 | Woody herb | Herbaceous |
| I. jurpia | Periderm, + | 4–5, – | 3–6, – | 2–5 vs. 2–6 | Cylinder | 0·12 | 2·7 | 0·04 | 1 | Woody herb | Herbaceous |
| I. keillii ssp. keillii | Epidermis, + | 0–2, – | 6–9, + | 0 vs. 2–3 | Island | – | 5·4 | – | – | Herbaceous | Herbaceous |
| I. kilimanjari | Epidermis, – | 3–6, – | 4–8, ± | 0 vs. 1–2 | Island | – | 6·1 | – | – | Herbaceous | Herbaceous |
| I. kilimanjari × pseudoviola | Initiating periderm, + | 3–5, – | 4–7, + | 8–10 vs. 15–20 | Cylinder | 0·46 | 4·93 | 0·09 | 3 | Woody herb | Herbaceous |
| I. latifolia | Periderm, + | ? | ? | 13–18 vs. 25–28 | Cylinder | 0·78 | 3·28 | 0·24 | 9 | Woody herb | Herbaceous |
| I. leschenaultii | Periderm, + | ? | ? | 24–36 vs. 40–48 | Cylinder | 1·81 | 3·95 | 0·46 | 19 | truly woody | Woody |
| I. lyallii | Periderm, + | ? | ? | 15–20 vs. 22–36 | Cylinder | 0·94 | 4·5 | 0·21 | 8 | Woody herb | Herbaceous |
| I. mackeyana ssp. claeri | Periderm, + | 5–10, – | 8–13, ± | 0 vs. 10–17 | Island | – | 15 | – | – | Herbaceous | Herbaceous |
| I. macrophylla | Periderm, + | 2–3, – | 6–10, – | 15–26 | Cylinder | 0·65 | 9 | 0·072 | 2 | Herbaceous | Herbaceous |
| I. masonii | Epidermis, – | 2–7, – | 3–7, – | 0 vs. 0–1 | Island | – | 2·1 | – | – | Herbaceous | Herbaceous |
| I. mengtszeana | Epidermis, – | 4–8, – | 7–10, – | 0 vs. 0–1 | Island | – | 1·8 | – | – | Herbaceous | Herbaceous |
| I. mirabilis | Periderm, + | 15–25, + | 20–25, ± | 0 vs. 1–2 | Island | – | 25 | – | – | Herbaceous | Herbaceous |
| I. namchabarwensis | Epidermis, + | 2–5, + | 1–5, + | 90–110 | Cylinder | 3·4 | 5·53 | 0·61 | 25 | Truly woody | Woody |
| I. niamniamensis | Periderm, + | 7–10, – | 12–16, + | 80–100 | Cylinder | 4·14 | 14 | 0·3 | 12 | Slightly woody | Woody |
| I. noli-tangere | Epidermis, + | 1–3, – | 2–4, ± | 7–15 vs. 16–25 | Cylinder | 1·4 | 10 | 0·14 | 5 | Woody herb | Herbaceous |
| I. nomenya | Epidermis, – | 2–3, – | 7–9, + | 0 vs. 2–5 | Island | – | 4·6 | – | – | Herbaceous | Herbaceous |
| I. omeiana | Epidermis, – | 3–5, – | 6–8, – | 0 vs. 1–6 | Island | – | 4 | – | – | Herbaceous | Herbaceous |
| I. opinata | Epidermis, – | 2–5, – | 2–6, – | 2–5 vs. 2–6 | Cylinder | 0·065 | 1·1 | 0·059 | 2 | Herbaceous | Herbaceous |
| I. parasitica | Epidermis, – | 11–14, – | 5–6, ± | 0 vs. 5–10 | Island | – | 10 | – | – | Herbaceous | Herbaceous |
| I. parviflora | Epidermis, – | 2–3, – | 3–5, + | 24–30 vs. 35–45 | Cylinder | 1·68 | 8·15 | 0·21 | 8 | Woody herb | Herbaceous |
| I. platypetala | Epidermis, + | 4–6, + | 6–11, + | 2–15 vs. 9–30 | Cylinder | 0·75 | 6·94 | 0·11 | 4 | Woody herb | Herbaceous |
| I. psittacina | Epidermis, – | 2–5, ± | 3–6, – | 0 vs. 1–4 | Island | – | 1·6 | – | – | Herbaceous | Herbaceous |
| I. pseudomacroptera | Initiating periderm, + | 4–6, – | 4–6, – | 12–20 vs. 18–30 | Cylinder | 1·09 | 6·16 | 0·18 | 7 | Woody herb | Herbaceous |
| I. pseudoviola | Initiating periderm, + | 4–9, + | 6–8, + | 0 vs. 2–4 | Island | – | 4·4 | – | – | Herbaceous | Herbaceous |
| I. purpureo-violacea | Initiating periderm, + | 2–5, – | 3–5, ± | 10–17 vs. 12–14 | Cylinder | 0·42 | 2·6 | 0·16 | 6 | Woody herb | Herbaceous |
| I. repens | Epidermis, + | 2–3, ± | 5–10, + | 0 vs. 2–10 | Island | – | 6·7 | – | – | Herbaceous | Herbaceous |
| I. shirensis | Periderm, + | ? | ? | 36–44 vs. 42–51 | Cylinder | 1·43 | 4·03 | 0·36 | 14 | Slightly woody | Woody |
| I. sodenii | Epidermis, + | 5–8, + | 9–14, + | 2–3 vs. 10–15 | Cylinder | 0·1 | 8·4 | 0·01 | 1 | Woody herb | Herbaceous |
| I. stenantha | Periderm, + | ? | ? | 17–25 vs. 32–52 | Cylinder | 1·6 | 3·55 | 0·45 | 18 | Truly woody | Woody |
| I. stuhlmannii | Epidermis, – | 4–7, – | 4–7, ± | 9–17 vs. 13–33 | Cylinder | 0·57 | 6·99 | 0·08 | 3 | Woody herb | Herbaceous |
| I. cf. stuhlmannii | Epidermis, – | 3–8, – | 4–5, – | 4–8 vs. 6–13 | Cylinder | 0·2 | 3·74 | 0·05 | 2 | Woody herb | Herbaceous |
| I. usambarensis | Epidermis, – | 4–9, + | 4–8, + | 1–6 vs. 5–12 | Cylinder | 0·32 | 6·68 | 0·05 | 1 | Woody herb | Herbaceous |
| I. vaughanii | Epidermis, – | 3–4, – | 5–10, ± | 0 vs. 1–2 | Island | – | 2·5 | – | – | Herbaceous | Herbaceous |
| I. violaeflora | Periderm, + | ? | ? | 21–39 | Cylinder | 1·64 | 3·58 | 0·46 | 19 | Truly woody | Woody |
| I. viscida | Epidermis, – | 2–3, – | 5–7, ± | 0 vs. 5–15 | Island | – | 5 | – | – | Herbaceous | Herbaceous |
| I. vitellina | Periderm, + | ? | ? | 52–64 vs. 72–118 | Cylinder | 2·71 | 4·75 | 0·57 | 23 | Truly woody | Woody |
Species are arranged alphabetically. +, present; ±, sometimes present; –, absent; IntraF, intrafascicular; InterF, interfascicular.
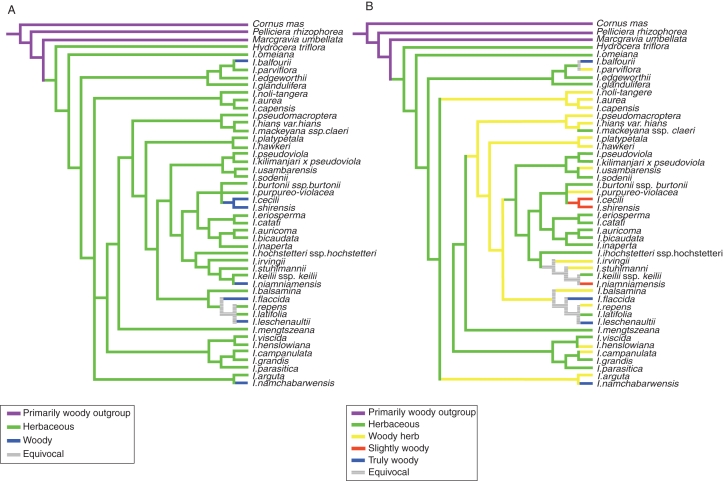
Fig. 7.
Phylogenetic tree showing multiple habit shifts towards secondary woodiness within Balsaminaceae. The primarily woody Cornus, Pelliciera and Marcgravia are chosen as the outgroup. Character optimization of the habit character on the strict consensus tree from chloroplast atpB-rbcL and nuclear ImpDEF1/ImpDEF2 sequences. (A) Two-character state analysis with two classes of species: herbaceous species (character states 1–9 plus all the species without a wood cylinder) and woody species (character states 12–26; Table 1). (B) Four-character state analysis with four classes of species: herbaceous species (species without a wood cylinder), ‘woody’ herbs (character states 1–9), slightly woody species (character states 12–14) and truly woody species (character states 17–26; Table 1).
RESULTS
Anatomical descriptions
The stem anatomy of Impatiens is described separately from the stem anatomy of Hydrocera (Table 1). The wood anatomical description of Impatiens is based on the ten most woody species having character states 17–26: I. balfourii, I. dewildeana, I. flaccida, I. gesneroidea, I. havilandii, I. leschenaultii, I. namchabarwensis, I. stenantha, I. violaeflora and I. vitellina (Figs 2F and 4; Table 2). For both genera examined, the numerator represents the number of species studied and the denominator includes the total number of species. Numbers without parentheses are ranges of means, while numbers between parentheses represent minimum or maximum values. A summary of selected wood features is shown in Table 2.
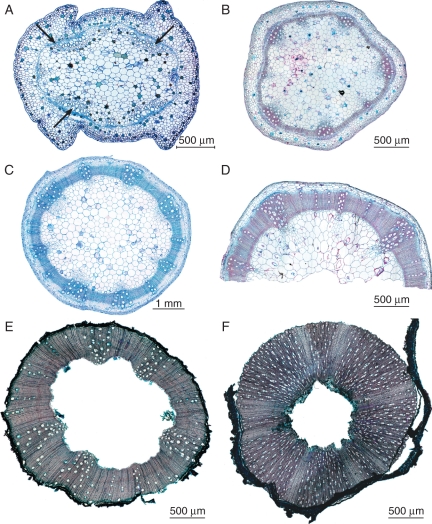
Fig. 2.
Transverse light microscope sections of Impatiens showing variation in wood development at the base of the stem. (A) Impatiens mansonii, clearly herbaceous stem without wood cylinder, intrafascicular cambium produces few wood cells (arrows). (B) Impatiens cf. stuhlmannii, herbaceous stem with narrow wood cylinder; character state after gap weighting = 2. (C) Impatiens hawkeri, herbaceous stem with slightly larger wood cylinder, character state after gap weighting = 4. (D) Impatiens latifolia, herbaceous stem with the most developed wood cylinder observed, but still limited compared with the entire stem diameter; character state after gap weighting = 9. (E) Impatiens eubotrya, woody stem, ratio of double wood cylinder thickness over stem diameter is significantly larger than in (D); wood cylinder also extends to the upper parts of the stem; character state after gap weighting = 14. (F) Impatiens gesneroidea, clearly woody stem; character state after gap weighting = 23.
Table 2.
Overview of selected wood anatomical characters within the ten most woody Impatiens species observed and Hydrocera triflora
| Taxon | Solitary vessels | Vessels in radial multiples | Vessels in clusters | Vessels confined to fascicular regions | Vessel diameter (μm) | Vessel density ( per mm2) | Vessel element length (μm) | Fibres septate | Ray-like areas present | Rays present | Ray width (no. of cells) | Multiseriate ray height (μm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrocera triflora | + | (2) | – | + | (25)-56-(80) | (60)-70-(80) | (150)-230-(400) | – | – | + | 1–2, 12–14 | >6200 |
| Impatiens balfourii | + | (2) | – | – | (30)-48-(60) | (16)-25-(36) | (100)-190-(270) | + | – | + | (1–2), 3–10 | (1200)-2020-(2600) |
| I. dewildeana | + | (2–3) | (3–5) | + | (30)-47-(70) | (5)-23-(42) | (225)-270-(350) | + | – | – | – | – |
| I. flaccida | + | (2–3) | – | – | (30)-60-(90) | (22)-36-(50) | (130)-220-(330) | – | – | + | 7–15 | >5000 |
| I. gesneroidea | ± | 2–3-(5) | – | – | (25)-41-(60) | (53)-64-(75) | (170)-225-(300) | + | + | – | – | – |
| I. havilandii | + | (2) | (3) | + | (30)-61-(90) | (3)-8-(17) | (175)-275-(350) | – | – | – | – | – |
| I. leschenaultii | + | (2–4) | – | – | (30)-45-(60) | (45)-66-(84) | (160)-220-(310) | ± | + | – | – | – |
| I. namchabarwensis | + | (2) | – | – | (30)-46-(60) | (8)-20-(38) | (150)-230-(350) | – | + | – | – | – |
| I. stenantha | + | (2) | – | + | (25)-39-(55) | (20)-27-(35) | (120)-175-(250) | – | – | – | – | – |
| I. violaeflora | ± | 2–3 | (3–5) | – | (30)-43-(70) | (48)-63-(80) | (190)-255-(320) | – | – | – | – | – |
| I. vitellina | + | (2–5) | – | + | (20)-27-(40) | (12)-29-(50) | (120)-175-(250) | – | + | – | – | – |
Species are arranged alphabetically. Numbers between hyphens are mean values flanked by minimum and maximum values. +, present; ±, sometimes present; – absent.
Impatiens (68/1000 + ; Figs 2–4; Tables 1 and 2; Supplementary Data Fig. S2)
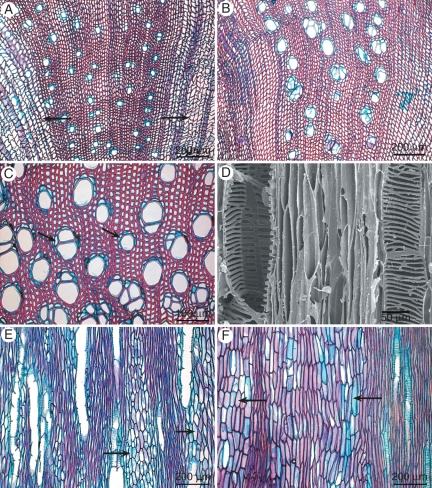
Fig. 4.
Wood anatomical illustrations of Impatiens using light microscopy (A–C, E, F) and scanning electron microscopy (D). (A) Impatiens vitellina, transverse section showing mainly solitary vessels and ray-like areas (arrows). (B) Impatiens dewildeana, transverse section vessels arranged solitarily or in clusters, rayless wood. (C) Impatiens shirensis, detail of transverse section showing scanty paratracheal parenchyma (arrows). (D) Impatiens flaccida, tangential section showing wide gaping intervessel pitting. (E) Impatiens balfourii, tangential section showing true rays with exclusively upright ray cells (arrows). (F) Impatiens vitellina, tangential section showing broad ray-like areas with fibre-like ray cells (arrows).
Continuous variation in wood development throughout the genus: ranging from islands of wood in the fascicular regions of some species (Fig. 2A), towards species with a small wood cylinder (Fig. 2B–D) over species with larger wood cylinders (Fig. 2E, F).
Stems hollow in I. aurea, I. balfouri, I. biflora, I. capensis, I. dewildeana, I. eubotrya, I. gesneroidea, I. glandulifera, I. griffithii, I. havilandii, I. jerdoniae, I. latifolia, I. lyallii, I. macrophylla, I. noli-tangere, I. pseudomacroptera and I. usumbarensis. Outer stem layer with (non-)meristematic epidermis attached in most species (Fig. 3A, B); initiating periderm observed in I. aurea, I. auricoma, I. bicaudata, I. clavigera, I. flaccida, I. kilimanjari × pseudoviola, I. pseudoviola, I. pseudomacroptera and I. purpureo-violacea, a more developed periderm present in most species that are considered to be slightly woody or truly woody except for I. capensis, I. griffithii, I. jurpia, I. latifolia, I. lyallii, I. mackeyana, I. macrophylla and I. mirabilis; enlarged cork cells in I. niamniamensis and I. stenantha. Collenchyma 3-6-(25) cell layers in width, meristematic activity mostly confined to species with large stem diameters (Fig. 3A, D). Cortex 3-7-(25) cell layers in width, meristematic activity mostly confined to species with large stem diameters (Fig. 3B). Cell groups radiating around remnants of primary xylem helical tracheids in pith observed in most species (Fig. 3D, E). Raphides born in mucilage sacs observed in collenchyma, cortex and pith parenchyma (Fig. 3F).
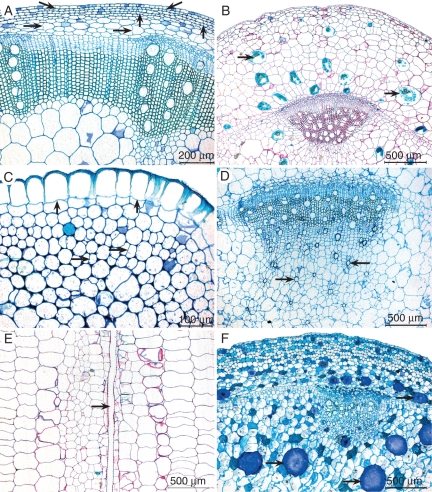
Fig. 3.
Transverse (A–D, F) and longitudinal (E) light microscope stem sections of Impatiens. (A) Impatiens hawkeri, meristematic activity in epidermis (oblique arrows), collenchyma (vertical arrows) and cortex (horizontal arrows). (B) Impatiens repens, epidermis at the outside, collenchyma narrow, broad cortex with abundant meristematic activity and enlarged idioblasts containing raphide bundles (arrows). (C) Impatiens pseudomacroptera, detail of periderm with phellogen (vertical arrows) forming enlarged cork cells at the outside; horizontal arrows point to meristematic activity in collenchyma. (D) Impatiens hochstetteri, cell groups radiating around lost primary xylem helical tracheids that resemble flowers (arrows). (E) Impatiens glandulifera, longitudinal section through such a flower-like structure; the arrow points to a lost primary xylem helical element. (F) Impatiens grandis, unusually enlarged idioblasts (arrows) in pith and cortex containing raphides in mucilage sacs.
Growth ring boundaries in wood absent. Wood diffuse-porous (Fig. 4A–C). Vessels (3)-8-70-(84) mm−2, usually solitary (Fig. 4A), sometimes in radial multiples of 2-3-(5) and/or occasionally in clusters of 3–5 (Fig. 4B, C), vessel outline angular. Vessel perforation plates simple. Lateral wall pitting typically wide gaping (pseudo-)scalariform (Fig. 4D) to sometimes reticulate, pits with minute borders, pit cavities 10–75 µm in horizontal size, in I. balfourii distinctly bordered alternate pitting, pit borders 6–12 µm in horizontal size, non-vestured. Tangential diameter of vessels (20)-27-61-(90) μm, vessel elements (100)-175-275-(400) μm long. Tracheids absent. Fibres septate in I. balfourii, I. dewildeana and I. gesneroidea, and also sometimes in I. leschenaultii, thin-walled, and relatively wide, (320)-420-640-(900) μm long, with mostly simple to occasionally minutely bordered pits equally distributed in radial and tangential walls, pits 2–3 µm in horizontal diameter. Axial parenchyma scanty paratracheal (Fig. 4C), 2–3 cells per strand. Rays absent in most species studied, tall and multiseriate in I. balfourii (Fig. 4E) and I. flaccida, 3–15 cells wide and (1200)-2020 to >5000 µm high, 0–2 rays mm−1, consisting of upright cells only, ray-like areas with fibre-like ray parenchyma cells observed in I. gesneroidea, I. leschenaultii, I. namchabarwensis and I. vitellina (Fig. 4F); sheath cells absent. Raphides observed in ray-like areas of I. vitellina.
Hydrocera (1/1; Fig. 5; Tables 1 and 2)
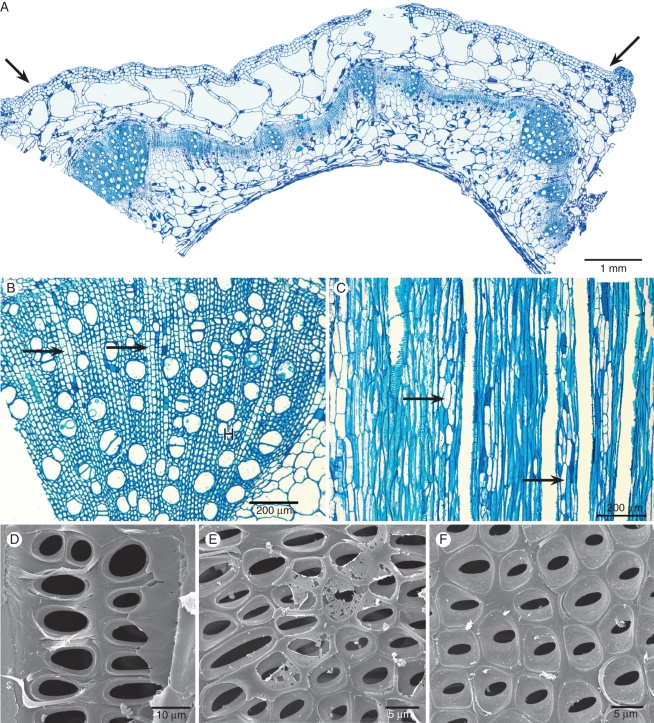
Fig. 5.
Stem anatomical pictures of Hydrocera triflora using light microscopy (A–C) and scanning electron microscopy (D–F). (A) Transverse section showing two stem ribs (arrows) and an aerenchyma region in the outer layer with large intercellular spaces. (B) Detail of transverse section showing wood formation at the rib region, vessels solitary or in small radial multiples, rays present (arrows). (C) Tangential section showing true rays with exclusively upright cells (arrows). (D) Tangential section near the pith showing enlarged intervessel pits with reduced pit borders. (E) Tangential section closer to the cambium; smaller intervessel pits with more pronounced pit borders. (F) Tangential section near the cambium; distinctly bordered intervessel pits in an opposite to alternate arrangement.
Wood formation is mainly restricted to the ribs of the stem. In between rib regions, the thin ring-like wood cylinder is interrupted at some places (Fig. 5A).
Stems hollow. Outer stem layer with epidermis still attached. Collenchyma 2–3 cell layers in width, without meristematic activity. Cortex modified into aerenchyma with large intercellular spaces (Fig. 5A). Raphides observed in collenchyma, aerenchyma and pith parenchyma.
Wood mostly confined to the ribs of the stem. Growth ring boundaries absent. Wood diffuse-porous (Fig. 5B). Vessels (60)-70-(80) mm−2, usually solitary and sometimes in radial multiples of two (Fig. 5B), vessel outline angular. Vessel perforation plates simple. Lateral wall pitting (pseudo-)scalariform near the primary xylem (Fig. 5D), 15–30 µm in horizontal size, and rapidly changing to an alternate pattern towards the cambium (Fig. 5E, F), pit border 5–6 µm in horizontal diameter, non-vestured. Tangential diameter of vessels (25)-56-(80) μm, vessel elements (200)-255-(400) μm long. Tracheids absent. Fibres non-septate, thin-walled, (400)-590-(800) μm long, with mostly simple to occasionally minutely bordered pits equally distributed in radial and tangential walls, pits 2-3 µm in diameter. Axial parenchyma scanty paratracheal, 2–3 cells per strand. Rays uniseriate and multiseriate, 12–14 cells wide and >6200 µm high, 0–2 rays mm−1, consisting of upright cells only (Fig. 5C); sheath cells absent. Raphides not observed in wood.
Phylogenetic analyses and character mapping
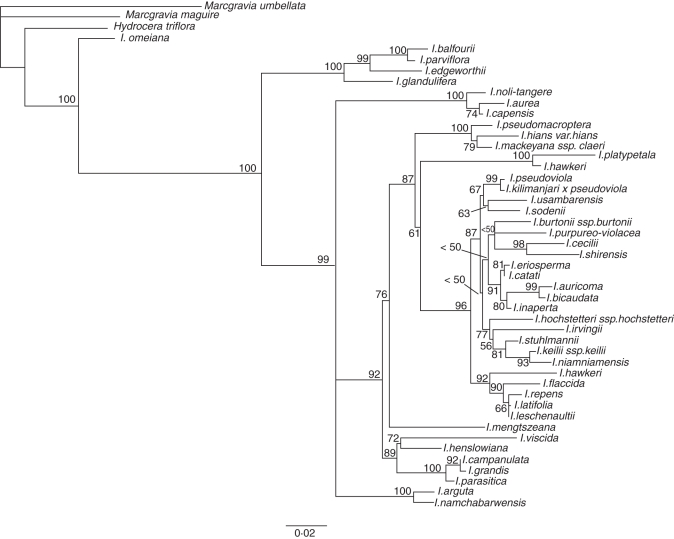
The combined data molecular matrix contains 3560 characters from which 961 (27 %) are variable. The chloroplast atpB-rbcL data matrix consist of 879 characters (213 variable characters), whereas the nuclear ImpDEF1 and ImpDEF2 data matrices contain 710 (197 variable) and 1970 (551 variable) characters, respectively. Despite the inability to amplify some loci for a few species, the missing data had no notable impact in the combined matrix. Ambiguously aligned nucleotides of microsatellite regions were removed from both chloroplast and nuclear data matrices. Despite the fact that ML analysis of the ImpDEF1/ImpDEF2 data sets resulted in a much better resolved topology than the atpB-rbcL data set, no incongruent relationships were found between the two analyses. This congruence is confirmed by the partition homogeneity test, which found no significant difference between both partitions of the combined data set (P > 0·05). The combined data set yielded a well-resolved topology in which most of the lineages are resolved (Fig. 6). The obtained topology corroborates the major clades found by Janssens et al. (2006, 2007, 2008, 2009). In total, only three unresolved lineages are found: (1) the clade consisting of I. arguta and I. namchabarwensis forming a polytomy with the clade including I. aurea, I. capensis and I. noli-tangere and the additional Impatiens species; (2) I. purpureo-violacea forming a polytomy with the I. burtonii–I. assurgens clade and the I. cecilii–I. shirensis clade; and (3) the group represented by I. repens, I. latifolia and I. leschenaultii. Bootstrap analysis shows relatively high support for many of the lineages. Nevertheless, some of the nodes have relatively low bootstrap value below 75, and thus interpretation of these phylogenetic relationships should be treated with caution (for a discussion of bootstrap values in phylogenetic analyses, see Hillis and Bull, 1993; Soltis and Soltis, 2003), but this does not change the number of habit shifts in this study.
Fig. 6.
Maximum likelihood analysis of the chloroplast marker atpB-rbcL and the nuclear markers ImpDEF1 and ImpDEF2, representing only species from which anatomical observations are also made. Numbers above or below branches indicate bootstrap support values.
Maximum parsimony optimization of four states (0 = herbaceous species, 1 = ‘woody’ herbs, 2 = slightly woody species, 3 = woody species) or only two states [0 = herbaceous species (combining 0 and 1, and species without a wood cylinder), 1 = woody species (combining 2 and 3)], representing variation in habit in Impatiens, indicates a similar pattern in which secondary woodiness originated at least five times from a herbaceous Balsaminaceae ancestor (Fig. 7).
DISCUSSION
Stem anatomical diversity within Impatiens
Based on a careful screening of >100 Impatiens species in the herbarium of the Netherlands Centre for Biodiversity Naturalis-section NHN (L), taking into account multiple flowering specimens per species with roots attached, we observed that more than about 70 % of the species had very thin and completely flattened stems, indicating that the amount of wood formation is negligible to nearly absent. This can be confirmed by the Impatiens literature mentioning that the genus can be characterized by herbaceous, semi-succulent stems (Grey-Wilson, 1980a; Yuan et al., 2004). However, amongst the >1000 species present in Impatiens, several of them show a continuous variation in wood development at the genus level (Table 1; Fig. 2, Supplementary Data Fig. S1): truly herbaceous species only show a few wood cells in the fascicular regions (Fig. 2A), while the woodiest species have a significant wood cylinder of >50 cell layers in thickness at the base of their stems (Fig. 2F). It is known that most so-called herbaceous eudicot species develop a limited amount of wood at the base of their stems, either confined to the fascicular regions or somewhat more developed into a tiny wood cylinder (Krumbiegel and Kästner, 1993; Schweingruber, 2007; Schweingruber et al., 2011; Lens et al., 2012). Consequently, the continuous range in wood formation within the genus Impatiens emphasizes once more the fuzzy boundary between the terms ‘herbaceousness’ and ‘woodiness’ – in line with authors advocating the continuum morphology (e.g. Sattler, 1996) – which makes it extremely difficult to decide at which point a species can be considered woody. Some authors even propose to abandon both terms and use the Raunkiær (1943) terminology instead, based on the presence/absence and the position of the surviving buds, to describe a plant's life form (Dulin and Kirchoff, 2010).
We believe that the terms herbaceousness and woodiness remain valid as long as two criteria are fulfilled: (1) ‘herbaceousness’ should not be interpreted as ‘without wood formation’; and (2) a detailed description of the entire stem anatomy should be provided so that it is clear how much wood is developed in relation to the rest of the stem. Based on the carefully measured ratio of double wood cylinder thickness over total stem diameter, we calculated a habit character that continuously varied among the 42 species having a wood cylinder at the base of their stems. Following the gap weighting method of Thiele, we could define either two- or four-character states (Fig. 7, Supplementary Data Fig. S1). According to us, the two-character state solution [herbaceous (no wood cylinder and character states 1–9) vs. woody (character states 12–26)] is more appropriate from an anatomical point of view (Fig. 7A), because one of the species with character state 12, I. niamniamensis, has one of the largest wood cylinders studied in terms of number of cell layers (Table 1). Moreover, the wood cylinder at the base of the stems in both I. niamniamensis and I. biflora – the two species investigated with character state 12 – also extends to the upper stem parts (results based on hand sections), which is also true for the woodier species. On the other hand, the narrower wood cylinders in species with character states 1–9 are restricted to the first few centimetres of the aboveground stem and are considered here as herbaceous.
In contrast to the large differences in amount of wood development within Impatiens, wood anatomical variation between the species observed is negligible. Impatiens species all have diffuse-porous wood with simple vessel perforation plates, very short vessel elements (frequently <300 µm), flat or continuously decreasing length-on-age curves (Supplementary Data Fig. S2), wide gaping scalariform vessel wall pitting (Fig. 4D), thin-walled fibres with simple to minutely bordered pits, and scanty paratracheal parenchyma (Fig. 4C). Rays are usually absent, but, when present, they are clearly visible as tall, multiseriate structures with exclusively upright ray cells (Fig. 4E, Table 2). In some species, transverse sections show radial zones of slightly different cells suggesting rays (Fig. 4A), but tangential sections of the same wood samples demonstrate that the shape and size of the cells in these so-called ray-like areas resemble those of libriform fibres (Fig. 4F).
When looking at the outer stem layers, many species with large stem diameters show meristematic activity in their cortex and collenchyma (Fig. 3A, B), explaining that secondary growth in Impatiens stems is not always triggered by a vascular cambium. For instance, the branch of I. mirabilis investigated – one of the species with the largest stems within the genus (Fig. 1C) – has a diameter of 25 mm, while the wood formation is negligible and only confined to the fascicular regions. In other words, stem diameter is not always a good proxy to distinguish between herbaceousness and woodiness. The presence of a periderm including a few layers of cork is better linked with woodiness, although some truly herbaceous species with large stem diameters have a periderm as well (I. macrophylla, I. mackeyana and I. mirabilis).
Two unusual features are found in the stems of Impatiens: ‘flower-like’ cells below the primary xylem regions observed in the pith in most species (Fig. 3D, E), and unusually large idioblasts in the pith of I. grandis (Fig. 3F) and I. parasitica. The flower-like structures can best be interpreted as remnants of primary xylem helical tracheids that have been removed from the primary xylem by meristematic activity, and are surrounded by elongated parenchymatous cells giving the appearance of a flower (cf. Solereder, 1899; Fig. 3D). This interpretation is confirmed by longitudinal sections (Fig. 3E). Some of the extremely large idioblasts in the pith of I. grandis bear raphide bundles embedded in mucilage sacs, and resemble smaller raphide-containing idioblasts in the cortex, which are often observed in Impatiens stems.
Stem anatomy of Hydrocera triflora
The stem anatomy of Hydrocera strongly resembles that of Impatiens, except for the aerenchyma that is derived from the normal parenchymatous cortex (Fig. 5A). Since H. triflora often grows in shallow water (Grey-Wilson, 1980b), the adapted cortex into a zone with large air-filled cavities allows low resistance transport of oxygen and other gases in the plant between stem parts above water and submerged parts. Wood formation in Hydrocera is largely confined to the ribs of the hollow stem (Fig. 5A) – interconnected by an extremely thin wood cylinder that is interrupted at some places – and is therefore interpreted as herbaceous in the present study. Although its wood anatomy is nearly identical to the one of Impatiens, Hydrocera wood can be distinguished from that of Impatiens by the presence of a gradual transition of scalariform to alternate intervessel pitting from the pith region towards the cambium (Fig. 5D–F).
Secondary origin of wood formation within Balsaminaceae
As discussed before, the wood anatomy of Balsaminaceae is characterized by a number of paedomorphic wood features, including flat or decreasing length-on-age curves for vessel elements in all the woodiest Impatiens observed and in Hydrocera (Supplementary Data Fig. S2). The relatively small wood cylinder observed in these woodiest species do not allow reconstruction of length-on-age curves over a long distance, but we feel confident that the curves generated are informative to assess secondary woodiness. This is based on the ideas of Bailey (1920) who demonstrated that the vessel element length remains almost constant with age in species having short vessel elements (<300 µm) with simple perforations (cf. Carlquist, 1962). However, from a hydraulic point of view, the length of entire vessels has proven to be more important in the water transport mechanism of plants, and greatly outweighs the importance of vessel element length (Sperry et al., 2006, 2007; Lens et al., 2011). A second paedomorphic wood feature that is often cited is the absence of rays or the presence of rays with exclusively upright ray cells (Fig. 4E; Carlquist, 1962, 1970, 1992, 2009), or the presence of ray-like areas containing fibre-like parenchyma cells (Fig. 4F). Whether or not the occurrence of wide gaping or gash-like intervessel pits in Impatiens (Fig. 4D) is a truly paedomorphic character and/or an adaptation to its parenchymatous semi-succulent stems is difficult to assess. The abundance of parenchyma cells in stems provides mechanical strength through cell turgor, which might compensate for the large intervessel pit apertures that weaken vessel walls (Dulin and Kirchoff, 2010). Alternatively, these wide gaping pits may be an adaptation to expansion and contraction of the slightly lignified wood during wet and drier periods, respectively (Carlquist, 2009), although this has to be experimentally evaluated.
Molecular evolutionary trees support our wood anatomical hypothesis that the most woody Impatiens species are secondarily woody. Whether or not one chooses to divide the character growth form into four or two character states, the woodiest species remain scattered in the Impatiens topology in at least five different clades (Fig. 7).
As has been mentioned by Grey-Wilson (1980a), the question of why secondary woodiness occurs within Impatiens remains unanswered. Various hypotheses on the origin of secondary woodiness have been put forward, such as intraspecific competition (Darwin, 1859; Tilman 1988; Givnish, 1995), ability to produce more seeds (Wallace, 1878), counter-selection against inbreeding (Böhle et al., 1996), uniform climate (especially absence of frost; Carlquist, 1974) and the absence of large native herbivores (Carlquist, 1974). Most of these hypotheses are based on secondarily woody plants that are native to islands (insular woodiness) or island-like regions on continents, a distribution which is also characteristic of many Impatiens species (Grey-Wilson, 1980a; Janssens et al., 2009, 2010, 2011). However, when the habitat of the woodiest Impatiens species is compared with that of the majority of the herbaceous species, none of these hypotheses applies to Impatiens.
Surprisingly, despite the presence of paedomorphic wood features in Balsaminaceae and the huge range of habit differences within the balsaminoid Ericales clade (lianas, mangroves, trees, small shrubs and woody herbs), we can list two phylogenetically informative wood anatomical resemblances between Balsaminaceae on the one hand and the related families Tetrameristaceae sensu lato and Marcgraviaceae on the other: septate libriform fibres and raphides in ray cells (Lens et al., 2005a). Furthermore, the three families share vessels in radial multiples and simple perforation plates, alternate intervessel pitting (although mainly absent in Impatiens) and paratracheal parenchyma, although this combination of characters is common in other woody flowering plant families as well.
Genetic background of secondary woodiness
Recently, Melzer et al. (2008) revealed the first evidence for the genetic mechanism triggering secondary or insular woodiness in a mutant of Arabidopsis thaliana. In this species, which only produces a negligible amount of wood in the fascicular regions under normal growth conditions (Lens et al., 2012), two flowering time control genes were knocked out: SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1) and FRUITFULL (FUL). Surprisingly, the resulting double mutant developed into a woody shrub after several months of growth, showing wood development throughout all stems and to a much larger extent than any A. thaliana mutant described so far. Moreover, the induced wood in this mutant showed the expected paedomorphic features, and strongly resembles other secondarily woody Brassicaceae native to islands, suggesting that knocking out SOC1 and FUL triggers the normal pathway leading to secondary woodiness in A. thaliana (Lens et al., 2012). Besides the shrub-like habit, the double mutants showed a combination of perennial-like features, such as a prolonged age up to 18 months, the co-occurrence of active vegetative and reproductive meristems, and recurrent flowering cycles (Melzer et al., 2008).
The fact that knocking out only two genes in a truly herbaceous species can result in a woody phenotype is not only amazing, but it may also offer an explanation as to how this relatively ‘simple’ genetic mechanism could perhaps trigger woodiness in many non-related flowering plant lineages on islands or island-like continental regions throughout the world (Carlquist, 1974; Böhle et al., 1996; Givnish, 1998; Lee et al., 2005; Lens et al., 2009). It also clearly demonstrates that herbaceous plants keep the genetic capability to develop woodiness, but the wood-forming genes need to be activated (Oh et al., 2003; Ko et al., 2004; Groover, 2005; Spicer and Groover, 2010). SOC1 and FUL might not be the only key players: other upstream, downstream or in parallel-acting (positive or negative) regulatory genes could be more important. Evidently, more genetic insights into wood development are required to better understand one of the most appealing developmental aspects of plants (Chaffey et al., 2002; Nieminen et al., 2004; Schrader et al., 2004; Dharmawardhana et al., 2010; Agusti et al., 2011).
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
S.B.J. is postdoctoral fellow of the Fund for Scientific Research-Flanders (Belgium) (FWO-Vlaanderen). We thank the National Botanic Garden of Belgium (BR) for providing us with material.
APPENDIX
List of taxa investigated in this study with reference to their locality and vouchers.
Hydrocera triflora (L.) Wight & Arn., grown at BR, 20060140-55; Impatiens amplexicaulis Edgew., India, Maas & Geesteranus s.n., NCB Naturalis, L0388626; I. arguta Hook.f. & Thomson, grown at BR, 20060001-13; I. aurea Muhl., USA, Meeuse 13, NCB Naturalis, L0388634; I. auricoma Baill., grown at BR, 20060034-46; I. balfourii Hk.f., France, Wright senior s.n., NCB Naturalis, L0388666; I. balsamina L., grown at BR, 20091329-10; I. bicaudata H.Perrier, grown at BR, 20020131-10; I. biflora Walter, France, student's expedition 210, NCB Naturalis (U 74807); I. burtonii Hook.f. ssp. burtonii, Uganda, collector unknown, grown at BR, 20090336-70; I. campanulata Wight, grown at BR, 20020132-11; I. capensis Meerb., France, Mennema s.n., NCB Naturalis, L0388795; I. catati Baill., grown at BR; I. cecili N.E.Br., grown at BR, 20060002-14; I. clavigera Hook.f., Thailand, Maxwell 1278, NCB Naturalis, L0201398; I. curvipes Hook.f., Thailand (Chiang Mai), Maxwell 654, NCB Naturalis, L0383007; I. dewildeana Grey-Wilson, Sumatra, de Wilde & de Wilde-Duyfjes 14030, NCB Naturalis, L001382; I. edgeworthii Hook.f., Germany, Janssens 003-2008 (LV); I. eriosperma H.Perrier, grown at BR, 20090397-55; I. eubotrya Miq., Sumatra, van Borssum Waalkes 2164, NCB Naturalis, L0388893; I. flaccida Arn., grown at BR, 19680576; I. gesneroidea Gilg., Laarman s.n., DR Congo, NCB Naturalis, L0388905; I. glandulifera Royle, Belgium, Lens & Janssens s.n, LV; I. glandulosa Tardieu, origin unknown, collector unknown, NCB Naturalis, L0383043; I. grandis B.Heyne, grown at BR, 20090002-41; I. griffithii Hook.f. & Thomson, Malaysia, Samsuri Amad. 299, NCB Naturalis, L0388920; I. havilandii Grey-Wilson, Borneo, Chew, Corner & Staiton 1050, NCB Naturalis, L0388925; I. hawkeri W.Bull, unknown origin, Janssens 006 (LV); I. henslowiana Arn., Sri-Lanka, collector unknown, grown at BR, 20090338-86; I. hians Hook.f. ssp. hians, grown at BR, 20060003-15; I. hochstetteri Warb. ssp. hochstetteri, grown at BR, 20091294-72; I. inaperta H.Perrier, Madagascar, grown at BR, 20090340-88; I. irvingii Hook.f. ex Oliv., grown at BR, 20081389-61; I. jurpia Buch.-Ham. ex Hook.f. & Thomson, Thailand (Chiang Mai), Maxwell 490, NCB Naturalis, L0383002; I. keilii Gilg ssp. keilii, Tanzania, grown at BR, 20090341-89; I. kilimanjari Oliv., grown at BR, 2006004-16; I. kilimanjari Oliv. × pseudoviola Gilg, grown at BR, JMG 94613; I. latifolia L. ssp. bipartita Grey-Wilson, Sri Lanka, Comanor 92, NCB Naturalis, L0389078; I. leschenaultii Wall., India, Hohenacker 1138, NCB Naturalis, L038990; I. lyallii Baker var. trichogyna H.Perrier, Madagascar, Bai & Vohinar s.n., NCB Naturalis, L0389107; I. mackeyana Hook.f. ssp. claeri (N.Hallé) Grey-Wilson, grown at BR, 20090003-42; I. macrophylla Gardner ex Hook.f., Sri Lanka, Kostermans 24608, NCB Naturalis, L0389876; I. masonii Hook.f., Thailand (Chiang Mai), Maxwell s.n., NCB Naturalis, L0799401; I. mengtszeana Hook.f., Thailand, Maxwell 1426, NCB Naturalis, L0201700; I. mirabilis Hook.f., Malaysia (Pulau Langkawi), van Balgooy 2361, NCB Naturalis, L0383038; I. namchabarwensis Morgan R.J., Yuan YM & Ge XJ, grown at LV; I. niamniamensis Gilg emend. G.M.Schulze, grown at BR, 19770093; I. noli-tangere L., Germany, Janssens 005-2008 (LV); I. nomenya Eb.Fish. & Raheliv., grown at BR, 20090349-00; I. omeiana Hook.f., grown at Botanic Garden in Ghent, 2002-1323; I. opinata Craib, Thailand (Kanchanaburi), Maxwell 1160, NCB Naturalis, L0382997; I. parasitica Bedd., grown at BR, 20020134-13; I. parviflora DC., Belgium, Lens & Janssens s.n., LV; I. platypetala Lindl., grown at BR, 20090005-44; I. pseudomacroptera Grey-Wilson, Gabon, Dessein 2023 (BR); I. pseudoviola Gilg, grown at BR; I. psittacina Hook.f., Thailand (Chiang Mai), Maxwell 1072, NCB Naturalis, L0382984; I. purpureo-violacea Gilg, Rwanda, grown at BR, 20090353-04; I. repens Moon, grown at BR; I. shirensis Baker F., Malawi, Brass 16423, NCB Naturalis, L0389737; I. sodenii Engl. & Warb. ex Engl., grown at BR, 20090006-45; I. stenantha Hook.f., India, Hooker 1239, NCB Naturalis, L0389743; I. stuhlmannii Warb., E. Africa, grown at BR, 16583; I. cf. stuhlmannii Warb., grown at BR, 20090356-07; I. usambarensis Grey-Wilson, grown at BR, 20090359-10; I. vaughanii Hook.f., Thailand (Nan), Maxwell 765, NCB Naturalis, L0201341; I. violaeflora Hook.f., Thailand, Maxwell s.n., NCB Naturalis, L0389801; I. viscida Wight, grown at BR, 20020133-12; I. vitellina Grey-Wilson, Sumatra, de Wilde & de Wilde-Duyfjes 13812, NCB Naturalis, L0389805.
LITERATURE CITED
- Agusti J, Lichtenberger R, Schwarz M, Nehlin L, Greb T. Characterization of transcriptome remodeling during cambium formation identifies MOL1 and RUL1 as opposing regulators of secondary growth. PLoS Genetics. 2011;7:e1001312. doi: 10.1371/journal.pgen.1001312. http://dx.doi.org/10.1371/journal.pgen.1001312 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey IW. The cambium and its derivative tissues. II. Size variations of cambial initials in gymnosperms and angiosperms. American Journal of Botany. 1920;7:355–367. [Google Scholar]
- Böhle UR, Hilger HH, Martin WF. Island colonization and evolution of the insular woody habit in Echium L. (Boraginaceae) Proceedings of the National Academy of Sciences, USA. 1996;93:11740–11745. doi: 10.1073/pnas.93.21.11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caris PL, Geuten KP, Janssens SB, Smets EF. Floral development in three species of Impatiens (Balsaminaceae) American Journal of Botany. 2006;93:1–14. [Google Scholar]
- Carlquist S. A theory of paedomorphosis in dicotyledonous woods. Phytomorphology. 1962;12:30–45. [Google Scholar]
- Carlquist S. Wood anatomy of insular species of Plantago and the problem of raylessness. Bulletin of the Torrey Botanical Club. 1970;97:353–361. [Google Scholar]
- Carlquist S. Island biology. New York: Columbia University Press; 1974. Insular woodiness; pp. 350–428. [Google Scholar]
- Carlquist S. Wood anatomy of sympetalous dicotyledon families: a summary, with comments on systematic relationships and evolution of the woody habit. Annals of the Missouri Botanic Garden. 1992;79:303–332. [Google Scholar]
- Carlquist S. Xylem heterochrony: an unappreciated key to angiosperm origin and diversifications. Botanical Journal of the Linnean Society. 2009;161:26–65. [Google Scholar]
- Chaffey N, Cholewa E, Regan S, Sundberg B. Secondary xylem development in Arabidopsis: a model for wood formation. Physiologia Plantarum. 2002;114:594–600. doi: 10.1034/j.1399-3054.2002.1140413.x. [DOI] [PubMed] [Google Scholar]
- Cheek M, Fischer E. A tuberous and epiphytic new species of Impatiens (Balsaminaceae) from southwest Cameroon. Kew Bulletin. 1999;57:669–674. [Google Scholar]
- Darwin C. On the origin of species by means of natural selection. London: John Murray; 1859. [Google Scholar]
- Dharmawardhana P, Brunner AM, Strauss SH. Genome-wide transcriptome analysis of the transition from primary to secondary stem development in Populus trichocarpa. BMC Genomics. 2010;11:150. doi: 10.1186/1471-2164-11-150. http://dx.doi.org/10.1186/1471-2164-11-150 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulin MW, Kirchoff BK. Paedomorphosis, secondary woodiness, and insular woodiness in plants. Botanical Review. 2010;76:405–490. [Google Scholar]
- Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5(113) doi: 10.1186/1471-2105-5-113. http://dx.doi.org/10.1186/1471-2105-5-113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco-Ortega F, Fuerte-Aguilar F, Kim SC, Santos-Guerra A, Crawford DJ, Jansen RK. Phylogeny of the Macaronsesian endemic Crambe section Dendrocrambe (Brassicaceae) based on internal transcribed spacer sequences of nuclear ribosomal DNA. American Journal of Botany. 2002;89:1984–1990. doi: 10.3732/ajb.89.12.1984. [DOI] [PubMed] [Google Scholar]
- Gerard AM. Recherches sur la spécification histologique de différents bois de Madagascar, avec étude comparative des principaux bois industriels d'Europe. France: Lons-Le-Saunier; 1917. [Google Scholar]
- Givnish TJ. Plant stems: biomechanical adaptation for energy capture and influence on species distributions. In: Gartner BL, editor. Plant stems: physiology and functional morphology. New York: Chapman and Hall; 1995. pp. 3–49. [Google Scholar]
- Givnish TJ. Adaptive plant evolution on islands: classical patterns, molecular data, new insights. In: Grant PR, editor. Evolution on islands. Oxford: Oxford University Press; 1998. pp. 281–304. [Google Scholar]
- Grey-Wilson C. Rotterdam: Balkema; 1980a. Impatiens of Africa. [Google Scholar]
- Grey-Wilson C. Hydrocera triflora, its floral morphology and relationship with Impatiens. Studies in Balsaminaceae. Kew Bulletin. 1980b;34:221–227. [Google Scholar]
- Groover AT. What genes make a tree a tree? Trends in Plant Science. 2005;10:210–214. doi: 10.1016/j.tplants.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Hamann TD, Smets E, Lens F. A comparison of paraffin and resin-based techniques used in bark anatomy. Taxon. 2011;60:841–851. [Google Scholar]
- Hillis DM, Bull JJ. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology. 1993;42:182–192. [Google Scholar]
- Hooker JD, Thomson T. Praecursores ad floram Indicam. Balsaminaceae. Botanical Journal of the Linnean Society. 1859;4:106–157. [Google Scholar]
- IAWA Committee. IAWA list of microscopic features for hardwood identification. International Association of Wood Anatomists Bulletin, New Series. 1989;10:219–332. [Google Scholar]
- Janssens SB, Geuten K, Yuan Y-M, Küpfer P, Smets EF. Phylogenetics of Impatiens and Hydrocera using chloroplast atpB-rbcL spacer sequences. Systematic Botany. 2006;31:171–180. [Google Scholar]
- Janssens SB, Geuten KP, Viaene T, Yuan Y-M, Song Y, Smets E. Phylogenetic utility of the AP3/DEF K-domain and its molecular evolution in Impatiens (Balsaminaceae) Molecular Phylogenetics and Evolution. 2007;43:225–239. doi: 10.1016/j.ympev.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Janssens SB, Viaene T, Huysmans S, Smets EF, Geuten KP. Selection on length mutations after frameshift can explain the origin and retention of the AP3/DEF-like paralogues in Impatiens. Journal of Molecular Evolution. 2008;66:424–435. doi: 10.1007/s00239-008-9085-5. [DOI] [PubMed] [Google Scholar]
- Janssens SB, Knox EB, Huysmans S, Smets EF, Merckx VSFT. Rapid radiation of Impatiens (Balsaminaceae) during Pliocene and Pleistocene: result of a global climate change. Molecular Phylogenetics and Evolution. 2009;52:806–824. doi: 10.1016/j.ympev.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Janssens SB, Fischer E, Stévart T. New insights on the origin of epiphytic Impatiens (Balsaminaceae) in West Equatorial Africa based on molecular phylogenetic and biogeographical analysis. Taxon. 2010;59:1508–1518. [Google Scholar]
- Janssens SB, Dessein S, Smets E. Portrayal of Impatiens nzabiana (Balsaminaceae): a morphological, molecular and biogeographic study of a new Gabonese species. Systematic Botany. 2011;36:440–448. [Google Scholar]
- Ko JH, Han KH, Yang J. Plant body weight-induced secondary growth in Arabidopsis and its transcription phenotype revealed by whole-transcriptome profiling. Plant Physiology. 2004;135:1069–1083. doi: 10.1104/pp.104.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek-Noorman J. Juvenile chracters in the wood of certain Rubiaceae with special emphasis to Rubia fruticosa Ait. International Association of Wood Anatomists Bulletin. 1976;3:38–42. [Google Scholar]
- Krumbiegel A, Kästner A. Sekundäres Dickenwachstum von Sproß und Wurzel bei annuellen dicotylen. 1993;4:1–49. Österreichische Akademie der Wissenschaften, Biosystematics and Ecology Series. [Google Scholar]
- Lee C, Kim S-C, Lundy K, Santos-Guerra A. Chloroplast DNA phylogeny of the woody Sonchus alliance (Asteraceae: Sonchinae) in the Macaronesian islands. American Journal of Botany. 2005;92:2072–2085. doi: 10.3732/ajb.92.12.2072. [DOI] [PubMed] [Google Scholar]
- Lens F, Caris P, Smets E, Serlet L, Jansen S. Comparative wood anatomy of the primuloid clade (Ericales s.l.) Systematic Botany. 2005a;30:162–182. [Google Scholar]
- Lens F, Dressler S, Jansen S, Van Evelghem L, Smets E. Relationships within balsaminoid Ericales: a wood anatomical approach. American Journal of Botany. 2005b;92:941–953. doi: 10.3732/ajb.92.6.941. [DOI] [PubMed] [Google Scholar]
- Lens F, Schönenberger J, Baas P, Jansen S, Smets E. The role of wood anatomy in phylogeny reconstruction of Ericales. Cladistics. 2007;23:229–254. doi: 10.1111/j.1096-0031.2006.00142.x. [DOI] [PubMed] [Google Scholar]
- Lens F, Groeninckx I, Smets E, Dessein S. Woodiness within the Spermacoceae–Knoxieae alliance (Rubiaceae): retention of the basal woody condition in Rubiaceae or recent innovation? Annals of Botany. 2009;103:1049–1064. doi: 10.1093/aob/mcp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens F, Sperry JS, Christman MA, Choat B, Rabaey D, Jansen S. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytologist. 2011;190:709–723. doi: 10.1111/j.1469-8137.2010.03518.x. [DOI] [PubMed] [Google Scholar]
- Lens F, Smets E, Melzer S. Stem anatomy supports Arabidopsis thaliana as a model for insular woodiness. New Phytologist. 2012;193:12–17. doi: 10.1111/j.1469-8137.2011.03888.x. [DOI] [PubMed] [Google Scholar]
- Maddison DR, Maddison WP. MacClade 4·04. Sunderland, MS: Sinauer Associates Inc; 2002. [Google Scholar]
- Melzer S, Lens F, Gennen J, Vanneste S, Rhode A, Beeckman T. Flowering time genes modulate meristem determinacy and growth form in Arabidopsis. Nature Genetics. 2008;40:1489–1492. doi: 10.1038/ng.253. [DOI] [PubMed] [Google Scholar]
- Newman MF. Impatiens pachycaulon (Balsaminaceae) a new species from Laos. Edinburgh Botanical Journal. 2008;65:23–26. [Google Scholar]
- Nieminen KM, Kauppinen L, Helariutta Y. A weed for wood? Arabidopsis as a genetic model for xylem development. Plant Physiology. 2004;135:635–659. doi: 10.1104/pp.104.040212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Park S, Han KH. Transcriptional regulation of secondary growth in Arabidopsis thaliana. Journal of Experimental Botany. 2003;54:2709–2722. doi: 10.1093/jxb/erg304. [DOI] [PubMed] [Google Scholar]
- Perrier de la Bathie H. Révision des Impatiens de Madagascar et des Comores. Mémoires de l'Académie des Sciences. 1948;67:1–16. [Google Scholar]
- Raunkiær C. Life forms of plants and statistical plant geography. Oxford: Clarendon; 1934. [Google Scholar]
- Sattler R. Classical morphology and continuum morphology: opposition and continuum. Annals of Botany. 1996;78:577–581. [Google Scholar]
- Schols P, D'Hondt C, Geuten K, Merckx V, Janssens S, Smets E. MorphoCode: coding quantitative data for phylogenetic analyses. Phyloinformatics. 2004;4:1–4. [Google Scholar]
- Schrader J, Nilsson J, Mellerowicz E, et al. A high-resolution transcript profile across the wood-forming meristem of poplar identifies potential regulators of cambial stem identity. The Plant Cell. 2004;16:2278–2292. doi: 10.1105/tpc.104.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweingruber FH. Wood structure and environment. Berlin: Springer; 2007. [Google Scholar]
- Schweingruber FH, Börner A, Schulze E-D. Atlas of stem anatomy in herbs, shrubs and trees. Vol. 1. Heidelberg: Springer; 2011. [Google Scholar]
- Solereder H. Systematische Anatomie der Dicotyledonen. Stuttgart: Verlag von Ferdinand Enke; 1899. [Google Scholar]
- Soltis PS, Soltis DE. Applying the bootstrap in phylogeny reconstruction. Statistical Science. 2:256–267. [Google Scholar]
- Sperry JS, Hacke UG, Pittermann J. Size and function in conifer tracheids and angiosperm vessels. American Journal of Botany. 2006;93:1390–1500. doi: 10.3732/ajb.93.10.1490. [DOI] [PubMed] [Google Scholar]
- Sperry JS, Hacke UG, Field TS, Sano Y, Sikkema EH. Hydraulic consequences of vessel evolution in angiosperms. International Journal of Plant Sciences. 2007;168:1127–1139. [Google Scholar]
- Spicer R, Groover A. Evolution of development of vascular cambia and secondary growth. New Phytologist. 2010;186:577–592. doi: 10.1111/j.1469-8137.2010.03236.x. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Ludwig T, Meier H. RAxML-III: a fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics. 2005;21:456–463. doi: 10.1093/bioinformatics/bti191. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods) Sunderland, MA: Sinauer Associates; 2002. Version 4·0b. [Google Scholar]
- Thiele K. The holy grail of the perfect character: the cladistic treatment of morphometric data. Cladistics. 1993;9:275–304. doi: 10.1111/j.1096-0031.1993.tb00226.x. [DOI] [PubMed] [Google Scholar]
- Tilman D. Resource competition and community structure. Princeton, NJ: Princeton University Press; 1988. [Google Scholar]
- Wallace AR. Tropical nature and other essays. London: Macmillan; 1878. [Google Scholar]
- Yuan Y-M, Song Y, Geuten K, et al. Phylogeny and biogeography of Balsaminaceae inferred from ITS sequence data. Taxon. 2004;53:391–404. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.