Abstract
Tendons regenerate and repair slowly and inefficiently after injury. Tendon-derived stem cells (TDSCs) have been isolated recently and have been shown to promote tendon repair. The ability to achieve sufficient numbers of cells for transplantation is essential for their clinical application. In this study, we aimed to study the effect of low oxygen (O2) tension (2%) on the clonogenicity, metabolic rate, DNA incorporation, population doubling time, β-galactosidase activity, immunophenotypes, multilineage differentiation potential, and tenocyte-like properties of human TDSCs (hTDSCs). hTDSCs were isolated from patellar tendon and characterized according to their adherence to plastic; colony-forming ability; multilineage differentiation potential; and high expression level of CD44, CD73, CD 90, and CD105 but low CD34, CD45, CD146, and Stro-1 at 20% O2 tension. Low O2 tension increased DNA incorporation but not metabolic rate of hTDSCs. It increased cell number 25% and the number of colonies but reduced the osteogenic, adipogenic, and chondrogenic differentiation potential of hTDSCs. The reduction in differentiation potential was associated with lower messenger RNA (mRNA) expression ratios of some lineage-related markers, including BGLAP, ALP, C/EBPα, PPARγ2, ACAN, and SOX9; the expression of a tendon-related marker, TNMD, was greater. There was no significant difference in the production of collagenous to noncollagenous protein ratio; the immunophenotypes and β-galactosidase activity were similar at 2% and 20% O2 tension. Hypoxia-preconditioned hTDSCs could successfully differentiate at 20% O2 tension, as shown by the return of the mRNA expression ratios of lineage-related markers to levels comparable to cells pre-incubated and differentiated at 20% O2 tension. In conclusion, hypoxia is advantageous for efficient expansion of hTDSCs in vitro for tendon tissue engineering.
Introduction
Tendon injuries are common in the workplace and sports activities.1 Tendons do not heal by a regenerative process but through the formation of a fibrotic scar. In a previous study, the crimp pattern of collagen fibers and fibrils of healing tendon was smaller than that of intact tendon, and the regenerated fibrotic scar tissue could not return to its original mechanical strength for a long time after injury, causing significant dysfunction and disability.2 The inability of tendons to self-repair and the general inefficiencies of current treatment regimens have spurred a demand for the development of tissue-engineering strategies for tissue replacement. Of particular interest in recent years is the use of adult mesenchymal stem cells (MSCs) to regenerate functional tendons,3–5 but ectopic bone formation after transplantation of bone marrow–derived MSCs (BMSCs) has been reported.3,6 There has also been evidence of tumor induction by undifferentiated BMSCs in some specific circumstances.7 Recently, multipotent stem cells, called tendon-derived stem cells (TDSCs), have been isolated from tendons.8,9 We fond that TDSCs promoted faster and better tendon repair histologically, biomechanically, and ultrasonographically than fibrin glue-only controls up to week 4 in a patellar tendon window injury rat model (unpublished work), indicating that TDSCs may be an appealing cell source for tendon tissue engineering.
Tendons have low cell density.10–12 The tendon fibroblasts occupy only a small fraction of the tendon volume.11,12 An efficient and high-yield process to obtain enough TDSCs for transplantation underlies cost-effective clinical application of TDSCs for tendon tissue engineering. The stem cell niches where stem cells reside essentially provide signals conducive to the maintenance of definitive stem cell properties.13 Physiological conditions, including biological factors, oxygen (O2) tension, mechanical loading, and extracellular matrix composition, are important regulators of stem and progenitor cell functions.8,14–16 Although the anatomical site of adult stem cells, including the tendon milieu, is relatively O2 deficient,17,18 cells isolated from these tissues are usually cultured under ambient condition, in which the “physiological hyperoxia” condition might alter the intrinsic stem cell properties during culture. It was reported that BMSCs proliferated faster and formed more colonies at 2% O2 tension, with an associated higher cellular metabolism and earlier start of cellular division, while maintaining MSC immunophenotype and multilineage differentiation potential.19 Tenocytes cultured at low O2 tension were also reported to enhance their proliferation capacity significantly without affecting their function and phenotype.20
Being a newly identified stem cell type, there has been no report on the behavior of TDSCs at low O2 tension. We hypothesized that culture of TDSCs under physiologically relevant low O2 tension may favor their in vitro expansion and maintenance of their undifferentiated stem cell characteristics, facilitating the application of TDSCs in tendon repair. In this study, we therefore aimed to study the effect of low O2 tension (2%) on the clonogenicity, metabolic rate, DNA incorporation, population doubling time, β-galactosidase activity, immunophenotypes, multilineage differentiation potential, and tenocyte-like properties of human TDSCs (hTDSCs).
Materials and Methods
Isolation and culture of hTDSCs
The clinical research ethics committee of the authors' institution approved the study. Human patellar tendons were collected from three patients undergoing anterior cruciate ligament reconstruction using bone–patellar tendon–bone autograft after obtaining their consent. Residual tissues from the patellar tendon autograft were collected, taking care not to collect tissue close to the tendon–bone junction. Peritendinous connective tissue was removed, and the tissue was sent to the laboratory in normal saline immediately for TDSC isolation. Human TDSCs (hTDSCs) were isolated from the patellar tendon tissue according to our established protocol, as described previously.9 Briefly, the tendon tissues with peritendinous connective tissue being removed were cut into small pieces and digested in type I collagenase (3 mg/mL; Sigma-Aldrich, St. Louis, MO) for 3 hours at 37°C. Undigested tissues and debris were filtered through a 70-μm cell strainer (Becton Dickinson, Franklin Lakes, IN) to yield single-cell suspension. The released cells in the filtrate were washed twice with phosphate-buffered saline (PBS; 137mM sodium chloride, 2.7mM potassium chloride, 100mM sodium pyrophosphate, and 2mM potassium dihydrogen phosphate, pH 7.4) by centrifugation at 300 g for 5 minutes and then resuspended in complete low-glucose Dulcecco's modified Eagle medium (LG-DMEM) supplemented with 10% fetal bovine serum (FBS), 50 μg/mL penicillin, 50 μg/mL streptomycin, and 100 μg/mL neomycin (all from Invitrogen, Carlsbad, CA). The isolated nucleated cells were plated at an optimal low plating density of 500 cells/cm2, as determined in previous experiments for the isolation of stem cells from human patellar tendon and cultured at 37°C, 5% carbon dioxide (CO2) to form colonies. At day 3 after initial plating, the cells were washed twice with PBS to remove the nonadherent cells. At day 7, the cells were trypsinized, pooled, and denoted as passage 0 (P0). The optimal initial seeding density for TDSCs isolation was determined using the colony-forming assay based on the following criteria: colony-to-colony contact inhibition did not affect colony size, and the greatest number of colonies per nucleated cell was obtained, with colonies that were smaller than 2mm in diameter and faintly stained being ignored. The optimal initial cell density thus determined was 500 cells/cm2 for the isolation of TDSCs from the human patellar tendon. TDSCs were subcultured when they reached 80% to 90% confluence. Medium was changed twice a week. Cells from passages 3 to 8 were used for subsequent experiments.
For the studies of clonogenicity, metabolic rate, DNA incorporation, population doubling time, β-galactosidase activity, and immunophenotypes, the cells were subcultured, incubated at 37°C under normoxic (20% O2, 5% CO2) conditions overnight, and then cultured under normoxic or hypoxic (2% O2, 5% CO2) conditions. For the study of multilineage differentiation potential and expression of tendon-like properties of hTDSCs under 20% or 2% O2 tension, the cells were cultured at 20% O2 tension until confluence and then subjected to induction and treatment at 20% or 2% O2 tension. To study the reversibility of the effect of hypoxia on the multilineage differentiation potential and expression of a tendon-related marker in hTDSCs, the cells were preconditioned under 20% or 2% O2 tension for at least 14 days before induction and treatment at 2% or 20% O2 tension. For maintaining the cells at hypoxic condition, we placed the cells in a hypoxic chamber (Galaxy 48R, C0-48-230, New Brunswick, Edison, NJ, kindly borrowed from Prof. Chao Wan, School of Biomedical Science, The Chinese University of Hong Kong) that was set at 37°C, 2% O2, 5% CO2.
Colony-forming unit assay
hTDSCs were seeded in a 10-cm culture dish at a seeding density of 100 cells/dish. At days 14 and 21, the cells were stained with 0.5% crystal violet (Sigma-Aldrich) for 15 minutes for counting of cell colonies. Colonies larger than 2 mm in diameter were counted.
Direct cell counting
Cells were seeded in a six-well plate at 500 or 5000 cells/cm2. At days 2, 4, 6, 8, and 10, the cells were harvested using 0.05% trypsin (Invitrogen) and resuspended in PBS after centrifugation at 300 g for 5 minutes at room temperature. Total cell number was counted using a hemocytometer. Doubling time (Td) at log phase was calculated according to the equation Td=0.3T/log(A/Ao), with T indicating the time elapsed, A the number of cells at the time of cell counting, and Ao the seeding cell number.
5-bromo-2′-deoxyruidine assay
The proliferation rate of hTDSCs was determined using the 5-bromo-2′-deoxyruidine (BrdU) assay (Roche, Mannheim, Germany) according to the manufacturer's instructions. In brief, hTDSCs were seeded on a 96-well plate from 1600 to 25 cells/cm2 in a twofold dilution manner. At days 4, 6, 8, and 10, the cells were labeled with BrdU, fixed, incubated with anti-BrdU antibodies conjugated with peroxidase, and then incubated with substrate. The proliferation rate was quantified by measuring the absorbance at 370 nm, with reference wavelength at 492 nm.
Alamar blue assay
The intracellular oxidative status and hence metabolic rate of hTDSCs was determined using alamarBlue cell viability reagent (Invitrogen). hTDSCs were seeded in a 96-well plate from 1600 to 25 cells/cm2 in a twofold dilution manner. At days 4, 6, 8, and 10, the cells were incubated with alamarBlue for 4 hours at 37°C. The metabolic rate of the cells was determined at 570 nm, with reference wavelength at 600 nm.
Senescence-associated β-galactosidase activity assay
hTDSCs were plated at 1×104 cells/cm2 in a six-well plate and incubated at 37°C, 5%CO2 at 2% or 20% O2 tension. At day 7, senescence-associated β-galactosidase activity was assessed using a mammalian β-galactosidase assay kit (Thermo Scientific, Inc., Rockford, IL) according to the manufacturer's instructions. Absorbance at 405 nm was measured and normalized with protein content.
Immunophenotypes
hTDSCs incubated at 20% or 2% O2 for 14 days were harvested using trypsinization, washed twice with PBS, pelleted using centrifugation at 350 g for 5 minutes at room temperature, and resuspended in staining buffer (Becton Dickson) at 2×106/mL for 15 minutes at 4°C. One hundred μL of cell suspension was incubated with primary antibodies against human CD44, CD90, CD73, CD34, and CD105 conjugated with phycoerythrin; CD45 conjugated with fluorescein isothiocyanate (FITC) (all from Becton Dickinson); CD146 (Abcam, Cambridge, UK); or stro-1 (R&D Systems, Inc., Minneapolis, MN) without conjugation for 15 minutes at 4°C. Unbound antibodies were removed by washing with ice-cold staining buffer. The cell pellet was resuspended in staining buffer containing antirabbit IgG conjugated with FITC (Santa Cruz Biotechnology, Santa Cruz, CA) for CD146 detection or antimouse immunoglobulin (Ig)M conjugated with allophycocyanin (R&D Systems, Inc.) for stro-1 detection, for at least 15 minutes at 4°C. The cells were washed with ice-cold PBS containing 2% bovine serum albumin before analysis using the LSRFortessa flow cytometer (Becton Dickinson). Rabbit IgG (Epitomics, Burlingame, CA), mouse IgG1 kappa (R&D Systems, Inc.) or mouse IgM (Becton Dickinson) antibodies were used as isotype controls.
Multilineage differentiation potential
For the induction of osteogenesis or adipogenesis, hTDSCs were seeded at 5000 cells/well in a six-well plate and cultured in completed LG-DMEM at 20% or 2% O2 tension before experiments. The cells were then cultured in complete LG-DMEM (basal group) or induction medium (induction group) for another 14 or 21 days at 20% or 2% O2 tension.
The osteogenic induction medium consisted of complete LG-DMEM supplemented with 100nM dexamethasone, 50μM L-ascorbic acid-2-phospate, and 20mM β-glycerol phosphate, and the adipogenic induction medium consisted of complete LG-DMEM supplemented with 500nM dexamethasone, 50μM indomethacin, 0.5mM 3-isobutyl-1-methylxanthine, and 10 μg/mL insulin. The cells were collected for RNA extraction at day 14 and fixed in 4% paraformaldehyde (pH 7.2) at day 21. Osteogenesis was examined according to the presence of calcium nodules stained with 0.5% (w/v) Alizarin red S (pH 4.1 adjusted using ammonia, Sigma) and adipogenesis according to the presence of intracellular oil droplets stained with 0.3% (w/v) fresh filtered Oil red O (Sigma-Aldrich) prepared in propylene glycol, as described previously.9 Briefly, for Alizarin red S staining of calcium nodules, the cell–matrix layer was washed with PBS, fixed with 4% paraformaldehyde (pH 7.2) for 15 minutes, and stained with 0.5% Alizarin red S for 15 minutes. For the Oil red O staining of oil droplets, the cells were washed with PBS, fixed with 4% paraformaldehyde (pH 7.2) for 15 minutes, and stained with 0.3% fresh Oil red O solution for 2 hours.
For chondrogenic induction, 5×105 hTDSCs that had been preincubated at 20% or 2% O2 tension before experiments were pelleted in a 15-mL centrifuge tube and cultured in complete LG-DMEM (basal group) or chondrogenic medium (induction group) (plain LG-DMEM supplemented with 100nM dexamethasone; 283.9 μM L-ascorbic acid-2-phospate; 1×insulin, human transferrin, and selenious acid (ITS) Premix (Becton Dickinson); 90.88μM sodium pyruvate; 34.75 μM L-Proline; 500 ng/mL bone morphogenetic protein (BMP-2); 10 ng/mL transforming growth factor beta 3 (TGF-β3) (both from R&D Systems, Inc.); 50 μg/mL penicillin; 50 μg/mL streptomycin; and 100 μg/mL neomycin (all other reagents, Sigma-Aldrich)) at 20% or 2% O2 tension. The cell pellets were collected for RNA extraction at day 14 and fixed in 4% paraformaldehyde, dehydrated, embedded in paraffin, sectioned at a 5-μm thickness, and stained with Safranin-O/fast green after deparaffination at day 21.
Determination of collagenous and noncollagenous protein production
The amount of collagenous and noncollagenous proteins that hTDSCs that had been precultured at 20% O2 tension produced was measured using Sirius Red F3BA and Fast Green FCF assays (Sigma-Aldrich), respectively, after incubating the cells at 20% or 2% O2 tension for 7 days, using previously described methods.21,22 Briefly, 5000 hTDSCs/cm2 were seeded in 24-well plates, cultured at 2% or 20% O2 tension for an additional 7 days after confluence, fixed with 70% (v/v) ethanol for 15 minutes, and stained with 0.1% (w/v) Sirius Red F3BA in saturated picric acid and 0.1% (w/v) Fast Green FCF in saturated picric acid for 15 minutes at room temperature for the quantification of collagenous and noncollagenous proteins, respectively. The staining solution was removed and the cells washed thoroughly with distilled water and air-dried overnight. The stain was extracted by the addition of 250 μL 1:1 (v/v) 0.1% sodium hydroxide and absolute methanol and measured at absorbance wavelengths of 540 nm and 610 nm for Sirius Red and Fast Green, respectively.
Expression of tendon-related markers
hTDSCs were cultured at 20% O2 tension until confluence and incubated at 20% or 2% O2 tension for 7 days for the assessment of mRNA expression of tendon-related markers. To study the reversibility of the effect of hypoxia on mRNA expression of tendon-related markers, mRNA expression of tendon-related markers in hTDSCs at 2% or 20% O2 tension that had been preconditioned at 20% or 2% O2 tension for at least 14 days was also measured.
Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed as previously described.16 The cells were harvested and homogenized for RNA extraction using an Rneasy mini kit (Qiagen, Hilden, Germany). Three hundred ng RNA was subjected to reverse transcription to complementary DNA (cDNA) using the First Strand cDNA kit (Promega, Madison, WI). Five μL total cDNA of each sample was amplified in a final volume of 25 μL reaction mixture containing Platinum SYBR Green qRT-PCR SuperMix-UDG ready-to-use reaction cocktail and specific primers for β-actin, alkaline phosphatase (ALP), osteocalcin (BGLAP), osteopontin (SPP1), CCAAT/enhancer binding protein alpha (C/EBPα), peroxisome proliferator-activated receptor gamma isoform 2 (PPARγ2), collagen type II α1 (COL2A1), SRY-related high-mobility group-box gene 9 (SOX9), aggrecan, tenascin C (TNC), tenomodulin (TNMD), or scleraxis (SCX) using the ABI StepOne Plus System (all from Applied Biosystems, Foster City, CA) (Table 1). The cycling conditions were denaturation at 95°C for 10 minutes, 40 cycles at 95°C for 15 seconds, optimal annealing temperature (Table 1) for 20 seconds, 72°C for 30 seconds, and 60°C to 95°C with a heating rate of 0.1°C/s. RNase-free water was used as a negative control for each set of experiments. The relative expression level of the gene of interest normalized to β-actin was calculated according to the 2-ΔΔCT formula. The difference in relative gene expression level between induction medium and basal medium was presented.
Table 1.
Primer Sequences and Annealing Temperature for Quantitative Real-Time Polymerase Chain Reaction
| Gene | Sequence (5′→3′) | Annealing temperature (°C) |
|---|---|---|
| β-actin | Forward: TGAAGTACCCCATCGAGCACG Reverse: CAAACATGATCTGGGTCATCTTCTC |
55 |
| Alkaline phosphatase | Forward: ACCATTCCCACGTCTTCACATTTG Reverse: AGACATTCTCTCTCGTTCACCGCC |
57 |
| Osteocalcin | Forward: ATGAGAGCCCTCACACTCCTC Reverse: GCCGTAGAAGCGCCGATAGGC |
57 |
| Osteopontin | Forward: TCCAACGAAAGCCATGACCA Reverse: TCCTCGCTTTCCATGTGTGA |
57 |
| CCAAT/enhancer binding protein alpha | Forward: AAGAAGTCGGTGGACAAGAACAG Reverse: GCAGGCGGTCATTGTCACT |
57 |
| Peroxisome proliferator-activated receptor gamma isoform 2 | Forward: AGCAAACCCCTATTCCATGCT Reverse: ATCAGTGAAGGAATCGCTTTCTG |
57 |
| Collagen type II alpha 1 | Forward: TGGCCTGAGACAGCATGAC Reverse: AGTGTTGGGAGCCAGATTGT |
58 |
| SRY-related high-mobility group-box gene 9 | Forward: TACGACTGGACGCTGGTGCC Reverse: CCGTTCTTCACCGACTTCCTCC |
60 |
| Aggrecan | Forward: AAGTATCATCAGTCCCAGAATCTAGC Reverse: CGTGGAATGCAGAGGTGGTT |
60 |
| Tenascin C | Forward: CCTAACCATTTCCGAC Reverse: GCACATAGGTAATCCG |
55 |
| Tenomodulin | Forward: CCATGCTGGATGAGAGAGGT Reverse: CTCGTCCTCCTTGGTAGCAG |
55 |
| Scleraxis | Forward: TCTCCAAGATTGAGACGCTG Reverse: TCTGTTTGGGCTGGGTGTTC |
60 |
Data analysis
The difference in cell number and the population doubling times were expressed as means±standard deviations. Differences in cell number, population doubling times, metabolic rate, DNA incorporation, β-galactosidase activity per μg protein, and mRNA expression of lineage-related markers are shown in boxplots. The representative histological photographs were shown. More than three O2 tension groups were compared using the Kruskal-Wallis test, followed by post hoc pair-wise comparison using the Mann-Whitney U-test; two O2 tension groups were compared using the Mann-Whitney U-test, and gene expression in induction medium and basal medium was compared using the Wilcoxon-signed rank test. All data analyses were performed using SPSS analysis software, version 16.0 (SPSS Inc, Chicago, IL). p≤0.05 was considered to be statistically significant.
Results
Cell proliferation
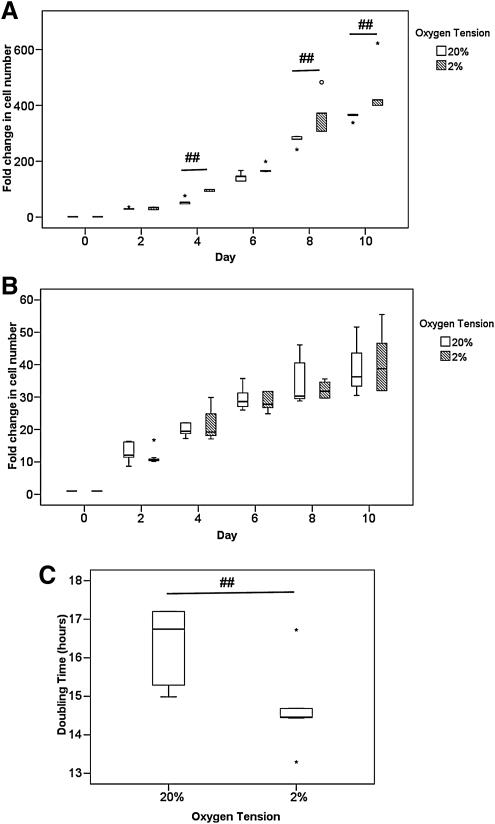
At a low plating density of 500 cells/cm2, hTDSCs proliferated faster at 2% than 20% O2 tension at days 4, 8, and 10 (all p=0.008) (Figure 1A). The cell number at 2% O2 tension was 450 times greater (452.5±95.6), which was 25% greater than that at 20% O2 tension (360.8±12.8 greater cell number) after incubation for 10 days (Figure 1A), but at a high plating density of 5000 cells/cm2, hTDSCs proliferated at a similar rate at 2% and 20% O2 tension up to day 10 (all p>0.05) (Figure 1B). The population doubling time of hTDSCs at low plating density at 2% O2 tension (14.67±1.02 hours) was significantly lower than at 20% O2 tension (16.36±0.97 hours; p=0.006) (Figure 1C).
FIG. 1.
Boxplots showing the fold change in the number of hTDSCs incubated at 20% or 2% oxygen (O2) tension at (A) low plating density (500 cells/cm2) and at (B) high plating density (5000 cells/cm2). (C) Boxplot comparing the doubling time of hTDSCs at the exponential phase at low plating density at 20% and 2% O2 tension. The data shown was the results of six independent experiments (N=6/group). ## indicated p≤0.01 for comparing 2% versus 20% O2 tension groups at each time point. “o” and “*” in boxplot represented outliner and extreme value, respectively. hTDSCs, human tendon-derived stem cells.
Metabolic rate and DNA incorporation
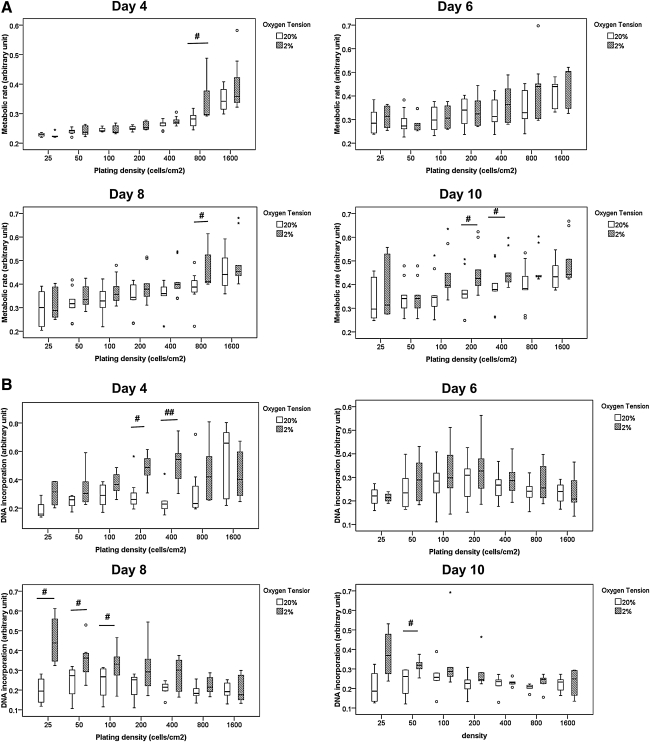
hTDSCs incubated at 20% and 2% O2 tension displayed similar metabolic rates except for a slight increase in the metabolic rates in the 2% O2 tension group at some seeding densities at days 4, 8, and 10. There was significantly higher metabolic rates in hTDSCs incubated at 2% O2 tension at 800 cells/cm2 at days 4 and 8 (both p=0.02), as well as at 200 and 400 cell/cm2 plating densities at day 10 than in cells incubated at 20% O2 tension (p=0.04 and 0.03, respectively) (Figure 2A), although the variation in data was large, and no specific pattern was seen. DNA incorporation in hTDSCs at 200 (p=0.02) and 400 (p=0.007) cell/cm2 plating densities at day 4; 25 (p=0.02), 50 (p=0.03), and 100 (p=0.048) cell/cm2 plating densities at day 8; and 50 cell/cm2 plating densities at day 10 (p=0.05) were significantly higher at 2% than 20% O2 tension (Figure 2B).
FIG. 2.
Boxplots showing the (A) metabolic rate as indicated by alamar blue assay and (B) DNA incorporation as indicated by BrdU assay of hTDSCs incubated at 20% or 2% oxygen (O2) tension at different plating densities at day 4, 6, 8 and 10. The data shown was the results of at least six independent experiments (N ≥6/group). # indicated p≤0.05; ## indicated p≤0.01 for comparing 2% versus 20% O2 tension groups at each time point. “o” and “*” in boxplot represented outliner and extreme value, respectively.
Clonogenicity
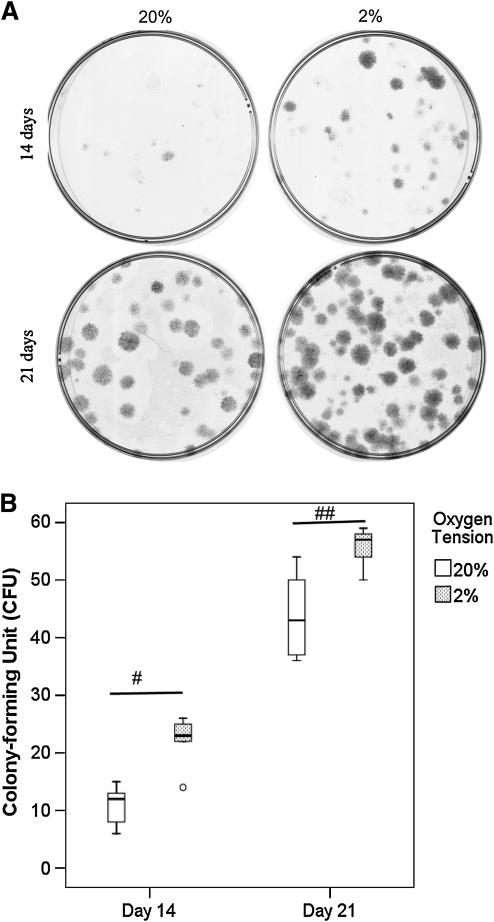
The colonies formed at 2% O2 tension were darker, indicating higher cell density, than those formed at 20% O2 tension (Figure 3A). More colonies were formed at 2% than 20% O2 tension at days 14 (p=0.02) and 21 (p=0.01) (Figure 3B).
FIG. 3.
(A) Photographs showing the clonogenicity of hTDSCs at day 14 and 21 after seeding at 100 cells/dish. The cells were stained with crystal violet before examination. (B) Boxplot showing the number of colonies upon incubation at 20% or 2% oxygen (O2) tension at day 14 and day 21. The data shown was the results of five independent experiments (N=5/group). # indicated p≤0.05 and ## indicated p≤0.01 for comparing 2% versus 20% O2 tension groups. “o” in boxplot (B) represented an outliner.
Senescence-associated β-galactosidase activity
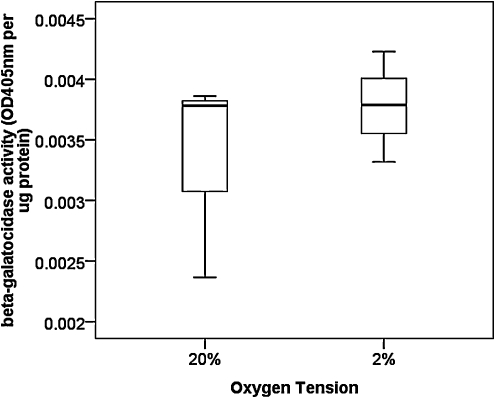
There was no significant difference in β-galactosidase activity between hTDSCs incubated at 20% and 2% O2 tension for 7 days (p=0.51) (Figure 4).
FIG. 4.
Boxplot showing the senescence-associated β-galactosidase activity in hTDSCs incubated at 20% or 2% oxygen (O2) tension. N=3/group.
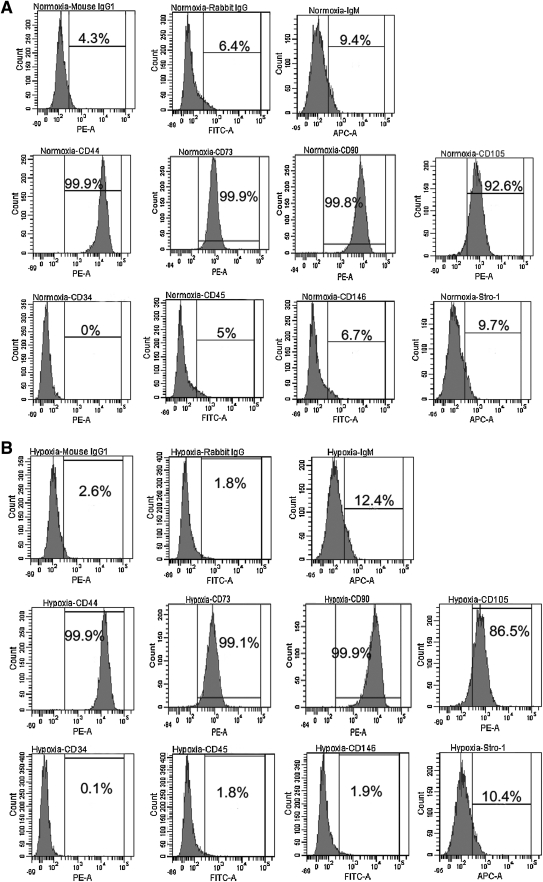
Immunophenotypes
hTDSCs expressed CD44, CD73, CD90, and CD105 but not CD34, CD45, CD146, or stro-1 at 20% O2 tension (Figure 5A). Similar expression patterns were observed for hTDSCs incubated at 2% O2 tension (Figure 5B).
FIG. 5.
Graphs showing the expression of surface markers on hTDSCs incubated at (A) 20% or (B) 2% oxygen tension. The upper three histograms were isotype controls for the corresponding primary antibodies. The middle panel showed those markers that were positively stained while the bottom panel showed those that were negatively stained in hTDSCs. N=3/group.
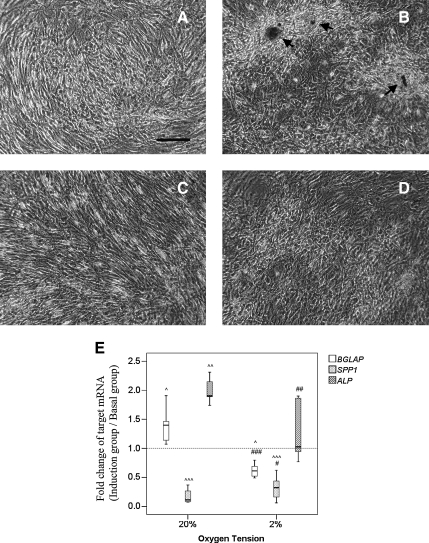
Osteogenic differentiation
hTDSCs were cultured at 20% O2 tension until confluence. Afterward, the cells were incubated in basal or osteogenic induction medium at 20% or 2% O2 tension. Calcium nodules were observed in hTDSCs at day 21 after osteogenic induction at 20% O2 tension (Figure 6B, arrows), but the formation of calcium nodules in hTDSCs was suppressed at 2% O2 tension (Figure 6D). The expression of BGLAP (p=0.01) and ALP (p=0.003) in hTDSCs were significantly upregulated 1.35 and 1.79 times, respectively, whereas the expression of SPP1 was significantly reduced by 90% (p=0.001) at 20% O2 tension at day 14 upon osteogenic induction (Figure 6E). For the cells incubated at 2% O2 tension during osteogenic induction, the expression of BGLAP (p=0.012) and SPP1 (p=0.001) were significantly downregulated, whereas the expression of ALP (p=0.81) was not significantly different from that in the basal group (Figure 6E). The relative expression ratios of BGLAP (p=0.001) and ALP (p=0.008) in hTDSCs incubated at 2% O2 tension were significantly lower than in cells incubated at 20% O2 tension (Figure 6E). The relative expression ratio of SPP1 in the 2% O2 tension group was significantly higher than that in the 20% O2 tension group after osteogenic induction (p=0.02) (Figure 6E).
FIG. 6.
Photographs showing the formation of calcium nodules in hTDSCs maintained at 20% oxygen (O2) tension until confluence and subsequently incubated at either (A, B) 20% or (C, D) 2% O2 tension in (A, C) basal or (B, D) osteogenic media for 21 days as indicated by Alizarin red S staining. N=6/group (E) Boxplot showing the relative mRNA expression ratios of BGLAP, SPP1 and ALP in hTDSCs maintained at 20% O2 tension until confluence and subsequently osteogenically induced at 20% or 2% O2 tension for 14 days. N=8/group for BGLAP, N=14/group for SPP1 and N=11/group for ALP. Arrow: calcium nodules; Scale bar: 50μm; # indicated p≤0.05, ## indicated p≤0.01 and ### indicated p≤0.001 for comparing 2% versus 20% O2 tension groups. ^ indicated p≤0.05, ^^ indicated p≤0.01 and ^^^ indicated p≤0.001 for comparing the induction group versus basal group.
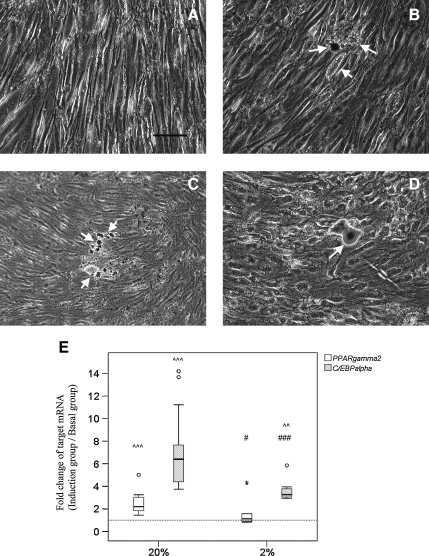
Adipogenic differentiation
hTDSCs were cultured at 20% O2 tension until confluence and then incubated in basal or adipogenic induction medium at 20% or 2% O2 tension. Intracellular oil droplets were seen in hTDSCs after 21 days of incubation in induction medium at 20% O2 tension (Figure 7B, arrows). Fewer cells with oil droplets were observed at 2% O2 tension after adipogenic induction (Figure 7D, arrows). Such difference was consistent with the mRNA results. The mRNA expression of PPARγ2 (p=0.001) and C/EBPα (p=0.001) in hTDSCs were significantly upregulated 2.56 and 7.18 times, respectively, at 20% O2 tension, and the relative mRNA expression ratios of PPARγ2 and C/EBPα in hTDSCs at 20% O2 tension were significantly higher than in cells incubated at 2% O2 tension (PPARγ2: p=0.02; C/EBPα: p<0.001) (Figure 7E). There was no significant difference between the expression of PPARγ2 in hTDSCs in the adipogenic induction medium and in cells in the basal medium at 2% O2 tension (p=0.21), whereas there was significantly greater expression of C/EBPα in hTDSCs after adipogenic induction at 2% O2 tension (p=0.003) (Figure 7E).
FIG. 7.
Photographs showing the formation of oil droplets in hTDSCs maintained at 20% oxygen (O2) tension until confluence and subsequently incubated at either (A, B) 20% or (C, D) 2% O2 tension in (A, C) basal or (B, D) adipogenic media for 21 days as indicated by Oil red O staining. N=6/group (E) Boxplot showing the relative mRNA expression ratios of PPARγ2 and C/EBPα in hTDSCs maintained at 20% O2 tension until confluence and subsequently adipogenically induced at 20% or 2% O2 tension for 14 days. N=9/group for PPARγ2 and N=11/group for C/EBP. Arrows: oil droplets; Scale bar: 25μm; # indicated p≤0.05, ## indicated p≤0.01 and ### indicated p≤0.001 for comparing 2% versus 20% O2 tension groups. ^^ indicated p≤0.01 and ^^^ indicated p≤0.001 for comparing the induction group versus the basal group. “o” and “*” in boxplot (E) represented outliner and extreme value, respectively.
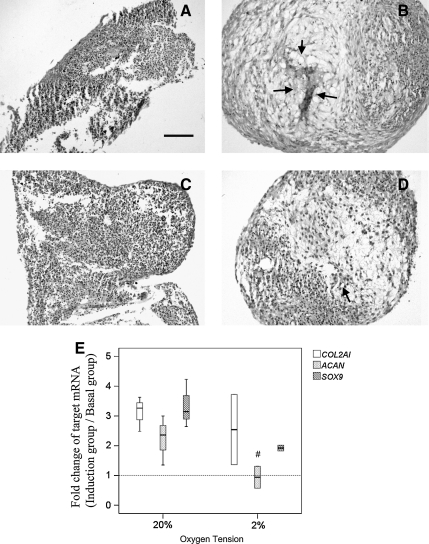
Chondrogenic differentiation
hTDSCs were cultured at 20% O2 tension until confluence and then pelleted and incubated in basal or chondrogenic induction medium at 20% or 2% O2 tension. Round and rigid pellets could not be formed in hTDSC culture in basal medium at 20% and 2% O2 tension (Figure 8A, C), although rigid pellets expressing proteoglycans could be observed after chondrogenic induction in both groups (Figure 8B, D). Pellets from culture at 2% O2 tension expressed fewer proteoglycans (Figure 8D) than those collected from hTDSC culture at 20% O2 tension (Figure 8B). Except the mRNA expression of ACAN at 2% O2 tension (p=0.28), the expression of COL2A1 (20%, p=0.07; 2%, p=0.11), ACAN (20%, p=0.07) and SOX9 (20%, p=0.11, 2%: p=0.07) in hTDSCs increased upon chondrogenic induction at 20% and 2% O2 tension though the increase was not statistically significant because of small sample size and large data variation (Figure 8E). The relative expression ratio of ACAN (p=0.03) in hTDSCs upon chondrogenic induction at 2% O2 tension was significantly lower compared to cells induced at 20% O2 tension (p=0.03, Figure 8E). The relative expression ratios of COL2A1 (p=0.72) and SOX9 (p=0.08) in hTDSCs were similar upon chondrogenic induction at 2% and 20% O2 tension (Figure 8E).
FIG. 8.
Photographs showing the production of proteoglycans in hTDSCs maintained at 20% oxygen (O2) tension until confluence and subsequently incubated in pellets at either (A, B) 20% or (C, D) 2% O2 tension in (A, C) basal or (B, D) chondrogenic media for 21 days as indicated by Safranin O (SO) / fast green staining. N=3/group (E) Boxplot showing the relative mRNA expression ratios of COL2A1, ACAN and SOX9 in hTDSCs maintained at 20% O2 tension until confluence and subsequently chondrogenically induced at 20% or 2% O2 tension for 14 days. N=3 or 4/group Arrows: SO-stained area; Scale bar: 100μm; # indicated p≤0.05 for comparing 2% versus 20% O2 tension groups.
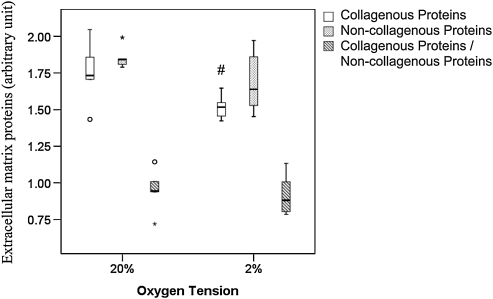
Collagenous and noncollagenous protein production
hTDSCs expressed a higher amount of collagenous protein at 20% O2 tension than at 2% O2 tension (p=0.04) (Figure 9 and supplementary information S1). There was no significant difference in the expression of noncollagenous protein (p=0.20) and collagenous to noncollagenous protein ratio (p=0.46) in hTDSCs incubated at 20% and 2% O2 tension (Figure 9 and supplementary information S1).
FIG. 9.
Boxplot showing the quantification of the amount of collagenous and non-collagenous protein in hTDSCs maintained at 20% oxygen (O2) tension until conference and subsequently incubated at 20% or 2% O2 tension for 7 days. # indicated p≤0.05 for comparing 2% versus 20% O2 tension groups. N=5/group at 20% and N=6/group at 2% O2 tension.
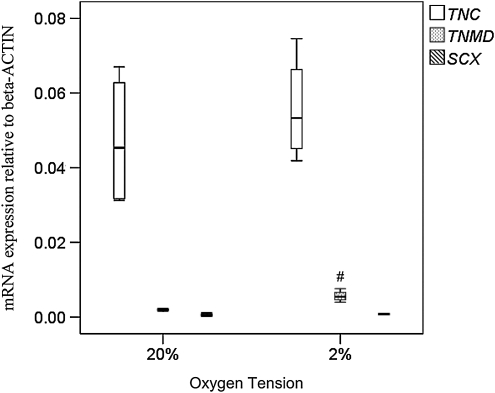
Expression of tendon-related markers
hTDSCs were cultured at 20% O2 tension until confluence and then incubated at 20% or 2% O2 tension for 7 days. hTDSCs expressed a significantly higher level of TNMD at 2% than at 20% O2 tension (p=0.02) (Figure 10). There was no significant difference in the expression of TNC (p=0.56) and SCX (p=0.24) in hTDSCs incubated at 2% and 20% O2 tension (Figure 10).
FIG. 10.
Boxplot showing the mRNA expression of TNC, TNMD and SCX in hTDSCs maintained at 20% until conference and subsequently incubated at 20% or 2% oxygen (O2) tension for 7 days. # indicated p≤0.05 for comparing 2% versus 20% O2 tension groups. N=4/group for TNC and TNMD, and N=3 or 4/group for SCX.
Ability of hTDSCs to maintain multilineage differentiation potential after hypoxic preconditioning
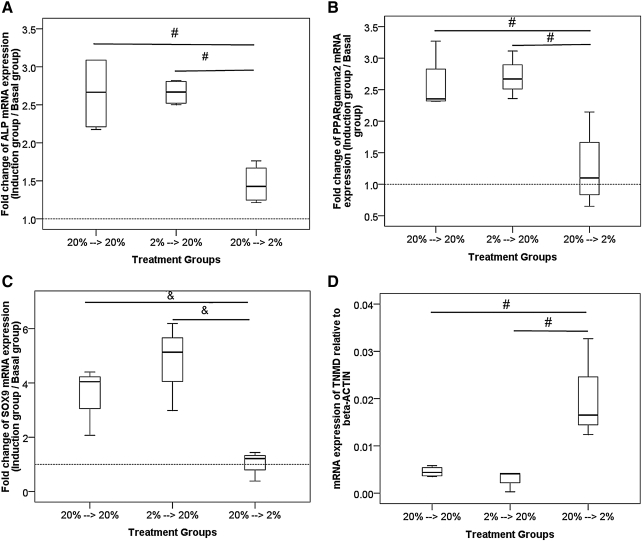
We measured the mRNA expression of ALP, PPARγ2, and SOX9 in hTDSCs preconditioned at 20% or 2% O2 tension for at least 14 days and then induced in osteogenic (for ALP), adipogenic (for PPARγ2), or chondrogenic (for SOX9) medium at 2% or 20% O2 tension. Consistent with the data shown in Figures 6 to 8, our results showed that induction at 2% O2 tension after preincubation at 20% O2 tension resulted in significantly lower expression ratios of ALP (overall p=0.02; post hoc p=0.02) (Figure 11A) and PPARγ2 (overall p=0.02; post hoc p=0.02) (Figure 11B) and nonsignificantly lower expression of SOX9 (overall p=0.05; post hoc p=0.05) (Figure 11C) than induction at 20% O2 tension. Preconditioning of hTDSCs at 2% O2 tension for at least 14 days before induction at 20% O2 tension reversed the effect of hypoxia. The expression ratios of ALP (post hoc p=0.02) (Figure 11A), PPARγ2 (post hoc p=0.02) (Figure 11B), and SOX9 (post hoc p=0.05) (Figure 11C) were significantly greater than with preconditioning of cells at 20% O2 tension and induction at 2% O2 tension. This resulted in expression ratios of ALP (post hoc p>.99) (Figure 11A), PPARγ2 (post hoc p=0.39) (Figure 11B), and SOX9 (post hoc p=0.275) (Figure 11C) similar to those with pre-incubation and induction of hTDSCs at 20% O2 tension.
FIG. 11.
Boxplots showing the relative mRNA expression ratios of (A) ALP, (B) PPARγ2, (C) SOX9 in hTDSCs maintained at 2% or 20% oxygen (O2) tension for at least 14 days and subsequently induced in (A) osteogenic, (B) adipogenic or (C) chondrogenic medium at 2% or 20% O2 tension for 14 days. (D) Boxplots showing the mRNA expression of TNMD in hTDSCs maintained at 2% or 20% O2 tension for at least 14 days and subsequently incubated at 2% or 20% O2 tension for 7 days. (N=3/group for SOX9; N=4/group for the other genes). # indicated post-hoc p≤0.05 with overall p≤0.05; & indicates post-hoc p≤0.05 with overall p>0.05.
We also measured the mRNA expression of TNMD at 20% or 2% O2 tension in hTDSCs preincubated at 20% or 2% O2 tension for at least 14 days. Our results showed that treatment of hTDSCs with 2% O2 tension for 7 days resulted in greater mRNA expression of TNMD than in hTDSCs treated with 20% O2 tension (p=0.03) (Figure 11D), consistent with the results shown in Figure 10. Preincubation of hTDSCs at 2% O2 tension for at least 14 days before treatment with 20% O2 tension resulted in significantly lower expression of TNMD than preincubation of cells at 20% O2 tension and treatment of cells with 2% O2 tension (p=0.03) (Figure 11D). This resulted in expression of TNMD similar to that of keeping hTDSCs at 20% O2 tension before and during the experiment (p=0.56) (Figure 11D).
Discussion
The number of stem cells required for tissue repair in the clinical setting is often greater than 1010 cells.23 For tendon repair, previous studies in rodents showed that at least 106 BMSCs were required,3,5,24 so it is not surprising that many more cells are needed in human patients. An optimized strategy for the in vitro expansion of stem cells while maintaining their undifferentiated stem cell characteristics is therefore essential. This study aimed to investigate the effect of low O2 tension (2%) on the in vitro expansion and maintenance of undifferentiated stem cell characteristics of hTDSCs. There has been no report on the measurement or mathematical estimation of O2 tension in human tendons, but it is reasonable to speculate that the O2 tension inside tendons is low, particularly after tissue injury, because tendons have a poor blood supply—only approximately one-third that of muscle.25 The O2 tension was reported to be 9% to 10% inside a sheep's patellar tendon,18 so the 2% O2 tension used in this study was expected to be below the physiological O2 tension inside tendons. We evaluated the behavior of hTDSCs only at 2% O2 tension in the present study; future study could optimize the in vitro culture condition by testing a wider range of O2 tension. We harvested and cultured hTDSCs at 20% O2 tension and then subjected the cells to different O2 tensions in each experiment rather than keeping the cells at different O2 tensions starting from cell harvesting. The effect on the experiments of exposing the cells to 20% O2 tension before subjecting them to 2% O2 tension is not known, although it is practically difficult to keep the cells at low O2 tension at all times from tissue harvesting until transplantation into the human body because this requires a special hood or culture room with O2 tension control. Hence our studies were designed with the cells harvested and cultured before experiments at the standard 20% O2 tension after considering this practical issue.
Our results showed that hypoxia increased clonogenicity and promoted hTDSC proliferation at a low seeding density. It took approximately 300 hours for 5000 hTDSCs to expand into 1010 cells at 2% O2 tension, and an additional 50 hours was required at 20% O2 tension. The degree of cell expansion is expected to be even greater with the use of a dynamic bioreactor with optimal supply of nutrients and removal of wastes instead of static tissue culture plates, as used in this study. The DNA incorporation and hence proliferation of hTDSCs were higher at 2% O2 tension at low initial seeding density at days 4, 8, and 10, consistent with the results of direct cell counting that significantly greater cell number was also observed on the same days at a low plating density of 500 cells/cm2. However, because the cell number increased with time, the plating density that showed greater DNA incorporation at 2% O2 tension compared to 20% O2 tension decreased on day 8 and day 10 compared to that at day 4. The culture of hTDSCs at a low plating density is hence critical for their in vitro expansion. This is consistent with previous studies that showed that stem cells, including TDSCs, grow faster at a very low seeding density.9,26,27
Hypoxia is a potent suppressor of mitochondrial oxidation28 and promoted “stemness” in adult and embryonic stem cells (ESCs).29–32 It has been suggested that the mitochondrial oxidative metabolism status is an indicator and regulator of the undifferentiated state of stem cells.33,34 A recent study showed that hypoxia substantially enhanced the reprogramming of mouse embryonic fibroblasts to induced pluripotent stem cells (iPSCs).32 Undifferentiated ESCs, iPSCs, and BMSCs were reported to display lower levels of mitochondrial mass and preferentially used nonoxidative glycolysis as a major source of energy.35–37 The preferential use of nonoxidative glycolysis to cover the bioenergetic needs of undifferentiated stem cells might therefore explain the similar mitochondrial activity and metabolic rate of hTDSCs as measured using the alamarBlue assay at 2% and 20% O2 tension despite the significantly grater cell number and DNA incorporation at 2% O2 tension.
There was no difference in senescence-associated β-galactosidase activity in hTDSCs at 2% and 20% O2 tension. This was different from the results of previous studies regarding the effect of hypoxia on maintaining self-renewal and inhibiting senescence of stem cells.31,38–41 This might be because of the short exposure of hTDSCs to 2% O2 tension (7 days) in our study. MSCs expanded under hypoxic condition were reported to expand by 100 population doublings, exhibiting telomerase activity with maintained telomere length, and did not form tumors.38 In another study, MSCs cultured under hypoxic condition (1% O2 tension) retained the ability to proliferate with an additional 8 to 20 population doublings, unlike cells cultured under normoxic condition (20% O2 tension).40 The MSCs of long-term hypoxic culture have higher multilineage differentiation potential than those cultured under normoxic condition.40 We have not examined the effects of hypoxia on the number of population doublings and the multilineage differentiation potential of long-term culture of hTDSCs. This requires further study.
The surface phenotypes of hTDSCs were maintained in hypoxic culture, although hTDSCs had less tendency to undergo osteogenic, adipogenic, and chondrogenic differentiation under hypoxic conditions. Our results are in concordance with those of previous study,42 in which hypoxic conditions produced opposite effects on stem cell proliferation and differentiation. It has been reported that low O2 tension maintains ESC pluripotency and minimizes spontaneous differentiation.29,30 Previous studies also showed that MSCs exhibited greater colony-forming potential,19,31,43–45 proliferated faster19,43,46–50 and longer,31,41 and maintained their undifferentiated characteristics better under low O2 conditions.31,45,46,50,51 Their immunophenotype remained unchanged,19,52 yet discrepancies in other studies were noticed in which low O2 tension reduced the proliferation and differentiation of human BMSCs.52 In several in vitro studies, low O2 concentrations were found to stimulate differentiation processes of MSCs,43,49,53,54 It enhancing the in vitro and in vivo bone-forming potential of rat MSCs.43 The inconsistency of these results was possibly due to variations in the experimental setup, including the use of different sources of MSCs from different species, different media composition, and subtle differences in O2 concentrations used.
Our results showed that hTDSCs preconditioned at 2% O2 tension could subsequently be stimulated to differentiate successfully at 20% O2 tension, as indicated by the reversal of tissue-specific gene expression upon induction or treatment at 20% O2 tension, suggesting that hTDSCs retained their differentiation potential at low O2 tension. This is consistent with the results of previous studies that showed that the differentiation potential of MSCs into mesenchymal tissue were maintained19,31,47,48 or improved19–21,25,45,55,56 in subsequent inductions under normoxic conditions.
Bone formation is a complex process that involves bone mineral deposition and bone resorption. Osteopontin is a glycoprotein abundant in the bone matrix produced by osteoblasts and osteoclasts. It has been suggested to play a role in bone remodeling by initiating osteoclasts to develop ruffled borders to begin bone resorption.57,58 It is an inhibitor of hydroxyapatite crystal formation when phosphorylated in vitro.59 The presence of osteopontin is a prerequisite for the activation of osteoclastic bone resorption and for the reduction in osteoblastic bone formation in tailed suspended mice.60 The expression of osteopontin therefore might decrease during the mineralization phase of bone formation, as shown in our data. The higher expression of SPP1 in hTDSCs at 2% than at 20% O2 tension therefore was consistent with the inhibition of osteogenic differentiation of the cells.
Sox9 is a transcription factor that functioned upstream of aggrecan and collagen type II. As a result, the expression of SOX9 is expected to increase earlier than the expression of ACAN and COL2A1 in hTDSCs during chondrogenic differentiation. The earlier transient upregulation of SOX9, which returned to baseline at day 14, or small sample size might therefore explain the insignificant decrease in the change in SOX9 in hTDSCs in Figure 8E. We observed significant decrease in the relative mRNA expression ratio of SOX9 in hTDSCs upon induction under hypoxic conditions in Figure 11C. Large data variation might explain the nonsignificantly lower relative mRNA expression ratio COL2A1 in hTDSCs at 2% O2 tension in Figure 8E. Further study of gene expression with larger sample sizes will confirm this speculation.
We assessed the production of collagenous and noncollagenous proteins and the expression of tendon-related markers in hTDSCs under hypoxic condition. hTDSCs exhibited comparable collagenous and noncollagenous protein-forming ability, albeit lower collagenous protein production, and significantly higher mRNA level of TNMD at 2% than 20% O2 tension, suggesting that culturing of hTDSCs at 2% O2 tension could maintain the spontaneous tenogenic differentiation potential while suppressing nontenogenic differentiation upon induction during in vitro culture. This has implications for tendon tissue engineering because ectopic bone formation3,6 and tumor formation in special circumstance7 were reported to be concerns in using MSCs as the cell source. The culture of tenocytes at low O2 tension was also reported to enhance their proliferation capacity significantly without affecting their function and phenotype.20
In conclusion, hTDSCs exhibited higher clonogenicity, cell proliferation, and DNA incorporation; showed lower but reversible osteogenic, chondrogenic, and adipogenic differentiation potential; displayed similar immunophenotypes, β-galactosidase activity, and collagenous and non-collagenous proteins production ratio, and expressed higher levels of a tendon-related marker, TNMD, at 2% than 20% O2 tension. Hypoxia is hence advantageous for efficient expansion of hTDSCs in vitro for tendon tissue engineering
Supplementary Material
Acknowledgments
This work was supported by equipment and resources donated by the Hong Kong Jockey Club Charities Trust and CUHK Direct Grant 010.2.038. The authors would like to thank Prof. Chao Wan, School of Biomedical Science, Chinese University of Hong Kong, for lending us the hypoxic chamber.
Disclosure Statement
No competing financial interests exist.
References
- 1.Maffulli N. Wong J. Almekinders L.C. Types and epidemiology of tendinopathy. Clin Sports EMd. 2003;22:675. doi: 10.1016/s0278-5919(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 2.Hampson K. Forsyth N.R. El Haj A. Maffulli N. Tendon tissue engineering. Ashammakhi N., editor; Reis R., editor; Chiellini F., editor. E-book: Topics in Tissue Engineering. 2008;4 chapter 3. [Google Scholar]
- 3.Awad H.A. Butler D.L. Dressler M.R. Smith F. Boivin G.P. Young R.G. Repair of patellar tendon injuries using mesenchymal stem cells and collagen scaffolds. J Orthop Res. 2003;21:420. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 4.Chong A.K. Ang A.D. Goh J.C. Hui J.H. Lim A.Y. Lee E.H. Lim B.H. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit Achilles tendon model. J Bone Joint Surg Am. 2007;89:74. doi: 10.2106/JBJS.E.01396. [DOI] [PubMed] [Google Scholar]
- 5.Ouyang H.W. Goh J.C.H. Thambyah A. Teoh S.H. Lee E.H. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit Achilles tendon. Tissue Eng. 2003;9:431. doi: 10.1089/107632703322066615. [DOI] [PubMed] [Google Scholar]
- 6.Harris M.T. Butler D.L. Boivin G.P. Florer J.B. Schantz E.J. Wenstrup R.J. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998. doi: 10.1016/j.orthres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Tasso R. Augell A. Carida'M Postiglione F. Tibiletti M.G. Bernasconi B. Astigiano S. Fais F. Truini M. Cancedda R. Pennesi G. Development of sacromas in mice implanted with mesenchymal stem cells seeded onto bioscaffolds. Carcinogenesis. 2009;30:150. doi: 10.1093/carcin/bgn234. [DOI] [PubMed] [Google Scholar]
- 8.Bi Y. Ehirchiou D. Kilts T.M. Inkson C.A. Embree M.C. Sonoyama W. Li L. Leet A.I. Seo B. M. Zhang L. Shi S. Young M. F. Identification of tendon stem / progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 9.Rui Y.F. Lui P.P.Y. Li G. Fu S.C. Lee Y.W. Chan K.M. Isolation and characterization of multi-potent rat tendon-derived stem cells. Tissue Eng Part A. 2010;16:1549. doi: 10.1089/ten.TEA.2009.0529. [DOI] [PubMed] [Google Scholar]
- 10.Shortkroff S. Spector M. Isolation and in vitro proliferation of chondrocytes, tenocytes and ligament cells. Methods Mol Med. 1999;18:195. doi: 10.1385/0-89603-516-6:195. [DOI] [PubMed] [Google Scholar]
- 11.Nordin M. Lorenz T. Campello M. Biomechanics of tendons and ligaments. In: Nordin M., editor; Frankel V.H., editor. Basic Biomechanics of the Musculoskeletal System. New York: Lippincott Williams & Wilkins; 2001. pp. 102–125. [Google Scholar]
- 12.Moore M.J. De Beaux A. A quantitative ultrastructural study of rat tendon from birth to maturity. J Anat. 1987;153:163. [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhn N.Z. Tuan R.S. Regulation of stemness and stem cell niche of mesenchymal stem cells: Implications in tumorigenesis and metastasis. J Cell Physiol. 2010;222:268. doi: 10.1002/jcp.21940. [DOI] [PubMed] [Google Scholar]
- 14.Thomas D. Vadas M. Lopez A. Regulation of haematopoiesis by growth factors – emerging insights and therapies. Expert Opin Biol Ther. 2004;4:869. doi: 10.1517/14712598.4.6.869. [DOI] [PubMed] [Google Scholar]
- 15.Mohyeldn A. Garzon-Muvdi T. Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Rui Y.F. Lui P.P. Ni M. Chan L.S. Lee Y.W. Chan K.M. Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells. J Orthop Res. 2010;29:390. doi: 10.1002/jor.21218. [DOI] [PubMed] [Google Scholar]
- 17.Chow D.C. Wenning L.A. Miller W.M. Papoutsakis E.T. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys J. 2001;81:685. doi: 10.1016/S0006-3495(01)75733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocca M. Giavaresi G. Nicoli Aldini N. Fini M. Marcacci M. Zaffagnini S. Giardino R. pO2 measurement in an experimental model of patellar tendon autograft pro-anterior cruciate ligament. Int J Artif Organs. 1998;21:174. [PubMed] [Google Scholar]
- 19.Dos Santo F. Andrade P.Z. Boura J.S. Abecasis M.M. da Silva C.L. Cabral J.M. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol. 2010;223:27. doi: 10.1002/jcp.21987. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y. Wang B. Zhang W.J. Zhou G. Cao Y. Liu W. Enhanced proliferation capacity of porcine tenocytes in low O2 tension culture. Biotechnol Lett. 2010;32:181. doi: 10.1007/s10529-009-0137-8. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-De Leon A. Rojkind M.A. A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. J Histochem Cytochem. 1985;33:737. doi: 10.1177/33.8.2410480. [DOI] [PubMed] [Google Scholar]
- 22.Helman Y. Natale F. Sherrell R.M. Lavigne M. Starovoytov V. Forbunov M.Y. Falkowski P.G. Extracellular matrix production and calcium carbonate precipitation by coral cells in vitro. Proc Natl Acad Sci U S A. 2008;105:54. doi: 10.1073/pnas.0710604105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirouac D.C. Zandstra P.W. The systematic production of cells for cell therapies. Cell Stem Cell. 2008;3:369. doi: 10.1016/j.stem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Ju Y.J. Muneta T. Yoshimura H. Koga H. Sekiya I. Synovial mesenchymal stem cells accelerate early remodeling of tendon-bone healing. Cell Tissue Res. 2008;332:469. doi: 10.1007/s00441-008-0610-z. [DOI] [PubMed] [Google Scholar]
- 25.Benjamin M. Ralphs J.R. Tendons and ligaments – an overview. Histol Histopathol. 1997;12:1135. [PubMed] [Google Scholar]
- 26.Colter D.C. Clas R. DiGirolamo C.M. Prockop D.J. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97:3213. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dezawa M. Ishikawa H. Itokazu Y. Yoshihara T. Hoshino M. Takeda S. Ide C. Nabeshima Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309(5732):314. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- 28.Scheffler I.E. Mitochonria. 2nd. Hoboken, NJ: Wiley-Liss; 2008. [Google Scholar]
- 29.Ezashi T. Das P. Roberts R.M. Low O2 tension and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102:4783. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forristal C.E. Wright K.L. Hanley N.A. Oreffo R.O.C. Houghton F.D. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010;139:85. doi: 10.1530/REP-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fehrer C. Brunauer R. Laschober G. Unterluggauer H. Reitinger S. Kloss F. Gully C. Babner R. Lepperinger G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida Y. Takahashi K. Okita K. Ichisaka T. Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Lonergan T. Brenner C. Bavister B. Differentiation-related changes in mitochondrial properties as indicators of stem cell competence. J Cell Physiol. 2006;208:149. doi: 10.1002/jcp.20641. [DOI] [PubMed] [Google Scholar]
- 34.Simon M.C. Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho Y.M. Kwon S. Pak Y.K. Seol H.W. Choi Y.M. Park do J. Park K.S. Lee H.K. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem Biophys Res Commun. 2006;348:1472. doi: 10.1016/j.bbrc.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Chen C.T. Shih Y.R. Kuo T.K. Lee O.K. Wei Y.H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26:960. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 37.Prigione A. Fauler B. Lurz R. Lhrach H. Adjaye J. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells. 2010;28:721. doi: 10.1002/stem.404. [DOI] [PubMed] [Google Scholar]
- 38.Tsai C.C. Chen Y.J. Yew T.L. Chen L.L. Wang J.Y. Chiu C.H. Hung S.C. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011;117:459. doi: 10.1182/blood-2010-05-287508. [DOI] [PubMed] [Google Scholar]
- 39.Davy P. Allsopp R. Hypoxia: are stem cells in it for the long run? Cell Cycle. 2011;10:206. doi: 10.4161/cc.10.2.14535. [DOI] [PubMed] [Google Scholar]
- 40.Jin Y. Kato T. Furu M. Nasu A. Kajita Y. Mitsui H. Ueda M. Aoyama T. Nakayama T. Nakamura T. Toguchida J. Mesenchymal stem cells cultured under hypoxia escape from senescence via down-regulation of p16 and extracellular signal regulated kinase. Biochem Biophys Res Commun. 2010;391:1471. doi: 10.1016/j.bbrc.2009.12.096. [DOI] [PubMed] [Google Scholar]
- 41.Buravkova L.B. Anokhina E.B. Effect of hypoxia on stromal precursors from rat bone marrow at the early stage of culturing. Bull Exp Biol Med. 2007;143:411. doi: 10.1007/s10517-007-0143-6. [DOI] [PubMed] [Google Scholar]
- 42.Nekanti U. Dastidar S. Venugopal P. Totey S. Ta M. Increased proliferation and analysis of differential gene expression in human Wharton's jelly-derived mesenchymal stromal cells under hypoxia. Int J Biol Sci. 2010;6:499. doi: 10.7150/ijbs.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lennon D.P. Edmison J.M. Caplan A.I. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. J Cell Physiol. 2001;187:345. doi: 10.1002/jcp.1081. [DOI] [PubMed] [Google Scholar]
- 44.Zscharnack M. Poesel C. Galle J. Bader A. Low oxygen expansion improves subsequent chondrogenesis of ovine bone-marrow-derived mesenchymal stem cells in collagen type I hydrogel. Cells Tissues Organs. 2009;190:81. doi: 10.1159/000178024. [DOI] [PubMed] [Google Scholar]
- 45.Grayson W.L. Zhao F. Izadpanah R. Bunnell B. Ma T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol. 2006;207:331. doi: 10.1002/jcp.20571. [DOI] [PubMed] [Google Scholar]
- 46.D'lippolito G. Diabira S. Howard G.A. Roos B.A. Schiller P.C. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39:513. doi: 10.1016/j.bone.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 47.Grayson W.L. Zhao F. Bunnell B. Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 48.Martin-Rendon E. Hale S.J.M. Ryan D. Baban D. Forde S.P. Roubelakis M. Sweeney D. Moukayed M. Harris A.L. Davies K. Watt S.M. Transcriptional profiling of human cord blood CD133(+) and cultured bone marrow mesenchymal stem cells in response to hypoxia. Stem Cell. 2007;25:1003. doi: 10.1634/stemcells.2006-0398. [DOI] [PubMed] [Google Scholar]
- 49.Ren H.Y. Cao Y. Zhao Q.J. Li J. Zhou C.X. Liao L.M. Jia M.Y. Zhao W. Cai H.G. Han Z.C. Yang R.C. Chen G.Q. Zhao R.C.H. Proliferation and differentiation of bone marrow stromal cells under hypoxic conditions. Biochem Biophys Res Commun. 2006;347:12. doi: 10.1016/j.bbrc.2006.05.169. [DOI] [PubMed] [Google Scholar]
- 50.Jee M.K. Kim J.H. Han Y.M. Jung S.J. Kang K.S. Kim D.W. Kang K.S. DHP-derivative and low oxygen tension effectively induces human adipose stromal cell reprogramming. PLos ONE. 2010;5:e9026. doi: 10.1371/journal.pone.0009026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou S. Lechpammer S. Greenberger J.S. Glowacki J. Hypoxia inhibition of adipocytogenesis in human bone marrow stromal cells requires transforming growth factor-β/Smad3 signaling. J Biol Chem. 2005;280:22688. doi: 10.1074/jbc.M412953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holzwarth C. Vaegler M. Gieseke F. Pfister S.M. Handgretinger R. Kerst G. Muller I. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biology. 2010;11:11. doi: 10.1186/1471-2121-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fink T. Abildtrup L. Fogd K. Abdallah B.M. Kassem M. Ebbesen P. Zachar V. Induction of adipocyte-like phenotype in human mesenchymal stem cells by hypoxia. Stem Cells. 2004;22:1346. doi: 10.1634/stemcells.2004-0038. [DOI] [PubMed] [Google Scholar]
- 54.Wang D.W. Fermor B. Gimble J.M. Awad H.A. Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005;204:184. doi: 10.1002/jcp.20324. [DOI] [PubMed] [Google Scholar]
- 55.Xu Y. Malladi P. Chiou M. Bekerman E. Giaccia A.J. Longaker M. T. In vitro expansion of adipose-derived adult stromal cells in hypoxia enhances early chondrogenesis. Tissue Eng. 2007;13:2981. doi: 10.1089/ten.2007.0050. [DOI] [PubMed] [Google Scholar]
- 56.Rosova I. Dao M. Capoccia B. Link D. Nolta J. A. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi S.T. Kim J.H. Kang E.J. Lee S.W. Park M.C. Park Y.B. Lee S.K. Osteopontin might be involved in bone remodelling rather than in inflammation in ankylosing spondylitis. Rheumatology (Oxford) 2008;47:1775. doi: 10.1093/rheumatology/ken385. [DOI] [PubMed] [Google Scholar]
- 58.Denhardt D.T. Guo X. Osteopontin: a protein with diverse function. FASEB J. 1993;7:1475. [PubMed] [Google Scholar]
- 59.Boskey A.L. Maresca M. Ullrich W. Doty S.B. Butler W.T. Prince C.W. Osteopontin-hydroxyapatite interactions in vitro: inhibition of hydroxyapatite formation and growth in a geltain-gel. J Bone Miner Res. 1993;22:147. doi: 10.1016/s0169-6009(08)80225-5. [DOI] [PubMed] [Google Scholar]
- 60.Ishijima M. Rittling S.R. Yamashita T. Tsuji K. Kurosawa H. Nifuji A. Denhardt D. T. Noda M. Enhancement of osteoclastic bone resorption and suppression of osteoblastic bone formation in response to reduced mechanical stress do not occur in the absence of osteopontin. J Exp Med. 2001;193:399. doi: 10.1084/jem.193.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.