Abstract
In order to direct antisense oligonucleotides to specific tissues or cell types in vivo, we are exploring the possibility to utilize lipoproteins as transport vehicles. A 16-mer oligonucleotide (ODN) was derivatized at the 5' prime through a 32P phosphate spacer with cholesterol, yielding a 32P-labeled amphiphatic cholesteryl-oligonucleotide (cholODN). Incubation of cholODN with low-density lipoprotein (LDL) for 2 hr at 37 degrees C resulted in the formation of a cholODN-LDL complex that migrates as a single peak on agarose gel electrophoresis. The cholODN was found to bind quantitatively to both high-density lipoproteins (HDL) and LDL, but not to albumin. Stable oligonucleotide-LDL particles with up to 50 molecules of cholODN per LDL particle could be obtained. In contrast, the control ODN did not show affinity for plasma lipoproteins. Upon injection into rats, cholODN became rapidly associated with plasma lipoproteins while control ODNs were recovered in the lipoprotein deficient serum fraction. The plasma half-life of cholODN (9-11 min) is considerably prolonged as compared with the control ODN (t1/2 less than 1 min). The cholODN-LDL was at least 5 min stable against degradation by rat plasma nucleases. It is concluded that derivatization of antisense oligonucleotides with cholesterol profoundly modifies their in vivo fate and opens possibilities for efficient and specific receptor-dependent targeting, mediated by lipoproteins coupled with specific recognition markers to various hepatic cell types.
Full text
PDF
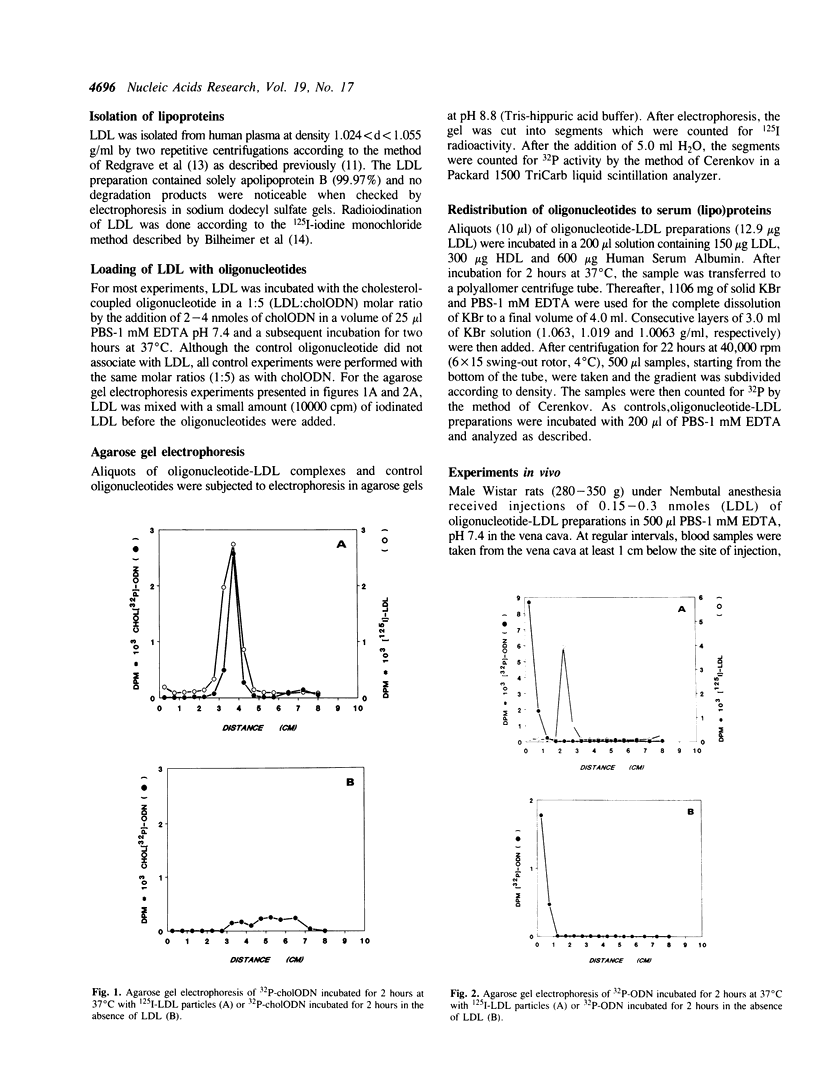
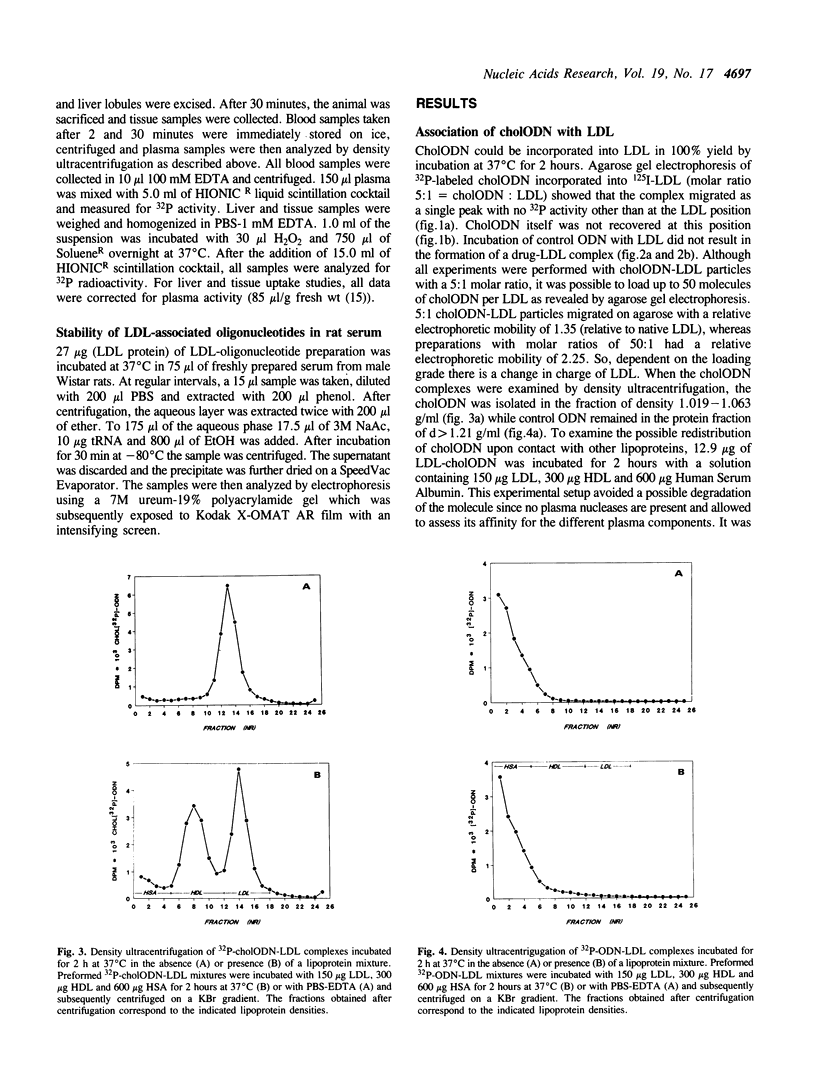
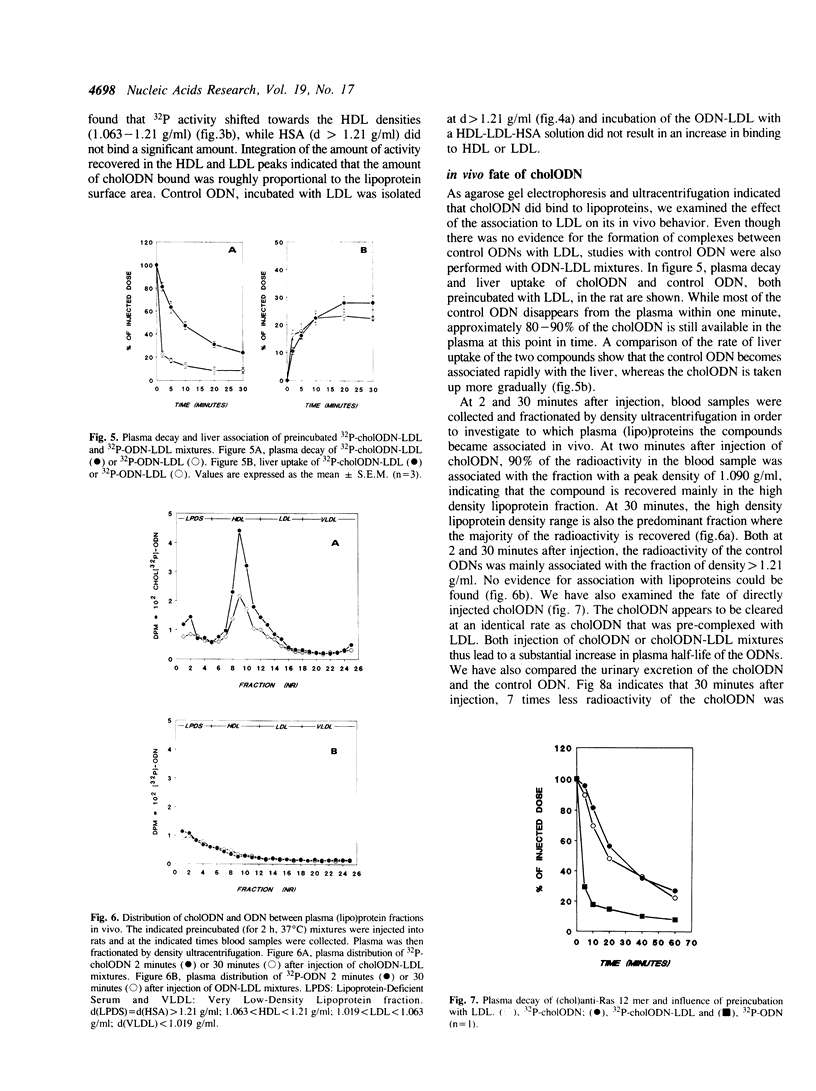
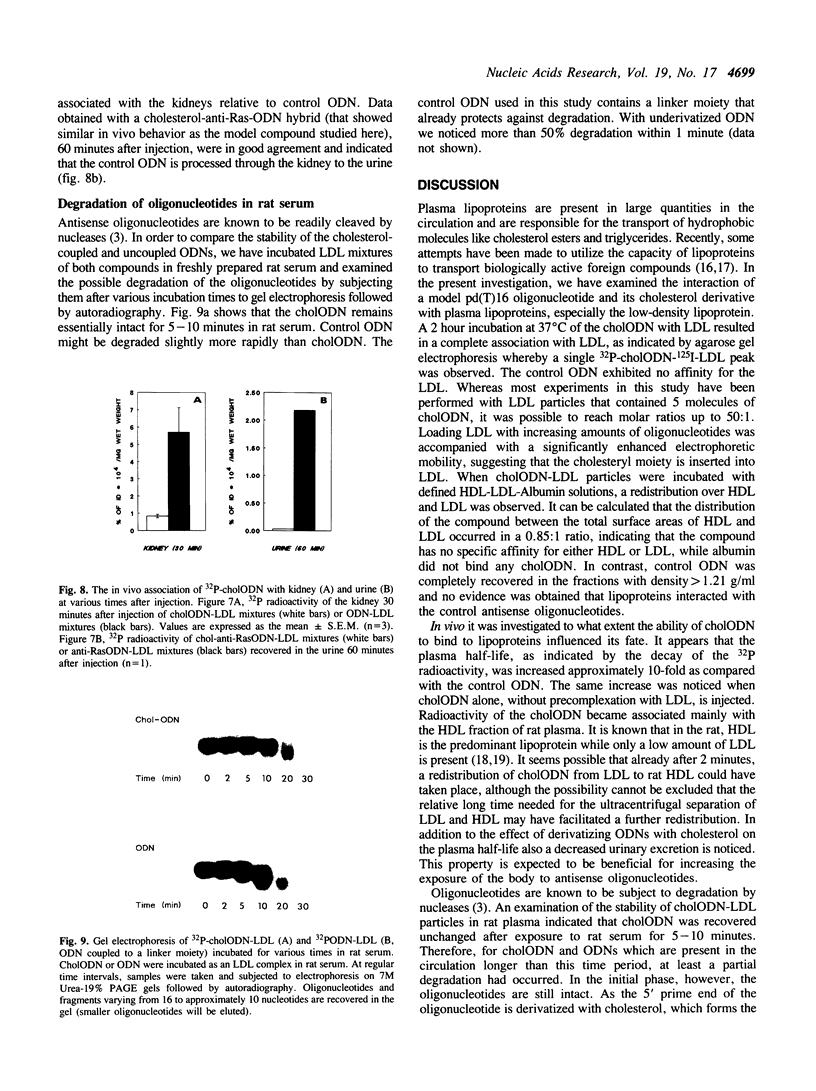

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bijsterbosch M. K., Ziere G. J., Van Berkel T. J. Lactosylated low density lipoprotein: a potential carrier for the site-specific delivery of drugs to Kupffer cells. Mol Pharmacol. 1989 Sep;36(3):484–489. [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Boutorine A. S., Le Doan T., Battioni J. P., Mansuy D., Dupré D., Hélène C. Rapid routes of synthesis of chemically reactive and highly radioactively labeled alpha- and beta-oligonucleotide derivatives for in vivo studies. Bioconjug Chem. 1990 Sep-Oct;1(5):350–356. doi: 10.1021/bc00005a009. [DOI] [PubMed] [Google Scholar]
- CASTER W. O., SIMON A. B., ARMSTRONG W. D. Evans blue space in tissues of the rat. Am J Physiol. 1955 Nov;183(2):317–321. doi: 10.1152/ajplegacy.1955.183.2.317. [DOI] [PubMed] [Google Scholar]
- Goodchild J. Conjugates of oligonucleotides and modified oligonucleotides: a review of their synthesis and properties. Bioconjug Chem. 1990 May-Jun;1(3):165–187. doi: 10.1021/bc00003a001. [DOI] [PubMed] [Google Scholar]
- Hobbelen P. M., Coert A., Geelen J. A., van der Vies J. Interactions of steroids with serum lipoproteins. Biochem Pharmacol. 1975 Jan 15;24(2):165–172. doi: 10.1016/0006-2952(75)90273-7. [DOI] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Kessel D., Thompson P., Saatio K., Nantwi K. D. Tumor localization and photosensitization by sulfonated derivatives of tetraphenylporphine. Photochem Photobiol. 1987 Jun;45(6):787–790. doi: 10.1111/j.1751-1097.1987.tb07883.x. [DOI] [PubMed] [Google Scholar]
- Knorre D. G., Vlassov V. V. Antisense oligonucleotide derivatives as gene-targeted drugs. Biomed Sci. 1990 Apr;1(4):334–343. [PubMed] [Google Scholar]
- Lemaire M., Tillement J. P. Role of lipoproteins and erythrocytes in the in vitro binding and distribution of cyclosporin A in the blood. J Pharm Pharmacol. 1982 Nov;34(11):715–718. doi: 10.1111/j.2042-7158.1982.tb06206.x. [DOI] [PubMed] [Google Scholar]
- Maziere J. C., Santus R., Morliere P., Reyftmann J. P., Candide C., Mora L., Salmon S., Maziere C., Gatt S., Dubertret L. Cellular uptake and photosensitizing properties of anticancer porphyrins in cell membranes and low and high density lipoproteins. J Photochem Photobiol B. 1990 Jun;6(1-2):61–68. doi: 10.1016/1011-1344(90)85074-7. [DOI] [PubMed] [Google Scholar]
- Oschry Y., Eisenberg S. Rat plasma lipoproteins: re-evaluation of a lipoprotein system in an animal devoid of cholesteryl ester transfer activity. J Lipid Res. 1982 Nov;23(8):1099–1106. [PubMed] [Google Scholar]
- Redgrave T. G., Roberts D. C., West C. E. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975 May 12;65(1-2):42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- Shu H. P., Nichols A. V. Benzo(a)pyrene uptake by human plasma lipoproteins in vitro. Cancer Res. 1979 Apr;39(4):1224–1230. [PubMed] [Google Scholar]
- Stein C. A., Cohen J. S. Oligodeoxynucleotides as inhibitors of gene expression: a review. Cancer Res. 1988 May 15;48(10):2659–2668. [PubMed] [Google Scholar]
- Toulmé J. J., Hélène C. Antimessenger oligodeoxyribonucleotides: an alternative to antisense RNA for artificial regulation of gene expression--a review. Gene. 1988 Dec 10;72(1-2):51–58. doi: 10.1016/0378-1119(88)90127-8. [DOI] [PubMed] [Google Scholar]
- Vitols S., Söderberg-Reid K., Masquelier M., Sjöström B., Peterson C. Low density lipoprotein for delivery of a water-insoluble alkylating agent to malignant cells. In vitro and in vivo studies of a drug-lipoprotein complex. Br J Cancer. 1990 Nov;62(5):724–729. doi: 10.1038/bjc.1990.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windler E., Chao Y., Havel R. J. Determinants of hepatic uptake of triglyceride-rich lipoproteins and their remnants in the rat. J Biol Chem. 1980 Jun 10;255(11):5475–5480. [PubMed] [Google Scholar]
- de Smidt P. C., van Berkel T. J. Prolonged serum half-life of antineoplastic drugs by incorporation into the low density lipoprotein. Cancer Res. 1990 Dec 1;50(23):7476–7482. [PubMed] [Google Scholar]
- van Berkel T. J., Kruijt J. K., Spanjer H. H., Nagelkerke J. F., Harkes L., Kempen H. J. The effect of a water-soluble tris-galactoside-terminated cholesterol derivative on the fate of low density lipoproteins and liposomes. J Biol Chem. 1985 Mar 10;260(5):2694–2699. [PubMed] [Google Scholar]



