Abstract
We have previously reported that DT40 cells deficient in the Y-family polymerase REV1 are defective in replicating G-quadruplex DNA. In vivo this leads to uncoupling of DNA synthesis from redeposition of histones displaced ahead of the replication fork, which in turn leads to loss of transcriptional repression due to failure to recycle pre-existing repressive histone post-translational modifications. Here we report that a similar process can also affect transcriptionally active genes, leading to their deactivation. We use this finding to develop an assay based on loss of expression of a cell surface marker to monitor epigenetic instability at the level of single cells. This assay allows us to demonstrate G4 DNA motif-associated epigenetic instability in mutants of three helicases previously implicated in the unwinding of G-quadruplex structures, FANCJ, WRN and BLM. Transcriptional profiling of DT40 mutants reveals that FANCJ coordinates two independent mechanisms to maintain epigenetic stability near G4 DNA motifs that are dependent on either REV1 or on the WRN and BLM helicases, suggesting a model in which efficient in vivo replication of G-quadruplexes often requires the established 5′–3′-helicase activity of FANCJ acting in concert with either a specialized polymerase or helicase operating in the opposite polarity.
INTRODUCTION
Maintaining epigenetic memory through somatic cell division is of critical importance in preserving stable gene expression and cell identity. Propagation of this memory is proposed to require the preservation of histone post-translational modifications, despite the fact that cell division requires incorporation of newly synthesized histones lacking the modifications characteristic of chromatin found at active or repressed genes [reviewed in (1)]. However, histone modifications linked to transcriptional states can be copied from parental to newly synthesized nucleosomes through the ability of chromatin modifying complexes to recognize the modification that they themselves introduce (2–4) suggesting that, following replication, the newly incorporated histones could be modified to reflect the pre-existing state of the parental histones [reviewed in (1,5)]. This model places stringent requirements on the continuity of replicative DNA synthesis as new histones must be deposited concurrently with parental histone recycling in order to maintain the registration between the histone code and underlying DNA sequence. Without this coordination, parental histones will not be deposited near to their original locations and the information carried by their post-translational modifications may therefore be lost.
Continuous DNA synthesis is challenged by replication impediments caused by exogenous DNA damaging agents, endogenous sources of DNA damage and structured DNA, all of which can cause replicative polymerases to pause or stall. Due to the inherent danger that a collapsed fork poses to genomic integrity, numerous proteins converge on stalled replication forks to protect them and enable rapid resumption of DNA synthesis [reviewed in (6)]. One important pathway to promote the resumption of continuous DNA replication is translesion synthesis, during which low fidelity polymerases of the Y-family bypass DNA damage thereby allowing conventional processive polymerases to continue replication [reviewed in (7)]. Importantly, bypass can take place at one of two temporally distinct points relative to histone displacement by the advancing replicative helicase. The first possibility is for the helicase to run ahead and for replication to restart downstream of the blockage, leaving a gap that can be filled in later on. This appears to be the dominant approach used by budding yeast (8). It is dependent on the ubiquitination of the sliding clamp PCNA by Rad6/Rad18, which recruits TLS polymerases or promotes recombination to assist in gap filling (9). PCNA ubiquitination-dependent gap filling also operates in vertebrate cells but does so alongside a second pathway operational at the replication fork, which is dependent on the unusual Y-family DNA polymerase REV1 (10,11). The deoxycytidyl transferase REV1 possesses a second, non-catalytic function that serves to recruit other TLS polymerases to the replication fork via its interaction with them (12) and the sliding clamp PCNA (13). Thus, in the absence of REV1, cells depend more heavily on gap-filling to complete replication of damaged DNA templates (10).
REV1 is also involved in replicating undamaged DNA at sequences capable of forming G-quadruplex secondary structures (14). G4 DNA motifs, of the general sequence L1–7-G3–5-L1–7-G3–5-L1–7-G3–5 (where L can be any base), can form a range of secondary structures at physiological pH and salt concentrations that comprise stacks of four planar Hoogsteen-bonded dG bases coordinated by monovalent metal ions (15,16). G4 DNA motifs are abundant in the vertebrate genome but do not appear to be randomly distributed, instead being found more frequently in the vicinity of promoters as well as at telomeres and in the immunoglobulin loci. There is increasingly strong evidence that these structures form in vivo and that they can significantly influence processes such as replication and transcription [reviewed in (17,18)]. Our previous data supports a model in which lack of REV1 leads to the interruption of processive replication at G-quadruplex structures. In turn this leads to uncoupling of DNA synthesis from recycling of parental histones at these sites, resulting in biased incorporation of newly synthesized histones lacking repressive modifications, and a loss of silencing in a subset of genes. (14).
As noted above, many proteins besides REV1 are important in maintaining replication fork integrity in response to replication stress. In particular, several DNA helicases active at the replication fork have also been implicated in processing G-quadruplex structures. FANCJ, deficiency of which gives rise to the human disease Fanconi anaemia (19), is recruited to stalled replication forks following DNA damage (20,21) but is also capable of unwinding G-quadruplex structures in vitro (22,23) and has been implicated in preventing deletions at G4 DNA motifs in vivo (24,25). The RecQ helicases mutated in Werner syndrome (WRN) and Bloom syndrome (BLM) are also capable of unwinding G-quadruplex structures (26–29), and play prominent roles in the response to replication stress [reviewed in (30)]. Therefore a logical question is whether REV1 acts alone in defending epigenetic stability at G4 DNA motifs or whether it collaborates with specialized helicases.
Here, we show that lack of REV1 also leads to silencing of a subset of G4 DNA motif-containing transcriptionally active loci. We also demonstrate that similar G4 DNA motif-associated epigenetic instability is seen in mutants of FANCJ, WRN and BLM helicases and further establish that FANCJ operates in two independent pathways for replicating G4 DNA motifs that depend on either REV1 or on WRN and BLM acting redundantly to each other. Our data suggest a model in which the ability to replicate G-quadruplex structures in a manner that preserves epigenetic information often requires the concerted action of enzymes acting at both the 5′- and 3′-end of the structure.
MATERIALS AND METHODS
DT40 strains culture and transfection
DT40 cells were cultured as previously described (31). The creation of the fancj line used in this study is described in Supplementary Figure S1. The wrn/blm double mutant was made by targeting the BLM locus in the WRN−/− (wrn) cells described previously (32). The origin of the other mutants used is listed in Supplementary Table S1.
Chromatin immunoprecipitation and antibodies
The method for ChIP (chromatin immunoprecipitation) and antibodies to H3K4me3, H4K9/14ac, H4 N-terminal tail ac and H3 have been described previously (14). H3K9me2 was immunoprecipitated using 20 µl anti-H3K9me2 from Cell Signalling Technology (Cat. No. 9753). In all cases the specific ChIP signal was normalized to total H3 and then to the signal at the promoter of the constitutively active GAS41 gene.
q-PCR and qRT–PCR
This was performed as previously described (14). Primers used are listed in Supplementary Table S5.
NMR spectroscopy
1H NMR spectra of CD72-F and BU1A-F were recorded at 25°C using a 500 MHz Bruker Avance 500 TCI spectrometer equipped with a cryogenic TCI ATM probe. The oligonucleotides were annealed in a 10 mM phosphate buffered saline (PBS) buffer (pH 7.0) supplemented with 70 mM KCl and 10% D2O at a final concentration of 0.1 mM for CD72-F and 0.2 mM for BU1A-F.
Circular dichroism spectroscopy
Circular dichroism (CD) experiments were conducted on a Chirascan spectropolarimeter using a quartz cuvette with an optical path length of 1 mm. Oligonucleotide solutions were prepared at a final concentration of 10 µM in 10 mM lithium cacodylate (pH 7.2) containing 1 mM of ethylenediaminetetraacetic acid (EDTA) and 100 mM of MCl (where M is either Li, Na or K). The samples were annealed by being heated at 95°C for 10 min and slowly cooled to room temperature. Scans were performed over the range of 200–320 nm at 20°C. Each trace is the result of the average of three scans taken with a step size of 1 nm, a time per point of 1 s and a bandwidth of 1 nm. A blank sample containing only buffer was treated in the same manner and subtracted from the collected data. The data were finally zero corrected at 320 nm.
Replicating plasmid assay
The basis and method for this assay have been described previously (14,33). Briefly, the efficiency of replication is ascertained by comparing the number of Escherichia coli colonies generated by transformation of a DpnI-treated Hirt supernatant of the G4 DNA motif-containing plasmid, which is ampicillin resistant, compared with a control plasmid that is kanamycin resistant recovered 24 h after transient transfection into the indicated DT40 line.
Microarrays and data analysis
Microarray experiments were performed using total RNA and the Affymetrix Chicken Genome array, with three repeats using independently isolated RNA for each mutant and for wild-type (WT) DT40. Data was processed using RMA to give log2 of probe intensity for each repeat, as described previously (14). The principal component analysis (PCA) was carried out on these data using XLSTAT. Probes which showed a change of >0.25 log2 units relative to the mean WT probe intensity, with a P-value of >0.05 (t-test), were identified as being statistically significantly altered. Macros written in Excel were used to identify genes co-regulated between different mutants. Significance for these overlaps was calculated using the hypergeometric distribution (stattrek.com/Tables/Hypergeometric.aspx). Codirectionality was also identified using Excel macros, and the significance of this was calculated using the χ2 test, with the expected values assuming complete independence for the mutants calculated using conditional probability. In order to determine the association of G4 DNA motifs to each gene set, a sample of 80–150 genes were randomly selected from the set and the coordinates, with 1500 bp either side of the transcription unit, were downloaded from Ensembl using Biomart. These coordinates were fed into Ensembl's genome browser, to which the coordinates of G4 DNA motifs had been uploaded from www.quadruplex.org, allowing the number of G4 DNA motifs within this window to be counted. The presence or absence of at least one G4 DNA motif, as well as the total number, were compared to a control set of genes whose expression did not change in any of the mutants.
Bu1a staining
Cells were washed in PBS and stained for 30 min at 4°C with anti-Bu1a (also known as chB6) directly conjugated with phycoerythrin (Santa Cruz clone 5K98, Cat. No. 70447) at a 1:10 dilution. Cells were then washed twice in PBS and resuspended in 500 µl PBS before being analyzed by flow cytometry using an LSRII cytometer (Becton–Dickinson). Although ligation of chb6/Bu-1 has previously been shown to induce apoptosis in DT40 (34), staining with this antibody did not induce apoptosis and cloning of Bu-1a-positive was achieved with the same efficiency as Bu1a-negative clones. DT40, being derived from an F1 hybrid bird, is heterozygous for Bu1a and Bu1b (35). To carry out fluctuation analysis single cells staining positive for Bu1a were sorted using a MOFLO (Dako-Cytomation) sorting cytometer and grown for 3 weeks before staining and analysis using flow cytometry as above.
RESULTS
Rev1-deficient DT40 cells exhibit deactivation of G4 DNA motif-containing genes, as well as derepression
We previously showed that rev1 DT40 cells exhibit derepression of a subset of genes, which correlated to the presence of G4 DNA motifs in the vicinity of their promoters (14). However, global analysis of gene expression in rev1 and WT DT40 revealed that there are an equal number of genes deactivated in rev1 relative to WT (Figure 1A and Supplementary Table S2). The dysregulated genes harbour a significant (P = 0.005) increase in the number of G4 DNA motifs relative to a control set of genes that were not perturbed in rev1 cells (Table 1).
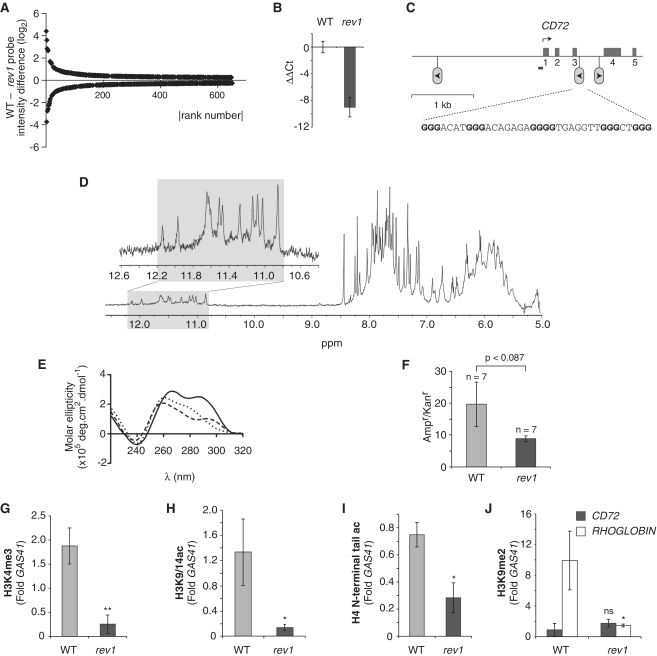
Figure 1.
Epigenetic instability in the CD72 locus of rev1 cells. (A) Plot of probe intensity differences between rev1 and WT ranked by absolute value (for probes whose expression is altered by >0.25 log2 with P < 0.05). (B) qRT-PCR confirming reduction of CD72 expression in a rev1 line independent of that used in the microarray analysis. Error bars show standard deviation. P < 0.05 (2-tailed T-test). (C) Map of the CD72 locus. The first five exons are shown as mid-gray boxes and the positions of the three G4 DNA motifs as inverted lollipops. The arrowhead within the lollipop indicates the direction that a replication fork would have to be travelling for the G-quadruplex structure to form on the leading strand template. The G4 DNA sequence (CD72-F) in the correct orientation to stall replication in rev1 cells is shown, with the dG repeats in bold. The position of the amplicon generated by the ChIP primers (CD72promF & R) is shown as a bar. (D) Part of the 1H NMR spectra of CD72-F. In the inset: an expansion of the spectrum focusing on the imino protons of the guanines involved in a tetrad (gray shaded area). (E) CD spectrum of CD72-F recorded in the presence of LiCl (dotted line), NaCl (dashed line) and KCl (solid line). (F) Replication efficiency of CD72-F incorporated into the leading strand template of the replicating plasmid pQ, shown as the ratio of Ampr to Kanr E. coli colonies. Ampr plasmid contains the CD72-F oligo while Kanr plasmid does not (14). The error bars represent standard error of the mean. P-value calculated with the unpaired t-test. (G) Trimethylation of H3K4 at the CD72 promoter in WT and rev1 cells. (H) Acetylation of H3K9 and K14 at the CD72 promoter in WT and rev1 cells. (I) Acetylation of the H4 N-terminal tail at the CD72 promoter in WT and rev1 cells. (J) Dimethylation of H3K9 at the CD72 and RHOGLOBIN promoters in WT and rev1 cells. Error bars in F–I represent standard error of the mean; P-values compared to WT (one-tailed, unpaired t-test): ns = not significant, *P < 0.075, **P < 0.01.
Table 1.
Association of dysregulated genes with G4 DNA motifs
| Control | rev1 | fancj | rev1/fancj | wrn/blm | rev1 and wrn/blm | fancj and wrn/blm | |
|---|---|---|---|---|---|---|---|
| Fraction of genes with ≥1 G4 DNA motif | 0.69 | 0.85 | 0.88 | 0.83 | 0.85 | 0.83 | 0.77 |
| n | 135 | 93 | 130 | 116 | 164 | 80 | 141 |
| P | 0.0013 | 0.0002 | 0.0075 | 0.0013 | 0.036 | 0.135 | |
| G4 DNA motifs per gene | 2.63 | 4.29 | 3.78 | 4.18 | 3.15 | 4.79 | 3.31 |
| P | 0.005 | 0.0003 | 0.0059 | 0.0186 | 0.0053 | 0.03 |
G4 DNA sequences were identified in the gene body plus 1 kb upstream using pre-existing annotations of the chicken genome in ENSEMBL uploaded from www.quadruplex.org. Genes were selected on the basis of an increase or decrease in probe intensity by ≥0.25 log2 units with P < 0.05. The control set comprises randomly selected probes that exhibit no change between WT and any of the mutants.
Loss of chromatin marks in the CD72 locus is associated with transcriptional deactivation
To investigate this phenomenon further, we initially focused on one gene, CD72, which we identified as being deactivated in rev1 cells from microarray data (Supplementary Table S2), an observation confirmed by Quantitative reverse transcription PCR (qRT–PCR) from an independent rev1 line (Figure 1B). CD72 is a transmembrane C-type lectin expressed constitutively on pro- to mature B cells (36). Examination of the CD72 locus in the vicinity of the promoter revealed three G4 DNA motifs, one of which is in the correct orientation to uncouple replication and histone recycling through the promoter (Figure 1C).
This G-rich sequence contains five runs of 3–4 dGs, separated by potential loops of 3–7 bases [5′-GGGACATGGGACAGAGAGGGGTGAGGTTGGGCTGGG]. Biophysical studies on this 37mer oligonucleotide confirmed G-quadruplex formation by the following observations: (i) clearly resolved G-tetrad imino resonance peaks in the 1H NMR spectrum (37) (Figure 1D). Imino protons of guanines (N1-H) are exchangeable with protons of the solvent during an NMR experiment in water. In a typical nucleic acid, when a guanine is unpaired, no peaks related to these protons are observable on the NMR spectra due to rapid exchange. When these protons are involved in any H-bonds, the exchange is slowed and some resonance peaks can be observed. In G:C Watson–Crick base pairs, imino protons appears lower field to 12.7 parts per million (ppm). In Hoogsteen hydrogen bond alignments, imino protons appear between 10.6 and 12 ppm. By accounting for the number of imino resonances for the particular sequence it is possible to ascertain if there are various species in solution and their relative populations. The 1H NMR spectrum of CD72-F reveals 12 resolved peaks in the range 10.8–12.2 ppm (gray shaded area) characteristic of a single conformation of quadruplex in solution. (ii) A reversible hypochromic melting transition at 295 nm (Supplementary Figure S2A), suggesting formation of an intramolecular structure. The high rate of melting used, 1°C/min, leads to a reversible melting curve which supports the formation of an intramolecular quadruplex. Indeed at this rate and this oligonucleotide concentration, 10 μM, the melting of intermolecular quadruplexes has been shown to be kinetically irreversible (38). (iii) Cation dependent melting temperatures in the order K+ > Na+ > Li+ (39) (Supplementary Figure S2A and Supplementary Table S3A) and (iv) a characteristic thermal difference spectrum (40) (Supplementary Figure S2C). Its melting temperature of 59.5°C in the presence of K+ supports its ability to form a stable structure under physiological conditions (Supplementary Table S2A). CD spectroscopy (Figure 1E and Supplementary Table S3B) was indicative of the formation of a hybrid quadruplex with both parallel and anti-parallel features (41).
We have previously shown that REV1 is required for efficient replication of a G4 DNA motif when placed on the leading strand template (14). To examine whether this was also the case for the CD72 G4 DNA motif, we cloned the sequence into the replicating plasmid pQ (33), placing it so the G-quadruplex structure would form on the leading strand template as previously described (14). The presence of the CD72 G4 DNA motif resulted in a reduction in the efficiency of recovery of replicated copies of the plasmid from rev1-deficient cells relative to WT cells, the borderline significance of which may reflect the heterogeneity of the structure seen in the CD experiments (Figure 1F). Together these results appear analogous to those we obtained for the RHOGLOBIN G4 DNA motif (14) and are consistent with a similar underlying model in which interruption of replication by a G-quadruplex structure in the CD72 locus of rev1 cells leads to loss of chromatin modifications associated with transcriptional activation.
To test this, we performed ChIP experiments to examine the histone modifications at the CD72 promoter. Consistent with the association of H3K4me3 with the promoter of active genes (42,43), we observed high levels of this modification at the CD72 promoter in WT DT40 cells. However, this enrichment was lost in rev1 cells (Figure 1G). H3K9/14ac, another mark associated with transcriptional activation (44), was also reduced (Figure 1H). Next we examined acetylation of the N-terminal tail of H4. This modification is associated with both transcriptional activation (44) and with newly deposited histones (45,46), which according to our previous results (14) would lead to the levels of this mark being subject to two opposing forces: loss of pre-existing, acetylated H4 associated with the loss of transcriptional activation but gain of newly synthesized H4 bearing the predeposition acetylations at H4K5 and K12 (45,46). We observed that rev1 cells exhibited a decrease in H4 N-terminal tail acetylation at the CD72 promoter relative to WT cells (Figure 1I) suggesting that the loss of acetylation of H4 at this locus due to loss of transcriptional activation is not compensated for by the acetylation introduced by new histone incorporation. Importantly, despite observing loss of histone modifications characteristic of active chromatin, we did not detect a significant increase in the level of the repressive modification H3K9me2 (Figure 1J). This suggests that the reduction in expression is caused by a loss of histone modifications associated with active transcription rather than an active silencing process mediated by the introduction of repression-associated marks.
Surface Bu1a staining as a readout for epigenetic instability in DT40 cells
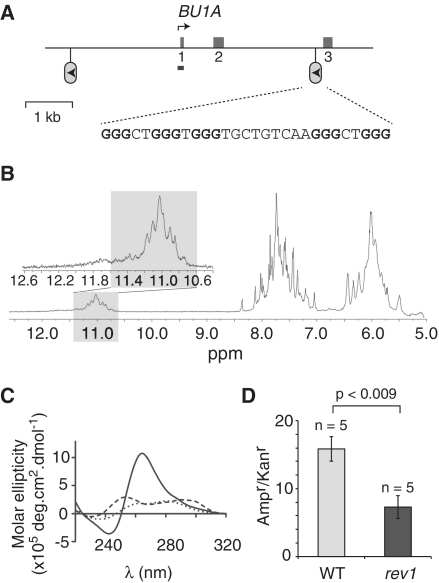
The loss of expression of CD72 in REV1-deficient cells suggested to us a facile assay for epigenetic instability, based on cell surface staining that would provide a more sensitive readout of loss of expression than Quantitative polymerase chain reaction (q-PCR) as small changes within a bulk population would be detected. As no antibody to chicken CD72 was available we sought another B cell surface marker whose locus contained a G4 DNA motif and whose expression we could monitor by flow cytometry. Bu1a is a cell surface receptor expressed on cells derived from the chicken bursa (47). Its locus contains a G-rich region positioned ∼3 kb from the transcription start site (Figure 2A). While this region [5′-GGGCTGGGTGGGTGCTGTCAAGGGCTGGG] contains two potential overlapping G4 DNA sequences, neither is predicted by available servers as both contain a non-dG loop of 9 bp. Nonetheless, an oligonucleotide comprising all five dG repeats was able to form a robust G-quadruplex structure in vitro. The UV-melting curves were reversible and display, in the presence of K+, a transition at 65.5°C, which was suggestive of an intramolecular quadruplex (Supplementary Figure S2B and C; Supplementary Table S3A). 1H NMR revealed seven broad peaks in the range 10.7–11.3 ppm suggesting conformational exchange between two equivalent quadruplex structures (Figure 2B, gray shaded area). The CD spectrum was characteristic of a parallel stranded G-quadruplex (Figure 2C and Supplementary Table S2B). Further, as for the CD72 G4 DNA motif, the sequence was replicated less efficiently in rev1 cells than in WT (Figure 2D).
Figure 2.
G-quadruplex formation by the G-rich region of the BU-1 A locus and its defective replication in rev1 cells. (A) Map of the BU-1 A locus. The first three exons are shown as mid-gray boxes. The position of the G-rich region is shown as a lollipop and sequence of the corresponding oligonucleotide BU1A-F shown below. The arrowhead within the lollipop indicates the direction that a replication fork will be travelling in to be stalled by the G-quadruplex in rev1 cells. The position of the amplicon generated by the ChIP primers (BU1ApromF & R) is shown as a bar. (B) Part of the 1H NMR spectra of BU1A-F. In the inset: an expansion of the spectrum focusing on the imino protons of the guanines involved in a tetrad (gray shaded area). (C) CD spectrum of BU1A-F recorded in the presence of LiCl (dotted line), NaCl (dashed line) and KCl (solid line). (D) Replication efficiency, in rev1 cells, of BU1A-F incorporated into the leading strand template of the replicating plasmid pQ, shown as the ratio of Ampr to Kanr E. coli colonies. Ampr plasmid contains the BU1A-F oligo while Kanr plasmid does not (14).
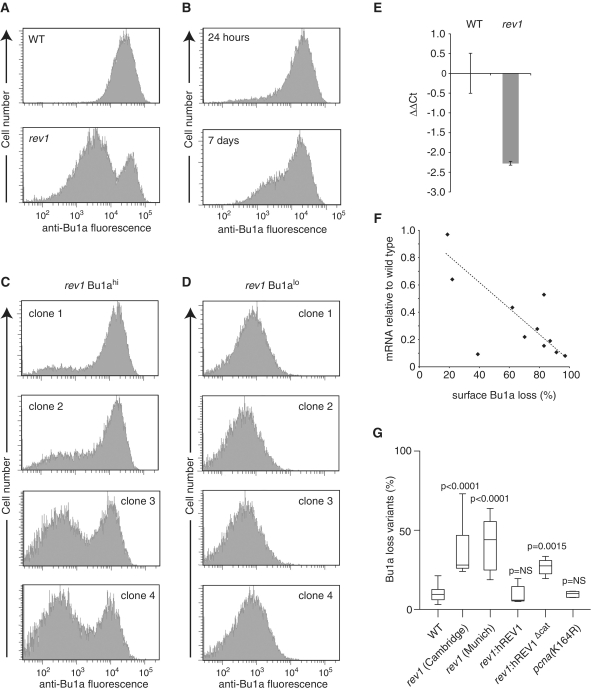
To assess whether loss of REV1 resulted in instability in Bu1a expression we compared surface expression of Bu1a in rev1 cells with WT. WT cells exhibit uniformly positive staining for surface Bu1a (Figure 3A). Strikingly, a significant subpopulation of a bulk rev1 culture exhibited low Bu1a surface staining (Figure 3A). To examine whether Bu1a loss is constitutive during culture, we sorted a population of Bu1a-positive rev1 by flow cytometry. A population of Bu1a-loss variants could be detected after 24 h and this population had increased by 7 days (Figure 3B). We then cloned single rev1 cells from the remaining Bu1a-positive population. After 3 weeks expansion we analyzed surface Bu1a levels by flow cytometry and demonstrated that the percentage of Bu1a-loss variants generated in this time varied between clones (Figure 3C), consistent with Bu1a loss being a stochastic process dependent on cell division. Cloning from the Bu1a-loss variant population demonstrated that loss of Bu1a expression was stable and heritable through cell division (Figure 3D). The loss of surface Bu1a in rev1 cells is associated with decreased mRNA levels (Figure 3E) and further, Bu1a gene expression, as measured by qPCR from different rev1 clones, correlated to the level of Bu1a surface staining, indicating that the change in surface Bu1a levels is caused by alteration in transcription of the BU1A locus (Figure 3F).
Figure 3.
Stochastic loss of surface Bu-1a in rev1-deficient cells. (A) A high-passage culture of rev1 cells exhibits a substantial population of Bu1a-loss variants. Flow cytometry histogram showing staining of WT and rev1 bulk populations with anti-Bu1a. (B) Constitutive loss of Bu1a from a Bu1a-positive population of rev1 cells. rev1 cells were enriched to >99% Bu1a positive by flow sorting and the population monitored for Bu1a loss at 24 h and 7 days. (C) Stochastic loss of Bu1a in Bu1a-positive subclones. Clones were expanded for 3 weeks before analysis by flow cytometry. (D) Bu1a loss is a stable phenotype. Expression of Bu1a in selected Bu1a-loss variant clones expanded for 3 weeks. (E) Reduction in Bu1a transcript in rev1 cells. qRT-PCR for Bu1a mRNA. Error bars show standard deviation. P = 0.05 (2-tailed T-test). (F) Loss of surface expression of Bu1a correlates with decreased mRNA. mRNA level in individual rev1 clones (WT = 1) assessed by qRT-PCR plotted against the percentage of cells showing negative surface Bu1a expression. (G) Complementation of the constitutive loss of Bu1a in rev1 cells by expression of full length, but not catalytically inactive hREV1. Individual Bu1a-positive subclones of each indicated genotype were expanded for 3 weeks and assessed for Bu1a loss by flow cytometry. rev1(Cambridge) and rev1(Munich) are two independently generated rev1 lines (Table S1). For each condition the graph shows the median (central line), range (whiskers) and interquartile range (boxes). The probability (Mann–Whitney U-test) that the distribution of loss is the same as WT is shown. NS = not significantly different. P for the difference between rev1:hREV1 and rev1:hREV1Δcat = 0.0104.
Loss of Bu1a expression could be prevented by a transgene driving expression of human REV1 (Figure 3G) as assessed in a fluctuation analysis, in which individual Bu1a-positive clones were propagated for a defined number of divisions. Interestingly, cells expressing a catalytically inactive human REV1 (hREV1[D570AE571A]) did not exhibit the same range of Bu1a loss as the rev1 mutants suggesting a possible role for the catalytic activity of REV1 in the replication of this G4 DNA motif, and in agreement with our previous observations on the RHOGLOBIN G4 DNA motif (14). Furthermore, supporting the idea that it is the maintenance of replication fork progression that is essential for maintaining epigenetic stability rather than an ability to carry out lesion bypass per se, a DT40 mutant deficient in the ubiquitination of PCNA (pcnaK164R) (48), and thus in gap-filling pathways of translesion synthesis (10), does not show elevated rates of Bu1a loss (Figure 3G).
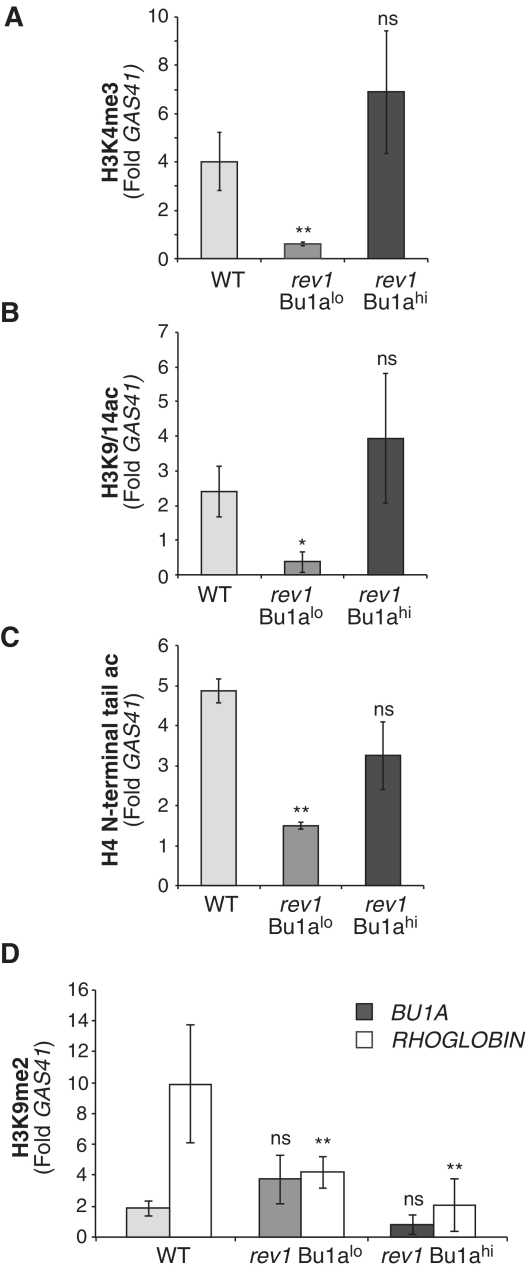
We next investigated whether changes in histone modifications were associated with loss of Bu1a expression in rev1 cells. ChIP analysis of a population sorted for Bu1a-positive cells showed WT levels of active chromatin modifications, while a Bu1a-low population showed reduced H3K4me3, H3K9ac and H4 N-terminal acetylation (Figure 4A–C). Further, there was no significant enrichment of H3K9me2 (Figure 4D). This pattern of histone modification changes is comparable to those seen at the CD72 locus in rev1 cells and suggests that a similar mechanism is responsible for the loss of expression. Further, since re-emergence of Bu1a-positive cells from Bu1a-low clones is not observed (Figure 3D), Bu1a loss is a stable epigenetic state.
Figure 4.
Loss of Bu1a expression gene of rev1 cells correlates with loss of histone modifications associated with transcriptional activation. (A) Trimethylation of H3K4 at the BU1A promoter in WT, Bu1a-negative rev1 cells (Bu1alo) and rev1 cells enriched for expression of surface Bu1a (Bu1ahi). (B) Acetylation of H3K9 and K14 at the BU1A promoter. (C) Acetylation of the H4 N-terminal tail at the BU1A promoter (D) Dimethylation of H3K9 at the BU1A (gray bars) and ρ-GLOBIN (white bars) promoters in WT, Bu1a-negative rev1 cells (Bu1alo) and rev1 cells enriched for expression of surface Bu1a (Bu1ahi). Error bars represent standard error of the mean, P-values compared to WT (one-tailed, unpaired t-test): ns = not significant,*P < 0.075, **P < 0.01.
Together, these data are consistent with loss of the Bu1a surface marker occurring as a result of replication fork stalling at the Bu1a G4 DNA motif, leading to loss of active chromatin modifications and epigenetic instability.
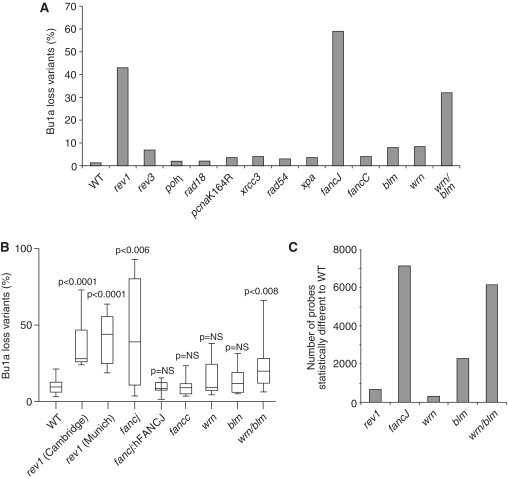
We next examined a range of DT40 DNA repair mutants for Bu1a loss, initially by monitoring Bu1a expression in the bulk population of high passage lines (Figure 5A). In contrast to rev1 cells, no significant increase in Bu1a loss was seen in lines deficient in the translesion polymerases η or ζ, or in cells defective in proliferating cell nuclear antigen (PCNA) ubiquitination, rad18 and pcnaK164R. Additionally, no significant loss was seen in cells lacking the recombination proteins XRCC3 and RAD54 or in a mutant for the key excision repair factor XPA.
Figure 5.
A screen for epigenetic instability. (A) Percentage of Bu1a-loss variants in high passage clones of DT40 mutants of the indicated genotype. (B) Constitutive instability of Bu1a expression confirmed by fluctuation analysis. Subclones were expanded for 3 weeks after which the percentage of Bu1a-loss variants was determined by flow cytometry. For each condition the graph shows the median percentage Bu1a loss (central line), range (error bars) and interquartile range (boxes). The WT and rev1 data is reproduced from Figure 4G for comparison. The loss of Bu1a in lines indicated with an asterisk is significantly (P < 0.01, Mann–Whitney U-test) different to WT. (C) Histogram showing the number of probes statistically significantly perturbed (P < 0.05) with a >0.25 log units difference in rev1, fancj, wrn, blm, wrn/blm lines compared to WT DT40.
Epigenetic instability in fancj mutants is associated with G4 DNA motifs and is independent of the Fanconi anaemia core complex
We then turned our attention to helicases implicated in G-quadruplex processing. Interestingly, a mutant deficient in the FANCJ helicase exhibited a striking loss of Bu1a expression (Figure 5A), which we confirmed in a fluctuation analysis (Figure 5B). Ongoing Bu1a loss could be prevented by expression of a human FANCJ transgene (Figure 5B). However, this role for FANCJ appears to be independent of the Fanconi anaemia core complex [reviewed in (49)] as a fancc mutant exhibited no significant increase in Bu1a loss compared to WT cells (Figure 5A and B). Thus, FANCJ could be involved in preventing epigenetic instability at G4 DNA motifs in a similar manner to REV1. Consistent with this idea, we also found a marked reduction in the expression of the CD72 gene in fancj mutants together with a corresponding decrease in H3K4me3 and H3K9/14ac, but no increase in H3K9me2 at the CD72 promoter (Supplementary Figure S3). Unexplained reproducibly poor recovery of even the control pQ plasmid from fancj cells (data not shown) precluded direct analysis of the requirement for FANCJ in G-quadruplex replication.
To confirm the association between altered gene expression and G4 DNA sequences we carried out genome-wide transcriptional profiling of fancj cells and identified probes whose mean signal intensity was increased or decreased in each mutant by >40% (>0.25 log2 units) relative to WT with a P < 0.05. This analysis revealed a large number of genes (7152 probes) that exhibit dysregulated expression in the fancj mutant (Figure 5C). These genes were significantly associated with G4 DNA motifs compared with an unaffected control set (Table 1). Although loss of FANCJ has been associated with deletions in the vicinity of G-rich sequences (22,24,25), a number of lines of evidence suggest that deletions alone are unlikely to account for the observed dysregulation of gene expression in fancj cells. First, we did not detect evidence of deletions around the G4 DNA sequences in fancj BU1A- or CD72-variants (Supplementary Figures S4 and S5). Second, we did not observe any alteration in the ChIP input signal from the BU1A or CD72 promoters, compared to the control GAS41 promoter. Third, it might be expected that a significant contribution of deletions to changes in gene expression would result in a bias to decreases in expression across the genome. This is not observed. In fancj cells, as for rev1 (Figure 1A), an equal number of genes show increased expression relative to WT as show decreased expression (Supplementary Figure S6). Finally, it is worth noting that the kinetics of Bu1a loss in fancj, and also rev1, cells is rapid, such that 30–40% of cells have lost surface Bu1a after 3 weeks. This is much more rapid than even the loss of immunoglobulin gene expression in fancj DT40 when augmented by the active mutator activation induced deaminase (AID) (50). Given our failure to detect either deletions or mutations of the BU1A locus, and particularly of the G4 DNA motif, it seems unlikely that the observed rate of loss of expression can be accounted for by genetic alterations, though they may make a small contribution.
The WRN and BLM helicases contribute redundantly to the preservation of epigenetic stability
We next examined the contribution of the BLM and WRN helicases, which have also been previously implicated in processing G-quadruplexes (26–29). Neither WRN nor BLM deficient cells showed significantly increased loss of surface Bu1a compared to WT. However, a mutant deficient in both WRN and BLM showed a dramatic increase in the level of Bu1a loss, both in a bulk culture (Figure 5A) and in a fluctuation assay (Figure 5B). Further wrn/blm double mutants replicated a G4 DNA motif-containing plasmid less efficiently than WT cells (Supplementary Figure S7). The apparent redundancy between WRN and BLM could also be seen in the pattern of genes dysregulated across the genome (Figure 5C). Again the genes dysregulated in each of these mutants are significantly associated with G4 DNA motifs (Table 1), in agreement with a previous report (51). wrn cells exhibited relatively little dysregulation of gene expression (304 probes significantly altered compared to WT). While blm cells exhibited more dysregulated genes than wrn cells (2284 probes), the number of genes whose expression was dysregulated in the wrn/blm double mutant was much greater than either single mutant (6152 probes). Further, there was a significant overlap both in the identity genes affected and the direction of change of expression between wrn and wrn/blm and blm and wrn/blm, but not between wrn and blm (Supplementary Figure S8). Together, these data suggest that WRN and BLM are also involved in maintaining epigenetic stability at G-quadruplex forming DNA, but that they act redundantly to each other.
FANCJ collaborates independently with REV1 and with WRN/BLM in maintaining epigenetic stability at G4 DNA motifs
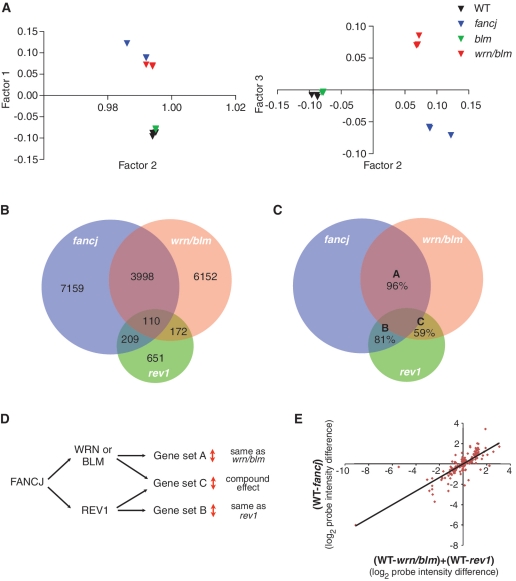
Since fancj, blm and wrn/blm mutants exhibited gross alterations in gene expression compared to WT, we carried out principal component analysis (PCA) on the array data from these lines. Intriguingly, we observed that the pattern of gene expression in the wrn/blm double mutant, rather than resembling the blm single mutant, resembles fancj (Figure 6A), an observation we confirmed by cluster analysis (data not shown). Combining this with the data from the BU1A locus (Figure 5A and B) led us to focus on the relationships between the mutants fancj, wrn/blm and rev1.
Figure 6.
Two pathways for the maintenance of epigenetic stability at G-quadruplex forming DNA sequences. (A) PCA on microarray probes from three independently growing fancj, wrn/blm, blm and WT DT40 lines. PCA is a statistical method for explaining a complex data set in a minimum number of factors, or principal components. In this case, three orthogonal factors were sufficient to explain >98% of the variation within the data set. (B) Venn diagram showing the number of probes statistically significantly perturbed (P < 0.05) with a >0.25 log units difference to WT DT40 in rev1, fancj and wrn/blm mutants, and the overlap between these sets. All overlaps are statistically significant (see text). (C) Venn diagram as in Figure 7C showing the percentage of probes, within each set, which show the same direction of change in each pair of mutants. The expected codirectionality assuming independence between the conditions is ∼50% for each set. (D) Hypothetical relationship between FANCJ, WRN or BLM and REV1 in maintaining epigenetic stability. Sets A, B and C refer to the pairwise overlaps between the mutants shown in Figure 6C. (E) Changes in probe intensity in the fancj mutant for probes overlapping with rev1 and wrn/blm. Log2 change in fancj is plotted against the sum of the log2 change in rev1 and the log2 change in wrn/blm. The regression line is indicated (R = 0.82).
We examined the overlap between each mutant in terms of which probes were perturbed and the direction of the perturbation, i.e. increased or decreased relative to WT. As predicted by the PCA, the genes dysregulated in fancj and wrn/blm show a high degree of overlap (P < 1 × 10−17, Fisher's hypergeometric distribution) (Figure 6B). Strikingly, 96% of genes within this overlap set change in the same direction in fancj and wrn/blm (P < 1 × 10−17, χ2 test) (Figure 6C), implying that the effect of deleting FANCJ or WRN and BLM is the same on the expression of these genes. There is also an increased propensity for G4 DNA sequences within this set (Table 1) implying that the presence of DNA with G-quadruplex forming potential is likely to underlie the requirement for FANCJ and WRN/BLM in epigenetic stability. At the same time, there is a strong overlap between the genes whose expression is perturbed in fancj and in rev1 (P < 1 × 10−17) (Figure 6B) and again there is a statistically significant codirectionality relationship (P < 1 × 10−14) (Figure 6C) and an increased propensity for G4 DNA sequences (Table 1). However, the overlap between genes perturbed in rev1 and wrn/blm, while still being statistically significant (P < 1 × 10−12) (Figure 6B), does not show the same directionality effect: only 59% change in the same direction, barely above that expected by chance (P > 0.2) (Figure 6C). This implies that while there are some genes whose expression is maintained through the action of REV1 and WRN or BLM, the processes involved are distinct.
We wanted to explore the idea of separate pathways for maintenance of epigenetic stability involving REV1 and WRN or BLM in more detail, so we looked at the set of genes that overlapped between the rev1 and the wrn/blm double mutants. If FANCJ is involved in separate pathways protecting epigenetic stability at G4 DNA motifs involving either REV1 or WRN/BLM, then the perturbations in fancj mutants in this set of genes (set C in Figure 6C) should reflect separate contributions from REV1 and from WRN/BLM. Thus, if a gene were to increase by 2-fold in rev1 and 2-fold in the wrn/blm double mutant then its expression would be >2-fold increased in fancj; equally, if a gene is increased in expression in rev1 and decreased in wrn/blm then it may not show an overall change in fancj. This predicts that the expression changes seen in this set in fancj mutants would be a function of the changes in rev1 mutants and changes in the wrn/blm double mutant (Figure 6D). To test this idea, we took the genes falling into set C (Figure 6C) and plotted the log2 change in fancj cells against the sum of the log2 change in wrn/blm and the log2 change in rev1. Strikingly, this graph revealed a linear correlation (Figure 6E) suggesting that the effect on gene expression of removing FANCJ is equivalent to the effect of removing both REV1 and WRN and BLM simultaneously. The correlation coefficient of 0.82 is significantly better than that seen between fancj and either wrn/blm or rev1 individually within gene set C (Supplementary Table S4); moreover, the relationship does not hold outside of gene set C (Supplementary Table S4). These observations strongly reinforce the idea that the REV1 and the WRN/BLM pathways to maintain epigenetic stability at G4 DNA motifs are distinct, but that both involve FANCJ.
DISCUSSION
A neutral state of gene expression?
We previously proposed that the loss of H3K9me2 around the promoter of repressed loci in rev1 cells is a passive consequence of uncoupling DNA synthesis from histone recycling induced by replication arrest at G-quadruplex structures. Supporting this model we did not observe any increase in the level of marks associated with transcriptional activation (e.g. H3K4me3 and H3K9/14ac) suggesting that there was no active induction of gene expression. The data we present here support an analogous model at active loci: G-quadruplex-induced uncoupling of DNA synthesis and histone recycling leads to the passive loss of active chromatin marks by biased incorporation of new histones. However, there is no active repression of affected loci, evidenced by an increase in H3K9me2. Thus, in cell lines defective for G-quadruplex replication both the derepression of a silent locus and the deactivation of an active locus produce a similar net result: a ‘neutral’ expression state with neither active nor repressive chromatin present. This model supports the notion that one or more ‘active’ histone modifications may be sensu stricto epigenetic, such that impaired recycling due to replication fork stalling leading to its reduction directly impairs the ability of the cell to maintain a transcriptionally active state. Of the ‘active’ histone modifications, we believe that H3K4me3 is the most likely candidate for an epigenetic mark, due to the long half-life of methylation on histones relative to acetylation (52), and its involvement in heritable gene activation in Drosophila development [reviewed in (53)].
Stochasticity of G4 DNA motif-associated epigenetic instability
At the BU1A locus we observed stochastic loss of expression over time, consistent with a mechanism involving replication fork stalling at G-quadruplex structures. It is noteworthy that the BU1A G4 DNA motif is further away from the transcriptional start site than is observed in CD72 or RHOGLOBIN. The post-replicative gap that would have to be left in order to lead to loss of epigenetic information at the promoter is therefore ∼3 kb in length, somewhat larger than the estimated average for human cells of ∼0.8 kb (54). However, this is within the range of lengths directly observed in DNA from Saccharomyces cerevisiae (8). Moreover, our previously described computer simulation (14) shows that increasing the variance of gap length increases the loss of epigenetic information suggesting that the system is sensitive to rare events in which long gaps are created (Supplementary Figure S9). It is possible therefore that the position of the G4 DNA sequence relative to the transcriptional start site may explain why we were able to successfully isolate single cells with high expression from a population of rev1 cells that had been grown for several months in culture as loss of Bu1a expression in any particular cell may be a relatively infrequent event.
Collaboration between REV1 and specialized helicases in G-quadruplex replication
Deletion of FANCJ, a helicase that has previously been shown to be able to unwind G-quadruplex structures (22,23), has been linked to deletions in G-rich sequences (22,24,25). We observed changes in gene expression both at the BU1A and CD72 loci, as well as genome wide. As discussed above deletions alone are unlikely to explain all of these effects. Because the set of dysregulated genes correlate significantly with an increased frequency of G4 DNA motifs, secondary effects due to alterations in transcription factors are also unlikely to explain the majority of these changes, though this may make some contribution.
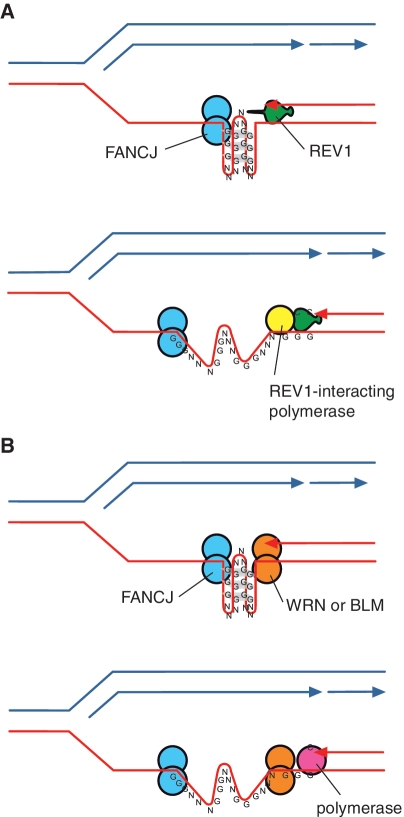
The overlap between genes dysregulated in fancj and rev1 cells, and the association of these genes with G4 DNA motifs, also suggests that REV1 is likely to operate with FANCJ at the fork to facilitate the replication of a subset of G-quadruplex-forming sequences. As FANCJ is a 5′–3′-DNA helicase (22,23) this suggests a model in which it acts from the opposite end of the G-quadruplex to new DNA synthesis (Figure 7A).
Figure 7.
Model for the cooperation of FANCJ with REV1 and with the WRN and BLM proteins in replicating a G-quadruplex. (A) Cooperation of FANCJ with REV1. We have previously suggested that REV1 may help destabilize G-quadruplex structures (14). This could facilitate unwinding by FANCJ, which will act from the opposite side of the G-quadruplex structure. (B) Cooperation of FANCJ with WRN or BLM. Since the helicase activities of WRN and BLM helicases have the opposite polarity to FANCJ, this suggests a model by which they also act from the other end of the quadruplex, possibly simultaneously with FANCJ. DNA synthesis across the remainder of the G4 DNA sequence may then be affected by one of the replicative polymerases.
Using G4 DNA motif-associated dysregulation of gene expression as a readout for effective G-quadruplex replication, we also show redundancy between the RECQ helicases WRN and BLM in maintaining epigenetic stability at G4 DNA motifs. Further, our data suggests that WRN and BLM act with FANCJ but in a different process from that involving FANCJ and REV1. Although WRN and BLM have been implicated in transcriptional regulation (51), they have much more extensively characterized roles in DNA replication [reviewed in (30)]. Therefore, we favour the idea that, as for FANCJ and REV1, this collaboration between FANCJ and WRN/BLM defends epigenetic stability by ensuring continuous replication at G-quadruplex-forming DNA sequences. Consistent with such a model, FANCJ and BLM have been shown to interact physically, and the two colocalize to sites of stalled replication (20). The fact that RECQ helicases act from the 3′–5′-direction suggests that WRN and BLM would access a G-quadruplex in the opposite direction to FANCJ, allowing them to collaborate in facilitating DNA replication through the quadruplex (Figure 7B).
Overall, our results offer a general model for in vivo G-quadruplex replication, in which the collaboration between two helicases, or a helicase and a polymerase, approaching from opposite ends of the G-quadruplex structure, could facilitate processive DNA synthesis. Further work will be needed to identify the factors, for instance the structure and sequence context of the G4 DNA motif, that determine which enzyme or combination of enzymes are used, and whether additional helicases and polymerases are involved. Finally, as WRN, BLM and FANCJ are all implicated in human diseases, our work may have significance for understanding the complex organismal phenotypes caused by mutations in these critical guardians of genome stability.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Tables 1–5 (including one Excel workbook), Supplementary Figures 1–9, Supplementary Methods and Supplementary References [55–67].
FUNDING
Work in J.E.S's lab is supported by the Medical Research Council (MC_US_A024_0039), Association for International Cancer Research (08-0017 & 11-0514) and The Fanconi Anaemia Research Fund. Work in S.B's lab is supported by programme funding from Cancer Research UK (C9681/A5709). Funding for open access charge: Medical Research Council.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Ian McFarlane and his team at the University of Cambridge School of Clinical Medicine Microarray Facility for carrying out the microarray hybridization, Maria Daly and Fan Zhang for cell sorting, Charlie Reams for advice on the Zippee simulation, Shunichi Takeda and Jean-Marie Buerstedde for sharing DT40 lines, Rebekka Schwab and Wojciech Niedzwiedz for the hFANCJ expression construct and Daniela Rhodes and members of the Sale lab for helpful discussions and comments on the article.
REFERENCES
- 1.Corpet A, Almouzni G. Making copies of chromatin: the challenge of nucleosomal organization and epigenetic information. Trends Cell Biol. 2009;19:29–41. doi: 10.1016/j.tcb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- 3.Lachner M, O'Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 4.Hansen K, Bracken A, Pasini D, Dietrich N, Gehani S, Monrad A, Rappsilber J, Lerdrup M, Helin K. A model for transmission of the H3K27me3 epigenetic mark. Nat. Cell Biol. 2008;10:1291–300. doi: 10.1038/ncb1787. [DOI] [PubMed] [Google Scholar]
- 5.Margueron R, Reinberg D. Chromatin structure and the inheritance of epigenetic information. Nat. Rev. Genet. 2010;11:285–296. doi: 10.1038/nrg2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Branzei D, Foiani M. Maintaining genome stability at the replication fork. Nat. Rev. Mol. Cell Biol. 2010;11:208–219. doi: 10.1038/nrm2852. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann AR, Niimi A, Ogi T, Brown S, Sabbioneda S, Wing JF, Kannouche PL, Green CM. Translesion synthesis: Y-family polymerases and the polymerase switch. DNA Repair. 2007;6:891–899. doi: 10.1016/j.dnarep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Lopes M, Foiani M, Sogo JM. Multiple mechanisms control chromosome integrity after replication fork uncoupling and restart at irreparable UV lesions. Mol. Cell. 2006;21:15–27. doi: 10.1016/j.molcel.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 10.Edmunds CE, Simpson LJ, Sale JE. PCNA ubiquitination and REV1 define temporally distinct mechanisms for controlling translesion synthesis in the avian cell line DT40. Mol. Cell. 2008;30:519–529. doi: 10.1016/j.molcel.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Jansen J, Tsaalbi-Shtylik A, Hendriks G, Gali H, Hendel A, Johansson F, Erixon K, Livneh Z, Mullenders L, Haracska L, et al. Separate domains of Rev1 mediate two modes of DNA damage bypass in mammalian cells. Mol. Cell. Biol. 2009;29:3113–3123. doi: 10.1128/MCB.00071-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo C, Fischhaber PL, Luk-Paszyc MJ, Masuda Y, Zhou J, Kamiya K, Kisker C, Friedberg EC. Mouse Rev1 protein interacts with multiple DNA polymerases involved in translesion DNA synthesis. EMBO J. 2003;22:6621–6630. doi: 10.1093/emboj/cdg626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross AL, Simpson LJ, Sale JE. Vertebrate DNA damage tolerance requires the C-terminus but not BRCT or transferase domains of REV1. Nucleic Acids Res. 2005;33:1280–1289. doi: 10.1093/nar/gki279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarkies P, Reams C, Simpson LJ, Sale JE. Epigenetic instability due to defective replication of structured DNA. Mol. Cell. 2010;40:703–713. doi: 10.1016/j.molcel.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundquist WI, Klug A. Telomeric DNA dimerizes by formation of guanine tetrads between hairpin loops. Nature. 1989;342:825–829. doi: 10.1038/342825a0. [DOI] [PubMed] [Google Scholar]
- 16.Williamson JR, Raghuraman MK, Cech TR. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell. 1989;59:871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]
- 17.Lipps HJ, Rhodes D. G-quadruplex structures: in vivo evidence and function. Trends Cell Biol. 2009;19:414–422. doi: 10.1016/j.tcb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Maizels N. Dynamic roles for G4 DNA in the biology of eukaryotic cells. Nat. Struct. Mol. Biol. 2006;13:1055–1059. doi: 10.1038/nsmb1171. [DOI] [PubMed] [Google Scholar]
- 19.Levitus M, Waisfisz Q, Godthelp BC, de Vries Y, Hussain S, Wiegant WW, Elghalbzouri-Maghrani E, Steltenpool J, Rooimans MA, Pals G, et al. The DNA helicase BRIP1 is defective in Fanconi anemia complementation group J. Nat. Genet. 2005;37:934–935. doi: 10.1038/ng1625. [DOI] [PubMed] [Google Scholar]
- 20.Suhasini AN, Rawtani NA, Wu Y, Sommers JA, Sharma S, Mosedale G, North PS, Cantor SB, Hickson ID, Brosh RM., Jr Interaction between the helicases genetically linked to Fanconi anemia group J and Bloom's syndrome. EMBO J. 2011;30:692–705. doi: 10.1038/emboj.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong Z, Kim JE, Leung CC, Glover JN, Chen J. BACH1/FANCJ acts with TopBP1 and participates early in DNA replication checkpoint control. Mol. Cell. 2010;37:438–446. doi: 10.1016/j.molcel.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.London TB, Barber LJ, Mosedale G, Kelly GP, Balasubramanian S, Hickson ID, Boulton SJ, Hiom K. FANCJ is a structure-specific DNA helicase associated with the maintenance of genomic G/C tracts. J. Biol. Chem. 2008;283:36132–36139. doi: 10.1074/jbc.M808152200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y, Shin-ya K, Brosh RM., Jr FANCJ helicase defective in Fanconia anemia and breast cancer unwinds G-quadruplex DNA to defend genomic stability. Mol. Cell. Biol. 2008;28:4116–4128. doi: 10.1128/MCB.02210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung I, Schertzer M, Rose A, Lansdorp PM. Disruption of dog-1 in Caenorhabditis elegans triggers deletions upstream of guanine-rich DNA. Nat. Genet. 2002;31:405–409. doi: 10.1038/ng928. [DOI] [PubMed] [Google Scholar]
- 25.Kruisselbrink E, Guryev V, Brouwer K, Pontier DB, Cuppen E, Tijsterman M. Mutagenic capacity of endogenous G4 DNA underlies genome instability in FANCJ-defective C. elegans. Curr. Biol. 2008;18:900–905. doi: 10.1016/j.cub.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Fry M, Loeb LA. Human werner syndrome DNA helicase unwinds tetrahelical structures of the fragile X syndrome repeat sequence d(CGG)n. J. Biol. Chem. 1999;274:12797–12802. doi: 10.1074/jbc.274.18.12797. [DOI] [PubMed] [Google Scholar]
- 27.Sun H, Karow JK, Hickson ID, Maizels N. The Bloom's syndrome helicase unwinds G4 DNA. J. Biol. Chem. 1998;273:27587–27592. doi: 10.1074/jbc.273.42.27587. [DOI] [PubMed] [Google Scholar]
- 28.Mohaghegh P, Karow JK, Brosh RM, Jr, Bohr VA, Hickson ID. The Bloom's and Werner's syndrome proteins are DNA structure-specific helicases. Nucleic Acids Res. 2001;29:2843–2849. doi: 10.1093/nar/29.13.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamath-Loeb AS, Loeb LA, Johansson E, Burgers PM, Fry M. Interactions between the Werner syndrome helicase and DNA polymerase delta specifically facilitate copying of tetraplex and hairpin structures of the d(CGG)n trinucleotide repeat sequence. J. Biol. Chem. 2001;276:16439–16446. doi: 10.1074/jbc.M100253200. [DOI] [PubMed] [Google Scholar]
- 30.Chu WK, Hickson ID. RecQ helicases: multifunctional genome caretakers. Nat. Rev. Cancer. 2009;9:644–654. doi: 10.1038/nrc2682. [DOI] [PubMed] [Google Scholar]
- 31.Simpson LJ, Sale JE. Rev1 is essential for DNA damage tolerance and non-templated immunoglobulin gene mutation in a vertebrate cell line. EMBO J. 2003;22:1654–1664. doi: 10.1093/emboj/cdg161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips LG, Sale JE. The Werner's Syndrome protein collaborates with REV1 to promote replication fork progression on damaged DNA. DNA Repair (Amst) 2010;9:1064–1072. doi: 10.1016/j.dnarep.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szüts D, Marcus AP, Himoto M, Iwai S, Sale JE. REV1 restrains DNA polymerase zeta to ensure frame fidelity during translesion synthesis of UV photoproducts in vivo. Nucleic Acids Res. 2008;36:6767–6780. doi: 10.1093/nar/gkn651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Funk PE, Pifer J, Kharas M, Crisafi G, Johnson A. The avian chB6 alloantigen induces apoptosis in DT40 B cells. Cell. Immunol. 2003;226:95–104. doi: 10.1016/j.cellimm.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Funk PE, Tregaskes CA, Young JR, Thompson CB. The avian chB6 (Bu-1) alloantigen can mediate rapid cell death. J. Immunol. 1997;159:1695–1702. [PubMed] [Google Scholar]
- 36.Parnes JR, Pan C. CD72, a negative regulator of B-cell responsiveness. Immunol. Rev. 2000;176:75–85. doi: 10.1034/j.1600-065x.2000.00608.x. [DOI] [PubMed] [Google Scholar]
- 37.Webba da Silva M. NMR methods for studying quadruplex nucleic acids. Methods. 2007;43:264–277. doi: 10.1016/j.ymeth.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Mergny JL, De Cian A, Ghelab A, Sacca B, Lacroix L. Kinetics of tetramolecular quadruplexes. Nucleic Acids Res. 2005;33:81–94. doi: 10.1093/nar/gki148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mergny JL, Phan AT, Lacroix L. Following G-quartet formation by UV-spectroscopy. FEBS Lett. 1998;435:74–78. doi: 10.1016/s0014-5793(98)01043-6. [DOI] [PubMed] [Google Scholar]
- 40.Mergny JL, Li J, Lacroix L, Amrane S, Chaires JB. Thermal difference spectra: a specific signature for nucleic acid structures. Nucleic Acids Res. 2005;33:e138. doi: 10.1093/nar/gni134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai J, Dexheimer TS, Chen D, Carver M, Ambrus A, Jones RA, Yang D. An intramolecular G-quadruplex structure with mixed parallel/antiparallel G-strands formed in the human BCL-2 promoter region in solution. J. Am. Chem. Soc. 2006;128:1096–1098. doi: 10.1021/ja055636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 43.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Litt MD, Simpson M, Recillas-Targa F, Prioleau MN, Felsenfeld G. Transitions in histone acetylation reveal boundaries of three separately regulated neighboring loci. EMBO J. 2001;20:2224–2235. doi: 10.1093/emboj/20.9.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sobel RE, Cook RG, Perry CA, Annunziato AT, Allis CD. Conservation of deposition-related acetylation sites in newly synthesized histones H3 and H4. Proc. Natl Acad. Sci. USA. 1995;92:1237–1241. doi: 10.1073/pnas.92.4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loyola A, Bonaldi T, Roche D, Imhof A, Almouzni G. PTMs on H3 variants before chromatin assembly potentiate their final epigenetic state. Mol. Cell. 2006;24:309–316. doi: 10.1016/j.molcel.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 47.Houssaint E, Diez E, Pink JR. Ontogeny and tissue distribution of the chicken Bu-1a antigen. Immunology. 1987;62:463–470. [PMC free article] [PubMed] [Google Scholar]
- 48.Arakawa H, Moldovan GL, Saribasak H, Saribasak NN, Jentsch S, Buerstedde JM. A role for PCNA ubiquitination in immunoglobulin hypermutation. PLoS Biol. 2006;4:e366. doi: 10.1371/journal.pbio.0040366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kee Y, D'Andrea AD. Expanded roles of the Fanconi anemia pathway in preserving genomic stability. Genes Dev. 2010;24:1680–1694. doi: 10.1101/gad.1955310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitao H, Nanda I, Sugino RP, Kinomura A, Yamazoe M, Arakawa H, Schmid M, Innan H, Hiom K, Takata M. FancJ/Brip1 helicase protects against genomic losses and gains in vertebrate cells. Genes Cells. 2011;16:714–727. doi: 10.1111/j.1365-2443.2011.01523.x. [DOI] [PubMed] [Google Scholar]
- 51.Johnson JE, Cao K, Ryvkin P, Wang LS, Johnson FB. Altered gene expression in the Werner and Bloom syndromes is associated with sequences having G-quadruplex forming potential. Nucleic Acids Res. 2010;38:1114–1122. doi: 10.1093/nar/gkp1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zee BM, Levin RS, Dimaggio PA, Garcia BA. Global turnover of histone post-translational modifications and variants in human cells. Epigenetics & Chromatin. 2010;3:22. doi: 10.1186/1756-8935-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu. Rev. Genet. 2004;38:413–443. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- 54.Lehmann AR. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J. Mol. Biol. 1972;66:319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- 55.Arakawa H, Lodygin D, Buerstedde JM. Mutant loxP vectors for selectable marker recycle and conditional knock-outs. BMC Biotechnol. 2001;1:7. doi: 10.1186/1472-6750-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ambrus A, Chen D, Dai J, Bialis T, Jones RA, Yang D. Human telomeric sequence forms a hybrid-type intramolecular G-quadruplex structure with mixed parallel/antiparallel strands in potassium solution. Nucleic Acids Res. 2006;34:2723–2735. doi: 10.1093/nar/gkl348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phan AT, Kuryavyi V, Burge S, Neidle S, Patel DJ. Structure of an unprecedented G-quadruplex scaffold in the human c-kit promoter. J. Am. Chem. Soc. 2007;129:4386–4392. doi: 10.1021/ja068739h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sonoda E, Okada T, Zhao GY, Tateishi S, Araki K, Yamaizumi M, Yagi T, Verkaik NS, van Gent DC, Takata M, et al. Multiple roles of Rev3, the catalytic subunit of polzeta in maintaining genome stability in vertebrates. EMBO J. 2003;22:3188–3197. doi: 10.1093/emboj/cdg308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawamoto T, Araki K, Sonoda E, Yamashita YM, Harada K, Kikuchi K, Masutani C, Hanaoka F, Nozaki K, Hashimoto N, et al. Dual roles for DNA polymerase eta in homologous DNA recombination and translesion DNA synthesis. Mol. Cell. 2005;20:793–799. doi: 10.1016/j.molcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 60.Yamashita YM, Okada T, Matsusaka T, Sonoda E, Zhao GY, Araki K, Tateishi S, Yamaizumi M, Takeda S. RAD18 and RAD54 cooperatively contribute to maintenance of genomic stability in vertebrate cells. EMBO J. 2002;21:5558–5566. doi: 10.1093/emboj/cdf534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang W, Seki M, Narita Y, Sonoda E, Takeda S, Yamada K, Masuko T, Katada T, Enomoto T. Possible association of BLM in decreasing DNA double strand breaks during DNA replication. EMBO J. 2000;19:3428–3435. doi: 10.1093/emboj/19.13.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niedzwiedz W, Mosedale G, Johnson M, Ong CY, Pace P, Patel KJ. The Fanconi anaemia gene FANCC promotes homologous recombination and error-prone DNA repair. Mol. Cell. 2004;15:607–620. doi: 10.1016/j.molcel.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 63.Bezzubova O, Silbergleit A, Yamaguchi-Iwai Y, Takeda S, Buerstedde JM. Reduced X-ray resistance and homologous recombination frequencies in a RAD54-/- mutant of the chicken DT40 cell line. Cell. 1997;89:185–193. doi: 10.1016/s0092-8674(00)80198-1. [DOI] [PubMed] [Google Scholar]
- 64.Takata M, Sasaki MS, Tachiiri S, Fukushima T, Sonoda E, Schild D, Thompson LH, Takeda S. Chromosome instability and defective recombinational repair in knockout mutants of the five Rad51 paralogs. Mol. Cell. Biol. 2001;21:2858–2866. doi: 10.1128/MCB.21.8.2858-2866.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okada T, Sonoda E, Yamashita YM, Koyoshi S, Tateishi S, Yamaizumi M, Takata M, Ogawa O, Takeda S. Involvement of vertebrate polkappa in Rad18-independent postreplication repair of UV damage. J. Biol. Chem. 2002;277:48690–48695. doi: 10.1074/jbc.M207957200. [DOI] [PubMed] [Google Scholar]
- 66.Wang J, Liu H, Lin CM, Aladjem MI, Epner EM. Targeted deletion of the chicken beta-globin regulatory elements reveals a cooperative gene silencing activity. J. Biol. Chem. 2005;280:23340–23348. doi: 10.1074/jbc.M501161200. [DOI] [PubMed] [Google Scholar]
- 67.Myers FA, Lefevre P, Mantouvalou E, Bruce K, Lacroix C, Bonifer C, Thorne AW, Crane-Robinson C. Developmental activation of the lysozyme gene in chicken macrophage cells is linked to core histone acetylation at its enhancer elements. Nucleic Acids Res. 2006;34:4025–4035. doi: 10.1093/nar/gkl543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.