Abstract
Subcutaneous abdominal adipose tissue is one of the largest fat depots and contributes the major proportion of circulating nonesterified fatty acids (NEFA). Little is known about aspects of human adipose tissue metabolism in vivo other than lipolysis. Here we collated data from 331 experiments in 255 healthy volunteers over a 23-year period, in which subcutaneous abdominal adipose tissue metabolism was studied by measurements of arterio-venous differences after an overnight fast. NEFA and glycerol were released in a ratio of 2.7:1, different (P < 0.001) from the value of 3.0 that would indicate no fatty acid re-esterification. Fatty acid re-esterification was 10.2 ± 1.4%. Extraction of triacylglycerol (TG) (fractional extraction 5.7 ± 0.4%) indicated intravascular lipolysis by lipoprotein lipase, and this contributed 21 ± 3% of the glycerol released. Glucose uptake (fractional extraction 2.6 ± 0.3%) was partitioned around 20–25% for provision of glycerol 3-phosphate and 30% into lactate production. There was release of lactate and pyruvate, with extraction of the ketone bodies 3-hydroxybutyrate and acetoacetate, although these were small numerically compared with TG and glucose uptake. NEFA release (expressed per 100 g tissue) correlated inversely with measures of fat mass (e.g., with BMI, rs = −0.24, P < 0.001). We examined within-person variability. Systemic NEFA concentrations, NEFA release, fatty acid re-esterification, and adipose tissue blood flow were all more consistent within than between individuals. This picture of human adipose tissue metabolism in the fasted state should contribute to a greater understanding of adipose tissue physiology and pathophysiology.
Keywords: lipolysis, reesterification, lipoprotein lipase action, adipose tissue glucose metabolism, ketone body metabolism
the subcutaneous abdominal adipose depot is often the largest single fat depot in humans, and has enormous capacity for expansion in obesity. Its metabolism dominates many aspects of whole-body substrate utilization. In particular, upper-body subcutaneous fat, of which the abdominal depot is the largest component by far, is the major supplier of nonesterified fatty acids (NEFA) to the systemic circulation (29), and the rate of NEFA release from this depot is therefore a key regulator of systemic plasma NEFA concentrations, and in turn of fat oxidation and resting metabolic rate (28, 49).
There are several approaches to studying the metabolic characteristics of this fat depot in vivo, including microdialysis (1), positron-emission tomography (70), and selective venous catheterization (45). The last of these allows for the quantitative estimation of exchange with the circulation of a number of substrates, including the non-water soluble substrates such as NEFA and triacylglycerols (TGs).
The first experiments examining the role of adipose tissue by selective venous catheterization were those of Gordon and colleagues, who examined NEFA release from subcutaneous thigh tissue by sampling blood from the saphenous vein (24). In 1989, a procedure was described for catheterization of veins draining the subcutaneous abdominal depot (17). This technique has been used over the years by several research groups (2, 10, 30, 47). In our laboratory, it has been in continuous use for over 20 years. During this time, we have amassed data on the characteristics of subcutaneous abdominal adipose tissue in the fasting state from a large number of volunteers of various body mass indices. This database offers the opportunity to explore in detail many facets of human adipose tissue physiology. Here we have used it in particular to examine factors that contribute to regulation of the rate of NEFA release after overnight fast.
We published some data from small numbers of volunteers in a similar way early in our studies (9). Since then, data on fasting characteristics have appeared in a large number of individual papers on selected groups of volunteers (references are given later) but not collated in this way.
EXPERIMENTAL PROCEDURES
Volunteers and protocol.
In all the experiments contributing to this database, volunteers were metabolically healthy (nondiabetic and without lipid disorders requiring medication), and all gave signed, informed consent. Experiments were approved by the Local Research Ethics Committees appropriate at the time, as detailed in individual papers. Methods of recruitment varied between studies and are reported in the individual papers (Table 1).
Table 1.
Subjects and studies included
| Characteristics | Protocol (after fasting period) | n Male/Female | Notes | References |
|---|---|---|---|---|
| Lean | OGTT and euglycemic clamp | 12/3 | No blood flow measurements | (8, 17) |
| Lean and overweight | Alcohol ingestion and control study | 5/2 | No blood flow measurements; two visits, short time apart | (18) |
| Lean and obese | Mixed meal ingestion | 13/14 | (6, 7) | |
| Lean and obese | High-fat meal ± insulin infusion | 6/1 | (21) | |
| Lean and obese | Controls for manipulation of GH, cortisol | 17/7 | (56–59) | |
| Lean and overweight | Intravenous lipid infusion | 6/0 | (55) | |
| Lean and overweight | Mixed meal/studies of TG-rich lipoprotein metabolism | 7/0 | (35) | |
| Lean and obese | Studies of structured TG metabolism | 4/15 | Two visits, short time apart | (65, 66) |
| Lean and overweight | Ingestion of meal rich in n-3 fatty acids | 9/0 | (64) | |
| Lean and overweight | Intralipid: oral vs. iv administration | 4/2 | Two visits, short time apart | (13) |
| Lean and overweight | Controls for study of familial combined hyperlipidemia | 5/5 | (11) | |
| Lean and obese | Dietary starch supplementation | 4/6 | (53) | |
| Lean and obese | Pulsatility of lipolysis | 6/3 | (33) | |
| Lean and obese | Studies of Pparg genetic variation | 42/0 | (68, 69) | |
| Lean and obese | Controls for study of insulin-resistant men | 10/0 | (2, 3) | |
| Lean and obese | Studies of metabolism over 24-h periods | 19/3 | (43, 54) | |
| Lean and overweight | Studies of abdominal vs. femoral fat metabolism | 8/4 | (44) | |
| Lean and obese | Various | 26/30 | Unpublished |
GH, growth hormone; TG, triacylglycerol; OGTT, oral glucose tolerance test.
Volunteers attended our Clinical Research Unit after an overnight fast, and selective venous catheterization of a branch of the superficial epigastric vein draining subcutaneous abdominal adipose tissue was performed as described previously (17, 45). In some of the studies, arterial blood samples were obtained by cannulation of either a radial artery or a femoral artery. In other experiments, arterialized venous blood was sampled by retrograde cannulation of a vein draining the hand, which was kept in a box with air temperature of 55–60°C (42). Differences between true arterial and arterialized venous blood are considered later.
After a period of 30–60 min rest, blood samples were drawn simultaneously from the arterial or arterialized site and from the adipose venous catheter. In most experiments, further fasting blood samples were taken after an interval of 20–30 min, and in some a further set was taken 30 min later.
In most of the experiments, adipose tissue blood flow (ATBF) was monitored during the period of blood sampling by the washout of 133Xe (38) following procedures described previously (60, 67).
Analytic techniques.
Hematocrit was measured using a microcapillary method. In most experiments, a small aliquot of blood was immediately deproteinized in ice-cold 7% wt/vol perchloric acid. Blood samples were kept on ice and rapidly centrifuged at 4°C. Some assays were made immediately, but some plasma aliquots were stored at −20°C or at −80°C before analysis.
Concentrations of metabolites were measured with enzymatic methods on a clinical chemistry analyzer. For glycerol, lactate, pyruvate, and ketone bodies, concentrations were measured in whole blood; for other substances measurements were made in plasma. Plasma insulin concentrations were measured by radioimmunoassay. Details of techniques have been given in individual papers (Table 1).
Calculations and statistical methods.
Data from experiments in which more than one fasting blood sample had been taken were averaged to give mean values for that individual on that day. As will be described further below, some volunteers took part in more than one experiment. For the first part of results, these multiple experiments were treated by averaging values for each metabolite on each occasion for each volunteer.
For substances extracted by the tissue, we calculated fractional extraction as the arteriovenous (A-V) difference divided by the arterial(ized) concentration, then expressed as a percentage. When comparing release of NEFA (measured in plasma) and glycerol (measured in whole blood), we calculated the equivalent whole blood NEFA concentration as NEFAwhole blood = NEFAplasma × (1 − hematocrit), where hematocrit is expressed as a fraction. The same was done when comparing TG extraction (measured in plasma) and glycerol release. Absolute release rates were calculated as whole-blood venoarterial (V-A) difference × ATBF.
Percent reesterification of fatty acids was calculated from the relative release of NEFA and glycerol, assuming that lipolysis of one mole of TG releases one mole of glycerol, which is not reutilized. The calculation was
%reesterification = 100 × [1 − (NEFA V-A difference/3 × glycerol V-A difference)]
where V-A differences are both expressed as whole blood concentrations. It should be noted that this calculation includes any fatty acids released in lipolysis that are oxidized; this is discussed further below.
Reesterification requires provision of glycerol 3-phosphate. The proportion of glucose uptake used for provision of glycerol 3-phosphate was calculated as
%glucose uptake for provision of glycerol 3-phosphate = 100 × [3 × glycerol V-A difference − NEFA V-A difference]/[glucose A-V difference × 1,000 × 2]
where A-V and V-A differences are expressed as whole blood concentrations. The factor 1,000 in the denominator converts glucose concentrations in mmol/l to μmol/l; the factor 2 allows for the fact that each molecule of glucose may give rise to two molecules of glycerol 3-phosphate.
The proportion of glucose released as lactate was calculated as
%glucose uptake released as lactate = 100 × [lactate V-A difference/glucose A-V difference × 1,000 × 2]
where again A-V and V-A differences are expressed as whole blood concentrations, and the factor 1,000 in the denominator converts glucose concentrations in mmol/l to μmol/l; the factor 2 allows for the fact that each molecule of glucose may give rise to two molecules of lactate.
All statistical analyses were made using SPSS Statistics v. 17.0. Comparisons between arterial(ized) and adipose venous concentrations were analyzed by Wilcoxon signed ranks test for paired samples. Comparisons between men and women were made using the Mann-Whitney test. Correlations were computed using Spearman's nonparametric coefficient. Other statistical techniques are described in the text. For the regression analysis for NEFA release, we used both untransformed and log-transformed values. Neither fitted the assumptions perfectly, but the results of the two analyses were identical. P values < 10−6 are shown as P ≪ 0.001.
Not all measurements were made in each volunteer, so numbers of measurements are presented with all data.
RESULTS
A total of 331 experiments were available for analysis, conducted in 255 individuals (167 male) with BMI range 18.3 to 53.4 kg/m2 (median 24.7 kg/m2), over a period of 23 yr. As noted in Statistical methods, all data in the first part of the paper have been averaged within individuals who had undergone more than study.
NEFA, glycerol, TG, and glucose.
Plasma or whole blood concentrations of the major metabolites measured are presented in Table 2 together with 95% confidence intervals for their A-V differences. For both NEFA and glycerol, there was clear release from the adipose tissue. Venous concentrations were 1.2 to 3.7 times those in arterial(ized) plasma for NEFA, 1.1 to 8.5 times those in arterial(ized) blood for glycerol. In contrast, there was extraction of both TG and glucose, with percentage extractions (means ± SE) of 5.7 ± 0.4 and 2.6 ± 0.3%, respectively.
Table 2.
Concentrations of metabolites and adipose tissue blood flow
| Metabolite and Matrix | Arterial(ized) | Adipose Venous | P Arterial(ized) vs. Venous | A-V Difference | CIs for A-V Difference |
|---|---|---|---|---|---|
| Plasma NEFA (μmol/l) | 575 ± 12 (253) | 1239 ± 26 (253) | <<0.001 | −665 | −628 to −701 |
| Blood glycerol (μmol/l) | 66.8 ± 1.9 (247) | 214.4 ± 5.2 (247) | <<0.001 | −148 | −139 to −156 |
| Plasma TG (mmol/l) | 1.20 ± 0.05 (238) | 1.14 ± 0.05 (238) | <<0.001 | 0.057 | 0.048–0.066 |
| Plasma glucose (mmol/l) | 5.25 ± 0.04 (242) | 5.11 ± 0.03 (236) | <<0.001 | 0.14 | 0.11–0.17 |
| Plasma insulin (mU/l) | 11.7 ± 0.7 (252) | ||||
| Blood 3OHB (μmol/l) | 120 ± 7 (204) | 104 ± 7 (199) | <<0.001 | 15 | 11–18 |
| Blood acetoacetate (μmol/l) | 80 ± 9 (31) | 64 ± 8 (31) | <0.001 | 16 | 11–20 |
| Blood lactate* (μmol/l) | 570 ± 34 (70) | 667 ± 32 (70) | <<0.001 | −97 | −73 to −121 |
| Blood pyruvate* (μmol/l) | 83 ± 9 (35) | 87 ± 8 (35) | 0.04 | −4.2 | −9.8–1.4 |
| ATBF (ml·100 g−1·min−1) | 3.53 ± 0.13 (240) |
Values are means ± SE (n) and are averaged within people who had undergone more than one study.
3OHB, 3-hydroxybutyrate; ATBF, adipose tissue blood flow. 95% confidence intervals (CIs) are shown.
Only presented for studies with true arterial samples.
There was a very strong correlation between the V-A difference for NEFA and that for glycerol release (Fig. 1). This was independent of ATBF analyzed as a covariate. The ratio of NEFA to glycerol release was 2.69 ± 0.04 (n = 247), highly significantly different from the value of 3.0 that would result if there were no reesterification (P ≪ 0.001, Wilcoxon signed ranks test). The percent reesterification of fatty acids was 10.2 ± 1.4% (n = 247).
Fig. 1.
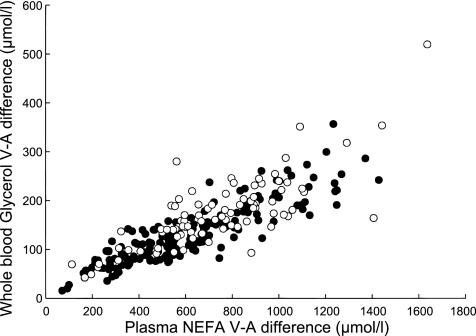
Relationship between veno-arterial (V-A) differences for glycerol (measured in whole blood) and NEFA (measured in plasma). Men are shown as solid points, women as open circles. Over both sexes, rs = 0.86, n = 246, P ≪ 0.001.
Since there was extraction of TG, reflecting the action of lipoprotein lipase (LPL) in the adipose tissue capillaries, some of the glycerol release will result from this reaction. Comparing TG extraction and glycerol release suggested that 21 ± 3% (n = 233) of the glycerol released from adipose tissue in the fasting state came from the action of LPL rather than from intracellular lipolysis.
Reesterification of fatty acids requires the provision of glycerol 3-phosphate. Assuming that this is produced in glycolysis, we can calculate the proportion of glucose uptake that is used for provision of glycerol 3-phosphate. This calculation (which involves the division of several small numbers) resulted in a wide range of values (−1,700 to 53,000%) with a wide skew: the median value was 15%, mean 330% (n = 234). We removed, arbitrarily, all nonphysiological values (>100% or <0%). For the remaining 137 values, the median was 23%, mean 29%.
Table 3 shows a comparison of men and women for arterial(ized) concentrations and for release rates and TG fractional extraction. As is well known, plasma NEFA concentrations were higher in women than in men, and the same was true for blood glycerol. TG concentrations were just significantly lower in women, and TG fractional extraction across adipose tissue was significantly higher in women than in men. In contrast, release rates of NEFA and glycerol (expressed per 100 g tissue) were not different between the sexes, nor was calculated reesterification of fatty acids.
Table 3.
Comparison of men and women
| Metabolite and Matrix | Men | Women | P |
|---|---|---|---|
| Plasma NEFA (μmol/l) | 532 ± 14 (166) | 655 ± 20 (87) | <<0.001 |
| Blood glycerol (μmol/l) | 62.5 ± 2.3 (162) | 75.2 ± 3.2 (85) | <0.001 |
| Plasma TG (mmol/l) | 1.24 ± 0.06 (155) | 1.12 ± 0.08 (83) | 0.05 |
| Blood 3OHB (μmol/l) | 109 ± 9 (141) | 146 ± 14 (63) | 0.003 |
| NEFA release, nmol·100 g−1·min−1 | 1357 ± 95 (156) | 1529 ± 135 (82) | 0.12 |
| Glycerol release, nmol·100 g−1·min−1 | 503 ± 35 (152) | 588 ± 59 (80) | 0.07 |
| Reesterification, % | 9.6 ± 1.7 (162) | 11.5 ± 2.7 (85) | 0.29 |
| TG fractional extraction, % | 4.9 ± 0.4 (155) | 7.1 ± 0.8 (155) | 0.004 |
Values are means ± SE (n) and are averaged within people who had undergone more than one study. Concentrations are in arterial(ized) blood or plasma.
We looked at relationships with measurements of adiposity. Overall, there was a negative relationship between NEFA release rate and BMI (Fig. 2A). This was strong in men (rs = −0.34, n = 156, P < 0.001), but nonsignificant in women (rs = −0.10, n = 82, P = 0.35). The ratio of NEFA to glycerol release was also negatively related to BMI (rs = −0.21, n = 247, P = 0.001), and consequently calculated reesterification (as a percentage) was positively related to BMI (rs = +0.21, n = 247, P = 0.001). ATBF was also negatively related to BMI (rs = −0.34, n = 240, P ≪ 0.001). TG fractional extraction was not related to BMI.
Fig. 2.
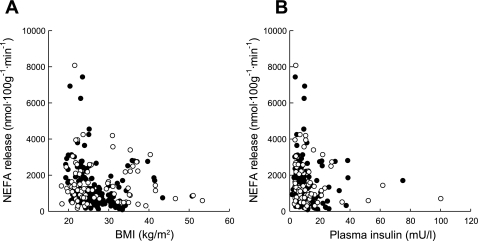
Relationship between NEFA release rate from adipose tissue and BMI (A), arterial(ized) insulin concentrations (B). Men are shown as solid points, women as open circles. Over both sexes, A: rs = −0.24, n = 238, P < 0.001; B: rs = −0.25, n = 236, P < 0.001.
We investigated factors determining NEFA release rate. We used the V-A difference for NEFA as the dependent variable rather than NEFA release rate, as we wished to include ATBF as a potential independent variable. We entered ATBF, glycerol V-A difference (as a measure of lipolysis), plasma insulin, percent reesterification, BMI, and age as independent variables in a linear regression using a backward stepping method. NEFA release was positively related to glycerol V-A difference (as a measure of lipolysis) and inversely to plasma insulin and to percent reesterification (P < 0.001 for each) after accounting for ATBF, BMI and age. In bivariate analysis, NEFA release was negatively related to arterial(ized) plasma insulin (Fig. 2B). Like the relationship with BMI, this was strong in men (rs = −0.29, n = 156, P < 0.001) but nonsignificant in women (rs = −0.19, n = 80, P = 0.10).
Ketone body, lactate, and pyruvate metabolism.
Concentrations of the ketone bodies 3-hydroxybutyrate (3OHB) and acetoacetate (AcAc) are shown in Table 2. There was significant extraction of both (fractional extraction, means ± SE: 3OHB, 12.8 ± 0.8%; AcAc, 20.1 ± 2.3%). The fractional extraction of AcAc was significantly greater than that of 3OHB (P = 0.001, Wilcoxon signed ranks test).
Concentrations of lactate are consistently different in arterial compared with arterialized blood (15). Therefore, lactate and pyruvate metabolism have only been analyzed in studies involving true arterial cannulation (Table 2). There was release of both lactate and pyruvate from the tissue. We asked what proportion of glucose uptake was released as lactate. This calculation resulted in physiologically plausible values (<100% and >0%) for all but one volunteer, in whom a value of −1,790% was found. After elimination of this one outlier, the mean value was 32 ± 7% (median 32%; n = 69). Arterial lactate concentrations correlated strongly with percent reesterfication of fatty acids (rs = 0.34, n = 70, P = 0.004). Adipose tissue NEFA release was also positively correlated with lactate release (rs = 0.41, n = 68, P < 0.001).
Analysis of repeated studies.
One volunteer had undergone nine studies over a period of 15 years. Two volunteers had undergone six studies over a period of ∼13 years each. Four individuals had been studied on four occasions over 2–5 years; nine had been studied three times, and 27 had been studied twice (usually within a few weeks, in the course of studies comparing one treatment with another, e.g., two different types of fat ingestion; see Table 1).
Some of the data are shown graphically in Fig. 3. BMI is shown as a “positive control”, as this would be expected to be more consistent within individuals than between individuals. It can be seen that BMI for each individual tends to track along a relatively constant value (or at least along a linear trend). However, for the V-A difference for NEFA release, the picture is less clear-cut, with considerable variation between repeated measures in the same subject.
Fig. 3.
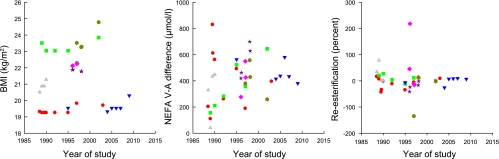
Analysis of repeated studies. Each symbol and color represents one individual studied on more than one occasion. Only people with ≥4 studies are shown. For BMI there is clearly more consistency within any one individual than between individuals. For statistics on other parameters see Table 4.
We therefore analyzed these data using a one-way analysis of variance, asking whether the variance within each individual was less than the variance between individuals (i.e., is there some consistency within individuals?). The results are shown in Table 4. BMI and age were entered as positive controls, and for both there was highly significantly less variation within than between individuals. Arterial(ized) concentrations of NEFA and TG were also highly significantly more consistent within than between individuals. The rate of NEFA release was significantly more consistent within than between volunteers, although this was not true for glycerol release. Percent reesterification was also significantly more consistent within than between individuals, as was ATBF. Fractional extraction of TG across adipose tissue was not more consistent within than between volunteers.
Table 4.
Analysis of repeated studies in the same individual
| Variable | Degrees of Freedom (total) | F | P |
|---|---|---|---|
| BMI | 117 | 150 | <<0.001 |
| Age | 117 | 55 | <<0.001 |
| ATBF | 100 | 2.5 | 0.001 |
| Plasma TG* | 100 | 16.2 | <<0.001 |
| Plasma NEFA* | 117 | 5.9 | <<0.001 |
| NEFA release | 96 | 2.1 | 0.004 |
| Glycerol release | 97 | 1.5 | 0.068 |
| Re-esterification (%) | 115 | 1.8 | 0.015 |
| TG fractional extraction | 98 | 0.99 | 0.506 |
Analysis by one-way ANOVA, with variance between studies within one individual compared with variance between individuals, using F-test.
Concentrations in arterial(ized) plasma.
DISCUSSION
These data, collected over a period of 23 years, form a unique dataset and have enabled us to examine several aspects of the metabolism of human subcutaneous adipose tissue after an overnight fast. Detailed analysis of A-V difference measurements can be difficult in non-steady-state situations (73), but here we examined the situation in a metabolic state that is as steady as any during the typical 24-h cycle of fasting and feeding, so it seems likely that these data give us a reliable window into adipose tissue metabolism. We found the expected release of NEFA and glycerol, indicating that we were indeed sampling adipose tissue drainage, and in other studies we and others have also shown release of leptin by use of this technique (12, 36, 45).
The ratio of NEFA to glycerol release was significantly less than 3:1. This indicates that some reesterification of fatty acids occurs in the fasting state, as has been shown also in tracer studies measuring the ratio of rates of appearance, and is usually found in isolated adipocytes (26). The value we found of 10% of fatty acids reesterified compares with ∼20% obtained from isotopic turnover methods (5, 72); in other words, two independent techniques imply that a small proportion (20% or less) of fatty acids released in lipolysis are reesterified in the fasting state. It should be noted that reesterification measured in vivo includes the fatty acids released from intravascular lipolysis (via LPL) as well as those released from intracellular lipolysis. Reesterification calculated in this way also includes any fatty acids released in lipolysis that were oxidized within the tissue. Although it is clear that fatty acid oxidation by human adipose tissue is low (19), it is difficult to speculate how much oxidation might contribute to the low level of reesterification observed.
Intravascular lipolysis (LPL action) is at its lowest in the usual 24-h cycle after an overnight fast (43) but was still readily measureable. We investigated previously whether LPL action released glycerol into the circulation in the same way that intracellular lipolysis does. LPL is an sn(1,3) TG lipase and causes release of sn-2 monoacylglycerols in vitro (51). However, we have shown that there is no release of monoacylglycerols from adipose tissue in vivo, even during times of high LPL action (14, 65). Furthermore, by using structured TGs with known fatty acids at the sn-2 position, we have shown that there is nothing distinctive about the metabolic fate of the sn-2 fatty acid (65, 66). Our conclusion is therefore that TG-rich lipoproteins are bound to the endothelial site of action of LPL [for which there is histological evidence (61)] long enough for nonenzymatic randomization of monoacylglycerol-fatty acids, resulting in complete lipolysis (with release of glycerol). Alternatively, it might be that sn-2 monoacylglycerols are taken up by adipocytes and immediately hydrolyzed by the active intracellular monoacylglycerol lipase, again presumably with release of glycerol, although the experiments with structured TGs argue against this. Measurements of interstitial glycerol concentrations seem to bear out the idea that glycerol release reflects both intracellular lipolysis and LPL action (63). The present data suggest that ∼20% of the glycerol released from adipose tissue in the fasting state arises from LPL action, a fact that is sometimes overlooked. There is little in the literature with which to compare this observation. Miles et al. (46) also found considerable release of LPL-derived glycerol in the forearm, although not complete, as forearm muscle undoubtedly also extracts glycerol.
Glucose uptake is a prominent feature of adipocyte metabolism and indeed is a parameter often measured in vitro. The present data allowed us to say something about the fate of the glucose taken up. Around 20–25% is used for synthesis of glycerol 3-phosphate used in reesterification (although this figure should be treated with some caution), and around 30% is released as lactate (with a very small amount of pyruvate). The remainder would be available for oxidation or other possible fates, although it would seem probable that de novo lipogenesis or glycogen synthesis, both known features of human adipocyte metabolism (41, 62), would be low after an overnight fast. The pathway for glycerol 3-phosphate formation might be the direct reduction of dihydroxyacetone phosphate formed in glycolysis, but it is also possible that 3-carbon intermediates are formed and used in the pathway known as glyceroneogenesis (4); our data cannot give information on that.
NEFA release from adipose tissue is an important driver of metabolic events, some potentially adverse, throughout the body. Our data strongly confirm the now widely accepted view that NEFA release is downregulated (per unit mass of adipose tissue) in obesity (32, 48), similarly in men and women. Our own interpretation of this observation is that it represents a physiological adaptation to prevent “fatty acid overload” to other organs (32, 43), although others have interpreted it as potentially causal for obesity (37). We have not attempted to adjust NEFA release per 100 g tissue for the total mass of the fat depot, as we do not have a reliable measure of this. We showed recently, however, that NEFA concentrations may be surprisingly similar in lean and abdominally obese men (43), suggesting that the downregulation per 100 g may in many cases appropriately correct for the greater mass of the depot. The downregulation of NEFA release with increasing BMI may reflect increasing adipocyte size. A wide range of cell sizes would be expected among participants with a BMI range from 18 to 53 kg/m2, suggesting that NEFA release per fat cell might be relatively constant over a range of BMI. Unfortunately, we did not have adequate numbers of measurements to test this idea. When we analyzed factors relating to NEFA release, BMI was not a significant contributor, but insulin was, implying, as we and others have suggested previously, that downregulation of adipose tissue NEFA release in obesity is largely driven by the associated hyperinsulinemia (31, 32). Reesterification also emerged as an independent (inverse) associate of NEFA release. We noted previously that reesterification is a characteristic of an individual in different adipose depots (44). We observed a strong positive relationship between the percent reesterification of fatty acids and arterial lactate concentrations, bearing out old observations of an antilipolytic effect of lactate mediated via reesterification (22). Recent studies showing that GPR81 is an antilipolytic lactate receptor in adipose tissue (39) might have suggested a direct relationship between arterial lactate and NEFA release, but we did not find this.
We confirm here the well-known observation that systemic NEFA concentrations are generally higher in women than in men (32), but we did not find a significantly greater rate of NEFA release, suggesting that the difference in concentrations simply reflects the greater fat mass of women. This would agree with the data of Mittendorfer et al. (48), although it conflicts somewhat with our own data showing a lower rate of appearance of NEFA (expressed per kg fat mass) in young women compared with men (40). It would have been interesting to analyze further differences between men and women in terms of different fat distributions, but for much of the database we did not have good data on this.
In most of the studies reported here, we sampled not true arterial blood but arterialized blood from a vein in a heated hand. This technique has a long history in metabolic research (42) and has been validated for a large number of metabolites (summarized in Ref. 20). However, there are consistent discrepancies between arterial and arterialized blood in blood gas and lactate concentrations (15); hence, we only analyzed studies with true arterial samples for lactate metabolism. Our data show that adipose tissue is a consistent producer of lactate and to a smaller extent of pyruvate. It is important to note here that we cannot exclude some venous drainage from skin with this preparation. It does not appear to be large, because skin is highly glycolytic, and lactate concentrations in superficial veins draining mainly skin are very high (reviewed in Ref. 16), but this could contribute toward the lactate release that we observed. However, in microdialysis studies, in which the skin contribution might be considered to be negligible, lactate release from adipose tissue is also observed (25, 52).
We confirmed previous observations (7, 9) of significant extraction of the ketone bodies 3OHB and AcAc by subcutaneous adipose tissue, with greater fractional extraction of the latter. The fate of these after removal from blood is not clear; they could in principle feed into de novo lipogenesis, but in the postabsorptive state oxidation seems more likely. However, it is important to note that the magnitude is small: the mean combined A-V difference was 30 μmol/l, whereas that for glucose (with 6 vs. 4 carbon atoms) was 125 μmol/l.
Fasting NEFA concentrations may show large day-to-day within-subjects variability (71), but this is reduced by close control of energy balance (49), such that, in general, some consistency is observable (32). In a recent study, the correlation coefficient between plasma NEFA concentrations on two occasions 4–5 wk apart was 0.70 (P < 0.001) (27). Here, we show that arterial(ized) plasma NEFA concentrations are highly significantly more consistent within than between individuals and that this reflects, in part, consistency of adipose tissue NEFA release measured with the A-V technique. ATBF was also relatively consistent within individuals, as we showed previously (34, 67), as was reesterification, reinforcing the point made above about this as an individual characteristic. We did not find consistency in TG extraction, but this may reflect the experimental error in measuring this small A-V difference in the relatively small subset of participants with repeated measurements. Nevertheless, measurements of TG fraction extraction were sufficiently robust to demonstrate greater efficiency of TG extraction in women than men.
The present dataset does not include information on blood gases and their exchange across adipose tissue. We did not collect reliable samples for this in many of our early studies. We have reported a small but consistent extraction of O2 and release of CO2 by adipose tissue (17). Recently, human adipose tissue O2 extraction has been quantified in obese and lean individuals and is lower in the obese (23). We have collected detailed information on adipose tissue O2 extraction and CO2 release in the fasting and postprandial states, which will be the subject of a separate paper.
An important limitation of this study is that we examined the metabolism of one specific adipose depot. The upper-body subcutaneous fat (of which subcutaneous abdominal is a major component) is a major contributor of systemic plasma NEFA (29); nevertheless, it is important to recognize that lipolysis (and, indeed, fat storage) are regulated somewhat differently in different adipose depots (44, 50).
We conclude that human adipose tissue metabolism and physiology are complex. Adipose tissue is not just a site for storage of excess fat; it participates in many metabolic activities. Its function is altered in obesity, most notably with a downregulation of NEFA release per unit weight of tissue. Our observations on the disposition of glucose taken up by adipose tissue are novel, as are our findings on the repeatability of measurements of adipose tissue function within individuals. We hope that the data provided here will provide a solid foundation for future explorations of adipose tissue physiology and pathophysiology.
GRANTS
This work has been supported by grants from many bodies including British Heart Foundation, Humane Research Trust, Medical Research Council, Wellcome Trust, and the European Commission. For studies conducted between 2007 and 2011, NIHR Oxford Biomedical Research Centre also provided support.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.N.F. and S.M.H. conception and design of research; K.N.F. and S.M.H. collated data; K.N.F. and S.M.H. analyzed data; K.N.F. and S.M.H. interpreted results of experiments; K.N.F. prepared figures; K.N.F. drafted manuscript; K.N.F. and S.M.H. edited and revised manuscript; K.N.F. and S.M.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all our colleagues who provided data as shown in Table 1, and particularly Konstantinos Manolopoulos, Louise Dennis, and Alex Bickerton, who provided the unpublished data. Fredrik Karpe and Derek Hockaday provided much support and stimulating discussion during the course of these studies.
REFERENCES
- 1. Arner P, Kriegholm E, Engfeldt P, Bolinder J. Adrenergic regulation of lipolysis in situ at rest and during exercise. J Clin Invest 85: 893–898, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bickerton AS, Roberts R, Fielding BA, Hodson L, Blaak EE, Wagenmakers AJ, Gilbert M, Karpe F, Frayn KN. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes 56: 168–176, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bickerton AST, Roberts R, Fielding BA, Tornqvist H, Blaak EE, Wagenmakers AJM, Gilbert M, Humphreys SM, Karpe F, Frayn KN. Adipose tissue fatty acid metabolism in insulin-resistant men. Diabetologia 51: 1466–1474, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Cadoudal T, Blouin JM, Collinet M, Fouque F, Tan GD, Loizon E, Beale EG, Frayn KN, Karpe F, Vidal H, Benelli C, Forest C. Acute and selective regulation of glyceroneogenesis and cytosolic phosphoenolpyruvate carboxykinase in adipose tissue by thiazolidinediones in type 2 diabetes. Diabetologia 50: 666–675, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Campbell PJ, Carlson MG, Hill JO, Nurjhan N. Regulation of free fatty acid metabolism by insulin in humans: role of lipolysis and reesterification. Am J Physiol Endocrinol Metab 263: E1063–E1069, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Coppack SW, Evans RD, Fisher RM, Frayn KN, Gibbons GF, Humphreys SM, Kirk MJ, Potts JL, Hockaday TDR. Adipose tissue metabolism in obesity: lipase action in vivo before and after a mixed meal. Metabolism 41: 264–272, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Coppack SW, Fisher RM, Gibbons GF, Humphreys SM, McDonough MJ, Potts JL, Frayn KN. Postprandial substrate deposition in human forearm and adipose tissues in vivo. Clin Sci 79: 339–348, 1990 [DOI] [PubMed] [Google Scholar]
- 8. Coppack SW, Frayn KN, Humphreys SM, Dhar H, Hockaday TDR. Effects of insulin on human adipose tissue metabolism in vivo. Clin Sci 77: 663–670, 1989 [DOI] [PubMed] [Google Scholar]
- 9. Coppack SW, Frayn KN, Humphreys SM, Whyte PL, Hockaday TDR. Arteriovenous differences across human adipose and forearm tissues after overnight fast. Metabolism 39: 384–390, 1990 [DOI] [PubMed] [Google Scholar]
- 10. Coppack SW, Horowitz JF, Paramore DS, Cryer PE, Royal HD, Klein S. Whole body, adipose tissue, and forearm norepinephrine kinetics in lean and obese women. Am J Physiol Endocrinol Metab 275: E830–E834, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Evans K, Burdge GC, Wootton SA, Collins JM, Clark ML, Tan GD, Karpe F, Frayn KN. Tissue-specific stable isotope measurements of postprandial lipid metabolism in familial combined hyperlipidaemia. Atherosclerosis 197: 164–170, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Evans K, Clark ML, Frayn KN. Carbohydrate and fat have different effects on plasma leptin concentrations and adipose tissue leptin production. Clin Sci 100: 493–498, 2001 [PubMed] [Google Scholar]
- 13. Evans K, Clark ML, Frayn KN. Effects of an oral and intravenous fat load on adipose tissue and forearm lipid metabolism. Am J Physiol Endocrinol Metab 276: E241–E248, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Fielding BA, Humphreys SM, Shadid S, Frayn KN. Arterio-venous differences across human adipose tissue for mono-, di- and tri-acylglycerols before and after a high-fat meal. Endocrinol Metab 2: 13–17, 1995 [Google Scholar]
- 15. Forster HV, Dempsey JA, Thomson J, Vidruk E, DoPico GA. Estimation of arterial PO2, PCO2, pH, and lactate from arterialized venous blood. J Appl Physiol 32: 134–137, 1972 [DOI] [PubMed] [Google Scholar]
- 16. Frayn KN, Coppack SW, Humphreys SM. Subcutaneous adipose tissue metabolism studied by local catheterization. Int J Obesity 17, Suppl 3: S18–S21, 1993 [PubMed] [Google Scholar]
- 17. Frayn KN, Coppack SW, Humphreys SM, Whyte PL. Metabolic characteristics of human adipose tissue in vivo. Clin Sci 76: 509–516, 1989 [DOI] [PubMed] [Google Scholar]
- 18. Frayn KN, Coppack SW, Walsh PE, Butterworth HC, Humphreys SM, Pedrosa HC. Metabolic responses of forearm and adipose tissues to acute ethanol ingestion. Metabolism 39: 958–966, 1990 [DOI] [PubMed] [Google Scholar]
- 19. Frayn KN, Langin D, Karpe F. Fatty acid-induced mitochondrial uncoupling in adipocytes is not a promising target for treatment of insulin resistance unless adipocyte oxidative capacity is increased. Diabetologia 51: 394–397, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Frayn KN, Macdonald IA. Methodological considerations in arterialization of venous blood (letter). Clin Chem 38: 316–317, 1992 [PubMed] [Google Scholar]
- 21. Frayn KN, Shadid S, Hamlani R, Humphreys SM, Clark ML, Fielding BA, Boland O, Coppack SW. Regulation of fatty acid movement in human adipose tissue in the postabsorptive-to-postprandial transition. Am J Physiol Endocrinol Metab 266: E308–E317, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Fredholm BB. The effect of lactate in canine subcutaneous adipose tissue in situ. Acta Physiol Scand 81: 110–123, 1970 [DOI] [PubMed] [Google Scholar]
- 23. Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JWE, Čajlakovič M, Ribitsch V, Clément K, Blaak EE. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation 124: 67–76, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Gordon RS. Unesterified fatty acid in human blood plasma. II. The transport function of unesterified fatty acid. J Clin Invest 36: 810–815, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hagström-Toft E, Enoksson S, Moberg E, Bolinder J, Arner P. Absolute concentrations of glycerol and lactate in human skeletal muscle, adipose tissue, and blood. Am J Physiol Endocrinol Metab 273: E584–E592, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Hammond VA, Johnston DG. Substrate cycling between triglyceride and fatty acid in human adipocytes. Metabolism 36: 308–313, 1987 [DOI] [PubMed] [Google Scholar]
- 27. Hodson L, Harnden KE, Roberts R, Dennis AL, Frayn KN. Does the DASH diet lower blood pressure by altering peripheral vascular function? J Hum Hypertens 24: 312–319, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Jelic K, Hallgreen CE, Colding-Jorgensen M. A model of NEFA dynamics with focus on the postprandial state. Ann Biomed Eng 37: 1897–1909, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Jensen MD. Adipose tissue and fatty acid metabolism in humans. J R Soc Med 95, Suppl 42: 3–7, 2002 [PMC free article] [PubMed] [Google Scholar]
- 30. Jocken JW, Goossens GH, van Hees AM, Frayn KN, van Baak M, Stegen J, Pakbiers MT, Saris WH, Blaak EE. Effect of beta-adrenergic stimulation on whole-body and abdominal subcutaneous adipose tissue lipolysis in lean and obese men. Diabetologia 51: 320–327, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jocken JWE, Langin D, Smit E, Saris WHM, Valle C, Hul GB, Holm C, Arner P, Blaak EE. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J Clin Endocrinol Metab 92: 2292–2299, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 60: 2441–2449, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Karpe F, Fielding BA, Coppack SW, Lawrence VJ, Macdonald IA, Frayn KN. Oscillations of fatty acid and glycerol release from human subcutaneous adipose tissue in vivo. Diabetes 54: 1297–1303, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Karpe F, Fielding BA, Ilic V, Macdonald IA, Summers LKM, Frayn KN. Impaired postprandial adipose tissue blood flow response is related to aspects of insulin sensitivity. Diabetes 51: 2467–2473, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Karpe F, Humphreys SM, Samra JS, Summers LKM, Frayn KN. Clearance of lipoprotein remnant particles in adipose tissue and muscle in humans. J Lipid Res 38: 2335–2343, 1997 [PubMed] [Google Scholar]
- 36. Klein S, Horowitz JF, Landt M, Goodrick SJ, Mohamed Ali V, Coppack SW. Leptin production during early starvation in lean and obese women. Am J Physiol Endocrinol Metab 278: E280–E284, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Large V, Reynisdottir S, Langin D, Fredby K, Klannemark M, Holm C, Arner P. Decreased expression and function of adipocyte hormone-sensitive lipase in subcutaneous fat cells of obese subjects. J Lipid Res 40: 2059–2066, 1999 [PubMed] [Google Scholar]
- 38. Larsen OA, Lassen NA, Quaade F. Blood flow through human adipose tissue determined with radioactive xenon. Acta Physiol Scand 66: 337–345, 1966 [DOI] [PubMed] [Google Scholar]
- 39. Liu C, Wu J, Zhu J, Kuei C, Yu J, Shelton J, Sutton SW, Li X, Yun SJ, Mirzadegan T, Mazur C, Kamme F, Lovenberg TW. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J Biol Chem 284: 2811–2822, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Marinou K, Adiels M, Hodson L, Frayn KN, Karpe F, Fielding BA. Young women partition fatty acids towards ketone body production rather than VLDL-TAG synthesis, compared with young men. Br J Nutr 105: 857–865, 2011 [DOI] [PubMed] [Google Scholar]
- 41. Markan KR, Jurczak MJ, Brady MJ. Stranger in a strange land: roles of glycogen turnover in adipose tissue metabolism. Mol Cell Endocrinol 318: 54–60, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McGuire EAH, Helderman JH, Tobin JD, Andres R, Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol 41: 565–573, 1976 [DOI] [PubMed] [Google Scholar]
- 43. McQuaid SE, Hodson L, Neville MJ, Dennis AL, Cheeseman J, Humphreys SM, Ruge T, Gilbert M, Fielding BA, Frayn KN, Karpe F. Down-regulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes 60: 47–55, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McQuaid SE, Humphreys SM, Hodson L, Fielding BA, Karpe F, Frayn KN. Femoral adipose tissue may accumulate the fat that has been recycled as VLDL and nonesterified fatty acids. Diabetes 59: 2465–2473, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McQuaid SE, Manolopoulos KN, Dennis AL, Cheeseman J, Karpe F, Frayn KN. Development of an arterio-venous difference method to study the metabolic physiology of the femoral adipose tissue depot. Obesity 18: 1055–1058, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Miles JM, Park YS, Walewicz D, Russell-Lopez C, Windsor S, Isley WL, Coppack SW, Harris WS. Systemic and forearm triglyceride metabolism: fate of lipoprotein lipase-generated glycerol and free fatty acids. Diabetes 53: 521–527, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Mitrou P, Boutati E, Lambadiari V, Maratou E, Komesidou V, Papakonstantinou A, Sidossis L, Tountas N, Katsilambros N, Economopoulos T, Raptis SA, Dimitriadis G. Rates of lipid fluxes in adipose tissue in vivo after a mixed meal in morbid obesity. Int J Obes 34: 770–774, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Mittendorfer B, Magkos F, Fabbrini E, Mohammed BS, Klein S. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity 17: 1872–1877, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nielsen S, Guo Z, Albu JB, Klein S, O'Brien PC, Jensen MD. Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest 111: 981–988, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 113: 1582–1588, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nilsson-Ehle P, Egelrud T, Belfrage P, Olivecrona T, Borgström B. Positional specificity of purified milk lipoprotein lipase. J Biol Chem 248: 6734–6737, 1973 [PubMed] [Google Scholar]
- 52. Qvisth V, Hagstrom-Toft E, Moberg E, Sjoberg S, Bolinder J. Lactate release from adipose tissue and skeletal muscle in vivo: defective insulin regulation in insulin-resistant obese women. Am J Physiol Endocrinol Metab 292: E709–E714, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Robertson MD, Bickerton AST, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr 82: 559–567, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Ruge T, Hodson L, Cheeseman J, Dennis AL, Fielding BA, Humphreys SM, Frayn KN, Karpe F. Fasted to fed trafficking of fatty acids in human adipose tissue reveals a novel regulatory step for enhanced fat storage. J Clin Endocrinol Metab 94: 1781–1788, 2009 [DOI] [PubMed] [Google Scholar]
- 55. Samra J, Giles S, Summers L, Evans R, Arner P, Humphreys S, Clark M, Frayn K. Peripheral fat metabolism during infusion of an exogenous triacylglycerol emulsion. Int J Obesity 22: 806–812, 1998 [DOI] [PubMed] [Google Scholar]
- 56. Samra JS, Clark ML, Humphreys SM, Macdonald IA, Bannister PA, Frayn KN. Effects of physiological hypercortisolemia on the regulation of lipolysis in subcutaneous adipose tissue. J Clin Endocr Metab 83: 626–631, 1998 [DOI] [PubMed] [Google Scholar]
- 57. Samra JS, Clark ML, Humphreys SM, Macdonald IA, Bannister PA, Matthews DR, Frayn KN. Suppression of the nocturnal rise in growth hormone reduces subsequent lipolysis in subcutaneous adipose tissue. Eur J Clin Invest 29: 1045–1052, 1999 [DOI] [PubMed] [Google Scholar]
- 58. Samra JS, Clark ML, Humphreys SM, Macdonald IA, Frayn KN. Regulation of lipid metabolism in adipose tissue during early starvation. Am J Physiol Endocrinol Metab 271: E541–E546, 1996 [DOI] [PubMed] [Google Scholar]
- 59. Samra JS, Clark ML, Humphreys SM, Macdonald IA, Matthews DR, Frayn KN. Effects of morning rise in cortisol concentration on regulation of lipolysis in subcutaneous adipose tissue. Am J Physiol Endocrinol Metab 271: E996–E1002, 1996 [DOI] [PubMed] [Google Scholar]
- 60. Samra JS, Frayn KN, Giddings JA, Clark ML, Macdonald IA. Modification and validation of a commercially available portable detector for measurement of adipose tissue blood flow. Clin Physiol 15: 241–248, 1995 [DOI] [PubMed] [Google Scholar]
- 61. Scow RO, Blanchette-Mackie EJ, Smith LC. Role of capillary endothelium in the clearance of chylomicrons. A model for lipid transport from blood by lateral diffusion in cell membranes. Circ Res 39: 149–162, 1976 [DOI] [PubMed] [Google Scholar]
- 62. Strawford A, Antelo F, Christiansen M, Hellerstein MK. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am J Physiol Endocrinol Metab 286: E577–E588, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Summers LKM, Arner P, Ilic V, Clark ML, Humphreys SM, Frayn KN. Adipose tissue metabolism in the postprandial period: microdialysis and arteriovenous techniques compared. Am J Physiol Endocrinol Metab 274: E651–E655, 1998 [DOI] [PubMed] [Google Scholar]
- 64. Summers LKM, Barnes SC, Fielding BA, Beysen C, Ilic V, Humphreys SM, Frayn KN. Uptake of individual fatty acids into adipose tissue in relation to their presence in the diet. Am J Clin Nutr 71: 1470–1477, 2000 [DOI] [PubMed] [Google Scholar]
- 65. Summers LKM, Fielding BA, Herd SL, Ilic V, Clark ML, Quinlan PT, Frayn KN. Use of structured triacylglycerols containing predominantly stearic and oleic acids to probe early events in metabolic processing of dietary fat. J Lipid Res 40: 1890–1898, 1999 [PubMed] [Google Scholar]
- 66. Summers LKM, Fielding BA, Ilic V, Quinlan PT, Frayn KN. The effect of triacylglycerol-fatty acid positional distribution on postprandial metabolism in subcutaneous adipose tissue. Br J Nutr 79: 141–147, 1998 [DOI] [PubMed] [Google Scholar]
- 67. Summers LKM, Samra JS, Humphreys SM, Morris RJ, Frayn KN. Subcutaneous abdominal adipose tissue blood flow: variation within and between subjects and relationship to obesity. Clin Sci 91: 679–683, 1996 [DOI] [PubMed] [Google Scholar]
- 68. Tan GD, Neville MJ, Liverani E, Humphreys SM, Currie JM, Dennis AL, Fielding BA, Karpe F. The in vivo effects of the Pro12Ala PPARγ2 polymorphism on adipose tissue NEFA metabolism: the first use of the Oxford Biobank. Diabetologia 49: 158–168, 2006 [DOI] [PubMed] [Google Scholar]
- 69. Tan GD, Savage DB, Fielding BA, Collins J, Hodson L, Humphreys SM, O'Rahilly S, Chatterjee K, Frayn KN, Karpe F. Fatty acid metabolism in patients with PPARγ mutations. J Clin Endocrinol Metab 93: 4462–4470, 2008 [DOI] [PubMed] [Google Scholar]
- 70. Virtanen KA, Peltoniemi P, Marjamäki P, Asola M, Strindberg L, Parkkola R, Huupponen R, Knuuti J, Lönnroth P, Nuutila P. Human adipose tissue glucose uptake determined using [18F]-fluoro-deoxy-glucose ([18F]FDG) and PET in combination with microdialysis. Diabetologia 44: 2171–2179, 2001 [DOI] [PubMed] [Google Scholar]
- 71. Widjaja A, Morris RJ, Levy JC, Frayn KN, Manley SE, Turner RC. Within- and between-subject variation in commonly measured anthropometric and biochemical variables. Clin Chem 45: 561–566, 1999 [PubMed] [Google Scholar]
- 72. Wolfe RR, Klein S, Carraro F, Weber JM. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am J Physiol Endocrinol Metab 258: E382–E389, 1990 [DOI] [PubMed] [Google Scholar]
- 73. Zierler KL. Theory of the use of arteriovenous concentration differences for measuring metabolism in steady and non-steady states. J Clin Invest 40: 2111–2125, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]


