Abstract
Recent publications link mitophagy mediated by PINK1 and Parkin with cardioprotection and attenuation of inflammation and cell death. The field is in need of methods to monitor mitochondrial turnover in vivo to support the development of new therapies targeting mitochondrial turnover.
Keywords: mitophagy, mitochondria, cardiac, ischemia, inflammation, Parkin, cytokine
Several recent publications converge on the importance of mitophagy in the heart and for control of inflammation. Huang et al. demonstrated a role for Parkin in cardioprotection mediated by ischemic preconditioning, an intervention in which brief ischemic stress rapidly elicits a cellular response that confers increased resistance to ischemia/reperfusion injury.1 While Parkin’s role in the brain has been extensively studied, this is the first report to demonstrate a physiological and beneficial role for Parkin in the heart. PARKIN’s collaborator, PINK1, was recently shown by Billia et al. to be essential for normal cardiac function: Pink1 knockout mice develop left ventricular dysfunction and pathological hypertrophy by 2 mo of age, and pressure overload leads to heart failure at an accelerated pace in the PINK1-null animals.2 Recently Tran et al. reported that TNFα, IL-1β and iNOS mRNA levels are higher in activated macrophages from PARKIN-null mice compared with wild-type macrophages.3 Cardiomyocytes are active components of inflammatory signaling; reactive oxygen species (ROS)-dependent NFκB signaling leads to cytokine production including TNFα, IL-1β and IL-6. Importantly, ROS derived from mitochondria are responsible for activating the NLRP3 inflammasome, and failure to remove mitochondria by autophagy exacerbates inflammasome activation.4 Damaged mitochondria may activate inflammatory signals through a second signal beyond NFκB: mitochondrial disintegration will release mitochondrial DNA (mtDNA), a key factor for activating the NLRP3 inflammasome required for production of IL-1β and IL-18.5 This is prevented by autophagic removal of dysfunctional mitochondria before mtDNA can be released into the cytosol. These studies beg the question of whether the inflammatory response to ischemia/reperfusion injury might be more severe in the PARKIN-null and PINK1-null mice. In the more acute setting, the benefits of mitophagy might derive from removal of individual damaged mitochondria before they can trigger a propagating wave of mitochondrial permeability transition pore (mPTP) opening that culminates in death of the cardiomyocyte (Fig. 1). Mitochondria are central to both pathological processes, and therefore mitophagy is likely to play a protective role as long as enough mitochondria are left intact to support cellular metabolic demands.
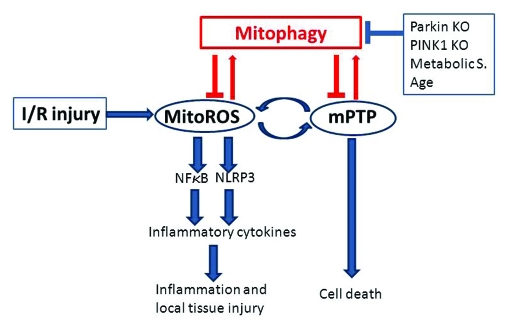
Figure 1.
Connection between mitophagy and inflammation in the heart. Ischemia/reperfusion injury triggers a burst of mitochondrial ROS followed by opening of the mPTP. Depolarization triggers autophagic removal (mitophagy), unless autophagy/mitophagy is impaired [as is the case in the Park2 (Parkin) and Pink1 knockout animals, or in the setting of metabolic syndrome (Metabolic S.) or advanced age]. Failure to eliminate damaged mitochondria leads to a vicious cycle of ROS-induced ROS release, which can trigger mPTP in many mitochondria (catastrophic mPTP), eventually culminating in cell death. ROS derived from mitochondria (mitoROS) activate NFκB, driving transcription of mRNA for inflammatory cytokines, and simultaneously activate the NLRP3 inflammasome responsible for processing the mature forms of IL-1β and IL-18. Released cytokines trigger inflammation and local tissue injury affecting adjacent “innocent bystander” cells. Mitophagy, by promptly eliminating damaged mitochondria, can prevent cell death and inflammatory signaling.
Epidemiological studies of Parkinson disease (PD) raise some intriguing questions. In a case-control study of PD patients, history of myocardial infarction and coronary artery disease was much lower in PD patients than in age-matched controls.6 Could this be due to premature cardiac mortality before the diagnosis of PD is made? The same study also noted a disproportionate increase in the incidence of congestive heart failure in the PD patients, a finding supported by a more recent study.7 Interestingly, PINK1 expression is diminished in end-stage human heart failure.2 PINK1 and PARKIN play a critical role in autophagic elimination of mitochondria; it is unclear what other roles they may play in the cell. Clearance of effete mitochondria is essential to preserve optimal function in long-lived cells, notably those of the heart and brain. Failure to remove damaged, ROS-producing mitochondria will inevitably result in cumulative oxidative damage. Taken together, these studies reveal an important role for mitophagy in cardiovascular homeostasis.
What other clinically relevant conditions might interfere with autophagy, and more specifically mitophagy? Many studies have shown that expression of rate-limiting autophagy proteins is downregulated in aged animals, resulting in impaired autophagy/mitophagy. Such changes might lead to increased ROS signaling (already known to be the case) and thus increased inflammation. Similarly, metabolic syndrome, through excessive mTOR signaling, may suppress autophagy, leading to increased mitochondrial ROS production and inflammatory signaling. The link between metabolic syndrome and inflammation is so well established that a new term has been coined: “meta-flammation.” Dysregulation of autophagy/mitophagy may contribute materially to various primary pathologies including ischemia/reperfusion and pressure overload. It will be important to consider the impact upon cardioprotection of factors affecting mitophagy, such as PARKIN, PINK1, metabolic syndrome and advanced age.
Given the abundance of mitochondria and the key role for mitophagy in the heart, it will be important to develop new technology to monitor mitochondrial turnover and to identify impaired mitophagy in the setting of disease. This could lead, in turn, to the development of entirely new approaches to the treatment of ischemia/reperfusion injury and post-infarction inflammation. Mitophagy, in and of itself, may represent the next frontier in the treatment of heart disease.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/18175
References
- 1.Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, Gottlieb RA. Preconditioning Involves Selective Mitophagy Mediated by Parkin and p62/SQSTM1. PLoS ONE. 2011;6:e20975. doi: 10.1371/journal.pone.0020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billia F, Hauck L, Konecny F, Rao V, Shen J, Mak TW. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc Natl Acad Sci USA. 2011;108:9572–7. doi: 10.1073/pnas.1106291108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran TA, Nguyen AD, Chang J, Goldberg MS, Lee J-K, Tansey MG. Lipopolysaccharide and tumor necrosis factor regulate Parkin expression via nuclear factor-Kappa B. PLoS ONE. 2011;6:e23660. doi: 10.1371/journal.pone.0023660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–5. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 5.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–30. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine RL, Jones J, Bee N. Stroke and Parkinson’s disease. Stroke. 1992;23:839–42. doi: 10.1161/01.STR.23.6.839. [DOI] [PubMed] [Google Scholar]
- 7.Zesiewicz TA, Strom JA, Borenstein AR, Hauser RA, Cimino CR, Fontanet HL, et al. Heart failure in Parkinson’s disease: analysis of the United States medicare current beneficiary survey. Parkinsonism Relat Disord. 2004;10:417–20. doi: 10.1016/j.parkreldis.2004.04.001. [DOI] [PubMed] [Google Scholar]