Abstract
Herkinorin is the first μ opioid (MOP) selective agonist derived from salvinorin A, a hallucinogenic natural product. Previous work has shown that, unlike other opioids, herkinorin does not promote the recruitment of β-arrestin-2 to the MOP receptor and does not lead to receptor internalization. This paper presents the first in vivo evaluation of herkinorin’s antinociceptive effects in rats, using the formalin test as a model of tonic inflammatory pain. Herkinorin was found to produce a dose-dependent decrease in the number of flinches evoked by formalin. These antinociceptive effects were substantially blocked by pretreatment with the nonselective antagonist naloxone, indicating that the antinociception is mediated by opioid receptors. Contralateral administration of herkinorin did not attenuate the number of flinches evoked by formalin, indicating that its effects are peripherally restricted to the site of injection. Following chronic administration (5-day), herkinorin maintained antinociceptive efficacy in both phases of the formalin test. Furthermore, unlike morphine, herkinorin was still able to inhibit flinching in both phases of the formalin test in animals made tolerant to chronic systemic morphine treatment. Collectively, these results suggest that herkinorin may produce peripheral antinociception with decreased tolerance liability and thereby represents a promising template for the development of agents for the treatment of a variety of pain states.
Keywords: MOP receptors, herkinorin, β-arrestin-2, morphine, tolerance, formalin
1. Introduction
Traditional μ opioid (MOP) receptor agonists provide highly efficacious pain relief but after extended use manifest several deleterious side-effects, such as respiratory depression, constipation, and antinociceptive tolerance (Mather and Smith, 1999). The mechanisms leading to tolerance are not yet fully understood, but several theories have been proposed, including those involving multiple neurotransmitter systems and those focused directly upon opioid receptor regulation. The MOP receptor is a G protein coupled receptor (GPCR) and is subject to regulation involving GPCR kinase (GRK)-mediated phosphorylation and subsequent βarrestin recruitment promoting the uncoupling of the receptor and the associated G-proteins (DeWire et al., 2007). Subsequently, receptor phosphorylation and β-arrestin binding can lead to receptor internalization. In its regulatory role, β-arrestin interactions with GPCRs prevent further activation of downstream effectors leading to desensitization of the receptor (Liu and Anand, 2001). Certain MOP agonists, such as DAMGO, fentanyl, etorphine and methadone, induce rapid receptor internalization when studied in cellular model systems. Morphine, and several closely related chemical derivatives, such as heroin, codeine, and oxycodone, produce much slower and diminished internalization in these same cellular models (Bushell et al., 2002). Initial in vitro studies indicated that morphine promotes very little receptor phosphorylation (Bohn et al., 2004a; Whistler and von Zastrow, 1998; Zhang et al., 1998) and thus subsequent βarrestin recruitment and receptor internalization. Further, overexpression of GRKs led to more robust morphine-initiated β-arrestin recruitment and internalization of MOP receptors (Bohn et al., 2004a) indicating that the intracellular availability of GRK levels can determine the overall impact of the agonist on the receptor fate.
The regulation of the receptor has been implicated as a pivotal point in determining opioid-induced behavioral adaptations in vivo. Following internalization of the MOP receptor, the receptor can be resensitized, whereby it is dephosphorylated and trafficked back to the membrane where agonist activation can recur. In this scenario, an agonist that would not promote internalization would also not promote resensitization and would therefore, upon chronic treatment, produce an “immobilized” inactive receptor. Morphine’s decreased ability to internalize MOP receptors has been proposed as a mechanism underlying morphine analgesic tolerance (Kim et al., 2008). However, agonists that do promote robust receptor internalization, such as methadone and fentanyl, also clinically produce analgesic tolerance. Therefore, other mechanisms aside from or in addition to internalization of the receptor must be considered.
The phosphorylated MOP receptor can recruit both β-arrestin-1 and β-arrestin-2; however, agonists, such as morphine, that promote little receptor phosphorylation, promote MOP receptor interactions with β-arrestin-2 over β-arrestin-1 (Bohn et al., 2004a). Moreover, the genetic deletion of βarrestin2 has dramatic consequence in vivo, producing enhanced morphine-induced antinociception and a concomitant attenuation of tolerance (Bohn et al., 2000; Bohn et al., 1999). Interestingly, the comparison of “highly phosphorylating/internalizing” MOP agonists (etorphine, fentanyl, and methadone) revealed equivalent antinociceptive responses in both wildtype (WT) and βarr2-KO mice demonstrating that the importance of β-arrestins in MOP receptor regulation is most apparent when agonist-induced phosphorylation of the receptor is minimal. Moreover, while βarr2-KO mice do not develop morphine tolerance in the hot plate assay following chronic morphine infusion, tolerance does ensue when the mutant mice are infused methadone or fentanyl (Raehal and Bohn, 2011).
In addition to its role in determining morphine-induced analgesic responses, β-arrestin-2 also plays a role in determining the extent of morphine-induced side effects. Mice lacking β-arrestin-2 display decreased constipation and respiratory suppression following morphine treatment (Raehal et al., 2005). The impact of β-arrestin-2 on gut function occurs primarily at peripheral sites; however the mechanism underlying morphine induced constipation involving β-arrestin-2 remains to be determined. Taken together, previous in vivo studies suggest that avoidance of the MOP receptor – β-arrestin-2 interaction may be a way to enhance antinociceptive responses while preventing the onset of tolerance and side effects.
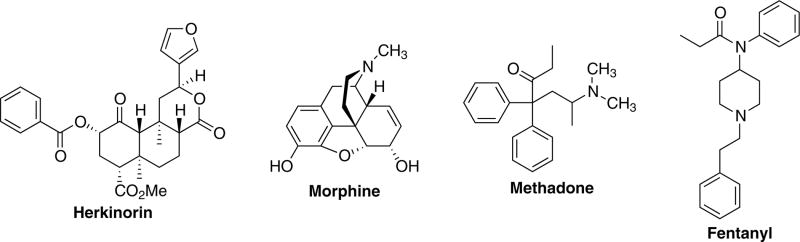
Herkinorin is the first MOP selective ligand from the neoclerodane diterpene salvinorin A (Harding et al., 2005). This is unique given that herkinorin bears little structural similarity to other MOP receptor agonists such as morphine, methadone, and fentanyl (Figure 1). The key structural difference is that herkinorin does not contain a basic nitrogen, a key structural feature of these clinically used opioids. Herkinorin has relative μ >κ>δ binding selectivity in vitro and can act as an agonist at both MOP and κ opioid (KOP) receptors, in vitro (Groer et al., 2007; Harding et al., 2005). Surprisingly, herkinorin was also found to activate G protein coupling and ERK1/2 in a naloxone reversible manner yet does not induce receptor-β-arrestin interactions (Groer et al., 2007). The development of ligands that activate ERK in the absence of β-arrestin–MOP receptor interactions may provide valuable tools for studying this pharmacology further and could possibly lead to the discovery of novel compounds for the treatment chronic pain. Additional studies in non-human primates showed that herkinorin has opioid receptor mediated effects using prolactin release, a neuroendocrine biomarker assay that is responsive to both MOP and KOP agonists, as well as to compounds with limited ability to cross the blood-brain barrier (Butelman et al., 2008). More recently, we described three new derivatives of herkinorin with similar properties and one that promotes recruitment of β-arrestin-2 to the MOP receptor and receptor internalization (Tidgewell et al., 2008).
Fig. 1.
Structures of herkinorin, morphine, methadone, and fentanyl.
The aim of the present work was to provide a preliminary investigation of the antinociceptive properties of herkinorin using a rodent model of nociception. The proposed studies were designed to provide valuable information on the suitability of herkinorin and related analogs for further preclinical development. In this study, we report that herkinorin has antinociceptive properties in the rat formalin paw withdrawal test, a model for peripheral antinociception in inflammatory pain. Further, we find that herkinorin has a reduced tolerance profile and remains efficacious in rats made tolerant to chronic morphine.
2. Methods
2.1. Animals
Male Sprague-Dawley rats (250 – 275 g) (Charles River Laboratories, Wilmington, MA), housed in pairs with free access to food and water, were maintained on a 12 hour light/dark cycle (lights on 6:00 am – 6:00 pm) in the Association for the Assessment of Laboratory Animal Care-approved animal care facility. The Institutional Animal Care and Use Committee of the University of Iowa approved all experimental procedures. All animals were used only once per treatment group.
2.2. Measurement of nociceptive activity
Testing was in accordance with protocols previously described (Joshi and Gebhart, 2003; Kaneko et al., 2000). Rats were allowed to acclimate in a screening chamber for 20 minutes prior to testing. Herkinorin’s antinociceptive effects were determined by evaluating its ability to quantitatively attenuate the number of flinches evoked following injection of formalin into the plantar surface of the right hind-paw (Wheeler-Aceto et al., 1990). Flinching of the paw following injection was a consistent component of formalin-induced behavior in our observations and was measured in this study (Joshi and Gebhart, 2003; Tjolsen et al., 1992). Discrete groups of rats received the indicated doses of herkinorin or morphine injected into the plantar of the right hindpaw 5 minutes prior to injection of 100 μL of 1.25%-concentrated formalin in an adjacent region of the plantar surface of the hindpaw. Rats were then placed in an elevated testing chamber with a mirror placed at a 45° angle below the testing stage to ensure an unobstructed view of the rat’s paws. Immediately following injection, rats were observed for one hour total in a series of 12, 5-minute bins during which flinches were recorded using an experimenter-controlled computer accounting program. The formalin assay shows a distinct biphasic nociceptive response with first (0–10 minutes) and second (15–60 minutes) phases wherein the phases are defined by a brief period of nonresponse (Dubuisson and Dennis, 1977; Tjolsen et al., 1992). Therefore the flinch response was divided into first and second phases by summing the total number of flinches occurring within the first 10 minutes and between 15 and 60 minutes, respectively. Additionally, the effects of herkinorin in the presence of the opioid receptor antagonist naloxone were evaluated using the formalin test.
2.3. Drugs
Herkinorin (2S,4aR,6aR,7R,9S,10aS,10bR)-9-(benzoyloxy)-2-(3-furanyl) dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho-[2,1-c]pyran-7-carboxylic acid methyl ester) was synthesized as described previously (Harding et al., 2005) and dissolved in dimethylacetamide (DMA) (Sigma-Aldrich, St. Louis, MO). Vehicle, in all cases, refers to the vehicle used to deliver herkinorin. Morphine hydrochloride (Mallinckdrot, St. Louis, MO), fentanyl (Sigma-Aldrich, St. Louis, MO) and naloxone (Sigma-Aldrich, St. Louis, MO) were dissolved in 0.9% saline.
2.4. Study design
Herkinorin was assessed for its ability to suppress the formalin-induced paw flinch response in rats when injected intraplantar (i.pl.) in the hind paw prior to formalin injection in the same paw. Observers were blinded to the treatment of each animal. To determine if herkinorin acts at opioid receptors, the opioid receptor antagonist naloxone was administered s.c. in the back of the neck 45 minutes before testing.
In order to assess if antinociceptive effects were centrally or peripherally mediated, animals received a dose of herkinorin (10 mg/kg) in either the ipsilateral or contralateral hindpaw 5 minutes before the formalin was injected and nociceptive behavior was observed. In order to assess if the antinociceptive effects of herkinorin diminished upon repeated administration, animals received an i.pl. injection into the right hindpaw of vehicle or herkinorin (10 mg/kg) for four days. In order to reduce sensitization, injections were alternated between plantar and dorsal side. On the fifth day, herkinorin or vehicle was given 5 minutes before formalin was injected into the right hindpaw and the effect on nociceptive behavior was assessed.
Chronic administration studies were performed to determine whether herkinorin effects would diminish over time (tolerance). Morphine was used as a standard of comparison in most studies. In order to assess the effects of morphine tolerance on the antinociceptive effect of herkinorin, animals were made tolerant to morphine by a subcutaneous implantation of an osmotic mini-pump (Alzet Osmotic Pumps, Cupertino, CA; Wiesenfeld and Gustafsson, 1982). The osmotic pumps were calibrated and loaded to infuse 15 mg/kg/day of morphine (Stevens and Yaksh, 1989). On the fifth day following implantation of the pumps, these animals received an i.pl. injection into the right hindpaw of either morphine (10 mg/kg), fentanyl (0.1 mg/kg), or herkinorin (10 mg/kg) 5 minutes prior to formalin and nociceptive behavior was assessed.
2.5. Data Analysis
The number of formalin-induced flinches was recorded within 5 minute bins per animal. Statistical anaylsis was performed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA). Data reflecting the response over time were analyzed using a repeated measures, two-way analysis of variance (ANOVA). Analyses of sums of flinches within each phase of the test were performed by Student’s t test or a one-way analysis of variance followed by Tukey-Kramer post-hoc analysis. A value of P < 0.05 was considered to be statistically significant in all tests. All data are expressed as the means ± SEM.
3. Results
3.1. Effect of herkinorin on formalin-induced nociception
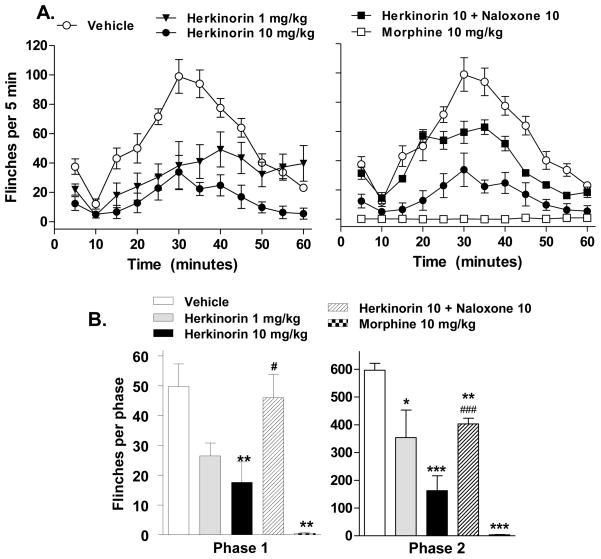
Two doses of herkinorin were initially chosen based upon its comparable efficacy and potency in biochemical measures of MOP receptor affinities and G protein coupling (Harding et al., 2005; Tidgewell et al., 2008). Overall, a pretreatment with herkinorin (1 or 10 mg/kg, i.pl.) produced a significant reduction in the number of flinches compared to vehicle (vehicle vs. herkinorin 1 mg/kg: p<0.0001, F(1,108) = 41.88; vehicle vs. herkinorin 10 mg/kg: p<0.0001, F(1,144) = 207.91; Figure 2A.) Furthermore, a higher dose of herkinorin was more effective at attenuating the flinch response (herkinorin 1 mg/kg vs. 10 mg/kg: p<0.0001, F(1,108) = 26.90; Figure 2A). For comparison, the classic opioid analgesic, morphine, was also tested (Figure 1B). Morphine (10 mg/kg, i.pl.) essentially eliminated the flinch response to formalin and at this dose, was more efficacious than herkinorin (vehicle vs. morphine: p<0.0001, F(1,108) = 389.56; morphine vs herkinorin: p<0.0001, F(1,108) = 36.05). To assess the role of opioid receptors in the antinociceptive response of herkinorin, animals were pretreated with the non-selective opioid antagonist naloxone (10 mg/kg, s.c.). The antinociceptive response elicited by herkinorin (10 mg/kg i.pl.) was attenuated by pretreatment with naloxone (p<0.0001, F(1,108) = 74.44, Figure 2B). Herkinorin was effective at blocking the nociceptive response in both phases of the test. Naloxone fully reversed herkinorin’s effects in the first phase (p<0.05 vs. herkinorin 10 mg/kg) and partially, but significantly blocked herkinorin effects in the second phase (p<0.05 vs. herkinorin 10 mg/kg).
Fig. 2.
Antinociceptive effects of herkinorin and morphine on flinch responses in the rat paw formalin test. Formalin (1.25%, 100 μL) was injected in the paw 5 minutes after drug injection (i.pl). A. Time course of formalin-induced flinching behavior for groups of rats treated with vehicle, morphine (10 mg/kg), or herkinorin (1 or 10 mg/kg). Morphine and herkinorin both blocked the flinch response. Herkinorin dose dependently attenuated the formalin-induced flinching response. Pretreatment with naloxone decreased the antinociceptive response elicited by a 10 mg/kg dose of herkinorin (p<0.0001). Data are shown as the mean ± SEM and analyzed by two-way ANOVA. B. First and second phase. First phase (left): Morphine and herkinorin significantly block the flinch response at 10 mg/kg (vs. vehicle, **p<0.01). Naloxone pretreatment (10 mg/kg, s.c.) significantly prevented herkinorin-induced antinociception (vs. herkinorin 10 mg/kg, #p<0.05). Second phase (right): Herkinorin prevented the formalin-induced response at both doses and naloxone reversed herkinorin effects (vs. vehicle, *p<0.05, **p<0.001, ***p<0.0001; vs. herkinorin 10 mg/kg, #p<0.05). Data are presented as the mean ± SEM of the sum of flinches in each phase and are analyzed by Student’s t test.
Importantly, the vehicle, dimethylacetamide did not produce any painful or tissue damaging effects on its own. This vehicle has been used previously to study the brain uptake of [11C]toluene (Gerasimov et al., 2002). In addition, dimethylacetamide has been used with other agents for intravenous formulations since 1961 (Spiegel and Noseworthy, 1963; Tesconi et al., 1999; Wiles and Narcisse, 1971) and cyclodextrins possess a similar pattern of metabolism and excretion following intravenous administration to rats (De Bie et al., 1998).
3.2. Effect of site of injection on formalin-induced nociception
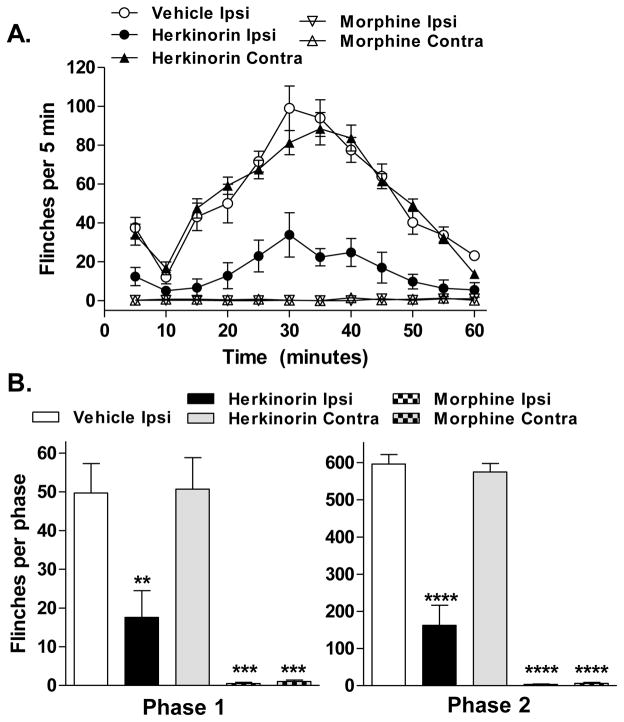
Upon injection to the paw, a compound may act locally at the site of injection or may be distributed via the bloodstream to target receptors in the spinal cord or central nervous system (DeHaven-Hudkins et al., 1999). An injection in the opposite paw (contralateral injection) should not produce an effect on the response in the test paw if the compound’s actions are restricted locally. An injection of herkinorin (10 mg/kg) into the contralateral paw mirrors the treatment with vehicle, showing no significant reduction of flinching throughout the entire time course of the assay (p=0.7236, F(1,132) = 0.13, Figure 3A). These observations suggest that the antinociception produced by this dose of herkinorin is peripherally localized and is effective at blocking responses to both the acute and inflammatory phases associated with this test (Figure 2B). In comparison, morphine (10 mg/kg, i.pl.), blocks formalin-induced flinches whether injected into the ipsilateral or contralateral paw suggesting that, at this dose, it is also systemically active (vehicle vs. contralateral morphine: p<0.0001, F(1,108) = 385.45; Figure 3).
Fig. 3.
Ipsilateral but not contralateral administration of herkinorin attenuates the formalin-induced flinch response. A. Timecourse of formalin-induced flinching behavior for groups of rats treated with vehicle, morphine (10 mg/kg), or herkinorin (10 mg/kg) at the ipsilateral or contralateral paw. Ipsilateral injections analyzed in figure 1 and shown here for comparison. Morphine is active on both the contralateral and ipsilateral sides while herkinorin is only active at the site of injection. Data are presented as mean ± SEM and are analyzed via two-way ANOVA. B. First and second phase analysis. Ipsilateral injections of morphine or herkinorin significantly block the flinch response at 10 mg/kg while only contralateral morphine injection produced antinociception (First phase (left): vs. vehicle, **p<0.01, ***p<0.001; Second phase (right): vs. vehicle: ***p<0.0001, n=4-7). Data are presented as the mean ± SEM of the sum of flinches in each phase and are analyzed by Student’s t test.
3.3 Effect of chronic herkinorin treatment on formalin-induced nociception
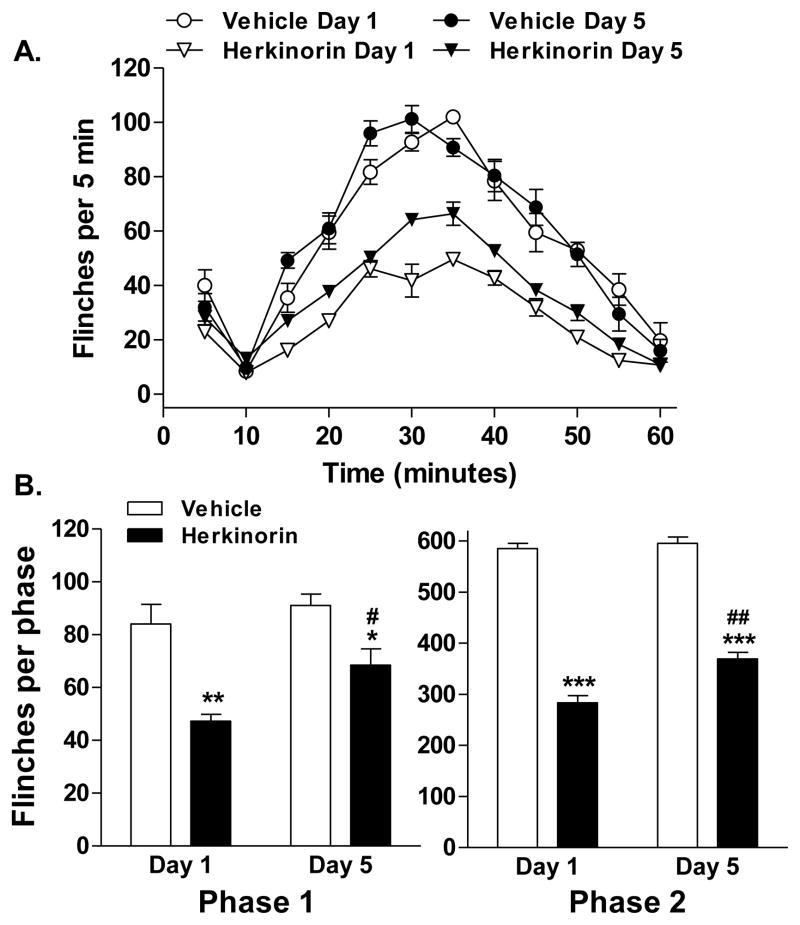
Repeated administration of most opioid narcotics results in decreased antinociceptive potency over time; such waning of responsiveness is considered to represent the development of antinociceptive tolerance. To determine if tolerance develops with repeated herkinorin dosing, herkinorin (10 mg/kg) was administered peripherally for 5 days with the formalin injection occurring on the fifth day of treatment. Following repeated administration, herkinorin produced significant inhibition of formalin-induced flinching when compared to vehicle on day 5 (p<0.0001, F(1,84) = 264.87; Figure 4A). Repeated administration of vehicle alone had no significant impact on the formalin-induced flinch response (p=0.4882, F(1,72) = 0.49). When compared to the acute administration of drug on day 1, repeated herkinorin injections produced a minor loss of efficacy as the two curves were significantly different (herkinorin day 1 vs. herkinorin day 5: p<0.0001; F(1,96) = 73.65). When assessed per phase of the formalin test, herkinorin continued to be effective in reducing flinches in both phases with only a slight but significant loss in efficacy over time (phase 1: herkinorin day 1 vs. day 5: p<0.05; phase 2: herkinorin day 1 vs. day 5: p<0.01; Figure 4B).
Fig. 4.
Herkinorin produces antinociception following chronic herkinorin treatment. Herkinorin or vehicle was given subcutaneously (i.pl.) for 4 days and testing was conducted on day 5. A. Timecourse of formalin-induced flinching behavior for groups of rats treated with vehicle or herkinorin. Herkinorin was antinociceptive prior to and following chronic treatment. Repeated vehicle treatments had no effect. Herkinorin had slightly decreased efficacy following the chronic treatment. Data are expressed as the mean ± SEM and are analyzed by two-way ANOVA. B. First and second phase analysis. Herkinorin maintained antinociceptive activity in both phases of the formalin test following chronic administration although the effects were slightly diminished following the chronic treatment regimen (First phase (left): vs. vehicle of the same day: *p<0.05, **p<0.01; vs. herkinorin day 1: #p<0.05; Second phase (right): vs. vehicle of the same day: ***p<0.0001; vs. herkinorin day 1: ##p<0.01, n=4-7). Data are presented as the mean ± SEM of the sum of flinches in each phase and are analyzed by Student’s t test.
3.4 Effect of morphine tolerance on herkinorin antinociception
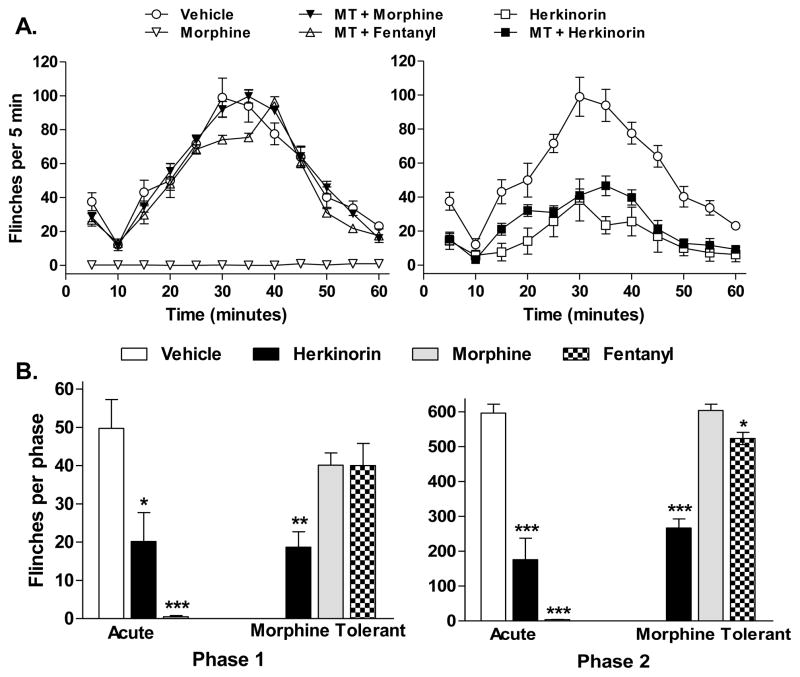
Chronic treatment of morphine via a systemic route was used to render rats tolerant to further morphine-induced antinociceptive responses. Under these conditions, herkinorin was tested for its ability to suppress the formalin-induced flinch response. Morphine was infused by osmotic minipump for 5 days; morphine tolerance was demonstrated with an acute dose of morphine (10 mg/kg) which produced no antinociceptive effects (vehicle vs. MT + morphine: p=0.9320, F(1,144) = 0.01; Figure 5A). Fentanyl, tested at a maximally efficacious dose (1 mg/kg, i.pl.; Seguin et al., 1995) in the morphine tolerant animals produced a minimal but significant decrease in the flinch responses over time when compared to vehicle or morphine in the morphine tolerant animals (vehicle vs. MT + fentanyl: p=0.0034, F(1,132) = 8.70; MT + fentanyl vs. MT + morphine: p<0.001, F(1,132) = 24.07). Herkinorin maintained its antinociceptive properties in morphine tolerant animals (vehicle vs. MT + herkinorin: p<0.0001, F(1,132) = 161.45), however, it was slightly less effective than observed following an acute dose of herkinorin in naïve animals (herkinorin vs. MT + herkinorin: P=0.0014, F(1,120) = 10.70). Phase analysis of data reveals that herkinorin maintains equal effectiveness in the first phase of the response in animals before and after morphine tolerance (acute herkinorin vs. MT + herkinorin, p=0.8646) while morphine and fentanyl responses in the morphine tolerant rats do not differ from vehicle. A similar profile was observed in the second phase of the test with herkinorin-induced responses not differing between naïve and morphine tolerant animals (acute herkinorin vs. MT + herkinorin, p=0.2031). In the second phase, morphine responses in morphine tolerant animals did not differ from vehicle treatments; fentanyl induced a slight reduction in flinches in morphine tolerant animals (p<0.05).
Fig. 5.
Herkinorin produces antinociception in morphine tolerant rats. Rats were made tolerant to morphine by continuous mini-osmotic pump infusion of morphine (75 mg/kg) for 5 days. Formalin-induced paw flinches were monitored following subsequent i.pl. injections of morphine (10 mg/kg), fentanyl (0.1 mg/kg), or herkinorin (10 mg/kg) and compared to responses obtained in drug naïve animals treated with vehicle analyzed in figure 1 and displayed here for comparison. A. Time course of drug effects on formalin-induced flinches in naïve and morphine-tolerant rats. Herkinorin retained antinociceptive efficacy in morphine tolerant rats while morphine did not. Fentanyl produced minimal but significant antinociception in the morphine tolerant rats. B. First and second phase analysis. Herkinorin maintained antinociceptive activity in both phases of the formalin test in morphine tolerant rats. Rats were tolerant to morphine in both phases; cross tolerance to fentanyl was significant in the first phase. (vs. vehicle: *p<0.05, **p<0.01; ***p<0.001, n=4-7). Data are presented as the mean ± SEM of the sum of flinches in each phase and are analyzed by Student’s t test.
4. Discussion
In this study, we report that herkinorin, a MOP-selective agonist, derived from salvinorin A, possesses antinociceptive properties in vivo that can be reversed by the non-selective opioid receptor antagonist, naloxone (Figure 2). Herkinorin activity in this assay appears to be localized to the site of injection as contralateral injections produced no antinociception (Figure 3). Repeated paw injections of herkinorin produced only marginally decreased responsiveness to the initial dose tested suggesting minimal antinociceptive tolerance (Figure 4). Importantly, while chronic systemic morphine treatment produced robust tolerance to subsequent paw doses of morphine, herkinorin remained efficacious in the morphine-treated rats (Figure 5).
These findings are encouraging as they indicate that a derivative of salvinorin A can produce antinociceptive responses in the rat formalin test indicating therapeutic potential for preventing pain responses to both noxious stimuli (first phase responses) and inflammatory pain (second phase responses). The antinociceptive effects of herkinorin in the formalin test appear to involve opioid receptors as the effects can be significantly attenuated by naloxone pretreatment. In in vitro receptor pharmacology studies, herkinorin displays a higher affinity for MOP over KOP receptors (~10-fold) with no appreciable affinity for δ opioid (DOP) receptors. However, its antinociceptive responses cannot solely be attributed to MOP receptor activation without excluding potential KOP receptor activity. Furthermore, the activity at KOP receptors may also affect the development of tolerance to herkinorin. It has been suggested that the opposing actions of KOP agonists on MOP opioid receptor actions may play a role in morphine tolerance as previous reports have shown that coadministration of KOP agonists, such as nalbuphine or U50,488H, with morphine reduced the development of morphine tolerance (Jang et al., 2006; Lee et al., 1997; Tokuyama et al., 2007). Such dual receptor interactions could also be at play when herkinorin is chronically administered thereby producing an overall apparent resistance to herkinorin tolerance.
Studies utilizing chronic morphine administration at the site of formalin injection have found that, under these conditions, morphine does not produce antinociceptive tolerance (Cohen et al., 1984). A study by Zoellner et al., demonstrated that chronic systemic morphine does not promote peripheral tolerance in a rat model in which inflammatory pain has already been induced by paw CFA injections (Zollner et al., 2008). The repeated paw injections of any substance may inadvertently produce a chronic inflammatory state, thereby creating an environment wherein opioid tolerance is reduced. The apparent reduction in opioid tolerance following chronic herkinorin treatments shown in Figure 4 could potentially be then attributed to the repeated paw injection paradigm. Therefore, a secondary approach was taken to determine if herkinorin could maintain antinociceptive properties when administered to an animal made tolerant to morphine by chronic systemic pump infusion of morphine. These studies (Figure 5) demonstrate that while rats develop peripheral tolerance to an acute challenge with morphine following systemic morphine treatment (Detweiler et al., 1995), herkinorin is still effective at reducing formalin-induced flinch responses while the effects of fentanyl were mostly attenuated.
Morphine produces antinociception whether it is administered into the test paw or into the opposite paw suggesting that at the dose of 10 mg/kg, it produces predominant effects at spinal cord and brain sites of action. The activity of herkinorin was restricted to the site of action as contralateral injections produced no antinociception. It is not clear however whether herkinorin acts only at the site of injection due to a selective activity at the periphery or rather, that it may not cross the blood brain barrier. Further studies regarding its bioavailability and pharmacokinetics must be undertaken to resolve these issues. Regardless, several studies have suggested that peripherally acting MOP agonists, such as loperamide, may represent a therapeutic approach for alleviating neuropathic pain when administered at the site of reported pain (Guan et al., 2008). Therefore, even while bioavailability may prove to be limiting for herkinorin in its current form, the antinociceptive properties associated with this compound may warrant further chemical characterization toward generation of novel pain therapeutics.
Herkinorin, and several initial chemical derivatives, have been shown to activate MOP receptors without recruiting βarrestins or internalizing the receptor (Groer et al., 2007; Tidgewell et al., 2008; Xu et al., 2007). Since βarrestin interactions have been strongly implicated in the receptor desensitization facet of morphine antinociceptive tolerance, an agonist that could activate the receptor without recruiting βarrestins may be useful for producing agonist activation without inducing desensitization and resulting drug tolerance (Bohn et al., 2004b; Bohn et al., 2000). Genetic deletion of β-arrestin-2 or chemical inhibition of β-arrestins may inadvertently effect the regulation of countless other G protein coupled receptors, producing a myriad of adverse side effects. Therefore the development of ligands that could direct signaling of the receptors toward G protein coupling without recruiting βarrestins may be therapeutically advantages for producing pain relief without tolerance. Herkinorin may represent the first compound of its kind, revealing a dramatic functional selectivity of MOP receptor activation without β-arrestin interactions. Our initial findings using this parent compound suggest that therapeutic efficacies may be attainable and that herkinorin-like compounds may be useful in morphine-tolerant peripheral pain treatment.
Acknowledgments
The authors thank Gerald F. Gebhart and Donna L. Hammond for helpful discussions and input on study design.
Role of funding source
Funding for this study was provided by NIDA Grants K01DA14600 (to LMB), R01DA18860 (to LMB), R01DA018151 (to TEP), and R01DA018151S1 (to TEP); the NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
This paper was presented in a symposium at the Behavior, Biology, and Chemistry: Translational Research in Addiction meeting on March 5, 2011 in San Antonio, TX entitled “New concepts in mu-opioid pharmacology -implications for addiction and its management.”
Conflict of Interest
The University of Iowa Research Foundation has several patents related to this research. T.E.P. and L.M.B. have consulted for Mencuro in the past year. L.M.B has consulted for Trevena, Inc. and Purdue Pharma in the past year. All other authors that they have no conflict of interests.
Contributors
Prisinzano and Bohn designed all experiments. Lamb and Tidgewell performed animal experiments. Tidgewell and Simpson provided reagents. Prisinzano and Bohn analyzed the data and obtained funding for work. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bohn LM, Dykstra LA, Lefkowitz RJ, Caron MG, Barak LS. Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol Pharmacol. 2004a;66:106–112. doi: 10.1124/mol.66.1.106. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Caron MG. G protein-coupled receptor kinase/beta-arrestin systems and drugs of abuse: psychostimulant and opiate studies in knockout mice. Neuromolecular Med. 2004b;5:41–50. doi: 10.1385/NMM:5:1:041. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Bushell T, Endoh T, Simen AA, Ren D, Bindokas VP, Miller RJ. Molecular components of tolerance to opiates in single hippocampal neurons. Mol Pharmacol. 2002;61:55–64. doi: 10.1124/mol.61.1.55. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Rus S, Simpson DS, Wolf A, Prisinzano TE, Kreek MJ. The effects of herkinorin, the first mu-selective ligand from a salvinorin A-derived scaffold, in a neuroendocrine biomarker assay in nonhuman primates. J Pharmacol Exp Ther. 2008;327:154–160. doi: 10.1124/jpet.108.140079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SR, Abbott FV, Melzack R. Unilateral analgesia produced by intraventricular morphine. Brain Res. 1984;303:277–287. doi: 10.1016/0006-8993(84)91214-9. [DOI] [PubMed] [Google Scholar]
- De Bie AT, Van Ommen B, Bar A. Disposition of [14C]gamma-cyclodextrin in germ-free and conventional rats. Regul Toxicol Pharmacol. 1998;27:150–158. doi: 10.1006/rtph.1998.1219. [DOI] [PubMed] [Google Scholar]
- DeHaven-Hudkins DL, Burgos LC, Cassel JA, Daubert JD, DeHaven RN, Mansson E, Nagasaka H, Yu G, Yaksh T. Loperamide (ADL 2-1294), an opioid antihyperalgesic agent with peripheral selectivity. J Pharmacol Exp Ther. 1999;289:494–502. [PubMed] [Google Scholar]
- Detweiler DJ, Rohde DS, Basbaum AI. The development of opioid tolerance in the formalin test in the rat. Pain. 1995;63:251–254. doi: 10.1016/0304-3959(95)00051-S. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Logan J, Ferrieri RA, Muller RD, Alexoff D, Dewey SL. Effect of vehicle on brain uptake of [11C]toluene. Nuclear Med Biol. 2002;29:607–612. doi: 10.1016/s0969-8051(02)00315-3. [DOI] [PubMed] [Google Scholar]
- Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. An opioid agonist that does not induce mu-opioid receptor-arrestin interactions or receptor internalization. Mol Pharmacol. 2007;71:549–557. doi: 10.1124/mol.106.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Johanek LM, Hartke TV, Shim B, Tao YX, Ringkamp M, Meyer RA, Raja SN. Peripherally acting mu-opioid receptor agonist attenuates neuropathic pain in rats after L5 spinal nerve injury. Pain. 2008;138:318–329. doi: 10.1016/j.pain.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, Prisinzano TE. Neoclerodane diterpenes as a novel scaffold for mu opioid receptor ligands. J Med Chem. 2005;48:4765–4771. doi: 10.1021/jm048963m. [DOI] [PubMed] [Google Scholar]
- Jang S, Kim H, Kim D, Jeong MW, Ma T, Kim S, Ho IK, Oh S. Attenuation of morphine tolerance and withdrawal syndrome by coadministration of nalbuphine. Arch Pharm Res. 2006;29:677–684. doi: 10.1007/BF02968252. [DOI] [PubMed] [Google Scholar]
- Joshi SK, Gebhart GF. Nonopioid actions of U50,488 enantiomers contribute to their peripheral cutaneous antinociceptive effects. J Pharmacol Exp Ther. 2003;305:919–924. doi: 10.1124/jpet.103.049023. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Mestre C, Sanchez EH, Hammond DL. Intrathecally administered gabapentin inhibits formalin-evoked nociception and the expression of Fos-like immunoreactivity in the spinal cord of the rat. J Pharmacol Exp Ther. 2000;292:743–751. [PubMed] [Google Scholar]
- Kim JA, Bartlett S, He L, Nielsen CK, Chang AM, Kharazia V, Waldhoer M, Ou CJ, Taylor S, Ferwerda M, Cado D, Whistler JL. Morphine-induced receptor endocytosis in a novel knockin mouse reduces tolerance and dependence. Curr Biol. 2008;18:129–135. doi: 10.1016/j.cub.2007.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Wang JJ, Ho ST, Tao PL. Nalbuphine coadministered with morphine prevents tolerance and dependence. Anesth Analg. 1997;84:810–815. doi: 10.1097/00000539-199704000-00021. [DOI] [PubMed] [Google Scholar]
- Liu JG, Anand KJ. Protein kinases modulate the cellular adaptations associated with opioid tolerance and dependence. Brain Res Brain Res Rev. 2001;38:1–19. doi: 10.1016/s0165-0173(01)00057-1. [DOI] [PubMed] [Google Scholar]
- Mather LE, Smith MT. Clincal pharmacology and adverse effects. In: Stein C, editor. Opioids in Pain Control: Basic and Clinical Aspects. Cambridge University Press; New York: 1999. pp. 188–211. [Google Scholar]
- Raehal KM, Bohn LM. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology. 2011;60:58–65. doi: 10.1016/j.neuropharm.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Walker JKL, Bohn LM. Morphine Side Effects in β-Arrestin 2 Knockout Mice. J Pharmacol Exp Ther. 2005;314:1195–1201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- Seguin L, Le Marouille-Girardon S, Millan MJ. Antinociceptive profiles of non-peptidergic neurokinin1 and neurokinin2 receptor antagonists: a comparison to other classes of antinociceptive agent. Pain. 1995;61:325–343. doi: 10.1016/0304-3959(94)00194-J. [DOI] [PubMed] [Google Scholar]
- Spiegel AJ, Noseworthy MM. Use of nonaqueous solvents in parenteral products. J Pharm Sci. 1963;52:917–927. doi: 10.1002/jps.2600521003. [DOI] [PubMed] [Google Scholar]
- Stevens CW, Yaksh TL. Potency of infused spinal antinociceptive agents is inversely related to magnitude of tolerance after continuous infusion. J Pharmacol Exp Ther. 1989;250:1–8. [PubMed] [Google Scholar]
- Tesconi MS, Bramer SL, Yalkowsky SH. The preparation of soft gelatin capsules for a radioactive tracer study. Pharm Dev Technol. 1999;4:507–513. doi: 10.1081/pdt-100101388. [DOI] [PubMed] [Google Scholar]
- Tidgewell K, Groer CE, Harding WW, Lozama A, Schmidt M, Marquam A, Hiemstra J, Partilla JS, Dersch CM, Rothman RB, Bohn LM, Prisinzano TE. Herkinorin analogues with differential beta-arrestin-2 interactions. J Med Chem. 2008;51:2421–2431. doi: 10.1021/jm701162g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Tokuyama S, Nagae R, Mashida E, Hamabe W. Involvement of kappa opioid receptors in formalin-induced inhibition of analgesic tolerance to morphine in mice. J Pharm Pharmacol. 2007;59:1109–1115. doi: 10.1211/jpp.59.8.0008. [DOI] [PubMed] [Google Scholar]
- Wheeler-Aceto H, Porreca F, Cowan A. The rat paw formalin test: comparison of noxious agents. Pain. 1990;40:229–238. doi: 10.1016/0304-3959(90)90073-M. [DOI] [PubMed] [Google Scholar]
- Whistler JL, von Zastrow M. Morphine-activated opioid receptors elude desensitization by beta-arrestin. Proc Natl Acad Sci USA. 1998;95:9914–9919. doi: 10.1073/pnas.95.17.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld Z, Gustafsson LL. Continuous intrathecal administration of morphine via an osmotic minipump in the rat. Brain Res. 1982;247:195–197. doi: 10.1016/0006-8993(82)91051-4. [DOI] [PubMed] [Google Scholar]
- Wiles JS, Narcisse JK., Jr The acute toxicity of dimethylamides in several animal species. Am Ind Hyg Assoc J. 1971;32:539–545. doi: 10.1080/0002889718506502. [DOI] [PubMed] [Google Scholar]
- Xu H, Partilla JS, Wang X, Rutherford JM, Tidgewell K, Prisinzano TE, Bohn LM, Rothman RB. A comparison of noninternalizing (herkinorin) and internalizing (DAMGO) mu-opioid agonists on cellular markers related to opioid tolerance and dependence. Synapse. 2007;61:166–175. doi: 10.1002/syn.20356. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci USA. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner C, Mousa SA, Fischer O, Rittner HL, Shaqura M, Brack A, Shakibaei M, Binder W, Urban F, Stein C, Schafer M. Chronic morphine use does not induce peripheral tolerance in a rat model of inflammatory pain. J Clin Invest. 2008;118:1065–1073. doi: 10.1172/JCI25911. [DOI] [PMC free article] [PubMed] [Google Scholar]