Abstract
Phytoestrogens are estrogenic compounds of plant origin classified into different groups including isoflavones, lignans, coumestans and stilbenes. Isoflavones such as genistein and daidzein are the most studied and most potent phytoestrogens, and are found mainly in soy based foods. The effects of phytoestrogens are partly mediated via estrogen receptors (ERs): ERα, ERβ and possibly GPER. The interaction of phytoestrogens with ERs is thought to induce both genomic and non-genomic effects in many tissues including the vasculature. Some phytoestrogens such as genistein have additional non-ER-mediated effects involving signaling pathways such as tyrosine kinase. Experimental studies have shown beneficial effects of phytoestrogens on endothelial cells, vascular smooth muscle, and extracellular matrix. Phytoestrogens may also affect other pathophysiologic vascular processes such as lipid profile, angiogenesis, inflammation, tissue damage by reactive oxygen species, and these effects could delay the progression of atherosclerosis. As recent clinical trials showed no vascular benefits or even increased risk of cardiovascular disease (CVD) and CV events with conventional menopausal hormone therapy (MHT), phytoestrogens are being considered as alternatives to pharmacologic MHT. Epidemiological studies in the Far East population suggest that dietary intake of phytoestrogens may contribute to the decreased incidence of postmenopausal CVD and thromboembolic events. Also, the WHO-CARDIAC study supported that consumption of high soybean diet is associated with lower mortalities from coronary artery disease. However, as with estrogen, there has been some discrepancy between the experimental studies demonstrating the vascular benefits of phytoestrogens and the data from clinical trials. This is likely because the phytoestrogens clinical trials have been limited in many aspects including the number of participants enrolled, the clinical end points investigated, and the lack of long-term follow-up. Further investigation of the cellular mechanisms underlying the vascular effects of phytoestrogens and careful evaluation of the epidemiological evidence and clinical trials of their potential vascular benefits would put forward the use of phytoestrogens as an alternative MHT for the relief of menopausal symptoms and amelioration of postmenopausal CVD.
Keywords: estrogen, endothelium, smooth muscle, calcium, blood pressure
INTRODUCTION
Estrogen (E2) deficiency during menopause is associated with perimenopausal symptoms such as hot flushes and night sweats which prompt women to seek menopausal hormone therapy (MHT). In addition to the relief of menopausal hot flushes and vaginal dryness, MHT may improve sleep quality and social well-being, retard bone loss and minimize osteoporotic fractures [1,2].
The risk of cardiovascular disease (CVD) also increases after menopause, suggesting vascular benefits of endogenous E2 [3-5]. Estrogen receptors (ERs) have been identified in the vasculature, and E2 has been shown to promote beneficial effects on the endothelium, vascular smooth muscle (VSM) and extracellular matrix (ECM) [3,4,6-8]. The vascular benefits of E2 observed in experimental studies have suggested potential benefits of MHT in CVD.
Studies of the vascular benefits of female sex hormones have mainly focused on natural and synthetic estrogens. Initial population-based observational studies showed 35% reduction in mortality and a 50% reduction in CV events among women using MHT [9]. Also, a meta-analysis of results from different studies demonstrated overall improvement of atherosclerotic biomarkers and suggested CV benefit of MHT [10]. However, randomized clinical trials (RCTs) did not demonstrate a decrease in CV events and instead showed increased risk of thromboembolic events. As of 2001, only 38% of postmenopausal women (Post-MW) in the United States used MHT [11]. This has prompted investigations of the possible causes of the discrepancies between the experimental vascular benefits of E2 and the results of the clinical trials. Other investigations have focused on alternative MHT.
In the past two decades, there has been an increasing interest in phytoestrogens as natural alternatives to MHT [12]. Phytoestrogens, or “dietary estrogens”, are a heterogeneous group of naturally occurring compounds with structural similarities to E2 that allow them to mimic the effects of E2. Phytoestrogens have several potential applications in different diseases. Phytoestrogens decrease bone resorption and delay the progression of osteoporosis in Post-MW [13], exert anti-androgenic effects which could be useful in benign prostatic hypertrophy [14], and may have protective effects in prostate and breast cancers [15], and neuroprotective effects that could improve cognitive functions of the brain [16]. Phytoestrogens also showed a potential to improve CV function and to decrease the risk of CVD associated with menopause [17].
Epidemiological evidence suggests potential protective effects of phytoestrogens. The incidence of CVD, diabetes, obesity and breast cancer are less in Asian than Western populations. Also, the incidence of hot flushes is 70-80% in menopausal Western women compared to 14-15% in Asian women [18]. Migration studies of the Japanese population moving to the United States showed that they developed an increased incidence in “Western Diseases” – mainly CV- after two generations. These observations suggest that the factors contributing to CVD are not only genetic, but could also involve environmental factors such as the diet. One important difference between Asian and Western diets is the high content of soy-rich in phytoestrogens- in the Asian diet (20-150 mg/d) compared to the Western diet (1-3 mg/d) [12]. In a study examining the relation between coronary artery disease (CAD) and dietary habits of 61 populations in 25 countries, the 24 hour urinary excretion of taurine and isoflavones, which are abundant in fish and soybean diets, was inversely related to mortality rates from CAD [19]. These dietary differences may contribute to the lower incidence of CAD among the Asian populations.
Research on the CV effects of phytoestrogens has progressed steadily, and the beneficial vascular effects demonstrated in some studies have suggested potential applications in CVD. Also, being natural, phytoestrogens have less side effects. However, phytoestrogens are a diverse group of compounds with different modes of metabolism, bioavailability and in vivo effects. Thus, after decades of research there is no definitive agreement as to the vascular effects of phytoestrogens and their benefit in CVD. In effect, some studies have suggested that phytoestrogens may not have any benefit in CVD, and other studies attributed the benefits of the soy-rich diet to food components other than phytoestrogens [20]. Also, most of the clinical studies of phytoestrogens have been limited in terms of the number of subjects enrolled, the compounds studied, the duration of dietary intake and the long-term follow-up of the participants.
This review discusses reports from the Pubmed database and highlights the sources, classification, and chemical structure of phytoestrogens, and their interaction with ERs, signaling pathways and vascular effects on the endothelium, VSM and ECM. Other vascular effects of phytoestrogens on lipid profile, angiogenesis, and inflammation, and how these effects could retard the progression of atherosclerosis will also be discussed. We will then highlight some of the clinical trials that evaluated the vascular effects of phytoestrogens, and their implications in CV medicine. Throughout the review we will discuss the reported benefits of phytoestrogens and suggest areas that need further investigation. To facilitate comparison, we will briefly describe the effects of E2 followed by the data on phytoestrogens.
Sources and Classification of Phytoestrogens
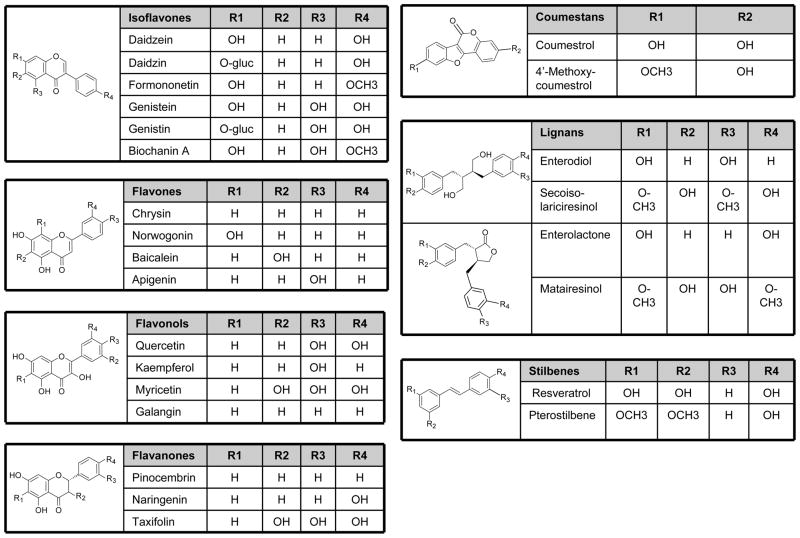
Phytoestrogens are polyphenolic non-steroidal compounds of estrogenic activity. While phytoestrogens are generally plant in origin, resorcylic acid lactones, which are produced by molds, exhibit estrogenic activity and hence termed mycoestrogens [21]. Major classes of phytoestrogens include isoflavones, lignans and coumestans. Other phytoestrogens include stilbenes, flavanones, flavonols, and flavones (Fig. 1). The most abundant, most studied and most potent phytoestrogens are isoflavones. There are more than 1000 types of isoflavones including genistein, daidzein, genistin, daidzin, formononetin, biochanin-A and equol. The most commonly studied isoflavones are genistein, daidzein and its metabolite equol.
Figure 1.
Classification of phytoestrogens. Phytoestrogens include isoflavones, flavanones, flavonols, flavones, lignans, coumestans, and stilbenes with different core structure and side chains.
Isoflavonoids are a subclass of flavonoids, where one phenolic ring has migrated from C-3 to C-2. Isoflavones are found in legumes such as soy, chickpeas, clover, lentils and beans (Table 1) [22]. Unextracted soy protein contains onaverage 1.105 mg genistein and 0.365 mg daidzein/g soy proteins isolate. However, total isoflavone content may vary up to 3-fold with growth of the same soy cultivar in different geographical areas and different years. Biochanin-A and formononetin are precursors of genistein and daidzein, respectively, and also have estrogenic properties. Formononetin is abundant in Astragalus mongholicus Bunge and Curcuma comosa Roxb. Glycitein and its conjugates are minor isoflavones in soybean cotyledons, but are major components in dietary supplements and foods made from the soybean hypocotyls.
Table 1.
Sources of Phytoestrogens
| Isoflavones | Coumestans | Lignans | ||
|---|---|---|---|---|
| Food | Daidzein | Genistein | Coumestrol | Secoisolariciresinol |
| Soy based foods | ||||
| Black bean sauce | 2304.0 | 2486.6 | tr | tr |
| Miso soup | 430.2 | 1009.8 | nd | tr |
| Soy beans | 56621.4 | 44213.4 | tr | 79.1 |
| Soy bean sprouts | 268.3 | 514.6 | nd | tr |
| Soy milk | 921.3 | 1852.2 | tr | tr |
| Soy nuts | 28351.2 | 36264.0 | tr | tr |
| Soy sauce | tr | 100.6 | tr | tr |
| Soy yogurt | 3364.4 | 6565.1 | tr | tr |
| Tempeh | 6974.8 | 10729.6 | tr | tr |
| Tofu | 9337.5 | 17050.2 | tr | tr |
| Veggie burger | 461.5 | 1111.5 | tr | tr |
| Vegetables and legumes | ||||
| Alfalfa sprouts | 151.7 | 117.6 | 105.3 | tr |
| Broccoli | tr | tr | tr | 414.0 |
| Clover sprouts | 71.3 | 70.9 | 97.7 | nd |
| Mung bean sprouts | 91.4 | 135.2 | 136.6 | 97.0 |
| Beans, green | tr | 32.9 | nd | 30.9 |
| Beans, white | tr | 25.3 | tr | 29.9 |
| Nuts and oil seeds | ||||
| Almonds | tr | tr | tr | 70.3 |
| Chestnuts | tr | tr | tr | 172.7 |
| Flaxseed | 58.2 | 173.2 | 46.8 | 375321.9 |
| Hazelnuts | tr | tr | tr | 60.5 |
| Pistachios | 73.1 | 103.3 | tr | tr |
| Sunflower seeds | nd | nd | nd | 127.8 |
| Walnuts | 35.2 | tr | tr | 78.0 |
| Peanut butter | tr | 38.2 | tr | 28.6 |
| Fruits | ||||
| Dried apricots | tr | tr | tr | 147.6 |
| Dried dates | tr | tr | tr | 106.2 |
| Dried prunes | tr | tr | tr | 103.8 |
| Strawberries | tr | tr | tr | 1210.0 |
| Cranberries | tr | tr | nd | 1500.0 |
| Blackberries | tr | tr | nd | 3710.0 |
| Breads | ||||
| Bread, flax | 85.0 | 212.3 | tr | 7208.3 |
| Bread, rye | tr | tr | nd | 122.0 |
| Bread, multigrain | tr | tr | tr | 4770.4 |
| Bread, whole wheat | 155.8 | 141.8 | tr | tr |
| Beverages | ||||
| Tea, black | na | na | na | 159.0 |
| Tea, green | na | na | na | 246.0 |
| Wine, red | tr | tr | nd | 29.4 |
Lignans are common in the plant kingdom and are the building block of lignin found in the plant cell wall. Food containing lignans include flaxseed, lentils, whole grains, beans, fruits, and vegetables (Table 1). Enterolactone and enterodiol are major lignans produced by the action of intestinal bacteria on matairesinol and secoisolariciresinol, respectively [23].
Coumestans such as coumestrol and 4-methoxycoumestrol are found in mung bean sprouts, brussel sprouts and spinach. Coumestrol, the most important coumestan consumed by humans, is found in clover sprouts, alfalfa sprouts, and other legumes.
The stilbenes family of phytoestrogens includes reseveratrol and pterostilbene which are commonly found in red wine and peanuts. Resveratrol has estrogenic activity only in the Trans form [24].
Flavanones include eriodictyol, naringenin, pinocembrin and are mainly found in citrus fruits. Flavonols include kaempferol, myricetin, quercetin, and quercetagetin, and are found abundantly in green tea and to a less extent in dark tea and chocolate. Flavones include apigenin, baicalain, chyrisin, norwogenin and are found mainly in cereals and herbs.
Phytoestrogens Metabolism
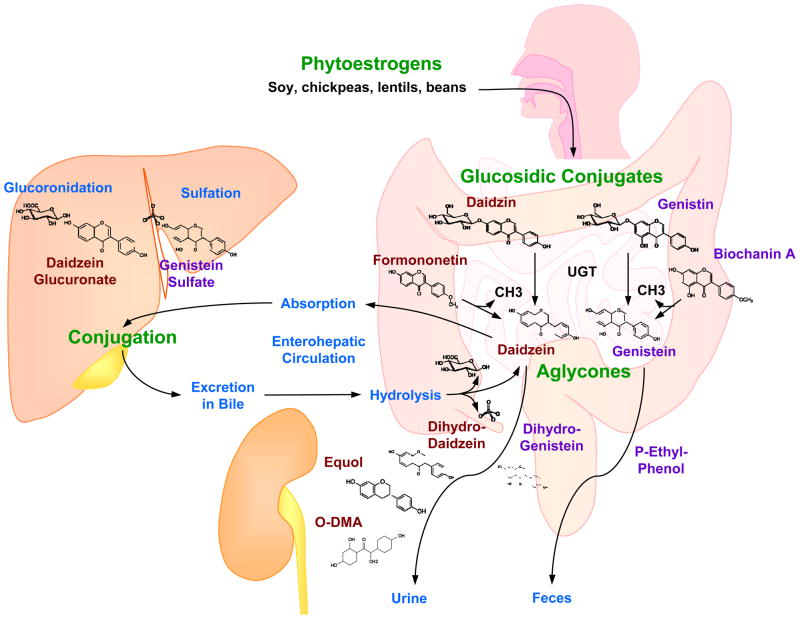
Phytoestrogens are present in plants as inactive glycosidic conjugates. In the intestine, they are hydrolyzed by the action of UDP-glucuronosyltransferase secreted by intestinal bacteria to the active forms aglycones (Fig. 2). The aglycones are then absorbed by the intestinal tract. On entering the circulation, aglycones may undergo extensive metabolism to other compounds through various reactions including demethylation, methylation, hydroxylation, chlorination, iodination, and nitration [25]. These metabolites are then transported to the liver where they undergo conjugation to form β-glucuronides and to a less extent sulfate esters. In the liver some glucuronides undergo further fermentation into other metabolites that vary depending on the class of phytoestrogen. The glucuronides are excreted in bile and partially reabsorbed via the enterohepatic circulation. Phytoestrogens are excreted in bile and urine as conjugated glucuronides and in feces in the unconjugated form (Fig. 2) [25,26].
Figure 2.
Absorption, metabolism and excretion of isoflavones. Phytoestrogens, found in diet as glucoconjugates (daidzin, genistin), are hydrolyzed in the intestine, by the action of UDP-glucuronosyltransferase (UGT) secreted by intestinal bacteria, into the active forms aglycones (daidzein and genistein). Genistein and daidzein are also produced from the demethylation of their precursors biochanin A and formononetin, respectively. The aglycones are absorbed from the intestinal tract to the liver where they are mainly conjugated with glucuronic acid and sufates. Some of the conjugated aglycones are excreted in the bile where they are hydrolyzed, and some of the unconjugated aglycones are excreted in the feces, while some are reabsorbed to the liver via enterohepatic circulation. In blood, Isoflavones are metabolized mainly into equol and O-desmethylangolensin (O-DMA) which are excreted in urine.
As with other phytoestrogens, isoflavones in food are bound to glucose. When ingested, they are enzymatically cleaved in the gut into active aglycones. Genistein and daidzein, the most active forms of isoflavones, are produced both by hydrolysis of their biologically inactive glucoconjugates, as well as from the demethylation of their precursors biochanin A and formononetin, respectively. The aglycone forms of isoflavones are easily transported across the intestinal epithelial cells to the blood or are further metabolized in the intestine [26]. In humans consuming soy-free diets, the plasma concentration of isoflavones is usually in the nanomolar range, ≤40 nM. Acute ingestion of dietary soy leads to a rapid increase in the plasma concentration of isoflavones up to the micromolar range [27]. The isoflavone serum concentration shows variability in different populations. In serum samples of Japanese men, the average concentration of genistein is 276 nmol/L and of daidzein is 107 nmol/L [28].
The majority of the genistein and daidzein consumed is eliminated from the body within 24 hours [26]. Genistein is transformed to dihydrogenistein and is further metabolized in the colon to 4-ethyl phenol. Daidzein is metabolized to dihydrodaidzein, which is further metabolized to both equol and O-desmethylangolensin (O-DMA). Genistein, daidzein, equol and O-DMA are the major isoflavones detected in blood and urine of humans and animals [29]. Interestingly, only 30-40% of humans –mostly Asians and vegetarians - are able to metabolize daidzein into equol, and the ability to produce equol may be associated with an increased benefit of isoflavones on bone mineral density and a lower risk of breast cancer [30,31].
Factors Affecting the Metabolism of Phytoestrogens
The metabolism and excretion of isoflavones after soy consumption show considerable variation among individuals. The average time taken after ingesting the aglycones to reach peak plasma concentration is 4–7 hr, and is delayed to 8–11 hr for the corresponding glycosidic conjugates. This suggests that the rate-limiting step for absorption is the initial hydrolysis of the glycosidic moiety. The half-lives of genistein and daidzein are 7.1 and 9.3 hr, respectively [32].
In addition to the inter-individual variations in phytoestrogen metabolism, sex may also play a role, with women metabolizing phytoestrogens more efficiently than men [12]. Other factors that could influence isoflavone bioavailability include the chemical composition, the administered dose, intestinal transit time, intestinal microflora and the individual ability to produce equol [26]. The source of the isoflavones and hence the food matrix in which the compound is delivered plays a minor role in their bioavailability. The effect of age on the bioavailability of isoflavones was also investigated, but no difference was found in the pharmacokinetics of either genistein or daidzein between Pre- and Post-MW [33]. Also, the frequency of ingestion does not appear to cause significant difference in the bioavailability of isoflavones [34].
Estrogen Receptor
ER has two major subtypes ERα and ERβ that differ in their C-terminal ligand-binding domain and in the N-terminal transactivation domain [3,35]. Several splice variants of ER subtypes have also been identified. The diversity among ER variants could be due to epigenetic changes, methylation of the genes encoding ERs, alternative RNA splicing leading to multiple ER mRNA isoforms, and multiple sites for initiation of translation of ER mRNA [36]. The two nuclear ER genes are located on separate chromosomes. ER1, the gene that encodes ERα, is located on chromosome 6q(25.1) and ER2, the gene that encodes ERβ, is located on chromosome 14q(23-24.1) [37]. Although E2 release patterns and plasma levels change with aging, little is known about the age-associated changes in ER expression and subtypes.
Similar to other members of the nuclear receptor superfamily, ERα and ERβ share a common structure with five functional domains A/B, C, D, E and F [3]. Domain A/B is involved in protein-protein interactions and transcriptional activation of target gene expression. Domain C is involved in DNA binding and ER dimerization. Domain D is the hinge domain linking domain C and E and is responsible for nuclear localization of ER. Domain E is the ligand-binding domain. Domain F contains co-factor recruitment regions [3]. Two acidic activation factors, AF-1 and AF-2, mediate the ligand-dependent transcriptional activity of ER. AF-1 is located within the N terminus. AF-1 in ERα is very active on a variety of E2-sensitive promoters whereas its activity in ERβ is minimal. Hormone-dependent AF-2 is located in the ligand-binding domain [38]. AF-1 and AF-2 may also be required for ligand independent receptor functions, including growth factor activation by AF-1 and cAMP activation by AF-2.
Nuclear ERs are 40 times more abundant than membrane ERs. The same DNA sequence is responsible for coding both nuclear and membrane ERs, but post-translational protein modifications are likely to be responsible for targeting ER to either the nucleus or plasma membrane [39]. ERα and ERβ have overlapping but not identical tissue distribution and expression levels, suggesting distinct biological roles. ERα is expressed abundantly in the uterus, vagina, ovaries, mammary gland, and hypothalamus [3,40]. ERβ is more active in the prostate and ovaries, with smaller number in the lungs, brain, and bones [3]. ERs have also been identified in ECs and VSM [3,6,41,42].
G protein-coupled receptor (GPR30) also termed G protein-coupled ER (GPER) is a novel membrane receptor that binds E2. GPER comprises 375 amino acids and shares little homology with the classical ERs. The gene coding for GPER is located on chromosome 7p22.3 and consists of three exons which code for three domains; an N-terminal domain, a 7-transmembrane domain and a C-terminal domain [43]. GPER is widely distributed in the brain and peripheral tissues and may play a functional role in the vasculature. GPER has been localized in the endoplasmic reticulum [44], and plasma membrane [45]. However, the cellular localization of GPER appears to vary depending on the cell type.
ERs display marked differences in binding affinity and activation by natural and synthetic ligands [37,46]. Endogenous natural estrogens are C18 steroids and include estrone (E1), estradiol (E2), and estriol (E3). They have 4 rings A, B, C, D, a hydroxyl group at C3, and either a hydroxyl or ketone group at C17. The phenolic A ring is responsible for selective high-affinity binding to ER. Only 5 chemicals without aromatic rings were found to be active. These 5 chemicals possess H-bond capability with a rigid hydrophobic backbone that matches the A, B and C rings of E2 [3,47].
Phytoestrogens and ERs
Different classes of phytoestrogens have distinct chemical structures that could allow them to bind to ERs (Fig. 1). The key structural elements which are essential for the estrogenic effects are the phenolic rings, low molecular weight, and optimal hydroxylation patterns [3]. Phytoestrogens could modulate ER function in several ways, including having both agonist and antagonist effects. Phytoestrogens bind both ERα and ERβ, and activate ER-dependent gene transcription. The affinity of most phytoestrogens to ERs is 1/100 to 1/10000 that of E2 but they may reach concentrations up to 10000 times that of E2 in the human body. Phytoestrogens also have different binding affinities to ER subtypes with generally higher affinity for ERβ than ERα, which explains why they may act differently from E2 [12,16]. Genistein has high affinity for ERβ, almost identical to that of E2, while its affinity for ERα is only 6% of E2. Daidzein has very weak binding affinity for both ERα and ERβ, but its relative affinity for ERβ is still higher than that for ERα. One study estimated that the maximal activity induced by isoflavone phytoestrogens is about half the activity of E2. Coumestrol has very high binding affinity for human ERα, but still a slightly higher affinity for ERβ. The actions of phytoestrogens at the cellular and molecular level are influenced by many factors including the phytoestrogen concentration, ER status, presence or absence of endogenous estrogens, and the type of target organ or cell [35,48].
ER-Mediated Genomic and Nongenomic Effects
Although life is possible without either or both ERs, the reproductive functions are severely impaired [49]. ERs also mediate multiple vascular, hematologic and metabolic effects through stimulation or inhibition of gene expression (genomic pathways) and via other pathways which do not involve gene transcription or new protein synthesis (nongenomic pathways).
E2/ER activate genomic pathways that regulate many transcriptional processes and require relatively longer time to show their effects. Upon binding E2, ER undergoes conformational changes resulting in the formation of a homo- or heterodimer with high affinity for E2 and DNA. This is followed by nuclear translocation of ER, binding to specific estrogen response elements (ERE) and regulation of target gene expression. Depending on the cell and promoter context, the DNA-bound ER exerts either positive or negative effects on the expression of downstream target gene(s). Ligand-bound ER may also interact with other transcription factor complexes to influence transcription of genes whose promoters do not harbor ERE [3,5].
The overall effects of E2 depend on the ratio between ERα and ERβ in different tissues. However, ERβ stimulation can produce some ERα effects in some organs [49]. Also, ERβ may interact in a ligand independent manner with EREs of target promoters and attenuate the ligand dependent transcriptional activity of ERα [50].
ERs can also regulate gene transcription without binding directly to DNA and thus regulate the expression of a large number of E2-responsive genes that do not contain ERE. The receptors in such cases are tethered through protein-protein interactions to a transcription factor complex that contacts the DNA [51].
ER function can also be modulated by extracellular signals in the absence of E2. Polypeptide growth factors such as epidermal growth factor and insulin-like growth factor-1 can activate ER and increase the expression of ER target genes. The mechanisms by which the E2 and growth factor pathways converge are not clear, but these pathways appear to be dependent on each other for the full manifestation of the ligand-mediated response [52].
Non-genomic effects are rapid responses that occur too quickly to be mediated by gene transcription, are independent of protein synthesis, and typically involve modulation of membrane bound and cytoplasmic regulatory proteins. For example, following E2 binding to GPER, the Gα-GTPase subunit dissociates from the G-protein complex and activates adenylyl cyclase and phospholipase C, which in turn generates second messengers such as cAMP, IP3 and Ca2+ [53]. Other E2-activated pathways include mitogen-activated protein kinase (MAPK), phosphatidylinositol trisphosphate kinase PI3K/Akt, and alteration of ion channel fluxes [3,54].
Phytoestrogens and Endothelium
Like E2, phytoestrogens may have beneficial effects on the CV system partly through effects on the vascular endothelium.
Effects of Phytoestrogens on Endothelial Cell Growth and Permeability
E2 stimulates endothelial cell (EC) proliferation via cytosolic and nuclear ERs [55]. E2 is also important for the integrity of the endothelium and consequently the vascular permeability. In cultured human umbilical vein endothelial cells (HUVECs) E2 has a biphasic effect on vascular permeability; at nanomolar concentrations E2 decreases the permeability, but at micromolar concentrations E2 increases it [56]. Animal studies also support a role of E2 on EC integrity and permeability. Studies have shown that the permeability of the blood brain barrier is 500% greater in ovariectomized (OVX) than intact female rats, and E2 replacement restores barrier properties [57]. The E2-induced decrease in EC permeability may be related to regulation of prostaglandin E2 (PGE2) levels [58]. Similar to E2, phytoestrogens regulate EC proliferation, maintain EC integrity and decrease vascular permeability. Several studies have shown that genistein regulates the proliferation of human endometrial ECs [59]. Genistein derivatives protect HUVEC-12 from H202 induced apoptosis [60]. Genistein also inhibits TNF-α-induced apoptosis in human aortic ECs [61]. Phytoestrogens also maintain the integrity and decrease the permeability of the endothelium. For example, in HUVECs equol improves EC function by reducing the generation of reactive oxygen species (ROS) [62]. Equol also has a protective effect against EC dysfunction induced by ritonavir, an antiprotease drug used in HIV patients [63]. Low concentrations of biochanin A also inhibit cell proliferation in the human EC line ECV304 [64]. Also, long-term oral administration of genistein inhibits retinal vascular leakage in experimentally-induced diabetes in rats, possibly via tyrosine kinase (TK) inhibition [65]. Genistein, also via TK inhibition, modulates bradykinin- and substance P-induced increase in macromolecular efflux from the hamster cheek pouch microcirculation [66]. In mouse skin, genistein inhibits vascular endothelial growth factor (VEGF)-induced increase in vascular permeability, possibly by inhibiting TK-mediated local production of NO and arachidonic acid metabolites [67]. Also, pretreatment of bovine aortic ECs with genistein inhibits thrombin-induced increase in EC permeability via activation of the PKA/cAMP pathway [68]. Thus, phytoestrogens regulate EC proliferation, maintain vascular integrity and decrease endothelial permeability; and further studies are needed to define the mechanisms and pathways involved.
Phytoestrogens and EC Function
Vascular tone is controlled by the ratio between vasodilators such as NO, PGI2 and EDHF and vasoconstrictors such as angiotensin II (Ang II) and endothelin (ET). Experimental data have shown beneficial vascular effects of E2. ERs mediate endothelium-dependent vascular relaxation, and E2 promotes NO, PGI2 and EDHF production and decreases ET release [5,8,69,70]. Phytoestrogens promote endothelium-mediated vascular relaxation via similar mechanisms.
Phytoestrogens and NO
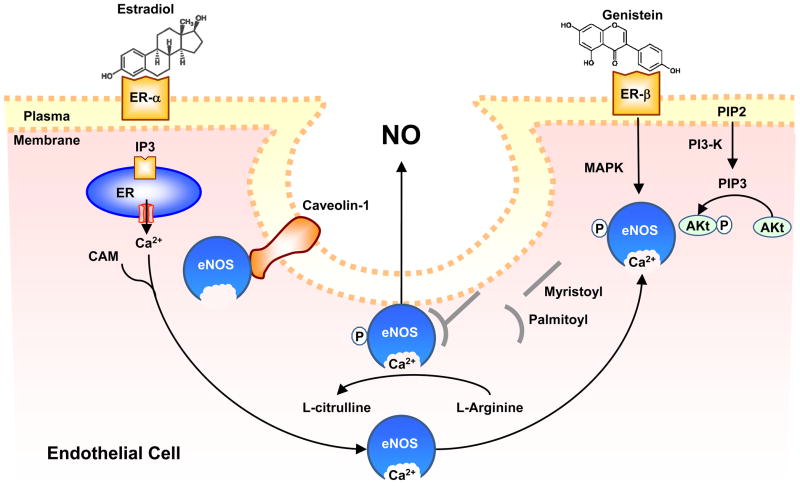
Endothelium-derived NO is a key regulator of vascular tone. In ECs, activation of eNOS leads to transformation of L-arginine to L-citrulline and NO production (Fig. 3). E2 upregulates eNOS in human ECs by increasing eNOS promoter activity and enhancing the binding activity of the transcription factor Sp1. E2 also increases EC Ca2+, MAPK and PI3K activity and thereby increases eNOS activity and NO production. E2 also reduces antioxidants which are known to decrease NO bioavailability [71]. Phytoestrogens may have similar effects on NO production and activity. Genistein stimulates NO release in human aortic ECs and HUVECs [72]. In human EA.hy926 EC line, biochanin A and formononetin and their metabolites genistein and daidzein increase eNOS promoter activity and NO release [73]. Studies suggested different mechanisms of phytoestrogen-induced increase in NO production. In bovine aortic ECs and HUVECs, genistein may act through a protein kinase A (PKA)-dependent pathway, as genistein-induced eNOS activation and phosphorylation was abolished by inhibition of PKA by H89 and was not blocked by ER antagonists, MAPK or PI3K/Akt-Kinase inhibitors [74]. In human aortic ECs and HUVECs, equol stimulates phosphorylation of ERK1/2 and PI3K/Akt, leading to the activation of NOS and increased NO production at resting cytosolic Ca2+ levels [75]. Animal studies also support that phytoestrogens increase NO production by increasing eNOS expression and activity [76]. In OVX female Sprague-Dawley rats, treatment with E2 reverses EC dysfunction and increases Ca2+ dependent NOS activity in lung homogenates, and treatment with genistein increases NOS activity and improves endothelial dysfunction to the same extent [77]. Also, in isolated rat carotid and basilar arteries both equol and daidzein possess vasodilator activity. Interestingly, in hypertensive rats the vasorelaxant response to equol, but not daidzein, is preserved [78]. Also, formononetin relaxes phenylephrine-preconstricted rat aorta via NO-dependent mechanism and other endothelium-independent mechanisms [79]. Several studies support a role of ER in phytoestrogen-induced vasodilatation. In anesthetized pigs, intracoronary infusion of genistein at constant heart rate and BP increases coronary blood flow as assessed by ultrasound flowmeters and induces the phosphorylation of eNOS and NO production through ERK 1/2, Akt and p38 MAPK pathways. The genistein-induced coronary vasodilation appears to involve ERα/ERβ and stimulation of β2-adrenoreceptors [80]. In mouse aorta, red wine polyphenols as well as ERα agonists stimulate endothelium-dependent NO pathway via activation of ERα [81]. Also, red clover extracts stimulate NO synthesis in cultured human ECs by recruiting ER-β [82]. In ECs, caveolin-1 is an anchoring protein that binds to eNOS and reduces its activity. The increase in Ca2+ together with calmodulin promotes the dissociation of eNOS from caveolin leading to increased eNOS activity. One study suggested that daidzein and E2 may not alter eNOS protein in rat aorta but reduce the expression of caveolin-1 and increase the expression of calmodulin, and thereby increase eNOS activity [83]. Thus phytoestrogens may promote vasodilation by increasing the expression and activity of eNOS and increasing NO production in ECs.
Figure 3.
Phytoestrogens-induced NO release from endothelial cells. Like estradiol, phytoestrogens bind to ER on EC and increase the formation of inositol 1,4,5-trisphosphate (IP3), which stimulates Ca2+ release from the endoplasmic reticulum. Ca2+ forms a complex with calmodulin (CAM), which in turn binds to and causes initial activation of eNOS, its dissociation from caveolin-1, and translocation to intracellular sites. Phytoestrogens may also activate phosphatidylinositol 3-kinase (PI3-K), leading to transformation of phosphatidylinositol-4,5-bisphosphate (PIP2) into phosphatidylinositol 3,4,5-trisphosphate (PIP3), which activates Akt. ER-mediated activation of Akt or MAPK pathway causes phosphorylation of cytosolic eNOS and its second translocation back to the cell membrane where it undergoes myristoylation and palmitoylation, a process required for its full activation. Activated eNOS promotes the transformation of L-arginine to L-citrulline and the production of NO, which is released by EC and causes VSM relaxation.
Phytoestrogens and cGMP
Biological signaling by NO is primarily mediated by cGMP. cGMP is synthesized by NO-activated guanylyl cyclase and is broken down by phosphodiesterase enzyme. cGMP activates PKG, which phosphorylates many cellular proteins, leading to activation or inactivation of various cellular processes. E2 induces cGMP production especially in ischemic tissues [84], cGMP may also mediate E2-induced stimulation of Ca2+ activated K+ channels (BKca) in porcine coronary artery [85]. Phytoestrogens may have similar cGMP-mediated vascular effects. In human coronary SMCs, resveratrol enhances cGMP formation and stimulates PKG activity. E2 has 46% lower maximal response than that of resveratrol. The cGMP formation by resveratrol or E2 is attenuated by the ER blocker ICI-182,780, even in endothelium-disrupted coronary arteries. Interestingly, combining E2 with resveratrol shows a competitive rather than an additive response [86].
Phytoestrogens and Prostacyclin (PGI2)
PGI2 is a prostaglandin (PG) produced from the metabolism of arachidonic acid by cyclooxygenase enzyme (COX) and in turn promotes endothelium-dependent vascular relaxation. E2-induced vascular relaxation is partly mediated by endothelium-derived PGI2 [87]. E2 induces upregulation of COX-1 expression and PGI2 synthesis in ECs, and increases urinary excretion of the PGI2 stable metabolite 6-keto-PGF1α. Interestingly, deletion of the PGI2 receptor diminishes the vascular protective effect of E2 in OVX female mice [88]. Phytoestrogens may have similar effects on the PGI2 pathway. Treatment of HUVECs with serum from Post-MW whose diet was supplemented with soy isoflavones and red clover increased the capacity of ECs to produce PGI2 [89]. Also, in HUVECs, genistein and daidzein increase PGI2 production through an ER-dependent mechanism involving increased COX-2 protein and activity, but not COX-1 [90]. In mesenteric microvessels isolated from female Wistar rats and preconstricted with norepinephrine, COX inhibitors abolish the vasodilatory effects of genistein, suggesting a role of PGs in genistein-induced vasorelaxation [76]. Phytoestrogens may also affect other PGs. In SHR aorta, isoflavones and E2 inhibit endothelium-dependent contraction to acetylcholine by reducing the release of PGH2 (unstable precursor of PGs and TXs) and its vasoconstrictor response [91]. Thus, phytoestrogens increase PGI2 production and may alter the level of other PGs as well.
Phytoestrogens and cAMP
Cyclic adenosine monophosphate (cAMP) is an intracellular second messenger derived from adenosine triphosphate (ATP) by activated adenylate cyclase. cAMP mediates some of the vascular effects of PGs [92,93]. E2 via ER increase cAMP production [94]. cAMP stimulates ER-mediated transcriptional activity in the absence of E2 by direct phosphorylation of the receptor [95]. Phytoestrogens may also enhance adenylate cyclase activity and affect cAMP-dependent pathways in ECs and VSM. In porcine coronary artery, genistein-induced vasodilatation is abolished by the cAMP-dependent protein kinase inhibitor Rp-8-Br-cAMP, suggesting a role of cAMP-dependent signal transduction [96]. Genistein also potentiates β1-adrenoceptor-induced relaxation in rat aortic rings mostly by inhibiting cAMP-phosphodiesterase activity [97]. In bovine aortic ECs, low concentrations of genistein, but not E2, increase intracellular cAMP by enhancing adenylate cyclase activity via a nongenomic mechanism [68]. Also, in porcine coronary artery, genistein causes VSM via a cAMP-dependent mechanism that does not involve Gs proteins or ERs [98]. Collectively, soy isoflavones appear to activate adenylate cyclase, increase cAMP and promote cAMP-dependent pathways. Further studies are needed to examine the effects of other phytoestrogens on cAMP-dependent pathways.
Phytoestrogens and EDHF
EDHF plays an important role in acetylcholine-induced endothelium-dependent hyperpolarization and relaxation of VSM [99]. E2 stimulates EDHF release [100]. EDHF may also play a role in phytoestrogen-induced vascular relaxation. In male Sprague-Dawley rats treatment with daidzein or E2 for one week stimulates aortic relaxation via a non-NO, non-PG factor acting through the opening of small conductance Ca2+ dependent K+ channels [SKCa] and intermediate Ca2+ dependent K+ channels [IKCa], and involving activation of Na/K-ATPase, inward rectifier K+ channel [KIR] and CYP450 epoxygenase, suggesting a role of EDHF in daidzein-induced vascular relaxation [83]. Further studies are needed to investigate the contribution of EDHF to the vasorelaxant effect of phytoestrogens.
Phytoestrogens and Endothelin (ET)
ET has three isoforms (ET-1, ET-2, and ET-3) acting on 2 receptors, ETA receptor which is found in VSMCs and induces vasoconstriction and ETB receptor which is found mainly in ECs and promotes vascular relaxation [101]. Changes in ET levels and metabolism are thought to contribute to CVD, and ET antagonists are used for treatment of pulmonary hypertension and are being investigated for treatment of other CVD [102]. E2 decreases ET production via an ER-dependent mechanism [70,103]. Phytoestrogens mimic the effects of E2 on ET. For example, genistein via ERs decreases ET production in rat arteries probably by inhibiting the expression of ET converting enzyme-1 [104].
Phytoestrogens and Vascular Smooth Muscle (VSM)
Phytoestrogens and Inhibition of VSM Proliferation
VSMC proliferation is involved in many pathological processes including vascular remodeling, neointimal hyperplasia and atherosclerosis. E2 inhibits VSMC proliferation via cytosolic/nuclear ERs and transcriptional genomic effects [105]. Phytoestrogens also inhibit VSMC proliferation. In human VSMCs, the red clover-derived isoflavone metabolite cis-tetrahydrodaidzein inhibits PDGF-induced extracellular receptor kinase (ERK-1) activation and cell proliferation [106]. Also, genistein regulates the activation of apoptosis-related molecules in TNFα-induced human aortic SMCs, leading to the suppression of proliferation and induction of apoptosis [107]. In endothelium-denuded rabbit aorta both genistein and daidzein inhibit VSMC proliferation via an effect independent from inhibition of TK activity by genistein [108]. Also, in aortic SMCs of stroke-prone SHR, genistein, daidzein and glycitein inhibit naturally and PDGF-induced VSMC proliferation and DNA synthesis [109]. In a study on cholesterol-fed mice, acute neointimal proliferation was induced in the iliac artery by mechanically damaging the endothelium and the damaged arteries were harvested after oral administration of dihydrodaidzein for 4 weeks. The study showed that dihydrodaidzein selectively inhibited neointimal proliferation, possibly by inhibiting VSMC migration and proliferation and/or enhancing endothelial proliferation and function [110]. Also, in rat aortic SMCs genistein inhibits PDGF-induced proliferation by blocking the progression from the G0/G1 to S phase of the cell cycle [111]. It has also been shown that TGF-β-stimulated clone-36, a matricellular protein induced by daidzein, inhibits human umbilical artery SMC proliferation and migration in vivo and in vitro, and causes accumulation of SMCs in G2 phase of the cell cycle [112]. Collectively, these studies support that phytoestrogens inhibit VSMC proliferation.
Phytoestrogens and VSM Function
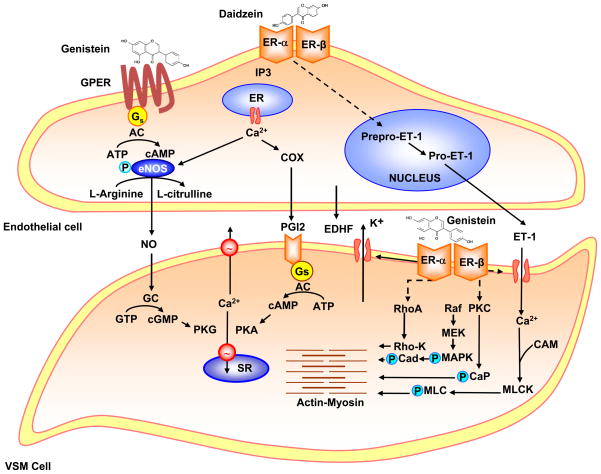
VSM contraction is triggered by increases in intracellular free Ca2+ concentration ([Ca2+]i) (Fig. 4). Activation of myosin light chain kinase (MLCK), Rho-K, protein kinase C (PKC) and MAPK also contribute to VSM contraction. E2 causes rapid relaxation of endothelium-denuded blood vessels [7,113]. Phytoestrogens may mimic the effects of E2 on VSM. Genistein supplements improve VSM function, vascular motor tone and systemic arterial compliance [61]. Phytoestrogens-induced vasodilation is mediated by different mechanisms. Systemically, phytoestrogens inhibit the renin-angiotensin system and Ang II production. At the cellular level, phytoestrogens regulate Ca2+ and K+ ion fluxes and other signaling pathways such as TK, Rho-K, PKC and MAPK.
Figure 4.
Effects of phytoestrogens on ECs and VSMCs. In ECs, phytoestrogens such as genistein or daidzein bind ERs and GPER and increase [Ca2+]i, PI3K/Akt, MAPK and cAMP which cause phosphorylation and activation of eNOS and promote the production of NO. NO release activates guanylate cyclase (GC) in VSM leading to increased cGMP and stimulation of cGMP-dependent protein kinase (PKG). PKG decreases [Ca2+]i by stimulating Ca2+ extrusion pump in the plasma membrane and Ca2+ uptake pump in the sarcoplasmic reticulum (SR) and/or decrease the sensitivity of the contractile myofilaments to [Ca2+]i. Phytoestrogens also activate cyclooxygenase (COX) to produce prostacyclin (PGI2) and in turn activate adenylate cyclase (AC) and the PGI2-cAMP-PKA pathway, leading to VSM relaxation. Phytoestrogens may also induce EDHF release and activate Ca2+-activated K+ channels causing hyperpolarization and relaxation of VSM. Phytoestrogens also inhibit ET-1 release and thereby decrease VSM contraction. In VSM, phytoestrogens may activate K+ channels, leading to membrane hyperpolarization, inhibition of Ca2+ entry through Ca2+ channels, and inhibition of Ca2+-dependent MLC phosphorylation and VSM contraction. Phytoestrogens through activation of plasma membrane ERs may also inhibit protein kinase C (PKC), Rho-K and/or the MAPK pathway and thereby further inhibit VSM contraction.
Phytoestrogens and Angiotensin
Ang II is a potent vasoconstrictor and an important regulator of electrolyte balance and blood pressure. E2 induces downregulation of vascular Ang II type 1 receptor mRNA and protein [114]. Phytoestrogens also affect the renin-angiotensin system. Genistein inhibits the expression of angiotensin converting enzyme (ACE) in rat aortic ECs via ER and ERK1/2 signaling pathway. The downregulation of ACE could, in turn, change the circulating levels of Ang II, the vasorelaxant Ang-(1-7) and bradykinin [115]. Also, in anaesthetized rats, genistein enhances the vasodilator response to bradykinin as a result of ACE inhibition. Genistein decreases ACE activity both in vivo and in vitro [116]. Further studies are needed to clarify the contribution of ACE inhibition to phytoestrogens-induced vasodilatation.
Phytoestrogens and VSM Ca2+
Activation of VSM by various agonists is associated with increases in [Ca2+]i due to Ca2+ release from the sarcoplasmic reticulum (SR) and Ca2+ entry from the extracellular space. E2 mainly inhibits Ca2+ influx rather than Ca2+ release from the intracellular stores, and a direct effect of E2 on Ca2+ channels has been suggested [117,118]. E2 may also decrease [Ca2+]i by stimulating Ca2+ extrusion via the plasmalemmal Ca2+ pump [119]. Phytoestrogens may inhibit VSM contraction by inhibiting Ca2+ influx or Ca2+ release from the SR, or by decreasing the Ca2+ sensitivity of the contractile apparatus. In pregnant women who consume soy-derived products in their meals, circulating isoflavones may play a role in the regulation of feto-maternal blood flow, possibly by inhibiting both Ca2+ influx and Ca2+ release from the SR and decreasing [Ca2+]i in umbilical SMCs [120]. Also, in swine carotid artery, genistein attenuates histamine-induced [Ca2+]I, myosin light chain (MLC) phosphorylation and isometric stress via TK inhibition [121]. However, the mechanisms by which phytoestrogens decrease [Ca2+]i may vary in different blood vessels. In rabbit basilar artery, genistein, daidzein, zearalanone and biochanin A cause vascular relaxation by blocking Ca2+ entry [122]. Also, in porcine coronary artery, genistein and E2 cause relaxation of KCl-, 5HT- and CaCl2-induced contractions mainly by inhibiting Ca2+ influx, and these effects may not be related to ER or classical genomic activities [123]. Other studies suggest that the vasorelaxant effects of phytoestrogens involve inhibition of intracellular Ca2+ release. In rat aortic SMCs, genistein mainly suppresses the transient phase of VSM contraction and slightly inhibits the sustained phase, suggesting that genistein decreases [Ca2+]i by inhibiting TK-linked Ca2+ release [124]. Also, in endothelium-denuded rat aorta, the effects of genistein are more pronounced on the norepinephrine-induced phasic contraction in the absence of extracellular Ca2+ than on the tonic contraction in the presence of extracellular Ca2+, suggesting that genistein inhibits contraction mainly by inhibiting intracellular Ca2+ release [125]. Other studies have shown enhanced vascular reactivity in cardiomyopathic hamster aorta possibly due to increased Ca2+ sensitivity of the contractile apparatus. The enhanced myofilament Ca2+ sensitivity by phenylephrine was markedly inhibited by genistein and to a less extent by daidzein [126]. Thus phytoestrogens appear to cause vasorelaxation mainly by decreasing Ca2+ influx and intracellular Ca2+ release, and may also decrease the Ca2+ sensitivity of the contractile apparatus.
Phytoestrogens and K+ Channels
K+ channels play a role in the regulation of VSM membrane potential and consequently the sensitivity to membrane depolarization and contraction. K+ efflux through the opening of K+ channels causes membrane hyperpolarization that closes voltage-gated Ca2+ channels, decreases Ca2+ entry, and lead to VSM relaxation. K+ channels include large conductance Ca2+-activated K+ channels [BKCa], intermediate-conductance [IKCa], small conductance [SKCa], inward rectifier [KIR], voltage-dependent [KV] and ATP-sensitive K+-channels [KATP] [127]. E2 and phytoestrogens cause VSM relaxation by activating K+ channels. In human coronary artery SMCs, E2 via ERα induces stimulation of BKCa causing membrane hyperpolarization and decreased Ca2+ influx [128]. Also, in rat aorta, treatment with daidzein or E2 stimulates the opening of SKCa and IKCa channels, and activation of Na/K-ATPase and KIR [83]. Also, in rat basilar artery SMCs, daidzein inhibits BKCa [129]. In rat mesenteric artery precontracted with norepinephrine, E2, genistein and daidzein cause relaxation that is antagonized by blockers of BKCa and SKCa [130]. Genistein also activates KCa in rat aortic SMCs [131]. Formononetin and biochanin A activate BKCa and KATP in rat aortic SMCs whereas daidzein is less potent [79]. Phytoestrogens may affect K+ channels via different signaling pathways. In rabbit portal vein SMC, TK may play a role in the regulation of KATP activity [132]. In contrast, in rabbit pulmonary artery genistein inhibits Kv current through a mechanism not involving TK inhibition or PKC activity [133]. Thus, the effects of phytoestrogens on different K+ channels may contribute to endothelium-independent vasodilation. Further studies are needed to define the intracellular signaling pathways mediating the effects of phytoestrogens on K+ channels.
Phytoestrogens and Tyrosine Kinase (TK)
Phosphorylation of tyrosine residues in certain proteins affects a wide range of their properties such as enzyme activity, subcellular localization, and interaction between molecules. TK activity in the nucleus is involved in the control of the cell cycle and various transcription factors. E2 and phytoestrogens inhibit TK activity. In OVX female SHR, treatment with E2 and low-dose genistein for 2 weeks is associated with decreased renal artery contraction to Ang II, but not to norepinephrine, KCl or ET-1, and these effects are likely due to TK inhibition [134]. Tyrosine phosphorylation maintains Ca2+ channels in a susceptible state for depolarization. In isolated rat portal vein SMC, genistein, which inhibits TK, decreases slow Ca2+ current (ICaL) in a concentration-dependent manner while superfusion with daidzein, which does not inhibit TK, had no inhibitory effect even at high concentrations [135]. Also, in rat aorta, genistein inhibits intracellular Ca2+ release via TK inhibition [125]. Thus, TK inhibition appears to play a role in the vascular relaxation induced by some phytoestrogens.
Phytoestrogens and Rho-Kinase
Rho-K is a downstream effector of the small GTP-binding protein Rho. Rho/Rho-K pathway plays an important role in the regulation of VSM contraction, and may be involved in the pathogenesis of vasospasm, arteriosclerosis, systemic and pulmonary hypertension, and stroke [136]. Studies have shown that long-term inhibition of Rho-K causes regression of coronary arteriosclerosis [137]. Also, in human coronary VSM, inflammatory stimuli such as Ang II and IL-1β, increase the expression and activity of Rho-K possibly via PKC and NF-κB [138]. In rat basilar artery, the Rho/Rho-K inhibitor Y-27632 is 3-fold more potent as vasodilator in males than females. Also, in OVX female rats, the vasodilator response to Y-27632 resembles the response in males, and treatment of OVX rats with E2 normalizes the vasodilator effects of Y-27632 to those observed in intact females. These observations suggest that endogenous E2 inhibits Rho/Rho-K [139]. Phytoestrogens may also inhibit Rho/Rho-K. In male rat aorta, genistein and daidzein cause relaxation of contraction induced by fluoride, a RhoA/Rho-K activator. The phytotestrogen-induced relaxation occurs in the absence of TK inhibition or functional endothelium, and is not antagonized by BKCa inhibitors, supporting that RhoA/Rho-K inhibition is involved in genistein-induced vasodilation [140].
Phytoestrogens and PKC
Protein kinase C (PKC) is a ubiquitous enzyme that comprises a family of Ca2+-dependent and Ca2+-independent isoforms, expressed in different proportions in VSM of various vascular beds. During cell activation, PKC translocation to the cell surface may trigger a cascade of protein kinases that ultimately interact with the contractile myofilaments and cause VSM contraction [141]. E2 inhibits PKC [142] and decreases the myofilament sensitivity to [Ca2+]i [143]. PKC activity may also be modulated by phytoestrogens [144], and the effects of phytoestrogens on PKC-dependent pathways need to be further investigated.
Phytoestrogens and MAPK
MAPKs are serine/threonine-specific protein kinases that respond to extracellular stimuli and regulate various cellular activities such as gene expression, mitosis, differentiation, proliferation, and cell survival/apoptosis [145]. MAPK also mediates some of the processes contributing to VSM contraction. Some of the effects of E2 are mediated by inhibiting MAPK [146], and phytoestrogens may also inhibit MAPK. Gene expression profiling revealed that MAPK signaling is one of the biological pathways affected by genistein. In human aortic SMCs, several phytoestrogens inhibit/downregulate MAPK activity in a concentration-dependent manner and in the following order of potency: biochanin A > genistein > equol > daidzein > formononetin [147]. Also, resveratrol inhibits ERK and p38 MAPK phosphorylation causing inhibition of IL-8 secretion in human monocytic cell line [148]. Further studies are needed to define the role of MAPK in phytoestrogens-induced vascular effects.
Phytoestrogens and Extracellular Matrix (ECM)
ECM is a major component of the blood vessel architecture, and plays an important role in the control of vascular wall integrity and vascular remodeling. Pathogenic changes in the ECM have been linked to elevated TGF-β levels, oxidative stress, and lipid accumulation. ECM proteins also play a role in the formation of the atherosclerotic plaque [149]. E2 plays a role in the regulation of the cellular cytoskeleton, ECM and vascular remodeling. For instance, ERα interacts with the G protein Gα 13 to induce activation of the RhoA/Rho-K pathway and phosphorylation of the actin-regulatory protein moesin, leading to remodeling of the actin cytoskeleton and EC migration [150]. Phytoestrogens also affect various components of ECM including collagens, elastin, glycoproteins, glycosaminoglycans and proteoglycans.
Collagen is secreted by fibroblasts and is present in the form of elongated fibrils mostly in fibrous tissues and also in blood vessels. Collagen is an essential component in the process of fibrosis and in the pathophysiology of atherosclerosis and atherosclerotic events [151]. Phytoestrogens suppress the synthesis of new collagen fibers. Genistein inhibits the proliferation of hypertrophic scar fibroblasts and ECM collagen synthesis via inhibition of TK [152]. In rodent renal mesangial cells cultured in a high-glucose environment, which stimulates collagen deposition, genistein attenuates the synthesis of type IV collagen and fibronectin [153]. However, in SMCs of mature pigs coronary arteries, genistein upregulates matrix protein expression [149].
While collagen fibers in blood vessels bear most of the strength at higher pressures, elastin fibers are essential in determining the mechanical strength of the vessels at lower pressures [3]. Heat-induced ROS may play a role in heat-induced expression of tropoelastin, a precursor of elastin. Pretreatment of human skin with genistein for 24 h inhibits heat-induced expression of tropoelastin in the epidermis [154]. More studies are needed to define the effects of phytoestrogens on the different components of the vascular ECM.
Phytoestrogens and MMPs
Matrix metalloproteases (MMPs) are zinc-dependent endopeptidases that play a role in vascular remodeling [155]. MMPs also degrade ECM within the atherosclerotic plaque, and may be involved in plaque instability and CV events. In Sprague-Dawley rats, E2 induces rapid ECM remodeling by upregulating different MMPs [156]. Although MHT E2 downregulates MMPs, it induces acute MMP modulation of vascular function [157]. Phytoestrogens may also regulate vascular remodeling via MMPs. Studies have examined the effects of 4.5 months of genistein treatment on atherosclerosis pattern and MMP expression in hypercholesterolemic rabbits. The average cross sectional area of atherosclerotic lesions in rabbit aortas progressed in rabbits on continuous hyperlipidemic diet (HD), increased mildly in genistein-treated rabbit on HD and decreased in rabbits on normal diet. Western blot analysis showed reduction of MMP-3 expression in HD+genistein and normal diet groups than HD group, and suggested that genistein stabilized the atherosclerotic lesions by inhibiting MMP-3 expression [158]. MMP-2 and MMP-9 play a role in the pathogenesis of atherosclerosis. In human aortic SMCs, the naturally occurring flavolignan deoxypodophyllotoxin (DPT) inhibits cell migration and MMP-2/9 activities, and MMP-9 transcription [159].
MMPs also play a role in enhancing cancer metastasis. Studies on cancer cell lines have supported an inhibitory effect of phytoestrogens on MMPs. Treatment of U87MG cancer cells with genistein and biochanin A induced decreases in the enzymatic activity of MMP-9 and the protein levels of MT1-MMP and urokinase plasminogen activation which are involved in the degradation of ECM proteins and subsequent tumor invasion [160]. Phytoestrogens also prevent the degradation of ECM and subsequent tumor invasion in breast cancer cells [161], prostate cancer [162] and melanomas [163]. Thus phytoestrogens regulate various ECM proteins, and further studies are needed to investigate the effects of phytoestrogens on different components of ECM, and the implications of these effects in postmenopausal CVD.
Phytoestrogens and Atherosclerosis
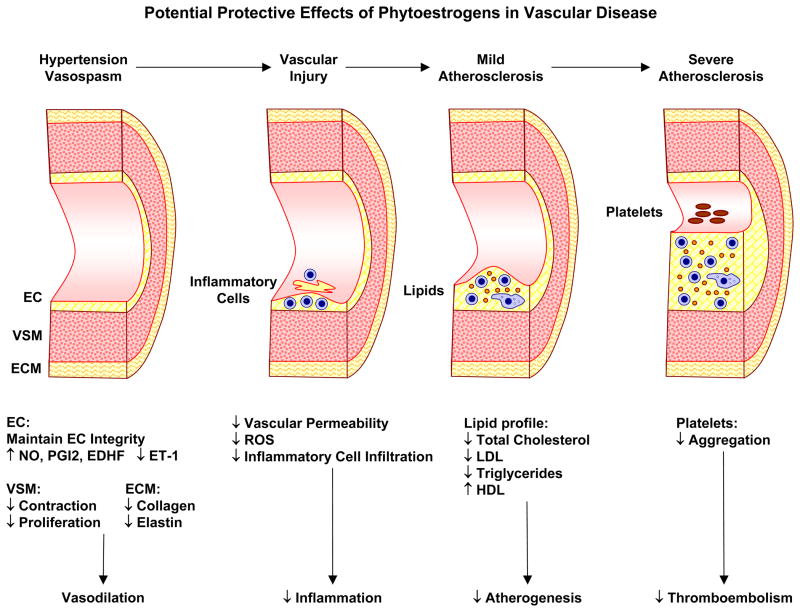
Atherosclerosis is a multifactorial vascular disease. Dysfunctional endothelium recruits different inflammatory pathways leading to intimal hyperplasia, VSMC proliferation, ox-LDL deposition, platelet activation and aggregation resulting in the formation of an atheroma of fat, collagen and elastin with a thin fibrous cap (Fig. 5). Hypertension is a major risk factor of endothelial dysfunction and atherosclerosis. E2 reduces atherogenesis by inhibiting SMC proliferation, LDL oxidation and deposition, and attenuating vascular inflammation by decreasing cell adhesion molecules (CAM), macrophage accumulation and monocyte adhesion [164]. E2-induced vasodilatation may also contribute to its anti-atherogenic properties. However, anti-atherogenic effects of E2 depend on the patient’s age and the stage of atherosclerosis. MHT containing E2 given early during perimenopause may decrease the development/progression of the atherosclerotic lesion. In contrast, in already established atheromatous plaques E2 may increase inflammation, MMP expression and neovascularization leading to lesion progression, plaque instability and rupture/hemorrhage. This may explain the reduced vascular benefits of MHT in Post-MW with preexisting CVD. Some studies suggest that dietary supplementation of phytoestrogens prevent the progression of atherosclerosis (Fig. 5). Grape phytoestrogens prevent cholesterol accumulation in cultured monocytes from Post-MW [165]. In HUVECs, genistein reverses homocysteine- and ox-LDL induced decrease in the anti-atherogenic proteins annexin V and lamin A [166]. Animal studies also support the anti-atherogenic properties of phytoestrogens. In the proximal left circumflex coronary artery of atherosclerotic rhesus monkeys, E2 and dietary soy isoflavones enhance the dilator response to acetylcholine [167]. Also, genistein inhibits atherogenesis in hypercholesterolemic rabbits mainly by improving EC dysfunction [158]. Compared with genistein, its derivative 7-difluoromethyl-5,4′-dimethoxygenistein has a better protective effect against EC damage in rabbits [168]. Also, resveratrol exhibits multiple anti-atherogenic effects [169] including inhibition of intimal hyperplasia [170], attenuation of TXA2-induced platelet aggregation [171] and inhibition of LDL oxidation [172]. However, other studies showed that isoflavone treatment of cholesterol fed rabbits failed to exert the same anti-atherogenic effects of E2 [164]. Whether phytoestrogens improve the clinical course of atherosclerosis and whether their effects are consistent throughout the different atherosclerotic stages needs to be further examined. Also, the different factors underlying the potential anti-atherogenic effect of phytoestrogens including inhibition of platelet aggregation, improvement of lipid metabolism, attenuation of vascular inflammation, anti-angiogenic and antioxidant effects need to be further examined.
Figure 5.
Potential protective effects of phytoestrogens in vascular disease. Increased vascular tone and blood pressure cause hypertension, leading to vascular injury, inflammatory cell infiltration, lipid deposition, atherosclerosis, narrowing of vessel lumen and platelets aggregation. Phytoestrogens promote vasodilation and may ameliorate hypertension. Phytoestrogens could also reduce vascular permeability, reactive oxygen species (ROS), and inflammatory cell infiltration. Phytoestrogens may also retard the progress of atherosclerosis by improving lipid profile, and reduce thromboembolism by decreasing platelets aggregation.
Phytoestrogens and Platelet Aggregation
Platelet aggregation plays a role in atherogenesis and thromboembolic events. An ex vivo study on platelets from 18 Post-MW showed that E2 inhibits the activity of thrombin-activated platelets by inhibiting Ca2+ influx and raising cAMP [173]. However, other studies have shown that E2 potentiates thrombin-induced platelet aggregation in platelets from healthy men through ERβ and Src kinase [174]. Phytoestrogens may inhibit platelet aggregation. Genistein suppresses platelet aggregation induced by collagen when administered intravenously in the mouse femoral artery [175]. Genistein also inhibits TXA2- and collagen analogs-induced platelet aggregation [176]. The mechanism of genistein-induced inhibition of platelet aggregation likely involves alteration of the early event signaling pathways involved in platelet activation [177]. NO production could play a role in genistein-induced inhibition of platelet aggregation, since the NOS inhibitor L-NAME suppresses the platelet anti-aggregation effect of genistein in rat aortic strips [178]. The inhibitory effects of phytoestrogens on platelet aggregation could also be due to their ability to compete for binding to the TXA2 receptor [179]. Resveratrol also inhibits collagen- and epinephrine-induced platelet aggregation in acetyl salicylic acid resistant platelets [180]. Thus studies showed inhibitory effects of phytoestrogens on platelet aggregation, but the pathways involved need to be further defined.
Phytoestrogens and Lipid Profile
Lipid deposition, especially the oxidized form, plays an integral part in atherogenesis. E2 has a favorable effect on the lipid profile [181]. Phytoestrogens may also improve the lipid profile. In mice fed a high fat diet (HFD), and then 6 weeks later either treated or not treated with a daidzein derivative, the daidzein-treated HFD group showed a reduction in body and fat pad weight and an improvement of HFD-induced hyperlipidemia. Daidzein ameliorates HFD-induced hyperlipidemia and reduces body fat by inhibiting the activity of both pancreatic lipase (which promotes lipid absorption) and lipoprotein lipase (which promotes fat tissue deposition). Daidzein also inhibits the differentiation of rat pre-adipocytes and stimulates lipolysis by activating hormone-sensitive lipase [182]. Genistein also inhibits the oxidation of LDL in human ECs and bovine aortic ECs in the presence of copper ions or superoxide/NO radicals [183]. Similar beneficial effects on lipid profile are observed with dietary fiber and lignans [184] possibly due to induction of adiponectin gene expression through an increase in PPAR-γ DNA binding activity [185]. However, a study in cholesterol fed OVX female rabbits found no significant effect of E2 or isoflavones on serum total cholesterol levels [164]. Also, genistein could transform synovial fibroblasts into adipocytes and enhance glucocorticoid-mediated synovial fibroblast adipogenesis [186]. Thus, while most of the experimental evidence suggests beneficial effects of phytoestrogens on lipid metabolism, few studies have not supported these findings.
Phytoestrogens and Angiogenesis
Angiogenesis involves EC proliferation and differentiation into new vascular capillaries. Angiogenesis is also involved in several disease conditions such as diabetic retinopathy, tumor growth and atherosclerosis [187]. E2 promotes angiogenesis through enhancing the release of VEGF and promoting its angiogenic effects [188]. E2 also disrupts adherens junctions which are important regulators of EC migration and proliferation and thereby enhances the angiogenic effect of VEGF [189]. Some phytoestrogens have similar angiogenic effects. Formononetin promotes early fracture healing and increases the number of vessels and expression of VEGF and VEGF receptor 2 in the early stage of chondrogenesis in rats [190]. Other studies suggest that phytoestrogens may suppress angiogenesis. Genistein inhibits cell proliferation, induces apoptosis, and suppresses in vivo angiogenesis in human renal carcinoma cells injected into rats [191]. Genistein also inhibits oxLDL-induced angiogenesis in HUVECs [192]. The anti-angiogenic effect of genistein may be due to downregulation of cell adhesion related genes and impairment of cell adhesion [193]. Genistein may also activate anti-angiogenic molecules such as tissue factor, endostatin and angiostatin [194]. Isoflavones may promote anti-angiogenic effects and cell growth arrest by inhibiting TK and targeting growth factors such as FGF, PDGF, EGF and VEGF. Flaxseed lignans may also inhibit E2-induced VEGF secretion in MCF-7 cancer cells [195]. Thus most of the experimental evidence supports anti-angiogenic effects of phytoestrogens which could be of benefit in CVD and neoplastic disease.
Phytoestrogens and Vascular Inflammation
Low-grade inflammation is implicated in atherogenesis, and E2 may affect the course of atherosclerosis by inhibiting vascular inflammation. E2 attenuates vascular expression of inflammation-associated genes and inhibits adhesion of monocytes to ECs [196]. CD40L activates antigen-presenting cells and regulates B cell function by engaging CD40 on the B cell surface. E2 via an ERα-mediated pathway blocks Interferon-γ induced CD40 and CD40L protein expression and prevents neutrophil adhesion [197]. E2 also attenuates TNF-α-induced mRNA expression of inflammatory mediators [198]. Similar to E2, phytoestrogens may exert vascular anti-inflammatory effects, and the anti-inflammatory effect of several medicinal herbs could be due to their phytoestrogen content [199]. Genistein protects against inflammatory factor-induced EC dysfunction and inhibits leukocyte-endothelium interaction [200]. Genistein, and to a lesser extent daidzein, decrease TNFα-induced secretion of monocyte chemotactic protein-1, a cytokine recruiting white blood cells to sites of inflammation [201]. In human brain microvascular ECs, genistein pretreatment reduces cytokine-mediated upregulation of blood leukocytes transmigration [202]. Also, in HUVECs, genistein inhibits TNFα-induced signaling and plasminogen activator inhibitor (PAI-1) transcription likely due to inhibition of TK because daidzein does not exert the same effect [203]. Genistein also reduces mRNA expression levels of E-selectin, CAM-1 and P-selectin which are elicited by the proinflammatory bacterial LPS [59]. Genistein also inhibits the activity of the key inflammatory enzyme secretory phospholipase A2 in mice [199]. Other studies support the anti-inflammatory properties of phytoestrogens and suggest the utilization of these properties in preventing graft rejection [204], treating arthritis [205], protection against UV rays-induced skin inflammation [206] and treating bronchial asthma [207]. In contrast to isoflavones, resveratrol may have a vascular pro-inflammatory activity as shown in normoglycemic and diabetic rat aortic SMCs [208]. Thus studies support that most phytoestrogens have anti-inflammatory effect in vascular ECs and several other tissues.
Phytoestrogens as Antioxidants
CVD is partly caused by decreased bioavailability of NO due to increased oxidative stress, ROS production and lipid peroxidation. E2 alters the expression of ROS-generating and -scavenging enzymes and decreases oxidative stress in different cells [209]. Phytoestrogens especially soy isoflavones have multiple protective effects against oxidative stress in vascular ECs [210]. In HUVECs, genistein inhibits the potential of glucose-oxidized LDL to increase tissue factor synthesis [211]. Bcl-2 protein is critical for regulation of cell proliferation and apoptosis under both normal and oxidative conditions. Soy isoflavones prevent oxidative stress-induced apoptosis via ERβ and Bcl-2/Bax expression and modulation of cell survival signaling [212]. Also, treatment of HUVECs with equol reduces O2•− production by NAD(P)H oxidase [62]. Animal studies have also supported antioxidant effects of isoflavones. Genistein and daidzein through ER-independent mechanisms restore EC function in male SHR by increasing NO production and protection of NO from O2•−-driven inactivation [91]. Also, in rat aortic ECs, soy and alfalfa extract potently inhibit the formation of ox-LDL [213]. In porcine coronary artery, the inhibitory effect of genistein and resveratrol on ROS-induced vasoconstriction is greater than that of E2 [214]. 8-Oxo-2′-deoxyguanosine (8-OHdG) is one of the major products of DNA oxidation and its concentration within a cell is used as a measure of oxidative stress. In SHR-Stroke Prone and Wistar-Kyoto rats, genistein, daidzein, and resveratrol decrease the levels of 8-OHdG and prevent oxidative DNA damage induced by advanced glycation end products. Phytoestrogens also increase the levels of the antioxidant glutathione in VSMCs [215]. The antioxidant effects of various phytoestrogens may vary in different tissues and cell types. In rat basilar artery where there is a high O2•− level, equol exerts weak antioxidant effects, and the effects of daidzein are insignificant [78]. However, in bovine aortic ECs, equol potently inhibits H2O2-induced cell death by reducing ROS production [216]. Collectively, studies support the antioxidant activity of phytoestrogens which adds to their potential benefits in CVD.
Epidemiological Evidence and Clinical Trials of Vascular Benefits of Phytoestrogens
The potential vascular benefits of phytoestrogens demonstrated in epidemiological and experimental studies have prompted more observational and interventional studies to further investigate the clinical effects of phytoestrogens on vascular function and CVD. However, the clinical trials have been limited in many aspects and showed inconsistent results.
Human studies examining the effects of phytoestrogens on the endothelium have suggested beneficial effects on endothelial functions and vasorelaxant effects of phytoestrogens (Table 2). However, these results have not been consistent in all studies. Of note, in the studies that showed no beneficial effects of phytoestrogens on endothelial functions, the cohort group had normal endothelial function at baseline.
Table 2.
Representative Human Studies Examining the Effects of Phytoestrogens on EC Function
| Phytoestrogen - Clinical Trial | Subjects | Study Design | Outcome | Ref |
|---|---|---|---|---|
| Isoflavones - Observational | 126 High risk CV patients | Dietary questionnaire | Isoflavone intake is associated with enhanced brachial artery FMD and reduced mean carotid intima-media thickness | [221] |
| Genistein - RCT | 79 Healthy Post-MW age 56±4 yr | 1 year of either E2/progesterone or genistein therapy | Genistein increased nitrites/nitrates levels, decreased plasma ET-1 levels in brachial artery and improved EC function to the same extent as E2/progesterone regimen | [222] |
| Genistein Resveratrol - RCT | 12 Post-MW with CHD & 14 age- matched controls | One hour incubation of resistance subcutaneous arteries with genistein, resveratrol, ERα agonist and E2. | Arterial dilatation to phytoestrogens was enhanced in CHD group as compared to controls. Inhibition of NO synthase had no effect on dilatation induced by the investigated compounds. ERβ expression was enhanced in the vascular wall from CHD women, while ERα predominated in controls. | [223] |
| Dehydroequol - Clinical trial | 6 Healthy males | Brachial artery infusion of dehydroequol in forearm resistance arteries in the absence and presence of eNOS inhibition | Dehydroequol demonstrated potent vasodilator properties in human forearm resistance arteries via a NO-dependent mechanism | [224] |
| Soy - RCT | 22 Healthy Post-MW | 6 wk of either daily raloxifene, soy phytoestrogens 55 mg or placebo in random sequence with intervening 6 wk wash-out periods | No change from baseline endothelial function | [225] |
| Lignan - RCT | 22 Healthy Post-MW | Daily consumption of a low- fat muffin enriched with a lignan complex, providing 500 mg/d of secoisolariciresinol diglucoside for 6 wk periods separated by a 6-wk wash-out intervals. | No difference in FMD and nitroglycerine-mediated endothelium-independent vasodilation, plasma nitrites/nitrates, ET-1, or asymmetric dimethylarginine between the lignan complex intervention period and the placebo period | [226] |
| Isoflavones - RCT | 62 Post-MW age 45-60 yr | 72 mg of soy-derived isoflavones or placebo | No effect on endometrial thickness or the pulsatile index of the uterine and cerebral arteries | [227] |
| Isoflavones - Meta-analysis | 17 RCTs | Isoflavones can modestly, but significantly improve endothelial function | [228] | |
| Isoflavones - Meta-analysis | 9 RCTs | Isoflavone supplementation improves endothelial function in Post-MW with low baseline FMD levels, but not in women with high baseline FMD levels | [229] |
FMD, flow-mediated dilation
Studies have also examined the effects of phytoestrogens on plasma lipid profile (Table 3). Observational population-based studies have shown better lipid profile in individuals with high dietary soy intake. However, these beneficial effects should be viewed with caution because individuals who consume soy as a source of protein may have a lower intake of animal proteins, causing further reduction in cholesterol and saturated fat intake [12]. Most clinical studies supported that phytoestrogens decrease LDL-C, total cholesterol and triglycerides, and increase HDL-C. However, some studies did not support beneficial effects of phytoestrogens on lipid profile (Table 3).
Table 3.
Representative Human Studies Examining the Effects of Phytoestrogens on Lipid Profile
| Phytoestrogen - Clinical Trial | Subjects | Study Design | Outcome | Ref |
|---|---|---|---|---|
| Soy Phytoestrogens - Epidemiological | 1242 Japanese M, 3592 F | Semiquantitative Food Frequency Questionnaire | Intake of soy products is associated with lower TC levels | [230] |
| Daidzein - Observational | 483 F with CHD risk factors | Blood genistein and daidzein levels, lipoprotein levels, E2 levels, and angiographic CAD | Higher blood levels of daidzein associated with lower TG, higher HDL-C levels, and an improved TC to HDL-C ratio | [231] |
| Isoflavones - RCT | 20 Healthy Post-MW Age 50-70 yr | After 3 wk stabilization on low fat diet, phytoestrogens or placebo intake for 8 wk in random order, separated by 8 wk washout | No effect on BP and plasma lipid or lipoprotein levels | [232] |
| Isoflavones - RCT | 156 Volunteers, mildly high TC | NCEP Step I diet, One of 5 daily diets: 25 g casein or 25 g isolated soy protein containing 3, 27, 37, or 62 mg of isoflavones | Isoflavones reduced plasma concentrations of TC and LDL-C without affecting concentrations of TG or HDL-C | [233] |
| Phytoestrogens - RCT | 19 Post-MW | 2 Month supplementation with Pueraria mirifica plant (rich in phytoestrogens) | Increased HDL-C & apolipoprotein A-1, decreased LDL-C and Apo B. and decreased LDL-C/HDL-C. Miroestrol and coumestrol enhanced ERα-& ERβ-mediated transactivation. Daidzein & genistein, preferentially enhanced ERβ-mediated transactivation | [234] |
| Soy Phytoestrogens - Clinical trial | 24 Post-MW with high TC | 25 g soy protein supplement or a mild protein placebo for 6 wk, separated by 4 wk washout. | Serum TG increased, TC and LDL levels decreased significantly, HDL showed mild change | [235] |
| Isoflavones - RCT | 30 Post-MW | Isoflavones or placebo for 3 month interrupted by a 2 month washout period. Cholesterol efflux from cells used as a marker of improved lipid metabolism. | No differences between the isoflavone and the placebo group | [236] |
| Lignans - RCT | 22 Healthy Post-MW | Daily low-fat muffin enriched with a lignan complex for 6 wk separated by a 6-wk washout. Different markers measured at the beginning and end of each intervention. | No effect on plasma lipid concentrations, serum lipoprotein oxidation resistance, or plasma antioxidant capacity | [237] |
| Isoflavones - RCT | 49 Post-MW 47-66 yrs in Brazil | 40 mg of isoflavone (n = 25) or 40 mg of casein placebo (n = 22). Lipid profile monitoring at baseline and after 6 month of treatment | No significant effects of isoflavone on LDL or TC | [238] |
TC, total cholesterol, NCEP, National Cholesterol Education program
Epidemiological studies demonstrated that even in the absence of other risk factors (e.g. diabetes, hypertension, hypercholesterolemia), advanced age increases CV morbidity by enhancing vascular oxidative stress and inflammation [217]. Studies suggest that dietary intake of phytoestrogens reduces vascular inflammation especially in Post-MW (Table 4).
Table 4.
Representative Human Studies Examining the Effects of Phytoestrogens on Vascular Inflammation
| Phytoestrogen - Clinical Trial | Subjects | Study Design | Outcome | Ref |
|---|---|---|---|---|
| Phytoestrogens- - Cross-sectional study | 151 Middle age elderly males and 91 Post-MW | Secoisolariciresinol, matairesinol, pinoresinol, lariciresinol intake was evaluated. Soluble ICAM-1 (biomarker of inflammation involving damage to the endothelium and platelets), insulin, CRP, glucose, TC, HDL-C and triglycerides measured in fasting blood samples. FMD (only in 56 M and 55 F) | Plasma concentrations of soluble ICAM-1 significantly decreased in the whole group. Marked decrease in soluble ICAM-1 accompanied with relevant improvement of FMD only in the matairesinol group. | [239] |
| Isoflavones - RCT | 60 Post-MW | Isoflavone or placebo tablets for 6 month | Significant improvement of endothelium dependent vasodilation in the isoflavone treatment group. Plasma ICAM-1, VCAM-1, and E-selectin decreased in the isoflavone group compared to placebo. | [240] |
| Genistein - Cross-sectional | 30 Males | Either a placebo or high-genistein treatment. Blood samples were collected before and within 5 min, and after 30 min of 80% peak O2 consumption exercise. Measurements were taken before and after 4-week supplementation. | Isoflavones decreased homocysteine levels. Phytoestrogens were shown to have antioxidant effects, but a limited ability to diminish an abrupt surge of oxidative stress due to acute exercise | [241] |
| Isoflavones - Crossover RCT | 117 Healthy Post-MW | Isoflavone-enriched or placebo cereals consumed for 8 wk, with washout of 8 wk | Lower CRP levels but no effect on other inflammatory markers | [242] |
| Lignan - RCT | 22 Healthy Post-MW | Low-fat muffin, with or without a lignan complex, for 6 weeks, separated by a 6-week washout period. | No differences between the lignans and placebo periods in IL-6, TNFα, ICAM-1, VCAM-1, and MCP-1 levels. Lower CRP in the lignans-added period. | [243] |
Phytoestrogens may delay the onset of atherosclerotic CVD by reducing vascular inflammation, oxidative stress, platelet aggregation and plasma lipid levels. Also, by improving vascular compliance, phytoestrogens may improve hypertension, a major atherosclerosis risk factor. Epidemiologic studies, Crossover RCT including the WHO-CARDIAC study have shown that phytoestrogens may have beneficial effect on CVD [19,218]. Clinical trials have examined the effect of phytoestrogens on different CVD risk factors and end points, but the results have not been consistent (Table 5).
Table 5.
Representative Human Studies Examining the Effects of Phytoestrogens on CVD
| Phytoestrogen - Clinical Trial | Subjects | Study Design | Outcome | Ref |
|---|---|---|---|---|
| Flavonoid - Observational | 34489 Healthy Post-MW age 55-69 yr | Flavonoid food composition data, 16 years follow-up | Inverse association between flavanones intake and CHD, and between flavones intake and total mortality. No association between flavonoid intake and stroke mortality. Individual flavonoid-rich foods associated with significant mortality reduction: bran, apples, pears, red wine (CHD and CVD), grapefruit (CHD), strawberries (CVD), and chocolate (CVD) | [244] |
| Lignan - Cross- sectional study | 301 Post-MW, 60-75 yrs in the Netherlands | Food frequency questionnaire covering the year prior to enrollment | Lower systolic & diastolic BP & lower prevalence of hypertension observed with lignan intake, but no association with ankle-arm BP index or EC function. | [245] |
| Biochanin A & formononetin - Clinical trial | 80 Healthy subjects, age 45-75 yr | Biochanin A& formononetin in two 6-week periods. Large artery stiffness, endothelial function, 24-hour ambulatory BP, and total peripheral resistance measured at baseline and after each intervention. | In normotensive men and Post-MW, formononetin reduced arterial stiffness and total vascular resistance, but had no effect on BP | [246] |
| Phytoestrogens - Meta-analysis | 133 Clinical trials | Structured search strategy using MEDLINE, EMBASE, and Cochrane databases | Chocolate increased FMD after acute & chronic intake and reduced systolic & diastolic BP. Soy protein isolate reduced diastolic BP and LDL-C. Acute black tea consumption increased systolic & diastolic BP. Green tea reduced LDL. | [17] |
Thus while several clinical studies have suggested a variety of beneficial vascular effects of phytoestrogens, there have been discrepancies in the results. The causes of these discrepancies may be related to the number of subjects, type of phytoestrogen, study duration, clinical end points, and subjects compliance. The number of subjects involved in the interventional trials was too low to extrapolate the results to the general population. The phytoestrogens examined in clinical trials have been mainly isoflavones, and other classes of phytoestrogens could have different or perhaps better outcomes. The duration of the clinical studies has been limited to few weeks or months, and more chronic studies are needed to investigate the long-term effects of phytoestrogens. The clinical studies used different parameters with no clear clinical end points, and used different and incomplete sets of diagnostic tools. Finally, as with other clinical trials involving a change in diet, the compliance of the enrolled subjects is an important issue due to the difficulty of changing dietary habits of a large population consistently over a long period of time.
Conclusions and Perspectives
Phytoestrogens are currently being evaluated for their potential vascular benefits and as alternatives for MHT. Like E2, phytoestrogens bind to ERs and induce both genomic and non-genomic vascular effects. Phytoestrogens maintain endothelial integrity and decrease vascular permeability, increase NO, PGI2 and/or EDHF release leading to endothelium-dependent vasodilation. Phytoestrogens also inhibit VSM proliferation, and inhibit VSM contraction by activating cAMP- and cGMP-dependent pathways, decreasing Ca2+ influx and release, activation of different K+ channels, regulating the RhoA/Rho-K dependent pathway, and inhibition of TK. Phytoestrogens improve lipid metabolism, reduce oxidative stress, inhibit angiogenesis and attenuate vascular inflammation. Several clinical studies support beneficial vascular effects of soy isoflavone extracts, and phytoestrogens may improve endothelial function, the lipid profile and vascular inflammation biomarkers especially in Post-MW. However, the data from clinical studies showed inconsistent results and have been limited due to factors related to the study design and the subjects’ compliance.
Thus phytoestrogens may improve the course of several diseases. The vasorelaxant effects of phytoestrogens could be of value in delaying the progression of hypertension in certain populations. Phytoestrogens could also alter the course of diseases characterized by severe vasoconstriction such as pulmonary hypertension and thromboangiitis obliterans. The vasorelaxant and anti-proliferative effects of phytoestrogens could be of benefit in preventing/delaying vascular stent restenosis. The vascular anti-inflammatory effects of phytoestrogens could be of value as a co-therapy in inflammatory vascular diseases and vasculitis. Phytoestrogens could also prevent or delay the progression of atherosclerosis and decrease the incidence of CV events.
Future studies are needed to investigate the effects of phytoestrogens other than isoflavones. Also, some of the signaling pathways of phytoestrogens are not clearly defined. The recently-discovered nongenomic effects of phytoestrogens are one of the areas that need further research. Also, the interactions between different phytoestrogens, E2, SERMs are yet to be investigated.
Future clinical trials need to enroll larger number of subjects, compare different phytoestrogens, include the other dietary components of the population enrolled, study the long-term effects of dietary modifications, and use clear clinical end points (e.g. myocardial infarctions, stroke). The results from these clinical trials could help in answering important questions regarding the relative potencies of different phytoestrogens, the proper quantity of dietary supplement needed to produce measurable effects and the differences in the effects depending on the subjects’ ethnicity, age, gender and preexisting CVD. As for now, while studies suggest several beneficial vascular and metabolic effects of phytoestrogens and a tendency to reduce the risk of CVD, there is insufficient evidence to recommend specific quantities or types of phytoestrogens for prevention or treatment of CVD.
Acknowledgments
This work was supported by grants from National Heart, Lung, and Blood Institute (HL-65998, HL-98724) and The Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD-60702). We thank Ms. Alexandra Fen for her assistance in collecting some of the information and in reviewing and proof-reading the manuscript.
List of abbreviations
- AF
activation factor
- AngII
angiotensin II
- [Ca2+]i
intracellular free Ca2+ concentration
- CAD
coronary artery disease
- CVD
cardiovascular diseases
- ACE
angiotensin converting enzyme
- EC
endothelial cell
- ECM
extracellular matrix
- EDHF
endothelium-derived hyperpolarizing factor
- ER
estrogen receptor
- E2
estradiol
- HUVECs
human umbilical vein endothelial cells
- MAPK
mitogen-activated protein kinase
- NO
nitric oxide
- eNOS
endothelial NO synthase
- PGI2
prostacyclin
- PG
prostaglandin
- PKC
protein kinase C
- Post-MW
postmenopausal women
- Pre-MW
premenopausal women
- RCT
randomized clinical trials
- ROS
reactive oxygen species
- SR
sarcoplasmic reticulum
- SHR
spontaneously hypertensive rat
- TK
tyrosine kinase
- VSM
vascular smooth muscle
References
- 1.Hays J, Ockene JK, Brunner RL, Kotchen JM, Manson JE, Patterson RE, Aragaki AK, Shumaker SA, Brzyski RG, LaCroix AZ, Granek IA, Valanis BG. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003;348(19):1839–1854. doi: 10.1056/NEJMoa030311. [DOI] [PubMed] [Google Scholar]
- 2.Stefanick ML. Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am J Med. 2005;118(Suppl 12B):64–73. doi: 10.1016/j.amjmed.2005.09.059. [DOI] [PubMed] [Google Scholar]
- 3.Smiley DA, Khalil RA. Estrogenic compounds, estrogen receptors and vascular cell signaling in the aging blood vessels. Curr Med Chem. 2009;16(15):1863–1887. doi: 10.2174/092986709788186093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubey RK, Imthurn B, Zacharia LC, Jackson EK. Hormone replacement therapy and cardiovascular disease: what went wrong and where do we go from here? Hypertension. 2004;44(6):789–795. doi: 10.1161/01.HYP.0000145988.95551.28. [DOI] [PubMed] [Google Scholar]
- 5.Ross RL, Serock MR, Khalil RA. Experimental benefits of sex hormones on vascular function and the outcome of hormone therapy in cardiovascular disease. Curr Cardiol Rev. 2008;4(4):309–322. doi: 10.2174/157340308786349462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286(2):R233–249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 7.Crews JK, Murphy JG, Khalil RA. Gender differences in Ca(2+) entry mechanisms of vasoconstriction in Wistar-Kyoto and spontaneously hypertensive rats. Hypertension. 1999;34(4 Pt 2):931–936. doi: 10.1161/01.hyp.34.4.931. [DOI] [PubMed] [Google Scholar]
- 8.Mendelsohn ME. Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol. 2002;90(1A):3F–6F. doi: 10.1016/s0002-9149(02)02418-9. [DOI] [PubMed] [Google Scholar]
- 9.Grodstein F, Stampfer MJ, Manson JE, Colditz GA, Willett WC, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335(7):453–461. doi: 10.1056/NEJM199608153350701. [DOI] [PubMed] [Google Scholar]
- 10.Stampfer MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev Med. 1991;20(1):47–63. doi: 10.1016/0091-7435(91)90006-p. [DOI] [PubMed] [Google Scholar]
- 11.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349(6):523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 12.Glazier MG, Bowman MA. A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Arch Intern Med. 2001;161(9):1161–1172. doi: 10.1001/archinte.161.9.1161. [DOI] [PubMed] [Google Scholar]
- 13.Salari Sharif P, Nikfar S, Abdollahi M. Prevention of bone resorption by intake of phytoestrogens in postmenopausal women: a meta-analysis. Age (Dordr) doi: 10.1007/s11357-010-9180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lund TD, Munson DJ, Adlercreutz H, Handa RJ, Lephart ED. Androgen receptor expression in the rat prostate is down-regulated by dietary phytoestrogens. Reprod Biol Endocrinol. 2004;2:5. doi: 10.1186/1477-7827-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21(2):113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 16.Zhao L, Mao Z, Brinton RD. A select combination of clinically relevant phytoestrogens enhances estrogen receptor beta-binding selectivity and neuroprotective activities in vitro and in vivo. Endocrinology. 2009;150(2):770–783. doi: 10.1210/en.2008-0715. [DOI] [PubMed] [Google Scholar]
- 17.Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88(1):38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 18.Adlercreutz H, Mazur W. Phyto-oestrogens and Western diseases. Ann Med. 1997;29(2):95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- 19.Yamori Y. Food factors for atherosclerosis prevention: Asian perspective derived from analyses of worldwide dietary biomarkers. Exp Clin Cardiol. 2006;11(2):94–98. [PMC free article] [PubMed] [Google Scholar]
- 20.Kurzer MS. Phytoestrogen supplement use by women. J Nutr. 2003;133(6):1983S–1986S. doi: 10.1093/jn/133.6.1983S. [DOI] [PubMed] [Google Scholar]
- 21.Pilsakova L, Riecansky I, Jagla F. The physiological actions of isoflavone phytoestrogens. Physiol Res. doi: 10.33549/physiolres.931902. [DOI] [PubMed] [Google Scholar]
- 22.Thompson LU, Boucher BA, Liu Z, Cotterchio M, Kreiger N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr Cancer. 2006;54(2):184–201. doi: 10.1207/s15327914nc5402_5. [DOI] [PubMed] [Google Scholar]
- 23.Glitso LV, Mazur WM, Adlercreutz H, Wahala K, Makela T, Sandstrom B, Bach Knudsen KE. Intestinal metabolism of rye lignans in pigs. Br J Nutr. 2000;84(4):429–437. [PubMed] [Google Scholar]
- 24.Schmitt E, Lehmann L, Metzler M, Stopper H. Hormonal and genotoxic activity of resveratrol. Toxicol Lett. 2002;136(2):133–142. doi: 10.1016/s0378-4274(02)00290-4. [DOI] [PubMed] [Google Scholar]
- 25.D’Alessandro TL, Boersma-Maland BJ, Peterson TG, Sfakianos J, Prasain JK, Patel RP, Darley-Usmar VM, Botting NP, Barnes S. Metabolism of phytoestrogen conjugates. Methods Enzymol. 2005;400:316–342. doi: 10.1016/S0076-6879(05)00019-4. [DOI] [PubMed] [Google Scholar]
- 26.Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131(4 Suppl):1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 27.King RA, Bursill DB. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am J Clin Nutr. 1998;67(5):867–872. doi: 10.1093/ajcn/67.5.867. [DOI] [PubMed] [Google Scholar]
- 28.Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342(8881):1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- 29.Setchell KD. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr. 1998;68(6 Suppl):1333S–1346S. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- 30.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132(12):3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 31.Duncan AM, Merz-Demlow BE, Xu X, Phipps WR, Kurzer MS. Premenopausal equol excretors show plasma hormone profiles associated with lowered risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2000;9(6):581–586. [PubMed] [Google Scholar]
- 32.Kurzer MS, Xu X. Dietary phytoestrogens. Annu Rev Nutr. 1997;17:353–381. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- 33.Faughnan MS, Hawdon A, Ah-Singh E, Brown J, Millward DJ, Cassidy A. Urinary isoflavone kinetics: the effect of age, gender, food matrix and chemical composition. Br J Nutr. 2004;91(4):567–574. doi: 10.1079/BJN20041087. [DOI] [PubMed] [Google Scholar]
- 34.Setchell KD, Cole SJ. Variations in isoflavone levels in soy foods and soy protein isolates and issues related to isoflavone databases and food labeling. J Agric Food Chem. 2003;51(14):4146–4155. doi: 10.1021/jf026199b. [DOI] [PubMed] [Google Scholar]
- 35.Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M, Masamune Y. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull. 2001;24(4):351–356. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J, Cidlowski JA. The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids. 2005;70(5–7):407–417. doi: 10.1016/j.steroids.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 38.Hall JM, Korach KS. Analysis of the molecular mechanisms of human estrogen receptors alpha and beta reveals differential specificity in target promoter regulation by xenoestrogens. J Biol Chem. 2002;277(46):44455–44461. doi: 10.1074/jbc.M200849200. [DOI] [PubMed] [Google Scholar]
- 39.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13(2):307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 40.Ascenzi P, Bocedi A, Marino M. Structure-function relationship of estrogen receptor alpha and beta: impact on human health. Mol Aspects Med. 2006;27(4):299–402. doi: 10.1016/j.mam.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60(2):210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERbeta has nongenomic action in caveolae. Mol Endocrinol. 2002;16(5):938–946. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- 43.Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- 44.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 45.Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148(7):3236–3245. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- 46.Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res Mol Brain Res. 1998;54(1):175–180. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- 47.Fang H, Tong W, Shi LM, Blair R, Perkins R, Branham W, Hass BS, Xie Q, Dial SL, Moland CL, Sheehan DM. Structure-activity relationships for a large diverse set of natural, synthetic, and environmental estrogens. Chem Res Toxicol. 2001;14(3):280–294. doi: 10.1021/tx000208y. [DOI] [PubMed] [Google Scholar]
- 48.Zhu BT, Han GZ, Shim JY, Wen Y, Jiang XR. Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology. 2006;147(9):4132–4150. doi: 10.1210/en.2006-0113. [DOI] [PubMed] [Google Scholar]
- 49.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 50.Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140(12):5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 51.Bjornstrom L, Sjoberg M. Estrogen receptor-dependent activation of AP-1 via non-genomic signalling. Nucl Recept. 2004;2(1):3. doi: 10.1186/1478-1336-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ignar-Trowbridge DM, Nelson KG, Bidwell MC, Curtis SW, Washburn TF, McLachlan JA, Korach KS. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc Natl Acad Sci U S A. 1992;89(10):4658–4662. doi: 10.1073/pnas.89.10.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16(8):362–367. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104(5):719–730. [PubMed] [Google Scholar]
- 55.Oviedo PJ, Sobrino A, Laguna-Fernandez A, Novella S, Tarin JJ, Garcia-Perez MA, Sanchis J, Cano A, Hermenegildo C. Estradiol induces endothelial cell migration and proliferation through estrogen receptor-enhanced RhoA/ROCK pathway. Mol Cell Endocrinol. doi: 10.1016/j.mce.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 56.Cho MM, Ziats NP, Pal D, Utian WH, Gorodeski GI. Estrogen modulates paracellular permeability of human endothelial cells by eNOS- and iNOS-related mechanisms. Am J Physiol. 1999;276(2 Pt 1):C337–349. doi: 10.1152/ajpcell.1999.276.2.C337. [DOI] [PubMed] [Google Scholar]
- 57.Cipolla MJ, Godfrey JA, Wiegman MJ. The effect of ovariectomy and estrogen on penetrating brain arterioles and blood-brain barrier permeability. Microcirculation. 2009;16(8):685–693. doi: 10.3109/10739680903164131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hass MA, Nichol P, Lee L, Levin RM. Estrogen modulates permeability and prostaglandin levels in the rabbit urinary bladder. Prostaglandins Leukot Essent Fatty Acids. 2009;80(2–3):125–129. doi: 10.1016/j.plefa.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 59.Sandoval MJ, Cutini PH, Rauschemberger MB, Massheimer VL. The soyabean isoflavone genistein modulates endothelial cell behaviour. Br J Nutr. 104(2):171–179. doi: 10.1017/S0007114510000413. [DOI] [PubMed] [Google Scholar]
- 60.Fu XH, Wang L, Zhao H, Xiang HL, Cao JG. Synthesis of genistein derivatives and determination of their protective effects against vascular endothelial cell damages caused by hydrogen peroxide. Bioorg Med Chem Lett. 2008;18(2):513–517. doi: 10.1016/j.bmcl.2007.11.097. [DOI] [PubMed] [Google Scholar]
- 61.Si H, Liu D. Isoflavone genistein protects human vascular endothelial cells against tumor necrosis factor-alpha-induced apoptosis through the p38beta mitogen-activated protein kinase. Apoptosis. 2009;14(1):66–76. doi: 10.1007/s10495-008-0283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamiyama M, Kishimoto Y, Tani M, Utsunomiya K, Kondo K. Effects of equol on oxidized low-density lipoprotein-induced apoptosis in endothelial cells. J Atheroscler Thromb. 2009;16(3):239–249. doi: 10.5551/jat.1057. [DOI] [PubMed] [Google Scholar]
- 63.Cheng C, Wang X, Weakley SM, Kougias P, Lin PH, Yao Q, Chen C. The soybean isoflavonoid equol blocks ritonavir-induced endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J Nutr. 140(1):12–17. doi: 10.3945/jn.109.110981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ying C, Hsu JT, Shieh SC. Growth inhibition of human endothelial cells by the phyto-oestrogen biochanin A, a metabolite of genistein. Br J Nutr. 2001;85(5):615–620. doi: 10.1079/bjn2000290. [DOI] [PubMed] [Google Scholar]
- 65.Nakajima M, Cooney MJ, Tu AH, Chang KY, Cao J, Ando A, An GJ, Melia M, de Juan E., Jr Normalization of retinal vascular permeability in experimental diabetes with genistein. Invest Ophthalmol Vis Sci. 2001;42(9):2110–2114. [PubMed] [Google Scholar]
- 66.Rubinstein I. Bradykinin- and substance P-induced edema formation in the hamster cheek pouch is tyrosine kinase dependent. J Appl Physiol. 2007;103(1):184–189. doi: 10.1152/japplphysiol.00941.2006. [DOI] [PubMed] [Google Scholar]
- 67.Fujii E, Irie K, Ohba K, Ogawa A, Yoshioka T, Yamakawa M, Muraki T. Role of nitric oxide, prostaglandins and tyrosine kinase in vascular endothelial growth factor-induced increase in vascular permeability in mouse skin. Naunyn Schmiedebergs Arch Pharmacol. 1997;356(4):475–480. doi: 10.1007/pl00005079. [DOI] [PubMed] [Google Scholar]
- 68.Liu D, Jiang H, Grange RW. Genistein activates the 3′,5′-cyclic adenosine monophosphate signaling pathway in vascular endothelial cells and protects endothelial barrier function. Endocrinology. 2005;146(3):1312–1320. doi: 10.1210/en.2004-1221. [DOI] [PubMed] [Google Scholar]
- 69.Kauser K, Rubanyi GM. Gender difference in endothelial dysfunction in the aorta of spontaneously hypertensive rats. Hypertension. 1995;25(4 Pt 1):517–523. doi: 10.1161/01.hyp.25.4.517. [DOI] [PubMed] [Google Scholar]
- 70.Christodoulakos G, Panoulis C, Kouskouni E, Chondros C, Dendrinos S, Creatsas G. Effects of estrogen-progestin and raloxifene therapy on nitric oxide, prostacyclin and endothelin-1 synthesis. Gynecol Endocrinol. 2002;16(1):9–17. doi: 10.1080/gye.16.1.9.17. [DOI] [PubMed] [Google Scholar]
- 71.Hernandez I, Delgado JL, Diaz J, Quesada T, Teruel MJ, Llanos MC, Carbonell LF. 17beta-estradiol prevents oxidative stress and decreases blood pressure in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2000;279(5):R1599–1605. doi: 10.1152/ajpregu.2000.279.5.R1599. [DOI] [PubMed] [Google Scholar]
- 72.Si H, Liu D. Phytochemical genistein in the regulation of vascular function: new insights. Curr Med Chem. 2007;14(24):2581–2589. doi: 10.2174/092986707782023325. [DOI] [PubMed] [Google Scholar]
- 73.Rathel TR, Leikert JF, Vollmar AM, Dirsch VM. The soy isoflavone genistein induces a late but sustained activation of the endothelial nitric oxide-synthase system in vitro. Br J Pharmacol. 2005;144(3):394–399. doi: 10.1038/sj.bjp.0706075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu D, Homan LL, Dillon JS. Genistein acutely stimulates nitric oxide synthesis in vascular endothelial cells by a cyclic adenosine 5′-monophosphate-dependent mechanism. Endocrinology. 2004;145(12):5532–5539. doi: 10.1210/en.2004-0102. [DOI] [PubMed] [Google Scholar]
- 75.Joy S, Siow RC, Rowlands DJ, Becker M, Wyatt AW, Aaronson PI, Coen CW, Kallo I, Jacob R, Mann GE. The isoflavone Equol mediates rapid vascular relaxation: Ca2+-independent activation of endothelial nitric-oxide synthase/Hsp90 involving ERK1/2 and Akt phosphorylation in human endothelial cells. J Biol Chem. 2006;281(37):27335–27345. doi: 10.1074/jbc.M602803200. [DOI] [PubMed] [Google Scholar]
- 76.Siriviriyakul P, Khemapech S, Monsiri K, Patumraj S. The vascular effect of genistein: what is its mechanism, nitric oxide or PGI2? Clin Hemorheol Microcirc. 2006;34(1–2):97–101. [PubMed] [Google Scholar]
- 77.Chanawirat A, Khemapech S, Patumraj S, Siriviriyakul P. Genistein replacement therapy on endothelial dysfunction and bone loss in bilateral ovariectomized rats. Clin Hemorheol Microcirc. 2006;34(1–2):309–314. [PubMed] [Google Scholar]
- 78.Jackman KA, Woodman OL, Chrissobolis S, Sobey CG. Vasorelaxant and antioxidant activity of the isoflavone metabolite equol in carotid and cerebral arteries. Brain Res. 2007;1141:99–107. doi: 10.1016/j.brainres.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 79.Wu JH, Li Q, Wu MY, Guo DJ, Chen HL, Chen SL, Seto SW, Au AL, Poon CC, Leung GP, Lee SM, Kwan YW, Chan SW. Formononetin, an isoflavone, relaxes rat isolated aorta through endothelium-dependent and endothelium-independent pathways. J Nutr Biochem. 21(7):613–620. doi: 10.1016/j.jnutbio.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 80.Grossini E, Molinari C, Mary DA, Uberti F, Caimmi PP, Surico N, Vacca G. Intracoronary genistein acutely increases coronary blood flow in anesthetized pigs through beta-adrenergic mediated nitric oxide release and estrogenic receptors. Endocrinology. 2008;149(5):2678–2687. doi: 10.1210/en.2007-1361. [DOI] [PubMed] [Google Scholar]
- 81.Chalopin M, Tesse A, Martinez MC, Rognan D, Arnal JF, Andriantsitohaina R. Estrogen receptor alpha as a key target of red wine polyphenols action on the endothelium. PLoS One. 5(1):e8554. doi: 10.1371/journal.pone.0008554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simoncini T, Fornari L, Mannella P, Caruso A, Garibaldi S, Baldacci C, Genazzani AR. Activation of nitric oxide synthesis in human endothelial cells by red clover extracts. Menopause. 2005;12(1):69–77. doi: 10.1097/00042192-200512010-00013. [DOI] [PubMed] [Google Scholar]
- 83.Woodman OL, Boujaoude M. Chronic treatment of male rats with daidzein and 17 beta-oestradiol induces the contribution of EDHF to endothelium-dependent relaxation. Br J Pharmacol. 2004;141(2):322–328. doi: 10.1038/sj.bjp.0705603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Palmon SC, Williams MJ, Littleton-Kearney MT, Traystman RJ, Kosk-Kosicka D, Hurn PD. Estrogen increases cGMP in selected brain regions and in cerebral microvessels. J Cereb Blood Flow Metab. 1998;18(11):1248–1252. doi: 10.1097/00004647-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 85.Darkow DJ, Lu L, White RE. Estrogen relaxation of coronary artery smooth muscle is mediated by nitric oxide and cGMP. Am J Physiol. 1997;272(6 Pt 2):H2765–2773. doi: 10.1152/ajpheart.1997.272.6.H2765. [DOI] [PubMed] [Google Scholar]
- 86.El-Mowafy AM, Alkhalaf M, Jaffal SM. Nongenomic activation of the GC-A enzyme by resveratrol and estradiol downstream from membrane estrogen receptors in human coronary arterial cells. Nutr Metab Cardiovasc Dis. 2007;17(7):508–516. doi: 10.1016/j.numecd.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 87.Barber DA, Miller VM. Gender differences in endothelium-dependent relaxations do not involve NO in porcine coronary arteries. Am J Physiol. 1997;273(5 Pt 2):H2325–2332. doi: 10.1152/ajpheart.1997.273.5.H2325. [DOI] [PubMed] [Google Scholar]
- 88.Egan KM, Lawson JA, Fries S, Koller B, Rader DJ, Smyth EM, Fitzgerald GA. COX-2-derived prostacyclin confers atheroprotection on female mice. Science. 2004;306(5703):1954–1957. doi: 10.1126/science.1103333. [DOI] [PubMed] [Google Scholar]
- 89.Garcia-Martinez MC, Hermenegildo C, Tarin JJ, Cano A. Phytoestrogens increase the capacity of serum to stimulate prostacyclin release in human endothelial cells. Acta Obstet Gynecol Scand. 2003;82(8):705–710. doi: 10.1080/j.1600-0412.2003.00178.x. [DOI] [PubMed] [Google Scholar]
- 90.Hermenegildo C, Oviedo PJ, Garcia-Perez MA, Tarin JJ, Cano A. Effects of phytoestrogens genistein and daidzein on prostacyclin production by human endothelial cells. J Pharmacol Exp Ther. 2005;315(2):722–728. doi: 10.1124/jpet.105.090456. [DOI] [PubMed] [Google Scholar]
- 91.Vera R, Galisteo M, Villar IC, Sanchez M, Zarzuelo A, Perez-Vizcaino F, Duarte J. Soy isoflavones improve endothelial function in spontaneously hypertensive rats in an estrogen-independent manner: role of nitric-oxide synthase, superoxide, and cyclooxygenase metabolites. J Pharmacol Exp Ther. 2005;314(3):1300–1309. doi: 10.1124/jpet.105.085530. [DOI] [PubMed] [Google Scholar]
- 92.Fukuyama K, Ichiki T, Ono H, Tokunou T, Iino N, Masuda S, Ohtsubo H, Takeshita A. cAMP-response element-binding protein mediates prostaglandin F2alpha-induced hypertrophy of vascular smooth muscle cells. Biochem Biophys Res Commun. 2005;338(2):910–918. doi: 10.1016/j.bbrc.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 93.Wang X, Klein RD. Prostaglandin E2 induces vascular endothelial growth factor secretion in prostate cancer cells through EP2 receptor-mediated cAMP pathway. Mol Carcinog. 2007;46(11):912–923. doi: 10.1002/mc.20320. [DOI] [PubMed] [Google Scholar]
- 94.Shingo AS, Kito S. Estrogen induces elevation of cAMP-dependent protein kinase activity in immortalized hippocampal neurons: imaging in living cells. J Neural Transm. 2002;109(2):171–174. doi: 10.1007/s007020200012. [DOI] [PubMed] [Google Scholar]
- 95.Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137(5):2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- 96.Lee MY, Leung SW, Vanhoutte PM, Man RY. Genistein reduces agonist-induced contractions of porcine coronary arterial smooth muscle in a cyclic AMP-dependent manner. Eur J Pharmacol. 2004;503(1–3):165–172. doi: 10.1016/j.ejphar.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 97.Satake N, Imanishi M, Keto Y, Yamada H, Ishikawa M, Shibata S. Genistein potentiates the relaxation induced by beta1-and beta2-adrenoceptor activation in rat aortic rings. J Cardiovasc Pharmacol. 2000;35(2):227–233. doi: 10.1097/00005344-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 98.Ng WW, Keung W, Xu YC, Ng KF, Leung GP, Vanhoutte PM, Choy PC, Man RY. Genistein potentiates protein kinase A activity in porcine coronary artery. Mol Cell Biochem. 2008;311(1–2):37–44. doi: 10.1007/s11010-007-9691-3. [DOI] [PubMed] [Google Scholar]
- 99.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23(8):374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 100.Villar IC, Hobbs AJ, Ahluwalia A. Sex differences in vascular function: implication of endothelium-derived hyperpolarizing factor. J Endocrinol. 2008;197(3):447–462. doi: 10.1677/JOE-08-0070. [DOI] [PubMed] [Google Scholar]
- 101.Dhaun N, Pollock DM, Goddard J, Webb DJ. Selective and mixed endothelin receptor antagonism in cardiovascular disease. Trends Pharmacol Sci. 2007;28(11):573–579. doi: 10.1016/j.tips.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 102.Kirkby NS, Hadoke PW, Bagnall AJ, Webb DJ. The endothelin system as a therapeutic target in cardiovascular disease: great expectations or bleak house? Br J Pharmacol. 2008;153(6):1105–1119. doi: 10.1038/sj.bjp.0707516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dubey RK, Jackson EK, Keller PJ, Imthurn B, Rosselli M. Estradiol metabolites inhibit endothelin synthesis by an estrogen receptor-independent mechanism. Hypertension. 2001;37(2 Part 2):640–644. doi: 10.1161/01.hyp.37.2.640. [DOI] [PubMed] [Google Scholar]
- 104.Rodrigo MC, Martin DS, Eyster KM. Vascular ECE-1 mRNA expression decreases in response to estrogens. Life Sci. 2003;73(23):2973–2983. doi: 10.1016/j.lfs.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 105.Cross JC. The genetics of pre-eclampsia: a feto-placental or maternal problem? Clin Genet. 2003;64(2):96–103. doi: 10.1034/j.1399-0004.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- 106.Ling S, Dai A, Williams MR, Husband AJ, Nestel PJ, Komesaroff PA, Sudhir K. The isoflavone metabolite cis-tetrahydrodaidzein inhibits ERK-1 activation and proliferation in human vascular smooth muscle cells. J Cardiovasc Pharmacol. 2004;43(5):622–628. doi: 10.1097/00005344-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 107.Kim H, Lee MJ, Kim JE, Park SD, Moon HI, Park WH. Genistein suppresses tumor necrosis factor-alpha-induced proliferation via the apoptotic signaling pathway in human aortic smooth muscle cells. J Agric Food Chem. 58(3):2015–2019. doi: 10.1021/jf903802v. [DOI] [PubMed] [Google Scholar]
- 108.Finking G, Wohlfrom M, Lenz C, Wolkenhauer M, Eberle C, Hanke H. The phytoestrogens Genistein and Daidzein, and 17 beta-estradiol inhibit development of neointima in aortas from male and female rabbits in vitro after injury. Coron Artery Dis. 1999;10(8):607–615. doi: 10.1097/00019501-199912000-00010. [DOI] [PubMed] [Google Scholar]
- 109.Pan W, Ikeda K, Takebe M, Yamori Y. Genistein, daidzein and glycitein inhibit growth and DNA synthesis of aortic smooth muscle cells from stroke-prone spontaneously hypertensive rats. J Nutr. 2001;131(4):1154–1158. doi: 10.1093/jn/131.4.1154. [DOI] [PubMed] [Google Scholar]
- 110.Shen J, White M, Husband AJ, Hambly BD, Bao S. Phytoestrogen derivatives differentially inhibit arterial neointimal proliferation in a mouse model. Eur J Pharmacol. 2006;548(1–3):123–128. doi: 10.1016/j.ejphar.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 111.Yu JY, Lee JJ, Lim Y, Kim TJ, Jin YR, Sheen YY, Yun YP. Genistein inhibits rat aortic smooth muscle cell proliferation through the induction of p27kip1. J Pharmacol Sci. 2008;107(1):90–98. doi: 10.1254/jphs.08001fp. [DOI] [PubMed] [Google Scholar]
- 112.Liu S, Wang L, Wang W, Lin J, Han J, Sun H, Guo H, Sun R, Wu Q. TSC-36/FRP inhibits vascular smooth muscle cell proliferation and migration. Exp Mol Pathol. 2006;80(2):132–140. doi: 10.1016/j.yexmp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 113.Crews JK, Khalil RA. Antagonistic effects of 17 beta-estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arterioscler Thromb Vasc Biol. 1999;19(4):1034–1040. doi: 10.1161/01.atv.19.4.1034. [DOI] [PubMed] [Google Scholar]
- 114.Dean SA, Tan J, O’Brien ER, Leenen FH. 17beta-estradiol downregulates tissue angiotensin-converting enzyme and ANG II type 1 receptor in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;288(3):R759–766. doi: 10.1152/ajpregu.00595.2004. [DOI] [PubMed] [Google Scholar]
- 115.Xu YY, Yang C, Li SN. Effects of genistein on angiotensin-converting enzyme in rats. Life Sci. 2006;79(9):828–837. doi: 10.1016/j.lfs.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 116.Montenegro MF, Pessa LR, Tanus-Santos JE. Isoflavone genistein inhibits the angiotensin-converting enzyme and alters the vascular responses to angiotensin I and bradykinin. Eur J Pharmacol. 2009;607(1–3):173–177. doi: 10.1016/j.ejphar.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 117.Murphy JG, Khalil RA. Decreased [Ca(2+)](i) during inhibition of coronary smooth muscle contraction by 17beta-estradiol, progesterone, and testosterone. J Pharmacol Exp Ther. 1999;291(1):44–52. [PubMed] [Google Scholar]
- 118.Ullrich ND, Koschak A, MacLeod KT. Oestrogen directly inhibits the cardiovascular L-type Ca2+ channel Cav1.2. Biochem Biophys Res Commun. 2007;361(2):522–527. doi: 10.1016/j.bbrc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 119.Prakash YS, Togaibayeva AA, Kannan MS, Miller VM, Fitzpatrick LA, Sieck GC. Estrogen increases Ca2+ efflux from female porcine coronary arterial smooth muscle. Am J Physiol. 1999;276(3 Pt 2):H926–934. doi: 10.1152/ajpheart.1999.276.3.H926. [DOI] [PubMed] [Google Scholar]
- 120.Speroni F, Rebolledo A, Salemme S, Roldan-Palomo R, Rimorini L, Anon MC, Spinillo A, Tanzi F, Milesi V. Genistein effects on Ca2+ handling in human umbilical artery: inhibition of sarcoplasmic reticulum Ca2+ release and of voltage-operated Ca2+ channels. J Physiol Biochem. 2009;65(2):113–124. doi: 10.1007/BF03179062. [DOI] [PubMed] [Google Scholar]
- 121.Gould EM, Rembold CM, Murphy RA. Genistein, a tyrosine kinase inhibitor, reduces Ca2+ mobilization in swine carotid media. Am J Physiol. 1995;268(6 Pt 1):C1425–1429. doi: 10.1152/ajpcell.1995.268.6.C1425. [DOI] [PubMed] [Google Scholar]
- 122.Torregrosa G, Burguete MC, Perez-Asensio FJ, Salom JB, Gil JV, Alborch E. Pharmacological profile of phytoestrogens in cerebral vessels: in vitro study with rabbit basilar artery. Eur J Pharmacol. 2003;482(1–3):227–234. doi: 10.1016/j.ejphar.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 123.Li HF, Zhang P, Tian ZF, Qiu XQ, Zhang YF, Wu JX, Jia ZJ. Differential mechanisms involved in effects of genistein and 17-beta-estradiol on porcine coronary arteries. Pharmazie. 2006;61(5):461–465. [PubMed] [Google Scholar]
- 124.Nelson SR, Chien T, Di Salvo J. Genistein sensitivity of calcium transport pathways in serotonin-activated vascular smooth musclecells. Arch Biochem Biophys. 1997;345(1):65–72. doi: 10.1006/abbi.1997.0247. [DOI] [PubMed] [Google Scholar]
- 125.Abebe W, Agrawal DK. Role of tyrosine kinases in norepinephrine-induced contraction of vascular smooth muscle. J Cardiovasc Pharmacol. 1995;26(1):153–159. doi: 10.1097/00005344-199507000-00024. [DOI] [PubMed] [Google Scholar]
- 126.Sato A, Hattori Y, Kanno M. Effects of genistein and daidzein on enhanced vascular contractile reactivity and Ca2+ sensitivity in cardiomyopathic hamsters. Methods Find Exp Clin Pharmacol. 2000;22(1):25–30. doi: 10.1358/mf.2000.22.1.795816. [DOI] [PubMed] [Google Scholar]
- 127.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268(4 Pt 1):C799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 128.Han G, Yu X, Lu L, Li S, Ma H, Zhu S, Cui X, White RE. Estrogen receptor alpha mediates acute potassium channel stimulation in human coronary artery smooth muscle cells. J Pharmacol Exp Ther. 2006;316(3):1025–1030. doi: 10.1124/jpet.105.093542. [DOI] [PubMed] [Google Scholar]
- 129.Zhang HT, Wang Y, Deng XL, Dong MQ, Zhao LM, Wang YW. Daidzein relaxes rat cerebral basilar artery via activation of large-conductance Ca2+-activated K+ channels in vascular smooth muscle cells. Eur J Pharmacol. 630(1–3):100–106. doi: 10.1016/j.ejphar.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 130.Nevala R, Paukku K, Korpela R, Vapaatalo H. Calcium-sensitive potassium channel inhibitors antagonize genistein- and daidzein-induced arterial relaxation in vitro. Life Sci. 2001;69(12):1407–1417. doi: 10.1016/s0024-3205(01)01233-4. [DOI] [PubMed] [Google Scholar]
- 131.Speroni F, Rebolledo A, Salemme S, Anon MC, Tanzi F, Milesi V. Genistein inhibits contractile force, intracellular Ca2+ increase and Ca2+ oscillations induced by serotonin in rat aortic smooth muscle. J Physiol Biochem. 2007;63(2):143–151. doi: 10.1007/BF03168225. [DOI] [PubMed] [Google Scholar]
- 132.Ogata R, Kitamura K, Ito Y, Nakano H. Inhibitory effects of genistein on ATP-sensitive K+ channels in rabbit portal vein smooth muscle. Br J Pharmacol. 1997;122(7):1395–1404. doi: 10.1038/sj.bjp.0701532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ko EA, Park WS, Son YK, Kim do H, Kim N, Kim HK, Choi TH, Jung ID, Park YM, Han J. The effect of tyrosine kinase inhibitor genistein on voltage-dependent K+ channels in rabbit coronary arterial smooth muscle cells. Vascul Pharmacol. 2009;50(1–2):51–56. doi: 10.1016/j.vph.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 134.Nevala R, Lassila M, Finckenberg P, Paukku K, Korpela R, Vapaatalo H. Genistein treatment reduces arterial contractions by inhibiting tyrosine kinases in ovariectomized hypertensive rats. Eur J Pharmacol. 2002;452(1):87–96. doi: 10.1016/s0014-2999(02)02270-7. [DOI] [PubMed] [Google Scholar]
- 135.Liu H, Li K, Sperelakis N. Tyrosine kinase inhibitor, genistein, inhibits macroscopic L-type calcium current in rat portal vein smooth muscle cells. Can J Physiol Pharmacol. 1997;75(9):1058–1062. doi: 10.1139/cjpp-75-9-1058. [DOI] [PubMed] [Google Scholar]
- 136.Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005;25(9):1767–1775. doi: 10.1161/01.ATV.0000176193.83629.c8. [DOI] [PubMed] [Google Scholar]
- 137.Zhou Q, Liao JK. Rho kinase: an important mediator of atherosclerosis and vascular disease. Curr Pharm Des. 2009;15(27):3108–3115. doi: 10.2174/138161209789057986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hiroki J, Shimokawa H, Mukai Y, Ichiki T, Takeshita A. Divergent effects of estrogen and nicotine on Rho-kinase expression in human coronary vascular smooth muscle cells. Biochem Biophys Res Commun. 2005;326(1):154–159. doi: 10.1016/j.bbrc.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 139.Chrissobolis S, Budzyn K, Marley PD, Sobey CG. Evidence that estrogen suppresses rho-kinase function in the cerebral circulation in vivo. Stroke. 2004;35(9):2200–2205. doi: 10.1161/01.STR.0000136951.85586.c8. [DOI] [PubMed] [Google Scholar]
- 140.Seok YM, Baek I, Kim YH, Jeong YS, Lee IJ, Shin DH, Hwang YH, Kim IK. Isoflavone attenuates vascular contraction through inhibition of the RhoA/Rho-kinase signaling pathway. J Pharmacol Exp Ther. 2008;326(3):991–998. doi: 10.1124/jpet.108.138529. [DOI] [PubMed] [Google Scholar]
- 141.Salamanca DA, Khalil RA. Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol. 2005;70(11):1537–1547. doi: 10.1016/j.bcp.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kang JH, Niidome T, Katayama Y. Role of estrogenic compounds (diethylstibestrol, 17beta-estradiol, and bisphenol A) in the phosphorylation of substrate by protein kinase Calpha. J Biochem Mol Toxicol. 2009;23(5):318–323. doi: 10.1002/jbt.20294. [DOI] [PubMed] [Google Scholar]
- 143.Kanashiro CA, Khalil RA. Gender-related distinctions in protein kinase C activity in rat vascular smooth muscle. Am J Physiol Cell Physiol. 2001;280(1):C34–45. doi: 10.1152/ajpcell.2001.280.1.C34. [DOI] [PubMed] [Google Scholar]
- 144.Park J, Chung SW, Kim SH, Kim TS. Up-regulation of interleukin-4 production via NF-AT/AP-1 activation in T cells by biochanin A, a phytoestrogen and its metabolites. Toxicol Appl Pharmacol. 2006;212(3):188–199. doi: 10.1016/j.taap.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 145.Pearson LL, Castle BE, Kehry MR. CD40-mediated signaling in monocytic cells: up-regulation of tumor necrosis factor receptor-associated factor mRNAs and activation of mitogen-activated protein kinase signaling pathways. Int Immunol. 2001;13(3):273–283. doi: 10.1093/intimm/13.3.273. [DOI] [PubMed] [Google Scholar]
- 146.Suzuki T, Yu HP, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. Mitogen activated protein kinase (MAPK) mediates non-genomic pathway of estrogen on T cell cytokine production following trauma-hemorrhage. Cytokine. 2008;42(1):32–38. doi: 10.1016/j.cyto.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dubey RK, Gillespie DG, Imthurn B, Rosselli M, Jackson EK, Keller PJ. Phytoestrogens inhibit growth and MAP kinase activity in human aortic smooth muscle cells. Hypertension. 1999;33(1 Pt 2):177–182. doi: 10.1161/01.hyp.33.1.177. [DOI] [PubMed] [Google Scholar]
- 148.Kang OH, Jang HJ, Chae HS, Oh YC, Choi JG, Lee YS, Kim JH, Kim YC, Sohn DH, Park H, Kwon DY. Anti-inflammatory mechanisms of resveratrol in activated HMC-1 cells: pivotal roles of NF-kappaB and MAPK. Pharmacol Res. 2009;59(5):330–337. doi: 10.1016/j.phrs.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 149.Vincent A, Ruan M, Fitzpatrick LA. Gender differences in the effect of genistein on vascular smooth muscle cells: a possible cardioprotective effect? J Gend Specif Med. 2001;4(1):28–34. [PubMed] [Google Scholar]
- 150.Simoncini T, Scorticati C, Mannella P, Fadiel A, Giretti MS, Fu XD, Baldacci C, Garibaldi S, Caruso A, Fornari L, Naftolin F, Genazzani AR. Estrogen receptor alpha interacts with Galpha13 to drive actin remodeling and endothelial cell migration via the RhoA/Rho kinase/moesin pathway. Mol Endocrinol. 2006;20(8):1756–1771. doi: 10.1210/me.2005-0259. [DOI] [PubMed] [Google Scholar]
- 151.Rekhter MD. Collagen synthesis in atherosclerosis: too much and not enough. Cardiovasc Res. 1999;41(2):376–384. doi: 10.1016/s0008-6363(98)00321-6. [DOI] [PubMed] [Google Scholar]
- 152.Cao C, Li S, Dai X, Chen Y, Feng Z, Zhao Y, Wu J. Genistein inhibits proliferation and functions of hypertrophic scar fibroblasts. Burns. 2009;35(1):89–97. doi: 10.1016/j.burns.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 153.Yuan WJ, Jia FY, Meng JZ. Effects of genistein on secretion of extracellular matrix components and transforming growth factor beta in high-glucose-cultured rat mesangial cells. J Artif Organs. 2009;12(4):242–246. doi: 10.1007/s10047-009-0479-y. [DOI] [PubMed] [Google Scholar]
- 154.Chen Z, Seo JY, Kim YK, Lee SR, Kim KH, Cho KH, Eun HC, Chung JH. Heat modulation of tropoelastin, fibrillin-1, and matrix metalloproteinase-12 in human skin in vivo. J Invest Dermatol. 2005;124(1):70–78. doi: 10.1111/j.0022-202X.2004.23550.x. [DOI] [PubMed] [Google Scholar]
- 155.Grandas OH, Mountain DH, Kirkpatrick SS, Cassada DC, Stevens SL, Freeman MB, Goldman MH. Regulation of vascular smooth muscle cell expression and function of matrix metalloproteinases is mediated by estrogen and progesterone exposure. J Vasc Surg. 2009;49(1):185–191. doi: 10.1016/j.jvs.2008.07.080. [DOI] [PubMed] [Google Scholar]
- 156.Russo LA, Peano BJ, Trivedi SP, Cavalcanto TD, Olenchock BA, Caruso JA, Smolock AR, Vishnevsky O, Gardner RM. Regulated expression of matrix metalloproteinases, inflammatory mediators, and endometrial matrix remodeling by 17beta-estradiol in the immature rat uterus. Reprod Biol Endocrinol. 2009;7:124. doi: 10.1186/1477-7827-7-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lekontseva O, Jiang Y, Davidge ST. Estrogen replacement increases matrix metalloproteinase contribution to vasoconstriction in a rat model of menopause. J Hypertens. 2009;27(8):1602–1608. doi: 10.1097/HJH.0b013e32832c41b5. [DOI] [PubMed] [Google Scholar]
- 158.Lee CS, Kwon SJ, Na SY, Lim SP, Lee JH. Genistein supplementation inhibits atherosclerosis with stabilization of the lesions in hypercholesterolemic rabbits. J Korean Med Sci. 2004;19(5):656–661. doi: 10.3346/jkms.2004.19.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Suh SJ, Kim JR, Jin UH, Choi HS, Chang YC, Lee YC, Kim SH, Lee IS, Moon TC, Chang HW, Kim CH. Deoxypodophyllotoxin, flavolignan, from Anthriscus sylvestris Hoffm. inhibits migration and MMP-9 via MAPK pathways in TNF-alpha-induced HASMC. Vascul Pharmacol. 2009;51(1):13–20. doi: 10.1016/j.vph.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 160.Puli S, Lai JC, Bhushan A. Inhibition of matrix degrading enzymes and invasion in human glioblastoma (U87MG) cells by isoflavones. J Neurooncol. 2006;79(2):135–142. doi: 10.1007/s11060-006-9126-0. [DOI] [PubMed] [Google Scholar]
- 161.Kousidou OC, Mitropoulou TN, Roussidis AE, Kletsas D, Theocharis AD, Karamanos NK. Genistein suppresses the invasive potential of human breast cancer cells through transcriptional regulation of metalloproteinases and their tissue inhibitors. Int J Oncol. 2005;26(4):1101–1109. doi: 10.3892/ijo.26.4.1101. [DOI] [PubMed] [Google Scholar]
- 162.Huang X, Chen S, Xu L, Liu Y, Deb DK, Platanias LC, Bergan RC. Genistein inhibits p38 map kinase activation, matrix metalloproteinase type 2, and cell invasion in human prostate epithelial cells. Cancer Res. 2005;65(8):3470–3478. doi: 10.1158/0008-5472.CAN-04-2807. [DOI] [PubMed] [Google Scholar]
- 163.Yan C, Han R. Genistein suppresses adhesion-induced protein tyrosine phosphorylation and invasion of B16-BL6 melanoma cells. Cancer Lett. 1998;129(1):117–124. doi: 10.1016/s0304-3835(98)00093-7. [DOI] [PubMed] [Google Scholar]
- 164.Haines C, James A, Sahota D, Chen ZY, Panesar N, Tomlinson B, Chow L, Benzie I, Husband A. Comparison between phytoestrogens and estradiol in the prevention of atheroma in ovariectomized cholesterol-fed rabbits. Climacteric. 2006;9(6):430–436. doi: 10.1080/13697130600863266. [DOI] [PubMed] [Google Scholar]
- 165.Nikitina NA, Sobenin IA, Myasoedova VA, Korennaya VV, Mel’nichenko AA, Khalilov EM, Orekhov AN. Antiatherogenic effect of grape flavonoids in an ex vivo model. Bull Exp Biol Med. 2006;141(6):712–715. doi: 10.1007/s10517-006-0260-7. [DOI] [PubMed] [Google Scholar]
- 166.Fuchs D, de Pascual-Teresa S, Rimbach G, Virgili F, Ambra R, Turner R, Daniel H, Wenzel U. Proteome analysis for identification of target proteins of genistein in primary human endothelial cells stressed with oxidized LDL or homocysteine. Eur J Nutr. 2005;44(2):95–104. doi: 10.1007/s00394-004-0499-6. [DOI] [PubMed] [Google Scholar]
- 167.Honore EK, Williams JK, Anthony MS, Clarkson TB. Soy isoflavones enhance coronary vascular reactivity in atherosclerotic female macaques. Fertil Steril. 1997;67(1):148–154. doi: 10.1016/s0015-0282(97)81872-9. [DOI] [PubMed] [Google Scholar]
- 168.Zhao H, Li C, Cao JG, Xiang HL, Yang HZ, You JL, Li CL, Fu XH. 7-Difluoromethyl-5,4′-dimethoxygenistein, a novel genistein derivative, has therapeutic effects on atherosclerosis in a rabbit model. J Cardiovasc Pharmacol. 2009;54(5):412–420. doi: 10.1097/FJC.0b013e3181bad280. [DOI] [PubMed] [Google Scholar]
- 169.Wang Z, Zou J, Cao K, Hsieh TC, Huang Y, Wu JM. Dealcoholized red wine containing known amounts of resveratrol suppresses atherosclerosis in hypercholesterolemic rabbits without affecting plasma lipid levels. Int J Mol Med. 2005;16(4):533–540. [PubMed] [Google Scholar]
- 170.Zou J, Huang Y, Cao K, Yang G, Yin H, Len J, Hsieh TC, Wu JM. Effect of resveratrol on intimal hyperplasia after endothelial denudation in an experimental rabbit model. Life Sci. 2000;68(2):153–163. doi: 10.1016/s0024-3205(00)00925-5. [DOI] [PubMed] [Google Scholar]
- 171.Olas B, Wachowicz B. Resveratrol, a phenolic antioxidant with effects on blood platelet functions. Platelets. 2005;16(5):251–260. doi: 10.1080/09537100400020591. [DOI] [PubMed] [Google Scholar]
- 172.Brito P, Almeida LM, Dinis TC. The interaction of resveratrol with ferrylmyoglobin and peroxynitrite; protection against LDL oxidation. Free Radic Res. 2002;36(6):621–631. doi: 10.1080/10715760290029083. [DOI] [PubMed] [Google Scholar]
- 173.Nakano Y, Oshima T, Ozono R, Ueda A, Oue Y, Matsuura H, Sanada M, Ohama K, Chayama K, Kambe M. Estrogen replacement suppresses function of thrombin stimulated platelets by inhibiting Ca(2+) influx and raising cyclic adenosine monophosphate. Cardiovasc Res. 2002;53(3):634–641. doi: 10.1016/s0008-6363(01)00410-2. [DOI] [PubMed] [Google Scholar]
- 174.Moro L, Reineri S, Piranda D, Pietrapiana D, Lova P, Bertoni A, Graziani A, Defilippi P, Canobbio I, Torti M, Sinigaglia F. Nongenomic effects of 17beta-estradiol in human platelets: potentiation of thrombin-induced aggregation through estrogen receptor beta and Src kinase. Blood. 2005;105(1):115–121. doi: 10.1182/blood-2003-11-3840. [DOI] [PubMed] [Google Scholar]
- 175.Wilcox JN, Blumenthal BF. Thrombotic mechanisms in atherosclerosis: potential impact of soy proteins. J Nutr. 1995;125(3 Suppl):631S–638S. doi: 10.1093/jn/125.suppl_3.631S. [DOI] [PubMed] [Google Scholar]
- 176.Kondo K, Suzuki Y, Ikeda Y, Umemura K. Genistein, an isoflavone included in soy, inhibits thrombotic vessel occlusion in the mouse femoral artery and in vitro platelet aggregation. Eur J Pharmacol. 2002;455(1):53–57. doi: 10.1016/s0014-2999(02)02449-4. [DOI] [PubMed] [Google Scholar]
- 177.Schoene NW, Guidry CA. Dietary soy isoflavones inhibit activation of rat platelets. J Nutr Biochem. 1999;10(7):421–426. doi: 10.1016/s0955-2863(99)00023-6. [DOI] [PubMed] [Google Scholar]
- 178.Polini N, Rauschemberger MB, Mendiberri J, Selles J, Massheimer V. Effect of genistein and raloxifene on vascular dependent platelet aggregation. Mol Cell Endocrinol. 2007;267(1–2):55–62. doi: 10.1016/j.mce.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 179.Guerrero JA, Lozano ML, Castillo J, Benavente-Garcia O, Vicente V, Rivera J. Flavonoids inhibit platelet function through binding to the thromboxane A2 receptor. J Thromb Haemost. 2005;3(2):369–376. doi: 10.1111/j.1538-7836.2004.01099.x. [DOI] [PubMed] [Google Scholar]
- 180.Stef G, Csiszar A, Lerea K, Ungvari Z, Veress G. Resveratrol inhibits aggregation of platelets from high-risk cardiac patients with aspirin resistance. J Cardiovasc Pharmacol. 2006;48(2):1–5. doi: 10.1097/01.fjc.0000238592.67191.ab. [DOI] [PubMed] [Google Scholar]
- 181.Fait T, Vrablik M, Zizka Z, Trnkova B, Masata J, Zivny J. Effect of early onset of transdermal and oral estrogen therapy on the lipid profile: a prospective study with cross-over design. Ceska Gynekol. 2006;71(3):226–230. [PubMed] [Google Scholar]
- 182.Guo Y, Wu G, Su X, Yang H, Zhang J. Antiobesity action of a daidzein derivative on male obese mice induced by a high-fat diet. Nutr Res. 2009;29(9):656–663. doi: 10.1016/j.nutres.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 183.Kapiotis S, Hermann M, Held I, Seelos C, Ehringer H, Gmeiner BM. Genistein, the dietary-derived angiogenesis inhibitor, prevents LDL oxidation and protects endothelial cells from damage by atherogenic LDL. Arterioscler Thromb Vasc Biol. 1997;17(11):2868–2874. doi: 10.1161/01.atv.17.11.2868. [DOI] [PubMed] [Google Scholar]
- 184.Bassett CM, Rodriguez-Leyva D, Pierce GN. Experimental and clinical research findings on the cardiovascular benefits of consuming flaxseed. Appl Physiol Nutr Metab. 2009;34(5):965–974. doi: 10.1139/H09-087. [DOI] [PubMed] [Google Scholar]
- 185.Fukumitsu S, Aida K, Ueno N, Ozawa S, Takahashi Y, Kobori M. Flaxseed lignan attenuates high-fat diet-induced fat accumulation and induces adiponectin expression in mice. Br J Nutr. 2008;100(3):669–676. doi: 10.1017/S0007114508911570. [DOI] [PubMed] [Google Scholar]
- 186.Relic B, Zeddou M, Desoroux A, Beguin Y, de Seny D, Malaise MG. Genistein induces adipogenesis but inhibits leptin induction in human synovial fibroblasts. Lab Invest. 2009;89(7):811–822. doi: 10.1038/labinvest.2009.41. [DOI] [PubMed] [Google Scholar]
- 187.Inoue M, Ishida T, Yasuda T, Toh R, Hara T, Cangara HM, Rikitake Y, Taira K, Sun L, Kundu RK, Quertermous T, Hirata K. Endothelial cell-selective adhesion molecule modulates atherosclerosis through plaque angiogenesis and monocyte-endothelial interaction. Microvasc Res. 80(2):179–187. doi: 10.1016/j.mvr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 188.Aberdeen GW, Wiegand SJ, Bonagura TW, Jr, Pepe GJ, Albrecht ED. Vascular endothelial growth factor mediates the estrogen-induced breakdown of tight junctions between and increase in proliferation of microvessel endothelial cells in the baboon endometrium. Endocrinology. 2008;149(12):6076–6083. doi: 10.1210/en.2008-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Groten T, Pierce AA, Huen AC, Schnaper HW. 17 beta-estradiol transiently disrupts adherens junctions in endothelial cells. FASEB J. 2005;19(10):1368–1370. doi: 10.1096/fj.04-2558fje. [DOI] [PubMed] [Google Scholar]
- 190.Huh JE, Kwon NH, Baek YH, Lee JD, Choi DY, Jingushi S, Kim KI, Park DS. Formononetin promotes early fracture healing through stimulating angiogenesis by up-regulating VEGFR-2/Flk-1 in a rat fracture model. Int Immunopharmacol. 2009;9(12):1357–1365. doi: 10.1016/j.intimp.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 191.Sasamura H, Takahashi A, Yuan J, Kitamura H, Masumori N, Miyao N, Itoh N, Tsukamoto T. Antiproliferative and antiangiogenic activities of genistein in human renal cell carcinoma. Urology. 2004;64(2):389–393. doi: 10.1016/j.urology.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 192.Ambra R, Rimbach G, de Pascual Teresa S, Fuchs D, Wenzel U, Daniel H, Virgili F. Genistein affects the expression of genes involved in blood pressure regulation and angiogenesis in primary human endothelial cells. Nutr Metab Cardiovasc Dis. 2006;16(1):35–43. doi: 10.1016/j.numecd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 193.Piao M, Mori D, Satoh T, Sugita Y, Tokunaga O. Inhibition of endothelial cell proliferation, in vitro angiogenesis, and the down-regulation of cell adhesion-related genes by genistein. Combined with a cDNA microarray analysis. Endothelium. 2006;13(4):249–266. doi: 10.1080/10623320600903940. [DOI] [PubMed] [Google Scholar]
- 194.Su SJ, Yeh TM, Chuang WJ, Ho CL, Chang KL, Cheng HL, Liu HS, Hsu PY, Chow NH. The novel targets for anti-angiogenesis of genistein on human cancer cells. Biochem Pharmacol. 2005;69(2):307–318. doi: 10.1016/j.bcp.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 195.Bergman Jungestrom M, Thompson LU, Dabrosin C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin Cancer Res. 2007;13(3):1061–1067. doi: 10.1158/1078-0432.CCR-06-1651. [DOI] [PubMed] [Google Scholar]
- 196.Gao H, Liang M, Bergdahl A, Hamren A, Lindholm MW, Dahlman-Wright K, Nilsson BO. Estrogen attenuates vascular expression of inflammation associated genes and adhesion of monocytes to endothelial cells. Inflamm Res. 2006;55(8):349–353. doi: 10.1007/s00011-006-5194-z. [DOI] [PubMed] [Google Scholar]
- 197.Geraldes P, Gagnon S, Hadjadj S, Merhi Y, Sirois MG, Cloutier I, Tanguay JF. Estradiol blocks the induction of CD40 and CD40L expression on endothelial cells and prevents neutrophil adhesion: an ERalpha-mediated pathway. Cardiovasc Res. 2006;71(3):566–573. doi: 10.1016/j.cardiores.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 198.Xu Y, Arenas IA, Armstrong SJ, Plahta WC, Xu H, Davidge ST. Estrogen improves cardiac recovery after ischemia/reperfusion by decreasing tumor necrosis factor-alpha. Cardiovasc Res. 2006;69(4):836–844. doi: 10.1016/j.cardiores.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 199.Dharmappa KK, Mohamed R, Shivaprasad HV, Vishwanath BS. Genistein, a potent inhibitor of secretory phospholipase A2: a new insight in down regulation of inflammation. Inflammopharmacology. 18(1):25–31. doi: 10.1007/s10787-009-0018-8. [DOI] [PubMed] [Google Scholar]
- 200.Trompezinski S, Denis A, Schmitt D, Viac J. Comparative effects of polyphenols from green tea (EGCG) and soybean (genistein) on VEGF and IL-8 release from normal human keratinocytes stimulated with the proinflammatory cytokine TNFalpha. Arch Dermatol Res. 2003;295(3):112–116. doi: 10.1007/s00403-003-0402-y. [DOI] [PubMed] [Google Scholar]
- 201.Gottstein N, Ewins BA, Eccleston C, Hubbard GP, Kavanagh IC, Minihane AM, Weinberg PD, Rimbach G. Effect of genistein and daidzein on platelet aggregation and monocyte and endothelial function. Br J Nutr. 2003;89(5):607–616. doi: 10.1079/BJN2003820. [DOI] [PubMed] [Google Scholar]
- 202.Lee YW, Lee WH. Protective effects of genistein on proinflammatory pathways in human brain microvascular endothelial cells. J Nutr Biochem. 2008;19(12):819–825. doi: 10.1016/j.jnutbio.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 203.van Hinsbergh VW, Vermeer M, Koolwijk P, Grimbergen J, Kooistra T. Genistein reduces tumor necrosis factor alpha-induced plasminogen activator inhibitor-1 transcription but not urokinase expression in human endothelial cells. Blood. 1994;84(9):2984–2991. [PubMed] [Google Scholar]
- 204.Schrepfer S, Deuse T, Schafer H, Koch-Nolte F, Braendle W, Reichenspurner H. The phytoestrogen biochaninA weakens acute cardiac allograft rejection without affecting the reproductive system. Transpl Immunol. 2005;15(1):45–53. doi: 10.1016/j.trim.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 205.Khanna D, Sethi G, Ahn KS, Pandey MK, Kunnumakkara AB, Sung B, Aggarwal A, Aggarwal BB. Natural products as a gold mine for arthritis treatment. Curr Opin Pharmacol. 2007;7(3):344–351. doi: 10.1016/j.coph.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 206.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 302(2):71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kalhan R, Smith LJ, Nlend MC, Nair A, Hixon JL, Sporn PH. A mechanism of benefit of soy genistein in asthma: inhibition of eosinophil p38-dependent leukotriene synthesis. Clin Exp Allergy. 2008;38(1):103–112. doi: 10.1111/j.1365-2222.2007.02862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cignarella A, Minici C, Bolego C, Pinna C, Sanvito P, Gaion RM, Puglisi L. Potential pro-inflammatory action of resveratrol in vascular smooth muscle cells from normal and diabetic rats. Nutr Metab Cardiovasc Dis. 2006;16(5):322–329. doi: 10.1016/j.numecd.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 209.Wing LY, Chen YC, Shih YY, Cheng JC, Lin YJ, Jiang MJ. Effects of oral estrogen on aortic ROS-generating and -scavenging enzymes and atherosclerosis in apoE-deficient mice. Exp Biol Med (Maywood) 2009;234(9):1037–1046. doi: 10.3181/0811-RM-332. [DOI] [PubMed] [Google Scholar]
- 210.Patel RP, Crawford J, Boersma B, Barnes S, Darley-Usmar VM. Antioxidant properties of phytoestrogens. J Med Food. 1999;2(3–4):163–166. doi: 10.1089/jmf.1999.2.163. [DOI] [PubMed] [Google Scholar]
- 211.Exner M, Hermann M, Hofbauer R, Kapiotis S, Quehenberger P, Speiser W, Held I, Gmeiner BM. Genistein prevents the glucose autoxidation mediated atherogenic modification of low density lipoprotein. Free Radic Res. 2001;34(1):101–112. doi: 10.1080/10715760100300101. [DOI] [PubMed] [Google Scholar]
- 212.Xu SZ, Zhong W, Ghavideldarestani M, Saurabh R, Lindow SW, Atkin SL. Multiple mechanisms of soy isoflavones against oxidative stress-induced endothelium injury. Free Radic Biol Med. 2009;47(2):167–175. doi: 10.1016/j.freeradbiomed.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 213.Hwang J, Hodis HN, Sevanian A. Soy and alfalfa phytoestrogen extracts become potent low-density lipoprotein antioxidants in the presence of acerola cherry extract. J Agric Food Chem. 2001;49(1):308–314. doi: 10.1021/jf0007028. [DOI] [PubMed] [Google Scholar]
- 214.Zhang P, Li HF, Tian ZF, Qiu XQ, Wu JX, Jia ZJ. Effects of phytoestrogens and 17beta-estradiol on vasoconstriction elicited by reactive oxygen species. Pharmazie. 2007;62(5):378–381. [PubMed] [Google Scholar]
- 215.Mizutani K, Ikeda K, Nishikata T, Yamori Y. Phytoestrogens attenuate oxidative DNA damage in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. J Hypertens. 2000;18(12):1833–1840. doi: 10.1097/00004872-200018120-00018. [DOI] [PubMed] [Google Scholar]
- 216.Chung JE, Kim SY, Jo HH, Hwang SJ, Chae B, Kwon DJ, Lew YO, Lim YT, Kim JH, Kim EJ, Kim MR. Antioxidant effects of equol on bovine aortic endothelial cells. Biochem Biophys Res Commun. 2008;375(3):420–424. doi: 10.1016/j.bbrc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 217.Labinskyy N, Csiszar A, Orosz Z, Smith K, Rivera A, Buffenstein R, Ungvari Z. Comparison of endothelial function, O2-* and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol Heart Circ Physiol. 2006;291(6):H2698–2704. doi: 10.1152/ajpheart.00534.2006. [DOI] [PubMed] [Google Scholar]
- 218.Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab. 2001;86(1):41–47. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- 219.Cornwell T, Cohick W, Raskin I. Dietary phytoestrogens and health. Phytochemistry. 2004;65(8):995–1016. doi: 10.1016/j.phytochem.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 220.Mazur W. Phytoestrogen content in foods. Baillieres Clin Endocrinol Metab. 1998;12(4):729–742. doi: 10.1016/s0950-351x(98)80013-x. [DOI] [PubMed] [Google Scholar]
- 221.Chan YH, Lau KK, Yiu KH, Li SW, Chan HT, Tam S, Shu XO, Lau CP, Tse HF. Isoflavone intake in persons at high risk of cardiovascular events: implications for vascular endothelial function and the carotid atherosclerotic burden. Am J Clin Nutr. 2007;86(4):938–945. doi: 10.1093/ajcn/86.4.938. [DOI] [PubMed] [Google Scholar]
- 222.Squadrito F, Altavilla D, Crisafulli A, Saitta A, Cucinotta D, Morabito N, D’Anna R, Corrado F, Ruggeri P, Frisina N, Squadrito G. Effect of genistein on endothelial function in postmenopausal women: a randomized, double-blind, controlled study. Am J Med. 2003;114(6):470–476. doi: 10.1016/s0002-9343(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 223.Cruz MN, Agewall S, Schenck-Gustafsson K, Kublickiene K. Acute dilatation to phytoestrogens and estrogen receptor subtypes expression in small arteries from women with coronary heart disease. Atherosclerosis. 2008;196(1):49–58. doi: 10.1016/j.atherosclerosis.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 224.Chin-Dusting JP, Boak L, Husband A, Nestel PJ. The isoflavone metabolite dehydroequol produces vasodilatation in human resistance arteries via a nitric oxide-dependent mechanism. Atherosclerosis. 2004;176(1):45–48. doi: 10.1016/j.atherosclerosis.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 225.Katz DL, Evans MA, Njike VY, Hoxley ML, Nawaz H, Comerford BP, Sarrel PM. Raloxifene, soy phytoestrogens and endothelial function in postmenopausal women. Climacteric. 2007;10(6):500–507. doi: 10.1080/13697130701750123. [DOI] [PubMed] [Google Scholar]
- 226.Hallund J, Tetens I, Bugel S, Tholstrup T, Ferrari M, Teerlink T, Kjaer A, Wiinberg N. Daily consumption for six weeks of a lignan complex isolated from flaxseed does not affect endothelial function in healthy postmenopausal women. J Nutr. 2006;136(9):2314–2318. doi: 10.1093/jn/136.9.2314. [DOI] [PubMed] [Google Scholar]
- 227.Penotti M, Fabio E, Modena AB, Rinaldi M, Omodei U, Vigano P. Effect of soy-derived isoflavones on hot flushes, endometrial thickness, and the pulsatility index of the uterine and cerebral arteries. Fertil Steril. 2003;79(5):1112–1117. doi: 10.1016/s0015-0282(03)00158-4. [DOI] [PubMed] [Google Scholar]
- 228.Beavers DP, Beavers KM, Miller M, Stamey J, Messina MJ. Exposure to isoflavone-containing soy products and endothelial function: A Bayesian meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. doi: 10.1016/j.numecd.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 229.Li SH, Liu XX, Bai YY, Wang XJ, Sun K, Chen JZ, Hui RT. Effect of oral isoflavone supplementation on vascular endothelialfunction in postmenopausal women: a meta-analysis of randomized placebo-controlled trials. Am J Clin Nutr. 91(2):480–486. doi: 10.3945/ajcn.2009.28203. [DOI] [PubMed] [Google Scholar]
- 230.Nagata C, Takatsuka N, Kurisu Y, Shimizu H. Decreased serum total cholesterol concentration is associated with high intake of soy products in Japanese men and women. J Nutr. 1998;128(2):209–213. doi: 10.1093/jn/128.2.209. [DOI] [PubMed] [Google Scholar]
- 231.Bairey Merz CN, Johnson BD, Braunstein GD, Pepine CJ, Reis SE, Paul-Labrador M, Hale G, Sharaf BL, Bittner V, Sopko G, Kelsey SF. Phytoestrogens and lipoproteins in women. J Clin Endocrinol Metab. 2006;91(6):2209–2213. doi: 10.1210/jc.2005-1853. [DOI] [PubMed] [Google Scholar]
- 232.Simons LA, von Konigsmark M, Simons J, Celermajer DS. Phytoestrogens do not influence lipoprotein levels or endothelial function in healthy, postmenopausal women. Am J Cardiol. 2000;85(11):1297–1301. doi: 10.1016/s0002-9149(00)00759-1. [DOI] [PubMed] [Google Scholar]
- 233.Crouse JR, 3rd, Morgan T, Terry JG, Ellis J, Vitolins M, Burke GL. A randomized trial comparing the effect of casein with that of soy protein containing varying amounts of isoflavones on plasma concentrations of lipids and lipoproteins. Arch Intern Med. 1999;159(17):2070–2076. doi: 10.1001/archinte.159.17.2070. [DOI] [PubMed] [Google Scholar]
- 234.Okamura S, Sawada Y, Satoh T, Sakamoto H, Saito Y, Sumino H, Takizawa T, Kogure T, Chaichantipyuth C, Higuchi Y, Ishikawa T, Sakamaki T. Pueraria mirifica phytoestrogens improve dyslipidemia in postmenopausal women probably by activating estrogen receptor subtypes. Tohoku J Exp Med. 2008;216(4):341–351. doi: 10.1620/tjem.216.341. [DOI] [PubMed] [Google Scholar]
- 235.Blum A, Lang N, Vigder F, Israeli P, Gumanovsky M, Lupovitz S, Elgazi A, Peleg A, Ben-Ami M. Effects of soy protein on endothelium-dependent vasodilatation and lipid profile in postmenopausal women with mild hypercholesterolemia. Clin Invest Med. 2003;26(1):20–26. [PubMed] [Google Scholar]
- 236.Tormala RM, Nikander E, Tiitinen A, Vaisanen-Tommiska M, Ylikorkala O, Mikkola TS. Serum cholesterol efflux potential in postmenopausal women treated with isolated isoflavones. Menopause. 2006;13(1):96–101. doi: 10.1097/01.gme.0000191210.13115.90. [DOI] [PubMed] [Google Scholar]
- 237.Hallund J, Ravn-Haren G, Bugel S, Tholstrup T, Tetens I. A lignan complex isolated from flaxseed does not affect plasma lipid concentrations or antioxidant capacity in healthy postmenopausal women. J Nutr. 2006;136(1):112–116. doi: 10.1093/jn/136.1.112. [DOI] [PubMed] [Google Scholar]
- 238.Rios DR, Rodrigues ET, Cardoso AP, Montes MB, Franceschini SA, Toloi MR. Lack of effects of isoflavones on the lipid profile of Brazilian postmenopausal women. Nutrition. 2008;24(11–12):1153–1158. doi: 10.1016/j.nut.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 239.Pellegrini N, Valtuena S, Ardigo D, Brighenti F, Franzini L, Del Rio D, Scazzina F, Piatti PM, Zavaroni I. Intake of the plant lignans matairesinol, secoisolariciresinol, pinoresinol, and lariciresinol in relation to vascular inflammation and endothelial dysfunction in middle age-elderly men and post-menopausal women living in Northern Italy. Nutr Metab Cardiovasc Dis. 20(1):64–71. doi: 10.1016/j.numecd.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 240.Colacurci N, Chiantera A, Fornaro F, de Novellis V, Manzella D, Arciello A, Chiantera V, Improta L, Paolisso G. Effects of soy isoflavones on endothelial function in healthy postmenopausal women. Menopause. 2005;12(3):299–307. doi: 10.1097/01.gme.0000147017.23173.5b. [DOI] [PubMed] [Google Scholar]
- 241.Chen CY, Bakhiet RM, Hart V, Holtzman G. Isoflavones improve plasma homocysteine status and antioxidant defense system in healthy young men at rest but do not ameliorate oxidative stress induced by 80% VO2pk exercise. Ann Nutr Metab. 2005;49(1):33–41. doi: 10.1159/000084175. [DOI] [PubMed] [Google Scholar]
- 242.Vafeiadou K, Hall WL, Williams CM. Does genotype and equol-production status affect response to isoflavones? Data from a pan-European study on the effects of isoflavones on cardiovascular risk markers in post-menopausal women. Proc Nutr Soc. 2006;65(1):106–115. doi: 10.1079/pns2005483. [DOI] [PubMed] [Google Scholar]
- 243.Hallund J, Tetens I, Bugel S, Tholstrup T, Bruun JM. The effect of a lignan complex isolated from flaxseed on inflammation markers in healthy postmenopausal women. Nutr Metab Cardiovasc Dis. 2008;18(7):497–502. doi: 10.1016/j.numecd.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 244.Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR., Jr Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85(3):895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 245.Kreijkamp-Kaspers S, Kok L, Bots ML, Grobbee DE, van der Schouw YT. Dietary phytoestrogens and vascular function in postmenopausal women: a cross-sectional study. J Hypertens. 2004;22(7):1381–1388. doi: 10.1097/01.hjh.0000125435.28861.d2. [DOI] [PubMed] [Google Scholar]
- 246.Teede HJ, McGrath BP, DeSilva L, Cehun M, Fassoulakis A, Nestel PJ. Isoflavones reduce arterial stiffness: a placebo-controlled study in men and postmenopausal women. Arterioscler Thromb Vasc Biol. 2003;23(6):1066–1071. doi: 10.1161/01.ATV.0000072967.97296.4A. [DOI] [PubMed] [Google Scholar]