Abstract
Germline mutation of the tumor suppressor gene CDC73 confers susceptibility to the hyperparathyroidism-jaw tumor syndrome associated with a high risk of parathyroid malignancy. Inactivating CDC73 mutations have also been implicated in sporadic parathyroid cancer, but are rare in sporadic benign parathyroid tumors. The molecular pathways that distinguish malignant from benign parathyroid transformation remain elusive. We previously showed that a hypomorphic allele of hyrax (hyx), the Drosophila homolog of CDC73, rescues the loss-of-ventral-eye phenotype of lobe, encoding the fly homolog of Akt1s1/ PRAS40. We report now an interaction between hyx and Tor, a central regulator of cell growth and autophagy, and show that eukaryotic translation initiation factor 4E-binding protein (EIF4EBP), a translational repressor and effector of mammalian target of rapamycin (mTOR), is a conserved target of hyx/CDC73. Flies heterozygous for Tor and hyx, but not Mnn1, the homolog of the multiple endocrine neoplasia type 1 (MEN1) tumor suppressor associated with benign parathyroid tumors, are starvation resistant with reduced basal levels of Thor/4E-BP. Human peripheral blood cell levels of EIF4EBP3 were reduced in patients with CDC73, but not MEN1, heterozygosity. Chromatin immunoprecipitation demonstrated occupancy of EIF4EBP3 by endogenous parafibromin. These results show that EIF4EBP3 is a peripheral marker of CDC73 function distinct from MEN1-regulated pathways, and suggest a model whereby starvation resistance and/or translational de-repression contributes to parathyroid malignant transformation.
Keywords: PAF1 complex, HRPT2, apoptosis, eIF4E, 4E-BP, s6k
Germline loss-of-function mutation of the tumor suppressor gene CDC73 (also known as HRPT2) confers susceptibility to the hyperparathyroidism-jaw tumor syndrome (HPT-JT), an autosomal dominant familial cancer syndrome associated with a high risk of parathyroid malignancy.1, 2, 3, 4, 5, 6 Analysis of multiple kindreds with HPT–JT by Carpten et al.7 led to the identification of CDC73/HRPT2 by positional candidate cloning. Inactivating somatic and/or germline CDC73/HRPT2 mutations have also been implicated in sporadic parathyroid cancer.8, 9 CDC73 encodes parafibromin,7 a 531-amino acid putative tumor suppressor protein with sequence homology to yeast Cdc73p.10 As was originally shown in yeast for Cdc73p,11 evidence in humans suggests that parafibromin interacts with RNA polymerase II as a part of the PAF1 complex.12, 13, 14 The components of the PAF1 complex are highly conserved in Drosophila as well, including hyrax (hyx) a homolog of CDC73.15
Despite its strong association with parathyroid cancer, inactivation of CDC73 is quite rare in sporadic, benign parathyroid tumors.16, 17 In contrast, inactivation of the multiple endocrine neoplasia type 1 (MEN1) tumor suppressor gene is nearly always associated with benign parathyroid tumors in the context of familial or sporadic MEN1,18 and somatic inactivation of MEN1 in sporadic benign parathyroid tumors is common.19, 20 Despite the identification of these key parathyroid tumor suppressor genes, the mechanisms and pathways that distinguish benign from malignant transformation of the parathyroid remain elusive. Furthermore, the development of a Cdc73/Hrpt2 knockout mouse model21 and other experimental approaches15, 22, 23 have so far yielded little insight into the pathway(s) whereby CDC73 loss-of-function might promote tumorigenesis.
Using Drosophila as a model system, we recently showed that hyx mutant flies were resistant to starvation and that hyx rescued the loss-of-ventral-eye phenotype of lobe, the fly homolog of Akt1 substrate 1 (AKT1S1).24 Akts1, also called 40 kDa proline-rich AKT substrate (PRAS40), is a negative regulator of mammalian target of rapamycin (mTOR) complex 1.25 The protein kinase mTOR functions as a cellular sensor of nutrient and energy availability, and regulates cell growth and autophagy.26
One well-characterized effector of mTOR is the eukaryotic translation initiation factor 4E-binding protein (eIF4EBP or 4E-BP) that competes with eIF-4G for binding to eIF-4E to inhibit mRNA cap-dependent translational initiation.27, 28 Phosphorylation of eIF4EBP by mTOR reduces its affinity for eIF-4E and promotes the initiation of protein synthesis from a pool of transcripts enriched in mRNAs encoding proteins involved in angiogenesis, growth, and survival.29 Enhanced function of eIF-4E, often a result of inactivation of eIF4EBP by phosphorylation, is linked to poor prognosis, tumor progression, and metastasis in squamous cell,30 breast,31, 32 ovarian, 33 and other cancers.
We demonstrate now that EIF4EBP is an evolutionarily conserved target of hyx/CDC73, and show that basal and serum starvation-stimulated levels of EIF4EBP3 transcript are reduced in peripheral mononuclear cells from patients heterozygous for CDC73, but not MEN1. We show that the expression of the EIF4EBP3 translational repressor represents a peripheral marker of CDC73 function and propose a model whereby starvation resistance, mediated at least in part by de-repression of protein translation, may contribute to parathyroid malignant transformation.
Results
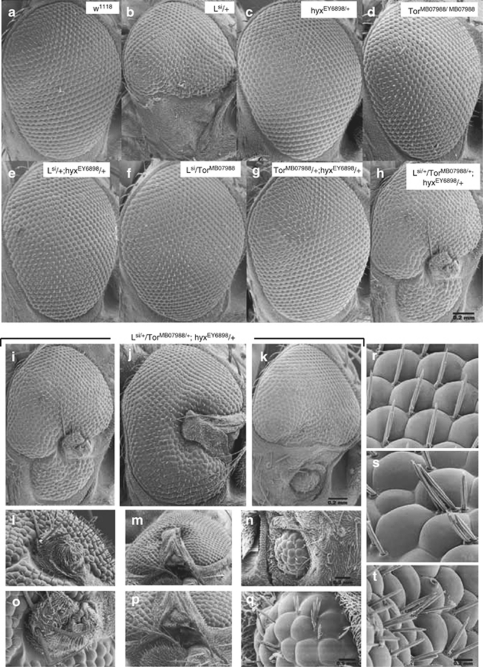
We previously showed that in Drosophila a hypomorphic allele of hyx rescues the eye phenotype of lobe and that hyx heterozygous flies were resistant to starvation.24 Thus, Lsi/+ hyxEY6898/+ double heterozygotes have normal eyes, even though heterozygous Lsi/+ flies show a loss-of-ventral-eye phenotype (Figure 1e, cf. 1a–c), and provide a sensitive genetic background to identify additional genes that interact with L, hyx, or both by eye phenotype screening.24
Figure 1.
Genetic interaction among lobe/Akt1s1, hyx/CDC73, and Tor evident from Drosophila eye phenotypes. Genetic interactions were recognized by the formation of novel NOG structures at the ventral part of the adult eye after crosses between flies with different genotypes. Shown are representative eye phenotypes captured by scanning electron microscopy for w1118 control (a), heterozygous Lsi mutant of lobe/AKT1S1 (b), heterozygous hyxEY6898 mutant of hyx/CDC73 (c), homozygous TorMB07988 mutant of Tor (d); double heterozygous mutants of lobe and hyx/CDC73 (e), lobe and Tor (f), and hyx/CDC73 and Tor (g); and triple heterozygous hybrid mutants of lobe, hyx/CDC73 and Tor strains (h). Images of additional eyes from triple heterozygous hybrid mutants of lobe, hyx/CDC73 and Tor strains are shown in (j) and (k) (image (i) is a duplicate of (h)). Higher magnification images of dysplastic overgrowth regions of (i–k) are shown in (l–n) and (o–q). Higher magnification images of the ommatidial and sensory bristle phenotype of the triple heterozygous lobe, hyx/CDC73 and Tor mutant are shown (s and t), with corresponding image of wild-type (r)
On the basis of the rescue of lobe by hyx and the enhanced resistance to starvation seen in hyx heterozygous flies,24 we used the eye phenotype screening assay to look for an interaction of hyx with Tor, a key regulator of cellular nutritional homeostasis26 and target of lobe/ Akt1S1/ PRAS40 inhibition.25 To this end we crossed doubly heterozygous (Lsi/+ hyxEY6898/+) flies with a TorMB07988 mutant strain (Figures 1h–q, s, and t; Table 1). Approximately, 40% of the triple heterozygote (Lsi/TorMB07988 hyxEY6898/+) flies had NOG eye phenotypes (notch and dysplastic overgrowth), including half eyes with dysplastic overgrowths24 (Figures 1h–k; Table 1, cross 8). The overgrowths typically appeared in or near the missing ventral eye region (Figures 1i–q). The ommatidia and the sensory bristles were deformed and in disarray (Figures 1s and t, cf. 1r). Testing of another Tor mutant allele, TorK17004, gave similar results (Table 1, cross 10). In contrast, 90% or more of the doubly heterozygous mutant flies carrying the Lsi allele (Lsi/TorMB07988L si/TorK17004 Lsi/+hyxEY6898/+) had normal eyes (Figures 1e and f; Table 1, crosses 3, 7, and 11). As the other possible doubly heterozygous mutant combinations from the same matings produced flies with normal eyes (Figure 1g; Table 1, crosses 9 and 12), the observed strong eye phenotype must result from the specific combination of hyx and Tor loss-of-function.
Table 1. Genetic interactions of L, hyrax, and Tor.
| Cross No. | Parent 1 | Parent 2 | F1 genotype | % flies with NOGa eye phenotype | % NOG in both eyes | % notch w/o OG | Molecular function |
|---|---|---|---|---|---|---|---|
| 1 | w1118 | w1118 | w1118 | 0 | 0 | 0 | |
| 2 | Lsi/si | w1118 | Lsi/+ | 0 | 0 | 100 | L (lobe) is the fly homolog of mammalian AKT1S1 (also called PRAS40) |
| 3 | hyxEY6898/+ | Lsi/+hyx EY6898/+ | 6 | 0 | 11 | Hyx (hyrax) is the fly homolog of CDC73/ HRPT2 | |
| 4 | hyxEY6898/+ | w1118 | hyxEY6898/+ | 0 | 0 | 0 | |
| 5 | TorMB07988/MB07988 | TorMB07988/MB07988 | TorMB07988/MB07988 | 0 | 0 | 0 | Tor is a key cellular kinase involved in the regulation of cell growth and nutritional homeostasis |
| 6 | w1118 | TorMB07988/+ | 0 | 0 | 0 | ||
| 7 | Lsi/+ | Lsi/TorMB07988 | 6 | 0 | 2 | ||
| 8 | Lsi/+hyx EY6898/+ | Lsi/TorMB07988; hyx EY6898/+ | 40*** | 7 | 3 | ||
| 9 | hyxEY6898/+ | TorMB07988/+ hyxEY6898/+ | 0 | 0 | 0 | ||
| 10 | TorK17004/+ | Lsi/+ hyx EY6898/+ | Lsi/TorK17004; hyxEY6898/+ | 45*** | 8 | 7 | |
| 11 | Lsi/TorK17004 | 10 | <1 | 9 | |||
| 12 | hyxEY6898/+ | TorK17004/+ hyxEY6898/+ | 0 | 0 | 0 |
NOG: notch and overgrowth (OG), with notches and variably sized dysplastic overgrowths in the ventral eye fields.
***P<0.0001 compared with w1118n, and all double and single heterozygote mutants
We previously showed that the eye discs of Lsi/+ hyxEY6898/orb2BG02373 triple heterozygotes with a propensity to later develop the NOG phenotype were characterized by an abnormal pattern of apoptosis at the larval stage.24 In contrast, the larval eye discs of Lsi/TorMB07988 hyxEY6898/+ triple heterozygotes showed no gross abnormalities in the pattern or extent of apoptosis compared with w1118 controls (data not shown).
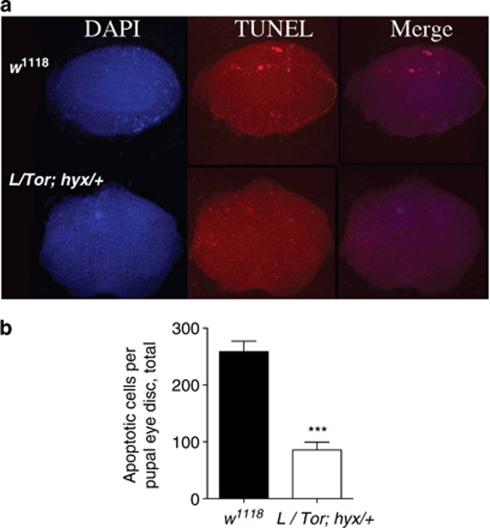
We, therefore, compared the pupal eye discs of wild-type and Lsi/TorMB07988 hyxEY6898/+ triple heterozygotes to look for developmental abnormalities that might account for the subsequent development of the NOG phenotype in the triple mutants. Normal eye development in Drosophila involves a wave of cell apoptosis between 35 and 50 h after pupation that eliminates a large fraction of undifferentiated interommatidial cells.34 The pupal eye discs of wild-type and Lsi/TorMB07988 hyxEY6898/+ triple heterozygote flies were, therefore, examined at 45 h after pupation using the terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL) assay to identify the nuclei of cells undergoing apoptosis (Figure 2). Eye discs from Lsi/TorMB07988 hyxEY6898/+ triple heterozygote pupae showed a disordered pattern of apoptosis as well as a significant decrease in TUNEL-positive apoptotic nuclei compared with eyes from w1118 controls (Figures 2a and b). Thus, the eye discs of Lsi/TorMB07988 hyxEY6898/+ triple heterozygotes with a propensity to later develop the NOG phenotype were characterized by a substantial reduction in the usual burst of apoptosis between 35 and 50 h after pupation.
Figure 2.
Loss of normal apoptosis during pupal development correlates with the NOG eye phenotype observed in the L/Tor;hyx triple heterozygous mutant flies. Normal eye development in Drosophila involves a wave of cellular apoptosis between 35 and 50 h after pupal formation (APF) that eliminates a large fraction of undifferentiated interommatidial cells. The eye discs of Lsi/TorMB07988 hyxEY6898/+ triple heterozygotes with a propensity to later develop the NOG phenotype were characterized by a substantial reduction in this usual burst of apoptosis APF compared with w1118 controls. (a) 4′,6-diamidino-2-phenylindole nuclear staining (left), TUNEL analysis (middle), and merged images (right) of pupal eye discs dissected ∼45 h APF reveal a loss and mis-localization of apoptotic-positive cell nuclei in Lsi/TorMB07988 hyxEY6898/+ triple heterozygote flies compared with w1118 controls. The difference in size between w1118 control and Lsi/TorMB07988 hyxEY6898/+ triple heterozygote eye discs shown represents a random variation and was not reflective of a consistent difference between the two. (b) Quantification of apoptotic cells per pupal eye disc shows significant (∼60%) reduction in total apoptotic-positive cell nuclei in Lsi/TorMB07988 hyxEY6898/+ triple heterozygotes compared with w1118 controls (***P<0.0001, unpaired Student's t-test; w1118 control, n=16; Lsi/TorMB07988 hyxEY6898/+, n=18)
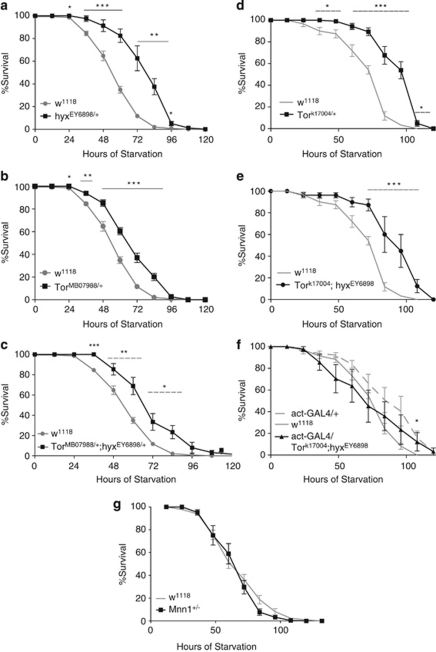
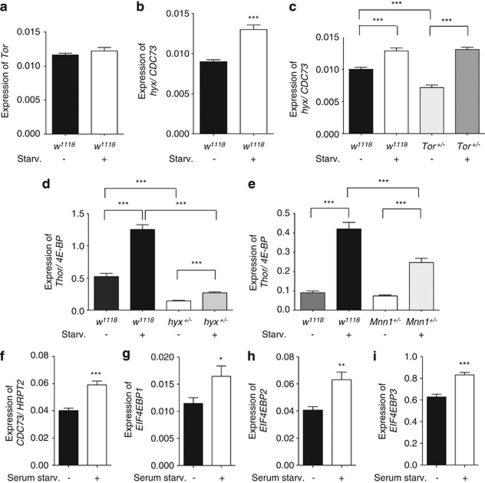
As current models suggest that Tor acts as a nutrient sensor, and regulator of cell growth and autophagy,26 we studied starvation resistance in Tor and hyx mutant flies. Consistent with our previous results,24 hyx heterozygous flies were resistant to starvation compared with w1118 controls (Figure 3a). Resistance to starvation was also found to be higher in Tor (TorMB07988/+) heterozygous flies (Figure 3b). Double hyx/ Tor heterozygotes (TorMB07988/+ hyxEY6898/+) were also resistant to starvation without any obvious additivity of the effect (Figure 3c).
Figure 3.
Enhanced starvation resistance in hyx/CDC73 and Tor mutant flies. Survival upon starvation of the indicated single or double hyx/CDC73 and Tor heterozygous mutant fly strains is shown. Flies a–g were supplied only with water to test starvation resistance. The number of surviving flies was recorded every 12 h. For each experiment, 20 or more vials were used and two or more independent experiments were conducted for each fly line. Vials contained 10 flies each. Each data point represents the pooled mean survival from 20 to 30 vials of the indicated genotype (*P<0.05; **P<0.001; ***P<0.0001; versus wt for the indicated time points, two–tailed P-values for unpaired Student's t-test). The experiment shown in (f) tested the rescue of the starvation resistance of the TorK17004/+ hyxEY6898/+ double mutant (cf. e) by overexpression of hyx from the hyxEY6898 allele upon mating with a driver strain expressing GAL4 from the 5C-actin promoter (act-GAL4), with the driver-only control shown (act-GAL4/+; dashed gray line). In panel f, the P values for the unpaired Student's t-test comparing the % survival of w1118 control strain with the act-Gal4/TorK17004/+ hyxEY6898/+ strain were: 84 h, P=0.32; 96 h, P=0.14; 108 h, P=0.036
A separate set of experiments used a different mutant allele of Tor (TorK17004) and showed that TorK17004/+ heterozygotes, like TorMB07988/+ heterozygous flies, exhibited resistance to starvation (Figure 3d). A double hyx/ Tor heterozygote strain demonstrated comparable resistance to starvation as seen with the single heterozygotes (Figure 3e, cf. 3a and d). We tested for rescue of the phenotype exploiting the fact that hyx can be overexpressed in strains carrying the GAL4-sensitive hyxEY6898 allele by mating to GAL4-overexpressing strains.15, 24 As shown in Figure 3f, overexpression of hyx in flies carrying the ubiquitously expressed act5C-Gal4 driver and doubly heterozygous for hyx/ Tor mutation restored starvation sensitivity to near control levels, whereas control flies with 5C-actin promoter-driven GAL4 expression only had slightly enhanced starvation resistance (Figure 3f). In contrast to the hyx mutants, flies heterozygous for Mnn1 (Mnn1DG30701/+) were sensitive to starvation like the w1118 controls (Figure 3g). Thus, heterozygosity of hyx and Tor, but not Mnn1, confers starvation resistance. Furthermore, hyx gene overexpression, at least at time points <100 h in a model system using the GAL4-sensitive hyxEY6898 allele and the act5C-Gal4 driver, can rescue the starvation-resistant phenotype of Tor.
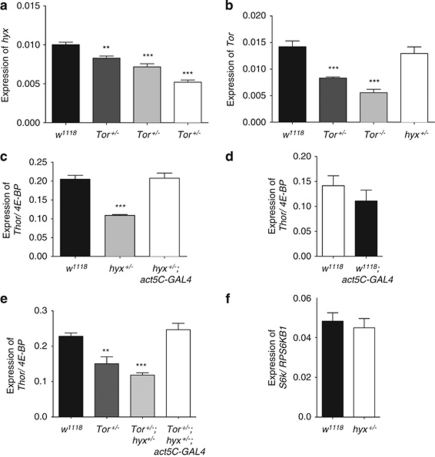
To better understand the interaction between hyx and Tor, quantitative RT-PCR was used to measure transcript levels in hyx or Tor mutant fly strains. Levels of hyx transcript were partially reduced in TorMB07988 heterozygotes and by some 30% in TorMB07988 homozygotes compared to with control (Figure 4a). In contrast, adult flies heterozygous for hyxEY6898 had normal levels of Tor transcript (Figure 4b), even though Tor message levels were successively reduced in heterozygous and homozygous TorMB07988 mutant flies (Figure 4b).
Figure 4.
Transcriptional regulation of Tor, hyx, and Thor in fly. Heterozygous Tor+/− (TorMB07988/+) and homozygous Tor−/− (TorMB07988/MB07988) mutant flies have a dose-dependent reduction in both Tor and hyx transcript compared with w1118 controls (a and b). Heterozygous hyx (hyxEY6898/+) mutant flies show a reduction in hyx, but not in Tor transcript expression (a and b). Thor/4E-BP transcript expression is significantly reduced in hyx/CDC73+/− flies compared with w1118 controls, and is rescued back to control levels following act5C-GAL4-driven overexpression of hyx/CDC73 from the hyxEY6898 allele (c). The act5C-GAL4/+ driver alone has no effect on Thor/4E-BP transcript levels (d). Tor+/− heterozygosity and Tor+/−;hyx+/− double heterozygosity results in a decrease in Thor/4E-BP transcript levels, and is similarly rescued following act5C-GAL4-driven overexpression of hyx/CDC73 from the hyxEY6898 allele in the Tor+/−;hyx+/− double heterozygous mutant background (e). Heterozygosity of hyx/CDC73+/− has no effect on transcript levels of s6k/RPS6KB1, which is, like 4E-BP, a major effector of Tor (f). For a, b, c, and e **P<0.005; ***P<0.0005 compared with w1118 control, using unpaired Student's t-test
To test the effect of Mtor inactivation on Cdc73 expression in a mammalian model, the expression of parafibromin in several tissues harvested from wild-type and Mtor heterozygous mice was compared by quantitative immunoblotting. In several tissues examined, including pancreas, liver, and kidney, the expression of parafibromin was reduced in Mtor heterozygous mice relative to β-actin (Supplementary Figure S1).
The translational repressor 4E-BP (eIF4EBP), which competes with eIF-4G for binding to eIF-4E to regulate translational initiation,35 is a downstream target of the Tor kinase in mammals28 and in flies.36 As our results indicate an interaction between hyx and Tor at the transcriptional level, we looked for a possible interaction between hyx and Thor, the fly ortholog of EIF4EBP. Transcript levels of Thor/EIF4EBP are reduced by ∼50% in hyx heterozygotes (hyxEY6898/+) (Figure 4c). This reduction in Thor/EIF4EBP expression was reversed by the GAL4-induced overexpression of hyx (Figure 4c). The level of Thor/EIF4EBP transcript in flies expressing the act5C-Gal4 driver alone was comparable to the controls (Figure 4d). The level of Thor/EIF4EBP transcript was reduced by ∼30% in Tor heterozygotes and additionally reduced to ∼50% in double hyx/Tor heterozygotes, a reduction rescued by overexpression of hyx through the hyxEY6898 allele (Figure 4e). Heterozygosity of hyx had no effect on the expression of S6k, the fly ortholog of RPS6KB1 encoding the ribosomal protein S6 kinase, another well-characterized downstream target of the Tor kinase in mammals37 and in flies36 (Figure 4f).
Next the effect of starvation on relevant transcript levels in control and mutant flies was determined (Figures 5a–e). Although starvation of flies had no effect on Tor transcript levels (Figure 5a), hyx expression was increased (Figure 5b). The induction of hyx by starvation persisted in Tor heterozygotes, suggesting nutritional cues may regulate hyx transcription independently of Tor activity (Figure 5c). Starvation also increased Thor/EIF4EBP transcript expression compared with fed controls; however, the level of Thor/EIF4EBP transcript in starved hyx heterozygous flies was reduced by ∼80% compared with starved w1118 controls (Figure 5d). There was no difference in the basal Thor/EIF4EBP transcript level between w1118 control and Mnn1 heterozygous flies, although the level of starvation-induced Thor/EIF4EBP was somewhat lower in the Mnn1 mutants (Figure 5e). These results suggest that the starvation resistance of both hyx and Tor heterozygous flies results, at least in part, from impaired upregulation of Thor/EIF4EBP in response to caloric deprivation.
Figure 5.
Transcriptional regulation of hyx/CDC73 and Thor/4E-BP in response to caloric or serum starvation. Upregulation in w1118 control or mutant flies of hyx/CDC73 and Thor/4E-BP, but not of Tor transcript, compared with fed controls following 48 h of starvation (a–d). The basal reduction of hyx transcript observed in Tor+/− (TorMB0798/+) heterozygous flies was lost following 48 h of starvation, suggesting that nutritional cues may regulate hyx transcription independently of Tor activity (c). Conversely, the basal reduction in Thor/4E-BP transcript observed in hyx+/− heterozygous flies persists following 48 h of starvation, suggesting that hyx is necessary for the starvation-induced increase in Thor/4E-BP transcript (d). Basal expression of Thor/4E-BP transcript was unchanged in Mnn1+/− (Mnn1DG30701/+) heterozygous flies compared with w1118 controls; however, the level of Thor transcript was somewhat reduced following 48 h of starvation (e). Expression of human CDC73, EIF4EBP1, EIF4EBP2, and EIF4EBP3 transcript was significantly increased in HEK293 cells following 48 h of serum starvation (f–i). For b–i *P<0.05, **P<0.005; ***P<0.0005 compared with control, using unpaired Student's t-test
To look for these interactions in human cellular models, the effect of serum starvation on the expression of CDC73 and the three mammalian EIF4EBP isoforms was determined in HEK293 human embryonic kidney cells and cultured human peripheral blood mononuclear cells (WBC). Serum starvation for 48 h significantly increased CDC73, EIF4EBP1, EIF4EBP2, and EIF4EBP3 transcript expression in HEK293 cells (Figures 5f–i). In control WBC, as in HEK293 cells, serum starvation significantly increased CDC73 transcript expression (Supplementary Figure S2).
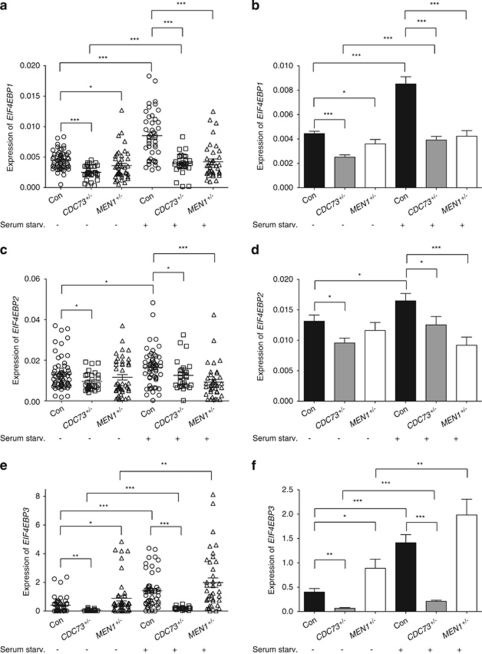
The expression of EIF4EBP isoforms was compared in WBC collected from normal healthy volunteer controls and from patients with the HPT-JT (CDC73 heterozygotes) or MEN1 (MEN1 heterozygotes) (Supplementary Table S1). Compared with control WBC, basal levels of all three EIF4EBP isoforms were reduced in CDC73 heterozygotes with the most striking reduction in EIF4EBP3 (Figure 6). In WBC from MEN1 heterozygous patients, the basal level of EIF4EBP1 transcript was reduced compared with control; however, there was no difference in the basal EIF4EBP2 level, and basal EIF4EBP3 was increased (Figure 6). Serum starvation-induced levels of EIF4EBP1 and EIF4EBP2 transcript did not discriminate between CDC73 and MEN1 heterozygous patients, and both groups of patients had lower induced transcript levels than the controls (Figure 6). In contrast, the serum starvation-induced level of EIF4EBP3 transcript from CDC73 heterozygous patients was markedly lower than that from both control and MEN1 heterozygous patient WBCs (Figure 6). Thus, reduced basal and serum starvation-induced EIF4EBP3 transcript in peripheral WBC represents a phenotype that correlates strongly with CDC73 heterozygosity, but not with that of MEN1.
Figure 6.
Heterozygosity of CDC73 results in a reduction of EIF4EBP transcript levels in human WBCs. WBCs were collected from healthy volunteers or from patients with HPT-JT (CDC73 heterozygotes) or MEN1 (MEN1 heterozygotes). The expression of transcripts for the indicated isoforms of EIF4EBP was determined by quantitative RT-PCR following 72 h of control culture conditions or serum starvation, as indicated. The same data are presented as scatter dot plots on the left (a, c and e) and histograms on the right (b, d and f). Control, n=43–62 transcript measurements from eight cell harvests from eight normal volunteers. CDC73+/−, n=22–34 transcript measurements from seven cell harvests from five patients. MEN1+/−, n=37–47 transcript measurements from 11 cell harvests from 9 patients. For a–f *P<0.05, **P<0.005; ***P<0.0005 for indicated comparisons, using unpaired Student's t-test
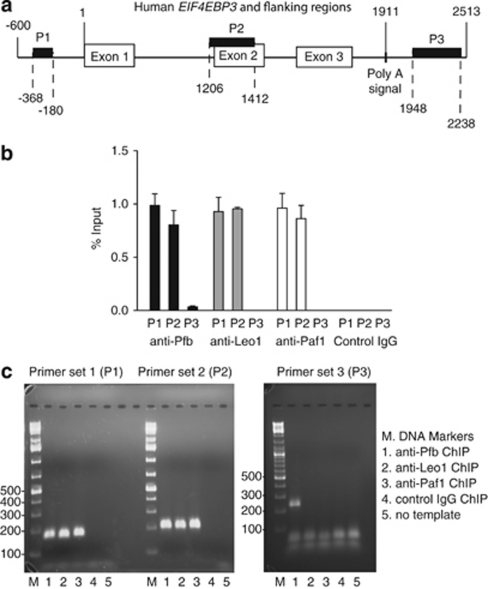
Chromatin immunoprecipitation (ChIP) was used in HEK293 cells to determine if the effect of CDC73 allele dosage on EIF4EBP3 transcript levels was consistent with regulation at the transcriptional level. Three pairs of primers were used to interrogate anti-parafibromin immunoprecipitates in a ChIP assay by quantitative PCR, with one primer set (P1) targeting the upstream EIF4EBP3 promoter region, one set (P2) targeting the internal gene coding sequence at exon 2, and one set (P3) directed at a flanking region downstream of the putative poly A signal (Figure 7a). Antibodies to other components of the PAF1 complex, Leo1, and Paf1 were also used. As shown in Figure 7b, primer pairs P1 and P2 produced significantly higher signals from the anti-parafibromin, anti-Leo1, and anti-Paf1 immunoprecipitates than from those using control IgG. The specific anti-parafibromin ChIP signal was much stronger in the P1 and P2 regions of EIF4EBP3 than in the P3 region, and no signal at all was detectable in the P3 region with anti-Leo1 or anti-Paf1 ChIP (Figure 7b). Taken together, these data suggest that the PAF1 complex might be involved in both EIF4EBP3 transcript initiation and elongation, consistent with PAF1 complex function at other gene loci.12, 13, 14, 38
Figure 7.
ChIP demonstrates occupancy at EIF4EBP3 by the PAF1 complex. The physical association of endogenous parafibromin and other components of the PAF1 complex (including the Paf1 and Leo1 proteins) with human EIF4EBP3 was examined by ChIP in HEK293 cells. (a) Schematic diagram showing the relative location of the three PCR primer sets used in the ChIP assay (P1, P2, and P3) along the human EIF4EBP3 gene (HGNC ID: 3290) and flanking regions (not to scale). The numbering shown is relative to the first base of exon 1 as +1 as indicated. (b) ChIP analysis using primer sets targeting upstream (P1), exon 2 (P2) and downstream (P3) sequence of EIF4EBP3 using antibodies against parafibromin, Leo1 or Paf11 or control rabbit IgG as shown. Results shown are mean percentage of input signal±S.E.M. (c) Qualitative analysis of ChIP PCR-derived DNA products by agarose gel electrophoresis and ethidium bromide staining. All experiments are representative of three or more independent biological repeats
Discussion
Gene expression profiling studies of parathyroid tumors by Haven et al.39 strongly suggest that sporadic parathyroid malignancies and tumors associated with CDC73 inactivation follow a pathway distinct from benign sporadic and MEN1-related parathyroid tumors. This dichotomy is reinforced by the clinical observations that inactivation of CDC73 is frequent in parathyroid cancer,8, 9 but rare in sporadic benign parathyroid tumors,16, 17 whereas mutation of MEN1 is nearly always associated with benign parathyroid adenomas in both sporadic and MEN1-associated cases.
We show here that EIF4EBP3 is distinctly regulated by CDC73 and MEN1, that parafibromin and other PAF1 complex components occupy the EIF4EBP3 promoter and coding regions, and that loss of EIF4EBP3 expression represents a marker of CDC73 haploinsufficiency and heterozygosity, but not MEN1 loss-of-function, in a human WBC model system. As current PCR-based CDC73 germline mutation testing methods may miss some carriers,7 our results indicate it may be possible to independently assess CDC73 allele dosage by the determination of EIF4EBP3 expression in a clinically practical manner.
The strong evolutionary conservation of the link between EIF4EBP and CDC73 is striking. Taken together with our previous finding that hyx opposes lobe/Akt1S1 in the eye phenotype assay,24 the present work strengthens the notion that TOR and CDC73 interact with an overlapping set of regulators and effector targets. Functioning as a sensor of nutrient availability, the protein kinase mTOR regulates cell growth and autophagy.26 Under physiological conditions, growth signals acting through mTOR promote the phosphorylation of eIF4EBP and reduce its affinity for eIF-4E; thus initiating protein synthesis from a pool of transcripts critical for cell growth and survival. Under pathological conditions, the enhanced function of eIF-4E, frequently a result of inactivation of eIF4EBP by mTor phosphorylation, is linked to poor prognosis and metastasis in multiple malignancies.30, 31, 32, 33 Unlike the inactivation of eIF4EBP by phosphorylation, via the canonical mTOR pathway, loss of CDC73 exerts its negative effect on EIF4EBP3 through loss of gene expression. Our findings suggest a model in which de-repression of cap-dependent protein translation and heightened survival resulting from CDC73 loss-of-function may enhance carcinogenesis in parathyroid tumors, conferring selective advantage to hypermetabolic neoplastic cells for clonal expansion in the face of nutritional stress.
Materials and Methods
Human subjects
All patients participated in the protocols approved by the Investigational Review Boards of the National Institute of Diabetes and Digestive and Kidney Diseases and each gave written consent. Normal volunteers had no personal or family history of hyperparathyroidism, HPT-JT, or MEN1. Patients with HPT-JT or MEN1 had documented heterozygous germline mutation of CDC73/HRPT2 or MEN1, respectively. Patient characteristics are summarized in Supplementary Table 1.
Fly stocks
The enhancer trapped fly lines from the Japanese NP Consortium Gal4 Enhancer Trap Insertion Database were obtained from the Drosophila Genetic Resource Center, Kyoto Institute of Technology (Kyoto, Japan). The fly line bearing the hypomorphic allele, hyxEY6898, which contains a P-element (P[EPgy2]) insertion located 36 bp upstream of the hyx translational start site in the 5′ untranslated transcript region (Stock No. 16768), and all the other fly lines used, including Mnn1DG30701 (Stock No. 21335), TorK17004/CyO (Stock No. 11218), TorMB07988 (Stock No. 25363), and Act5C-GAL4/CyO (Stock no. 4414), were from the Bloomington Drosophila Stock Center at Indiana University (Bloomington, IN, USA).
Scanning electron microscopy
Flies were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA). Before examination, the samples were dehydrated through 100% ethanol, and then critically point dried (Tousimis model Samdri-795, Rockville, MD, USA). A 10-nm gold coating was deposited in a sputter coater (Electron Microscopy Sciences model 575 × ), and the detailed structure of the fly eyes were studied at 5 kV on a scanning electron microscope (Hitachi S-3400N, Pleasanton, CA, USA).
Pupal eye disc apoptosis analysis
The ApopTag Red In situ Apoptosis Detection Kit (Millipore, Billerica, MA, USA) was used for TUNEL analysis. Eye discs collected ∼45 h following pupation were dissected in PBS and analyzed for apoptosis following the same method as previously described for the eye discs of third instar fly larvae.24
Fly starvation resistance
For starvation stress tests, flies with desired genotypes that eclosed within 24 h were collected. After ageing for 8 days at 25°C, mated female flies were placed into vials containing paper discs (d=2.3 cm) soaked in 400 μl of H2O (Whatman, Piscataway, NJ, USA). H2O was replenished throughout the experiment as needed. A total of 20–30 vials (10 flies per vial) were used for each test, and at least two independent experiments were performed. Dead flies were recorded every 12 h. For data analysis, each vial was treated as a data point.
mRNA quantification
Gene expression levels were estimated on the basis of the transcript abundance as measured by quantitative RT-PCR. Quantitative RT-PCR was performed with one-step quantitative RT-PCR master mix (Agilent Technologies, Santa Clara, CA, USA) using a Stratagene MX 3005P real time PCR machine (Agilent Technologies) and analyzed using the accompanying software. Each reaction was conducted in triplicate and three to nine biological samples prepared independently were used in data analysis. The Prism software version 5.0c (GraphPad Software, Inc., La Jolla, CA, USA) was used for graphing the analyzed data set. Total RNA was prepared from both mutant and control samples using QIAGEN RNeasy kit (Valencia, CA, USA). Primer sequences were developed using PrimerQuest software (Integrated DNA Technologies, Coralville, IA, USA), and were as follows: Drosophila Tor forward (5′-TGG CAA AGC AAC TGG GCA AGA A-3′) and reverse (5′-TCG GGA TCC ACA ATG CGA TGT T-3′); hyx forward (5′-TTT GCG TGC CAT CCT GGA CTA T-3′) and reverse (5′-TCT TGC CCG TGC TTT GCA GAA T-3′); Mnn1 forward (5′-CAA ATG GAT AGA CGG ACT GCT CGT-3′) and reverse (5′GTC AAC AGT TCG TAA CAA GGA TTT GC-3′); Thor forward (5′-CCA TGA TCA CCA GGA AGG TTG TCA-3′) and reverse (5′-TCT TCA TGA AAG CCC GCT CGT AGA-3′); s6k forward (5′-ATG CGG CGG CTG TTC AAA TAC A-3′) and reverse (5′-TGG CAC TTT CGC TTA GCG TTG T-3′). Human CDC73 forward (5′-AGA TGC AAC CAG GGG GCA CTG-3′) and reverse (5′-GCA GGA CCC TGC ACA AAA ACG G-3′); EIF4EBP1 forward (5′-TGG ACA AGA ACG AAC CCT TCC T-3′) and reverse (5′-AGG GAG CTT TCC CAA GCA CAT-3′); EIF4EBP2 forward (5′-TTT GCA TTC ACC CTC CTT CCC A-3′) and reverse (5′-AGG GCA CCA AAT CCA ACC AGA A-3′); EIF4EBP3 forward (5′-AAG TTC CTG CTG GAG TGC AAG A-3′) and reverse (5′-TCT CCT GCT CCT TCA GCT CCT C-3′).
Immunoblotting and infrared imaging
Tissues from 6-week old female wild-type C57BL/6 and Mtor heterozygous mice (Jackson Laboratory, Bar Harbor, ME, USA, strain B6.129S5-MtorMtorGt(OST92090)Lex/J, Stock No. 013190) were stored at −80° and then thawed on ice. For every 50 mg of mouse tissue, 1 ml of 1 × RIPA cell lysis buffer (Cell Signaling, Beverly, MA, USA, Cat. No. 9806) including 1 × protease inhibitors (Calbiochem, EMD Serono, Inc., Rockland, MA, USA, Cat. No. 539134) was added followed by homogenization on ice in a 1.5-ml microcentrifuge tube using a tissue homogenizer (Omni International, Kennesaw, GA, USA, Model TH-115) at high speed for 30 s. Homogenates were combined with an equal volume of Laemmli's 2 × gel loading buffer, vortexed, and heated at 95°C for 10 min before gel loading. Electrophoresis by SDS-PAGE was followed by the transfer of the proteins on to 0.45-micron nitrocellulose membrane. Membranes were blocked with TBS or PBS (pH 7.4) containing 0.1% Tween 20 and 5% nonfat dry milk (blocking buffer), and incubated overnight with primary antibodies in the same buffer. Primary antibodies used were rabbit anti-parafibromin polyclonal (GRAPE antibody40) and mouse monoclonal anti-β- actin (Sigma-Aldrich, St. Louis, MO, USA, Cat. No. A5316). Membranes were then washed, and IR-labeled secondary antibodies (dilution 1 : 20 000) were used for detecting the protein signals in conjunction with the Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA). Quantification of parafibromin immunoreactivity used the β-actin signal in the same lane for normalization. IR secondary antibodies (anti-rabbit IR 800 and anti-mouse Green) were from LI-COR Biosciences.
Mammalian cell culture
Human embryonic kidney HEK293 cells (American Type Culture Collection, Manassas, VA, USA, Cat. No. CRL-1573) were grown in 75-cm2 flasks in DMEM supplemented with 10% fetal bovine serum (FBS), 4 mM-glutamine and penicillin/streptomycin at 37°C and 5% CO2. For serum-starvation experiments, HEK293 cells newly seeded the night before were either fed with medium supplemented with 10% FBS (control) or not (starved) for 48 h. Cells were then detached using Trypsin–EDTA, pelleted in microcentrifuge tubes, and either used immediately or stored at −80°C.
A mononuclear cell fraction was isolated from a morning whole blood sample drawn from non-fasting normal volunteers, CDC73 or MEN1 heterozygotes (collected at room temperature in heparinized tubes) processed using Lymphocyte Separation Medium (Lonza, Walkersville, MD, USA, Cat. No. 17–829E). The buffy layer, enriched in lymphocytes and mononuclear cells, was collected, washed once in PBS after which the cells were resuspended in complete RPMI medium 1640 (GIBCO, Life Technologies, Grand Island, NY, USA) containing 10% FBS, plated on to 12-well cell culture plates and incubated at 37°C with 5% CO2. After 24 h of incubation, non-adherent cells were gently removed and the adherent cell population, enriched in mononuclear cells, was divided and further incubated with either fresh complete RPMI medium (control cells) or the RPMI medium without serum (serum-starved cells) for an additional 72 h. The cells were then detached by trypsin–EDTA treatment, pelleted in microcentrifuge tubes, and stored at −80°C until use.
ChIP assay
ChIP assay kit from Millipore (Cat. No. 17–295) was used in the analysis of HEK293 cells following the manufacturer's instructions except that the QIAquick PCR purification kit (Qiagen, Cat. No. 28104) was used for DNA purification. Purified DNA was used as a template for the amplification of selected regions of EIF4EBP3 and its flanking sequences using the following primer pairs (Figure 7a): P1 (upstream promoter region, PCR product 191 bp, forward 5′-TCC CTT CCT GCA AGA TGT GTG ACT-3′, reverse 5′-TGG CCC AGC GCT GGC CAA CCT GCC-3′); P2 (exon 2 region, PCR product 206 bp, forward 5′-TCC TGA CTC TTA CCT CAG TCC CAA-3′, reverse 5′-TAC CGG GTA TCT CTT CCT CTG TCT-3′); P3 (downstream region following putative polyA signal, PCR product 288 bp, forward 5′-TCT CAT CTC AGC CAC ACA GCT GAA-3′, reverse 5′-ATA GGC TGG GAC AGC ACC TCT T-3′). Antibodies used for the ChIP assay were anti-parafibromin (GRAPE antibody40), anti-Leo1 (Bethyl Laboratories, Montgomery, TX, USA, Cat. No. A300-175A) and anti-Paf1 (Upstate (Millipore), Cat. No. 07–653), and control rabbit IgG (Santa Cruz Biotechnologies, Santa Cruz, CA, USA, Cat. No. sc-2027). Real-time quantitative PCR was performed using 2 × Brilliant II SYBR green QPCR master mix (Agilent Technologies, Cat. No. 600828-51) using the Stratagene Mx3005P QPCR system. PCR parameters used were 95°C 10 min, 40 cycles of 94°C 30 s, 60°C 1 min, and 72°C 1 min, followed by 1 cycle of dissociation curve analysis. The size and quality of the PCR-amplified DNA was assessed by agarose gel electrophoresis and ethidium bromide staining in comparison to DNA markers (GeneRuler DNA Ladder Mix, Fermentas, Glen Burnie, MD, USA, Cat. No. SM0333). Data analysis and graphing were performed using Prism software version 5.0c (GraphPad Software, Inc.) with results expressed as percentage input signal after normalizing Ct values from each primer set.
Statistical analysis
Data were analyzed by Prism software version 5.0c. (GraphPad Software, Inc.). Unpaired Student's t-test was used to evaluate for statistical significance.
Acknowledgments
We are grateful to Sunita Agarwal and Stephen Marx for encouragement and helpful discussions. This research was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases, the National Heart, Lung and Blood Institute, and the National Institute of Allergy and Infectious Diseases.
Glossary
- HPT-JT
hyperparathyroidism-jaw tumor syndrome
- Hyx
hyrax
- EIF4EBP
eukaryotic translation initiation factor 4E-binding protein
- MEN1
multiple endocrine neoplasia type 1
- PRAS40
proline-rich AKT substrate of 40 kDa
- AKT1S1
Akt1 substrate 1
- mTOR
mammalian target of rapamycin
- FBS
fetal bovine serum
- APF
after pupal formation
- TUNEL
terminal deoxynucleotidyl transferase mediated dUTP nick end labeling
- ChIP
chromatin immunoprecipitation
- WBC
cultured human peripheral blood mononuclear cells
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Stephanou
Supplementary Material
References
- Jackson CE, Norum RA, Boyd SB, Talpos GB, Wilson SD, Taggart RT, et al. Hereditary hyperparathyroidism and multiple ossifying jaw fibromas: a clinically and genetically distinct syndrome. Surgery. 1990;108:1006–1012. [PubMed] [Google Scholar]
- Mallette LE, Malini S, Rappaport MP, Kirkland JL. Familial cystic parathyroid adenomatosis. Ann Intern Med. 1987;107:54–60. doi: 10.7326/0003-4819-107-1-54. [DOI] [PubMed] [Google Scholar]
- Teh BT, Farnebo F, Kristoffersson U, Sundelin B, Cardinal J, Axelson R, et al. Autosomal dominant primary hyperparathyroidism and jaw tumor syndrome associated with renal hamartomas and cystic kidney disease: linkage to 1q21-q32 and loss of the wild type allele in renal hamartomas. J Clin Endocrinol Metab. 1996;81:4204–4211. doi: 10.1210/jcem.81.12.8954016. [DOI] [PubMed] [Google Scholar]
- Teh BT, Farnebo F, Twigg S, Höög A, Kytölä S, Korpi-Hyövälti E, et al. Familial isolated hyperparathyroidism maps to the hyperparathyroidism-jaw tumor locus in 1q21-q32 in a subset of families. J Clin Endocrinol Metab. 1998;83:2114–2120. doi: 10.1210/jcem.83.6.4896. [DOI] [PubMed] [Google Scholar]
- Simonds WF, James-Newton LA, Agarwal SK, Yang B, Skarulis MC, Hendy GN, et al. Familial isolated hyperparathyroidism: Clinical and genetic characteristics of thirty-six kindreds. Medicine (Baltimore) 2002;81:1–26. doi: 10.1097/00005792-200201000-00001. [DOI] [PubMed] [Google Scholar]
- Simonds WF, Robbins CM, Agarwal SK, Hendy GN, Carpten JD, Marx SJ. Familial isolated hyperparathyroidism is rarely caused by germline mutation in HRPT2, the gene for the hyperparathyroidism-jaw tumor syndrome. J Clin Endocrinol Metab. 2004;89:96–102. doi: 10.1210/jc.2003-030675. [DOI] [PubMed] [Google Scholar]
- Carpten JD, Robbins CM, Villablanca A, Forsberg L, Presciuttini S, Bailey-Wilson J, et al. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet. 2002;32:676–680. doi: 10.1038/ng1048. [DOI] [PubMed] [Google Scholar]
- Shattuck TM, Valimaki S, Obara T, Gaz RD, Clark OH, Shoback D, et al. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N Engl J Med. 2003;349:1722–1729. doi: 10.1056/NEJMoa031237. [DOI] [PubMed] [Google Scholar]
- Cetani F, Pardi E, Borsari S, Viacava P, Dipollina G, Cianferotti L, et al. Genetic analyses of the HRPT2 gene in primary hyperparathyroidism: germline and somatic mutations in familial and sporadic parathyroid tumors. J Clin Endocrinol Metab. 2004;89:5583–5591. doi: 10.1210/jc.2004-0294. [DOI] [PubMed] [Google Scholar]
- Wade PA, Werel W, Fentzke RC, Thompson NE, Leykam JF, Burgess RR, et al. A novel collection of accessory factors associated with yeast RNA polymerase II. Protein Expr Purif. 1996;8:85–90. doi: 10.1006/prep.1996.0077. [DOI] [PubMed] [Google Scholar]
- Shi X, Chang M, Wolf AJ, Chang CH, Frazer-Abel AA, Wade PA, et al. Cdc73p and Paf1p are found in a novel RNA polymerase II-containing complex distinct from the Srbp-containing holoenzyme. Mol Cell Biol. 1997;17:1160–1169. doi: 10.1128/mcb.17.3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt-Rosen O, Hughes CM, Nannepaga SJ, Shanmugam KS, Copeland TD, Guszczynski T, et al. The parafibromin tumor suppressor protein is part of a human Paf1 complex. Mol Cell Biol. 2005;25:612–620. doi: 10.1128/MCB.25.2.612-620.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yart A, Gstaiger M, Wirbelauer C, Pecnik M, Anastasiou D, Hess D, et al. The HRPT2 tumor suppressor gene product parafibromin associates with human PAF1 and RNA polymerase II. Mol Cell Biol. 2005;25:5052–5060. doi: 10.1128/MCB.25.12.5052-5060.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Mandal SS, Pham AD, Zheng Y, Erdjument-Bromage H, Batra SK, et al. The human PAF complex coordinates transcription with events downstream of RNA synthesis. Genes Dev. 2005;19:1668–1673. doi: 10.1101/gad.1292105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosimann C, Hausmann G, Basler K. Parafibromin/Hyrax activates Wnt/Wg target gene transcription by direct association with beta-catenin/Armadillo. Cell. 2006;125:327–341. doi: 10.1016/j.cell.2006.01.053. [DOI] [PubMed] [Google Scholar]
- Krebs LJ, Shattuck TM, Arnold A. HRPT2 mutational analysis of typical sporadic parathyroid adenomas. J Clin Endocrinol Metab. 2005;90:5015–5017. doi: 10.1210/jc.2005-0717. [DOI] [PubMed] [Google Scholar]
- Bradley KJ, Cavaco BM, Bowl MR, Harding B, Cranston T, Fratter C, et al. Parafibromin mutations in hereditary hyperparathyroidism syndromes and parathyroid tumours. Clin Endocrinol. 2006;64:299–306. doi: 10.1111/j.1365-2265.2006.02460.x. [DOI] [PubMed] [Google Scholar]
- Gagel RF, Marx SJ.Multiple endocrine neoplasiaIn: Kronenberg HM, Melmed S, Polonsky KS, Larsen PR (eds)Williams Textbook of Endocrinology11th edn. WB Saunders & Co: Philadelphia; 20071705–1746. [Google Scholar]
- Heppner C, Kester MB, Agarwal SK, Debelenko LV, Emmert-Buck MR, Guru SC, et al. Somatic mutation of the MEN1 gene in parathyroid tumours. Nat Genet. 1997;16:375–378. doi: 10.1038/ng0897-375. [DOI] [PubMed] [Google Scholar]
- Farnebo F, Teh BT, Kytola S, Svensson A, Phelan C, Sandelin K, et al. Alterations of the MEN1 gene in sporadic parathyroid tumors. J Clin Endocrinol Metab. 1998;83:2627–2630. doi: 10.1210/jcem.83.8.4846. [DOI] [PubMed] [Google Scholar]
- Wang P, Bowl MR, Bender S, Peng J, Farber L, Chen J, et al. Parafibromin, a component of the human PAF complex, regulates growth factors and is required for embryonic development and survival in adult mice. Mol Cell Biol. 2008;28:2930–2940. doi: 10.1128/MCB.00654-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Zhang JH, Panicker LM, Simonds WF. The parafibromin tumor suppressor protein inhibits cell proliferation by repression of the c-myc proto-oncogene. Proc Natl Acad Sci U S A. 2008;105:17420–17425. doi: 10.1073/pnas.0710725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt-Rosen O, Nagaike T, Francis JM, Kaneko S, Glatt KA, Hughes CM, et al. The tumor suppressor Cdc73 functionally associates with CPSF and CstF 3′ mRNA processing factors. Proc Natl Acad Sci U S A. 2009;106:755–760. doi: 10.1073/pnas.0812023106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Panicker LM, Seigneur EM, Lin L, House CD, Morgan W, et al. Cytoplasmic polyadenylation element binding protein is a conserved target of tumor suppressor HRPT2/CDC73. Cell Death Differ. 2010;17:1551–1565. doi: 10.1038/cdd.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert F, Pelletier J. Translation initiation: a critical signalling node in cancer. Expert Opin Ther Targets. 2009;13:1279–1293. doi: 10.1517/14728220903241625. [DOI] [PubMed] [Google Scholar]
- Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–6422. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- Graff JR, Zimmer SG. Translational control and metastatic progression: enhanced activity of the mRNA cap-binding protein eIF-4E selectively enhances translation of metastasis-related mRNAs. Clin Exp Metastasis. 2003;20:265–273. doi: 10.1023/a:1022943419011. [DOI] [PubMed] [Google Scholar]
- Yeh CJ, Chuang WY, Chao YK, Liu YH, Chang YS, Kuo SY, et al. High expression of phosphorylated 4E-binding protein 1 is an adverse prognostic factor in esophageal squamous cell carcinoma. Virchows Arch. 2011;458:171–178. doi: 10.1007/s00428-010-0994-5. [DOI] [PubMed] [Google Scholar]
- Li BD, Liu L, Dawson M, De Benedetti A. Overexpression of eukaryotic initiation factor 4E (eIF4E) in breast carcinoma. Cancer. 1997;79:2385–2390. [PubMed] [Google Scholar]
- Rojo F, Najera L, Lirola J, Jimenez J, Guzman M, Sabadell MD, et al. 4E-binding protein 1, a cell signaling hallmark in breast cancer that correlates with pathologic grade and prognosis. Clin Cancer Res. 2007;13:81–89. doi: 10.1158/1078-0432.CCR-06-1560. [DOI] [PubMed] [Google Scholar]
- Castellvi J, Garcia A, Rojo F, Ruiz-Marcellan C, Gil A, Baselga J, et al. Phosphorylated 4E binding protein 1: a hallmark of cell signaling that correlates with survival in ovarian cancer. Cancer. 2006;107:1801–1811. doi: 10.1002/cncr.22195. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Cell death in normal and rough eye mutants of Drosophila. Development. 1991;113:825–839. doi: 10.1242/dev.113.3.825. [DOI] [PubMed] [Google Scholar]
- Lasko P. Gene regulation at the RNA layer: RNA binding proteins in intercellular signaling networks. Sci STKE. 2003;2003:RE6. doi: 10.1126/stke.2003.179.re6. [DOI] [PubMed] [Google Scholar]
- Miron M, Lasko P, Sonenberg N. Signaling from Akt to FRAP/TOR targets both 4E-BP and S6 K in Drosophila melanogaster. Mol Cell Biol. 2003;23:9117–9126. doi: 10.1128/MCB.23.24.9117-9126.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller CL, Jaehning JA. Ctr9, Rtf1, and Leo1 are components of the Paf1/RNA polymerase II complex. Mol Cell Biol. 2002;22:1971–1980. doi: 10.1128/MCB.22.7.1971-1980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haven CJ, Howell VM, Eilers PH, Dunne R, Takahashi M, van Puijenbroek M, et al. Gene expression of parathyroid tumors: molecular subclassification and identification of the potential malignant phenotype. Cancer Res. 2004;64:7405–7411. doi: 10.1158/0008-5472.CAN-04-2063. [DOI] [PubMed] [Google Scholar]
- Lin L, Czapiga M, Nini L, Zhang JH, Simonds WF. Nuclear localization of the parafibromin tumor suppressor protein implicated in the hyperparathyroidism-jaw tumor syndrome enhances its proapoptotic function. Mol Cancer Res. 2007;5:183–193. doi: 10.1158/1541-7786.MCR-06-0129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.