Abstract
Precuneus responds to a wide range of cognitive processes. Here, we examined how the patterns of resting state connectivity may define functional subregions in the precuneus. Using a K-means algorithm to cluster the whole-brain “correlograms” of the precuneus in 225 adult individuals, we corroborated the dorsal-anterior, dorsal-posterior, and ventral subregions, each involved in spatially guided behaviors, mental imagery, and episodic memory as well as self-related processing, with the ventral precuneus being part of the default mode network, as described extensively in earlier work. Furthermore, we showed that the lateral/medial volumes of dorsal anterior and dorsal posterior precuneus are each connected with areas of motor execution/attention and motor/visual imagery, respectively. Compared to the ventral precuneus, the dorsal precuneus showed greater connectivity with occipital and posterior parietal cortices, but less connectivity with the medial superior frontal and orbitofrontal gyri, anterior cingulate cortex as well as the parahippocampus. Compared to dorsal-posterior and ventral precuneus, the dorsal-anterior precuneus showed greater connectivity with the somatomotor cortex, as well as the insula, supramarginal, Heschl’s, and superior temporal gyri, but less connectivity with the angular gyrus. Compared to ventral and dorsal-anterior precuneus, dorsal-posterior precuneus showed greater connectivity with the middle frontal gyrus. Notably, the precuneus as a whole has negative connectivity with the amygdala and the lateral and inferior orbital frontal gyri. Finally, men and women differed in the connectivity of precuneus. Men and women each showed greater connectivity with the dorsal precuneus in the cuneus and medial thalamus, respectively. Women also showed greater connectivity with ventral precuneus in the hippocampus/parahippocampus, middle/anterior cingulate gyrus, and middle occipital gyrus, compared to men. Taken together, these new findings may provide a useful platform upon which to further investigate sex-specific functional neuroanatomy of the precuneus and to elucidate the pathology of many neurological illnesses.
Keywords: functional connectivity, fMRI, resting state, precuneus, default network
Introduction
A part of the medial posterior parietal cortex, the precuneus is engaged in reflective, self-related processing (Kjaer et al., 2002, Lou et al., 2004), awareness and conscious information processing (Kjaer et al., 2001, Vogt and Laureys, 2005), episodic memory (Dorfel et al., 2009, Lundstrom et al., 2005, Lundstrom et al., 2003), and visuospatial processing (Kawashima et al., 1995, Wenderoth et al., 2005), as well as showing greater activity during resting as compared to responding to an external task (Fransson and Marrelec, 2008) (see also (Cavanna, 2007, Cavanna and Trimble, 2006) for review). These different processes may selectively involve subregions within the precuneus. Indeed, axonal tracings in macaque monkeys revealed distinct patterns of anatomical connectivity for three subdivisions of the precuneus (Buckwalter et al., 2008, Colby et al., 1988, Leichnetz, 2001, Morecraft et al., 2004, Pandya and Seltzer, 1982). The dorsal-anterior precuneus is connected with medial somatomotor regions (Morecraft et al., 2004); the posterior precuneus with visual areas (Colby et al., 1988); and the ventral precuneus with the dorsolateral prefrontal cortex, the inferior parietal lobule, and the superior temporal sulcus (Morecraft et al., 2004, Pandya and Seltzer, 1982).
Numerous studies have suggested connectivity analysis of resting state fMRI data as a useful alternative to characterize functional subdivisions of a brain region. This approach parceled brain areas on the basis that each subregion has a unique pattern of connectivities – a “functional fingerprint” (Passingham et al., 2002). Specifically, low frequency blood oxygenation level dependent (BOLD) signal fluctuations reflect connectivity between functionally related brain regions (Biswal et al., 1995, Fair et al., 2007, Fox and Raichle, 2007). Studies of this “spontaneous” activity have provided insight into the intrinsic functional architecture of the brain and shown that the spontaneous fluctuations are present in many neuroanatomical systems, including motor, visual, auditory, default mode, memory, language, dorsal attention, and ventral attention systems (Fox and Raichle, 2007). Based on the findings that regions with similar functionality tend to be correlated in their spontaneous BOLD activity, investigators described subareal boundaries for the thalamus (Zhang et al., 2008, Zhang et al., 2010), basal ganglia (Barnes et al., 2010), medial superior frontal cortex (Kim et al., 2010, Zhang et al., 2011), anterior cingulate cortex (Margulies et al., 2007), cerebellum (O’Reilly et al., 2010), as well as precuneus (Cauda et al., 2010, Margulies et al., 2009).
In accord with anatomical studies, a recent work confirmed three functional subdivisions of the precuneus in anesthetized monkeys and awake humans on the basis of resting state connectivity analysis of BOLD signals (Margulies et al., 2009). In the study, however, the seed regions (spheres of 3mm in radius) covered only the medial wall of the precuneus and provided little information on brain volumes laterally beyond the seeds. This is in contrast to a wider extent of precuneus activation (x=−23 to +15) during cognitive performance as has been reported in the literature (Cavanna and Trimble, 2006). Another recent study characterized functional connectivity of the posterior medial cortex but similarly focused only on the medial wall of the precuneus (Cauda et al., 2010).
We have three specific aims in this study. First, we sought to investigate functional subdivisions of the entire precuneus by characterizing both cortical and subcortical connectivities of a large resting state fMRI data set. In particular, we characterized the connectivity of the lateral versus medial parts of the precuneus and examined whether or which part of the precuneus belongs to the default mode network. Second, we examined the differences in regional connectivities and highlighted the opposing pattern of connectivities between the three precuneus subdivisions. Third, recent works suggested sex differences in functional connectivity of brain areas including those of the default mode network (Biswal et al., 2010, Weissman-Fogel et al., 2010). We thus explored sex differences in precuenus connectivities.
Materials and methods
Resting state data
Resting-state fMRI scans were pooled from three datasets (Leiden_2180/Leiden_2200, Newark, and Beijing_Zang, n=144) downloadable from the 1000 Functional Connectomes Project (Biswal et al., 2010) and our own data (n=81). Individual subjects’ images were viewed one by one to ensure that the whole brain was covered. A total of 225 healthy subjects’ resting state data (3-Tesla magnet; 18–53 (mean = 24) years of age; 109 men; one scan per participant; duration: 4.5–10 minutes; eyes closed during resting) were analyzed (Table 1). Figure 1 illustrates the analyses step by step with the details described as follows.
Table 1.
Available demographic data and imaging parameters for the selected resting-state functional MRI datasets from the image repository for the 1000 Functional Connectomes Project and for our own dataset.
| Dataset | Subjects | Ages (years) | Timepoints | TR (s) | Slice acquisition order |
|---|---|---|---|---|---|
| Beijing_Zang | 31 M/66 F | 18 – 26 | 225 | 2 | Interleaved ascending |
| Leiden_2180 | 10 M/0 F | 20 – 27 | 215 | 2.18 | Sequential descending |
| Leiden_2200 | 11 M/8 F | 18 – 28 | 215 | 2.2 | Sequential descending |
| Newark | 9 M/9 F | 21 – 39 | 135 | 2 | Interleaved ascending |
| Our own | 48 M/33 F | 19 – 53 | 295 | 2 | Interleaved ascending |
Note: M, males; F, females.
Figure 1.
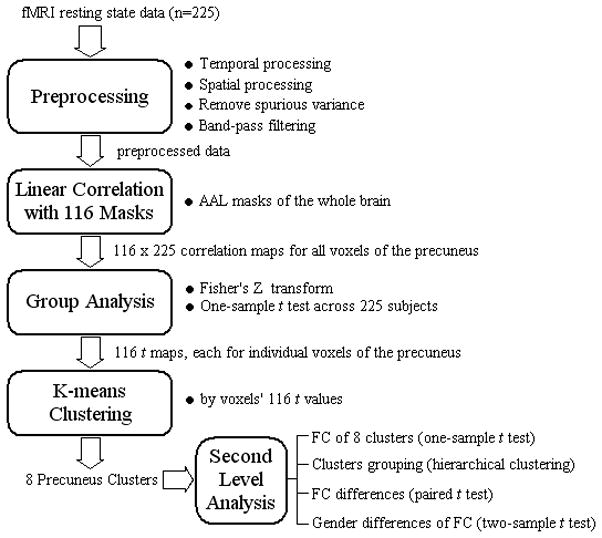
A flow chart of data analysis in this study. FC: functional connectivity;
Imaging data preprocessing
Brain imaging data were preprocessed using Statistical Parametric Mapping (SPM 8, Wellcome Department of Imaging Neuroscience, University College London, U.K.), as described in our previous work (Zhang et al., 2011). Briefly, images of each individual subject were first realigned (motion corrected) and corrected for slice timing. Individual structural image was normalized to an MNI (Montreal Neurological Institute) EPI (echo-planar imaging) template with affine registration followed by nonlinear transformation (Ashburner and Friston, 1999, Friston et al., 1995). The normalization parameters determined for the structural volume were then applied to the corresponding functional image volumes for each subject. Finally, the images were smoothed with a Gaussian kernel of 8 mm at Full Width at Half Maximum. In a separate analysis, we used 4 mm Gaussian kernel smoothing and obtained nearly identical results (Supplementary Materials).
Additional preprocessing was applied to reduce spurious BOLD variances that were unlikely to reflect neuronal activity (Fair et al., 2007, Fox and Raichle, 2007, Fox et al., 2005, Rombouts et al., 2003). The sources of spurious variance were removed through linear regression by including the signal from the ventricular system, the white matter, and the whole brain, in addition to the six parameters obtained by rigid body head motion correction. First-order derivatives of the whole brain, ventricular and white matter signals were also included in the regression.
Cordes and colleagues suggested that BOLD fluctuations below a frequency of 0.1Hz contribute to regionally specific BOLD correlations (Cordes et al., 2001). The majority of resting state studies low-pass filtered BOLD signal at a cut-off of 0.08 or 0.1 Hz (Fox and Raichle, 2007). Thus, we applied a temporal band-pass filter (0.009Hz < f < 0.08Hz) to the time course in order to obtain low-frequency fluctuations (Fair et al., 2007, Fox and Raichle, 2007, Fox et al., 2005, Lowe et al., 1998).
Linear correlations with 116 anatomical masks
We used the anatomical parcelation algorithm to delineate 116 anatomical masks from the Montreal Neurological Institute (MNI) template (Tzourio-Mazoyer et al., 2002). The BOLD time courses were averaged spatially each for all 116 seed regions. We computed the correlation coefficient between the averaged time course of each mask and the time courses of each individual voxels of the precuneus for individual subjects.
To assess and compare the resting state “correlograms,” we converted these image maps, which were not normally distributed, to z score maps by Fisher’s z transform (Berry and Mielke, 2000, Charles F. Bond and Richardson, 2004, Jenkins and Watts, 1968): z = 0.5 loge [(1 + r)/(1 − r)]. The z maps were used in group, random effect analyses (Penny et al., 2004). A one-sample t-test was applied to the “z maps” across 225 subjects for each of the 116 correlograms for further analysis.
Parcelation of the precuneus based on functional connectivity
Voxels within the precuneus mask, which was obtained from the 116 anatomical masks of an MNI template created by Tzourio-Mazoyer et al. (2002), were subject to functional connectivity based segmentation, with each voxel represented by 116 t values. A K-means algorithm was applied to cluster the voxels within the precuneus on the bases of the 116 t values.
As an unsupervised learning algorithm, K-means clustering classifies a given data set into an a-priori set of K clusters by minimizing an objective squared error function as shown in Eq. (1):.
| (1) |
where is a distance measure between a data point and the cluster center cj (MacQueen, 1967). The algorithm was executed by:
Placing K points into the space represented by the objects that are being clustered. These points represent initial group centroids.
Assigning each object to the group that has the closest centroid.
When all objects have been assigned, recalculating the positions of the K centroids.
Repeating Steps 2 and 3 until the centroids no longer move. This produces a separation of the objects into groups from which the metric to be minimized can be calculated.
In order to determine the optimal number of clusters that best described the data set, we used the Bayesian Information Criterion (BIC) (Gentle et al., 2004, Schwarz, 1978), which is widely used for model identification in time series and linear regression:
| (2) |
where n is the number of observations (=116); k is the number of class; RSS is the residual sum of squares from the K-means model. Given any two clustering number k’s, the one with lower BIC value was preferred. Furthermore, because the K-means algorithm is sensitive to the initial, randomly selected cluster centers, we repeated this algorithm 1,000 times to alleviate the effect of the initial conditions.
Furthermore, we also employed spectral clustering analysis, which yielded similar results as K-means clustering (Supplementary Materials).
Results
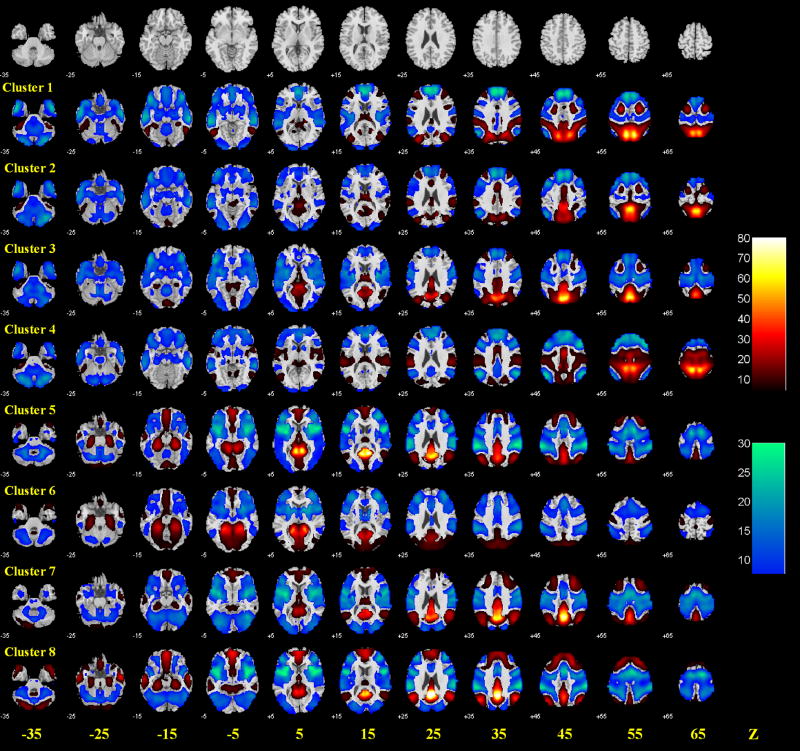
The results of 1,000 runs of K-means clustering suggested an optimal cluster number of 8 according to the BIC (Supplementary Figure 2a). Figure 2 and Figure 3 each shows these 8 clusters and the t statistic connectivity map of individual clusters.
Figure 2.
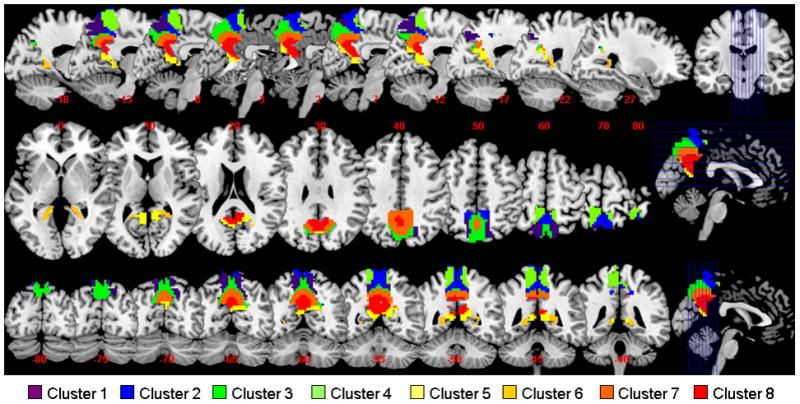
K-means clustering segments the precuneus based on functional connectivities of individual voxels within the region. Eight clusters were represented in axial, sagittal, and coronal sections with different colors.
Figure 3.
Group results of voxel-wise functional connectivity of each of the eight clusters of the precuneus. Positive (warm color) and negative (cold color) correlations were superimposed on axial slices at Z = −35, −25, −15, −5, 5, 15, 25, 35, 45, 55, 65 mm of a structural image. n = 225, p<0.05, corrected for family-wise error or FWE of multiple comparisons. Color scales reflect T values of one-sample t test.
To examine the relationship of the 8 clusters identified from K-means clustering, we applied hierarchical clustering to their connectivity maps (see Supplement for methodological details). The results showed that the 8 clusters were broadly divided into the dorsal (clusters 1–4) and ventral precuneus (clusters 5–8) (Supplementary Figure 2b). We thus re-ran K means clustering for two- and three- cluster solutions. The results confirmed the findings that the precuneus could be separated into dorsal and ventral parts, and the dorsal part could be further separated into dorsal-anterior and dorsal-posterior subregions (Supplementary Figure 3).
The dorsal precuneus (clusters 1–4)
The dorsal precuneus comprised the lateral dorsal-anterior precuneus (cluster 4), the medial dorsal-anterior precuneus (cluster 2), the lateral dorsal-posterior precuneus (cluster 1), and the medial dorsal-posterior precuneus (cluster 3) (Figure 2).
The dorsal precuneus (clusters 1–4) showed positive connectivity with the superior parietal cortex, and negative connectivity with the middle, left medial, and right lateral parts of the orbital frontal gyri, left superior frontal gyrus, orbital part of the inferior frontal gyrus, medial superior frontal gyrus, gyrus rectus, amygdala, as well as cerebellar lobule IX and right cerebellar lobule VIII (Schmahmann et al., 1999, Schmahmann et al., 2000) (Figure 3 and Supplementary Table 1).
Other than these connectivities shared by all four clusters of the dorsal precuneus, the dorsal-anterior precuneus (clusters 2 and 4) also showed positive connectivity with the supplementary motor area, right supramarginal gyrus and paracentral lobule, and negative connectivity with the left lateral part of the orbital frontal gyrus, left triangular part of inferior frontal gyrus, right medial part of the orbital frontal gyrus, left angular gyrus, inferior temporal gyrus, and right cerebellar crus II and lobule VII b. The dorsal-posterior precuneus (clusters 1 and 3) showed positive connectivity with the right posterior cingulate cortex, right cuneus and superior occipital gyrus, and negative connectivity with the rolandic operculum, insula, Heschl’s gyrus, superior and middle temporal gyri and temporal pole, as well as left cerebellar lobule X and cerebellar vermis lobule VI, VII, VIII, IX X (Figure 3 and Supplementary Table 1).
Positive connectivity was observed between the lateral dorsal-anterior precuneus (cluster 4) and the right primary motor cortex, rolandic operculum, postcentral lobule, left supramarginal gyrus, Heschl’s and superior temporal gyri; between the medial dorsal-anterior precuneus (cluster 2) and the middle and posterior cingulate cortices, cuneus, superior occipital gyrus, and cerebellar vermis lobule III; between the lateral dorsal-posterior precuneus (cluster 1) and the middle occipital gyrus, intraparietal sulcus, and right paracentral lobule; and between the medial dorsal-posterior precuneus (cluster 3) and the middle and posterior cingulate cortices, calcarine sulcus, cuneus, lingual, right angular, and cerebellar vermis lobule III, IV V (Figure 3 and Supplementary Table 1).
Negative connectivity was observed between the lateral dorsal-anterior precuneus (cluster 4) and middle frontal gyrus, right triangular part of inferior frontal gyrus, left posterior cingulate cortex, right angular, anterior cingulate cortex, and right cerebellar crus II; between the medial dorsal-anterior precuneus (cluster 2) and the left primary motor cortex, left opercular part of the inferior frontal gyrus, left hippocampus and parahippocampus, inferior occipital gyrus, left middle temporal gyrus and temporal pole, left cerebellar lobule VIII, X, and vermis lobule VIII, IX,X; between the lateral dorsal-posterior precuneus (cluster 1) and the left olfactory bulb, right medial part of the orbital frontal gyrus, anterior cingulate cortex and parahippocampus; and between the medial dorsal-posterior precuneus (cluster 3) and the primary motor cortex, right superior frontal gyrus, left lateral part of orbital frontal gyrus, opercular and triangular parts of the inferior frontal gyri, supplementary motor area, hippocampus, inferior occipital gyrus, postcentral gyrus, supramarginal gyrus, left paracentral lobule, inferior temporal gyrus, and cerebellar lobule VIII, X.
The ventral precuneus (clusters 5–8)
The ventral precuneus (clusters 5–8) showed positive connectivity with the left medial part of the orbital frontal gyrus, posterior cingulate cortex, calcarine sulcus, cuneus, left cerebellar lobule IV, V and cerebellar vermis lobule III, IV, V, and negative connectivity with the left primary motor cortex, lateral part of the orbital frontal gyrus, opercular, triangular, and orbital parts of inferior frontal gyri, supplementary motor area, insula, amygdala, supramarginal gyrus, superior temporal pole, cerebellar lobule VIII (Figure 3 and Supplementary Table 1). Clusters 5, 7, and 8 shared a similar pattern of connectivity (Supplementary Figure 2b), showing positive connectivity with the right medial part of the orbital frontal gyrus and angular gyrus, and negative connectivity with the right primary motor cortex, rolandic operculum, inferior occipital gyrus, postcentral lobule, left paracentral lobule, Heschl’s gyrus, superior temporal gyrus, right middle temporal pole, right cerebellar crus II, and cerebellar vermis lobule VIII, IX, X (Figure 3 and Supplementary Table 1).
Positive connectivity can be found in the right olfactory bulb, gyrus rectus, parahippocampus, lingual, superior occipital gyrus, fusiform gyrus, cerebellar hemisphere lobule III, IV, V, and left cerebellar lobule VI for the medial-ventral part of ventral precuneus (cluster 5); in the rectus gyrus, parahippocampus, lingual, superior, middle, and inferior occipital gyri, fusiform gyrus, cerebellar lobule III, IV, V, VI, X as well as vermis lobuleI, II, VI for lateral-ventral part of ventral precuneus (cluster 6); in the middle cingulate cortex and left superior occipital gyrus for dorsal part of ventral precuneus (cluster 7); and in the middle part of the orbital frontal gyrus, olfactory bulb, medial superior frontal gyrus, rectus gyrus, middle cingulate cortex, parahippocampus, and cerebellar lobule III, IV, V for middle part of ventral precuneus (cluster 8) (Figure 3 and Supplementary Table 1). Negative connectivity was observed in the superior and middle frontal gyri, superior parietal gyurs, intraparietal sulcus, left middle temporal gyrus, inferior temporal gyrus, left cerebellar crus II, and cerebellar lobule VIIb, IX for medial-ventral part of ventral precuneus (cluster 5); in the superior and middle frontal gyri, medial superior frontal gyrus, anterior and middle cingulate cortices, intraparietal sulcus, left cerebellar crus II, and right cerebellar lobule VIIb for lateral-ventral part of ventral precuneus (cluster 6); in the right hippocampus, left middle occipital gyrus, left middle temporal gyrus and temporal pole, right inferior temporal gyrus, cerebellar crus I and lobule VI, IX, as well as vermis lobule VI, VII for dorsal part of ventral precuneus (cluster 7); and in the right middle frontal gyrus, middle occipital gyrus, superior parietal gyrus, intraparietal sulcus, cerebellar crus I,II and lobule VI, VIIb and vermis lobule VI, VII for middle part of ventral precuneus (cluster 8).
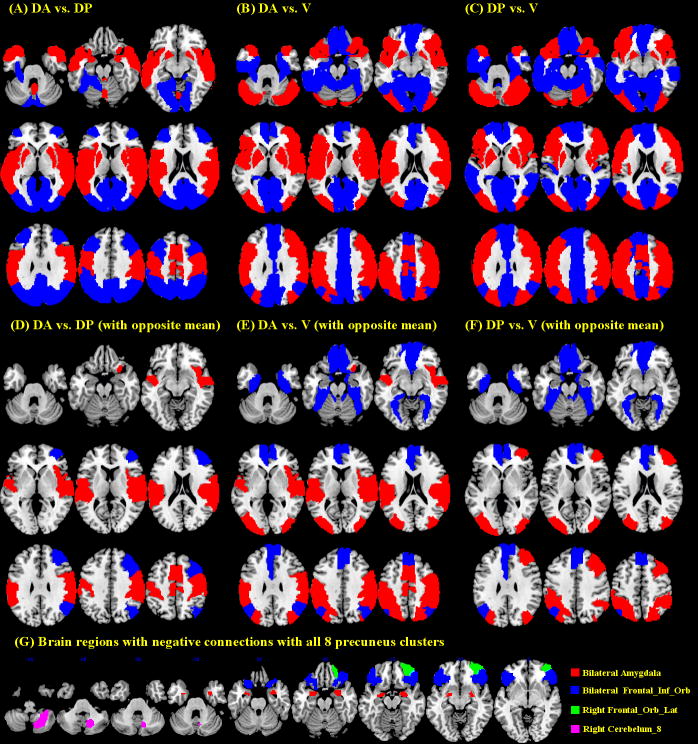
Notably, all eight precuneus clusters showed negative connectivity with right lateral part of the orbital frontal gyrus, orbital part of inferior frontal gyrus, amygdala, and right cerebellar lobule VIII (Figure 3, Figure 4G and Supplementary Table 1).
Figure 4.
Differences in functional connectivity between dorsal-anterior (DA), dorsal-posterior (DP), and ventral (V) precuneus. (A-C, upper row) Connectivities with each of the 116 AAL masks was examined with pairwise paired t tests. Results at p < 0.00014 (p<0.05, corrected for multiple comparisons) are superimposed on axial slices at Z= −30, −20, −10, 0, 10, 20, 30, 40, 50 mm of a structural image. Red: DA > DP (A), DA > V (B), and DP > V (C); blue: DP > DA, V > DA, and V > DP. (D-F, bottom row) Significant differences are shown only for regions with an opposite pattern of connectivity between DA and DP (D), DA and V (E), and DP and V (F). See Supplementary Tables 1, 2, and 3 for additional information. (G) Brain regions with negative connectivity with all eight precuneus clusters. Frontal_Inf_Orb: inferior part of the orbital frontal gyrus; Frontal_Orb_Lat: lateral part of the orbital frontal gyrus; Cerebelum_8: cerebellum part 8;
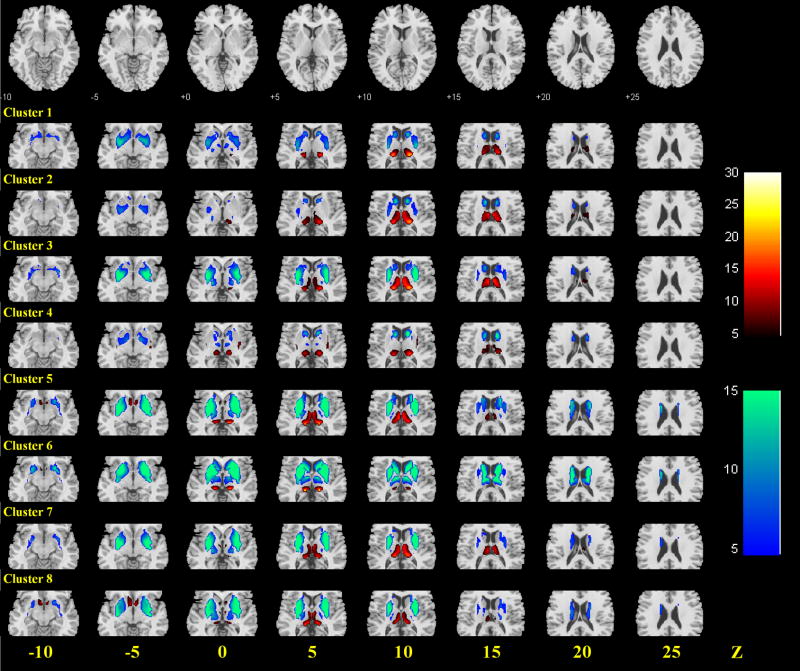
Functional connectivity with subcortical regions
We used a subcortical mask that encompassed the thalamus, caudate, putamen, and pallidum to describe subcortical connectivities of each of 8 clusters (Figure 5). Overall, the precuneus showed positive connectivity with the pulvinar and little or negative connectivity with the sensory thalamic nuclei as documented in studies of non-human primates (Schmahmann and Pandya, 1990, Yeterian and Pandya, 1993). Furthermore, the medial dorsal-anterior precuneus (cluster 2), medial dorsal-posterior precuneus (cluster 3), and the ventral-posterior precuneus (clusters 5, 7, and 8 but not cluster 6) showed positive connectivity with the mediodorsal (MD) nucleus of the thalamus.
Figure 5.
Group results of each of the 8 clusters, masked to show the subcortical connectivities. Positive (warm color) and negative (cold color) correlations were superimposed on eight axial slices at Z = −10, −5, 0, 5, 10, 15, 20, 25 mm of a structural image. n = 225, p<0.05, corrected for family-wise error or FWE of multiple comparisons. Color scales represents T values of one-sample t test.
In the basal ganglia, we observed negative connectivity between middle and dorsal parts of caudate and all eight clusters, and positive connectivity between the ventral part of the caudate and clusters 5 and 8 of ventral precuneus. The ventral (clusters 5–8) as well as dorsal-posterior precuneus (clusters 1 and 3) also had negative connectivity with the putamen and pallidum.
Differences in functional connectivity between the three precuneus clusters
We quantified the differences in functional connectivity of the dorsal-anterior, dorsal-posterior, and ventral precuneus. To better represent the results, we separated the whole brain into 116 regions based on the AAL atlas and examined the differences in functional connectivity with each region with paired t tests across the entire cohort of subjects. Results were summarized in Figure 4, and Supplementary Table 2, 3, and 4. In the following, we described those brain regions showing not only significant differences but also an opposite pattern of connectivity between the three precuneus subdivisions (Figure 4, D, E, and F, and Table 2).
Table 2.
Differences in functional connectivity (p < 0.00014, or p<0.05, corrected for multiple comparisons) between the dorsal-anterior and dorsal-posterior precuneus (DA vs. DP), between dorsal-anterior and ventral precuneus (DA vs. V), and between dorsal-posterior and ventral precuneus (DP vs. V). Only brain regions showing an opposite pattern of connectivity between the two respective clusters are shown here. “>” indicates DA>DP, DA>V, or DP>V, and “<” indicates DA<DP, DA<V, or DP<V.
| DA vs. DP | DA vs. V | DP vs. V | |
|---|---|---|---|
| Right primary motor cortex | > | > | |
| Bilateral middle part of orbital frontal gyrus | < | < | |
| Right middle frontal gyrus | < | > | |
| Bilateral rolandic operculum | > | > | |
| Bilateral supplementary motor area | > | > | |
| Bilateral olfactory bulb | < | < | |
| Bilateral medial superior frontal gyrus | < | < | |
| Bilateral medial part of orbital frontal gyrus | < | < | |
| Bilateral gyrus rectus | < | < | |
| Right insula | > | > | |
| Left anterior cingulated gyrus | < | < | |
| Bilateral paraHippocampal | < | < | |
| Bilateral middle occipital gyrus | > | > | |
| Bilateral fusiform gyrus | < | < | |
| Bilateral postcentral gyrus | > | > | |
| Bilateral superior parietal lobule | > | > | |
| Bilateral intraparietal sulcus | > | > | |
| Bilateral supramarginal gyrus | > | > | |
| Left angular | < | < | |
| Right angular | < | < | |
| Bilateral paracentral gyrus | > | > | |
| Bilateral heschl’s gyrus | > | > | |
| Bilateral superior temporal gyrus | > | > | |
| Left cerebellum part 3 | < | ||
| Right cerebellum part 3 | < | < | |
| Left cerebellum part 10 | < | < | |
| Right cerebellum part 10 | < | ||
| Vermis part 1 and 2 | < |
Compared to both the dorsal-posterior and and ventral precuneus, the dorsal-anterior precuneus showed greater connectivity with the right primary motor cortex (PMC), rolandic operculum, SMA, right insula, postcentral gyrus, supramarginal gyrus, Heschl’s gyrus, superior temporal gyrus, as well as less connectivity with the right angular gyrus. Compared to ventral precuneus, both dorsal-anterior and dorsal-posterior precuneus showed greater connectivity with middle occipital, inferior/superior parietal, and paracentral gyri. Conversely, compared to the dorsal precuneus, the ventral precuneus showed greater connectivity in middle part of orbital frontal gyrus, olfactory bulb, medial superior frontal gyrus, medial part of orbital frontal gyrus, gyrus rectus, left anterior cingulate gyrus, parahippocampal, left angular, and fusiform gyri, as well as right cerebellar lobule III and left cerebellar lobule X. Compared to the dorsal-anterior and ventral precuneus, the dorsal-posterior precuneus showed greater connectivity in the middle frontal gyrus. Compared to the ventral precuneus, the dorsal-anterior precuneus showed less connectivity in the left cerebellar lobule III and the dorsal-posterior precuneus showed less connectivity in the right cerebellar lobule X and vermis lobule I, II.
Sex differences in precuneus connectivity
We examined the functional connectivities of the precuneus separately for men (n = 109) and women (n = 116). Although men showed an optimal cluster number of 6 rather than 8, the patterns of connectivity of the major clusters were very similar between men and women and to those obtained with men and women combined (Supplementary Figures 4 and 5). Thus, to compare precuneus connectivity between men and women, we focused on the two-cluster solution of dorsal (clusters 1–4) and ventral (clusters 5–8) precuneus, as well as the three-cluster solution with additional subdivisions of the dorsal-posterior (clusters 1 and 3) and dorsal-anterior precuneus (clusters 2 and 4).
The results of two-sample t tests (cluster-level threshold of P<0.05, FWE corrected) are shown in Figure 6 and Table 3. Compared to women, men showed greater connectivity in the cuneus for dorsal precuneus. Conversely, compared to men, women showed greater connectivity in the thalamus and hypothalamus for dorsal precuneus and in hippocampus, parahippocampus, middle/anterior cingulate gyrus, and middle occipital gyrus for ventral precuneus.
Figure 6.
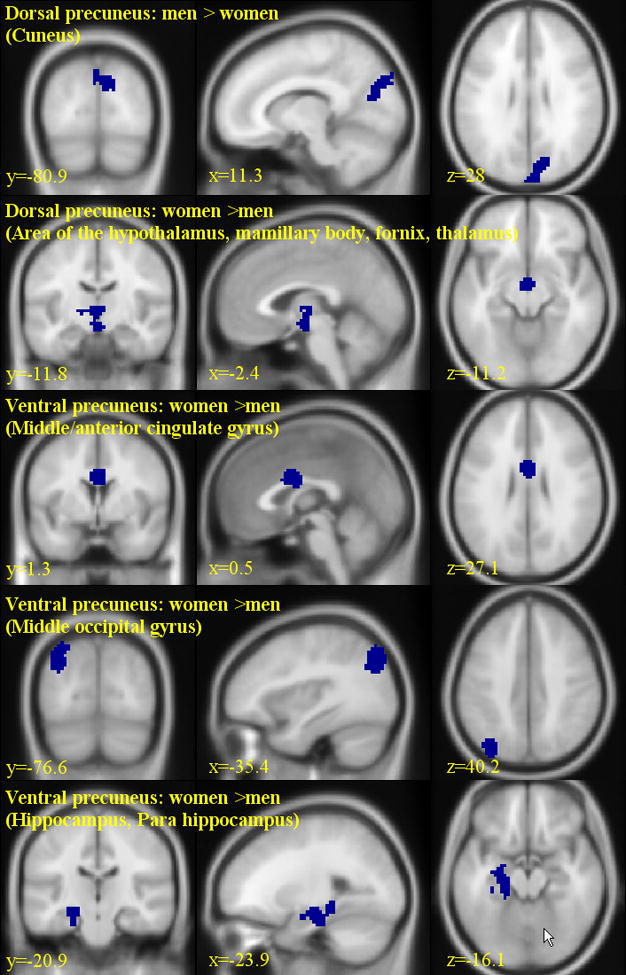
Gender differences (109 men vs. 116 women) in the functional connectivity of the precuneus (cluster-level threshold: p<0.05, FWE corrected). Significant regional differences were identified for dorsal precuneus of men > women (cuneus) and of women > men (area of the hypothalamus, mamillary body, fornix, and thalamus), as well as for ventral precuneus of women > men (middle/anterior cingulated gyrus, middle occipital gyrus, and hippocampus/para hippocampus) as shown in Tables 3.
Table 3.
Gender differences in functional connectivity of the dorsal precuneus (clusters 1–4) and ventral precuneus (clusters 5–8); cluster-level threshold: p<0.05, FWE corrected.
| Cluster size (voxels) | Voxel Z value | MNI coordinate (mm)
|
Identified region and approximate BA | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Dorsal precuneus: men > women | |||||
| 289 | 4.53 | 3 | −88 | 37 | Cuneus, BA 18/19 |
| Dorsal precuneus: women > men | |||||
| 116 | 4.99 | 0 | −10 | −14 | Area of the hypothalamus, MB, Fx |
| 4.14 | 0 | −13 | 1 | Thalamus | |
| Ventral precuneus: men > women | |||||
| None | |||||
| Ventral precuneus: women > men | |||||
| 104 | 5.02 | 0 | −1 | 25 | Middle/Anterior cingulate G, BA 24 |
| 154 | 4.74 | −36 | −76 | 40 | Middle occipital G, BA 7/19 |
| 197 | 4.37 | −24 | −19 | −14 | Hippocampus, BA 20 |
| 4.18 | −21 | −34 | −11 | Para hippocampus, BA 30 | |
Note: P < 0.001, uncorrected, and 20 voxels in extent of activation. G, gyrus; BA, Brodmann area; MB: mamillary body; Fx: fornix
Discussion
We presented resting state functional connectivity maps of the human precuneus. The patterns of connectivity were distinct for the dorsal-anterior, dorsal-posterior, and ventral precuneus, consistent with previous anatomical and functional mappings (Buckwalter et al., 2008, Cauda et al., 2010, Colby et al., 1988, Leichnetz, 2001, Margulies et al., 2009, Morecraft et al., 2004, Pandya and Seltzer, 1982). The three subdivisions not only showed statistically significant differences in but sometimes an opposing pattern of regional connectivities. Furthermore, women and men differed in these connectivities. Altogether, these results highlighted anatomical and functional heterogeneity of the precuneus and suggested the utility of connectivity mapping in the delineation of functional divisions of a brain area. We summarize the pattern of connectivities in Figure 7 and discussed the main findings in the following.
Figure 7.
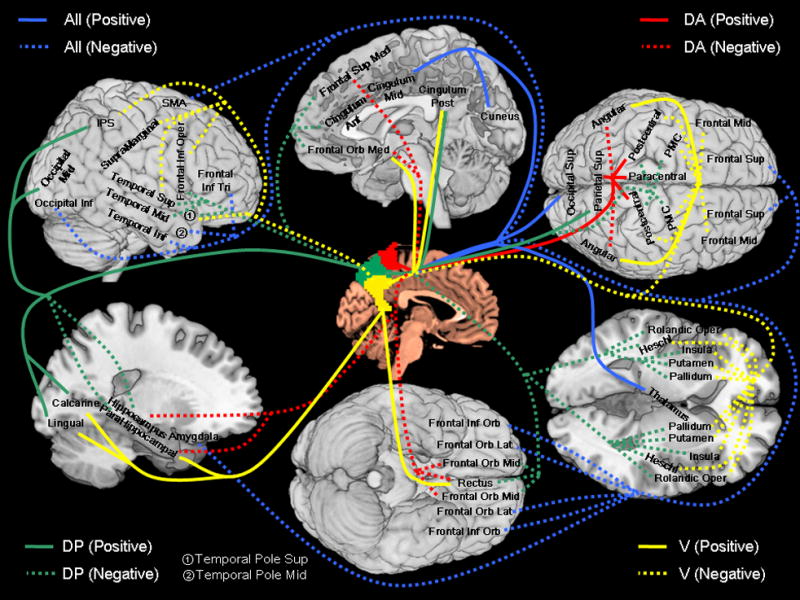
A summary of functional connectivity of the dorsal-anterior (DA, red), dorsal-posterior (DP, green), and ventral (V, yellow) precuneus, with blue indicating connectivity with all three regions. Positive and negative connectivities are each indicated by solid and dashed line. Abbreviations: PMC: primary motor cortex; Frontal Sup: superior frontal gyrus; Frontal Orb Mid: middle part of orbital frontal gyrus; Frontal Mid: middle frontal gyrus; Frontal Orb Lat: lateral part of orbital frontal gyrus; Frontal Inf Oper: opercular part of inferior frontal gyrus; Frontal Inf Tri: triangular part of inferior frontal gyrus; Frontal Inf Orb: orbital part of inferior frontal gyrus; Rolandic Oper: rolandic operculum; SMA: supplementary motor area; Frontal Sup Med: medial superior frontal gyrus; Frontal Orb Med: medial part of orbital frontal gyrus; Rectus: gyrus rectus; Cingulum Ant: anterior cingulated gyrus; Cingulum Mid: middle cingulated gyrus; Cingulum Post: posterior cingulated gyrus; Occipital Sup: superior occipital gyrus; Occipital Mid: middle occipital gyrus; Occipital Inf: inferior occipital gyrus; Postcentral: postcentral gyrus; Parietal Sup: superior parietal lobule; IPS: intraparietal sulcus; SupraMarginal: supramarginal gyrus; Paracentral: paracentral gyrus; Heschl: Heschl’s gyrus; Temporal Sup: superior temporal gyrus; Temporal Pole Sup: superior temporal pole; Temporal Mid: middle temporal gyrus; Temporal Pole Mid: middle temporal pole; Temporal Inf: inferior temporal gyrus;
The dorsal-anterior/posterior precuneus
We observed significant functional connectivity of the dorsal-anterior precuneus (clusters 2 and 4) with the superior parietal cortex, consistent with co-activation of these structures in the execution or preparation of spatially guided behaviors (Cavanna and Trimble, 2006, Wenderoth et al., 2005). Both lateral (cluster 1) and medial (cluster 3) dorsal-posterior precuneus showed strong connections with the superior occipital and parietal cortices, known to be involved in visual-spatial information processing (Cavada and Goldman-Rakic, 1989, Leichnetz, 2001), and responded to imagery during visual rotation (Suchan et al., 2002), deductive reasoning (Knauff et al., 2003), music processing (Platel et al., 1997, Satoh et al., 2001), spatial navigation (Ghaem et al., 1997), as well as motor imagery (Hanakawa et al., 2003, Malouin et al., 2003, Ogiso et al., 2000). Altogether, the dorsal-anterior and dorsal-posterior precuneus are each connected with networks involved in spatially guided behaviors and mental imagery.
Furthermore, extending previous work, we described distinct connectivities of the lateral and medial precuneus. The lateral dorsal-anterior region showed negative or little correlation, while the medial region showed positive correlation, with the posterior cingulate cortex, suggesting that the medial but not the lateral precuneus plays a role of a transition from the parieto-occipital to parieto-limbic cortex (Cavanna and Trimble, 2006, Margulies et al., 2009). The primary motor cortex showed strong connection with the lateral dorsal-anterior precuneus (cluster 4) but not with the medial dorsal-anterior precuneus (cluster 2), while the anterior cingulate cortex showed connection with the medial dorsal-anterior precuneus but not with the lateral dorsal-anterior precuneus. These contrasting patterns of connectivity suggest that the lateral and medial dorsal-anterior precuneus may each play a more important role in the execution and attentional monitoring of spatial behavior. Imaging studies support these functional distinctions (Astafiev et al., 2003, Simon et al., 2002). For instance, an earlier study showed that the lateral superior parietal cortex and precuneus responded to saccadic eye movements and manual pointing, but the left medial dorsal-anterior precuneus responded during attention to a peripheral visual target (Simon et al., 2002).
The lateral dorsal-posterior precuneus (cluster 1) was particularly responsive during motor imagery. For instance, in an fMRI study, Hanakawa et al. (2003) identified lateral dorsal-posterior precuneus activation during imagery as compared to execution of visually guided sequential finger-tapping. In a PET study, Malouin et al. (2003) showed activation in lateral dorsal-posterior precuneus during mental simulation of locomotion. Using magnetoencephalographic recording, (Ogiso et al., 2000) localized the dipole source in lateral dorsal-posterior precuneus when participants imagined themselves hurdling in self-centered space.
Taken together, the current results extended the roles of the dorsal anterior and posterior precuneus in spatially guided behavior and mental imagery: the medial/lateral dorsal anterior precuens are each specific to attentional monitoring/execution of spatially guided behavior; and the medial/lateral dorsal posterior precuens are each specific to visual/motor imagery.
The ventral precuneus
In reviewing functional imaging findings, Cavanna and Trimble (2006) suggested a central role for the precuneus in episodic memory retrieval and self-related processing. The MNI coordinates of the brain regions reviewed in Cavanna and Trimble (2006) were mostly within the ventral precuneus (especially clusters 7 and 8, as described here). For instance, recognition of meaningful sentences (Tulving et al., 1994), and retrieval of word-pairs (Shallice et al., 1994) as well as autobiographical information (Addis et al., 2004, Gilboa et al., 2004) activated dorsal part of ventral precuneus (cluster 7); recollection of previously studied words (Henson et al., 1999), retrieval of musical melody (Platel et al., 2003), and self-related processes such as empathy, intentionality judgment, attribution of emotional state and perspective taking (den Ouden et al., 2005, Farrow et al., 2001, Kircher et al., 2002, Ochsner et al., 2004, Ruby and Decety, 2001) activated middle part of ventral precuneus (cluster 8).
Notably, clusters 5, 6, 8 and part of the cluster 7 (Brodmann area 31) are often suggested to belong to both posterior cingulate and precuneate cortices and appear to be a cortical transition zone from the medial parietal areas to the posterior cingulate cortex (Cavanna, 2007). Our results confirmed this view by demonstrating strong functional connectivity of the ventral precuneus with the posterior cingulate cortex.
The precuneus and the default network
We observed a distinct pattern of positive connectivity with the default network for ventral but not dorsal precuneus, replicating (Cauda et al., 2010). This is also consistent with our recent study of independent component analyses delineating two networks each involving the dorsal (cuneus-precuneus network) and ventral (default network) precuneus (Zhang and Li, 2011). Similarly, Buckner et al. (2008) suggested that the dorsal precuneus, or Brodmann area 7, may not be part of the default network. A recent study also supported this view by showing decreased regional cerebral blood flow (CBF) in the posterior cingulate gyrus, medial frontal cortex and ventral but not dorsal precuneus, in participants performing a spatial working memory task, as compared to rest (Pfefferbaum et al., 2011). In contrast, our previous work suggested a role of the dorsal precuneus in mediating behavioral engagement (Zhang and Li, 2010). We observed that the fractional amplitude of low frequency fluctuation (fALFF) of the dorsal precuneus accounted for approximately 10% of the variance in prefrontal activations related to attentional monitoring and response inhibition in a stop signal task. Thus, although both the dorsal and ventral precuneus appear to be involved in behavioral engagement to an external task, their specific roles remained to be specified.
Precuneus connectivity with the inferior parietal lobule
We observed a distinct pattern of functional connectivity with the inferior parietal lobule, suggesting functional differentiation of the supramarginal gyrus, angular gyrus, and intraparietal sulcus (Caspers et al., 2008, Caspers et al., 2006, Uddin et al., 2010, Wu et al., 2009). The ventral precuneus was positively connected with the angular gyrus but not other parts of the inferior parietal lobule. With the exception of the medial dorsal-anterior precuneus, the dorsal and ventral precuneus were each positively and negatively connected with the intraparietal sulcus.
The angular gyrus was a key parietal node of the default network as described in both fMRI and positron emission tomography studies (Greicius et al., 2003, Raichle et al., 2001, Uddin et al., 2009, Uddin et al., 2010). A recent study showed that the angular but not supramarginal gyrus or intraparietal sulcus responded differently to task difficulty in mental arithmetic, consistent with its integral role in the default network (Wu et al., 2009). During cue-directed spatial attention the intraparietal sulcus showed greater activation during the early than late phase of the task while the opposite was true of the ventral precuneus (Schultz and Lennert, 2009). Moreover, in a learning task, the intraparietal sulcus responded more to untrained than trained epochs, while ventral precuneus as well as angular gyrus responded more to the opposite contrast (Delazer et al., 2005). In a task of object and place recognition, the ventral precuneus and intraparietal sulcus each responded more to place and object (Sugiura et al., 2005). Additionally, many studies showed that supramarginal gyrus, intraparietal sulcus, and dorsal precuneus, but not the angular gyrus or ventral precuneus, often activated concurrently to the same contrasts, under a variety of different experimental contexts (Filimon et al., 2009, Loayza et al., 2011, Stilla et al., 2007, Van de Winckel et al., 2005). Taken together, these studies suggested that the ventral precuneus and angular gyrus belong to a functional network in distinction to the one that comprises the dorsal precuneus, supramarginal gyrus, and intraparietal sulcus.
Subcortical connectivity of the precuneus
By interconnecting with cortical areas, subcortical structures play an important role in motor and cognitive control (Barnes et al., 2010, Draganski et al., 2008, Lehericy et al., 2004a, Lehericy et al., 2004b, Robinson et al., 2009, Schmahmann and Pandya, 1990, Yeterian and Pandya, 1993, Zhang et al., 2008, Zhang et al., 2010). The precuneus projects to dorsal thalamus, the intralaminar nucleus, and pulvinar, but not sensory thalamus, such as the ventral posterior lateral nucleus (Cavanna and Trimble, 2006, Parvizi et al., 2006, Schmahmann and Pandya, 1990, Yeterian and Pandya, 1993, Zhang et al., 2008). In accord with these previous studies, the current findings suggested that the precuneus does not share the thalamic connectivity with the parietal somatosensory cortical regions (Cavanna and Trimble, 2006). As with Cauda et al. (2010), we also showed positive connectivity between the ventral precuneus and mediodorsal thalamus, and negative connectivity between the ventral precuneus and sensory thalamic nuclei. We observed positive connectivity between the precuneus (all 8 clusters) and the pulvinar, consistent with both human and monkey tract-tracing studies (Parvizi et al., 2006, Zhang et al., 2008, Zhang et al., 2010).
We observed positive connectivity between ventral precuneus (clusters 5 and 8) and the ventral caudate, and negative connectivity between all precuneus clusters and middle/dorsal caudate, as well as between ventral precuneus (clusters 5–8) and dorsal-posterior precuneus (clusters 1 and 3) and the putamen/pallidum, in line with recent findings (Barnes et al., 2010, Cauda et al., 2010, Di Martino et al., 2008, Draganski et al., 2008, Leh et al., 2007, Parvizi et al., 2006, Yeterian and Pandya, 1991). For instance, Di Martino et al. (2008) showed that the dorsal caudate was positively connected with the dorsolateral prefrontal cortex (DLPFC) and negatively connected with the precuneus, while the ventral caudate was positively connected with the ventrolateral prefrontal cortex (VLPFC), ventral precuneus, as well as the limbic areas. The VLPFC but not the DLPFC appears to have a specific role in memory retrieval (Kostopoulos and Petrides, 2003, Petrides, 2002), which is consistent with the role of the ventral precuneus. Other studies reported that the putamen and dorsal caudate activated, while ventral precuneus deactivated to positive emotions (Bartels and Zeki, 2000, 2004). During working memory of spatial and nonspatial aspects of a visual scene, precuneus responded more to spatial than nonspatial cues, while dorsal caudate demonstrated the opposite pattern of responses (Wallentin et al., 2006). Taken together, these results are in line with our findings of negative connectivity between the ventral precuneus and dorsal caudate/putamen.
Global connectivity of the precuneus
All eight clusters of the precuneus showed negative connectivity with the lateral and inferior orbital frontal gyri, and the amygdala. These results are consistent with earlier reports identifying negative connectivities between the precuneus and amygdala (Hahn et al., In Press, Kim et al., 2010, Roy et al., 2009, Xie et al., 2011, Yan et al., 2009), between the precuneus and lateral/inferior orbital frontal gyrus (Fox et al., 2005, Liu et al., In Press, Xie et al., 2011, Yan et al., 2009), as well as positive connectivities between the amygdala and lateral/inferior orbital frontal gyrus (Dannlowski et al., 2009, Lang et al., 2009, Robinson et al., 2010, Roy et al., 2009). Notably, in a study of contextual conditioning and extinction, amygdala responded more to acquisition of contextual cues than extinction, while precuneus responded more to the opposite contrast (Lang et al., 2009). Another study showed activation of the precuneus and inhibition of the amygdala and orbital frontal gyrus during virtual violence in a first-person shooter game (Mathiak and Weber, 2006). Using resting state functional connectivity MRI, recent studies showed that the precuneus, amygdala, as well as orbital frontal gyrus are the major cortical and subcortical hubs or the brain’s most globally connected areas (Cole et al., 2010, Tomasi and Volkow, In Press). Taken together, these findings suggest a general role of the opposing functional connectivity between the precuneus and amygdale/orbitofrontal cortex in mediating cognitive and affective functions.
A methodological note on negative connectivity
In addition to positive correlations between functionally related brain regions, negative correlations have also been observed between brain regions with theoretically opposed functional roles (Kelly et al. 2008; Fox et al. 2005; Fransson 2005; Greicius et al. 2003; Uddin et al. 2009; Wang et al. 2006). However, recent studies suggested that the global signal regression, a common data preprocessing step in seed region based functional connectivity analyses, is a likely cause of anti-correlation functional networks (Murphy et al., 2009; Weissenbacher et al., 2009). On the other hand, it has also been demonstrated that the multiple characteristics of anti-correlation networks, which include cross-subject consistency, spatial distribution, as well as presence with modified whole brain masks and before global signal regression, are not determined by global regression (Fox et al., 2009). We also examined this issue by repeating the same analysis only without the global signal regression in our previous work using the same data set as we used in this study (Zhang et al., 2011). The results showed a very similar pattern of functional connectivity as in the analyses with global signal regression, suggesting that the negative connectivities are not a result of image preprocessing (e.g., global signal regression).
Furthermore, these negative connectivities are represented anatomically in tract tracing studies of monkey. For instance, amygdala, which showed negative connections with all eight precuneus clusters, was connected with both dorsal (Leichnetz, 2001) and ventral (Parvizi et al., 2006) precuneus in macaques. Orbitofrontal and ventromedial prefrontal cortices were connected with the dorsal precuneus (Parvizi et al., 2006), while the superior temporal cortex was connected with the ventral precuneus (Morris et al., 1999). Connections with putamen were also observed in monkeys for dorsal precuneus (Cavada and Goldman-Rakic, 1991, Leichnetz, 2001, Parvizi et al., 2006). Overall, the findings suggested that both positive and negative functional connectivity are represented anatomically.
Sex differences of the functional connectivity in precuneus
Sex differences in regional brain activation of the precuneus have been observed in word generation (Bell et al., 2006, Gizewski et al., 2006), spatial attention (Bell et al., 2006), visual word learning (Chen et al., 2007), working memory (Bell et al., 2006, Goldstein et al., 2005, Mitchell, 2007, Schweinsburg et al., 2005), execution of a visuospatial plan (Boghi et al., 2006, Unterrainer et al., 2005), spatial navigation (Maguire et al., 1999), and target and novelty detection (Gur et al., 2007), covering sensory motor processing and complex cognitive and emotive functions (Hamann and Canli, 2004, Kaiser et al., 2008, Li et al., 2006, Li et al., 2009, Wager and Ochsner, 2005, Wager et al., 2003). For instance, men showed greater precuneus activation than women during cognitive planning in the Tower of London task (Boghi et al., 2006), and during spatial perspective taking (Kaiser et al., 2008). On the other hand, while some investigators reported decreased connectivity between the precuneus and other structures in the default network (Qiu et al., 2010), others do not (Weissman-Fogel et al., 2010).
Here, we observed greater connectivity in men than women between the hippocampus/parahippocampus and the ventral precuneus – a structure implicated in episodic memory and self-related processing (see discussions in the above), consistent with sex differences in the role of hippocampus in learning and memory (Cahill, 2006, Jackson et al., 2006, Maren et al., 1994, Rucker et al., 2004). Women also showed greater connectivity between dorsal precuneus and thalamus/hypothalamus, consistent with earlier studies (Swaab et al., 2003, Swaab et al., 2001, Tomasi et al., 2008, Wager et al., 2003), although the functional implications of this finding remained to be established.
Methodological and anatomical considerations
A potential issue of the medial/lateral differences concerned the 8 mm Gaussian kernel used on data smoothing, which may induce artefactual results far more laterally than the precuneus itself. We thus ran the analyses using the data smoothed with a 4 mm Gaussian kernel and provided the results in Supplementary Figure 6. Although the best cluster number was 7 instead 8 according to the BIC, the results of the 7- and 8- cluster solutions both suggested differences between the medial and lateral precuneus.
Another issue concerns that the precuneus is primarily a medial cortical area while the lateral structures as described here represented sulcal extensions of neighboring regions. As shown in Supplementary Figure 7, while the AAL precuneus mask covers largely medial areas, two clusters involved sulci of other parietal and visual cortices. Specifically, the lateral dorsal-posterior precuneus (cluster 1) appeared to cover some medial posterior extensions of the intraparietal sulci (y=−60 and −65) and cluster 6 of the ventral precuneus appeared to cover the lingual sulci. We feel that these anatomical considerations do not negate the current findings, since the sulcal extensions represent a small part of the cluster, particularly in the case of the lateral dorsal-posterior precuneus, and did not form a functional sub-cluster in our analyses. Nevertheless, that the AAL precuneus mask appears to involve some of these “non-medial” parietal and occipital structures warrants caution in defining areal activation and connectivity in functional imaging studies. In particular, what we referred to here as lateral/medial precuneus should most appropriately be termed the lateral/medial aspects of the precuneus, to avoid confusion in anatomical nomenclature.
Potential clinical implications
Structural and functional abnormalities of the precuneus were observed in many neurological conditions including multiple sclerosis (Bendfeldt et al., 2009, Cohen-Adad et al., In Press, Prinster et al., 2006), Huntington’s disease (Rosas et al., 2008), geriatric depression (Gunning-Dixon et al., 2008), Alzheimer’s disease (Dickerson and Sperling, 2009, Matsuda, 2001, Petrella et al., 2007, Ryu et al., 2010, Sperling et al., 2010), mild cognitive impairment (Dai et al., 2009, Matsuda, 2007, Petrella et al., 2007, Pihlajamaki et al., 2009), and in non-demented older individuals with increased amyloid burden (Drzezga et al., In Press). For instance, by combining positron emission tomography and resting state functional magnetic resonance imaging (fMRI), the latter study showed significant disruptions of whole-brain connectivity in amyloid-positive patients with mild cognitive impairment in the posterior cingulate cortex and precuneus, strongly overlapping with regional hypometabolism (Drzezga et al., In Press). Many of these studies have described an altered pattern of functional connectivity of the precuneus as a pathognomonic marker of the early Alzheimer’s disease (Dai et al., 2009, Dickerson and Sperling, 2009, Pihlajamaki et al., 2009, Sperling et al., 2010). Characterizing the connectivity would further elucidate the functions of precuneus and shed new lights on how dysfunctions of the precuneus may contribute to the clinical manifestations of these neurological illnesses.
Supplementary Material
HIGHLIGHTS.
Precuneus contains dorsal anterior, dorsal posterior, and ventral subregions.
Precuneus is negatively connected with the amygdala and orbitofrontal cortex.
Men and women show different patterns of precuneus connectivity.
Acknowledgments
This study was supported by NIH grants R01DA023248, K02DA026990, R21AA018004. The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. We thank investigators of the 1000 Functional Connectomes Project and those who shared the data set for making this study possible, and Olivia Hendrick and Sarah Bednarski for their help in editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP. Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach. Neuroimage. 2004;23:1460–1471. doi: 10.1016/j.neuroimage.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes KA, Cohen AL, Power JD, Nelson SM, Dosenbach YB, Miezin FM, Petersen SE, Schlaggar BL. Identifying Basal Ganglia divisions in individuals using resting-state functional connectivity MRI. Front Syst Neurosci. 2010;4:18. doi: 10.3389/fnsys.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000;11:3829–3834. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Bell EC, Willson MC, Wilman AH, Dave S, Silverstone PH. Males and females differ in brain activation during cognitive tasks. Neuroimage. 2006;30:529–538. doi: 10.1016/j.neuroimage.2005.09.049. [DOI] [PubMed] [Google Scholar]
- Bendfeldt K, Kuster P, Traud S, Egger H, Winklhofer S, Mueller-Lenke N, Naegelin Y, Gass A, Kappos L, Matthews PM, Nichols TE, Radue EW, Borgwardt SJ. Association of regional gray matter volume loss and progression of white matter lesions in multiple sclerosis - A longitudinal voxel-based morphometry study. Neuroimage. 2009;45:60–67. doi: 10.1016/j.neuroimage.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Berry KJ, Mielke PW., Jr A Monte Carlo investigation of the Fisher Z transformation for normal and nonnormal distributions. Psychol Rep. 2000;87:1101–1114. doi: 10.2466/pr0.2000.87.3f.1101. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boghi A, Rasetti R, Avidano F, Manzone C, Orsi L, D’Agata F, Caroppo P, Bergui M, Rocca P, Pulvirenti L, Bradac GB, Bogetto F, Mutani R, Mortara P. The effect of gender on planning: An fMRI study using the Tower of London task. Neuroimage. 2006;33:999–1010. doi: 10.1016/j.neuroimage.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA, Parvizi J, Morecraft RJ, van Hoesen GW. Thalamic projections to the posteromedial cortex in the macaque. J Comp Neurol. 2008;507:1709–1733. doi: 10.1002/cne.21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, Amunts K. The human inferior parietal lobule in stereotaxic space. Brain Struct Funct. 2008;212:481–495. doi: 10.1007/s00429-008-0195-z. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Cauda F, Geminiani G, D’Agata F, Sacco K, Duca S, Bagshaw AP, Cavanna AE. Functional connectivity of the posteromedial cortex. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Topographic segregation of corticostriatal projections from posterior parietal subdivisions in the macaque monkey. Neuroscience. 1991;42:683–696. doi: 10.1016/0306-4522(91)90037-o. [DOI] [PubMed] [Google Scholar]
- Cavanna AE. The precuneus and consciousness. CNS Spectr. 2007;12:545–552. doi: 10.1017/s1092852900021295. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Charles F, Bond J, Richardson K. Seeing the fisher z-transformation. Psychometrika. 2004;69(2):291–303. [Google Scholar]
- Chen C, Xue G, Dong Q, Jin Z, Li T, Xue F, Zhao L, Guo Y. Sex determines the neurofunctional predictors of visual word learning. Neuropsychologia. 2007;45:741–747. doi: 10.1016/j.neuropsychologia.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Cohen-Adad J, Benner T, Greve D, Kinkel RP, Radding A, Fischl B, Rosen BR, Mainero C. In vivo evidence of disseminated subpial T2* signal changes in multiple sclerosis at 7T: A surface-based analysis. Neuroimage. doi: 10.1016/j.neuroimage.2011.04.009. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Gattass R, Olson CR, Gross CG. Topographical organization of cortical afferents to extrastriate visual area PO in the macaque: a dual tracer study. J Comp Neurol. 1988;269:392–413. doi: 10.1002/cne.902690307. [DOI] [PubMed] [Google Scholar]
- Cole MW, Pathak S, Schneider W. Identifying the brain’s most globally connected regions. Neuroimage. 2010;49:3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Mild cognitive impairment and alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology. 2009;250:856–866. doi: 10.1148/radiol.2503080751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Konrad C, Domschke K, Bauer J, Kugel H, Hohoff C, Schoning S, Kersting A, Baune BT, Mortensen LS, Arolt V, Zwitserlood P, Deckert J, Heindel W, Suslow T. Reduced amygdala-prefrontal coupling in major depression: association with MAOA genotype and illness severity. Int J Neuropsychopharmacol. 2009;12:11–22. doi: 10.1017/S1461145708008973. [DOI] [PubMed] [Google Scholar]
- Delazer M, Ischebeck A, Domahs F, Zamarian L, Koppelstaetter F, Siedentopf CM, Kaufmann L, Benke T, Felber S. Learning by strategies and learning by drill--evidence from an fMRI study. Neuroimage. 2005;25:838–849. doi: 10.1016/j.neuroimage.2004.12.009. [DOI] [PubMed] [Google Scholar]
- den Ouden HE, Frith U, Frith C, Blakemore SJ. Thinking about intentions. Neuroimage. 2005;28:787–796. doi: 10.1016/j.neuroimage.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Sperling RA. Large-scale functional brain network abnormalities in Alzheimer’s disease: insights from functional neuroimaging. Behav Neurol. 2009;21:63–75. doi: 10.3233/BEN-2009-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfel D, Werner A, Schaefer M, von Kummer R, Karl A. Distinct brain networks in recognition memory share a defined region in the precuneus. Eur J Neurosci. 2009;30:1947–1959. doi: 10.1111/j.1460-9568.2009.06973.x. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJ, Deichmann R, Ashburner J, Frackowiak RS. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzezga A, Becker JA, Van Dijk KR, Sreenivasan A, Talukdar T, Sullivan C, Schultz AP, Sepulcre J, Putcha D, Greve D, Johnson KA, Sperling RA. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain. doi: 10.1093/brain/awr066. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow TF, Zheng Y, Wilkinson ID, Spence SA, Deakin JF, Tarrier N, Griffiths PD, Woodruff PW. Investigating the functional anatomy of empathy and forgiveness. Neuroreport. 2001;12:2433–2438. doi: 10.1097/00001756-200108080-00029. [DOI] [PubMed] [Google Scholar]
- Filimon F, Nelson JD, Huang RS, Sereno MI. Multiple parietal reach regions in humans: cortical representations for visual and proprioceptive feedback during on-line reaching. J Neurosci. 2009;29:2961–2971. doi: 10.1523/JNEUROSCI.3211-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Friston K, Ashburner J, Frith C, Polone J, Heather J, Frackowiak R. Spatial registration and normalization of images. Hum Brain Mapp. 1995;2:165–189. [Google Scholar]
- Gentle JE, Härdle W, Mori Y. Handbook of computational statistics: concepts and methods. Springer 2004 [Google Scholar]
- Ghaem O, Mellet E, Crivello F, Tzourio N, Mazoyer B, Berthoz A, Denis M. Mental navigation along memorized routes activates the hippocampus, precuneus, and insula. Neuroreport. 1997;8:739–744. doi: 10.1097/00001756-199702100-00032. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cereb Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Gizewski ER, Krause E, Wanke I, Forsting M, Senf W. Gender-specific cerebral activation during cognitive tasks using functional MRI: comparison of women in mid-luteal phase and men. Neuroradiology. 2006;48:14–20. doi: 10.1007/s00234-005-0004-9. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Anagnoson R, Breiter HC, Makris N, Goodman JM, Tsuang MT, Seidman LJ. Sex differences in prefrontal cortical brain activity during fMRI of auditory verbal working memory. Neuropsychology. 2005;19:509–519. doi: 10.1037/0894-4105.19.4.509. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Hoptman MJ, Lim KO, Murphy CF, Klimstra S, Latoussakis V, Majcher-Tascio M, Hrabe J, Ardekani BA, Alexopoulos GS. Macromolecular white matter abnormalities in geriatric depression: a magnetization transfer imaging study. Am J Geriatr Psychiatry. 2008;16:255–262. doi: 10.1097/JGP.0b013e3181602a66. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Loughead J, Waxman J, Snyder W, Ragland JD, Elliott MA, Bilker WB, Arnold SE, Gur RE. Hemodynamic responses in neural circuitries for detection of visual target and novelty: An event-related fMRI study. Hum Brain Mapp. 2007;28:263–274. doi: 10.1002/hbm.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, Kasper S, Lanzenberger R. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. doi: 10.1016/j.neuroimage.2011.02.064. In Press. [DOI] [PubMed] [Google Scholar]
- Hamann S, Canli T. Individual differences in emotion processing. Curr Opin Neurobiol. 2004;14:233–238. doi: 10.1016/j.conb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Immisch I, Toma K, Dimyan MA, Van Gelderen P, Hallett M. Functional properties of brain areas associated with motor execution and imagery. J Neurophysiol. 2003;89:989–1002. doi: 10.1152/jn.00132.2002. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ED, Payne JD, Nadel L, Jacobs WJ. Stress differentially modulates fear conditioning in healthy men and women. Biol Psychiatry. 2006;59:516–522. doi: 10.1016/j.biopsych.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Jenkins GM, Watts DG. Spectral Analysis and Its Applications. Holden-Day; San Francisco: 1968. [Google Scholar]
- Kaiser S, Walther S, Nennig E, Kronmuller K, Mundt C, Weisbrod M, Stippich C, Vogeley K. Gender-specific strategy use and neural correlates in a spatial perspective taking task. Neuropsychologia. 2008;46:2524–2531. doi: 10.1016/j.neuropsychologia.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Roland PE, O’Sullivan BT. Functional anatomy of reaching and visuomotor learning: a positron emission tomography study. Cereb Cortex. 1995;5:111–122. doi: 10.1093/cercor/5.2.111. [DOI] [PubMed] [Google Scholar]
- Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee JM, Jo HJ, Kim SH, Lee JH, Kim ST, Seo SW, Cox RW, Na DL, Kim SI, Saad ZS. Defining functional SMA and pre-SMA subregions in human MFC using resting state fMRI: functional connectivity-based parcellation method. Neuroimage. 2010;49:2375–2386. doi: 10.1016/j.neuroimage.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety Dissociates Dorsal and Ventral Medial Prefrontal Cortex Functional Connectivity with the Amygdala at Rest. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher TT, Brammer M, Bullmore E, Simmons A, Bartels M, David AS. The neural correlates of intentional and incidental self processing. Neuropsychologia. 2002;40:683–692. doi: 10.1016/s0028-3932(01)00138-5. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Kjaer KW, Lou AR, Lou HC. Precuneus-prefrontal activity during awareness of visual verbal stimuli. Conscious Cogn. 2001;10:356–365. doi: 10.1006/ccog.2001.0509. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage. 2002;17:1080–1086. [PubMed] [Google Scholar]
- Knauff M, Fangmeier T, Ruff CC, Johnson-Laird PN. Reasoning, models, and images: behavioral measures and cortical activity. J Cogn Neurosci. 2003;15:559–573. doi: 10.1162/089892903321662949. [DOI] [PubMed] [Google Scholar]
- Kostopoulos P, Petrides M. The mid-ventrolateral prefrontal cortex: insights into its role in memory retrieval. Eur J Neurosci. 2003;17:1489–1497. doi: 10.1046/j.1460-9568.2003.02574.x. [DOI] [PubMed] [Google Scholar]
- Lang S, Kroll A, Lipinski SJ, Wessa M, Ridder S, Christmann C, Schad LR, Flor H. Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. Eur J Neurosci. 2009;29:823–832. doi: 10.1111/j.1460-9568.2009.06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leh SE, Ptito A, Chakravarty MM, Strafella AP. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci Lett. 2007;419:113–118. doi: 10.1016/j.neulet.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Krainik A, Francois C, Van de Moortele PF, Ugurbil K, Kim DS. 3-D diffusion tensor axonal tracking shows distinct SMA and pre-SMA projections to the human striatum. Cereb Cortex. 2004a;14:1302–1309. doi: 10.1093/cercor/bhh091. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 2004b;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR. Connections of the medial posterior parietal cortex (area 7m) in the monkey. Anat Rec. 2001;263:215–236. doi: 10.1002/ar.1082. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Gender differences in the neural correlates of response inhibition during a stop signal task. Neuroimage. 2006;32:1918–1929. doi: 10.1016/j.neuroimage.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Li CS, Zhang S, Duann JR, Yan P, Sinha R, Mazure CM. Gender Differences in Cognitive Control: an Extended Investigation of the Stop Signal Task. Brain Imaging Behav. 2009;3:262–276. doi: 10.1007/s11682-009-9068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kaneko Y, Ouyang X, Li L, Hao Y, Chen EY, Jiang T, Zhou Y, Liu Z. Schizophrenic Patients and Their Unaffected Siblings Share Increased Resting-State Connectivity in the Task-Negative Network but Not Its Anticorrelated Task-Positive Network. Schizophr Bull. doi: 10.1093/schbul/sbq074. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loayza FR, Fernandez-Seara MA, Aznarez-Sanado M, Pastor MA. Right parietal dominance in spatial egocentric discrimination. Neuroimage. 2011;55:635–643. doi: 10.1016/j.neuroimage.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, Sackeim HA, Lisanby SH. Parietal cortex and representation of the mental Self. Proc Natl Acad Sci U S A. 2004;101:6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage. 2005;27:824–834. doi: 10.1016/j.neuroimage.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Petersson KM, Andersson J, Johansson M, Fransson P, Ingvar M. Isolating the retrieval of imagined pictures during episodic memory: activation of the left precuneus and left prefrontal cortex. Neuroimage. 2003;20:1934–1943. doi: 10.1016/j.neuroimage.2003.07.017. [DOI] [PubMed] [Google Scholar]
- MacQueen JB. Some Methods for classification and Analysis of Multivariate Observations. Proceedings of the 5-th Berkeley Symposium on Mathematical Statistics and Probability; Berkeley: University of California Press; 1967. pp. 281–297. [Google Scholar]
- Maguire EA, Burgess N, O’Keefe J. Human spatial navigation: cognitive maps, sexual dimorphism, and neural substrates. Curr Opin Neurobiol. 1999;9:171–177. doi: 10.1016/s0959-4388(99)80023-3. [DOI] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J. Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapp. 2003;19:47–62. doi: 10.1002/hbm.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661:25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci U S A. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiak K, Weber R. Toward brain correlates of natural behavior: fMRI during violent video games. Hum Brain Mapp. 2006;27:948–956. doi: 10.1002/hbm.20234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Cerebral blood flow and metabolic abnormalities in Alzheimer’s disease. Ann Nucl Med. 2001;15:85–92. doi: 10.1007/BF02988596. [DOI] [PubMed] [Google Scholar]
- Matsuda H. The role of neuroimaging in mild cognitive impairment. Neuropathology. 2007;27:570–577. doi: 10.1111/j.1440-1789.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- Mitchell RL. fMRI delineation of working memory for emotional prosody in the brain: commonalities with the lexico-semantic emotion network. Neuroimage. 2007;36:1015–1025. doi: 10.1016/j.neuroimage.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Cipolloni PB, Stilwell-Morecraft KS, Gedney MT, Pandya DN. Cytoarchitecture and cortical connections of the posterior cingulate and adjacent somatosensory fields in the rhesus monkey. J Comp Neurol. 2004;469:37–69. doi: 10.1002/cne.10980. [DOI] [PubMed] [Google Scholar]
- Morris R, Petrides M, Pandya DN. Architecture and connections of retrosplenial area 30 in the rhesus monkey (Macaca mulatta) Eur J Neurosci. 1999;11:2506–2518. doi: 10.1046/j.1460-9568.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20:953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ogiso T, Kobayashi K, Sugishita M. The precuneus in motor imagery: a magnetoencephalographic study. Neuroreport. 2000;11:1345–1349. doi: 10.1097/00001756-200004270-00039. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. J Comp Neurol. 1982;204:196–210. doi: 10.1002/cne.902040208. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci U S A. 2006;103:1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE, Stephan KE, Kotter R. The anatomical basis of functional localization in the cortex. Nat Rev Neurosci. 2002;3:606–616. doi: 10.1038/nrn893. [DOI] [PubMed] [Google Scholar]
- Penny WD, Holmes AP, Friston K. Random-effects analysis. In: Frackowiak R, et al., editors. Human Brain Function. Academic Press; 2004. pp. 843–850. [Google Scholar]
- Petrella JR, Wang L, Krishnan S, Slavin MJ, Prince SE, Tran TT, Doraiswamy PM. Cortical deactivation in mild cognitive impairment: high-field-strength functional MR imaging. Radiology. 2007;245:224–235. doi: 10.1148/radiol.2451061847. [DOI] [PubMed] [Google Scholar]
- Petrides M. The mid-ventrolateral prefrontal cortex and active mnemonic retrieval. Neurobiol Learn Mem. 2002;78:528–538. doi: 10.1006/nlme.2002.4107. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Chanraud S, Pitel AL, Muller-Oehring E, Shankaranarayanan A, Alsop DC, Rohlfing T, Sullivan EV. Cerebral blood flow in posterior cortical nodes of the default mode network decreases with task engagement but remains higher than in most brain regions. Cereb Cortex. 2011;21:233–244. doi: 10.1093/cercor/bhq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamaki M, Jauhiainen AM, Soininen H. Structural and functional MRI in mild cognitive impairment. Curr Alzheimer Res. 2009;6:179–185. doi: 10.2174/156720509787602898. [DOI] [PubMed] [Google Scholar]
- Platel H, Baron JC, Desgranges B, Bernard F, Eustache F. Semantic and episodic memory of music are subserved by distinct neural networks. Neuroimage. 2003;20:244–256. doi: 10.1016/s1053-8119(03)00287-8. [DOI] [PubMed] [Google Scholar]
- Platel H, Price C, Baron JC, Wise R, Lambert J, Frackowiak RS, Lechevalier B, Eustache F. The structural components of music perception. A functional anatomical study. Brain. 1997;120(Pt 2):229–243. doi: 10.1093/brain/120.2.229. [DOI] [PubMed] [Google Scholar]
- Prinster A, Quarantelli M, Orefice G, Lanzillo R, Brunetti A, Mollica C, Salvatore E, Morra VB, Coppola G, Vacca G, Alfano B, Salvatore M. Grey matter loss in relapsing-remitting multiple sclerosis: a voxel-based morphometry study. Neuroimage. 2006;29:859–867. doi: 10.1016/j.neuroimage.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Qiu WQ, Claunch J, Kong J, Nixon EE, Fang J, Li M, Vangel M, Hui KK. The effects of acupuncture on the brain networks for emotion and cognition: an observation of gender differences. Brain Res. 2010;1362:56–67. doi: 10.1016/j.brainres.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum Brain Mapp. 2010;31:173–184. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Basso G, Soldati N, Sailer U, Jovicich J, Bruzzone L, Kryspin-Exner I, Bauer H, Moser E. A resting state network in the motor control circuit of the basal ganglia. BMC Neurosci. 2009;10:137. doi: 10.1186/1471-2202-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Stam CJ, Kuijer JP, Scheltens P, Barkhof F. Identifying confounds to increase specificity during a “no task condition”. Evidence for hippocampal connectivity using fMRI. Neuroimage. 2003;20:1236–1245. doi: 10.1016/S1053-8119(03)00386-0. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Salat DH, Lee SY, Zaleta AK, Pappu V, Fischl B, Greve D, Hevelone N, Hersch SM. Cerebral cortex and the clinical expression of Huntington’s disease: complexity and heterogeneity. Brain. 2008;131:1057–1068. doi: 10.1093/brain/awn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nat Neurosci. 2001;4:546–550. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Rucker B, Pereira GS, Furstenau CR, Izquierdo I, Bonan CD, Sarkis JJ. Inhibitory avoidance task reveals differences in ectonucleotidase activities between male and female rats. Neurochem Res. 2004;29:2231–2237. doi: 10.1007/s11064-004-7030-6. [DOI] [PubMed] [Google Scholar]
- Ryu SY, Kwon MJ, Lee SB, Yang DW, Kim TW, Song IU, Yang PS, Kim HJ, Lee AY. Measurement of precuneal and hippocampal volumes using magnetic resonance volumetry in Alzheimer’s disease. J Clin Neurol. 2010;6:196–203. doi: 10.3988/jcn.2010.6.4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Takeda K, Nagata K, Hatazawa J, Kuzuhara S. Activated brain regions in musicians during an ensemble: a PET study. Brain Res Cogn Brain Res. 2001;12:101–108. doi: 10.1016/s0926-6410(01)00044-1. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, Kabani N, Toga A, Evans A, Petrides M. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage. 1999;10:233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Toga A, Petrides M, Evans A. MRI atlas of the human cerebellum. Academic Press; San Diego (CA): 2000. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Anatomical investigation of projections from thalamus to posterior parietal cortex in the rhesus monkey: a WGA-HRP and fluorescent tracer study. J Comp Neurol. 1990;295:299–326. doi: 10.1002/cne.902950212. [DOI] [PubMed] [Google Scholar]
- Schultz J, Lennert T. BOLD signal in intraparietal sulcus covaries with magnitude of implicitly driven attention shifts. Neuroimage. 2009;45:1314–1328. doi: 10.1016/j.neuroimage.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Annals of Statistics. 1978;6(2):461–464. [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SF. fMRI reveals alteration of spatial working memory networks across adolescence. J Int Neuropsychol Soc. 2005;11:631–644. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RS, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;368:633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron. 2002;33:475–487. doi: 10.1016/s0896-6273(02)00575-5. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA. Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilla R, Deshpande G, LaConte S, Hu X, Sathian K. Posteromedial parietal cortical activity and inputs predict tactile spatial acuity. J Neurosci. 2007;27:11091–11102. doi: 10.1523/JNEUROSCI.1808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchan B, Yaguez L, Wunderlich G, Canavan AG, Herzog H, Tellmann L, Homberg V, Seitz RJ. Hemispheric dissociation of visual-pattern processing and visual rotation. Behav Brain Res. 2002;136:533–544. doi: 10.1016/s0166-4328(02)00204-8. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Shah NJ, Zilles K, Fink GR. Cortical representations of personally familiar objects and places: functional organization of the human posterior cingulate cortex. J Cogn Neurosci. 2005;17:183–198. doi: 10.1162/0898929053124956. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Chung WC, Kruijver FP, Hofman MA, Hestiantoro A. Sex differences in the hypothalamus in the different stages of human life. Neurobiol Aging. 2003;24(Suppl 1):S1-16. doi: 10.1016/s0197-4580(03)00059-9. discussion S17–19. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Chung WC, Kruijver FP, Hofman MA, Ishunina TA. Structural and functional sex differences in the human hypothalamus. Horm Behav. 2001;40:93–98. doi: 10.1006/hbeh.2001.1682. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Chang L, Caparelli EC, Ernst T. Sex differences in sensory gating of the thalamus during auditory interference of visual attention tasks. Neuroscience. 2008;151:1006–1015. doi: 10.1016/j.neuroscience.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Association between Functional Connectivity Hubs and Brain Networks. Cereb Cortex. doi: 10.1093/cercor/bhq268. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Markowitsch HJ, Craik FI, Habib R, Houle S. Neuroanatomical correlates of retrieval in episodic memory: auditory sentence recognition. Proc Natl Acad Sci U S A. 1994;91:2012–2015. doi: 10.1073/pnas.91.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Xavier Castellanos F, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, Menon V. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb Cortex. 2010;20:2636–2646. doi: 10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterrainer JM, Ruff CC, Rahm B, Kaller CP, Spreer J, Schwarzwald R, Halsband U. The influence of sex differences and individual task performance on brain activation during planning. Neuroimage. 2005;24:586–590. doi: 10.1016/j.neuroimage.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Van de Winckel A, Sunaert S, Wenderoth N, Peeters R, Van Hecke P, Feys H, Horemans E, Marchal G, Swinnen SP, Perfetti C, De Weerdt W. Passive somatosensory discrimination tasks in healthy volunteers: differential networks involved in familiar versus unfamiliar shape and length discrimination. Neuroimage. 2005;26:441–453. doi: 10.1016/j.neuroimage.2005.01.058. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Ochsner KN. Sex differences in the emotional brain. Neuroreport. 2005;16:85–87. doi: 10.1097/00001756-200502080-00001. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Wallentin M, Roepstorff A, Glover R, Burgess N. Parallel memory systems for talking about location and age in precuneus, caudate and Broca’s region. Neuroimage. 2006;32:1850–1864. doi: 10.1016/j.neuroimage.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Wang K, Jiang T, Liang M, Wang L, Tian L, Zhang X, Li K, Liu Z. Discriminative analysis of early Alzheimer’s disease based on two intrinsically anti-correlated networks with resting-state fMRI. Med Image Comput Comput Assist Interv. 2006;9:340–347. doi: 10.1007/11866763_42. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Weissman-Fogel I, Moayedi M, Taylor KS, Pope G, Davis KD. Cognitive and default-mode resting state networks: do male and female brains “rest” differently? Hum Brain Mapp. 2010;31:1713–1726. doi: 10.1002/hbm.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderoth N, Debaere F, Sunaert S, Swinnen SP. The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. Eur J Neurosci. 2005;22:235–246. doi: 10.1111/j.1460-9568.2005.04176.x. [DOI] [PubMed] [Google Scholar]
- Wu SS, Chang TT, Majid A, Caspers S, Eickhoff SB, Menon V. Functional heterogeneity of inferior parietal cortex during mathematical cognition assessed with cytoarchitectonic probability maps. Cereb Cortex. 2009;19:2930–2945. doi: 10.1093/cercor/bhp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Li SJ, Shao Y, Fu L, Goveas J, Ye E, Li W, Cohen AD, Chen G, Zhang Z, Yang Z. Identification of hyperactive intrinsic amygdala network connectivity associated with impulsivity in abstinent heroin addicts. Behav Brain Res. 2011;216:639–646. doi: 10.1016/j.bbr.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Zuo XN, Wang D, Wang J, Zhu C, Milham MP, Zhang D, Zang Y. Hemispheric asymmetry in cognitive division of anterior cingulate cortex: a resting-state functional connectivity study. Neuroimage. 2009;47:1579–1589. doi: 10.1016/j.neuroimage.2009.05.080. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Prefrontostriatal connections in relation to cortical architectonic organization in rhesus monkeys. J Comp Neurol. 1991;312:43–67. doi: 10.1002/cne.903120105. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Striatal connections of the parietal association cortices in rhesus monkeys. J Comp Neurol. 1993;332:175–197. doi: 10.1002/cne.903320204. [DOI] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol. 2008;100:1740–1748. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010;20:1187–1194. doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Ide JS, Li CS. Resting state functional connectivity of the medial superior frontal cortex. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CS. A neural measure of behavioral engagement: task-residual low-frequency blood oxygenation level-dependent activity in the precuneus. Neuroimage. 2010;49:1911–1918. doi: 10.1016/j.neuroimage.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CS. Functional networks for cognitive control in a stop signal task: independent component analysis. Human Brain Mapping. 2011 doi: 10.1002/hbm.21197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.