SUMMARY
Nuclear pore complexes (NPCs) are built from ~30 different proteins called nucleoporins. Previous studies have shown that several Nups exhibit cell-type-specific expression and that mutations in NPC components result in tissue-specific diseases. Here we show that a specific change in NPC composition is required for both myogenic and neuronal differentiation. The transmembrane nucleoporin Nup210 is absent in proliferating myoblasts and embryonic stem (ES) cells but becomes expressed and incorporated into NPCs during cell differentiation. Preventing Nup210 production by RNAi blocks myogenesis and the differentiation of ES cells into neuroprogenitors. We found that the addition of Nup210 to NPCs does not affect nuclear transport but is required for the induction of genes that are essential for cell differentiation. Our results identify a single change in NPC composition as an essential step in cell differentiation and establish a role for Nup210 in gene expression regulation and cell fate determination.
INTRODUCTION
Nuclear pore complexes (NPCs) are large multiprotein channels that penetrate the nuclear envelope (NE) at sites where the inner and the outer nuclear membranes are fused. Because NPCs are the sole gateway between the nucleus and the cytoplasm, it has been historically assumed that their main and only function is to regulate nucleo-cytoplasmic transport. It has now become evident, however, that NPCs are highly dynamic complexes with many transport-independent functions such as chromatin organization and the regulation of gene expression (D’Angelo and Hetzer, 2008; Strambio-De-Castillia et al., 2010).
Only three of the NPC components, Pom121, NDC1 and Nup210, are integral membrane proteins. These proteins have been proposed to anchor the NPC to the NE and to initiate nuclear membrane fusion during nuclear pore assembly (Antonin et al., 2005; Doucet et al., 2010; Stavru et al., 2006a; Stavru et al., 2006b). Increasing evidence supports the role of Pom121 and NDC1 in these processes. However, the function of Nup210 at the NPC is less clear. Nup210 is recruited late during nuclear pore assembly and exhibits cell-type-specific expression, indicating that it is not required for NPC formation (Bodoor et al., 1999; Olsson et al., 2004). Additionally, Nup210 was found to be important for NE breakdown in C. elegans (Galy et al., 2008), but the absence of Nup210 in several mammalian cell types suggests that its role in NE breakdown is not universally conserved. Thus, the function of Nup210 and the physiological significance of its tissue-specific expression remain to be determined.
The expression of several NPC components has recently been shown to vary among different cell types and tissues (Cho et al., 2009; Guan et al., 2000; Olsson et al., 2004), and also during development (D’Angelo et al., 2009; Lupu et al., 2008). These findings, together with the fact that mutations in certain NPC components result in tissue-specific diseases (Basel-Vanagaite et al., 2006; Cronshaw and Matunis, 2003; Neilson et al., 2009), suggest that NPC composition may play an important cellular function. However, whether different cell types have pores of different composition, and the importance of such variations is uncertain.
Here we show that the expression of Nup210 is induced during myogenic and neural differentiation, and that the addition of this nucleoporin to NPCs is required for the differentiation process. Interestingly, our data shows that Nup210 recruitment to the NPC does not affect nucleo-cytoplasmic transport or the targeting of inner nuclear membrane proteins to the NE. Using genome-wide expression analysis, we found that Nup210 regulates myogenesis through changes in the expression patterns of genes involved in differentiation. Remarkably, the ectopic expression of Nup210 is sufficient to increase the mRNA levels of these genes and to accelerate myoblast differentiation. Our results indicate that a single change in NPC composition is required for cell differentiation along two distinct lineages, and point towards nuclear pore specification as a key regulator of developmental gene expression.
RESULTS
Nup210 expression is induced during myogenic differentiation
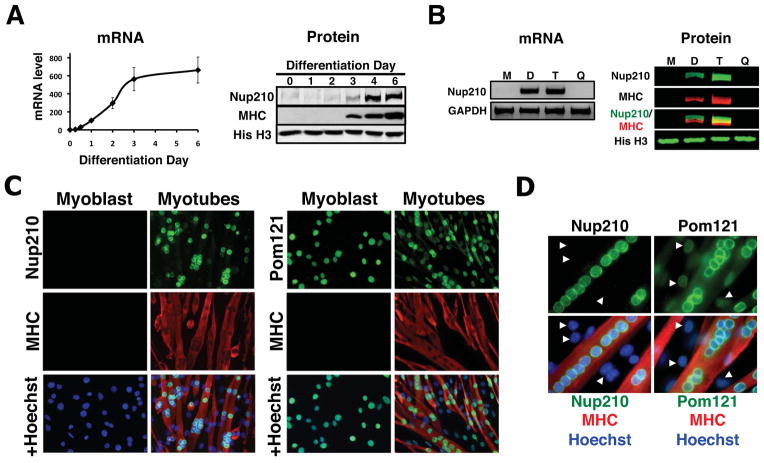
Using the well-characterized C2C12 in vitro myogenic model, we have recently reported changes in the gene expression profiles of several pore components during myoblast differentiation (D’Angelo et al., 2009). Briefly, we found that most scaffold nucleoporins were strongly down-regulated during cell-cycle exit, while the majority of dynamic Nups analyzed maintained their expression levels. By expanding our analysis to include all Nups, we found that the transmembrane nucleoporin Nup210 was the only NPC component that was absent in proliferating myoblasts but strongly expressed during myogenic differentiation (Figure 1A and S1). During C2C12 differentiation, a portion of myoblasts remain as quiescent cells that do not differentiate and maintain the potential to re-enter the cell cycle when exposed to growth factors. To determine whether Nup210 induction occurs during differentiation or upon cell-cycle exit, myotubes were separated from non-dividing quiescent cells, and Nup210 mRNA and protein levels were analyzed in each fraction. We found that Nup210 was only detectable in post-mitotic myotubes (Figure 1B). This differential expression was also evident in immunofluorescence assays. While Pom121, another transmembrane Nup, was detected in the NE of every cell before and after differentiation, Nup210 signal was restricted to post-mitotic nuclei and was absent in proliferating and quiescent myoblasts (Figure 1C, D). These results suggest that the expression of the Nup210 gene occurs during myoblast differentiation. Furthermore, Nup210 expression is induced after Myogenin (Figure S1A), an early regulator of myogenic differentiation, and sequence analysis of the Nup210 promoter identified two classical Myogenin binding sites (data not shown), suggesting that Nup210 is a downstream target of this transcription factor.
Figure 1. Nup210 expression is induced during myogenic differentiation.
(A) mRNA and protein levels of Nup210 during C2C12 differentiation were analyzed by qPCR and western blot respectively (n=3). Nup210 mRNA levels in differentiating cells were normalized to the expression of dividing cells (Day 0). Myosin Heavy Chain (MHC) was used as a differentiation marker and Histone H3 (His H3) as loading control. Data are presented as average values ± SD. (B) Nup210 mRNA and protein levels in dividing myoblasts (M), differentiated C2C12 cells (D), myotubes (T) and quiescent myoblasts (Q) were analyzed by semi-quantitative PCR and western blot respectively. (C) Immunofluorescence against Nup210 and Pom121 in dividing and differentiated (Day 4) C2C12 cells. MHC was used as a maker for differentiated myotubes and nuclei were stained with Hoechst. (D) Higher magnification of immunofluorescence against Nup210 and Pom121. Arrows indicate quiescent myoblasts. See also Figure S1.
Depletion of Nup210 during differentiation inhibits myotube formation
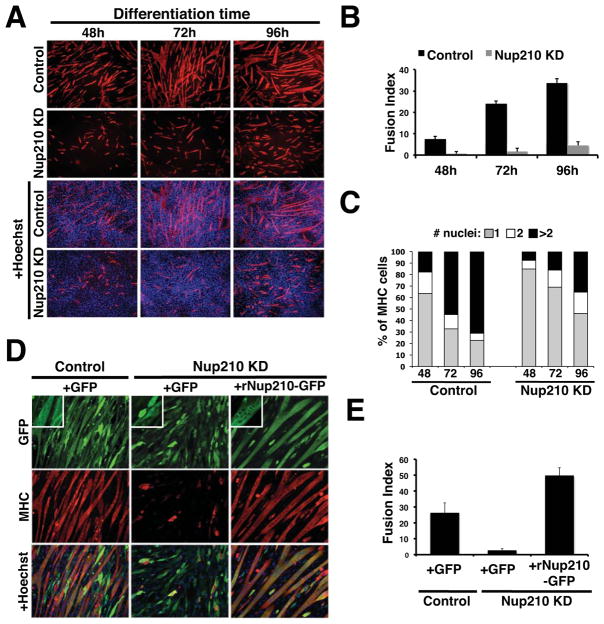
Next we wanted to determine whether the induction of Nup210 expression was required for cell differentiation. To test this, we generated stable knock-down cell lines by infecting proliferating myoblasts with lentivirus carrying control or Nup210-specific shRNAs (Figure S2A). As expected from the lack of Nup210 expression in dividing myoblasts, the presence of the Nup210 shRNA had no effect on myoblast proliferation or survival (Figure S2B). However, preventing Nup210 induction during differentiation resulted in a strong inhibition of myotube formation (Figure 2A–C and S2C, D). The lack of Nup210 under differentiation conditions resulted in fewer cells positive for the myogenic marker myosin heavy-chain. Most of these cells did not progress in the differentiation process and remained in the mononucleated stage (Figure 2A–C and S2D), suggesting that cells were able to exit the cell cycle but failed to initiate cell-cell fusion, a later event in muscle differentiation. Depleting Nup210 was also associated with increased cell mortality likely due to the activation of the apoptotic pathway as judged by activated caspase-3 staining (Figure S2E). A similar inhibition of myoblast differentiation was observed when using an shRNA directed to Nup210 3′ untranslated region (Figure S2F). In contrast, down-regulation of the other transmembrane nucleoporins Pom121 and NDC1 had no effect on differentiation indicating that the observed inhibition was specific to Nup210 depletion (Figure S2G).
Figure 2. Nup210 is required for myoblast differentiation.
(A) C2C12 cells were infected with lentivirus carrying control or Nup210-specific shRNAs. Infected cells were selected and induced to differentiate. Differentiated myotubes were stained against MHC at 48, 72, and 96 hours after differentiation. Nuclei were stained with Hoechst. (B) The percentage of nuclei in multinucleated cells (>2 nuclei) to the total number of nuclei in the field (Fusion Index) in panel (A) was quantified. Values represent the average of 4 different independent experiments ± SD. (C) Quantification of MHC positive cells shown in panel (A). Percentage of cells containing 1 (mononuclated), 2 (binucleated), or more nuclei (multinucleated) were determined at different times of differentiation. Data are presented as average values of 3 independent experiments. (D) C2C12 cell lines carrying control or Nup210 shRNAs were transfected with GFP or an RNAi resistant Nup210-GFP. Cells were reselected, induced to differentiate and stained against MHC on Day 4. Inlets show a higher magnification of GFP and Nup210-GFP expressing cells. (E) Fusion Index for the experiment in panel (E) was quantified as described. See also Figure S2.
To further verify the specificity of the Nup210 knock-down, we performed Nup210 rescue experiments. Stable cell lines carrying control or Nup210 shRNAs were transfected with a plasmid encoding either GFP or a Nup210-GFP fusion resistant to RNAi (rNup210-GFP). These cells were re-selected and their ability to differentiate was tested. As shown in Figure 2D and 2E, myoblasts expressing rNup210-GFP but not GFP alone were able to differentiate into multinucleated myotubes, confirming the essential role of Nup210 in the myogenic process.
Nup210 functions at the NPC of myotubes
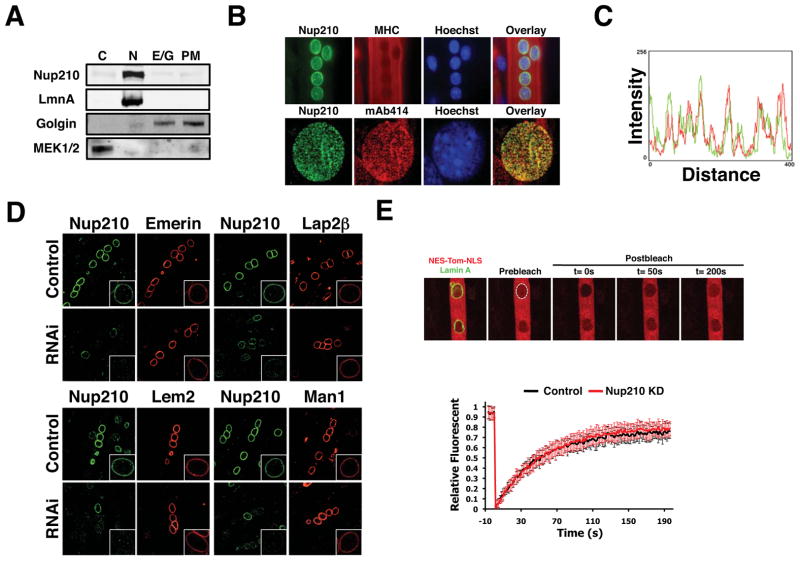
Nup210 is a transmembrane nucleoporin with a long lumenal domain, a single transmembrane segment and a short 55 aminoacid nuclear/cytoplasmic tail, a structure that resembles that of viral membrane fusion proteins (Figure S3A) (Greber et al., 1990). Therefore, it was possible that a fraction of Nup210 could function as a fusogenic protein at the plasma membrane. To investigate this, post-mitotic myotubes were fractionated by sequential centrifugation and the presence of Nup210 in the different fractions was analyzed by western blot. Like the NE marker Lamin A, Nup210 was only detected in the nuclear fraction (Figure 3A). In immunofluorescence studies, we found that endogenous Nup210 was specifically located at the NPC, colocalizing with other pore components, and not present at the plasma membrane (Figure 3B, C). Furthermore, exogenously expressing Nup210-GFP in myoblasts resulted in its localization to the NE and, at high expression levels, also to the ER but was undetectable at the cell surface (Figure S3B). These results indicate that endogenous Nup210 is present only at the NE and regulates myogenic differentiation through its association with NPCs.
Figure 3. NPC-associated Nup210 does not affect INMP targeting or global nucleo-cytoplasmic transport.
(A) Post-mitotic myotubes were fractionated by sequential centrifugation and Nup210 protein leveles were analyzed in the different subcellular fractions by western blot (C, Cytoplasm; N, nucleus; E/G, Endoplasmic Reticulum/Golgi; PM, Plasma Membrane). (B) Nup210 localization in post-mitotic myotubes was analyzed by immunofluorescence. MHC was used as a marker of differentiation and mAb414 as a marker for NPCs. (C) Co-localization of Nup210 and mAb414 signals at the nuclear envelope was determined using Image J. (D) C2C12 myoblasts were transfected with Emerin-V5, V5-Lap2B, Lem2-V5 and V5-Man1 and induced to differentiate. Cells were infected with lentivirus carrying control or Nup210 shRNAs at 36 hours after differentiation and stained for Nup210 and V5 at 96h post-infection. Inlets show higher magnification of myotube nuclei. (E) C2C12 myoblasts were transfected with NES-Tomato-NLS and Lamin A-EGFP and induced to differentiate. Cells were infected with lentivirus carrying control or Nup210 shRNAs at 36 hours after differentiation and nuclear transport was analyzed by FRAP of NES-Tomato-NLS 96h post-infection. Values represent the average of 3 different independent experiments ± SD. See also Figures S3 and S4.
Nup210 depletion does not affect targeting of inner nuclear membrane proteins
Microinjection of antibodies against the cytoplasmic domain of Nup210 in HeLa cells has been shown to block the passage of an inner nuclear membrane protein (INMP) reporter through the nuclear pore (Ohba et al., 2004). Interestingly, several INMPs have been shown to be required for myogenic differentiation (Huber et al., 2009; Kandert et al., 2009), suggesting that Nup210 could be acting by regulating the transport or accumulation of INMPs. To directly investigate whether Nup210 depletion affected the localization of INMPs we transfected proliferating myoblasts with vectors expressing V5-Tagged Emerin, Lem2 and MAN1, proteins known to play a role in C2C12 differentiation; or Lap2β, which is not required for this process. Myoblasts were then induced to differentiate and the post-mitotic myotubes were infected with lentivirus carrying control or Nup210 shRNAs. The distribution of the INMPs in Nup210-depleted myotubes was then analyzed by immunofluorescence using an α-V5 antibody. Infected post-mitotic cells showed decreased levels of Nup210 at 48–72h post-infection and induced an apoptotic response by 96–120h (Figure S3C–E), indicating that Nup210 expression is also required for the maintenance of the differentiated state. When infected myotubes were stained with the V5 antibody, no differences in localization or intensity of the four INMPs were observed compared to control cells (Figure 3D). More importantly, we found that unlike the depletion of INMPs (Datta et al., 2009; Huber et al., 2009), down-regulation of Nup210 before differentiation did not prevent myoblast cell-cycle exit or the induction of early muscle transcription factors such as Myogenin (Figure S3F, G). These results suggest that Nup210 does not regulate the transport or accumulation of INMPs at the NE, and indicate that the initial steps of myogenesis are properly executed in the absence of this nucleoporin.
Depletion of Nup210 does not affect global nuclear transport rates
A primary function of the NPC is to regulate the passage of molecules between the nucleus and the cytoplasm (D’Angelo and Hetzer, 2008). A previous study reported that antibodies against the lumenal domain of Nup210 affect NLS-dependent nuclear protein import (Greber and Gerace, 1992). We therefore investigated whether Nup210 recruitment to the NPC would affect general nucleo-cytoplasmic transport. Proliferating myoblasts were transfected with the transport reporter NES-Tomato-NLS and the NE marker Lamin A-GFP, induced to differentiate and then infected with lentivirus carrying control or Nup210 shRNAs as previously described. Nuclear transport was measured in post-mitotic myotubes 72h after infection by photo-bleaching the intranuclear reporter signal and measuring its recovery over time. Even though Nup210 levels at the NE were strongly reduced in infected myotubes (Figure S4A), no difference in nuclear import rates was observed between control and Nup210 knock-down cells (Figure 3E). Alterations in nuclear export can also be detected using this reporter as they lead to changes in its intranuclear accumulation (Figure S4B). Yet, no changes in the nuclear accumulation of the transport reporter were observed between control and Nup210-depleted cells, indicating that Nup210 knock-down does not affect nuclear export (Figure S4C). Altogether, these results suggest that Nup210 does not regulate myogenesis through major changes in nucleo-cytoplasmic transport.
Nup210 regulates the expression of genes essential for cell differentiation
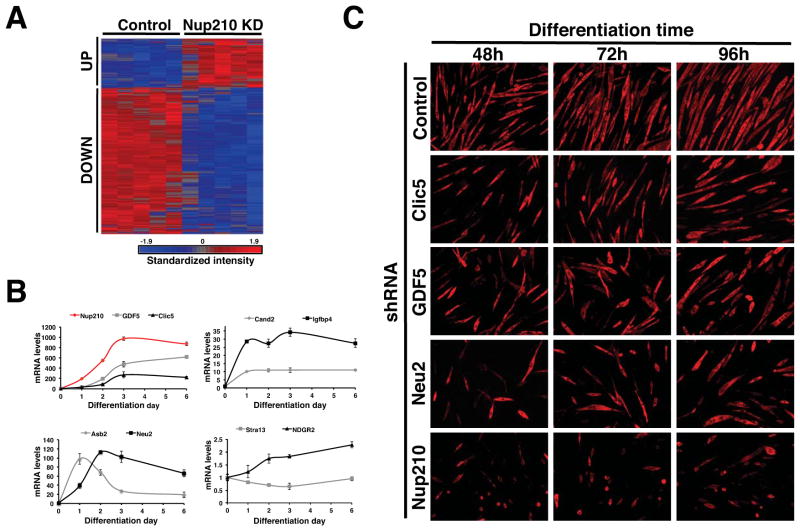
NPCs have been recently shown to play an important role in gene expression regulation of higher eukaryotes (Capelson et al., 2010; Yi et al., 2002). In the absence of evidence for a role of Nup210 in nuclear transport, we hypothesized that Nup210 might control the expression of genes relevant for myoblast differentiation. To address this possibility, we infected myotubes with control or Nup210 shRNA lentiviral particles and analyzed whole-genome gene expression at 48 or 72h post-infection, when Nup210 expression was at least 4-fold down-regulated but cell viability was not significantly affected. Of the 255 known genes that showed a greater than 1.5-fold change (P<0.05) between control and Nup210 knock-down, 64 genes were transcriptionally up-regulated while 191 genes were reduced (Figure 4A, Table S1).
Figure 4. Nup210 regulates gene expression in post-mitotic myotubes.
(A) C2C12 cells were infected with lentivirus carrying control or Nup210 shRNAs at 36 hours after differentiation and whole genome expression was analyzed by microarray. Heat map shows the microarray expression profile of altered expression in Nup210 knock-down myotubes. See also Table 1 and S1. (B) The expression of Nup210, GDF5, Clic5, Asb2, Neu2, Cand2, Igfbp4, Stra13 and NDRG2 during myoblast differentiation was analyzed by qPCR. mRNA levels in differentiating cells were normalized to the levels of dividing cells. Values represent average ± SD of 3 different independent experiments. (C) C2C12 myoblasts were infected with lentivirus carrying control, Clic5, GDF5, Neu2 or Nup210 shRNAs and induced to differentiate. Immunofluorescence against MHC was performed at 48, 72, and 96h post differentiation. See also Figure S5.
Up-regulated genes were notably enriched (20%) for cell-cycle/cell-growth related genes (Table 1). Immunofluorescence analysis of Cyclin A at 96hrs post-infection showed that the induction of Cyclin A by Nup210 RNAi occurred in quiescent cells and not in post-mitotic tubules (data not shown), suggesting that quiescent cells try to re-enter the cell cycle as Nup210-depleted myotubes are lost. This is supported by the findings that physically removing mytoubes from differentiated C2C12 cultures induces the activation of Cyclin A expression in quiescent cells (Figure S5A). These results support the hypothesis that Nup210 does not affect myoblast cell cycle exit and is not required for the early steps of myogenesis.
Table 1.
Selected genes affected by Nup210 depletion in post-mitotic myotubes
| Gene Symbol | Accession Number | Fold-Change | p-Value | Function |
|---|---|---|---|---|
|
UP
| ||||
| Hist1h3d | NM_178204 | 1.99 | 0.0003 | Nucleosome remodeling in cell-cycle |
| Psrc1 | NM_019976 | 1.86 | 8 E-05 | Mitosis progression, spindle dynamics regulation |
| Hgf | NM_178204 | 1.85 | 0.0004 | Proliferation, cell growth, and cell motility |
| Ccnb1 | NM_010427 | 1.62 | 0.0061 | Regulation of cell-cycle |
| Casc5 | NM_024245 | 1.63 | 0.0040 | Spindle assembly and chromosome alignment |
| Mki67 | NM_001081117 | 1.62 | 0.0048 | Cell proliferation |
| Kif11 | NM_001081117 | 1.58 | 0.0158 | Chromosome positioning, centrosome separation and bipolar spindle assembly in mitosis |
| Bub1 | NM_009773 | 1.55 | 0.0118 | Mitotic spindle assembly checkpoint, kinetochore assembly |
| Ccna2 | NM_009828 | 1.54 | 0.0136 | Cell-cycle regulation |
| Knl2 | NM_172578 | 1.54 | Chromosome segregation during mitosis | |
| Kif23 | NM_024245 | 1.54 | 0.0022 | Cell-cycle progression, cytokinesis. |
| Mad2l1 | NM_019499 | 1.53 | 0.0005 | Mitotic spindle assembly checkpoint |
| Parp14 | NM_001039530 | 1.50 | 0.0019 | Survival of injured proliferating cells |
|
| ||||
|
DOWN
| ||||
| Nkd2 | NM_028186 | 2.54 | 0.0004 | Development regulation through Wnt signaling |
| Tmem119 | NM_146162 | 2.19 | 0.0002 | Promotes osteoblast differentiation |
| L1cam | NM_008478 | 2.18 | 5 E-05 | Nervous system development, neuronal migration and differentiation |
| Gpr56 | NM_018882 | 1.95 | 0.0007 | Testis and nervous system development |
| Kpna3 | NM_008466 | 1.90 | 2 E-06 | Development regulation through Wnt signaling, spermatogenesis |
| Neu2 | NM_001160165 | 1.90 | 0.0013 | Myogenic differentiation |
| Grhl3 | NM_001013756 | 1.89 | 0.0021 | Epidermal differentiation |
| Wnt10a | NM_009518 | 1.86 | 0.0014 | Limb development, cartilage and odontoblast differentiation |
| Nefl | NM_010910 | 1.85 | 0.0026 | Nervous system development |
| Ablim3 | NM_198649 | 1.79 | 0.0049 | Embryonic development, cell lineage determination* |
| Ndrg2 | NM_013864 | 1.76 | 0.0128 | Myoblast proliferation and neural differentiation |
| Crim1 | NM_015800 | 1.74 | 0.0011 | Nervous system development |
| Cmklr1 | NM_008153 | 1.74 | 1 E-05 | Regulates adipogenesis and osteoblastogenesis |
| Apobec2 | NM_009694 | 1.73 | 1 E-06 | Stem cell differentiation |
| Mfap5 | NM_015776 | 1.72 | 0.0014 | Angiogenesis |
| Mir145 | NR_029557 | 1.70 | 0.0003 | Growth arrest and differentiation |
| Edn1 | NM_010104 | 1.69 | 0.0004 | Nervous system development |
| Nox4 | NM_015760 | 1.68 | 0.0072 | Myofibroblast, osteoblast and chondrogenic differentiation, angiogenesis |
| Gdf5 | NM_008109 | 1.67 | 0.0007 | Limb morphogenesis, embryonic cell differentiation, skeletal and joint development |
| Stra13 | NM_016665 | 1.66 | 3 E-08 | Satellite cell activation, myogenic and trophoblast differentiation |
| Clic5 | NM_172621 | 1.64 | 0.0018 | Myoblast proliferation and differentiation |
| Igfbp4 | NM_010517 | 1.64 | 0.0115 | Inhibitor of myoblast differentiation |
| Asb2 | NM_177407 | 1.63 | 8 E-05 | Muscle differentiation |
| Camk2a | NM_177407 | 1.63 | 8 E-05 | Nervous system development, osteoblast differentiation |
| Rrad | NM_019662 | 1.59 | 5 E-05 | Stem cell differentiation |
| Tspan12 | NM_173007 | 1.57 | 1 E-05 | Vascular development |
| Cand2 | NM_025958 | 1.56 | 0.0008 | Myogenic differentiation |
| Fam136a | BC103775 | 1.55 | 3 E-05 | Gastrointestinal development |
| Wnt10b | NM_011718 | 1.54 | 0.0026 | Skeletal development, epithelial and osteoblast differentiation, adipogenesis |
| Noxp20 | NM_026667 | 1.53 | 0.0001 | Neuronal differentiation |
| Bmp2k | NM_080708 | 1.5 | 6 E-05 | Osteoblast differentiation |
| Ptprf | NM_011213 | 1.5 | 0.0002 | Adipogenic, thymocite differentiation, nervous system development |
Conversely, down-regulated genes showed a significant enrichment (17%) in cell differentiation and development genes (Table 1). Among these genes Asb2, Cand2, Clic5, GDF5, Igfbp4, Neu2, Ndrg2 and Stra13 have been directly linked to myogenesis and skeletal system development. For example, increased expression of Clic5 and Neu2 in C2C12 cells results in accelerated myotube formation (Fanzani et al., 2003; Li et al., 2010). Additionally, Asb2 promotes myogenesis by inducing the ubiquitin-dependent degradation of the actin binding protein filamin B (Cortes et al., 2010); while Stra13 and Cand2 regulate myogenesis by inhibiting NOTCH signaling and suppressing the ubiquitination/degradation of myogenin respectively (Shiraishi et al., 2007; Sun et al., 2007). In contrast, Igfbp4 has been suggested to play a negative role during myoblast differentiation (Damon et al., 1998; Foletta et al., 2009) and GDF5 involvement in this process remains unclear. Using qPCR, we confirmed that the transcriptional activity of these 8 genes decreased with Nup210 down-regulation (Figure S5B) and that their expression, with the exception of Stra13, increased during myogenic differentiation (Figure 4B).
Among these 8 genes, only the role of GDF5 in C2C12 differentiation was previously uncharacterized. We therefore tested whether its down-regulation would affect myogenesis. We predicted that if GDF5 was downstream of Nup210 in the differentiation process, depletion of GDF5 should also inhibit myotube formation To address this, we generated stable cell lines expressing control shRNA, GDF5 shRNA or Neu2 and Clic5 shRNAs, which as mentioned had been already suggested to play a role in myoblast differentiation, and analyzed their ability to differentiate. Strikingly, reduced levels of the three genes resulted in a strong inhibition of myotube formation (Figure 4C and S5C), indicating a positive role for these factors in myogenic differentiation. The inhibition of myoblast differentiation for each single gene was weaker than the inhibition observed with Nup210 depletion, particularly for Clic5, supporting a model in which Nup210 regulates myogenesis by simultaneously controlling the expression of several genes critical for cell differentiation and/or the maintenance of the differentiated state.
Over-expression of Nup210 accelerates myoblast differentiation
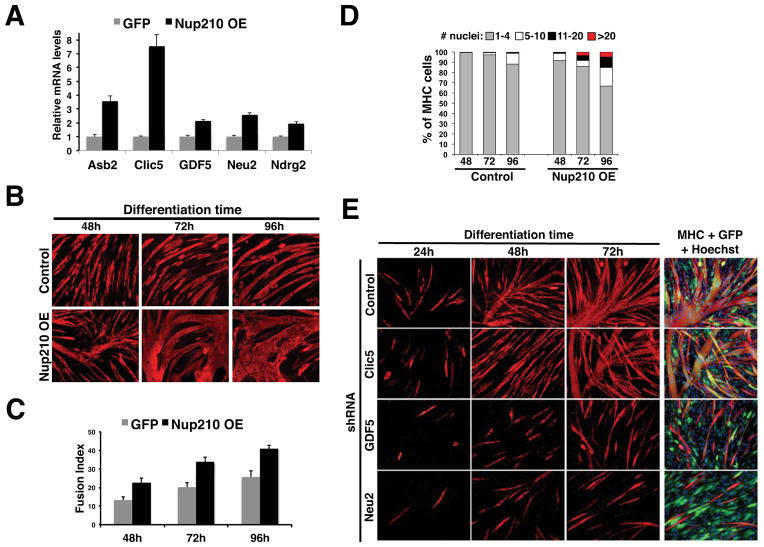
The ectopic expression of critical master myogenic regulatory factors such as MyoD has been shown to be sufficient to induce the expression of muscle differentiation genes and trigger muscle formation. The finding that Nup210 depletion resulted in the down-regulation of critical myogenic genes prompted us to investigate whether its over-expression in myoblasts was sufficient to activate these genes in the absence of differentiation conditions. To test this, we generated stable C2C12 cell lines expressing GFP or a Nup210-GFP fusion under a constitutively active CMV promoter (Figure S6A). Total RNA was isolated from control and Nup210-GFP expressing cells and the mRNA levels for the previously identified genes were quantified by qPCR. We found that 5 of the 8 genes were up regulated by exogenously expressing Nup210 (Figure 5A), suggesting that Nup210 is sufficient to induce the expression of developmentally regulated genes. Strikingly, the ectopic expression of Nup210 accelerated the formation of myotubes (Figure 5B, C). Accelerated differentiation was evident as early as 12h after differentiation (Figure S6B–C), and Nup210 over-expression was able to induce tubule formation in confluent myoblasts grown in high serum, growth factor-rich, conditions that normally do not trigger myoblast fusion (Figure S6D). This phenotype required the full-length Nup210 protein as myoblasts expressing either the lumenal N-terminal domain or the cytoplasmic/nuclear C-terminal domain of Nup210 showed a strong inhibition of myoblast differentiation (Figure S6E). The dominant negative effect of these fragments indicates that both domains of Nup210 are essential for its function and further confirms the direct role of this nucleoporin in myogenesis. The accelerated myogenic phenotype of Nup210 over-expression resembles that of Clic5 and Neu2 over-expression (Fanzani et al., 2003; Li et al., 2010), and supports the idea that Nup210 acts upstream of these genes. To test this hypothesis, we determined the effect of depleting GDF5, Neu2 and Clic5 on the differentiation of C2C12 cells over-expressing Nup210. We found that down-regulation of Neu2 and GDF5 completely blocked the acceleration of myogenesis by this nucleoporin, confirming that these are downstream targets of Nup210 (Figure 5E). Depletion of Clic5, on the other hand, did not significantly inhibited Nup210 induction of myotube formation. As Clic5 is strongly up-regulated in Nup210 overexpressing cells (Figure 5A), its possible that remaining levels of this protein after knock-down are sufficient to maintain the differentiation properties of the Nup210-expressing cell line. An alternatively explanation is that Nup210 overexpression induces the activity of other myogenic genes that can compensate Clic5 depletion. Taken all together, these findings demonstrate that Nup210 acts as an upstream regulator of key cell differentiation genes and indicate that its expression is sufficient to accelerate myogenic differentiation.
Figure 5. Ectopic expression of Nup210 in myoblasts increases the expression levels of differentiation genes and accelerates myotube formation.
(A) The expression levels of Asb2, Clic5, GDF5, Neu2 and Ndrg2 in GFP and Nup210-GFP expressing myoblasts were analyzed by qPCR. mRNA levels for each gene was normalized to the levels of GFP expressing cells. Values represent average ± SD of 3 different independent experiments. (B) GFP and Nup210-GFP C2C12 expressing myoblasts were induced to differentiate. Immunofluorescence against MHC was performed at 48, 72, and 96 hours post differentiation. (C) Fusion Index (percentage of nuclei in multinucleated cells (>2 nuclei) to the total number of nuclei in the field) for experiment in panel B. Values represent the average of 4 different independent experiments ± SD. (D) Percentage of cells containing 1–4, 5–10, 11–20 or >20 nuclei in panel B were determined at different times of differentiation. Data are presented as average values of 3 independent experiments. (E) C2C12 myoblasts ectopically expressing Nup210-GFP were infected with lentivirus carrying control, Clic5, GDF5 or Neu2 shRNAs and induced to differentiate. Immunofluorescence against MHC was performed at 24, 48 and 72h post differentiation. GFP overlay shows the expression of Nup210-GFP. See also Figure S6.
Nup210 regulates the differentiation of stem cells into neuroprogenitors
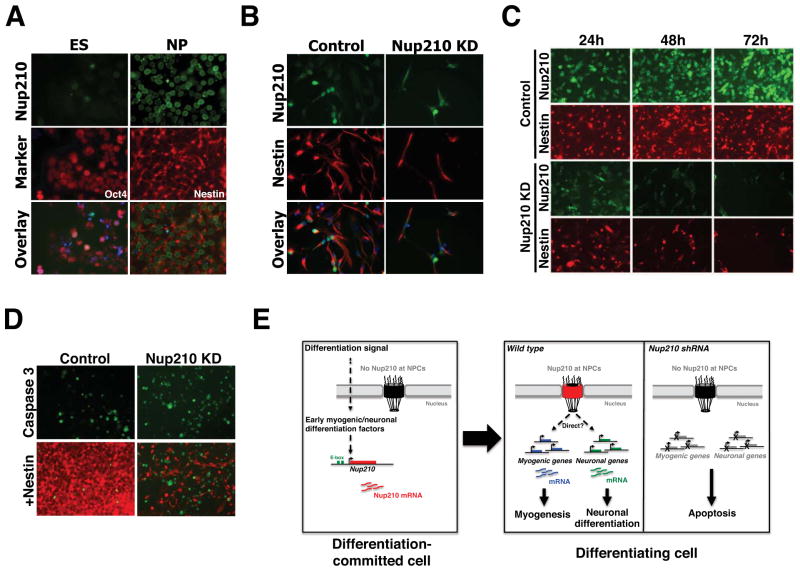
Nup210 was originally identified for its differential expression during metanephric epithelial differentiation (Olsson et al., 1999). In addition, Nup210 has a tissue-specific expression, with higher levels in kidney, skin, lung, gut, pancreas and brain (Olsson et al., 1999). Interestingly, some of the genes identified in our genome-wide expression analysis, such as Grhl3 and Wnt10b, play a role in epithelial differentiation, while others like Nefl, Crim1, L1CAM, NOXP20 and Wnt10a are involved in neuronal development. This raised the question of whether Nup210 might be involved in other differentiation processes. To test this hypothesis, we utilized the highly characterized ES cell to neuroprogenitor (NP) cell differentiation system. Briefly, ES cells of the Hb9-GFP line were induced to differentiate into NP cells and the expression of Nup210 was analyzed by immunofluorescence. Consistent with our findings using the myogenic model, Nup210 was not present in ES cells but was strongly expressed in neuroprogenitors (Figure 6A). These findings are in agreement with a previous genome-wide study in which Nup210 mRNA levels were reported to increase during ES to NP cell differentiation (Bain et al., 2000). To investigate whether Nup210 was also required for NP cell differentiation, we infected ES cells with lentivirus carrying control or Nup210 shRNAs and evaluated their ability to differentiate. Depletion of Nup210 did not affect stem cell survival (data not shown) but strongly inhibited differentiation into neuroprogenitors (Figure 6B). Under differentiation conditions, the absence of Nup210 was associated with strong cell death and a reduced number of Nestin (a neuroprogenitor marker) positive cells (Figure 6B). In agreement with our observations in myotubes, depletion of Nup210 in already differentiated NP cells resulted in cell death, likely through activation of the apoptotic pathway (Figure 6C, D). Altogether, these results indicate that the expression of Nup210 is essential for neural differentiation and the maintenance of the neuroprogenitor state, and confirm that Nup210 plays a critical role in diverse cellular differentiation processes.
Figure 6. Nup210 is essential for neuronal differentiation.
(A) Embryonic stem (ES) cells were induced to differentiate into neuroprogenitor (NP) cells and stained for Nup210. Oct4 and Nestin were used as specific markers for ES and NP cells respectively. Hoechst was used to stain nuclei. (B) ES cells were infected with lentivirus carrying control or Nup210 shRNAs and induced to differentiate into NP cells. Differentiated cells were stained against Nup210 and Nestin. Hoechst was used to stain nuclei. (C) Differentiated NP cells were infected with lentivirus carrying control or Nup210 shRNA and stained against Nup210 and Nestin. (D) Control or Nup210 knockdown NP cells were stained with Nestin and Cleaved Caspase-3 (Caspase 3) antibodies. (E) Schematic model of Nup210 regulation of cell differentiation. In undifferentiated myoblast the expression of Nup210 is repressed. Early differentiation signals activate Nup210 gene expression. In myoblast, Nup210 induction is likely carried out by Myogenin/MyoD binding to its promoter E-boxes. Nup210 protein is then recruited to the NPC where it regulates the expression of genes required for myogenic and neuronal differentiation. Prevention of Nup210 addition to the NPC by shRNAs prevents the activation of Nup210 regulated genes and leads to the death of the differentiation-committed cell by apoptosis.
DISCUSSION
Our data provide direct evidence for the existence of cell type-specific nuclear pore complexes and indicate that a single change in NPC composition is sufficient to regulate a complex cellular process such as cell differentiation. At the functional level, the transmembrane nucleoporin Nup210 is activated in a differentiation-dependent manner and acts to induce the expression of downstream targets. The finding that Nup210 addition to the NPC is required for two distinct differentiation processes indicates that this nucleoporin is a key regulator of cell fate determination, and further supports the idea that the NPC plays a critical role in organismal development.
Our findings suggest that cells employ NPCs of different composition to regulate diverse cellular processes. Considering that the NPC is built by multiple copies of 32 proteins, we can envision that unique combinations of nucleoporins will result in NPCs with different functionality. Supporting the existence of NPCs of different composition/function, the expression of several nucleoporins has been shown to be cell-type specific and/or to vary among different tissues (Cho et al., 2009; Guan et al., 2000; Lupu et al., 2008; Olsson et al., 2004). Furthermore, mutations in at least four nucleoporins, Aladin, Nup62, Nup155 and Nup358 give rise to diseases with tissue-specific phenotypes (Basel-Vanagaite et al., 2006; Cronshaw and Matunis, 2003; Neilson et al., 2009; Zhang et al., 2008). Finally, studies in transgenic knock-out animals have shown that the depletion of several nucleoporins specifically affect the function of distinct tissues or cells (de Jong-Curtain et al., 2009; Faria et al., 2006; Lupu et al., 2008; Smitherman et al., 2000). The existence of cell-type specific nuclear pores was previously suggested in a study where the tissue-specific expression of the scaffold nucleoporin Nup133 was determined by in situ hybridization (Lupu et al., 2008). Although this is an interesting finding, it was recently shown that the lack of gene expression for scaffold nucleoporins does not necessarily reflect their absence from NPCs and might not be a suitable measurement for tissue-specific nuclear pores (D’Angelo et al., 2009). In this work, it was found that the expression of Nup133 and other scaffold Nups is also cell-type specific in developing C. elegans embryos and even absent in adult worms; but despite the lack of gene expression, the scaffold proteins are still present in all embryonic and adult cells. This indicates that the NPC scaffold components are extremely stable proteins that remain incorporated at NPCs even when their expression is strongly down-regulated.
Work from our laboratory and others have shown that the NPC and its components bind and regulate the activity of several genes (Ahmed et al., 2010; Brickner and Brickner, 2010; Capelson et al., 2010; Casolari et al., 2004; Kalverda et al., 2010). Interestingly, the genes that associate with this structure are highly enriched in developmental genes (Kalverda et al., 2010). This suggests that the NPC might play an important role in differentiation and development by regulating the activity of genes critical for these processes. The identification that Nup210 regulates myogenesis by controlling the activity of key muscle differentiation genes supports this model. Interestingly, we found that depleting Nup210 also affects the activity of genes involved in neuronal differentiation and that this protein plays an essential role in this process. Our findings support a model in which early differentiation factors, likely Myogenin in myoblasts, would activate the expression of Nup210 gene in cells committed to differentiate (Figure 6E). The addition of Nup210 to the NPC will then regulate the expression of key genes specific for different differentiation processes. This would explain the tissue-specific expression of Nup210. The lack of Nup210 at the NPCs of differentiation-committed cells will prevent them to further proceed with the differentiation process and trigger cell death by apoptosis.
It is not immediately obvious how a transmembrane nucleoporin could regulate gene expression. We cannot rule out that Nup210 mediates a specific transport event that triggers the expression of it target genes. However, we think this is unlikely for two reasons. First, we did not observe any changes in general nucleo-cytoplasmic transport. Second, only 55 aa of Nup210 are exposed to interact with the NPC. This makes it unlikely that Nup210 would reach and change the properties of the pore transport channel or be able to interact with transport receptors. One possible mechanism for Nup210 regulation of gene activity is that its incorporation into the NPC could affect the dynamic properties of other nucleoporins known to regulate gene expression in a transport-independent manner. A promising candidate would be Nup98, a nucleoporin that shuttles between the NPC and the nucleoplasm in a transcription-dependent manner (Griffis et al., 2002) and that has been recently shown to regulate the activity of several developmental (Capelson et al., 2010; Kalverda et al., 2010). Our microarray data shows that 75% of the total altered genes we identify are down-regulated while only 25% are up-regulated. This suggests that Nup210 is more likely involved in gene expression activation than repression. This is consistent with the current view of the NPC as a site of active transcription. Alternatively, Nup210 could recruit and modulate the activity of gene/chromatin-regulatory factors and, as a consequence, control gene expression at the NPC. In support of such model, it was recently reported that the Nup155 nucleoporin recruits HDAC4 to the NPC, and that this association is important for the localization and activity of specific genes (Kehat et al., 2011). Finally, Nup210 might help to recruit specific genes to the NPC. The relocation of genes to the NPC in order to regulate their activity has been described for a small number of yeast genes (Brickner and Walter, 2004; Luthra et al., 2007; Taddei et al., 2006; Tan-Wong et al., 2009). Although, NPCs were initially associated with silent chromatin regions (Feuerbach et al., 2002; Galy et al., 2000) increasing evidences indicates that they mostly act as a gene-activating domains (Ahmed et al., 2010; Brickner and Brickner, 2010; Capelson et al., 2010; Casolari et al., 2004; Kalverda et al., 2010). A positive role of the NPC in gene activation is consistent with our findings that Nup210 depletion is mostly associated with gene down-regulation. Yet, arguing against a role of Nup210 in gene tethering to the NPC, using fluorescent in situ hybridization we have not found changes in the intranuclear localization of GDF5 and Clic5 genes during myoblast differentiation (data not shown).
Our findings that Nup210 plays a key role in cell differentiation provides insights into its functions and address the longstanding question of what is the biological significance of Nup210 tissue-specific expression.
EXPERIMENTAL PROCEDURES
Semi-quantitative RT–PCR and quantitative Q-PCR
C2C12 total RNA and cDNA was prepared with Qiagen RNeasy and QuantiTect Rev Transcription kits respectively. qPCR was performed using SYBR Green. Primers are listed in Supplementary experimental procedures.
Western blots and immunofluorescence
For protein extracts, proliferating myoblasts or differentiated myotubes were resuspended in 1X SDS buffer preheated to 95°C, incubated at 95°C for 5 min and passed 5–10 times though a 27G syringe. Protein concentration was determined using the BCA reagent (Pierce), normalized and 4% β-mercaptoethanol + 0.01% bromophenol blue were added. For western blot analysis 40–80μg of protein were resolved in SDS-PAGE gels and transfer to Immobilon-FL membranes (Millipore). Membranes were blocked with PBS-0.05% Tween (PBS-T) + 5% non-fat milk for 1h, washed and incubated with primary antibody for 1h at room temperature (RT) or over-night (ON) at 4°C. The secondary antibody was added after 3 washes with PBS-T for 1h at RT. Membranes were analyzed with an Odyssey infrared imaging system (LI-COR Biosciences).
For immunofluorescence, myoblasts and myotubes were cultured in μ-slide 8-well plates (Ibidi #80826). For Nup210 staining cells were fixed in −20°C methanol for 2 minutes and then permeabilized in 1XPBS/1% Triton-X100 for 1 min. For MHC, cells were either fixed in methanol when co-stained with Nup210 or in 4% PFA for 5 min. For all other antibodies and cells, fixation was done in 4% PFA for 5 min. Fixed cells were blocked using IF-buffer (1XPBS, 10mg/ml BSA, 0.02% SDS, 0.1% Triton-X100) and incubated with primary antibody in IF buffer for 1h or at RT or ON at 4°C (for Nup210, Nestin and Oct4 antibodies). Cells were washed in IF buffer and incubated with secondary antibody for an additional hour at RT. Cell were washed in IF buffer and incubated with Hoechst for 5 min before mounting using Vectashield (Vector labs). Images were taken with a Leica SP2 confocal microscope and a Zeiss Axio Observer Z1 motorized microscope.
Rescue experiment and Nup210 over expression
Cell lines expressing control or Nup210 shRNAs were transfected on 6 well plates at 30% confluency using 16μl of Optifect and 4μg of GFP, Nup-210GFP or rNup210-GFP plasmid DNA. After 48h, cells were split into 10 cm plates and exposed to 1mg/ml of G418 for two weeks. Resistant cells were sorted and GFP positive cells were collected and grown in proliferative media containing 500μg/ml of G418 for maintenance. Cell lines were grown in 2μg/ml of puromycin to maintain knockdown. The same protocol was used to generate the GFP and Gp210-GFP over-expressing cell lines in wild type C2C12.
Cell fractionation
For subcellular fractionation, cells were collected on day 3 after differentiation and resuspended in 1 volume of fractionation buffer (250mM sucrose, 20mM Hepes pH 7.4, 10mM KCl, 1.5mM MgCl2, 1mM EDTA, 1mM EGTA, 1mM DTT, protease inhibitors). Cells were placed on ice for 15 min and lyzed with a 27G syringe (5–10 strokes). Sub-cellular fractions were separated by sequential centrifugation at 4°C as follows: Nuclei (750×g 10 min), mitochondria (5.000×g 15 min), ER/Golgi (30.000×g 1h), and plasma membrane/microsomes (100.000×g 1h). After each centrifugation step, pellets were washed with 1ml of fractionation buffer and then resuspended in 1×SDS buffer preheated to 95°C, incubated at 95°C for 5 min. Protein was quantified as described and 10μg of proteins were used for western blot analysis.
BrdU incorporation
Approximately 100,000 cells were plated onto 24-well plates. 24h hours later, cultures were either left in proliferating media or shifted to differentiation media for the times indicated. Cells were incubated in media containing 10μM BrdU (BD Pharmingen Catalog # 550891) for 30 min, washed twice in 1X PBS and fixed for 5 min in 4% PFA at RT. Immunofluorescence against BrdU was carried out over-night at 4ºC and samples were counterstained with Hoechst 33342. Total number and BrdU-positive nuclei were counted manually by using Count Tool from Adobe Photopshop.
FACS
For sorting, cultures were dissociated with tripsin for 5 min, centrifuged at 1000Xg and resuspended in 1XPBS + 2% FBS. Positive cells were collected in 100% FBS and plated on DMEM + 20% FBS.
shRNA production, infection and selection
Lentivirus were packaged in 293T cells grown in DMEM + 20% FBS and 1X penicillin/streptomycin. 293T cells were transfected in 10 cm plates with 5.2μg of shRNA vector and 2.8μg of packaging mix using 24μl of lipofectamine 2000. Media was replaced 12h after transfection and virus were collected at 36 and 60h. Proliferating myoblasts at 30% confluency were infected with the 36h and 60h supernatants 24h later. Myoblasts were then split and cultured in media containing 2μg/ml of puromycin. For myotube infections highly concentrated viral stocks (at least 1–5×1010TU/ml) were generated in the Viral Vector Salk facility. For post-mitotic myotube infections cells were infected with 2×108TU at 36h after initiation of differentiation.
INM protein localization and nuclear transport analysis
Wild type C2C12 cells were tranfected at 30% confluency in μ-slide 8 well plates (Ibidi #80826) using 4μl of Optifect (Invitrogen) and 1μg of DNA. Differentiated cells were infected with shRNA against scramble and Nup210 KD 36h after shifting the cultures to differentiating media. Samples were fixed and stained against V5 and Nup210 at 96h and 120h post-infection. Images were taken with a Leica SP2 confocal microscope.
Microarray
For microarray analysis, shRNA-infected myotubes were resuspended in Trizol reagent at 48h or 72h post-infection. RNA was purified following the manufacturer specifications and after the addition of ethanol 70% the RNA was loaded on an RNeasy purification column (Qiagen) and further purified following the kit instructions. Before using the RNA for microarray analysis, Nup210 down regulation was confirmed by qPCR. For gene expression analysis in Nup210 knock-down, four independent myotube infections for control and Nup210 shRNA were used. Samples were compared using Affymetrix Mouse 1.0ST arrays as specified by the manufacturer.
Analysis of genes expression was preformed with Partek Genomics Suite software. RMA background correction, quantile normalization, log2 transformation and mean polished probeset summarization was performed to generate intensity values were. Due to the low number of genes affected, gene categories were mostly poorly defined. Thus, functional categorization of genes was performed by manually searching the published literature for each gene.
Supplementary Material
Highlights.
Identification of cell-type specific nuclear pore complexes
Nuclear pore composition changes during cell differentiation
Nup210 expression is critical for myoblast and neuroprogenitor cell differentiation
Nup210 regulates the expression of key differentiation genes
Acknowledgments
We thank Dr. F. Gage for reagents and technical assistance with ES differentiation. We thank the members of the Hetzer lab and Marcela Raices for critical discussion and reading of the manuscript. This work was supported by a grant from the National Institutes of Health (RO1 GM57438) and by an award from the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson DJ, Vargas JD, Hsiao JP, Hetzer MW. Recruitment of functionally distinct membrane proteins to chromatin mediates nuclear envelope formation in vivo. J Cell Biol. 2009;186:183–191. doi: 10.1083/jcb.200901106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Brickner DG, Light WH, Cajigas I, McDonough M, Froyshteter AB, Volpe T, Brickner JH. DNA zip codes control an ancient mechanism for gene targeting to the nuclear periphery. Nat Cell Biol. 2010;12:111–118. doi: 10.1038/ncb2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonin W, Franz C, Haselmann U, Antony C, Mattaj IW. The Integral Membrane Nucleoporin pom121 Functionally Links Nuclear Pore Complex Assembly and Nuclear Envelope Formation. Mol Cell. 2005;17:83–92. doi: 10.1016/j.molcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Bain G, Mansergh FC, Wride MA, Hance JE, Isogawa A, Rancourt SL, Ray WJ, Yoshimura Y, Tsuzuki T, Gottlieb DI, et al. ES cell neural differentiation reveals a substantial number of novel ESTs. Funct Integr Genomics. 2000;1:127–139. doi: 10.1007/s101420000014. [DOI] [PubMed] [Google Scholar]
- Basel-Vanagaite L, Muncher L, Straussberg R, Pasmanik-Chor M, Yahav M, Rainshtein L, Walsh CA, Magal N, Taub E, Drasinover V, et al. Mutated nup62 causes autosomal recessive infantile bilateral striatal necrosis. Ann Neurol. 2006;60:214–222. doi: 10.1002/ana.20902. [DOI] [PubMed] [Google Scholar]
- Bodoor K, Shaikh S, Salina D, Raharjo WH, Bastos R, Lohka M, Burke B. Sequential recruitment of NPC proteins to the nuclear periphery at the end of mitosis. J Cell Sci. 1999;112(Pt 13):2253–2264. doi: 10.1242/jcs.112.13.2253. [DOI] [PubMed] [Google Scholar]
- Brickner DG, Brickner JH. Cdk phosphorylation of a nucleoporin controls localization of active genes through the cell cycle. Mol Biol Cell. 2010;21:3421–3432. doi: 10.1091/mbc.E10-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickner JH, Walter P. Gene recruitment of the activated INO1 locus to the nuclear membrane. PLoS Biol. 2004;2:e342. doi: 10.1371/journal.pbio.0020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelson M, Liang Y, Schulte R, Mair W, Wagner U, Hetzer MW. Chromatin-bound nuclear pore components regulate gene expression in higher eukaryotes. Cell. 2010;140:372–383. doi: 10.1016/j.cell.2009.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casolari JM, Brown CR, Komili S, West J, Hieronymus H, Silver PA. Genome-wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell. 2004;117:427–439. doi: 10.1016/s0092-8674(04)00448-9. [DOI] [PubMed] [Google Scholar]
- Cho AR, Yang KJ, Bae Y, Bahk YY, Kim E, Lee H, Kim JK, Park W, Rhim H, Choi SY, et al. Tissue-specific expression and subcellular localization of ALADIN, the absence of which causes human triple A syndrome. Exp Mol Med. 2009;41:381–386. doi: 10.3858/emm.2009.41.6.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes R, Rosello-Lleti E, Rivera M, Martinez-Dolz L, Salvador A, Azorin I, Portoles M. Influence of heart failure on nucleocytoplasmic transport in human cardiomyocytes. Cardiovascular research. 2010;85:464–472. doi: 10.1093/cvr/cvp336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronshaw JM, Matunis MJ. The nuclear pore complex protein ALADIN is mislocalized in triple A syndrome. Proc Natl Acad Sci U S A. 2003;100:5823–5827. doi: 10.1073/pnas.1031047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo MA, Hetzer MW. Structure, dynamics and function of nuclear pore complexes. Trends Cell Biol. 2008;18:456–466. doi: 10.1016/j.tcb.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo MA, Raices M, Panowski SH, Hetzer MW. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136:284–295. doi: 10.1016/j.cell.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damon SE, Haugk KL, Birnbaum RS, Quinn LS. Retrovirally mediated overexpression of insulin-like growth factor binding protein 4: evidence that insulin-like growth factor is required for skeletal muscle differentiation. J Cell Physiol. 1998;175:109–120. doi: 10.1002/(SICI)1097-4652(199804)175:1<109::AID-JCP12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Datta K, Guan T, Gerace L. NET37, a nuclear envelope transmembrane protein with glycosidase homology, is involved in myoblast differentiation. J Biol Chem. 2009;284:29666–29676. doi: 10.1074/jbc.M109.034041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong-Curtain TA, Parslow AC, Trotter AJ, Hall NE, Verkade H, Tabone T, Christie EL, Crowhurst MO, Layton JE, Shepherd IT, et al. Abnormal nuclear pore formation triggers apoptosis in the intestinal epithelium of elys-deficient zebrafish. Gastroenterology. 2009;136:902–911. doi: 10.1053/j.gastro.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet CM, Talamas JA, Hetzer MW. Cell cycle-dependent differences in nuclear pore complex assembly in metazoa. Cell. 2010;141:1030–1041. doi: 10.1016/j.cell.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanzani A, Giuliani R, Colombo F, Zizioli D, Presta M, Preti A, Marchesini S. Overexpression of cytosolic sialidase Neu2 induces myoblast differentiation in C2C12 cells. FEBS Lett. 2003;547:183–188. doi: 10.1016/s0014-5793(03)00709-9. [DOI] [PubMed] [Google Scholar]
- Faria AM, Levay A, Wang Y, Kamphorst AO, Rosa ML, Nussenzveig DR, Balkan W, Chook YM, Levy DE, Fontoura BM. The nucleoporin Nup96 is required for proper expression of interferon-regulated proteins and functions. Immunity. 2006;24:295–304. doi: 10.1016/j.immuni.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Feuerbach F, Galy V, Trelles-Sticken E, Fromont-Racine M, Jacquier A, Gilson E, Olivo-Marin JC, Scherthan H, Nehrbass U. Nuclear architecture and spatial positioning help establish transcriptional states of telomeres in yeast. Nat Cell Biol. 2002;4:214–221. doi: 10.1038/ncb756. [DOI] [PubMed] [Google Scholar]
- Foletta VC, Prior MJ, Stupka N, Carey K, Segal DH, Jones S, Swinton C, Martin S, Cameron-Smith D, Walder KR. NDRG2, a novel regulator of myoblast proliferation, is regulated by anabolic and catabolic factors. J Physiol. 2009;587:1619–1634. doi: 10.1113/jphysiol.2008.167882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy V, Antonin W, Jaedicke A, Sachse M, Santarella R, Haselmann U, Mattaj I. A role for gp210 in mitotic nuclear-envelope breakdown. J Cell Sci. 2008;121:317–328. doi: 10.1242/jcs.022525. [DOI] [PubMed] [Google Scholar]
- Galy V, Olivo-Marin JC, Scherthan H, Doye V, Rascalou N, Nehrbass U. Nuclear pore complexes in the organization of silent telomeric chromatin. Nature. 2000;403:108–112. doi: 10.1038/47528. [DOI] [PubMed] [Google Scholar]
- Greber UF, Gerace L. Nuclear protein import is inhibited by an antibody to a lumenal epitope of a nuclear pore complex glycoprotein. J Cell Biol. 1992;116:15–30. doi: 10.1083/jcb.116.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber UF, Senior A, Gerace L. A major glycoprotein of the nuclear pore complex is a membrane-spanning polypeptide with a large lumenal domain and a small cytoplasmic tail. EMBO J. 1990;9:1495–1502. doi: 10.1002/j.1460-2075.1990.tb08267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis ER, Altan N, Lippincott-Schwartz J, Powers MA. Nup98 is a mobile nucleoporin with transcription-dependent dynamics. Mol Biol Cell. 2002;13:1282–1297. doi: 10.1091/mbc.01-11-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan T, Kehlenbach RH, Schirmer EC, Kehlenbach A, Fan F, Clurman BE, Arnheim N, Gerace L. Nup50, a nucleoplasmically oriented nucleoporin with a role in nuclear protein export. Mol Cell Biol. 2000;20:5619–5630. doi: 10.1128/mcb.20.15.5619-5630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber MD, Guan T, Gerace L. Overlapping functions of nuclear envelope proteins NET25 (Lem2) and emerin in regulation of extracellular signal-regulated kinase signaling in myoblast differentiation. Mol Cell Biol. 2009;29:5718–5728. doi: 10.1128/MCB.00270-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalverda B, Pickersgill H, Shloma VV, Fornerod M. Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell. 2010;140:360–371. doi: 10.1016/j.cell.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Kandert S, Wehnert M, Muller CR, Buendia B, Dabauvalle MC. Impaired nuclear functions lead to increased senescence and inefficient differentiation in human myoblasts with a dominant p.R545C mutation in the LMNA gene. Eur J Cell Biol. 2009;88:593–608. doi: 10.1016/j.ejcb.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Kehat I, Accornero F, Aronow BJ, Molkentin JD. Modulation of chromatin position and gene expression by HDAC4 interaction with nucleoporins. J Cell Biol. 2011;193:21–29. doi: 10.1083/jcb.201101046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Yin J, Yue T, Liu L, Zhang H. The CLIC5 (chloride intracellular channel 5) involved in C2C12 myoblasts proliferation and differentiation. Cell Biol Int. 2010;34:379–384. doi: 10.1042/CBI20090334. [DOI] [PubMed] [Google Scholar]
- Lupu F, Alves A, Anderson K, Doye V, Lacy E. Nuclear pore composition regulates neural stem/progenitor cell differentiation in the mouse embryo. Dev Cell. 2008;14:831–842. doi: 10.1016/j.devcel.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthra R, Kerr SC, Harreman MT, Apponi LH, Fasken MB, Ramineni S, Chaurasia S, Valentini SR, Corbett AH. Actively transcribed GAL genes can be physically linked to the nuclear pore by the SAGA chromatin modifying complex. J Biol Chem. 2007;282:3042–3049. doi: 10.1074/jbc.M608741200. [DOI] [PubMed] [Google Scholar]
- Neilson DE, Adams MD, Orr CM, Schelling DK, Eiben RM, Kerr DS, Anderson J, Bassuk AG, Bye AM, Childs AM, et al. Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am J Hum Genet. 2009;84:44–51. doi: 10.1016/j.ajhg.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba T, Schirmer EC, Nishimoto T, Gerace L. Energy- and temperature-dependent transport of integral proteins to the inner nuclear membrane via the nuclear pore. J Cell Biol. 2004;167:1051–1062. doi: 10.1083/jcb.200409149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Ekblom M, Fecker L, Kurkinen M, Ekblom P. cDNA cloning and embryonic expression of mouse nuclear pore membrane glycoprotein 210 mRNA. Kidney Int. 1999;56:827–838. doi: 10.1046/j.1523-1755.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- Olsson M, Scheele S, Ekblom P. Limited expression of nuclear pore membrane glycoprotein 210 in cell lines and tissues suggests cell-type specific nuclear pores in metazoans. Exp Cell Res. 2004;292:359–370. doi: 10.1016/j.yexcr.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Shiraishi S, Zhou C, Aoki T, Sato N, Chiba T, Tanaka K, Yoshida S, Nabeshima Y, Tamura TA. TBP-interacting protein 120B (TIP120B)/cullin-associated and neddylation-dissociated 2 (CAND2) inhibits SCF-dependent ubiquitination of myogenin and accelerates myogenic differentiation. J Biol Chem. 2007;282:9017–9028. doi: 10.1074/jbc.M611513200. [DOI] [PubMed] [Google Scholar]
- Smitherman M, Lee K, Swanger J, Kapur R, Clurman BE. Characterization and targeted disruption of murine Nup50, a p27(Kip1)-interacting component of the nuclear pore complex. Mol Cell Biol. 2000;20:5631–5642. doi: 10.1128/mcb.20.15.5631-5642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavru F, Hulsmann BB, Spang A, Hartmann E, Cordes VC, Gorlich D. NDC1: a crucial membrane-integral nucleoporin of metazoan nuclear pore complexes. J Cell Biol. 2006a;173:509–519. doi: 10.1083/jcb.200601001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavru F, Nautrup-Pedersen G, Cordes VC, Gorlich D. Nuclear pore complex assembly and maintenance in POM121- and gp210-deficient cells. J Cell Biol. 2006b;173:477–483. doi: 10.1083/jcb.200601002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strambio-De-Castillia C, Niepel M, Rout MP. The nuclear pore complex: bridging nuclear transport and gene regulation. Nat Rev Mol Cell Biol. 2010;11:490–501. doi: 10.1038/nrm2928. [DOI] [PubMed] [Google Scholar]
- Sun H, Li L, Vercherat C, Gulbagci NT, Acharjee S, Li J, Chung TK, Thin TH, Taneja R. Stra13 regulates satellite cell activation by antagonizing Notch signaling. J Cell Biol. 2007;177:647–657. doi: 10.1083/jcb.200609007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei A, Van Houwe G, Hediger F, Kalck V, Cubizolles F, Schober H, Gasser SM. Nuclear pore association confers optimal expression levels for an inducible yeast gene. Nature. 2006;441:774–778. doi: 10.1038/nature04845. [DOI] [PubMed] [Google Scholar]
- Tan-Wong SM, Wijayatilake HD, Proudfoot NJ. Gene loops function to maintain transcriptional memory through interaction with the nuclear pore complex. Genes Dev. 2009;23:2610–2624. doi: 10.1101/gad.1823209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J, Kloeker S, Jensen CC, Bockholt S, Honda H, Hirai H, Beckerle MC. Members of the Zyxin family of LIM proteins interact with members of the p130Cas family of signal transducers. J Biol Chem. 2002;277:9580–9589. doi: 10.1074/jbc.M106922200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen S, Yoo S, Chakrabarti S, Zhang T, Ke T, Oberti C, Yong SL, Fang F, Li L, et al. Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell. 2008;135:1017–1027. doi: 10.1016/j.cell.2008.10.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.