Abstract
Polyphenols are a large family of naturally occurring plant products and are widely distributed in plant foods, such as, fruits, vegetables, nuts, flowers, bark and seeds, etc. These polyphenols contribute to the beneficial health effects of dietary products. Clinical and epidemiological studies suggest that exposure of the skin to environmental factors/pollutants, such as solar ultraviolet (UV) radiation induce harmful effects and leads to various skin diseases including the risk of melanoma and non-melanoma skin cancers. The incidence of non-melanoma skin cancer, comprising of squamous cell carcinoma and basal cell carcinoma, is a significant public health concern world-wide. Exposure of the skin to solar UV radiation results in inflammation, oxidative stress, DNA damage, dysregulation of cellular signaling pathways and immunosuppression thereby resulting in skin cancer. The regular intake of natural plant products, especially polyphenols, which are widely present in fruits, vegetables, dry legumes and beverages have gained considerable attention as protective agents against the adverse effects of UV radiation. In this article, we first discussed the impact of polyphenols on human health based on their structure-activity relationship and bioavailability. We then discussed in detail the photoprotective effects of some selected polyphenols on UV-induced skin inflammation, proliferation, immunosuppression, DNA damage and dysregulation of important cellular signaling pathways and their implications in skin cancer management. The selected polyphenols include: green tea polyphenols, pomegranate fruit extract, grape seed proanthocyanidins, resveratrol, silymarin, genistein and delphinidin. The new information on the mechanisms of action of these polyphenols supports their potential use in skin photoprotection and prevention of photocarcinogenesis in humans.
Keywords: Polyphenols, Photoprotection, Photocarcinogenesis, Proliferation, Skin Cancer, Inflammation, Immunosuppression, DNA repair, Cellular signaling pathway, Reactive oxygen species, Ultraviolet radiation
1. INTRODUCTION
Since early in the history of medicine, an association between diet and disease has persisted. Also, from the ancient time, natural products, herbs and spices have been used for preventing several diseases, including cancer. These beliefs have been supported by the father of modern medicine, Hippocrates. He proclaimed “Let food be thy medicine and medicine be thy food”, and it is true. Plant polyphenols are vital part of the human diet and the total intake is approximately 1gm/day [1, 2]. The main dietary sources of polyphenols are fruits, vegetables, dry legumes, chocolate, cereals, and beverages (such as tea, coffee, juice, wine and beer). The most common polyphenols are phenolic acids, flavonoids, catechins, stilbenes, proanthocyanidins, ellagitannins and anthocyanins. Phenolic acids are simple molecules and form a diverse group that includes the widely distributed hydroxybenzoic and hydroxycinnamic acids. Hydroxycinnamic acid compounds occur most frequently as simple esters with hydroxy carboxylic acids or glucose, while the hydroxybenzoic acid compounds are present mainly in the form of glucosides. Flavonoids are widely distributed in nature and its polyphenolic structure renders them quite sensitive to oxidative enzymes. Catechins are primarily found in tea leaves and grape seeds and have the monomeric flavan-3-ols catechin, epicatechin, gallocatechin, epigallocatechin, epicatechingallate and epigallocatechin-3-gallate. Stilbene, such as resveratrol is found in the skin of dark colored grapes. Proanthocyanidins are found in grape seeds, red wine and pine bark, and share common properties with other polyphenols, in particular their reducing capacity and ability to chelate metal ions. Ellagitannins are large molecules and are found in red wine, tea, nuts and pomegranate. Anthocyanins are a large group of water-soluble pigments found in pigmented fruits and vegetables [3]. The biological properties of polyphenols depend on their molecular structure.
The skin is the largest organ of the body situated at the interface between the body and its environment. Skin protects the internal organs of the body by acting as an effective barrier against the deleterious effects of solar ultraviolet (UV) radiation [3–5]. Solar UV radiation is the main cause for the vast majority of skin disorders including skin cancer. The incidence of UV-induced non-melanoma skin cancer has increased dramatically worldwide accounting for more than 40% of all human cancers in the United States, with about 1.3 million new cases being diagnosed annually. The basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) collectively known as non-melanoma skin cancer, are by far the most common form of cancers in humans and account for approximately 80% and 16% of all skin cancers, respectively, [6]. Although the incidence of skin cancer is increasing constantly, it is imperative to mention that among all the cancers, it is considered one of the most preventable types of cancer [3–5].
In this review article, we have first discussed the impact of polyphenols on human health based on their structure-activity relationship, their bioavailability and the factors controlling their bioavailability. Since experimental and epidemiologic studies have suggested that polyphenols protect the skin from the adverse effects of UV radiation [3, 7, 8], we have discussed this issue in detail. For this purpose, we have selected some well known and extensively studied polyphenols which have significant photoprotective effects. These are: green tea polyphenols, pomegranate fruit extract, resveratrol, silymarin, grape seed proanthocyanidins, genistein and delphinidin. Molecular targets include: (i) regulation of anti-inflammatory activity, (ii) modulation of immunosuppression, (iii) prevention of DNA damage and regulation of DNA repair, and (iv) modulation of cell signaling pathways critically involved in different stages of photocarcinogenesis (i.e. initiation, promotion and progression).
2. STRUCTURE-ACTIVITY RELATIONSHIP OF POLYPHENOLS
The antioxidant activity of polyphenols is due to their interactions with metal ions both in vitro and in vivo [9, 10]. Metal ions are the main cause of reactive oxygen species (ROS) generation and play an important role in generation of oxidative stress, DNA damage and cell death [10]. It is well documented that catechol and gallol are effective metal ion chelators [9, 10]. Polyphenols in tea chelate copper ions and is known to protect low-density lipoproteins from peroxidation [11]. Because of their chelating properties polyphenols may also protect against heavy metal toxicity [9]. The reactivities of proanthocyanidins and gallate esters with hydroxyl radicals, azide radicals, or superoxide anions correlate with catechol and pyrogallol groups in their molecular structures that provide evidence of the antioxidant properties of these agents [12, 13]. It is well known that the scavenging activity of different tea catechin molecules is related to the number of o-dihydroxy and o-hydroxyketo groups, C2-C3 double bonds, concentration and solubility, the accessibility of the active group to the oxidant and on the stability of the reaction product [14]. Consumption of tea polphenols lowers absorption of dietary iron and decreases body iron balance indicating chelating activity [9]. Polyphenols also affect signal transduction pathways, modulate many endocrine systems, and alter hormones and other physiological processes as a result of their binding to metal ions and enzyme cofactors [15]. Recently, Cao et al. [16] have shown that green tea catechins and the hydrolyzable tannins are effective in inhibiting DNA adduct formation at least, in part, due to direct interaction of adjacent hydroxyl groups in their molecular structures.
3. BIOAVAILABILITY OF POLYPHENOLS
Biological activity of polyphenols largely depends on their bioavailability. The molecular diversity influences the bioavailability of the polyphenols and they differ by their glycosylation, esterification, hydroxylation, and polymerization. Studies have suggested that the rate and extent of intestinal absorption and their levels in plasma depends on the chemical structure of polyphenols. The maximum concentration of polyphenols in human plasma is often reached within 1 and 2 hours after ingestion [2, 17]. Studies have shown that human consumption of 1.5, 3.0, or 4.5 g of decaffeinated green tea in 500 ml of water resulted in the maximum plasma concentrations of (−)-epigallocatechin-3-gallate (EGCG), (−)-epigallocatechin (EGC) and (−)-epicatechin (EC) to be 326 ng/ml, 550 ng/ml, 190 ng/ml respectively, as observed at 1.4-2.4 h after ingestion [18]. Suganuma et al. [19] studied the distribution of radiolabeled [3H]EGCG in mouse by directly administering the solution into the stomachs of CD-1 female or male mice. Radioactivity was found in many organs, including skin. These studies suggested that frequent consumption of green tea enables the body to maintain a high level of tea polyphenols, indicating a wide range of target organs for cancer prevention in humans. Administration of EGCG or Polyphenon E (EGCG rich green tea polyphenolic mixture) at a daily dose of 800 mg (based on the EGCG content) for 4 weeks resulted in more than 60% increase in the systemic availability of free EGCG, and is safe and also well tolerated in healthy human subjects [20]. Female HWY/Slc hairless rats reduced UVB-mediated increase in epidermal thickness and transepidermal water loss and also restored UVB-mediated decrease in total antioxidant capacity in both skin and blood [21]. Administration of punicalagin (6%), an antioxidant ellagitannin of pomegranate juice, in the diet of mice is absorbed as such in animals and its level is detected in plasma [22]. Pharmacokinetic studies carried out in 18 healthy volunteers that received 180 ml of pomegranate juice concentrate showed ellagic acid metabolites, including dimethylellagic acid glucuronidein and hydroxy-6H-benzopyran-6-one derivatives in the plasma and urine, in conjugated and free forms [23].
4. ULTRAVIOLET RADIATION AND MULTISTAGE MODEL OF UV-INDUCED CARCINOGENESIS
Solar light is an indispensable commodity of life and plays an important role in maintaining a balanced ecosystem. Solar light provides energy needed for photosynthesis by plant, a good source of vitamin D synthesis in human skin, and several other beneficial effects in human life. However, excessive exposure to solar light, especially its UV component, has been linked to skin cancers and other skin related disorders [4, 5, 7, 24]. Development of skin cancer depends on the cumulative amount and form of the UV radiation, as well as on the skin type of the individual exposed. Factors that influence UV exposure include higher altitude, close proximity to the equator, outdoor occupation, recreational activity and use of tanning parlous.
Chronic exposure of the skin to solar UV radiation is a major etiologic factor for the risk of non-melanoma skin cancers comprising of BCCs and SCCs. According to the International Commission on Illumination, UV radiation is divided into three categories: short wave UVC (200–280 nm), mid wave UVB (280–320 nm) and long wave UVA (320–400 nm). UVC is extremely damaging to the skin because these wavelengths of light have enormous energy and can penetrate the skin to a depth of approximately 60–80 micrometer. However, UVC in solar radiation is prevented from reaching the earth, as it is almost completely absorbed by ozone layer and therefore its role in human pathogenesis is minimal. Both UVB and, to a lesser extent, UVA radiation are responsible for various skin disorders including skin cancer [3, 25]. UVB radiation constitutes about 5% of UV radiation and is thought to be the most active constituent of solar radiation responsible for a variety of skin diseases including nonmelanoma and melanoma skin cancers. UVB is more genotoxic and capable of causing much more cell damage than UVA and acts mainly on the epidermal basal layer of the skin. It has a less penetrating power than UVA and penetrates the skin to a depth of approximately 160–180 micrometer. UVB radiation can induce both direct and indirect adverse biologic effects including DNA damage, oxidative stress, depletion of cutaneous defense system, inflammation, immunosuppression, and premature aging of the skin. In addition, UVB can act as a tumor initiator, tumor promoter, and co-carcinogen [3, 26]. UVA on the other hand constitutes about 90–95% of solar radiation reaching the earth and due to its longer wavelength has high penetrating ability. UVA can penetrate deep into the skin to a depth of approximately 1000 micrometer. UVA exposure also leads to the generation of singlet oxygen, hydrogen peroxide (H2O2), and hydroxyl radical that can cause damage to cellular proteins, lipids and DNA [3, 25].
Solar UV radiation-induced skin cancer or photocarcinogenesis is a complex multistage phenomenon involving three distinct stages initiation-promotion-progression mediated via alterations in various cellular, biochemical, and molecular changes. Tumor initiation, the first step of carcinogenesis and irreversible process in which genetic alterations occur in genes that ultimately leads to DNA mutation in normal cells. These mutations are in the form of C to T and CC to TT transition and have been detected in tumor suppressor gene p53 in human SCCs, BCCs and actinic keratosis. Tumor promotion is considered to be slow and reversible process involving clonal expansion of initiated cells giving rise to premalignant lesions, essentially by alterations in signal transduction pathways. Tumor progression involves the conversion of premalignant lesions into an invasive and potentially metastatic malignant tumor [3, 27].
5. SKIN PHOTOPROTECTION AND ANTI-PHOTOCARCINOGENIC EFFECTS OF POLYPHENOLS
Dietary polyphenols have gained considerable attention for the prevention of UV-induced skin photodamage including the risk of skin cancer. Polyphenols possessing anti-inflammatory, immunomodulatory, DNA repair capability, and can correct undesired cellular functions are among the most promising group of compounds that can be exploited as ideal chemopreventive agents. Chemoprevention offers a practical approach to delineate substances, either components of food or pharmaceuticals, which can prevent, delay or completely halt the process of photocarcinogenesis. For a variety of reasons, among many chemopreventive agents known, there is greater emphasis on substances present in diet and beverages commonly consumed by humans. In this respect, chemoprevention offers a realistic strategy for controlling the risk of skin cancers because agents can be targeted for intervention at the initiation, promotion, or progression stage of the multistage photocarcinogenesis. An ideal chemopreventive agent for human use should have: (i) little or no toxicity, (ii) anti-mutagenic and anti-carcinogenic activities, (iii) striking inhibitory effects on diverse cellular events associated with multistage photocarcinogenesis, (iv) high efficacy in multiple sites, (v) capability of oral consumption, (vi) a known mechanism of action, (vii) affordable low cost, and (viii) human acceptance [28]. Furthermore, the individuals can modify their dietary habits and lifestyle in combination with a careful use of skin care products to prevent UVB-mediated skin damage. In this Mini Review Article, we have summarized and discussed the molecular targets or mechanism(s) of action of some of the selected polyphenols (such as green tea polyphenols, pomegranate fruit extract, grape seed proanthocyanidins, silymarin, resveratrol, genistein and delphinidin) against UV radiation-induced inflammation, immunosppression, prevention of DNA damage, DNA repair, modulation of cell signaling pathways critically involved in different stages of photocarcinogenesis (Table 1).
Table 1.
A summary of molecular mechanism(s)/cellular targets of some selected polyphenols in skin photoprotection.
| Polyphenols, Structure & Source | Category | In vitro Studies | In vivo Studies | Molecular Mechanism(s)/Cellular Targets | References |
|---|---|---|---|---|---|
|
Catechins | Keratinocytes | Inhibition of UVB-mediated phosphorylation of ERK1/2, JNK1/2 and p38 proteins. | 52 | |
| Inhibition of UVB-mediated activation of NF-κB signaling and NF-κB DNA binding activity. | 53 | ||||
| Inhibition of UVB-induced AP-1 activity. | 56 | ||||
| Fibroblasts XPA-proficient cells | Reduction in UVB-mediated DNA damage through IL-12 dependent functional NER mechanism. | 43, 109 | |||
| SKH-1 mice | Inhibition of UVB-mediated phosphorylations of MAPKs and activation of NF-κB and NF-κB/p65 DNA binding activity. | 54,55 | |||
| Reduction in UVB-mediated increase in protein expression of MMP-2, MMP-9, CD31, VEGF and PCNA. | 40,55 | ||||
| Inhibition of UVB-mediated increase in infiltration of leukocytes, protein oxidation and lipid peroxidation. | 54,55 | ||||
| C3H/HeN mice | Reduction in UVB-induced infiltration of CD11b+ cells and IL-10 production. Induction in UVB-mediated decrease in IL-12 production. | 85 | |||
| Inhibition of UVB-mediated increase in COX-2, PGE2, cyclin D1, TNFα, IL-6, IL-1β. | 39 | ||||
| Reduction in UVB-mediated increase in H2O2 producing cells, inducible nitric oxide synthase expressing cells, H2O2 and nitric oxide production. | 72 | ||||
| Reduction in UVB-mediated DNA damage through IL-12 dependent functional NER mechanism. | 43 | ||||
| XPA-positive mice | Reduction in UVB-mediated formation of CPD+ cells. | 109 | |||
| Human Reconstituted skin | Reduction in UVB-mediated DNA damage through IL-12 dependent induction of DNA repair. | 102 | |||
| Human skin | Inhibition of UVB-mediated increase in H2O2 and nitric oxide production. | 73 | |||
| Inhibition of UVB-mediated increase in the production of PGE2, infiltration of leukocytes and myeloperoxidase activity. | 74 | ||||
|
| |||||

|
Anthocyanins, hydrolyzable tannins and Ellagitannins | Keratinocytes | Inhibition of UVB-mediated phosphorylation MAPKs, degradation and phosphorylation of IκBα, activation of IKKα, nuclear translocation and phosphorylation of NF- κB/p65 at Ser536. | 57 | |
| Fibroblasts | Inhibition of UVB-mediated activation of NF-κB, downregulation of proapoptotic caspase-3, and accumulation of cells in G0/G1 phase of the cell cycle. | 59 | |||
| SKH-1 mice | Inhibition of UVB-mediated phosphorylation of MAPKs, phosphorylation of STAT3 (Tyr705) and NF-κB/p65 (Ser536), activation of IKKα and phosphorylation and degradation of IκBα. | 44,45 | |||
| Inhibition of UVB-mediated expression of inducible nitric oxide synthase and COX-2. | 44 | ||||
| Reduction in UVB-mediated formation of CPDs and 8-oxodG and H2O2. | 44 | ||||
| Human Reconstituted skin | Inhibition of UVB-mediated increase in protein expression of c-Fos and phosphorylation of c-Jun, 1), gelatinase (MMP-2, MMP-9), stromelysin (MMP-3), marilysin (MMP-7), and elastase (MMP-12). | 58 | |||
| Reduction in UVB-mediated formation of CPDs and 8-oxodG. | 58 | ||||
|
| |||||
|
Proanthocyanidin | Keratinocytes Fibroblasts XPA-proficient cells | Inhibition of UVB-mediated phosphorylation of MAPKs and activation of NF-κB signaling. | 30 | |
| Reduction in UVB-mediated DNA damage through IL-12 dependent functional NER mechanism. | 80 | ||||
| SKH-1 mice | Inhibition of UVB-mediated phosphorylation of MAPKs, activation and nuclear translocation of NF-κB. | 60 | |||
| Inhibition of UVB-mediated infiltration and accumulation of activated macrophages and neutrophils, myeloperoxidase activity, COX-2, cyclin D1, PCNA, PGE2, TNF-α, IL-6 and IL-1β. | 75 | ||||
| C3H/HeN mice | Reduction in the levels of CPD+ cells through through IL-12 dependent induction of DNA repair. | 80 | |||
| Protection against UVB-mediated enhanced production of IL-10 by the epidermal and dermal cells of the skin, and in the draining lymph nodes. | 84 | ||||
| Inhibition of UVB-induced immunosuppression by activating CD8+ effector T cells and inhibiting CD4+ T regulatory cells. | 80 | ||||
|
| |||||
|
Flavonoid | JB6 cells | Inhibition of UVB-induced mitogenic and cell survival signaling involving AP-1 and NF-κB. | 62 | |
| SKH-1 mice | Inhibition of UVB-induced phosphorylation of MAPKs and AKT. | 61 | |||
| Induction of UVB-mediated E2F1 protein expression. | 63 | ||||
| Suppression of UVB-mediated increase in inducible nitric oxide synthase and COX-2 protein expression, phosphorylation of STAT3 and NF-κB/p65. | 50 | ||||
| C3H/HeN mice | Inhibition of UVB-induced infiltrating leukocytes, myeloperoxidase activity, IL-10 producing cells and its production. | 78,79 | |||
| Inhibition of UV-induced oxidative stress by targeting CD11b+ cell type in the skin. | 79 | ||||
|
| |||||
|
Stilbene | Keratinocytes | Inhibition of UVB-mediated activation of IKKα and NF-κB, and phosphorylation and degradation of IκBα. | 29 | |
| A431 cell | Reduction in the phosphorylation of Akt and pCREB, and downregulation of TGF-β2. | 48 | |||
| SKH-1 mice | Inhibition in UVB-mediated infiltration of leukocytes, H2O2 production and PG metabolites, especially PGE2 and PGD2. | 76 | |||
| p53+/−/SKH-1 mice | Reduction in UVB-mediated expression of TGF-β2. | 48 | |||
|
| |||||
|
Isoflavones | A431 cells | Reduction in UVB-induced EGFR tyrosine phosphorylation and PGE2 synthesis. | 64 | |
| Fibroblasts | Inhibition of UVB-mediated phosphorylated p66Shc at Ser36, and FKHRL1 at Thr32. | 65 | |||
| SENCAR mice | Reduction in UVB-mediated protein expression c-fos and c-jun. | 64 | |||
| Human Reconstituted skin | Reduction in UVB-mediated formation of CPDs. | 111 | |||
|
| |||||
|
Anthocyanidin | JB6 P+ cells | Suppression of UVB-induced transactivation of AP-1 and NF-κB and phosphorylation of JNKs, p38 and Akt. | 66 | |
| HaCaT cells | Reduction in UVB-mediated formation of 8-oxodG. | 110 | |||
| SKH-1 mice | Reduction in UVB-mediated DNA damage in the form of CPDs and 8-oxodG. | 110 | |||
It is well established that exposure of the skin to UV radiation contributes to the development of skin cancers. Epidemiological, clinical and pre-clinical studies have implicated that solar UV radiation is the major etiological factor in the development of cutaneous malignancy [4, 27, 29, 30], including the nonmelanoma skin cancers which represent the most common malignant neoplasms in humans [31, 32]. Various animal models have been employed to examine the anti-photocarcinogenic effects of plant polyphenols. The plant polyphenols possess anti-inflammatory, immunomodulatory, anti-oxidant properties and DNA repair activities, and that can be exploited for the prevention of variety of skin disorders caused by excessive exposure to solar UV light. Recent advances in our understanding at the cellular and molecular levels of photocarcinogenesis have led to the development of promising strategies for the skin photoprotection including photocarcinogenesis. Studies have shown the photoprotective potential of several plant polyphenols, such as green tea polyphenols (GTPs), silymarin, retinoids, grape seed proanthocyanidins (GSPs), and delphinidin, etc. against UV radiation-induced adverse effects [33–35]. Here, we will summarize and discuss the recent developments in the area of anti-photocarcinogenic potential of some selected polyphenols which were studied extensively.
Following standard photocarcinogenesis protocols, topical application or oral administration of green tea polyphenols (GTPs) in drinking water of mice resulted in lower tumor burden in terms of tumor incidence and tumor multiplicity in these animals compared to non-GTPs-fed control group of mice [36–40]. Oral feeding of green tea or injection of GTPs fraction or EGCG to mice was found to inhibit the growth and/or caused the regression of established experimentally-induced nonmalignant skin papilloma in mice [41]. Histopathological examination of each tumor showed that oral administration of green tea had a marked inhibitory effect on the formation of UVB-induced keratoacanthomas and carcinomas [42]. Meeran et al. [43] employed interleukin-12 knockout (IL-12 KO) mice to elucidate whether the induction of IL-12 by EGCG is associated for its protective effect against photocarcinogenesis. It was tested because IL-12 has been shown to have anti-tumor activity and DNA repair ability in mice. Topical application of EGCG to wild-type mice resulted in a significant reduction in UVB-induced skin tumorigenesis in terms of tumor incidence and tumor multiplicity compared with non–EGCG-treated wild-type mice. However, topical application of EGCG to IL-12 KO mice did not protect photocarcinogenesis. These observations suggest that chemopreventive effect of green tea against photocarcinogenesis requires IL-12 or mediated through IL-12-based mechanism.
Pomegranate fruit extract (PFE) is a rich source of anthocyanins, ellagitannins and hydrolyzable tannins and possesses strong antioxidant activity [44]. Oral feeding of PFE to SKH-1 hairless mice in a UVB initiation-promotion protocol resulted in reduced tumor incidence, delay in the latency period of tumor appearance, and lower tumor body burden compared to that of non-PFE-treated and UVB-irradiated control animals [45]. Dietary grape seed proanthocyanidins (GSPs) supplemented with AIN76 control diet significantly lowered the tumor multiplicity and growth or size of the tumor in SKH-1 hairless mouse model in UVB-induced complete (both initiation + promotion) photocarcinogenesis protocol. Dietary GSPs also resulted in prevention and delay of malignant transformation of UVB-induced papillomas to carcinomas in terms of carcinoma incidence, carcinoma multiplicity and carcinoma growth [46]. Aziz et al. [47] have shown that topical application of resveratrol both pre- and post-UVB irradiation of SKH-1 hairless mice resulted in a significant inhibition in tumor incidence, and delay in the onset of tumorigenesis. Post-application of resveratrol was found to impart almost equal protection compared to pre-application, suggesting that resveratrol-mediated chemopreventive effects may not be due to sunscreen effects. Treatment of p53+/−/SKH-1 mice with resveratrol by oral gavage reduced the average number of skin tumors and the average tumor volume compared with non-resveratrol treated and UV-exposed control group of mice. In addition, the number of SCCs in resveratrol-treated mice was less than non-resveratrol treated control mice [48].
Topical treatment of SKH-1 hairless mouse skin with silymarin, a flavonoid from milk thistle (Silybum marianum), significantly reduced UVB-induced tumor incidence, tumor multiplicity, and average tumor volume per mouse compared to non-silymarin treated UVB-exposed mice. Silymarin was effective in protecting the skin against all the stages of photocarcinogenesis, such as UV-induced tumor initiation, tumor promotion, and complete carcinogenesis (initiation + promotion) protocols [49]. Topical treatment of mouse skin with silibinin, a major component of silymarin, before or immediately after UVB irradiation or given in diet afforded protection against photocarcinogenesis in terms of delay in tumor appearance, tumor multiplicity and tumor volume [50, 51]. Multiple in vitro and in vivo studies suggest anti-photocarcinogenic potential of plant polyphenols, we will briefly summarize and discuss the molecular mechanisms responsible for anti-photocarcinogenic effect of these selected polyphenols.
6. MECHANISM AND CELLULAR TARGETS OF PHOTOPROTECTION OF POLYPHENOLS
As the understanding of molecular mechanism(s) or molecular targets is essential in designing strategies for the skin photoprotection, we are describing some recent developments associated with the mechanism of photoprotection of the skin by selected polyphenols which are extensively studied.
6.1. MODULATION OF CELLULAR SIGNALING PATHWAYS RESPONSIBLE FOR PHOTOCARCINOGENESIS
UV radiation is known to activate multiple cellular signaling pathways responsible for various skin diseases. Of particular has been the activation of nuclear factor kappa B (NF-κB), and members of the activator protein-1 (AP-1) complex, mitogen activated protein kinases (MAPKs), phosphatidylinositol-3-kinase (PI3K)/Akt, and signal transducers and activators transcription 3 (STAT3) [4, 5, 8, 24, 50]. UV-induced responses depend on dose, cell type, duration of activation of the pathways and crosstalk between pathways that determine the course of direction (e.g. cell survival, apoptosis, proliferation, invasion and migration) adopted by the cells. Exposure of normal human epidermal keratinocytes (NHEK) to UVB radiation resulted in the phosphorylation of MAPKs, degradation and phosphorylation of IκBα, activation of IKKα, nuclear translocation of NF-κB/p65, and induction of NF-κB DNA binding activity. However, treatment of NHEK with EGCG reduced UVB-mediated phosphorylation of MAPKs and activation NF-κB signaling pathway. These findings demonstrate that EGCG has the potential to inhibit UVB-mediated activation of cellular signaling pathway in human keratinocytes, and which are deleterious for the normal skin cells [52, 53]. Studies were also designed to establish the relevance of these findings in vivo situation in mouse model. Topical treatment of the mouse skin with GTPs reduced UVB-induced phosphorylation of MAPKs, activation of IKKα, and phosphorylation and degradation of IαBα. GTPs also inhibited UVB-induced nuclear translocation of NF-κB/p65 and NF-κB/p65 DNA-binding activity. These studies suggest that beneficial or photoprotective effects of GTPs are mediated through the inhibition of these pathways and provide molecular basis for its photoprotective effect on the skin [54]. Topical application of GTPs or its active constituent EGCG in hydrophilic ointment-based topical formulation resulted in marked inhibition of UVB-induced phosphorylation of ERK1/2, JNK and p38 proteins of MAPK family [55]. Administration of GTPs in drinking water of mice reduced UVB-mediated increase in matrix metalloproteinases (MMP)-2 and MMP-9, CD31 (a biomarker of neo-vasculature), vascular endothelial growth factor, and proliferating cell nuclear antigen (PCNA). In addition, there were more cytotoxic CD8+ T cells and greater activation of caspase-3 in the tumors of mice that received GTPs, indicating the death of the tumor cells after treatment with GTPs [40]. Using cultured human keratinocytes, Barthelman et al. [56] have demonstrated that EGCG is effective at inhibiting AP-1 activity when applied before, after or both before and after UVB irradiation.
Similarly, treatment of NHEK with PFE resulted in a dose- and time-dependent inhibition of UVB-induced phosphorylation of ERK1/2, JNK1/2 and p38 proteins. PFE treatment to NHEK also resulted in a dose- and time-dependent inhibition of UVB-induced degradation and phosphorylation of IκBα, activation of IKKα, nuclear translocation and phosphorylation of NF-κB/p65 at Ser536 [57]. Similar effects were found when mice were given PFE in drinking water [44]. These findings demonstrate that PFE has a potential to inhibit UVB-induced oxidative stress-mediated activation of MAPKs and NF-κB signaling pathways both in vitro and in vivo models. Oral feeding of PFE also showed decrease in the phosphorylation of STAT3 (Tyr705) and NF-κB/p65 (Ser536) in UVB-induced skin tumors and tumor uninvolved-skin compared to skin tumors and tumor un-involved skin samples from non PFE-treated and UVB-exposed animals. Additionally, a concomitant decrease in the protein expression of inducible nitric oxide synthase and cyclooxygenase-2 (COX-2) was observed in UVB-exposed skin and tumors that are potential downstream targets of STAT3 and NF-κB. There was a decrease in the protein levels of hypoxia inducible factor-1α in skin tumors of mice that received PFE compared to non-PFE-treated animals [45]. The effect of PFE was also examined in human reconstituted skin. Treatment of human reconstituted skin with pomegranate derived products, such as juice, whole extract and oil, reduced UVB-induced protein expression of c-Fos and phosphorylation of c-Jun. In addition, pomegranate derived products inhibited UVB-induced expressions of collagenase (MMP-1), gelatinase (MMP-2, MMP-9), stromelysin (MMP-3), marilysin (MMP-7), and elastase (MMP-12) [58]. These observations indicate the photoprotective potential of pomegranate derived products against UVB-induced adverse effects on human skin. Treatment of human skin fibroblasts with PFE decreased UVB-induced cell death and this may be likely due to reduced activation of NF-κB, downregulation of proapoptotic caspase-3, and accumulation of cells in G0/G1 phase of the cell cycle [59]. This effect of PFE in UVB-exposed human fibroblasts is viewed as a photoprotective effect of PFE on normal cells
Treatment of NHEK with GSPs reduced UVB-mediated phosphorylation of MAPKs, activation of IKKα, degradation of IκBα, and phosphorylation and nuclear translocation of NF-κB/p65 [30]. Administration of dietary GSPs inhibited acute and chronic UVB irradiation-induced phosphorylation of ERK1/2, JNK1/2 and p38 proteins of MAPK family, which seems to be mediated through reactivation of MAPK phosphatases in the mouse skin compared with non-GSP-treated but UVB-exposed mice. It also reduced UVB-induced activation and nuclear translocation of NF-κB/p65 a downstream target of MAPK signal transduction pathways [60]. Topical application of silibinin, an active and major constituent of silymarin, induced MAPKs cascades in UVB-induced skin tumor, but reduced the phosphorylation of all three MAPKs in uninvolved skin. These data suggest that silibinin is enhancing apoptotic death of tumorigenic skin cells by further activating MAPKs and protecting uninvolved skin cells against UVB-caused MAPK activation that coorelates with proliferation and transformation [51]. Topical treatment of silibinin prior to or immediately after acute and chronic UVB exposure protected mouse skin from UVB-induced phosphorylation of MAPKs and Akt suggesting that these effects of silibinin possibly lead to a decrease in UVB-caused proliferation and apoptosis [61]. Treatment of tumor promoter-sensitive JB6 mouse epithelial cells with silibinin before or immediately after UVB exposure reduced UVB-induced phosphorylation of ERK1/2 and Akt, and activation of AP-1 and NF-κB, suggesting that silibinin possibly prevents skin tumor promotion by inhibiting UVB-induced mitogenic and cell survival signaling [62]. Topical treatment of mouse skin with silibinin or dietary administration of silibinin increased the levels of E2F1; however the expression of E2F2 and E2F3 proteins was decreased. Enhanced E2F1expression may have a role in inhibition of UVB-induced apoptosis in the epidermis of chronically UVB-exposed mouse skin [63].
The photoprotective effect of resveratrol was also examined using NHEK as an in vitro model. Similar to other polyphenols, resveratrol also blocked UVB-induced activation of NF-κB [29]. Kim et al. [48] have shown that topical treatment of mice with resveratrol reduced UVB-induced expression of transforming growth factor-β2 (TGF-β2) in skin as well as in SCCs. Downregulation of TGF-β2 by resveratrol led to the inhibition of both TGF-β2/Smad-dependent and -independent pathways. In addition, resveratrol treatment reduced the phosphorylation of Akt and pCREB in human epidermoid carcinoma A431 cells. These data suggest that resveratrol decreased the invasiveness of human A431 cells through Akt-mediated downregulation of TGF-β2. Studies have shown that exposure of keratinocytes to UVB increases prostaglandin (PG) synthesis through tyrosine kinase dependent pathway, and treatment of cells with genistein reduced the levels of UVB-induced epidermal growth factor receptor (EGFR) tyrosine phosphorylation and PGE2 synthesis. Topical application of mouse skin with genistein reduced UVB-enhanced expressions of c-fos and c-jun in mouse skin [64]. This study suggests that suppression of UVB-induced c-fos and c-jun expressions in mouse skin by genistein, at least in part, may be due to inhibition of tyrosine protein kinase activities and phosphorylation of EGFR. Treatment of human dermal fibroblasts with genistein also reduced UVB-mediated phosphorylated p66Shc at Ser36, and FKHRL1 at Thr32 [65]. Treatment of JB6 P+ cells with delphinidin suppressed UVB-induced transactivation of AP-1 and NF-κB and phosphorylation of JNKs, p38 and Akt. The study also revealed that delphinidin binds directly with MAPKK4 and PI3K in an adenosine triphosphate-competitive manner [66]. Overall, these studies suggest that polyphenols modulate UVB-mediated activation of cellular signaling pathways both in vitro and in vivo situations leading to photoprotection of skin cells.
6.2. ANTI-INFLAMMATORY ACTIVITY
Erythema or redness of the skin is the most prominent visible sign of inflammation in skin resulting from UVB exposure. UV-induced inflammatory responses are characterized by dilation of dermal blood vessels, vascular hyperpermeability, cutaneous edema, hyperplasia, infiltration of leukocytes, increase in pro-inflammatory cytokines, generation of ROS, increase in the enzyme and protein expression of COX-2 and PG metabolites, especially PGE2 [3, 26, 67, 68]. PGE2 is the primary mediator of erythema in human skin, as demonstrated in its reduction by specific inhibitors of prostaglandin synthesis [69]. UV-induced inflammation plays a crucial role in all three stages of tumor development, i.e., initiation, promotion and progression [3, 68]. COX-2 is over expressed in hyperplastic skin, benign papillomas and in SCCs after chronic UV exposure of the mouse skin [70]. Similarly in human skin, elevated levels of COX-2 is found in actinic keratoses, SCCs and BCCs [71, 72]. UV irradiation of both cultured keratinocytes and SKH-1 hairless mouse skin activates numerous cellular pathways including MAPK, PI3K/AKT, STAT and NF-κB leading to transcriptional activation of the COX-2 gene [3, 26].
Studies conducted by Meeran et al. [39] have found that the levels of biomarkers of inflammation (COX-2, PGE2, PCNA, cyclin D1) and proinflammatory cytokines (TNF-α, IL-6, IL-1β) were higher in chronically UVB-exposed skin and skin tumors of IL-12 KO mouse skin than in the UVB-exposed skin of their wild-type counterparts. This study suggests that IL-12 deficiency may be a contributing factor to inflammation and that contribute to the early occurrence and rapid development of skin tumors in IL-12 KO mice. Oral administration of GTPs in drinking water of mice significantly reduced the levels of inflammation and proinflammatory cytokines in UVB-exposed skin and skin tumors in the wild-type mice but had a non-significant effect in IL-12-KO mice. This study indicates that photoprotective effect of GTPs on UV-induced skin tumor development requires IL-12. Topical treatment of mouse skin with GTPs resulted in reduction of UVB-mediated edema and infiltration of leukocytes [54]. Topical treatment of mouse skin with EGCG or GTPs significantly inhibited UVB-induced protein oxidation (protein carbonyls formation) and lipid peroxidation in mouse skin [55]. EGCG inhibited UVB-induced infiltration of leukocytes (CD11b+ cell type), myeloperoxidase activity, the number of H2O2-producing cells and inducible nitric oxide synthase-expressing cells as well as the production of H2O2 and nitric oxide in both epidermis and dermis of the mouse skin [72]. Similar photoprotective effects of EGCG were also found in human skin [73]. Topical treatment of human skin with EGCG inhibits UVB-induced production of prostaglandin metabolites, particularly PGE2, which play a critical role in inflammatory disorders and in proliferative skin diseases [74].
Dietary GSPs inhibited UVB-mediated infiltration and accumulation of activated macrophages and neutrophils, myeloperoxidase activity, expression of COX-2, cyclin D1 and PCNA proteins, and the levels of PGE2 in the skin and skin tumors. Dietary GSPs also inhibited the levels of proinflammatory cytokines such as TNF-α, IL-6 and IL-1β in the skin and skin tumors [75]. These data collectively suggest that anti-photocarcinogenic activity of GSPs is associated with the inhibition of UVB-induced inflammation and inhibition of inflammatory mediators in mouse skin. Topical application of resveratrol to SKH-1 hairless mouse skin reduced UVB-mediated infiltration of leukocytes, skin edema, H2O2 production and PG metabolites, especially PGE2 and PGD2 [76]. Dietary supplement and topical formulations containing oligomeric proanthocyanidins reduced erythema and skin hydration in humans [77]. Treatment of JB6 P+ mouse epidermal cells with delphinidin reduced UVB-induced COX-2 expression and PGE2 production, and this was associated with the suppression of AP-1 and NF-κB transcriptional activities [66].
Topical treatment of mouse skin with silymarin inhibited UVB-induced oxidative stress through inhibition of infiltrating CD11b+ cells. UV radiation induced infiltrating leukocytes are the major source of ROS production [78]. In addition, the analysis of myeloperoxidase level also indicated that silymarin significantly decreased UVB-induced infiltration of leukocytes, and this effect of silymarin was similar to that of intraperitoneal treatment of mice with CD11b monoclonal antibodies. Analytical data revealed that CD11b+ cell population from UV-irradiated skin are the major source of ROS production in both epidermis and dermis than CD11b− cell population, and silymarin inhibited UV-induced oxidative stress by targeting CD11b+ cell type in the mouse skin [79]. Topical or dietary silibinin treatment reduced UVB-induced inducible nitric oxide synthase and COX-2 protein expression by targeting STAT3 and NF-κB/p65 [50]. All these studies suggest that the photoprotective effects and anti-photocarcinogenic activity of polyphenols are associated with the inhibition of UVB-induced inflammatory mediators and associated signaling molecules that regulate these events.
6.3. PREVENTION OF PHOTOCARCINOGENESIS THROUGH THE INDUCTION OF CYTOKINE INTERLEUKIN-12
UVB-induced immunosuppression has been implicated in photocarcinogenesis and the role of IL-12 in photocarcinogenesis has been extensively studied with GTPs and GSPs. Topical treatment of the skin with EGCG inhibited UVB-induced immunosuppression in mice through the induction of IL-12, an immunoregulatory cytokine. To verify the role of IL-12 in skin photoprotection by GSPs and GTPs, IL-12 KO mouse model was used. Topical application of EGCG resulted in a significant reduction in UVB-induced skin tumor development compared to non-EGCG treated wild-type mice of IL-12 KO mice. However, the treatment of IL-12 KO mice with EGCG did not inhibit tumor development in these mice [43]. The analysis of tumor data indicates that mice deficient in IL-12 are at greater risk of photocarcinogenesis, and suggest that the chemopreventive effect of EGCG against skin cancer is mediated through the induction of IL-12. Identical information were also observed when IL-12 KO and their wild-type counterparts were given GTPs in drinking water of mice and subjected to photocarcinogenesis protocol [39]. Similar to the polyphenols from green tea, the effect of GSPs was also examined on photocarcinogenesis using the identical IL-12 KO mouse model [80]. To examine the effect of dietary GSPs on photocarcinogenesis, GSPs were supplementation with AIN76A control diet. Using identical photocarcinogenesis protocol, it was noticed that dietary GSPs did not prevent photocarcinogenesis in those mice which were deficient in IL-12 [80]. These data suggest that IL-12 is required for the prevention of photocarcinogenesis by GTPs or GSPs.
6.4. PREVENTION OF UVB-INDUCED IMMUNOSUPPRESSION
The immunosuppressive effects of solar UVB radiation are well established and have been implicated in skin cancer [81, 82]. Some of the adverse effects of solar UV radiation on human health, including exacerbation of infectious diseases and initiation of skin cancer, are mediated at least in part by the ability of UV radiation to induce immunosuppression [24]. This assumption is supported by the facts that chronically immunosuppressed patients living in regions of intense sun exposure experience an exceptionally high rate of skin cancer [83]. Therefore, the prevention of UV-induced immunosuppression can be considered as a potential strategy for the prevention of photocarcinogenesis. In that context the effect of dietary GSPs was examined on UVB-induced immunosuppression in C3H/HeN mice. Intake of dietary GSPs resulted in significant protection against UVB-induced suppression of contact hypersensitivity (CHS) response [84]. Topical application of EGCG on the UVB-exposed skin of mice also inhibited UVB-induced suppression of CHS response to contact sensitizer, 2,4-dinitrofluorbenzene (DNFB) [85]. Topical treatment of EGCG or consumption of GTPs in drinking water of mice also resulted in inhibition of UV-induced inflammation, which suggests an association between the induction of inflammation and CHS after UV irradiation of mouse skin [39, 85]. Topical treatment of mouse skin with silymarin inhibited UVB-induced suppression of CHS response and this was found to be associated with the inhibition of infiltrating leukocytes, particularly CD11b+ cell type, and myeloperoxidase activity [78, 86, 87]. In addition, silymarin was found to reduce UVB-mediated increase in the immunosuppressive cytokine IL-10-producing cells and the levels of IL-10 cytokine [78]. This study was further extended to determine whether topical application of silymarin or silibinin, a major component of silymarin, has effect on UVB–induced suppression of CHS response in local and in systemic models of CHS. Both silymarin and silibinin inhibited UVB-induced local and systemic immunosuppression. However, the magnitude of the immunoprotective effect of silymarin or silibinin in the systemic CHS model was lower than that in the local CHS model [86]. Topical application of silymarin was found to reduce UVB-mediated increase in the level of IL-10 in the skin and draining lymph nodes and enhanced the levels of IL-12. In addition, treatment of mice with anti-IL-12 antibody abrogated the ability of silymarin to protect against UVB-induced suppression of the CHS response in a local model of CHS. Moreover, topical application of silymarin to mice did not protect against UVB-induced immunosupression of the CHS response in IL-12 KO mice but prevented it in their wild-type mice [86]. These studies suggest that prevention of UVB-induced immunosuppression by silymarin requires IL-12. This mechanism of protection by silymarin is identical with the mechanism found with GTPs and GSPs.
6.5. PREVENTION OF UVB-INDUCED IMMUNOSUPPRESSION THROUGH ADOPTIVE TRANSFER OF T-CELLS
To further elucidate the mechanisms by which GSPs counteract UVB-induced immunosuppression, it was determined whether immune cells were capable of transferring this protection. In vivo experiments suggested that the GSPs-induced prevention of immunosuppression is transferable to naïve mice [88]. Further, adoptive transfer approaches were used to characterize the cells that mediate the chemopreventive effects of GSPs on immune system in vivo. Studies revealed that dietary GSPs prevent UVB-induced immunosuppression through stimulation and/or enhanced development of CD8+ effector T cells and that the dietary GSPs enhance the ability of the CD8+ T cells to secrete Th1 cytokines. Thus, these results suggest that GSPs stimulate the development and the activity of CD8+ effector T cells. The studies conducted by these authors also suggested that inhibition of the development of regulatory T-cells and/or inactivation of CD4+ suppressor T cells also plays a role in the prevention of UVB-induced suppression of the CHS response by GSPs and this was borne out by the significant inhibitory effects of dietary GSPs on the ability of the CD4+ T cells to produce Th2 cytokines (IL-4 and IL-10). Thus, CD8+ T cells are the critical effector cells, a finding that is in accordance with the findings of other investigators [89, 90]. Xu et al. [89] reported that CHS is mediated through CD8+ T cells, whereas CD4+ Th2 cells exhibit an inhibitory effect on CHS, and this observation was supported by the findings of Gocinski and Tigelaar [90] and Anderson et al. [91] who reported that depletion of CD4+ T cells before sensitization results in an enhanced ear swelling response. Moreover, the transfer of CD4+ regulatory T cells from those donor mice that were treated with IL-12 into naïve mice resulted in an enhanced CHS response further suggests that IL-12 plays a role in inhibiting CD4+ T cells with this concept being supported by the inhibitory effects of IL-12 on the secretion of Th2 type cytokines, and this effect was found to be similar in magnitude to the effects of treatment of donor mice with GSPs+ UVB treatment.
As cytokines play a critical role in immune system, the cytokine profiles of CD8+ and CD4+ T cells obtained from mice that were exposed to UVB but not treated with GSPs and those that were GSPs treated and UVB exposed were determined, and to delineate the relationship of these profiles with the inhibitory effect of dietary GSPs on the UVB-induced immunosuppression [88]. It was observed that the levels of Th1 cytokines (IFNγ, IL-2) were much higher in CD8+ T cells from GSPs-treated mice, whereas the Th2 cytokines (IL-4 and IL-10) were hardly detectable. These alterations in cytokine profile of CD8+ T cells under the influence of GSPs may have a role in the enhancement of immune reactions. IFNγ-producing T cells are important effector cells in the CHS response and also are involved in reducing the development of UVB-induced skin tumors [88]. These observations also suggest that the immunopreventive effect of GSPs against UVB-induced immunosuppression are mediated, at least in part, through the inhibition of the development and/or inactivation of CD4+ regulatory T cells, which was evident in the significant reduction of Th2 cytokine (IL-4 and IL-10) levels in the cell supernatants obtained from bone marrow derived dendritic cells-stimulated CD4+ T cells as compared to the CD4+ T cells obtained from UVB irradiated mice that were not treated with GSPs. The Th2 cytokines, IL-4 and IL-10, have been implicated in the immunosuppressive effects and the development of Th2 or CD4+ cells [68]. This property of GSPs can be used as an alternative strategy to augment the induction of CD8+ effector T cells and to diminish the development of CD4+ regulatory T cells and that may lead to the prevention of photocarcinogenesis in humans.
6.6. UV-INDUCED DNA DAMAGE: A MOLECULAR TRIGGER FOR IMMUNOSUPPRESSION AND INITIATION OF PHOTOCARCINOGENESIS
UV irradiation results in DNA damage or DNA photoproducts in the skin which initiates an important cascade of signaling. The DNA photoproducts are altered DNA structures that activate a cascade of responses, beginning with the initiation of cell cycle arrest and activation of DNA repair mechanisms. The biologically harmful effects associated with UV irradiation are largely the result of errors in DNA repair, which can lead to oncogenic mutations [reviewed in 92]. UV-induced DNA damage in the form of cyclobutane pyrimidine dimers (CPDs) is a molecular trigger for the induction of immunosuppression and initiation of photocarcinogenesis [93, 94]. The CPDs form immediately in the skin after the interaction of photons with the DNA molecule.
Camouse et al. [95] found that topical application of green tea or white tea extracts provided human skin protection from solar-simulated UV light. These tea extracts were shown to provide protection against the detrimental effects of UV light on cutaneous immunity. These investigators also concluded that these protective effects were not due to direct UV absorption or sunscreen effects. Studies on the effects of GTPs and GSPs on the DNA repair kinetics and repair mechanisms of UV-induced CPDs have been carried out using in vitro cell culture and in vivo models [39, 43, 80]. Studies showed that dietary GSPs do not inhibit UVB-induced CPDs formation immediately after UVB irradiation. However; in skin samples obtained at 24 h or 48 h after UVB exposure, the numbers of CPD-positive cells were significantly reduced or repaired in the GSPs-treated mouse skin compared to the control group of mice which were not treated with GSPs [80]. Studies of the DNA repair mechanisms suggested that the rapid repair of UV-induced CPDs by GSPs was mediated through stimulation of IL-12 on treatment of the mice with GSPs [80]. IL-12 has been shown to possess potent antitumor activity in a wide variety of tumor models [96–98], and has the capacity to induce DNA repair [99–101] and this concept was verified by testing the effect of GSPs on UV-induced CPDs formation in IL-12 knockout mice. Dietary GSPs do not remove or repair UV-induced CPDs in the skin of IL-12 KO mice but repaired in the skin of their wild-type counterparts, further confirming the role of IL-12 in rapid repair of DNA damage by GSPs [80]. Studies were also conducted with the oral administration of GTPs in the drinking water of mice on UVB-induced DNA damage, and it was found that UV-induced DNA damage in the form of CPDs was resolved rapidly in the GTPs-treated mice compared to non-GTPs-treated mice [39]. Schwarz et al. [102] observed that treatment of normal human keratinocytes and “human skin equivalent” with GTPs reduced UVB-induced DNA damage in the form of CPDs and that this effect was mediated through the stimulation of IL-12 production. These investigations suggest that the difference in the DNA repair capacity between IL-12 knockout mice and their wild-type counterparts may be due to the absence of IL-12 in the IL-12 KO mice. Zhao et al. [103] demonstrated that application of green tea extract to Epiderm, a reconstituted human skin equivalent, also inhibited psoralen-UVA-induced formation of 8-methoxypsoralen-DNA adducts. Morley et al. [104] have shown that EGCG or drinking green tea protects human cellular DNA from UV and visible radiation-induced DNA damage, and also protect DNA damage in human peripheral blood cells after tea ingestion. These observations demonstrate the potential chemopreventive effects of plant polyphenols against UVB-induced DNA damage.
UV-induced DNA damage is also an important molecular trigger for the migration of antigen presenting cells from the skin to the draining lymph nodes. DNA damage in antigen presenting cells impairs their capacity to present Ag, which in turn results in a lack of sensitization [97]. CPD-containing epidermal antigen presenting cells have been found in the draining lymph nodes of UV-exposed mice [98], and exhibited an impaired Ag presentation capacity. As the treatment of EGCG induces IL-12 in mice [85], and IL-12 has the capacity to induce DNA repair [100, 101, 105], the effect of EGCG on the migration of CPD-positive cells from the UV-exposed skin to the draining lymph nodes was studied [106]. Immunohistochemical analysis of CPD-positive cells in lymph nodes after UV irradiation revealed that the numbers of CPD-positive cells in the lymph nodes of the UV-exposed IL-12 KO mice were higher than in the lymph nodes of their wild-type counterparts. The lower numbers of CPD-positive cells in the lymph nodes of UV-exposed wild-type mice compared to IL-12 KO mice may be attributable to the presence of endogenous IL-12 in the wild-type mice at levels that are capable of partial repair of the damaged DNA in the migrating cells. Treatment of mice with EGCG resulted in a significant reduction in the numbers of CPD-positive cells in the lymph nodes of UV-exposed wild-type mice compared to UV-exposed wild-type mice that did not receive EGCG. In contrast, there was no significant difference in the number of CPD-positive cells in the lymph nodes between EGCG-treated and non-EGCG-treated IL-12 KO mice which were exposed to UV radiation. This observation supports the evidence that the reduction in the numbers of CPD-positive cells in the lymph nodes of wild-type mice after EGCG treatment may be due to stimulation of IL-12 and IL-12-mediated DNA repair.
6.7. POLYPHENOLS REPAIR UV-INDUCED DNA DAMAGE THROUGH ENHANCEMENT OF NUCLEOTIDE EXCISION REPAIR (NER) GENES
Studies revealed that application of DNA repair enzymes that reduce the numbers of CPD-positive cells prevents UV-induced immunosuppression and risk of skin cancer [107, 108]. It was found that administration of GTPs in drinking water of mice has the ability to prevent UVB-induced immunosuppression in xeroderma pigmentosum complementation group A-positive (XPA+/+) mice but not in XPA-deficient mice, which are devoid of NER genes and that is necessary for the repair of UV-induced DNA damage in mammalian cells. Further studies were conducted to examine whether the GTPs-mediated repair of UV-induced DNA damage requires NER gene. For this purpose, XPA-positive and XPA-deficient mice were exposed to UVB with and without the treatment of mice with GTPs in drinking water, and mice were sacrificed 72 h later. In skin samples obtained from XPA-deficient mice, no significant difference in the staining pattern of CPDs was observed whether or not they were treated with GTPs. In contrast, in UVB-exposed skin samples obtained from XPA-positive mice, the numbers of CPD+ cells were significantly lower in the GTPs-treated mice than those mice which were not treated with GTPs [109]. To determine whether the NER mechanism is required for the polyphenols-enhanced repair of UVB-induced DNA damage, NER-deficient fibroblasts from a person suffering from xeroderma pigmentosum complementation group A (XPA) and NER-proficient fibroblasts from a healthy person (XPA-proficient) were exposed to UVB with or without prior treatment with EGCG. It was found that the numbers of CPD-positive cells were significantly lower in EGCG-treated group at 24 h after UVB exposure in the XPA-proficient cells compared to non-EGCG-treated cells. However, EGCG did not significantly remove or repair UVB-induced CPDs+ cells in NER-deficient cells [109]. This in vitro observation indicated that EGCG-induced DNA repair requires functional NER mechanism.
6.8. POLYPHENOLS PREVENT UVB-INDUCED IMMUNOSUPPRESSION FOLLOWING NER MECHANISM
To determine whether NER-mediated mechanism is required in the prevention of UVB-induced immunosuppression by GTPs, XPA-deficient and their wild-type XPA-proficient mice were subjected to CHS experiment with and without the treatment of GTPs in drinking water of mice [109]. Following the CHS protocol, it was observed that in the absence of treatment with GTPs, the CHS response was significantly lower in XPA-deficient mice that were UVB-irradiated than those XPA-deficient mice that were not UVB-irradiated, indicating the immunosuppressive effect of UVB radiation in XPA-deficient mice. The group of mice that were treated with GTPs in drinking water also exhibited a significant UVB-induced suppression of CHS response which was similar to non-GTPs-treated UVB-exposed mice. It suggests that administration of GTPs did not prevent UVB-induced suppression of CHS response to a contact sensitizer in XPA-deficient mice. In contrast, the administration of GTPs to the wild-type counterparts significantly induces contact sensitization reaction and ear swelling response to contact sensitizer was significantly higher than those mice which were not given GTPs in drinking water and exposed to UVB radiation [109]. The change in ear skin thickness in XPA-deficient mice in response to contact sensitizer in GTPs +UVB was also compared to the change in ear skin thickness in XPA-proficient mice in response to GTPs +UVB. The increase in ear skin thickness was greater in the XPA-proficient mice treated with GTPs +UVB as compared to increase in ear skin thickness after sensitization to contact sensitizer in XPA-deficient mice treated with GTPs +UVB. These observations suggest that prevention of UVB-induced immunosuppression by GTPs requires NER genes, which have a role in DNA repair.
NER is the main mechanism of repair in mammalian cells for the removal of UV radiation-induced DNA damage. Since the treatment of GTPs enhances the removal or repair of UVB-induced DNA damage, it was of interest to examine whether the removal or repair of UV-induced CPDs by GTPs is mediated via induction of NER genes. Subsequent analysis of data reveals that treatment of mice with GTPs in drinking water of mice increases the levels of some NER genes (e.g., XPA, XPC and RPA1) in UVB-exposed skin sites compared to non-GTPs-fed mice and that may have contributed to the rapid repair of damaged DNA in mouse skin [109]. The role of NER was further confirmed by assessing the effect of GTPs on UVB-induced immunosuppression in XPA-deficient mice and data were compared with the XPA-proficient mice. Administration of GTPs prevents UVB-induced suppression of CHS response in XPA-proficient mice but do not prevent in XPA-deficient mice further support the observations that inhibition of UVB-induced immunosuppression by GTPs requires functional NER genes. This observation was important as the treatment of GTPs do not remove or repair UVB-induced DNA damage in XPA-deficient or NER-deficient mice but repair in XPA-proficient (NER-proficient or wild-type) mice which were exposed to UVB. These findings have important implications for the chemopreventive mechanism of photocarcinogenesis by GTPs, and identify a novel mechanism by which GTPs prevent UV-induced immunosuppression.
Together, the studies conducted with green tea polyphenols indicate that the prevention of UV radiation-induced immunosuppression and subsequently the prevention of photocarcinogenesis by GTPs either through topical application or in drinking water of mice are mediated through rapid repair of UVB-induced DNA damage, as summarized in Figure 1. As UV-induced DNA damage and immunosuppression play an important role in photocarcinogenesis, it is tempting to suggest that the role of polyphenols discussed in this communication should be further investigated for the photoprotection of the skin in humans, and their possible use in future practice of complementary and alternative medicine.
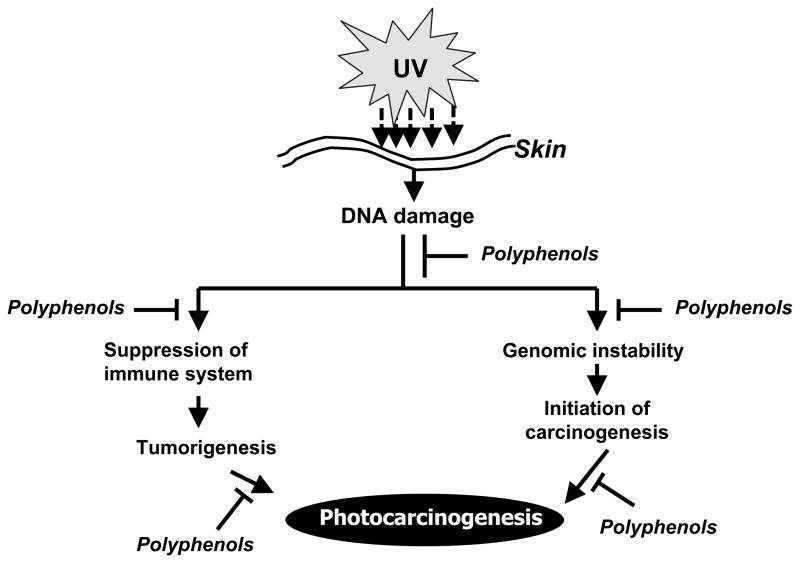
Figure 1.
Schematic diagram depicting the chemopreventive mechanism of plant polyphenols on photocarcinogenesis. Exposure of the skin to solar UV radiation results in suppression of immune system and DNA damage of the skin cells, which may result in the development of UV radiation-induced skin cancer. Experimental evidences suggest that regular dietary intake or topical application of polyphenols may inhibit UVB-induced immunosuppression and initiation of skin cancer through rapid repair of UVB-induced DNA damage in the skin.
Employing human reconstituted skin, Afaq et al. [58] determined the effect of pomegranate derived polyphenolic products against UVB-induced DNA damage using reconstituted human skin. It was found that pomegranate derived products were effective in reducing UVB-induced CPDs and 8-oxodG formation. Oral feeding of PFE to SKH-1 hairless mice reduced the number of CPDs-positive and 8-oxodG-positive cells in the mouse skin and that may be due to enhanced DNA repair. PFE was also found to enhance UVB-mediated increase in p53 and p21 proteins in the mouse skin, therefore shutting off cell replication and DNA synthesis and allowing extended time for DNA repair [44]. Similar effects of delphinidin were also observed when HaCaT cells were treated with delphinidin and exposed to UV radiation [110]. Treatment of human reconstituted skin with isoflavone genistein prior to UVB irradiation reduced UVB-mediated formation of CPDs [111].
6.9. POLYPHENOLS: FUTURE PROSPECTS IN SKIN PHOTOPROTECTION
The mechanistic studies which have been described briefly in this mini review article indicate the potential beneficial effects of polyphenols in the skin photoprotection including photocarcinogenesis in laboratory animals. Depending on the nature of the compounds, polyphenols showed beneficial effects in the animal models whether they were administered in the drinking water, as dietary supplements, or applied topically. Based on extensive in vitro and in vivo data, it is suggested that the supplementation of sunscreens with these polyphenols may provide an effective strategy for skin photoprotection in humans. The dietary polyphenols which have been discussed are considered to be non-toxic and pharmacologically safe for human consumption. Clinical trials are needed to validate the photoprotective and anti-photocarcinogenic potential of these plant polyphenols, either alone or in combination with existing therapies for skin cancers, in high-risk human populations.
Acknowledgments
This work was supported by funds from the National Institutes of Health [(R21 AT002429-02, F.A.) (CA104428, CA140832, AT002536, S.K.K.)] and Veterans Administration Merit Review Award (S.K.K.). The content of this article does not necessarily reflect the views or policies of the funding agencies.
References
- 1.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc. 1996;96:1027–39. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 2.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130S:2073S–85S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 3.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat Rev Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 5.Afaq F, Mukhtar H. Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp Dermatol. 2006;15:678–84. doi: 10.1111/j.1600-0625.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 7.Adhami VM, Syed DN, Khan N, Afaq F. Phytochemicals for prevention of solar ultraviolet radiation-induced damages. Photochem Photobiol. 2008;84:489–500. doi: 10.1111/j.1751-1097.2007.00293.x. [DOI] [PubMed] [Google Scholar]
- 8.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 9.Liao S, Kao YH, Hiipakka RA. Green tea: biochemical and biological basis for health benefits. Vitam Horm. 2001;62:1–94. doi: 10.1016/s0083-6729(01)62001-6. [DOI] [PubMed] [Google Scholar]
- 10.Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53:75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- 11.Yokozawa T, Dong E, Liu ZW, Shibata T, Hasegawa M, Watanabe H, Oura H. Magnesium lithospermate B ameliorates cephaloridine-induced renal injury. Exp Toxicol Pathol. 1997;49:337–41. doi: 10.1016/S0940-2993(97)80100-5. [DOI] [PubMed] [Google Scholar]
- 12.Bors W, Michel C. Antioxidant capacity of flavanols and gallate esters: pulse radiolysis studies. Free Radic Biol Med. 1999;27:1413–26. doi: 10.1016/s0891-5849(99)00187-2. [DOI] [PubMed] [Google Scholar]
- 13.Bors W, Michel C, Stettmaier K. Electron paramagnetic resonance studies of radical species of proanthocyanidins and gallate esters. Arch Biochem Biophys. 2000;374:347–55. doi: 10.1006/abbi.1999.1606. [DOI] [PubMed] [Google Scholar]
- 14.Sergediene E, Jonsson K, Szymusiak H, Tyrakowska B, Rietjens IM, Cenas N. Prooxidant toxicity of polyphenolic antioxidants to HL-60 cells: description of quantitative structure-activity relationships. FEBS Lett. 1999;462:392–6. doi: 10.1016/s0014-5793(99)01561-6. [DOI] [PubMed] [Google Scholar]
- 15.Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000;141:980–7. doi: 10.1210/endo.141.3.7368. [DOI] [PubMed] [Google Scholar]
- 16.Cao P, Cai J, Gupta RC. Effect of green tea catechins and hydrolyzable tannins on benzo[a]pyrene-induced DNA adducts and structure-activity relationship. Chem Res Toxicol. 2010;23:771–7. doi: 10.1021/tx900412a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee MJ, Wang ZY, Li H, Chen L, Sun Y, Gobbo S, Balentine DA, Yang CS. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol Biomarkers Prev. 1995;4:393–9. [PubMed] [Google Scholar]
- 18.Yang CS, Chen L, Lee MJ, Balentine D, Kuo MC, Schantz SP. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev. 1998;7:351–4. [PubMed] [Google Scholar]
- 19.Suganuma M, Okabe S, Oniyama M, Tada Y, Ito H, Fujiki H. Wide distribution of [3H](−)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998;19:1771–6. doi: 10.1093/carcin/19.10.1771. [DOI] [PubMed] [Google Scholar]
- 20.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, Dorr RT, Hara Y, Alberts DS. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–9. [PubMed] [Google Scholar]
- 21.Jeon HY, Kim JK, Seo DB, Cho SY, Lee SJ. Beneficial effect of dietary epigallocatechin-3-gallate on skin via enhancement of antioxidant capacity in both blood and skin. Skin Pharmacol, Physiol. 2010;23:283–9. doi: 10.1159/000313542. [DOI] [PubMed] [Google Scholar]
- 22.Cerda B, Llorach R, Ceron JJ, Espin JC, Tomas-Barberan FA. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur J Nutr. 2003;42:18–28. doi: 10.1007/s00394-003-0396-4. [DOI] [PubMed] [Google Scholar]
- 23.Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr. 2006;136:2481–5. doi: 10.1093/jn/136.10.2481. [DOI] [PubMed] [Google Scholar]
- 24.Katiyar SK. UV-induced immune suppression and photocarcinogenesis: chemoprevention by dietary botanical agents. Cancer Lett. 2007;255:1–11. doi: 10.1016/j.canlet.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachelor MA, Bowden GT. UVA-mediated activation of signaling pathways involved in skin tumor promotion and progression. Semin Cancer Biol. 2004;14:131–8. doi: 10.1016/j.semcancer.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Afaq F. Natural agents: Cellular and molecular mechanisms of photoprotection. Arch Biochem Biophys. 2010 Dec 11; doi: 10.1016/j.abb.2010.12.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afaq F, Adhami VM, Mukhtar H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat Res. 2005;571:153–73. doi: 10.1016/j.mrfmmm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000;71S:1698S–1702S. doi: 10.1093/ajcn/71.6.1698S. [DOI] [PubMed] [Google Scholar]
- 29.Adhami VM, Afaq F, Ahmad N. Suppression of ultraviolet B exposure-mediated activation of NF-kappaB in normal human keratinocytes by resveratrol. Neoplasia. 2003;5:74–82. doi: 10.1016/s1476-5586(03)80019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantena SK, Katiyar SK. Grape seed proanthocyanidins inhibit UV-radiation-induced oxidative stress and activation of MAPK and NF-kappaB signaling in human epidermal keratinocytes. Free Radic Biol Med. 2006;40:1603–14. doi: 10.1016/j.freeradbiomed.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 31.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774–8. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 32.Urbach F. Incidences of nonmelanoma skin cancer. Dermatol Clin. 1991;9:751–5. [PubMed] [Google Scholar]
- 33.Baliga MS, Katiyar SK. Chemoprevention of photocarcinogenesis by selected dietary botanicals. Photochem Photobiol Sci. 2006;5:243–53. doi: 10.1039/b505311k. [DOI] [PubMed] [Google Scholar]
- 34.Surh Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res. 1999;428:305–27. doi: 10.1016/s1383-5742(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 35.Pinnell SR. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J Am Acad Dermatol. 2003;48:1–19. doi: 10.1067/mjd.2003.16. [DOI] [PubMed] [Google Scholar]
- 36.Wang ZY, Agarwal R, Bickers DR, Mukhtar H. Protection against ultraviolet B radiation-induced photocarcinogenesis in hairless mice by green tea polyphenols. Carcinogenesis. 1991;12:1527–30. doi: 10.1093/carcin/12.8.1527. [DOI] [PubMed] [Google Scholar]
- 37.Katiyar SK, Mohan RR, Agarwal R, Mukhtar H. Protection against induction of mouse skin papillomas with low and high risk of conversion to malignancy by green tea polyphenols. Carcinogenesis. 1997;18:497–502. doi: 10.1093/carcin/18.3.497. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal R, Katiyar SK, Khan SG, Mukhtar H. Protection against ultraviolet B radiation-induced effects in the skin of SKH-1 hairless mice by a polyphenolic fraction isolated from green tea. Photochem Photobiol. 1993;58:695–700. doi: 10.1111/j.1751-1097.1993.tb04954.x. [DOI] [PubMed] [Google Scholar]
- 39.Meeran SM, Akhtar S, Katiyar SK. Inhibition of UVB-induced skin tumor development by drinking green tea polyphenols is mediated through DNA repair and subsequent inhibition of inflammation. J Invest Dermatol. 2009;129:1258–70. doi: 10.1038/jid.2008.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantena SK, Meeran SM, Elmets CA, Katiyar SK. Orally administered green tea polyphenols prevent ultraviolet radiation-induced skin cancer in mice through activation of cytotoxic T cells and inhibition of angiogenesis in tumors. J Nutr. 2005;135:2871–7. doi: 10.1093/jn/135.12.2871. [DOI] [PubMed] [Google Scholar]
- 41.Wang ZY, Huang MT, Ho CT, Chang R, Ma W, Ferraro T, Reuhl KR, Yang CS, Conney AH. Inhibitory effect of green tea on the growth of established skin papillomas in mice. Cancer Res. 1992;52:6657–65. [PubMed] [Google Scholar]
- 42.Wang ZY, Huang MT, Lou YR, Xie JG, Reuhl K, Newmark HL, Ho CT, Yang CS, Conney AH. Inhibitory effects of black tea, green tea, decaffeinated black tea, and decaffeinated green tea on ultraviolet B light-induced skin carcinogenesis in 7, 12-dimethylbenz[a]anthracene-initiated SKH-1 mice. Cancer Res. 1994;54:3428–35. [PubMed] [Google Scholar]
- 43.Meeran SM, Mantena SK, Elmets CA, Katiyar SK. (−)-Epigallocatechin-3-gallate prevents photocarcinogenesis in mice through interleukin-12-dependent DNA repair. Cancer Res. 2006;66:5512–20. doi: 10.1158/0008-5472.CAN-06-0218. [DOI] [PubMed] [Google Scholar]
- 44.Afaq F, Khan N, Syed DN, Mukhtar H. Oral feeding of pomegranate fruit extract inhibits early biomarkers of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse epidermis. Photochem Photobiol. 2010;86:1318–26. doi: 10.1111/j.1751-1097.2010.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Afaq F, Zaid MA, Khan N, Syed DN, Yun JM, Sarfaraz S, Suh Y, Mukhtar H. Inhibitory effect of oral feeding of pomegranate fruit extract on UVB-induced skin carcinogenesis in SKH-1 hairless mice. Proc Amer Assoc Cancer Res; The Annual Meeting of the American Association for Cancer Research; San Diego, CA, USA. 2008. p. 1246. [Google Scholar]
- 46.Mittal A, Elmets CA, Katiyar SK. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: relationship to decreased fat and lipid peroxidation. Carcinogenesis. 2003;24:1379–88. doi: 10.1093/carcin/bgg095. [DOI] [PubMed] [Google Scholar]
- 47.Aziz MH, Reagan-Shaw S, Wu J, Longley BJ, Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J. 2005;19:1193–5. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- 48.Kim KH, Back JH, Zhu Y, Arbesman J, Athar M, Kopelovich L, Kim AL, Bickers DR. Resveratrol targets transforming growth factor-β2 signaling to block UV-induced tumor progression. J Invest Dermatol. 2011;131:195–202. doi: 10.1038/jid.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katiyar SK, Korman NJ, Mukhtar H, Agarwal R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J Natl Cancer Inst. 1997;89:556–66. doi: 10.1093/jnci/89.8.556. [DOI] [PubMed] [Google Scholar]
- 50.Gu M, Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin inhibits inflammatory and angiogenic attributes in photocarcinogenesis in SKH-1 hairless mice. Cancer Res. 2007;67:3483–91. doi: 10.1158/0008-5472.CAN-06-3955. [DOI] [PubMed] [Google Scholar]
- 51.Mallikarjuna G, Dhanalakshmi S, Singh RP, Agarwal C, Agarwal R. Silibinin protects against photocarcinogenesis via modulation of cell cycle regulators, mitogen-activated protein kinases, and Akt signaling. Cancer Res. 2004;64:6349–56. doi: 10.1158/0008-5472.CAN-04-1632. [DOI] [PubMed] [Google Scholar]
- 52.Katiyar SK, Afaq F, Azizuddin K, Mukhtar H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (−)-epigallocatechin-3-gallate. Toxicol Appl Pharmacol. 2001;176:110–7. doi: 10.1006/taap.2001.9276. [DOI] [PubMed] [Google Scholar]
- 53.Afaq F, Adhami VM, Ahmad N, Mukhtar H. Inhibition of ultraviolet B-mediated activation of nuclear factor kappaB in normal human epidermal keratinocytes by green tea Constituent (−)-epigallocatechin-3-gallate. Oncogene. 2003;22:1035–44. doi: 10.1038/sj.onc.1206206. [DOI] [PubMed] [Google Scholar]
- 54.Afaq F, Ahmad N, Mukhtar H. Suppression of UVB-induced phosphorylation of mitogen-activated protein kinases and nuclear factor kappa B by green tea polyphenol in SKH-1 hairless mice. Oncogene. 2003;22:9254–64. doi: 10.1038/sj.onc.1207035. [DOI] [PubMed] [Google Scholar]
- 55.Vayalil PK, Elmets CA, Katiyar SK. Treatment of green tea polyphenols in hydrophilic cream prevents UVB-induced oxidation of lipids and proteins, depletion of antioxidant enzymes and phosphorylation of MAPK proteins in SKH-1 hairless mouse skin. Carcinogenesis. 2003;24:927–36. doi: 10.1093/carcin/bgg025. [DOI] [PubMed] [Google Scholar]
- 56.Barthelman M, Bair WB, 3rd, Stickland KK, Chen W, Timmermann BN, Valcic S, Dong Z, Bowden GT. (−)-Epigallocatechin-3-gallate inhibition of ultraviolet B-induced AP-1 activity. Carcinogenesis. 1998;19:2201–4. doi: 10.1093/carcin/19.12.2201. [DOI] [PubMed] [Google Scholar]
- 57.Afaq F, Malik A, Syed D, Maes D, Matsui MS, Mukhtar H. Pomegranate fruit extract modulates UV-B-mediated phosphorylation of mitogen-activated protein kinases and activation of nuclear factor kappa B in normal human epidermal keratinocytes. Photochem Photobiol. 2005;81:38–45. doi: 10.1562/2004-08-06-RA-264. [DOI] [PubMed] [Google Scholar]
- 58.Afaq F, Zaid MA, Khan N, Dreher M, Mukhtar H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp Dermatol. 2009;18:553–61. doi: 10.1111/j.1600-0625.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pacheco-Palencia LA, Noratto G, Hingorani L, Talcott ST, Mertens-Talcott SU. Protective effects of standardized pomegranate (Punica granatum L.) polyphenolic extract in ultraviolet-irradiated human skin fibroblasts. J Agric Food Chem. 2008;56:8434–41. doi: 10.1021/jf8005307. [DOI] [PubMed] [Google Scholar]
- 60.Sharma SD, Meeran SM, Katiyar SK. Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative stress and activation of mitogen-activated protein kinases and nuclear factor-kappaB signaling in in vivo SKH-1 hairless mice. Mol Cancer Ther. 2007;6:995–1005. doi: 10.1158/1535-7163.MCT-06-0661. [DOI] [PubMed] [Google Scholar]
- 61.Gu M, Dhanalakshmi S, Mohan S, Singh RP, Agarwal R. Silibinin inhibits ultraviolet B radiation-induced mitogenic and survival signaling, and associated biological responses in SKH-1 mouse skin. Carcinogenesis. 2005;26:1404–13. doi: 10.1093/carcin/bgi096. [DOI] [PubMed] [Google Scholar]
- 62.Singh RP, Dhanalakshmi S, Mohan S, Agarwal C, Agarwal R. Silibinin inhibits UVB- and epidermal growth factor-induced mitogenic and cell survival signaling involving activator protein-1 and nuclear factor-kappaB in mouse epidermal JB6 cells. Mol Cancer Ther. 2006;5:1145–53. doi: 10.1158/1535-7163.MCT-05-0478. [DOI] [PubMed] [Google Scholar]
- 63.Gu M, Singh RP, Dhanalakshmi S, Mohan S, Agarwal R. Differential effect of silibinin on E2F transcription factors and associated biological events in chronically UVB-exposed skin versus tumors in SKH-1 hairless mice. Mol Cancer Ther. 2006;5:2121–9. doi: 10.1158/1535-7163.MCT-06-0052. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Zhang X, Lebwohl M, DeLeo V, Wei H. Inhibition of ultraviolet B (UVB)-induced c-fos and c-jun expression in vivo by a tyrosine kinase inhibitor genistein. Carcinogenesis. 1998;19:649–54. doi: 10.1093/carcin/19.4.649. [DOI] [PubMed] [Google Scholar]
- 65.Wang YN, Wu W, Chen HC, Fang H. Genistein protects against UVB-induced senescence-like characteristics in human dermal fibroblast by p66Shc down-regulation. J Dermatol Sci. 2010;58:19–27. doi: 10.1016/j.jdermsci.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Kwon JY, Lee KW, Kim JE, Jung SK, Kang NJ, Hwang MK, Heo YS, Bode AM, Dong Z, Lee HJ. Delphinidin suppresses ultraviolet B-induced cyclooxygenases-2 expression through inhibition of MAPKK4 and PI-3 kinase. Carcinogenesis. 2009;30:1932–40. doi: 10.1093/carcin/bgp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yano K, Kajiya K, Ishiwata M, Hong YK, Miyakawa T, Detmar M. Ultraviolet B-induced skin angiogenesis is associated with a switch in the balance of vascular endothelial growth factor and thrombospondin-1 expression. J Invest Dermatol. 2004;122:201–8. doi: 10.1046/j.0022-202X.2003.22101.x. [DOI] [PubMed] [Google Scholar]
- 68.Mukhtar H, Elmets CA. Photocarcinogenesis: Mechanisms, models and human health implications. Photochem Photobiol. 1996;63:355–447. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 69.Gupta N, Levy L. Delayed manifestation of ultraviolet reaction in the guinea-pig caused by anti-inflammatory drugs. Br J Pharmacol. 1973;47:240–8. doi: 10.1111/j.1476-5381.1973.tb08321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buckman SY, Gresham A, Hale P, Hruza G, Anast J, Masferrer J, Pentland AP. COX-2 expression is induced by UVB exposure in human skin: Implications for the development of skin cancer. Carcinogenesis. 1998;19:723–9. doi: 10.1093/carcin/19.5.723. [DOI] [PubMed] [Google Scholar]
- 71.An KP, Athar M, Tang X, Katiyar SK, Russo J, Beech J, Aszterbaum M, Kopelovich L, Epstein EH, Jr, Mukhtar H, Bickers DR. Cyclooxygenase-2 expression in murine and human nonmelanoma skin cancers: Implications for therapeutic approaches. Photochem Photobiol. 2002;76:73–80. doi: 10.1562/0031-8655(2002)076<0073:ceimah>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 72.Katiyar SK, Mukhtar H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen-presenting cells, and oxidative stress. J Leukoc Biol. 2001;69:719–26. [PubMed] [Google Scholar]
- 73.Katiyar SK, Afaq F, Perez A, Mukhtar H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis. 2001;22:287–94. doi: 10.1093/carcin/22.2.287. [DOI] [PubMed] [Google Scholar]
- 74.Katiyar SK, Matsui MS, Elmets CA, Mukhtar H. Polyphenolic antioxidant (−)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem Photobiol. 1999;69:148–53. [PubMed] [Google Scholar]
- 75.Sharma SD, Katiyar SK. Dietary grape seed proanthocyanidins inhibit UVB-induced cyclooxygenase-2 expression and other inflammatory mediators in UVB-exposed skin and skin tumors of SKH-1 hairless mice. Pharm Res. 2010;27:1092–102. doi: 10.1007/s11095-010-0050-9. [DOI] [PubMed] [Google Scholar]
- 76.Afaq F, Adhami VM, Ahmad N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol Appl Pharmacol. 2003;186:28–37. doi: 10.1016/s0041-008x(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 77.Hughes-Formella B, Wunderlich O, Williams R. Anti-inflammatory and skin-hydrating properties of a dietary supplement and topical formulations containing oligomeric proanthocyanidins. Skin Pharmacol Physiol. 2007;20:43–9. doi: 10.1159/000096171. [DOI] [PubMed] [Google Scholar]
- 78.Katiyar SK. Treatment of silymarin, a plant flavonoid, prevents ultraviolet light-induced immune suppression and oxidative stress in mouse skin. Int J Oncol. 2002;21:1213–22. [PubMed] [Google Scholar]
- 79.Katiyar SK, Meleth S, Sharma SD. Silymarin, a flavonoid from milk thistle (Silybum marianum L.), inhibits UV-induced oxidative stress through targeting infiltrating CD11b+ cells in mouse skin. Photochem Photobiol. 2008;84:266–71. doi: 10.1111/j.1751-1097.2007.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vaid M, Sharma SD, Katiyar SK. Proanthocyanidins inhibit photocarcinogenesis through enhancement of DNA repair and xeroderma pigmentosum group A-dependent mechanism. Cancer Prev Res (Phila) 2010;3:1621–9. doi: 10.1158/1940-6207.CAPR-10-0137. [DOI] [PubMed] [Google Scholar]
- 81.Meunier L, Raison-Peyron N, Meynadier J. UV-induced immunosuppression and skin cancers. Rev Med Interne. 1998;19:247–54. doi: 10.1016/S0248-8663(97)89326-5. [DOI] [PubMed] [Google Scholar]
- 82.Yoshikawa T, Rae V, Bruins-Slot W. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–6. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- 83.Kinlen L, Sheil A, Peta J. Collaborative United Kingdom-Australia study of cancer in patients treated with immunosuppressive drugs. Br J Med. 1979;II:1461–6. doi: 10.1136/bmj.2.6203.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharma SD, Katiyar SK. Dietary grape-seed proanthocyanidin inhibition of ultraviolet B-induced immune suppression is associated with induction of IL-12. Carcinogenesis. 2006;27:95–102. doi: 10.1093/carcin/bgi169. [DOI] [PubMed] [Google Scholar]
- 85.Katiyar SK, Challa A, McCormick TS, Cooper KD, Mukhtar H. Prevention of UVB-induced immunosuppression in mice by the green tea polyphenol (−)-epigallocatechin-3-gallate may be associated with alterations in IL-10 and IL-12 production. Carcinogenesis. 1999;20:2117–24. doi: 10.1093/carcin/20.11.2117. [DOI] [PubMed] [Google Scholar]
- 86.Meeran SM, Katiyar S, Elmets CA, Katiyar SK. Silymarin inhibits UV radiation-induced immunosuppression through augmentation of interleukin-12 in mice. Mol Cancer Ther. 2006;5:1660–8. doi: 10.1158/1535-7163.MCT-06-0095. [DOI] [PubMed] [Google Scholar]
- 87.Katiyar SK, Meleth S, Sharma SD. Silymarin, a flavonoid from milk thistle (Silybum marianum L.), inhibits UV-induced oxidative stress through targeting infiltrating CD11b+ cells in mouse skin. Photochem Photobiol. 2008;84:266–71. doi: 10.1111/j.1751-1097.2007.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vaid M, Singh T, Li A, Katiyar N, Sharma S, Elmets CA, Xu H, Katiyar SK. Proanthocyanidins inhibit UV-induced immunosuppression through IL-12-dependent stimulation of CD8+ effector T cells and inactivation of CD4+ T cells. Cancer Prev Res. 2011;4:238–47. doi: 10.1158/1940-6207.CAPR-10-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu H, DiIulio NA, Fairchild RL. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (IL) 4/IL-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996;183:1001–12. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gocinski BL, Tigelaar RE. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J Immunol. 1990;144:4121–8. [PubMed] [Google Scholar]
- 91.Anderson C, Hehr A, Robbins R, Hasan R, Athar M, Mukhtar H, Elmets CA. Metabolic requirements for induction of contact hypersensitivity to immunotoxic polyaromatic hydrocarbons. J Immunol. 1995;155:3530–7. [PubMed] [Google Scholar]
- 92.Timares L, Katiyar SK, Elmets CA. DNA damage, apoptosis and Langerhans cells-activators of UV-induced immune tolerance. Photochem Photobiol. 2008;4:422–36. doi: 10.1111/j.1751-1097.2007.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiated systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci USA. 1992;89:7516–20. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yarosh D, Alas LG, Yee V, Oberyszyn A, Kibitel JT, Mitchell D, Rosenstein R, Spinowitz A, Citron M. Pyrimidine dimer removal enhanced by DNA repair liposomes reduces the incidence of UV skin cancer in mice. Cancer Res. 1992;52:4227–31. [PubMed] [Google Scholar]
- 95.Camouse MM, Domingo DS, Swain FR, Conrad EP, Matsui MS, Maes D, Declercq L, Cooper KD, Stevens SR, Baron ED. Topical application of green and white tea extracts provides protection from solar-simulated ultraviolet light in human skin. Exp Dermatol. 2009;18:522–6. doi: 10.1111/j.1600-0625.2008.00818.x. [DOI] [PubMed] [Google Scholar]
- 96.Colombo MP, Vagliani M, Spreafico F, Parenza M, Chiodoni C, Melani C, Stoppacciaro A. Amount of interleukin 12 available at the tumor site is critical for tumor regression. Cancer Res. 1996;56:2531–4. [PubMed] [Google Scholar]
- 97.Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of interleukin-12 against murine tumors. J Exp Med. 1993;78:1223–30. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Robertson MJ, Ritz J. Interleukin-12: basic biology and potential applications in cancer treatment. Oncologist. 1996;1:88–97. [PubMed] [Google Scholar]
- 99.Meeran SM, Mantena SK, Meleth S, Katiyar SK. Interleukin-12-deficient mice are at greater risk of ultraviolet Radiation-induced skin tumors and malignant transformation of papillomas to carcinomas. Mol Cancer Ther. 2006;5:825–32. doi: 10.1158/1535-7163.MCT-06-0003. [DOI] [PubMed] [Google Scholar]
- 100.Schwarz A, Maeda A, Kernebeck K, van Steeg H, Beissert S, Schwarz T. Prevention of UV radiation-induced immunosuppression by IL-12 is dependent on DNA repair. J Exp Med. 2005;201:173–9. doi: 10.1084/jem.20041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schwarz A, Stander S, Berneburg M, Böhm M, Kulms D, van Steeg H, Grosse-Heitmeyer K, Krutmann J, Schwarz T. Interleukin-12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nat Cell Biol. 2002;4:26–31. doi: 10.1038/ncb717. [DOI] [PubMed] [Google Scholar]
- 102.Schwarz A, Maeda A, Gan D, Mammone T, Matsui MS, Schwarz T. Green tea phenol extracts reduce UVB-induced DNA damage in human cells via interleukin-12. Photochem Photobiol. 2008;84:350–5. doi: 10.1111/j.1751-1097.2007.00265.x. [DOI] [PubMed] [Google Scholar]
- 103.Zhao JF, Zhang YJ, Jin XH, Athar M, Santella RM, Bickers DR, Wang ZY. Green tea protects against psoralen plus ultraviolet A-induced photochemical damage to skin. J Invest Dermatol. 1999;113:1070–5. doi: 10.1046/j.1523-1747.1999.00806.x. [DOI] [PubMed] [Google Scholar]
- 104.Morley N, Clifford T, Salter L, Campbell S, Gould D, Curnow A. The green tea polyphenol (−)-epigallocatechin gallate and green tea can protect human cellular DNA from ultraviolet and visible radiation-induced damage. Photodermatol Photoimmunol Photomed. 2005;21:15–22. doi: 10.1111/j.1600-0781.2005.00119.x. [DOI] [PubMed] [Google Scholar]
- 105.Katiyar SK. Interleukin-12 and photocarcinogenesis. Toxicol Appl Pharmacol. 2007;224:220–7. doi: 10.1016/j.taap.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meeran SM, Mantena SK, Katiyar SK. Prevention of ultraviolet radiation-induced immunosuppression by (−)-epigallocatechin-3-gallate in mice is mediated through interleukin 12-dependent DNA repair. Clin Cancer Res. 2006;12:2272–80. doi: 10.1158/1078-0432.CCR-05-2672. [DOI] [PubMed] [Google Scholar]
- 107.Yarosh D, Alas LG, Yee V, Oberyszyn A, Kibitel JT, Mitchell D, Rosenstein R, Spinowitz A, Citron M. Pyrimidine dimer removal enhanced by DNA repair liposomes reduces the incidence of UV skin cancer in mice. Cancer Res. 1992;52:4227–31. [PubMed] [Google Scholar]
- 108.Yarosh D, Klein J, O’Connor A, Hawk J, Rafal E, Wolf P. Effect of topically applied T4 endonuclease V in liposomes on skin cancer in xeroderma pigmentosum: a randomised study. Xeroderma Pigmentosum Study Group. Lancet. 2001;357:926–9. doi: 10.1016/s0140-6736(00)04214-8. [DOI] [PubMed] [Google Scholar]
- 109.Katiyar SK, Vaid M, van Steeg H, Meeran SM. Green tea polyphenols prevent UV-induced immunosuppression by rapid repair of DNA damage and enhancement of nucleotide excision repair genes. Cancer Prev Res. 2010;3:179–89. doi: 10.1158/1940-6207.CAPR-09-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Afaq F, Syed DN, Malik A, Hadi N, Sarfaraz S, Kweon MH, Khan N, Zaid MA, Mukhtar H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J Invest Dermatol. 2007;127:222–32. doi: 10.1038/sj.jid.5700510. [DOI] [PubMed] [Google Scholar]
- 111.Moore JO, Wang Y, Stebbins WG, Gao D, Zhou X, Phelps R, Lebwohl M, Wei H. Photoprotective effect of isoflavone genistein on ultraviolet B-induced pyrimidine dimer formation and PCNA expression in human reconstituted skin and its implications in dermatology and prevention of cutaneous carcinogenesis. Carcinogenesis. 2006;27:1627–35. doi: 10.1093/carcin/bgi367. [DOI] [PubMed] [Google Scholar]