Abstract
The role of glia maturation factor (GMF) in myelin oligodendrocyte glycoprotein (MOG) 35-55 peptide-induced experimental autoimmune encephalomyelitis (EAE) was investigated using GMF-deficient (GMF-KO) mice. We demonstrate that GMF-KO mice were resistant to the MOG 35-55 peptide-induced EAE as compared to wild type (Wt) mice (two in eight versus ten in ten). Next, we examined the effect of administration of recombinant human GMF (rGMF) on MOG 35-55 peptide-induced EAE in mice. Daily administration of rGMF, staring day 1 to 14, resulted in significant exacerbation of clinical symptoms. Following rGMF injections, both GMF-KO (six in eight) and Wt mice (eight in eight) developed severe EAE (maximal clinical score of 3.5–4.0) with high frequency. The histological examination revealed severe infiltration of inflammatory cells in the spinal cord of MOG-immunized Wt mice while the resistance to EAE in GMF-KO mice was characterized by the absence of inflammatory cells. Administration of rGMF in Wt mice and GMF-KO mice resulted in a significant increase in infiltrating cells in the spinal cord following MOG-immunizations. We also evaluated cytokines and chemokines production as parameters of severity of inflammation in the spinal cord of Wt versus GMF-KO mice with and without GMF-reconstitution following MOG-immunizations. Cytokines (TNF-α, IFN-γ, IL-1β, IL-6) and chemokines (CCL2, CCL3, CXCL10, GM-CSF) production were significantly greater in Wt mice than in GMF-KO mice following MOG-immunization. Furthermore, the reconstitution experiment with rGMF showed that the administration of rGMF in both, Wt mice and GMF-KO mice produced significant increase in the GMF-mediated cytokine/chemokine production.
Keywords: Glia maturation factor (GMF), Experimental autoimmune encephalomyelitis (EAE), Myelin oligodendrocyte glycoprotein 35–55 (MOG 35-55), Cytokine/chemokine, Neuro inflammation
1. INTRODUCTION
Multiple sclerosis (MS) is a disabling, chronic relapsing inflammatory demyelinating disease of the central nervous system (CNS); and according to the National Multiple Sclerosis Society of USA, affecting 400,000 Americans and over two million individuals worldwide. Most people are diagnosed between the ages of 20 and 50, although individuals as young as 2 and as old as 75 have developed it. MS progresses in two phases; the earlier phase starts with an autoimmune inflammatory attack against myelin sheath components followed by a chronic phase of neuro-degeneration in which both the myelin sheath and the underlying axons are damaged (Steinman, 2001). The loss of axons and spinal cord atrophy result in paralysis in MS patients (Trapp et al., 1998). Since the pathogenesis of MS is not clear, no definitive treatment is yet available. Much of our current knowledge about contributing factors of MS is based on experimental autoimmune encephalomyelitis (EAE), an animal model with clinical and pathological similarities to MS (Martin et al., 1992, Steinman, 1996). Several theories for pathogenesis of MS implicate infiltrating T cells, pro-inflammatory cytokines, chemokines, antibody-mediated toxicity, activated macrophages, microglia and astrocytes (Cannella and Raine, 1995, Glabinski et al., 1999, Glabinski and Ransohoff, 1999b, a, Iglesias et al., 2001). Other inflammatory mediators implicated in EAE include highly reactive oxygen, nitrogen species, and nuclear transcription factor NF-kB (Smith et al., 1999, Baldwin, 2001). Despite significant advances, the mechanism by which autoimmune dysfunction results in tissue destruction in EAE remains unresolved. In the present study, we demonstrate for the first time that the administration of exogenous recombinant human GMF resulted in exacerbation of clinical symptoms of MOG-induced EAE in wild type mice. We also show, for the first time, that the delivery of exogenous recombinant human GMF could restored full-blown EAE in EAE-resistant GMF-deficient (GMF-KO) mice. We also show that the administration of exogenous recombinant human GMF enhanced proinflammatory cytokine/chemokine production in the CNS of MOG-immunized mice, suggesting that GMF accelerates progression of EAE by regulating GMF-mediated proinflammatory environment in the CNS of MOG-immunized mice. These observations extend the pathological role of GMF in the progression of EAE.
2. EXPERIMENTAL PROCEDURES
2.1. Reagents
Myelin oligodendrocyte glycoprotein peptide 35-55 (MOG35-55), complete Freund’s adjuvant and pertussis toxin were purchased from Sigma-Aldrich, St. Louis, MO. ELISA kits for mouse TNF-α (Cat # KMC3011), IFN-γ (Cat # KMC4021), IL-1β (Cat # KMC0011), IL-6 (Cat # KMC0061), CCL2 (MCP-1, monocyte chemoattractant protein-1, Cat # KMC1011), CCL3 (MIP-1, macrophage inflammatory protein-1, Cat # KMC 2291), and GM-CSF (granulocyte-macrophage colony-stimulating factor, Cat # KMC2021) were obtained from BioSource International, CA. CXCL10 (IP-10, interferon gamma-induced protein 10, Cat # ab100675) was from Abcam, Cambridge, MA. Recombinant human GMF (rGMF) was prepared essentially as described earlier (Lim et al., 1989, Lim et al., 1990, Kaplan et al., 1991, Lim and Zaheer, 1991).
2.2. GMF-deficient mice
GMF-deficient (GMF knockout) mice were originally produced by homologous recombination with over 80% of the amino acid sequence deleted, as previously described (Lim et al., 2004, Zaheer et al., 2004). GMF- knockout (GMF-KO) mice were maintained by backcross breeding to C57BL/6 for twelve generations at the University of Iowa, Animal Care and Use facility. Control wild type (C57BL/6) mice were purchased from Harlan Sprague Dawley, Inc., Indianapolis, IN. The animals were cared for in accordance with the guidelines approved by the IACUC and National Institutes of Health.
2.3. Induction of EAE
C57BL/6 mice were purchased from Harlan Sprague Dawley, Inc., Indianapolis, IN. Mice were maintained in the animal colony at The University of Iowa and used in accordance with the guidelines approved by the IACUC and National Institutes of Health. For active induction of EAE, C57BL/6, 8–10 week-old, female mice were immunized with subcutaneous injection of 150 μg encephalitogenic myelin oligodendrocyte glycoprotein peptide 35-55 (MOG35-55) in 100 μl PBS and mixed with 100 μl of complete Freund’s adjuvant (CFA). Mice were boosted day 0 and day 2 with i.p. injection of 300 ng pertussis toxin. Control mice received identical injections without MOG35-55. The mice were observed for 36 days following immunization and weighed and scored daily in a double blinded fashion according to the scoring scale of 0 to 5, score 0, no disease; score 1, tail weakness; score 2, weakness in hind limb; score 3, complete hind limb paralysis; score 4, hind limb paralysis with fore limb weakness or paralysis; and score 5, moribund or deceased. For reconstitution experiments, wild type (Wt) and GMF-deficient (GMF-KO) mice were injected i.p. with recombinant human GMF (Lim et al., 1989, Lim et al., 1990, Kaplan et al., 1991, Lim and Zaheer, 1991) (0.5 μg/gram body weight) every day, starting at day 0 until day 14 after immunization with MOG35–55. Mice were compared for the disease with respect to rapidity of onset, severity, and disease duration.
2.4. Histological assessment
At the peak of the disease, three mice from each experimental group were anesthetized by intraperitoneal injection of sodium pentobarbital and transcardially perfused with PBS and by 4% paraformaldehyde in phosphate buffer as described earlier (Zaheer et al., 2007c, Thangavel et al., 2008a, Thangavel et al., 2008b, Thangavel et al., 2009a, Thangavel et al., 2009b). Spinal cords were assessed for inflammation essentially as described earlier (Zaheer et al., 2007c). Five micrometer thick transverse sections (five sections per mouse) were taken from lumbar region of spinal cord. The sections were stained with hematoxylin and eosin to reveal infiltrating inflammatory cells (with morphological characteristics of lymphocytes, granulocytes, macrophages, microglia and astrocytes).
2.5. Enzyme-linked immunosorbent assay (ELISA)
The analysis of TNF-α, IFN-γ, IL-1β, IL-6, CCL2 (MCP-1), CCL3 (MIP-1), CXCL10 (IP-10) and GM-CSF protein concentration was estimated by sandwich immuno-assay procedure as specified in the manufacturer’s protocol. Briefly, to 96-well microtiter ELISA plates pre-coated with anti-cytokine capture antibodies, the cytokine standard and samples were added and incubated overnight at 4°C followed by washing. Corresponding biotinylated antibodies, horseradish peroxidase-conjugated streptovidin and TMB substrate used to develop a yellow color and read by a microplate reader at 450 nm. The concentration of cytokine was estimated from a standard curve generated with each run. The lower detection limits of these ELISA are in the range of 8–12 pg/ml. ELISA data are presented as mean values ± standard deviations and represent more than three independent experiments with similar results.
2.6. Statistical analysis
Statistical significance was assessed with one-way ANOVA followed by Tukey’s procedure using SigmaStat software (SPP, Chicago, IL). A value of p< 0.05 was considered statistically significant.
3. RESULTS
3.1. Myelin oligodendrocyte glycoprotein (MOG) 35–55 peptide induced experimental autoimmune encephalomyelitis (EAE) is aggravated by glia maturation factor (GMF)
We examined the effect of recombinant human GMF (rGMF) on EAE induced by MOG 35-55 peptide in C57BL/6 control (Wt) mice and in GMF-deficient (GMF-KO) mice. We injected rGMF (0.5 μg/g/day) intraperitoneally from days 1 to 14. The clinical course of MOG-induced EAE in mice is summarized in Table 1 and shown in Fig. 1. Wild type mice immunized with MOG 35–55 developed typical disease course in all ten mice used (100% incidence), starting 13.0 ±1.5 days (onset) and reached a maximal clinical score of 3.2 ± 0.5 by day 20. Where as GMF-KO mice developed significantly less severe EAE (clinical score 1.5 ± 0.25 by day 26) at a lower frequency (2 in 8, 25%) with delayed onset (24.0 ± 2.0 days) following MOG 35–55 immunization. Daily administration (days 1 to 14) of rGMF resulted in a significant exacerbation of clinical symptoms. Following rGMF injections, both GMF-KO (six in eight) and Wt mice (eight in eight) developed severe EAE (maximal clinical score of 3.5–4.0) at a high frequency (75–100% respectively). There was no mortality in GMF-KO mice following MOG-immunization where as Wt mice had mortality rate of 30% (three in ten). Both Wt and GMF-KO following rGMF administration and MOG-immunization had slightly higher mortality rate, 37.7% (three in eight) and 33.3% (two in six), respectively. The results show a significant increase in incidence and severity of EAE following rGMF administration in mice. Thus, these results suggest that GMF plays a crucial role in the progression of EAE.
Table 1.
Clinical features of active EAE induced by MOG 35–55 in mice
| Wt | Wt + rGMFa | GMF (−/−) | GMF (−/−) + rGMFa | |
|---|---|---|---|---|
| Incidence (sick/total) | 10/10 (100%) | 8/8 (100%) | 2/8 (25%)b | 6/8 (75%)c |
| Day of onset (mean ± SEM) | 13.0 ± 1.5 | 12.0 ±2.5 | 24.0 ± 2.0 | 16.0 ± 2.0 |
| Maximal clinical score (mean ± SEM) | 3.2 ± 0.5 | 4.0 ± 0.6 | 1.5 ± 0.25 | 3.5 ± 0.5 |
| Mortality (death/sick) | 3/10 (30%) | 3/8 (37.5%) | 0/2 (0%) | 2/6 (33.3%) |
Mice immunized with MOG 35–55 were treated with recombinant GMF from day 1 to day 14;
p=0.01 vs. Wt mice;
p=0.01 vs. GMF (−/−) mice
Fig. 1.
Mean clinical disease score in GMF-KO and Wt mice treated with rGMF (0.5 μg/g/day) was injected i.p. every day, starting at day 0 until day 14 after immunization with MOG35–55 peptide. EAE in mice was scored as follows: score 0, no disease; score 1, tail weakness; score 2, weakness in hind limb; score 3, complete hind limb paralysis; score 4, hind limb paralysis with fore limb weakness or paralysis; and score 5, moribund or deceased. Each graph presents mean clinical severity scores from eight to ten mice per group.
3.2. Histological findings in the spinal cords from mice with MOG-induced EAE
Consistent with the clinical features, histological examination following hematoxylin and eosin (H & E) staining revealed prominent inflammatory cell infiltration throughout the white matter of the lumbar spinal cords of Wt mice (Fig. 2A) at the peak of EAE (clinical core 3.5, day 20). Administration of rGMF in Wt mice significant increased infiltrating cells (Fig. 2B) as seen in the representative sections of the lumbar spinal cord (clinical score 4.0 at day 20 post MOG-immunization). A drastic reduction in infiltrating cells was observed at day 26 (clinical score 1) post MOG-immunized GMF-KO mice (Fig. 2C) as compared to at day 26 (clinical score 3.0) in GMF-KO mice following rGMF administration (Fig. 2D).
Fig. 2.

Histopathology of paraffin sections from the lumbar spinal cords of mice immunized with MOG35–55. H&E staining showing typical inflammatory cellular infiltrates of spinal cord white matter at the peak of EAE in (A) Wt mice (20 days post immunization, clinical score 3), (B) Wt mice following rGMF administration (19 days post immunization, clinical score 4), (C) GMF-KO mice (26 days post immunization, clinical score 1.5), and (D) GMF-KO mice following rGMF administration (22 days post immunization, clinical score 3). Images at 20X magnification
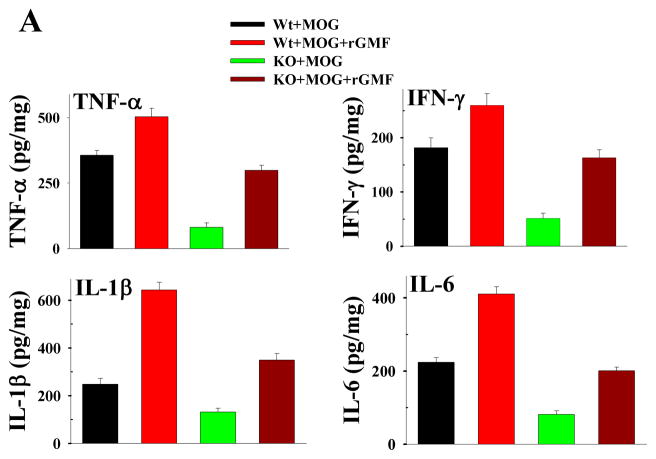
3.3. Quantitative analysis for proinflammatory cytokines and chemokines in the spinal cord
Quantitative estimation of proinflammatory cytokines and chemokines by ELISA was carried out in the spinal cord tissue homogenates from Wt mice at day 20 (clinical score 3) after MOG-immunization, Wt mice after rGMF administration at day 20 (clinical score 4), GMF-KO mice at day 26 (clinical score 1), and GMF-KO mice after rGMF administration at day 26 (clinical score 3). The results of quantitative ELISA for proinflammatory cytokines and chemokines in the spinal cord of MOG-induced EAE mice are shown in Fig. 3. All proinflammatory cytokine and chemokine levels in the spinal cord of mice with EAE were elevated. There was significant up-regulation of several proinflammatory cytokines (TNF-α, IFN-γ, IL-1β, and IL-6) and chemokines (CCL2, CCL3, CXCL10 and GM-CSF) concentrations in the spinal cord of MOG-induced EAE in Wt, and both Wt and GMF-KO mice following rGMF administration, suggesting severe inflammatory responses. The production of proinflammatory cytokines and chemokines was significantly lower in MOG-immunized GMF-KO mice. Thus, rGMF injection enhanced the severity of EAE as well as the production of proinflammatory cytokines and chemokines mice.
Fig. 3.
Production of cytokines (A) and chemokines (B) in the spinal cord from MOG35–55 immunized Wt mice, Wt mice injected with rGMF, GMF-KO mice and GMF-KO mice reconstituted with rGMF. Cytokine/chemokine concentrations were measured by quantitative ELISA in samples obtained from Wt mice (20 days post immunization, clinical score 3), Wt mice following rGMF administration (19 days post immunization, clinical score 4), GMF-KO mice (26 days post immunization, clinical score 1.5), and GMF-KO mice following rGMF administration (22 days post immunization, clinical score 3).
4. DISCUSSION
The glia maturation factor is a highly conserved protein, which was isolated, sequenced and cloned in our laboratory (Lim et al., 1989, Lim et al., 1990, Kaplan et al., 1991, Lim and Zaheer, 1991, Zaheer et al., 1993, Zaheer et al., 1995). We reported earlier a potent immunomodulatory function for GMF in EAE. In our previous reports, we uncovered mechanistic and functional interactions between GMF and multiple proinflammatory pathways in brain cells. We established that GMF over-expressing astrocytes secreted enough granulocyte-macrophage-colony stimulating factor (GM-CSF) in culture medium for the production of pro inflammatory cytokines/chemokines in microglia and resulted in the subsequent destruction of both oligodendroglia, the myelin producing cells, and neurons (Zaheer et al., 2002, Zaheer et al., 2007b). We provided evidence for the GMF-dependent GM-CSF induction via p38 MAPK and NF-kB signaling pathways in astrocytes. We also demonstrated that small interfering RNA-mediated GMF knockdown completely blocked the activation of p38 MAPK, NF-κB, and induced expression of proinflammatory mediators (Zaheer et al., 2007b). Based on GMF’s ability to activate microglia and induce several well-established inflammatory cytokines/chemokines, we hypothesize that intracellular GMF is involved in the pathogenesis of inflammatory diseases of the central nervous system. Our experimental evidence that the absence of endogenous GMF in GMF-deficient (GMF-KO) mice delayed the onset and drastically reduced the severity of EAE induced by MOG 35-55 peptide supports our hypothesis.
Experimental autoimmune encephalomyelitis (EAE) is an animal model of MS, produced in laboratory animals by immunization with myelin-derived antigens; and believed to be mediated by activation of myelin-reactive CD4+ T cells. Expression of high levels of proinflammatory cytokines and chemokines (small chemotactic cytokines) in the brain are thought to contribute to the initiation and maintenance of EAE (Godiska et al., 1995, Ransohoff et al., 1996). The activated T cells, microglia and astrocytes produce a variety of proinflammatory molecules such as tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-1 beta (IL-1β), IL-12, IL-23, and granulocyte macrophage-colony stimulating factor (GM-CSF). GM-CSF produced by activated astrocytes has a specific effect on the proliferation of microglia. Cytokines play a critical role in defining the Th1 or Th2 nature of immune response and in initiating and propagating inflammation in EAE irrespective of the underlying etiology. Therefore, it is important to understand the mechanism of cytokine/chemokine overproduction in EAE. In the present paper we investigated the role of GMF in propagation of inflammation in MOG-induced EAE by administration of exogenous GMF in mice. We demonstrated that GMF accelerates progression of EAE by regulating GMF-mediated production of proinflammatory cytokines/chemokines in the CNS of MOG-immunized mice. Our results show severe infiltration of inflammatory cells in the spinal cord of both Wt and Wt mice injected with exogenous rGMF with acute EAE, while the resistance to EAE in GMF-KO mice was characterized by the absence of inflammatory cells in the spinal cord. The clinical scores of EAE were well correlated with the histological manifestation of inflammation. These findings were consistent with our previous observation that GMF-KO mice display significantly less inflammation in the CNS (Zaheer et al., 2007a). The reconstitution experiment with rGMF showed that the administration of rGMF in mice significantly influence the course of EAE and histopathological findings. The Wt mice developed typical EAE course where as GMF-KO mice developed significantly less severe disease with delayed onset and at a lower frequency following MOG 35–55 immunization. Daily administration, starting day 1 to day 14, with rGMF resulted in a significant exacerbation of clinical symptoms. Following rGMF injections, both GMF-KO and Wt mice not only developed severe EAE but also at a high frequency and with higher mortality rate as compared with non-injected mice. These results suggest that GMF plays a crucial role in the progression of EAE. The overall results demonstrate that GMF plays an important role in the inflammation and progression of EAE and that GMF-mediated increased in Th1 response might explain the exacerbation of EAE in GMF- reconstituted mice.
Further studies, especially preparation of floxed GMF mouse, analysis of types of inflammatory infiltrates and studies to differentiate central and peripheral aspects of GMF in EAE pathology are required to clarify the pathophysiological roles of GMF in the disease course.
In summary, we have shown for the first time that the administration of exogenous recombinant human GMF resulted in exacerbation of clinical symptoms of MOG-induced EAE in wild type mice. We also provided data, for the first time to show that the delivery of exogenous recombinant human GMF restored full-blown EAE in EAE-resistant GMF-deficient (GMF-KO) mice. Additionally, our results demonstrated that the administration of exogenous recombinant human GMF enhanced proinflammatory cytokine/chemokine production in the CNS of MOG-immunized mice. We also provided data suggesting that GMF accelerates progression of EAE by regulating GMF-mediated proinflammatory environment in the CNS of MOG-immunized mice.
HIGHLIGHTS.
GMF plays a crucial role in the progression of EAE pathology in MOG-immunized mice
GMF accelerates progression of EAE by regulating production of proinflammatory cytokines/chemokines in MOG-immunized mice
Administration of exogenous rGMF resulted in a significant exacerbation of clinical symptoms of EAE in MOG-immunized mice
Acknowledgments
We thank Lavanya Ramamoorthy, John Newman, and Krishnakumar Menon for excellent technical help. This work was supported by VA Merit Review award (to A.Z.) and by the National Institute of Neurological Disorders and Stroke grant NS-47145 (to A.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldwin AS., Jr Series introduction: the transcription factor NF-kappaB and human disease. J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella B, Raine CS. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann Neurol. 1995;37:424–435. doi: 10.1002/ana.410370404. [DOI] [PubMed] [Google Scholar]
- Glabinski AR, Krakowski M, Han Y, Owens T, Ransohoff RM. Chemokine expression in GKO mice (lacking interferon-gamma) with experimental autoimmune encephalomyelitis. J Neurovirol. 1999;5:95–101. doi: 10.3109/13550289909029750. [DOI] [PubMed] [Google Scholar]
- Glabinski AR, Ransohoff RM. Chemokines and chemokine receptors in CNS pathology. J Neurovirol. 1999a;5:3–12. doi: 10.3109/13550289909029740. [DOI] [PubMed] [Google Scholar]
- Glabinski AR, Ransohoff RM. Sentries at the gate: chemokines and the blood-brain barrier. J Neurovirol. 1999b;5:623–634. doi: 10.3109/13550289909021291. [DOI] [PubMed] [Google Scholar]
- Godiska R, Chantry D, Dietsch GN, Gray PW. Chemokine expression in murine experimental allergic encephalomyelitis. J Neuroimmunol. 1995;58:167–176. doi: 10.1016/0165-5728(95)00008-p. [DOI] [PubMed] [Google Scholar]
- Iglesias A, Bauer J, Litzenburger T, Schubart A, Linington C. T- and B-cell responses to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis and multiple sclerosis. Glia. 2001;36:220–234. doi: 10.1002/glia.1111. [DOI] [PubMed] [Google Scholar]
- Kaplan R, Zaheer A, Jaye M, Lim R. Molecular cloning and expression of biologically active human glia maturation factor-beta. J Neurochem. 1991;57:483–490. doi: 10.1111/j.1471-4159.1991.tb03777.x. [DOI] [PubMed] [Google Scholar]
- Lim R, Miller JF, Zaheer A. Purification and characterization of glia maturation factor beta: a growth regulator for neurons and glia. Proc Natl Acad Sci U S A. 1989;86:3901–3905. doi: 10.1073/pnas.86.10.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Zaheer A. Structure and function of glia maturation factor beta. Adv Exp Med Biol. 1991;296:161–164. doi: 10.1007/978-1-4684-8047-4_16. [DOI] [PubMed] [Google Scholar]
- Lim R, Zaheer A, Khosravi H, Freeman JH, Jr, Halverson HE, Wemmie JA, Yang B. Impaired motor performance and learning in glia maturation factor-knockout mice. Brain research. 2004;1024:225–232. doi: 10.1016/j.brainres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Lim R, Zaheer A, Lane WS. Complete amino acid sequence of bovine glia maturation factor beta. Proc Natl Acad Sci U S A. 1990;87:5233–5237. doi: 10.1073/pnas.87.14.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Glabinski A, Tani M. Chemokines in immune-mediated inflammation of the central nervous system. Cytokine Growth Factor Rev. 1996;7:35–46. doi: 10.1016/1359-6101(96)00003-2. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- Thangavel R, Sahu SK, Van Hoesen GW, Zaheer A. Modular and laminar pathology of Brodmann’s area 37 in Alzheimer’s disease. Neuroscience. 2008a;152:50–55. doi: 10.1016/j.neuroscience.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangavel R, Sahu SK, Van Hoesen GW, Zaheer A. Loss of nonphosphorylated neurofilament immunoreactivity in temporal cortical areas in Alzheimer’s disease. Neuroscience. 2009a;160:427–433. doi: 10.1016/j.neuroscience.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangavel R, Van Hoesen GW, Zaheer A. Posterior parahippocampal gyrus pathology in Alzheimer’s disease. Neuroscience. 2008b;154:667–676. doi: 10.1016/j.neuroscience.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangavel R, Van Hoesen GW, Zaheer A. The abnormally phosphorylated tau lesion of early Alzheimer’s disease. Neurochemical research. 2009b;34:118–123. doi: 10.1007/s11064-008-9701-1. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Zaheer A, Fink BD, Lim R. Expression of glia maturation factor beta mRNA and protein in rat organs and cells. J Neurochem. 1993;60:914–920. doi: 10.1111/j.1471-4159.1993.tb03237.x. [DOI] [PubMed] [Google Scholar]
- Zaheer A, Mathur SN, Lim R. Overexpression of glia maturation factor in astrocytes leads to immune activation of microglia through secretion of granulocyte-macrophage-colony stimulating factor. Biochem Biophys Res Commun. 2002;294:238–244. doi: 10.1016/S0006-291X(02)00467-9. [DOI] [PubMed] [Google Scholar]
- Zaheer A, Sahu SK, Wu Y, Haas J, Lee K, Yang B. Diminished cytokine and chemokine expression in the central nervous system of GMF-deficient mice with experimental autoimmune encephalomyelitis. Brain Res. 2007a;1144:239–247. doi: 10.1016/j.brainres.2007.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaheer A, Yang B, Cao X, Lim R. Decreased copper-zinc superoxide dismutase activity and increased resistance to oxidative stress in glia maturation factor-null astrocytes. Neurochemical research. 2004;29:1473–1480. doi: 10.1023/b:nere.0000029558.82943.00. [DOI] [PubMed] [Google Scholar]
- Zaheer A, Zaheer S, Sahu SK, Knight S, Khosravi H, Mathur SN, Lim R. A novel role of glia maturation factor: induction of granulocyte-macrophage colony-stimulating factor and pro-inflammatory cytokines. J Neurochem. 2007b;101:364–376. doi: 10.1111/j.1471-4159.2006.04385.x. [DOI] [PubMed] [Google Scholar]
- Zaheer A, Zaheer S, Sahu SK, Yang B, Lim R. Reduced severity of experimental autoimmune encephalomyelitis in GMF-deficient mice. Neurochemical research. 2007c;32:39–47. doi: 10.1007/s11064-006-9220-x. [DOI] [PubMed] [Google Scholar]
- Zaheer A, Zhong W, Uc EY, Moser DR, Lim R. Expression of mRNAs of multiple growth factors and receptors by astrocytes and glioma cells: detection with reverse transcription-polymerase chain reaction. Cell Mol Neurobiol. 1995;15:221–237. doi: 10.1007/BF02073330. [DOI] [PMC free article] [PubMed] [Google Scholar]