Abstract
This study describes the effect of zinc on monocyte adhesion to endothelial cells under different shear stress regimens, which may trigger atherogenesis. Human umbilical vein endothelial cells were exposed to steady shear stress (15 dynes/cm2 or 1 dyne/cm2) or reversing shear stress (time average 1 dyne/cm2) for 24 hours. In all shear stress regimes, zinc deficiency enhanced THP-1 cell adhesion, while heparinase III reduced monocyte adhesion following reversing shear stress exposure. Unlike other shear stress regimes, reversing shear stress alone enhanced monocyte adhesion, which may be associated with increased H2O2 and superoxide together with relatively low levels of nitric oxide (NO) production. L-NG-Nitroarginine methyl ester (L-NAME) treatment increased monocyte adhesion under 15 dynes/cm2 and under reversing shear stress. After reversing shear stress monocyte adhesion dramatically increased with heparinase III treatment followed by a zinc scavenger. Static culture experiments supported the reduction of monocyte adhesion by zinc following endothelial cell cytokine activation. These results suggest that endothelial cell zinc levels are important for the inhibition of monocyte adhesion to endothelial cells, and may be one of the key factors in the early stages of atherogenesis.
Keywords: mechanical forces, vascular endothelial cells, heparan sulfate proteoglycans, and reactive oxygen species
Introduction
The activation or dysfunction of endothelial cells (EC) plays an important role in the development and progression of atherosclerosis. The formation of atherosclerotic plaques starts with the recruitment of leukocytes to the vessel wall by activated EC27. Risk factors for activating EC include disturbed shear stress, reactive oxygen species, and inflammatory mediators. In athero-prone areas of arteries, disturbed or reversing flow activates EC and leads to leukocyte adhesion and eventual atherosclerotic plaque development17. EC activated by reversing shear stress increase the amount of heparan sulfate proteoglycans (HSPG) in the glycocalyx layer8. HSPG with its high sulfate content increases cell surface negativity and is important for EC adhesion and mechanotransduction4, 28. However, this net negative charge would normally resist adhesion of blood cells, which are also negatively charged under physiological conditions. The disruption or degradation of endothelial HSPG impairs L-selectin-mediated functions such as leukocyte trafficking, homing, and adhesion2, 48. Activated EC recruit leukocytes, mainly monocytes8, 13. Monocytes then bind tightly to leukocyte adhesion receptors (e.g. E-selectin, VCAM-1, and ICAM-1) overexpressed by activated EC, and migrate into the vessel wall30, 47. Nitric oxide (NO) is also involved in monocyte adhesion. L-arginine, a key precursor to NO, reduces monocyte adhesion to endothelial cells1. The expression of argininosuccinate synthetase 1 which increases NO production, decreased monocyte adhesion stimulated by tumor necrosis factor-α (TNF-α)29.
Zinc is an essential mineral in cells and is required for the function of more than 300 enzymes and 2000 transcription factors. Zinc plays an important role in the defense against oxidative stress and apoptosis, precursors of atherosclerosis20, 26. Zinc protects a variety of cell types against apoptosis including EC by inhibiting the pathways of signal transduction that lead to upregulation of caspase genes20. Although zinc, a non-redox active ion, cannot scavenge reactive oxygen species directly, it behaves as an antioxidant by attenuating oxidative stress-sensitive transcription factors in activated EC6, 21, 34.
In humans, zinc deficiency results in lowered levels of the antioxidant enzyme superoxide dismutase, which increases oxidative stress, pro-inflammatory compounds, and the risk for atherosclerosis39. Physiological free zinc levels are strongly regulated by zinc-binding proteins. Because excess free zinc is toxic, most plasma zinc is bound to albumin and α2-macroglobulin.
Zinc transporters and zinc-binding metallothionein (MT) proteins function as zinc reservoirs10. NO or reactive oxygen species can induce the cleavage of zinc-sulphur bonds, releasing intracellular zinc from MT-Zn complexes23. Intracellular free zinc activates metal-responsive transcription factor-1, which increases gene transcription including the expression of MT isoforms and zinc transporter-1 (ZnT-1). Our recent microarray and qRT-PCR studies demonstrated that human umbilical vein endothelial cells (HUVEC) increased MTs and ZnT-1 mRNAs when the cells were exposed to reversing shear stress compared to cells exposed to 15 dynes/cm2 7, 8. Intracellular MTs and ZnT-1 protein levels change in response to shear stress7, 8. Despite the evidence of association of zinc with atherosclerosis, the effect of zinc on monocyte adhesion to EC under shear stress is not clear.
Herein we exposed EC to 15 dynes/cm2 steady shear stress, 1 dyne/cm2 steady shear stress, or reversing shear stress for 24 hours. Subsequent to all shear stress regimes, we perfused EC with THP-1 cells, and found increased adhesion of the monocyte cell line only to EC exposed to reversing shear stress. Also, production of reactive oxygen species was increased after reversing shear stress. Post-shear stress removal of zinc by chelation enhanced THP-1 adhesion to HUVEC regardless of shear stress regime. Heparinase III treatment after reversing shear stress exposure reduced THP-1 cell adhesion. When heparinase III was followed by zinc chelation, reversing shear stress increased monocyte adhesion significantly. These results suggest that athero-prone regions of the vasculature incur enhanced monocyte adhesion due in part to decreased free zinc levels.
Materials and Methods
Cell Culture and Shear Stress Conditions
HUVEC (Lonza, Walkersville, MD), passage 4, were grown at 37 °C under a humidified atmosphere of 5 % CO2 in “complete M199” containing M199 (Mediatech, Herndon, VA), 20 % (v/v) FBS (Hyclone, Logan, UT), 50 μg/ml endothelial mitogen (Biomedical Technologies, Stoughton, MA), 50 U/ml penicillin, 50 μg/ml streptomycin, 2mM L-glutamine (Mediatech, Herndon, VA), and 2.5 U/ml heparin sodium (American Pharmaceutical Partners, Schaumberg, IL). Glass slides were pretreated with 1 % (w/v) gelatin (Sigma-Aldrich, St. Louis, MO) for 40 minutes followed by 0.5 % glutaraldehyde (Electron Microscopy Science, Hatfield, PA) for 30 minutes and rinsed 3 times in PBS. Cells were seeded at 20,000 cells/cm2 on pretreated glass slides.
After 48 hours, HUVEC were subjected to one of three shear stress conditions, or maintained in static conditions, for 24 hours. Shear stress conditions included 1) 1 dyne/cm2 steady shear stress, 2) 15 dynes/cm2 steady shear stress, or 3) reversing shear (non-harmonic, +11 dynes/cm2 maximum, −11 dynes/cm2 minimum, 1 dyne/cm2 time average, 1 Hz, a flow pattern characteristic of the human carotid sinus8). The apparatus used to produce reversing shear stress has been described in detail8, and is briefly summarized here. Two 30 ml syringes mounted on a reciprocating syringe pump (Harvard 33 Dual Syringe Pump, Holliston, MA) provided 1 dyne/cm2 to parallel plate chambers, one side of which was the cell cultured slide. The reversing component was superimposed on the steady flow profile using a 1 ml glass syringe driven by a programmed linear motor. Devices supplying 15 dynes/cm2 to parallel plate chambers were driven by hydrostatic pressure14. Shear stress experiments were carried out at 37 °C under 5 % CO2.
Following shear stress, cells were treated with either 5 mU/mL heparinase III (Sigma-Aldrich, St. Louis, MO) at 37°C for 3 hours, or with the cell-permeable zinc specific chelator, 30 μM N,N,N’,N’-tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN) (Calbiochem, La Jolla, CA), at 37°C for 30 minutes, or with heparinase III followed by TPEN, and washed with complete M199 prior to monocyte perfusion.
Monocyte Adhesion Assay
THP-1 cells (human acute monocytic leukemia cell line, ATCC, Manassas, VA) were grown in RPMI-1640 (Mediatech, Herndon, VA) containing 10 % (v/v) FBS, 50 U/ml penicillin, 50 μg/ml streptomycin, and 2 mM L-glutamine. For adhesion assays, THP-1 cells were activated by cytokines (10 ng/mL TNF-α plus 1 ng/mL IL-1β) for 1 hour then stained with CellTracker Green CMFDA (Invitrogen, Carlsbad, CA) in serum free RPMI-1640 media for 30 minutes. THP-1 cells were washed and resuspended in complete M199.
THP-1 cells (6.7 × 105 cells/mL) in complete M199 were perfused across HUVEC at 1 dyne/cm2 for 5 minutes, flow was stopped for 30 seconds, and then cells were perfused with complete M199 media without monocytes for an additional 5 minutes at 1 dyne/cm2. For each condition, the number of adherent THP-1 cells was counted in 7 frames (1 mm2/frame) per slide under a fluorescence microscope (Nikon Eclipse TE2000-U, Tokyo, Japan), and at least 3 slides from each experiment were counted.
In the experiments indicated, HUVEC were activated by the addition of 10 ng/mLTNF-α plus 1 ng/mL IL-1β for 24 hours under static conditions. Zinc sulfate (100 μM) was added to both activated and control cells for 24 hours at the same time as the cytokines. TPEN (30 μM) was added to non-activated control cells for 30 minutes.
To assess the role of NO production under different shear stress regimes, 100 μM L-NG-Nitroarginine methyl ester (L-NAME), an inhibitor of NO synthase, was added to the media at the beginning of application of shear stress. The effect of ROS on HUVEC under shear stress was also investigated by adding exogenous 50 μM H2O2 or catalase (50 U/mL) (which decomposes H2O2 to water and oxygen), plus tempol (100 μM) (a superoxide dismutase mimetic which scavenges superoxide), to the media at the beginning of shear stress. In the indicated experiments, exogenous ROS were induced in statically cultured HUVEC by a solution of 100 μM H2O2 or 0.5 μg/mL KO2 (0.49 nmol superoxide/mL)35 for 24 hours.
Measurement of cell surface negativity
Cell surface charge was determined by photometric measurement of toluidine blue (Sigma-Aldrich, St. Louis, MO)43, 44. After shear stress and/or heparinase III or TPEN, HUVEC were washed in 0.25 M sucrose three times and stained with 0.001 % (w/v) toluidine blue in 0.25 M sucrose solution for 1 hour at 4°C. HUVEC were washed five times and then maintained in 0.1 mg/ml protamine sulfate (type X) (Sigma-Aldrich, St. Louis, MO) for 30 minutes at 4°C. Protamine sulfate extracts the toluidine blue combined with the glycocalyx, due to its higher affinity for carboxyl groups and sulfate groups than toluidine blue43. The absorbance of extracted toluidine blue (λabs = 630 nm) was measured on a Spectra MAX Plus microplate reader (Molecular Devices, Sunnyvale, CA).
Measurement of Hydrogen Peroxide, Superoxide, and Nitric Oxide
Following shear stress, HUVEC were trypsinized, washed with PBS and stained with either 5 μM 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) (Invitrogen, Carlsbad, CA) for H2O2 or 5 μM dihydroethidium (hydroethidine) (DHE) (Invitrogen, Carlsbad, CA) for superoxide. After 20 minutes, cells were washed 3 times with ice cold PBS and filtered into flow cytometry tubes. Fluorescence was measured by BD LSR flow cytometer (BD Bioscience) (San Jose, CA) using a laser for either CM-H2DCFDA (λex/λem = 488/530 nm) or DHE (λex/λem = 518/605 nm). Nitric oxide in circulating media from shear stressed cells was measured with the Nitrite/Nitrate Fluorometric Assay Kit (Cayman Chemical, Ann Arbor, MI) following the manufacturer’s instructions.
Apoptosis and Cell Death Assays
After TPEN treatment, apoptotic and dead HUVEC were identified by Annexin V-Alexa Fluor 488 (Invitrogen, Carlsbad, CA) and propidium iodide (Invitrogen, Carlsbad, CA) respectively, following the manufacturer’s instructions. Briefly, 1.5×105 cells were trypsinized, washed in cold PBS, and resuspended in 100 μl binding buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, pH=7.4). Annexin V-Alexa Fluor 488 (5 μL) and 100 μg/mL propidium iodide (1 μL) were added to the cell suspension and incubated at room temperature for 15 min. Fluorescence of stained cells was then analyzed by BD LSR flow cytometer (BD Bioscience) (San Jose, CA) using a laser for Alexa Fluor 488 (λex/λem = 495/519 nm) and propidium iodide (λex/λem = 535/617 nm).
Although TPEN is a zinc-specific chelator, 10 μM TPEN for 48 h can induce fundamental apoptotic events such as DNA fragmentation, caspase-3 activation26 and the translocation of negative phosphatidyl serines. In order to minimize the apoptotic and toxic effects of TPEN, we determined the optimum TPEN concentration to be 30 μM TPEN for 30 min, which scavenges intracellular zinc with no apoptotic effects or cell death (Supplemental Figure 1 A&B), to scavenge zinc in our shear stress experiments.
Statistics
Each bar graph represents an average ± SEM of at least three independent experiments. Statistical analysis was performed using paired Student’s t-test, comparing each treatment to static conditions unless otherwise mentioned. In all statistical analyses p<0.05 (*) was considered significant.
Results
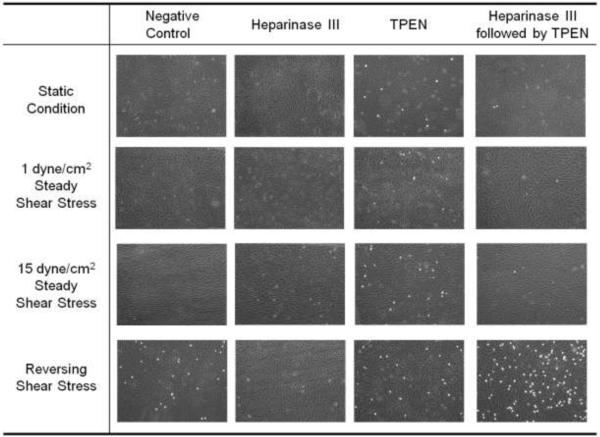
We exposed HUVEC to steady shear stress (either 15 dynes/cm2 or 1 dyne/cm2), reversing shear stress (± 11 dynes/cm2, 1 Hz, time average 1 dyne/cm2) for 24 hours, or static conditions, and subsequently assessed their adhesiveness to THP-1 cells. Figure 1 shows that only reversing shear stress enhanced THP-1 monocyte adhesion to HUVEC in the absence of post-shear stress treatment. TPEN addition, which chelates zinc, increased monocyte adhesion after all shear stress regimes; while heparinase III, which cleaves HSPG from the endothelial cell surface and removes monocyte-binding ligands, reduced monocyte binding after reversing shear stress exposure. Interestingly, after reversing shear stress, monocyte adhesion was dramatically increased when HUVEC were treated with heparinase III followed by TPEN.
Figure 1. THP-1 monocyte adhesion to HUVEC is enhanced by reversing shear stress and zinc removal.
HUVEC were subjected to 24 hours of one of four conditions: static condition, low steady shear stress (1 dyne/cm2), high steady shear stress (15 dynes/cm2), or reversing shear stress (± 11 dynes/cm2, time average 1 dyne/cm2). Following shear stress, cells were treated with complete M199 (negative control), 5 mU/mL heparinase III at 37°C for 3 hours, 30 μM TPEN at 37°C for 30 minutes, or heparinase III followed by TPEN. Adherent THP-1 cells (white cells) and HUVEC (background cells) were imaged using a fluorescence microscope.
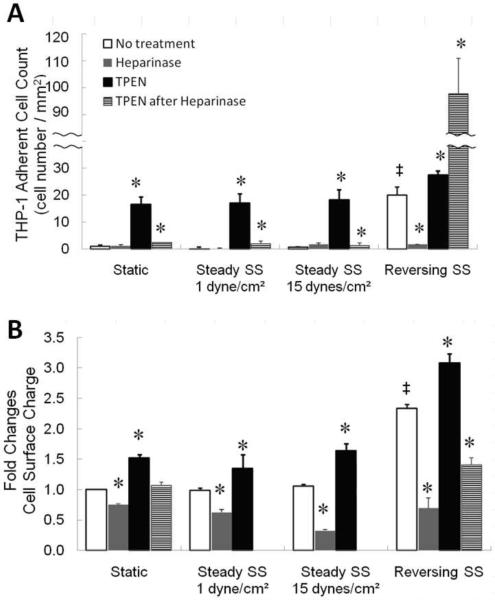
Figure 2A quantitates THP-1 adhesion. Under static and both steady shear stress conditions, control (no treatment) and heparinase III-treated EC had minimal THP-1 adhesion, and TPEN increased THP-1 adhesion 16-18 fold relative to other treatments. Both static and 1 dyne/cm2 elicited small, but significant increases in THP-1 adhesion when treated with heparinase III followed by TPEN. The no-treatment control group under reversing shear stress was 20 fold more adhesive to THP-1 cells. Although heparinase III minimized adherence of THP-1 to EC after reversing shear stress, TPEN increased adherence 27 fold, while heparinase III followed by TPEN (which had only small effects in the other regimens), increased adherence 108 fold.
Figure 2. Quantitation of THP-1 adhesion and surface negativity.
Following shear stress, cells were treated with complete M199 (white bars), 5 mU/mL heparinase III at 37°C for 3 hours (gray bars), 30 μM TPEN (black bars), or heparinase III followed by TPEN (hatched bars). A: THP-1 adherent cell counts per mm2. B: Cell surface negativity determined by toluidine blue. Higher values on the Y-axis indicate more negativity. (n=3, * p<0.05 compared to negative control of each shear stress regime, ‡ p<0.05 compared to static culture, white bars only).
Figure 2B demonstrates that regardless of shear stress or static conditions, heparinase III reduced the cell surface negativity while TPEN treatment enhanced cell surface negativity, (note that heparinase III followed by TPEN condition was not included for 15 dynes/cm2 or 1 dyne/cm2conditions). While Figure 2A indicates that treatment of EC with heparinase III and TPEN following reversing shear stress induces monocyte adhesion via a mechanism not involving HSPG, Figure 2B further demonstrates that this monocyte adhesion cannot be explained by surface charge.
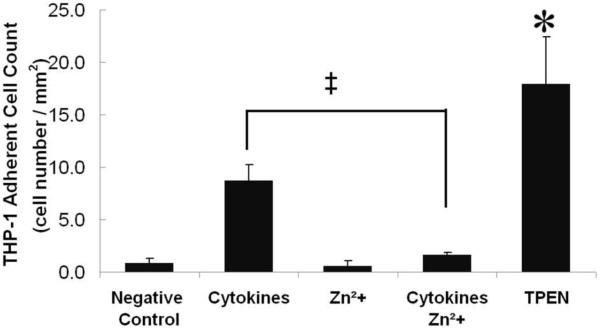
We examined whether zinc plays a role in monocyte adhesion to HUVEC under static conditions. In Figure 3, HUVEC that were activated by cytokine treatment for 24 hours showed enhanced THP-1 adhesion over the negative control. Exogenous zinc alone did not alter THP-1 adhesion, while cytokines plus zinc reduced THP-1 adhesion to negative control. THP-1 adhesion was significantly increased by zinc removal (TPEN treatment), suggesting that zinc inhibited THP-1 adhesion to activated HUVEC under static conditions.
Figure 3. In static culture, exogenous zinc attenuates increase in THP-1 adherence.
HUVEC were pre-treated with complete M199 (negative control), cytokines (10 ng/mL TNF-α plus 1 ng/mL IL-1β) for 24 hours, exogenous zinc sulfate (100 μM) for 24 hours, cytokines plus zinc sulfate (100 μM) for 24 hours, or TPEN (30 μM) for 30 minutes. (n=3, * p<0.05 compared to negative control, ‡ p<0.05 compared to cytokines (positive control)).
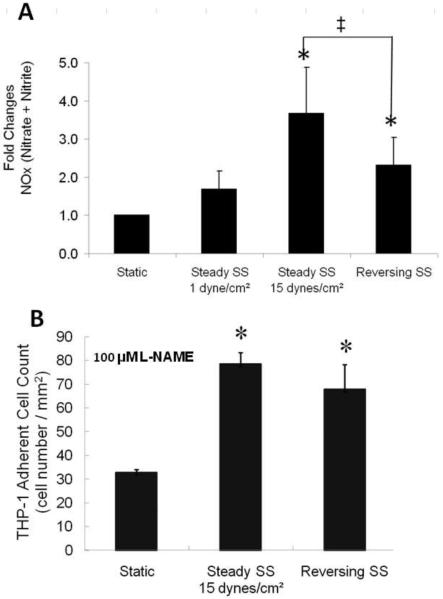
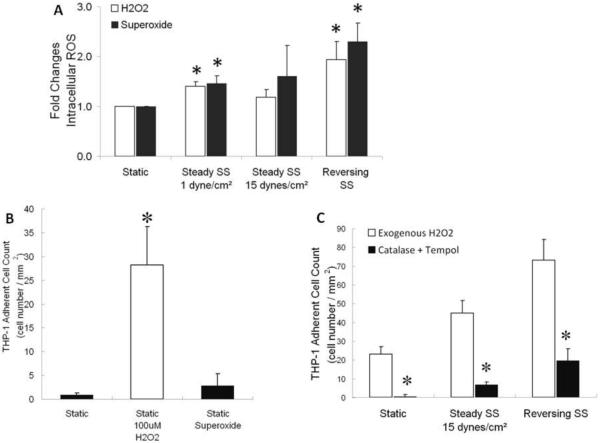
NO production (Figure 4A) was increased in EC subjected to 15 dynes/cm2 (3.7 fold) or to reversing shear stress (2.3 fold) compared to EC subjected to 1 dyne/cm2 or grown under static conditions. Addition of L-NAME during shear stress (Figure 4B) enhanced monocyte adhesion to EC subjected to 15 dynes/cm2 (87 fold) or reversing shear stress (3.4 fold) when compared to ECs under these same conditions in the absence of L-NAME (compare Figure 4B and Figure 2A). Interestingly, L-NAME pretreatment for 24 h also greatly increased monocyte adhesion to static EC. In addition to increasing NO, reversing shear stress also increased the generation of H2O2 and superoxide (Figure 5A). Levels of reactive oxygen species (ROS) between 15 dyne/cm2 steady shear stress and static culture are not statistically different, while increases in ROS under 1 dyne/cm2 steady shear stress were significantly higher. Under reversing shear stress, more H2O2 and superoxide were produced which would require more NO production to counteract. In order to investigate this phenomenon further, we exposed HUVEC to exogenous ROS under static conditions, and found that only H2O2 (100 μM) enhanced monocyte binding (Figure 5B). H2O2 (50 μM) also enhanced monocyte adhesion under shear stress, whereas ROS scavengers catalase (50 U/mL) plus tempol (100 μM) reduced monocyte adhesion (Figure 5C).
Figure 4. HUVEC exposed to 15 dynes/cm2 produces more NO and L-NAME enhances monocyte adhesion to HUVEC.
A: NO level measured by the Nitrate/Nitrite Fluorometric Assay Kit (n=6, * p<0.05 compared to static culture, ‡ p<0.05 comparing 15 dynes/cm2 to reversing shear stress). B: HUVEC were exposed to 100 μM L-NAME, simultaneously with shear stress for 24 h, then perfused with THP-1 cells (n=3, * p<0.05 compared to static culture)
Figure 5. HUVEC exposed to reversing shear stress generate more reactive oxygen species, and H2O2 enhances THP-1 cell adhesion to HUVEC.
A: Intracellular H2O2 level measured by CM-H2DCFDA (white bars) and intracellular superoxide level measured by DHE (black bars) (n=6, * p<0.05 compared to static culture), B: In static conditions, THP-1 cell adhesion to HUVEC was measured after 24 h treatment of HUVEC with exogenous H2O2 (100 μM) or superoxide (0.49 μM) (n=3, * p<0.05 compared to negative control), C: HUVEC were treated with either H2O2 (50 μM) (white bars) or catalase (50 U/mL) plus tempol (100 μM) (black bars) during 24 h shear stress (n=3, * p<0.05 compared to static culture).
Discussion
We examined the possible roles that shear stress, zinc, HSPG, NO production, and ROS production play in the adhesion of THP-1 cells to EC. To understand the mechanism by which reversing shear stress increases monocyte adhesion, and the role played by intracellular zinc in this process, we employed 3 post-shear stress treatments, 1) decreasing HSPG using heparinase III, 2) reducing zinc levels using the zinc-specific cell-permeable chelator TPEN, and 3) heparinase III treatment followed by TPEN, (which depleted zinc in the absence of HSPG). After exposure to reversing shear stress, monocyte adhesion dramatically increased (108 fold) when HUVEC were treated with heparinase III followed by TPEN. Without post-shear stress treatment, EC exposed to reversing shear stress are 20 fold more adhesive to THP-1 cells relative to 1 dyne/cm2 or 15 dynes/cm2 steady shear stress (compare reversing shear stress levels, Figure 2A). Other studies have reported increased monocyte adhesion to cultured EC under oscillatory shear stress produced by a cone-and-plate viscometer, and under reversing shear stress regimes delivered in a parallel plate chamber. Whether or not the wave form of oscillatory shear stress (sinusoidal) as delivered via the cone-and-plate viscometer, and that of the reversing shear stress profile used in the present study(which mimics that in the carotid sinus), is reflected in EC production of NO vs. ROS has not been determined. The advantages and disadvantages of these approaches and devices for delivering shear stress are discussed in a recent review5.
The fact that heparinase III treatment prevents THP-1 adhesion to EC under reversing shear stress demonstrates the significance of HSPG as the main component of the glycocalyx in mediating adhesion between THP-1 and EC under reversing shear conditions. We found through immunostaining that EC exposed to reversing shear stress had more HSPG than EC exposed to steady shear stresses8. If EC are treated with heparinase III prior to exposure to 15 dynes/cm2 shear stress for 24 h, they do not align 3, 51. Tarbell and collaborators present evidence that suggests that HSPG transduce fluid shear stress signals into the cell, which would explain why no alignment of EC occurs when they are treated with heparinase III prior to shear stress12, 40. These findings led Tarbell to label HSPG “mechanotransducers”. Herein we demonstrate the crucial role of HSPG in monocyte adhesion after reversing shear stress exposure.
We found that NO production under reversing shear stress is 2.3 fold greater, and NO production under 15 dynes/cm2 is 3.7 fold greater, than under static conditions (Figure 4A). However, intracellular ROS production under reversing shear stress exceeds that under 15 dynes/cm2 significantly (Figure 5A). Therefore, while more H2O2 and superoxide are produced under reversing shear stress, more NO is produced under 15 dynes/cm2. This may account for the resistance of ECs under 15 dynes/cm2 to monocyte adhesion. Others have shown that the balance between NO and ROS production is key to EC resistance to monocyte adhesion5, 19. Under static conditions, exposure to NO has been shown to increase free zinc in EC 50, and intracellular free zinc release by NO protects EC from H2O2-induced toxicity. 9
Harrison and collaborators have shown that laminar shear stress increases NO production through activation and nuclear translocation of NFĸB, which has a binding site in the endothelial nitric oxide synthase promoter. Oscillatory shear stress increases EC production of superoxide and H2O2, in addition to the increased production of NO by a different mechanism than laminar shear stress which is in part due to H2O219. Also, KLF-2 has been shown to be a mechanically activated transcription factor for eNOS in shear stressed EC11, 33.
As observed by others19, 31, we found that NO production was maximally stimulated by 15 dynes/cm2 (Figure 4A). We also demonstrated that the addition of L-NAME to cells subjected to 15 dynes/cm2 shear stress or reversing shear stress enhanced monocyte adhesion significantly (Figure 4B). The striking increase (87 fold) in monocyte adhesion after L-NAME treatment under 15 dynes/cm2 (compared to 15 dynes/cm2 with no treatment in Figure 2A) emphasizes the importance of the interplay of NO production and shear stress in determining regions of the vasculature prone to monocyte adhesion. Previously we showed that L-NAME inhibits the generation of NO and NO-induced zinc release from MT-zinc complexes under 15 dynes/cm2 shear stress7.
Our motivation for examining the effect of exogenous zinc on the EC response to shear stress is based on microarray studies of EC under different shear stress regimes, in which we found that metallothionein gene expression is extremely sensitive to shear stress 8, 52. These genes produce intracellular proteins that bind principally zinc and are responsible for the tight regulation of free intracellular zinc 22, 24. Our measurements of the intracellular zinc levels in shear stressed EC were based on flow cytometry profiles after FluoZin 3-AM uptake15. Such studies provide a relative measure of intracellular zinc levels. To obtain absolute measures of intracellular zinc requires ratiometric techniques, using FRET (fluorescent resonance energy transfer) measurements45. These probes show ratiometric changes upon zinc binding (e.g. with TPEN), and reveal that the intracellular free zinc levels in most cells is ~0.4 nM. Such studies would not be possible to use in shear stressed cells, due to the rapid equilibration that occurs after shear stress cessation. The extracellular levels of free zinc are higher than intracellular (micromolar). Most labile zinc in plasma is bound to albumin37. Exogenous zinc supplementation in static conditions demonstrated that zinc prevents monocyte adhesion to EC activated by cytokines (Figure 3). Previously we have shown that exogenous zinc supplementation under reversing shear stress increases intracellular free zinc and inhibits THP-1 adhesion to HUVEC7.
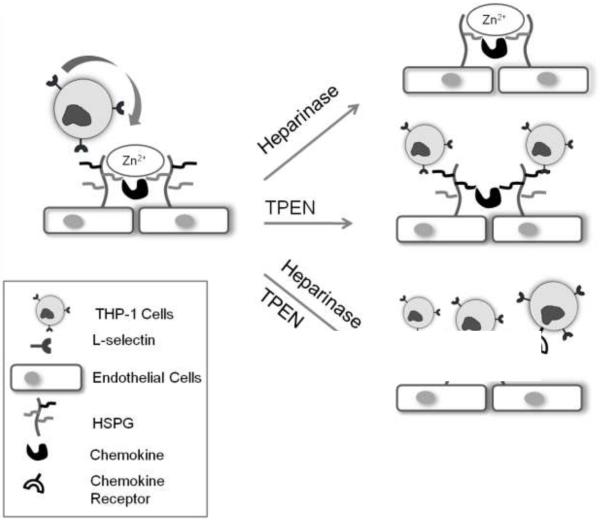
A possible explanation for the dramatic increase in THP-1 adhesion to EC under reversing shear stress after treatment with heparinase III followed by TPEN, is that adhesion increased as a result of stimulation of an inflammatory mediator. There is abundant evidence that activated EC increase the expression of leukocyte adhesion receptors such as VCAM-1 and ICAM-1 in vivo16, 30, 32 and enhance the secretion of cytokines42, 46, which could interact with HSPG and zinc. HSPG binds cytokines and protects them from proteolysis18, 38, 49 and zinc deficiency increases the levels of circulating cytokines25. Thus, the function of an inflammatory mediator may be suppressed by HSPG and zinc, and may be activated when both HSPG and zinc are removed by heparinase III and TPEN (Figure 6). As a result, heparinase III treatment followed by TPEN after reversing shear stress enhanced monocyte adhesion dramatically (Figure 6, bottom picture). Identification of which monocyte binding ligands could be presented by the treatment of post-shear stress heparinase III followed by TPEN is an interesting area for future research.
Figure 6. Proposed mechanism for monocyte adhesion to endothelial cells under reversing shear stress.
Under reversing shear stress, endothelial cells express more HSPG and chemokines that recruit and activate leukocytes including monocytes. HSPG can mediate monocyte adhesion, and the cleavage of HSPG with heparinase III reduces monocyte binding sites (top right picture). TPEN, a cell-permeable zinc chelator, eliminates resistance to monocyte adhesion (middle picture). TPEN treatment after heparinase III hypothetically releases chemokines loosely bound to HSPG (bottom right picture).
There are possible links between zinc and NO and/or ROS in explaining monocyte adhesion to EC (as noted above). NO increases free zinc in ECs, and intracellular free zinc release by NO protects ECs from H2O2-induced toxicity9. Lack of NO increases ICAM-1 levels on EC as well as monocyte adhesion41.
In addition, zinc is required for the function of superoxide dismutase (SOD-1), an antioxidant enzyme, and the catalytic constant of Cu2+/Zn2+ SOD-1 containing decreased amounts of copper and/or zinc has been shown to be 15 times lower than that of the native enzyme36. We found maximal levels of both superoxide and H2O2 under reversing shear stress (Figure 5A). Inflammatory ROS, enhanced by reversing shear stress could participate, in addition to HSPG, in monocyte adhesion. Exposing EC to H2O2 in static culture (Figure 5B) showed that H2O2 alone could increase THP-1 adhesion. It is possible that exogenous superoxide did not increase THP-1 adhesion due to the inactivation by serum in the medium, or the inability of superoxide to pass through the plasma membrane. This differs from the activating and proinflammatory effect of endogenously generated superoxide.
Exposing EC to H2O2 under 15 dynes/cm2 shear stress or reversing shear stress (Figure 5C) increases THP-1 adhesion; whereas catalase plus tempol, which decrease H2O2 and superoxide respectively, decrease THP-1 adhesion.
Herein we demonstrated an effect of zinc on the prevention of monocyte adhesion to HUVEC under shear stress. We also demonstrated that ROS and lack of NO can enhance monocyte adhesion, and that zinc decreases adhesion of monocytes. One of the possible roles of zinc is to inhibit monocyte adhesion by direct interaction with HSPG and cytokines or chemokines. Future work should emphasize which cytokines or chemokines and receptors are involved in monocyte adhesion to EC under reversing shear stress.
Supplementary Material
Footnotes
Conflict of Interest: None
References
- 1.Adams MR, Jessup W, Hailstones D, Celermajer DS. L-arginine reduces human monocyte adhesion to vascular endothelium and endothelial expression of cell adhesion molecules. Circulation. 1997;95:662–668. doi: 10.1161/01.cir.95.3.662. [DOI] [PubMed] [Google Scholar]
- 2.Bao X, Moseman EA, Saito H, Petryanik B, Thiriot A, Hatakeyama S, Ito Y, Kawashima H, Yamaguchi Y, Lowe JB, von Andrian UH, Fukuda M. Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity. 2010;33:817–829. doi: 10.1016/j.immuni.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brower JB, Targovnik JH, Caplan MR, Massia SP. High glucose-mediated loss of cell surface heparan sulfate proteoglycan impairs the endothelial shear stress response. Cytoskeleton (Hoboken) 2010;67:135–141. doi: 10.1002/cm.20430. [DOI] [PubMed] [Google Scholar]
- 4.Celie JW, Rutjes NW, Keuning ED, Soininen R, Heljasvaara R, Pihlajaniemi T, Drager AM, Zweegman S, Kessler FL, Beelen RH, Florquin S, Aten J, van den Born J. Subendothelial heparan sulfate proteoglycans become major l-selectin and monocyte chemoattractant protein-1 ligands upon renal ischemia/reperfusion. Am J Pathol. 2007;170:1865–1878. doi: 10.2353/ajpath.2007.070061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connell P, Young VM, Toborek M, Cohen DA, Barve S, McClain CJ, Hennig B. Zinc attenuates tumor necrosis factor-mediated activation of transcription factors in endothelial cells. J Am Coll Nutr. 1997;16:411–417. doi: 10.1080/07315724.1997.10718706. [DOI] [PubMed] [Google Scholar]
- 7.Conway DE, Lee S, Eskin SG, Shah AK, Jo H, McIntire LV. Endothelial metallothionein expression and intracellular free zinc levels are regulated by shear stress. Am J Physiol Cell Physiol. 2010;299:C1461–1467. doi: 10.1152/ajpcell.00570.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components: Low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol. 2010;298:H367–374. doi: 10.1152/ajpheart.00565.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortese-Krott MM, Suschek CV, Wetzel W, Kroncke KD, Kolb-Bachofen V. Nitric oxide-mediated protection of endothelial cells from hydrogen peroxide is mediated by intracellular zinc and glutathione. Am J Physiol Cell Physiol. 2009;296:C811–820. doi: 10.1152/ajpcell.00643.2008. [DOI] [PubMed] [Google Scholar]
- 10.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281:24085–24089. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- 11.Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, Gimbrone MA., Jr. Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/akt-dependent activation of nrf2. Circ Res. 2007;101:723–733. doi: 10.1161/CIRCRESAHA.107.152942. [DOI] [PubMed] [Google Scholar]
- 12.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93:e136–142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 13.Floris S, van den Born J, van der Pol SM, Dijkstra CD, De Vries HE. Heparan sulfate proteoglycans modulate monocyte migration across cerebral endothelium. J Neuropathol Exp Neurol. 2003;62:780–790. doi: 10.1093/jnen/62.7.780. [DOI] [PubMed] [Google Scholar]
- 14.Frangos JA, McIntire LV, Eskin SG. Shear stress induced stimulation of mammalian cell metabolism. Biotechnol Bioeng. 1988;32:1053–1060. doi: 10.1002/bit.260320812. [DOI] [PubMed] [Google Scholar]
- 15.Gee KR, Zhou ZL, Ton-That D, Sensi SL, Weiss JH. Measuring zinc in living cells. A new generation of sensitive and selective fluorescent probes. Cell Calcium. 2002;31:245–251. doi: 10.1016/S0143-4160(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 16.Hahn C, Schwartz MA. The role of cellular adaptation to mechanical forces in atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:2101–2107. doi: 10.1161/ATVBAHA.108.165951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halden Y, Rek A, Atzenhofer W, Szilak L, Wabnig A, Kungl AJ. Interleukin-8 binds to syndecan-2 on human endothelial cells. Biochem J. 2004;377:533–538. doi: 10.1042/BJ20030729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med. 2006;259:351–363. doi: 10.1111/j.1365-2796.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 20.Hennig B, Meerarani P, Ramadass P, Toborek M, Malecki A, Slim R, McClain CJ. Zinc nutrition and apoptosis of vascular endothelial cells: Implications in atherosclerosis. Nutrition. 1999;15:744–748. doi: 10.1016/s0899-9007(99)00148-3. [DOI] [PubMed] [Google Scholar]
- 21.Hennig B, Meerarani P, Toborek M, McClain CJ. Antioxidant-like properties of zinc in activated endothelial cells. J Am Coll Nutr. 1999;18:152–158. doi: 10.1080/07315724.1999.10718843. [DOI] [PubMed] [Google Scholar]
- 22.Kang Y, Metallothionein J. redox cycle and function. Exp Biol Med (Maywood) 2006;231:1459–1467. doi: 10.1177/153537020623100903. [DOI] [PubMed] [Google Scholar]
- 23.Kroncke KD, Fehsel K, Schmidt T, Zenke FT, Dasting I, Wesener JR, Bettermann H, Breunig KD, Kolb-Bachofen V. Nitric oxide destroys zinc-sulfur clusters inducing zinc release from metallothionein and inhibition of the zinc finger-type yeast transcription activator lac9. Biochem Biophys Res Commun. 1994;200:1105–1110. doi: 10.1006/bbrc.1994.1564. [DOI] [PubMed] [Google Scholar]
- 24.Maret W, Krezel A. Cellular zinc and redox buffering capacity of metallothionein/thionein in health and disease. Mol Med. 2007;13:371–375. doi: 10.2119/2007-00036.Maret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariani E, Cattini L, Neri S, Malavolta M, Mocchegiani E, Ravaglia G, Facchini A. Simultaneous evaluation of circulating chemokine and cytokine profiles in elderly subjects by multiplex technology: Relationship with zinc status. Biogerontology. 2006;7:449–459. doi: 10.1007/s10522-006-9060-8. [DOI] [PubMed] [Google Scholar]
- 26.Meerarani P, Ramadass P, Toborek M, Bauer HC, Bauer H, Hennig B. Zinc protects against apoptosis of endothelial cells induced by linoleic acid and tumor necrosis factor alpha. Am J Clin Nutr. 2000;71:81–87. doi: 10.1093/ajcn/71.1.81. [DOI] [PubMed] [Google Scholar]
- 27.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18:228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moon JJ, Matsumoto M, Patel S, Lee L, Guan JL, Li S. Role of cell surface heparan sulfate proteoglycans in endothelial cell migration and mechanotransduction. J Cell Physiol. 2005;203 doi: 10.1002/jcp.20220. [DOI] [PubMed] [Google Scholar]
- 29.Mun GI, Kim IS, Lee BH, Boo YC. Endothelial argininosuccinate synthetase 1 regulates nitric oxide production and monocyte adhesion under static and laminar shear stress conditions. J Biol Chem. 2011;286:2536–2542. doi: 10.1074/jbc.M110.180489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of vcam-1 and icam-1 at atherosclerosis-prone sites on the endothelium in the apoe-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–851. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- 31.Noris M, Morigi M, Donadelli R, Aiello S, Foppolo M, Todeschini M, Orisio S, Remuzzi G, Remuzzi A. Nitric oxide synthesis by cultured endothelial cells is modulated by flow conditions. Circ Res. 1995;76:536–543. doi: 10.1161/01.res.76.4.536. [DOI] [PubMed] [Google Scholar]
- 32.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates nf-kappab activation by flow: A potential role in atherosclerosis. J Cell Biol. 2005;169:191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr., Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powell SR. The antioxidant properties of zinc. J Nutr. 2000;130:1447S–1454S. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- 35.Rao AK, Ziegler YS, McLeod IX, Yates JR, Nardulli AM. Effects of cu/zn superoxide dismutase on estrogen responsiveness and oxidative stress in human breast cancer cells. Mol Endocrinol. 2008;22:1113–1124. doi: 10.1210/me.2007-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi L, Marchese E, De Martino A, Rotilio G, Ciriolo MR. Purification of a fully metal-depleted cu, zn superoxide dismutase from copper-deficient rat liver. Biometals. 1997;10:257–262. doi: 10.1023/a:1018364130807. [DOI] [PubMed] [Google Scholar]
- 37.Rowe DJ, Bobilya DJ. Albumin facilitates zinc acquisition by endothelial cells. Proc Soc Exp Biol Med. 2000;224:178–186. doi: 10.1046/j.1525-1373.2000.22418.x. [DOI] [PubMed] [Google Scholar]
- 38.Sadir R, Imberty A, Baleux F, Lortat-Jacob H. Heparan sulfate/heparin oligosaccharides protect stromal cell-derived factor-1 (sdf-1)/cxcl12 against proteolysis induced by cd26/dipeptidyl peptidase iv. J Biol Chem. 2004;279:43854–43860. doi: 10.1074/jbc.M405392200. [DOI] [PubMed] [Google Scholar]
- 39.Shen H, Oesterling E, Stromberg A, Toborek M, MacDonald R, Hennig B. Zinc deficiency induces vascular pro-inflammatory parameters associated with nf-kappab and ppar signaling. J Am Coll Nutr. 2008;27:577–587. doi: 10.1080/07315724.2008.10719741. [DOI] [PubMed] [Google Scholar]
- 40.Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med. 2006;259:339–350. doi: 10.1111/j.1365-2796.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 41.Tarin C, Gomez M, Calvo E, Lopez JA, Zaragoza C. Endothelial nitric oxide deficiency reduces mmp-13-mediated cleavage of icam-1 in vascular endothelium: A role in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:27–32. doi: 10.1161/ATVBAHA.108.169623. [DOI] [PubMed] [Google Scholar]
- 42.Tedgui A, Mallat Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 43.Ueda A, Shimomura M, Ikeda M, Yamaguchi R, Tanishita K. Effect of glycocalyx on shear-dependent albumin uptake in endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H2287–2294. doi: 10.1152/ajpheart.00808.2003. [DOI] [PubMed] [Google Scholar]
- 44.Van Damme MP, Blackwell ST, Murphy WH, Preston BN. The measurement of negative charge content in cartilage using a colloid titration technique. Anal Biochem. 1992;204:250–257. doi: 10.1016/0003-2697(92)90235-y. [DOI] [PubMed] [Google Scholar]
- 45.Vinkenborg JL, Nicolson TJ, Bellomo EA, Koay MS, Rutter GA, Merkx M. Genetically encoded fret sensors to monitor intracellular zn2+ homeostasis. Nat Methods. 2009;6:737–740. doi: 10.1038/nmeth.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von der Thusen JH, Kuiper J, van Berkel TJ, Biessen EA. Interleukins in atherosclerosis: Molecular pathways and therapeutic potential. Pharmacol Rev. 2003;55:133–166. doi: 10.1124/pr.55.1.5. [DOI] [PubMed] [Google Scholar]
- 47.Walpola PL, Gotlieb AI, Cybulsky MI, Langille BL. Expression of icam-1 and vcam-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler Thromb Vasc Biol. 1995;15:2–10. doi: 10.1161/01.atv.15.1.2. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs l-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005;6:902–910. doi: 10.1038/ni1233. [DOI] [PubMed] [Google Scholar]
- 49.Webb LM, Ehrengruber MU, Clark-Lewis I, Baggiolini M, Rot A. Binding to heparan sulfate or heparin enhances neutrophil responses to interleukin 8. Proc Natl Acad Sci U S A. 1993;90:7158–7162. doi: 10.1073/pnas.90.15.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiseman DA, Wells SM, Wilham J, Hubbard M, Welker JE, Black SM. Endothelial response to stress from exogenous zn2+ resembles that of no-mediated nitrosative stress, and is protected by mt-1 overexpression. Am J Physiol Cell Physiol. 2006;291:C555–568. doi: 10.1152/ajpcell.00509.2005. [DOI] [PubMed] [Google Scholar]
- 51.Yao Y, Rabodzey A, Dewey CF., Jr. Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am J Physiol Heart Circ Physiol. 2007;293:H1023–1030. doi: 10.1152/ajpheart.00162.2007. [DOI] [PubMed] [Google Scholar]
- 52.Yee A, Bosworth KA, Conway DE, Eskin SG, McIntire LV. Gene expression of endothelial cells under pulsatile non-reversing vs. Steady shear stress; comparison of nitric oxide production. Ann Biomed Eng. 2008;36:571–579. doi: 10.1007/s10439-008-9452-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.