Abstract
Whereas cholecystokinin (CCK) has long been known to exert anxiogenic effects in both animal anxiety models and humans, the underlying cellular and molecular mechanisms are ill-defined. CCK interacts with CCK-1 and CCK-2 receptors resulting in up-regulation of phospholipase C (PLC) and protein kinase C (PKC). However, the roles of PLC and PKC in CCK-mediated anxiogenic effects have not been determined. We have shown previously that CCK facilitates glutamate release in the hippocampus especially at the synapses formed by the perforant path and dentate gyrus granule cells via activations of PLC and PKC. Here we further demonstrated that CCK enhanced NMDA receptor function in dentate gyrus granule cells via activation of PLC and PKC pathway. At the single-channel level, CCK increased NMDA single-channel open probability and mean open time, reduced the mean close time and had no effects on the conductance of NMDA channels. Because elevation of glutamatergic functions results in anxiety, we explored the roles of PLC and PKC in CCK-induced anxiogenic actions using the Vogel Conflict Test (VCT). Our results from both pharmacological approach and knockout mice demonstrated that microinjection of CCK into the dentate gyrus concentration-dependently increased anxiety-like behavior via activation of PLC and PKC. Our results provide a novel unidentified signaling mechanism whereby CCK increases anxiety.
Keywords: NMDA receptor, hippocampus, anxiety, synaptic transmission, peptide, glutamate
INTRODUCTION
Cholecystokinin (CCK) is one of the most abundant neuropeptides in the brain (Beinfeld et al., 1981) where it interacts with two G protein-coupled receptors: CCK-1 and CCK-2 (Wank, 1995). Activation of both CCK receptors increases the activity of phospholipase C (PLC) leading to the hydrolysis of phosphatidylinositol 4, 5-bisphosphate (PIP2) into inositol trisphosphate (IP3) to increase intracellular Ca2+ release, and diacylglycerol to activate protein kinase C (PKC) although CCK-1 receptors also increase adenylyl cyclase activity which enhances the generation of cyclic AMP and subsequent activation of protein kinase A (Wank, 1995). Although CCK-1 receptors are distributed in the peripheral tissues and in limited brain regions including the postrema, interpeduncular nucleus and nucleus tractus solitarius (Hill et al., 1987; Hill et al., 1990; Moran et al., 1986), CCK-2 receptors are the predominant CCK receptors in the brain (Van Dijk et al., 1984). Interestingly, CCK is highly expressed in the limbic structures including the hippocampus (Greenwood et al., 1981; Hefft and Jonas, 2005), cingulate gyrus (Beinfeld et al., 1981), subiculum (Kohler and Chan-Palay, 1982) and entorhinal cortex (Beinfeld et al., 1981; Greenwood et al., 1981; Kohler and Chan-Palay, 1982; Lotstra and Vanderhaeghen, 1987). Consistent with the distribution of CCK, high density of CCK binding sites, possibly CCK-2 receptors, have also been detected in the limbic structures (Kohler and Chan-Palay, 1988; Kritzer et al., 1988). The selective expression of CCK and its receptors in the limbic system likely explains the physiological and pathological functions of CCK in the brain because CCK has been demonstrated to modulate physiological functions including satiety, analgesia, learning and memory (Beinfeld, 2001; Rehfeld, 2000; Sebret et al., 1999) and pathological disorders such as anxiety (Bradwejn and Koszycki, 1994; Crawley and Corwin, 1994; Noble and Roques, 1999; Rehfeld, 2000; Rodgers and Johnson, 1995). For example, application of CCK or CCK-2 receptor agonists or elevating CCKergic tone in the brain produces significant anxiogenic effects (Chen et al., 2006) whereas application of CCK-2 receptor antagonists (Hughes et al., 1990; Singh et al., 1991; Wang et al., 2005) or down-regulation of endogenous CCK precursor (Cohen et al., 1998) generates anxiolytic actions. Paradoxically, the cellular and molecular mechanisms whereby CCK exerts the anxiogenic effects remain to be determined.
Recent development in the neurobiology of anxiety has highlighted glutamate as a mediator in anxiety (Bergink et al., 2004; Gorman, 2003; Kent et al., 2002). Presynaptically released glutamate binds to three classes of ionotropic glutamate receptors including NMDA, AMPA and kainate receptors (Dingledine et al., 1999; Hollmann and Heinemann, 1994; Seeburg, 1993). Preclinical data in rodents and human subjects have shown that compounds that inhibit glutamatergic function either by blocking glutamate receptors or by reducing glutamate release elicit anxiolytic actions (Bergink et al., 2004) indicating that elevation in glutamatergic function underlies anxiety. Consistently, we have recently demonstrated that CCK facilitates glutamate release in the hippocampus via activation of PLC/PKC pathway (Deng et al., 2010). However, the roles of CCK-induced facilitation of glutamatergic transmission in CCK-mediated anxiogenic effects have not been determined. In the present study, we first investigated the effects of CCK on NMDA type of glutamate receptors and our results demonstrate that CCK facilitates NMDA receptor function via activation of PLC/PKC pathway. Because PLC/PKC pathway is required for CCK-mediated facilitation of both glutamate release and NMDA receptor function, we further explored the roles of this pathway in CCK-mediated facilitation of anxiety using the Vogel Conflict Test (VCT). Our results demonstrate that CCK-induced increases in anxiety-like behavior require the functions of CCK-2 receptors, PLC and PKC. Our results provide a novel signaling mechanism whereby CCK augments anxiety.
METHODS
Preparation of hippocampal slices
Horizontal brain slices (300 μm) were cut using a vibrating blade microtome (VT1000S; Leica, Wetzlar, Germany) from 15- to 22-day-old Sprague Dawley rats as described previously (Deng and Lei, 2007; Deng et al., 2006). After being deeply anesthetized with isoflurane, rats were decapitated and their brains were dissected out in ice-cold cutting solution containing (in mM) 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 5.0 MgCl2 and 10 glucose, saturated with 95% O2 and 5% CO2, pH 7.4. Slices were initially incubated in the above solution at 35°C for 40 min for recovery and then kept at room temperature (~24°C) until use. All animal procedures conformed to the guidelines approved by the University of North Dakota Animal Care and Use Committee.
Recordings of AMPA and NMDA receptor-mediated EPSCs at the synapses formed between the perforant path and dentate gyrus granule cells
Whole-cell patch-clamp recordings using a Multiclamp 700B amplifier in voltage-clamp mode from hippocampal slices were used to record AMPA and NMDA receptor-mediated EPSCs from dentate gyrus granule cells visually identified with infrared video microscopy and differential interference contrast optics (Deng and Lei, 2006; Deng et al., 2010). Recording electrodes were filled with the solution containing (in mM) 100 Cs-gluconate, 0.6 EGTA, 5 MgCl2, 8 NaCl, 2 ATP2Na, 0.3 GTPNa, 40 HEPES and 1 QX-314, pH 7.3. The holding potential was at -60 mV for both AMPA and NMDA EPSCs. For AMPA EPSCs, the extracellular solution comprised (in mM) 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 2.5 CaCl2, 1.5 MgCl2, 10 glucose and 0.01 bicuculline, saturated with 95% O2 and 5% CO2, pH 7.4. For NMDA EPSCs, Mg2+ in the above extracellular solution was omitted and DNQX (10 μM) was included to block AMPA EPSCs. Synaptic currents were evoked by placing a stimulation electrode in the middle to the inner one third of molecular layer of dentate gyrus to stimulate the medial perforant path. Series resistance was rigorously monitored by the delivery of 5 mV voltage steps after each evoked current. Experiments were discontinued if the series resistance changed by >15%. Data were filtered at 2 kHz, digitized at 10 kHz, acquired on-line and analyzed after-line using pCLAMP 9 software (Molecular Devices, Sunnyvale, CA). To avoid potential desensitization induced by repeated bath applications of CCK, one slice was limited to only one application of CCK and only one cell was recorded from each slice.
Preparation of dissociated cells and whole-cell recordings of NMDA currents
Granule cells in the dentate gyrus were acutely isolated using a method modified from the procedures described previously (Lei et al., 2001). Slices cut with the above methods were digested in the above cutting solution supplemented with 0.5 μM tetrodotoxin and 4 mg/ml papain (papaya latex, Sigma) at room temperature (~24°C) for 40 min. After washing with the cutting solution without papain for at least three times, slices were kept in this solution oxygenated with 95% O2 and 5% CO2 at room temperature until use. The dentate gyrus granule cell layer was micro-dissected out under a microscope and then triturated with a fire-polished glass pipette. The dissociated cells were adhered to the bottom of a 35 mm Petri dish and placed under a phase contrast microscope (Olympus 1X70) for recordings. The cells were bathed in an extracellular solution containing (in mM) 140 NaCl, 1.3 CaCl2, 5 KCl, 25 HEPES, 33 glucose and 0.0005 tetrodotoxin (pH 7.4). The internal solution for the recording electrodes consisted of (in mM) 70 Cs methylsulphonate, 70 CsF, 35 CsOH, 10 HEPES, 2 MgCl2, 2 tetraethylammonium, 1.1 EGTA, 0.25 CaCl2, 0.3 GTPNa and 4 Na2ATP, pH 7.3 (osmolarity, 300 mOsm). A computer-controlled, multi-barreled fast perfusion system (SF-77B Perfusion Fast Step, Warner) was used to achieve exchange of solutions. NMDA (50 μM) and glycine (3 μM) was applied to neurons via one barrel for 2 sec to evoke NMDA receptor-mediated currents. CCK and other drugs were applied to the cells through both the control and the agonists-containing barrels. After formation of whole-cell configuration, the cells were voltage-clamped at -60 mV and lifted into the stream of the solution from the control barrel. In this condition, the recorded currents were completely blocked by DL-APV (50 μM) confirming that they were mediated by NMDA receptors. To monitor access resistance, a voltage step of -10 mV was made before the application of each agonist. Data were included for analysis only from the cells showing <15% changes of series resistance. Data were filtered at 2 kHz, digitized at 10 kHz, acquired on-line and analyzed after-line using pCLAMP 9 software (Molecular Devices, Sunnyvale, CA).
Cell-attached single channel recordings
Detailed methods for recording and analyzing NMDA single channel currents were described in previous publication (Lei et al., 2001). Dissociated dentate gyrus granule cells were bathed in and perfused with the above extracellular solution. After being coated with Sylgard, the electrodes (~10 MΩ) were filled with the above extracellular solution containing NMDA (5 μM) and glycine (3 μM). Cell-attached patch was formed on the isolated granule cells. The holding potential of patches was usually 0 mV, but various potentials were used when acquiring data to calculate the single-channel conductance. NMDA single channel currents were recorded with Clampex 9. Recordings were filtered at 2 kHz and analyzed off-line with Clampfit 9. Channel openings and closings were determined by using a 50% crossing threshold. Mean open time and mean close time were calculated by integration of the mixture of exponential components fitted to open-time and close-time distributions, respectively. The open probability (Po) was calculated by dividing the mean open time by the sum of mean open time and mean close time. Single-channel current amplitudes were calculated from means of Gaussian fits to all-point amplitude histograms. Single channel conductance was computed by linearly fitting the amplitudes recorded at different holding potentials versus the corresponding voltages. Open dwell-time histograms were best fit with two to three components, and close dwell-time histograms were best fit with four to five components. Adequacy of the number of components fitting the histogram was determined by the program. For a better comparison, the mean open or close dwell time was calculated as the weighted dwell time.
Animals used for behavioral experiments
Sprague-Dawley rats weighing 220-260 g (male, 7-9 weeks), wild-type and knock-out mice weighing 20-23 g (8-10 weeks) at the time of surgery were housed with free access to food and water in a room with a 12:12 h light/dark cycle and controlled temperature (~23°C). Heterozygous mating pairs (F1 hybrid crosses from 129 PLC-β1+/- × C57BL/6J PLC-β1+/-) were obtained from Korea Institute of Science and Technology and used to derive wild-type, heterozygous and homozygous pups for experimental analysis. PCR genotyping from purified genomic DNA was performed as described previously (Deng et al., 2006; Kim et al., 1997). Wild-type (WT) and homozygous knockout (KO) mice were used for experiments and the heterozygous mice were used for breeding. Because of the scarcity of the homozygous KO and WT mice, both male and female mice of this strain were used for experiments. However, the ratio of male and female in each group was kept approximately the same to minimize the influence of sex. Pairs of WT (C57BL/6J/129sv) and homozygous KO mice for CCK-2 receptors were provided by Kobe University (Japan) and detailed methods for the generation and genotyping of KO mice for CCK-2 receptors were described previously (Nagata et al., 1996). Pups derived from the homozygous KO or WT mating pairs were bred in the animal facility of the University of North Dakota and used for experiments. Only male WT and male homozygous CCK-2 KO mice were used for experiments to exclude the influences of sex on the results. WT and homozygous PKCγ KO mice (002466B6; 129P2-Prkcc<tm1Stl>/J) were purchased from The Jackson Laboratory. Only male WT and male homozygous PKCγ KO mice were used for experiments. To minimize individual variation of the animals from different batches, animals of the same batches were equally and randomly assigned to each experimental group for each experiment. To avoid the influences of drugs and experiences of the animals on the results, each animal was limited to only one experiment.
Stereotaxic surgery and microinjection
Detailed procedures for stereotaxic cannulation and microinjection were described previously (Deng et al., 2009). Animals were anesthetized intraperitoneally with pentobarbital (50 mg/kg) and placed in a stereotaxic instrument. An adaptor purchased from World Precision Instruments was used for mice. Stainless steel guide cannulae (23-gauge for rats, 26-gauge for mice) were implanted to the right and left dentate gyrus. For rats, stereotaxic coordinates (-6.0 mm posterior to bregma, ±4.0 mm lateral to the midline and -4.2 mm in depth from the dura) for injection to dentate gyrus region were based on the brain atlas (Paxinos and Watson, 2009) and our preliminary experiments. For mice, stereotaxic coordinates were -3.3 mm posterior to bregma, ±2.0 mm lateral to the midline on each side and -2.3 mm in depth from the dura. The cannulae were fixed to the skull with acrylic dental cement. Animals were allowed for 5-7 days to recover from surgery. For microinjection, animals were anesthetized briefly with isoflurane to prevent struggling. Drugs in the volume of 0.5 to1 μl were infused into the dentate gyrus bilaterally by means of an internal cannula terminating 1 mm below the tip of the guides connected by polyethylene tubing to a 5-μl Hamilton syringe over a period of 2 min with a syringe pump (WPI, SP230IW). The inner cannula was left in place for an additional 60 s to allow diffusion of the solution. Intra-dentate gyrus injections were made ~15 min before testing. For those experiments requiring injection of CCK with other drugs, the interval between the two injections was ~5 min.
Vogel Conflict Test (VCT)
VCT was conducted in polycarbonate cages (42 × 25 × 20 cm for rats and 42 × 25 × 10 cm for mice) possessing a grid floor (Dekeyne et al., 2000). The metallic spout of a drinking bottle containing water projected into the box. Both the grid and the spout were connected to an Anxiometer (Columbus Instruments, Ohio, USA). The contact of the animal with the spout and the grid floor closed an electrical circuit that was counted as a lick by the Anxiometer. The animal received a mild electrical shock (0.3 mA for 0.5 s) every 20 licks. During the 3 days preceding the test, animals were restricted to 1 hr-per-day access to tap water (from 9:00 to 10:00 am). On day 4, just after water delivery, they were isolated in cages with a grid-floor. Testing took place on day 5. Each animal was placed in the test cage and the session was initiated after the animal had made 20 licks and received the first shock through the spout. Thereafter, a shock was delivered to the animal every twentieth lick during a period of 3 min. Data were the number of licks emitted by the animal during the 3-min session. When test was completed, animals were given overdose of pentobarbital and pontamine sky blue (Sigma) was microinjected through the same guide cannulae to mark the injection site. The brains were intracardially perfused with 0.1 M phosphate buffered saline (PBS) followed by 4% paraformaldehyde prepared in 0.1 M PBS. The brains were taken out and kept in 4% paraformaldehyde prepared in 0.1 M PBS for 24 h. Coronal brain sections (40 μm) were cut using a Leica cryostat (CM3050S). The sections were counterstained with neutral red, dehydrated in graded ethanol (30, 50, 70, 90, 95 and 100%) cleared in xylene, mounted with clarion mounting medium and coverslipped. The injection sites were examined and photographed under a light microscope. Data were included for analysis only from those animals showing that the injection sites were in the dentate gyrus.
Statistical Analysis
Data were presented as the means ± S.E.M. For the electrophysiological data, paired t-test or Two-Way ANOVA were used for statistical analysis as appropriate. For the behavioral experiments, data obtained by application of the inhibitors were analyzed by One-Way ANOVA followed by Dunnett or Tukey post-tests. Two-Way ANOVA (Drugs × Strains) was used for analysis of the data obtained from the experiments involving the KO mice. Post-hoc analysis was performed using the Bonferroni test. Significance was defined as p<0.05.
RESULTS
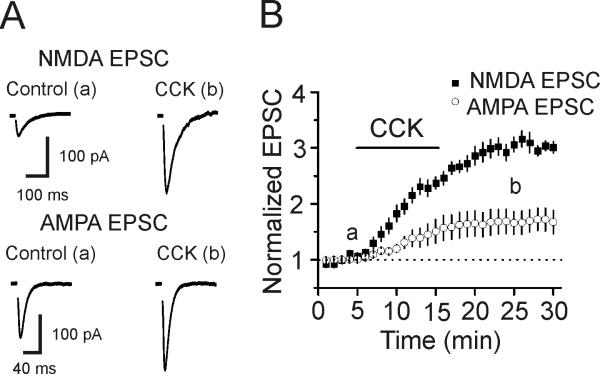
CCK induces a greater extent of facilitation of NMDA EPSCs than AMPA EPSCs at the perforant path-granule cell synapses
We have previously shown that application CCK facilitates glutamate release assessed by measuring the evoked AMPA EPSCs at the perforant path-granule cell synapses (Deng et al., 2010). Whereas CCK does not modulate AMPA receptors (Deng et al., 2010), a potential effect of CCK on NMDA receptors at this synapse type has not been determined. We therefore examined the effects of CCK on glutamatergic transmission assessed by measuring NMDA receptor-mediated EPSCs evoked by stimulation of the medial perforant pathway. Application of CCK (0.5 μM) significantly increased NMDA EPSCs to 306±13% of control (n=9, p<0.001, Fig. 1) whereas the AMPA EPSCs recorded at this synapse in an interleaved manner were facilitated only to 166±21% of control (n=10, p=0.011, Fig. 1). The extent of CCK-induced increases in NMDA EPSCs was significantly larger than that of the AMPA EPSCs (p<0.001, two-way ANOVA, Fig. 1). One explanation for the discrepancy of CCK-mediated facilitation of AMPA and NMDA EPSCs is that CCK also facilitates NMDA channel function in addition to increasing glutamate release at this synapse type.
Fig. 1.
CCK induces a larger scale of increase in NMDA EPSCs than AMPA EPSCs at the perforant path-granule cell synapses of the hippocampus. A, Representative NMDA (upper) and AMPA (lower) EPSCs recorded from a perforant path-granule cell synapse before (left) and after (right) application of CCK (0.5 μM). B, Summarized data for CCK-mediated facilitation of NMDA (n=9 cells) and AMPA (n=10 cells) EPSCs.
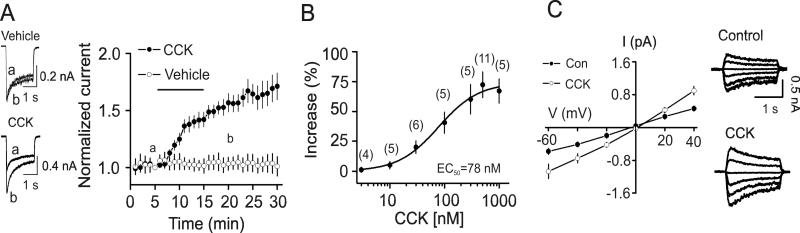
CCK increases NMDA receptor-mediated currents in acutely isolated granule cells
To avoid the influence of CCK-mediated facilitation of presynaptic glutamate release on postsynaptic NMDA receptors at the perforant path-granule cell synapses, we examined the effects of CCK on NMDA currents recorded from acutely isolated dentate granule cells by exogenous application of NMDA. Application of CCK (0.5 μM) significantly increased the peak of NMDA currents (172±11 % of control, n=11, p<0.001, Fig. 2A) whereas application of vehicle (0.004% NH4OH) used to dissolve CCK did not significantly change NMDA currents (104±8% of control, n=5, p=0.64, Fig. 2A). The EC50 of CCK was measured to be 78 nM (Fig. 2B). CCK-mediated increases in NMDA currents were not voltage-dependent (Fig. 2C).
Fig. 2.
Exogenous application of CCK increases NMDA currents recorded from acutely dissociated dentate gyrus granule cells. A, Left, NMDA currents recorded from the same cells before (a) and after (b) the application of vehicle (upper) or CCK (lower). Right, Summarized time course of the NMDA currents from cells treated with vehicle (n=5 cells) and CCK (n=11 cells). B, Concentration-response curve of CCK-mediated enhancement of NMDA currents. Numbers in the parenthesis were numbers of cells recorded for each concentration. C, Voltage-current relationship of CCK-mediated facilitation of NMDA currents averaged from 5 cells. Currents recorded at different voltages from the same cell before (upper) and after (lower) the application of CCK are shown on right panel.
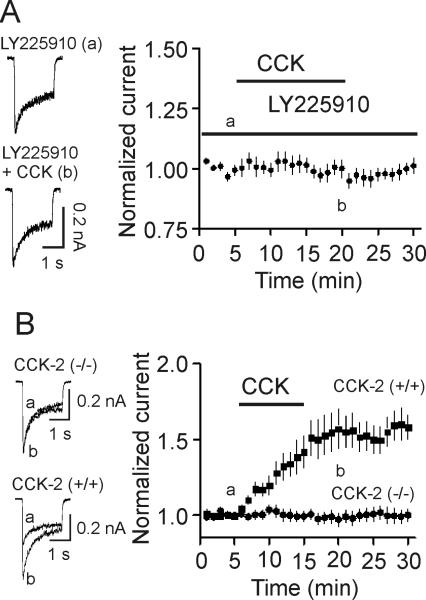
CCK increases NMDA currents via activation of CCK-2 receptors
CCK interacts with CCK-1 and CCK-2 receptors and CCK-2 receptors are expressed in the hippocampus (Shigeyoshi et al., 1994). We next examined the role of CCK-2 receptors in CCK-mediated increases in NMDA currents. Acutely dissociated granule cells were pretreated with LY225910 (5 μM), a selective CCK-2 receptor blocker, for ~20 min and the same concentration of LY225910 was continuously bath-applied. CCK failed to significantly increase NMDA currents in the presence of LY225910 (99±4% of control, n=8, p=0.98, Fig. 3A). Furthermore, application of CCK (0.5 μM) did not significantly increase NMDA currents (97±5% of control, n=13 cells, p=0.54) in the granule cells acutely isolated from 4 CCK-2 KO mice (Fig. 3B) whereas application of the same concentration of CCK still enhanced the peak of NMDA currents (155±11% of control, n=9 cells, p<0.001) in granule cells isolated from 3 WT mice (Fig. 3B). Together, these results indicate that CCK enhances NMDA currents via activation of CCK-2 receptors.
Fig. 3.
CCK enhances NMDA receptor currents via activation of CCK-2 receptors. A, Pretreatment of cells with and continuous bath application of LY225910 (5 μM) blocked CCK-induced enhancement of NMDA currents. Left, NMDA currents recorded before (upper) and after (lower) the application of CCK in the presence of LY225910. Right, Pooled data from 8 cells. B, Application of CCK failed to facilitate NMDA currents in granule cells (n=13 cells) isolated from 4 CCK-2 KO mice whereas application of CCK still significantly increased NMDA currents in granule cells (n=9 cells) isolated from 3 WT mice.
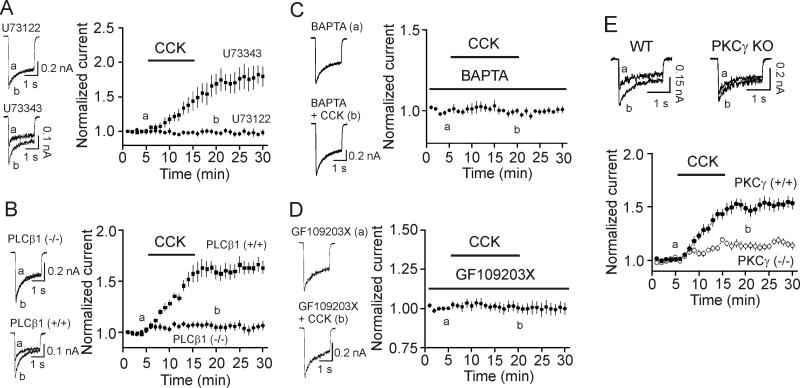
CCK-mediated increases in NMDA currents require the functions of PLC, intracellular Ca2+ release and PKC
Activation of CCK-2 receptors results in up-regulation of PLC generating IP3 to increase intracellular Ca2+ release and diacyglycerol to activate PKC. We further examined the role of this pathway in CCK-mediated enhancement of NMDA currents. The dissociated cells were pretreated with the PLC inhibitor U73122 (10 μM) for 20 min and the same concentration of U73122 was applied in the bath. Under this circumstance, application of CCK (0.5 μM) failed to significantly increase NMDA currents (98±3% of control, n=6, p=0.57, Fig. 4A) whereas application of CCK to the dissociated granule cells treated with the inactive analog U73343 (10 μM) in the same fashion still increased NMDA currents (169±15% of control, n=7, p=0.003, Fig. 4A). Among the 4 isoforms of PLCβ, PLCβ1 is expressed in the hippocampus (Watanabe et al., 1998). We thereby examined the role of PLCβ1 in CCK-mediated enhancement of NMDA currents using PLCβ1 KO mice (Deng et al., 2006; Deng et al., 2010; Kim et al., 1997; Lei et al., 2007). Application of CCK (0.5 μM) failed to significantly increase NMDA currents (108±4% of control, n=11 cells, p=0.09, Fig. 4B) in granule cells isolated from PLCβ1 KO (n=3) mice whereas CCK still significantly increased NMDA currents (166±8% of control, n=9 cells, p<0.001, Fig. 4B) in WT (n=3) mice. These data together indicate that PLCβ1 is necessary for CCK-mediated increases in NMDA currents.
Fig. 4.
CCK-mediated augmentation of NMDA currents requires the functions of PLC, intracellular Ca2+ and PKC. A, Pretreatment of cells with and continuous bath application of U73122 blocked CCK-induced increases in NMDA currents (n=7) whereas application of U73343 (the inactive analog, n=6) in the same fashion had no effects on NMDA currents. B, Application of CCK did not increase NMDA currents in granule cells (n=11 cells) isolated from PLCβ1(-/-) mice but still enhanced NMDA currents in granule cells (n=9 cells) isolated from WT mice. C, Inclusion of BAPTA (10 mM) in the recording pipettes blocked CCK-mediated enhancement of NMDA currents (n=6 cells). D, Pretreatment of cells with and inclusion of GF109203X (0.5 μM) in the recording pipettes blocked CCK-induced increases in NMDA currents (n=7 cells). E, Application of CCK induced a significantly smaller scale of increase in NMDA currents in dentate granule cells (n=12 cells) isolated from PKCγ KO mice compared with CCK-induced facilitation of NMDA currents (n=11 cells) in granule cells from WT mice.
We then examined the roles of intracellular Ca2+ release and PKC in CCK-mediated increases in NMDA currents. Inclusion of BAPTA (10 mM) in the recording pipettes blocked CCK-mediated increases in NMDA currents (99±2% of control, n=6, p=0.64, Fig. 4C) suggesting that intracellular Ca2+ release is required for the effect of CCK on NMDA currents. Pretreatment of cells with and inclusion of GF109203X (0.5 μM) in the recording pipettes blocked CCK-induced increases in NMDA currents (99±4% of control, n=7, p=0.87, Fig. 4D) suggesting that the conventional, Ca2+-dependent PKC is required for the effect of CCK on NMDA currents.
The conventional PKCs include PKCα, PKCβ and PKCγ. We next examined the roles of PKCγ in CCK-mediated facilitation of NMDA currents by using PKCγ KO mice based on the following two reasons. First, the expressions of PKCγ in dentate gyrus granule cells are much higher than those of the PKCα and PKCβ (Van der Zee et al., 2004) and if PKC is required, PKCγ should be the major type to be involved. Second, we have shown previously that CCK increases glutamate release at the perforant path-granule cell synapses via activation of PKCγ (Deng et al., 2010). In granule cells isolated from PKCγ KO mice, application of CCK (0.5 μM) increased NMDA currents only to 113±3% of control (n=12 cells from 3 PKCγ KO mice, p=0.001, Fig. 4E) which was significantly smaller compared with CCK-mediated increases in NMDA currents from WT mice (153±6% of control, n=11 cells from 3 WT mice, p<0.001, towway ANOVA, Fig. 4E) demonstrating that PKCγ is involved in CCK-mediated increases in NMDA currents in dentate gyrus granule cells.
CCK increases the open probability of NMDA receptors in cell-attached patches
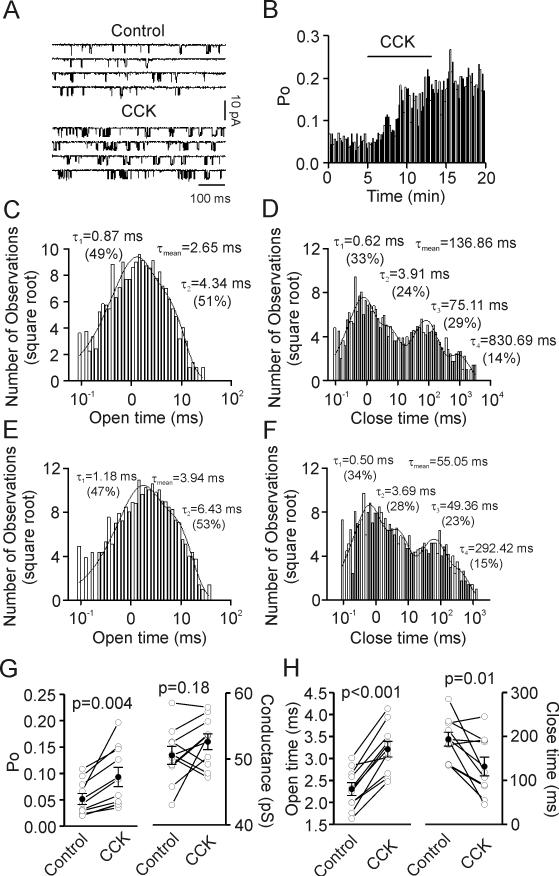
We then examined the effects of CCK on the properties of NMDA single channels recorded from dissociated granule cells with cell-attached patches (see methods). Bath application of CCK (0.5 μM) significantly increased NMDA single-channel open probability (PO) to 187±15% of control (n=10 patches, p=0.004, Fig. 5A, 5B and 5G left) but did not alter the conductance of NMDA receptors (Control: 50.5±1.4 pS, CCK: 52.6±1.2 pS, n=10 patches, p=0.18, Fig. 5G right). Further analysis of the kinetics of single channels showed that CCK significantly increased the mean open dwell time (n=10 patches, p<0.001, Fig. 5C, 5E and 5H left) but reduced the mean close dwell time (n=10 patches, p=0.01, Fig. 5D, 5F and 5H right).
Fig. 5.
Bath application of CCK increases open probability and open time, decreases close time and has no effects on the conductance of NMDA single channels in cell-attached patches. A, NMDA single-channel currents recorded from an acutely dissociated granule cell before (upper) and during (lower) the application of CCK. B, A continuous record of NMDA single-channel open probability (PO) recorded from a granule cell in cell-attached patch before, during and after application of CCK in the bath. NMDA single-channel PO was calculated in bins of 10 sec duration. C, Dwell-time histogram of open time in a patch before application of CCK. The time constant and the relative percentage of each component, as well as the weighted mean open time are displayed. D, Dwell-time histogram of close time from the same patch before application of CCK. The time constant and the relative percentage of each component, as well as the weighted mean close time are shown. E, Dwell-time histogram of open time from the same patch after application of CCK. F, Dwell-time histogram of close time from the same patch after application of CCK. G, Summarized data for NMDA single-channel PO (left) and conductance (right) from 10 patches before and after the application of CCK. Solid circles represent average values. H, Summarized data for NMDA single-channel weighted open time (left) and close time (right) from 10 patches before and after application of CCK. Note that CCK significantly increased PO and weighted open time, decreased weighted close time and had no effects on the conductance of NMDA single channels.
CCK increases anxiety-like behavior via activation of PLC/PKC pathway
Our previous study and present results demonstrate that CCK increases glutamate release at the perforant path-granule cell synapses (Deng et al., 2010) and NMDA channel function in dentate granule cells via activation of CCK-2 receptors and PLC/PKC pathway. We next examined the functions of CCK-mediated facilitation of glutamatergic transmission in vivo. CCK has been known for decades to exert anxiogenic effects (Bradwejn and Koszycki, 1994; Crawley and Corwin, 1994; Noble and Roques, 1999; Rehfeld, 2000; Rodgers and Johnson, 1995). However, the cellular and signaling mechanisms whereby CCK exerts the anxiogenic effects remain to be determined. Because elevation of glutamatergic function results in anxiety (Bergink et al., 2004; Gorman, 2003; Kent et al., 2002) and CCK elevates glutamatergic function in the dentate gyrus via activation of PLC/PKC pathway, we examined the roles of this pathway in CCK-induced anxiogenic effects by microinjecting CCK and other drugs into the dentate gyrus and then assessed the anxiety-like behavior of the animals. Because the anxiogenic effects of CCK have already been tested in animal anxiety models like the elevated-plus maze and open-field test (Fink et al., 1998; Wang et al., 2005) and multiple tests are required for assessing the effects of drugs on anxiety (Ramos, 2008), we used the VCT, another widely used anxiety model. We chose dentate gyrus for the following reasons. First, the dentate gyrus expresses the highest density of CCK receptors (Kohler and Chan-Palay, 1988; Kritzer et al., 1988). Second, our previous results have shown that the highest responsive ratio of CCK-induced augmentation of glutamate release was observed at the synapses formed between the perforant path and dentate gyrus granule cells (Deng et al., 2010). Third, our present study showed that CCK increased NMDA receptor-mediated glutamatergic transmission at the perforant path-granule cell synapses by enhancing NMDA receptor function in the granule cells. Fourth, dentate gyrus is involved in the modulation of anxiety (Chuang et al., 2011; Spolidorio et al., 2007; Tsetsenis et al., 2007). Finally, anxiety is related to changes of neural network activities majorly mediated by the limbic system in which dentate gyrus is the first station of the synaptic connection in the hippocampus and hippocampus is an important component of the limbic system.
Fig. 6A shows the representative photographs of microinjection sites from a rat and a mouse. Experiments were divided into 4 groups and each group comprised 7-10 animals; Control group was microinjected with vehicle (4% NH4OH) used to dissolve CCK and the other 3 groups were microinjected with 0.01, 0.1 and 0.5 nmol CCK, respectively. Microinjection of CCK at 0.01 nmol did not significantly alter the number of licks in the VCT (F(3,24)=1.96, p>0.05, Fig. 6B) whereas the number of licks was significantly reduced in rats microinjected with CCK at 0.1 nmol (F(3,24)=3.71, p<0.01, Fig. 6B) and 0.5 nmol (F(3,24)=5.23, p<0.01, Fig. 6B). These data together demonstrate that microinjection of CCK into the dentate gyrus exerts anxiogenic effects. For the rest of the experiments, we microinjected CCK at 0.5 nmol to further determine the mechanisms whereby CCK facilitates anxiety-like behavior.
Fig. 6.
Microinjection of CCK into the dentate gyrus concentration-dependently increases anxiety-like behavior via activation of CCK-2 receptors. A, Representative coronal sections showing microinjection sites for rats (left) and mice (right). Arrow shows the point of microinjection. CT: Cannula Track. B, CCK reduced the number of licks at 0.1 nmol and 0.5 nmol without effect at 0.01 nmol measured by VCT. ** p<0.01 (One-way ANOVA followed by post hoc Dunnett test). C, Microinjection of the CCK-2 receptor antagonist, LY225910, at 0.05 nmol did not, but at 0.2 nmol and 1 nmol blocked CCK-mediated reduction of the number of licks. * p<0.05. D, Microinjection of CCK significantly reduced the number of licks in WT mice (** p<0.01) but had no significant effect in CCK-2 KO mice. Note that CCK-2 KO mice injected with vehicle showed significant higher number of licks than the WT counterparts injected with vehicle (* p<0.05).
Because our data showed that CCK-induced increases in glutamate release and facilitation of NMDA receptor function are mediated via activation of CCK-2 receptors (Deng et al., 2010), we tested the involvement of CCK-2 receptors in CCK-induced enhancement of anxiety-like behavior by microinjecting the selective CCK-2 receptor inhibitor, LY225910. The effective dose of LY225910 for in vivo microinjection was reported to be 0.05-1 nmol (Bertoglio et al., 2006; Bertoglio and Zangrossi, 2005; Rezayat et al., 2005). Microinjection of LY225910 at 0.05 nmol failed to block CCK-induced reduction of the number of licks statistically (F(5,43)=3.53, p<0.05, Fig. 6C) whereas CCK-mediated reduction of the number of licks was counteracted by injection of LY225910 at 0.2 nmol (F(5,43)=1.45, p>0.05, Fig. 6C) and 1 nmol (F(5,43)=0.75, p>0.05, Fig. 6C) demonstrating that CCK-2 receptors are required for CCK-induced anxiogenic effects. Furthermore, microinjection of CCK into the dentate gyrus of WT mice significantly reduced the number of licks (F(1,24)=3.78, p<0.01, Fig. 6D) whereas it had no statistically significant effects on the number of licks in CCK-2 KO mice (F(1,24)=0.56, p>0.05, Fig. 6D). The number of licks in CCK-2 KO mice was significantly larger than that of the WT mice (F(1,24)=2.86, p<0.05, Fig. 6D). One explanation for the difference between the WT and CCK-2 KO mice is that endogenous CCK exerts an anxiogenic effect. Together, these results indicate that CCK-2 receptors are required for CCK-mediated anxiogenic effects.
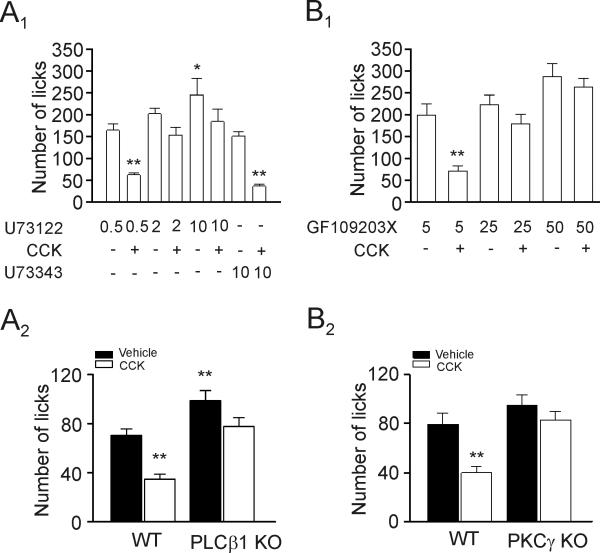
Activation of CCK-2 receptors increases PLC activity and CCK-mediated augmentations of glutamate release (Deng et al., 2010) and NMDA currents are mediated via activation of PLC. Accordingly, we probed the roles of PLC in CCK-induced increases in anxiety-like behavior. Microinjection of U73122, a selective PLC inhibitor, at a low dose (0.5 nmol), did not block CCK-induced reduction of the number of licks (F(7,50)=3.84, p<0.01, Fig. 7A1). However, CCK-induced reduction of the number licks was blocked by injection of U73122 at 2 nmol (F(7,50)=1.83, p>0.05, Fig. 7A1) and 10 nmol (F(7,50)=2.24, p>0.05, Fig. 7A1) whereas CCK still reduced significantly the number of licks in rats injected with the U73343 (the inactive analog) at 10 nmol (F(7,50)=4.18, p<0.01, Fig. 7A1). Rats injected with high-dose of U73122 (10 nmol) showed significantly larger number of licks than those injected with the same concentration of U73343 (F(7,50)=3.46, p<0.05, Fig. 7A1) suggesting a role for endogenous PLC in the modulation of anxiety. We further used PLCβ1 KO mice to test the roles of PLC in CCK-induced increases in anxiety-like behavior. Microinjection of CCK reduced significantly the number of licks in WT rats (F(1,24)=3.99, p<0.01, Fig. 7A2) whereas application of CCK failed to change significantly the number of licks in PLCβ1 KO mice (F(1,24)=2.37, p>0.05, Fig. 7A2). In addition, PLCβ1 KO mice licked significantly more than the WT mice (F(1,24)=3.17, p<0.01, Fig. 7A2) suggesting that endogenous PLCβ1 activity exerts control of anxiety. Consistent with our results, deletion of PLCβ1 gene has been found to result in reduction of anxiety (McOmish et al., 2008). Together, these data support a critical role of PLCβ1 in CCK-mediated facilitation of anxiety-like behavior.
Fig. 7.
CCK-mediated increases in anxiety-like behavior require the functions of PLC and PKC. A1, Microinjection of the PLC inhibitor, U73122, at 0.5 nmol did not (** p<0.01), but at 2 nmol and 10 nmol blocked CCK-induced reduction of the number of licks. Application of CCK following microinjection of the inactive analog, U73343 (10 nmol), still significantly reduced the number of licks (** p<0.01). The number of licks for the group of rats injected with 10 nmol U73122 was significantly higher than that of rats injected with 10 nmol U73343 (* p<0.05). A2, Microinjection of CCK significantly reduced the number of licks in WT mice (** p<0.01) but had no effect in PLCβ1 KO mice. The number of licks of WT mice injected with vehicle was significantly lower than that of the PLCβ1 KO mice (** p<0.01). B1, Microinjection of the PKC inhibitor, GF109203X, at 5 pmol did not, but at 25 pmol and 50 pmol blocked CCK-induced reduction of the number of licks. B2, Microinjection of CCK reduced the number of licks in WT mice but did not significantly alter the number of licks in PKCγ KO mice.
Activation of PLC results in diacylglycerol which further activates PKC. We have demonstrated that PKCγ is required for CCK-induced facilitation of glutamate release (Deng et al., 2010) and NMDA receptor function. We therefore tested whether PKC is involved in CCK-induced anxiogenic effect by using the selective and potent PKC inhibitor, GF109203X. The reported effective dose of GF109203X for microinjection in vivo is 25 pmol (Johnston et al., 2002). Microinjection of GF109203X at 5 pmol did not block CCK-mediated reduction of the number of licks (F(5,39)=3.82, p<0.01, Fig. 7B1) whereas CCK-mediated reduction of the number of licks was blocked by microinjection of GF109203X at 25 pmol (F(5,39)=1.38, p>0.05, Fig. 7B1) and 50 pmol (F(5,39)=0.77, p>0.05, Fig. 7B1) demonstrating the requirement of PKC for CCK-mediated facilitation of anxiety-like behavior. Our results are consistent with the notion that PKC is an important target for emotional control (Bowers et al., 2000; DiazGranados and Zarate, 2008; Szabo et al., 2009; Varadarajulu et al., 2011).
We also tested the roles of PKC by using PKCγ KO mice. Microinjection of CCK significantly reduced the number of licks in WT mice (F(1,30)=3.61, p<0.01, Fig. 7B2) but had no significant effect on the number of licks in PKCγ KO mice (F(1,30)=1.06, p>0.05, Fig. 7B2). Consistently, PKCγ (Bowers et al., 2000) and PKCε (Van Kolen et al., 2008) KO mice exhibit decreased anxiety-like behavior.
DISCUSSION
Whereas CCK facilitates glutamate release at the synapses formed between the perforant path and dentate gyrus granule cells, its potential effects on NMDA receptors in the granule cells have not been determined. We have shown for the first time that CCK exerts a larger scale of facilitation of glutamatergic transmission assessed by measuring NMDA receptor-mediated EPSCs by up-regulating NMDA receptor function in the granule cells. CCK-mediated facilitation of NMDA receptor function requires the functions of CCK-2 receptors, PLC and PKC. At the level of single channels, CCK increases PO and mean open time, decreases mean close time but has no effects on the conductance of NMDA channels. Because CCK-mediated augmentations of both glutamate release and NMDA receptor function are mediated via PLC/PKC pathway and up-regulation of glutamatergic function exacerbates anxiety, we have further explored the roles of these signaling molecules in CCK-induced facilitation of anxiety-like behavior. To our knowledge, this is the first study demonstrating that two intracellular signaling molecules, PLC and PKC, are essential for CCK-mediated exacerbation of anxiety-like behavior although CCK has been known to exert anxiogenic effects for decades.
High density of CCK-binding sites has been detected in the entorhinal cortex and the molecular layer of the dentate gyrus (Kohler and Chan-Palay, 1988; Kritzer et al., 1988). Because the perforant pathway encompasses the axons of entorhinal neurons which form synapses with the dendrites of the granule cells in the molecular layer of the dentate gyrus, the localization of CCK receptors in the presynaptic terminals suggests a presynaptic function for CCK at the perforant path-granule cell synapses. Consistent with the anatomical distribution of CCK receptors, we have shown previously that CCK facilitates glutamate release at the perforant path-granule cell synapses (Deng et al., 2010). However, high-density of CCK receptors has also been detected in the granule cell layer of the dentate gyrus (Kritzer et al., 1988) suggesting that CCK may also exert postsynaptic effects at this synapse type. In line with the expression of CCK receptors in dentate granule cells, we found that CCK facilitates glutamatergic transmission to a larger scale if assessed by measuring NMDA instead of AMPA EPSCs. This result prompted us to examine further the effects of CCK on NMDA receptors. To avoid the contaminant effect arisen from CCK-induced increases in presynaptic glutamate release, we measured NMDA currents evoked by exogenous application of NMDA from acutely isolated granule cells. In this preparation, we demonstrate that CCK facilitates NMDA currents via activation of CCK-2 receptors. Consistent with our results, another neuropeptide, substance P, also enhances NMDA receptor function in the dentate gyrus granule cells of the hippocampus (Lieberman and Mody, 1998) suggesting that the NMDA receptors in dentate gyrus granule cells are subjected to functional up-regulation by neuropeptides.
Our results indicate that both PLCβ1 and PKCγ are involved in CCK-mediated enhancement of NMDA currents. Consistent with our results, NMDA currents are up-regulated by the PLC/PKC signals in response to a series of G protein-coupled receptors including the group I metabotropic glutamate (Skeberdis et al., 2001), corticotrophin-releasing factor (CRF) (Ungless et al., 2003), somatostatin (Pittaluga et al., 2005), orexin A (Borgland et al., 2006), muscarinic (Lu et al., 1999) and pituitary adenylate cyclase-activating peptide (PACAP) (Macdonald et al., 2005; Yang et al., 2010) receptors. It is interesting to note that activation of these receptors also generates anxiogenic effects. For example, activation of group I metabotropic glutamate receptors in the dorsolateral periaqueductal gray facilitates defensive responses and anxiety-like behavior (Lima et al., 2008). It is well established that CRF is involved in stress and anxiety (Binder and Nemeroff, 2009; Hauger et al., 2009). Microinjection of orexin in the paraventricular nucleus of the thalamus region elicits anxiety-like response (Li et al., 2010). Genetic deletion of muscarinic M4 receptors produces anxiolytic effects (Degroot and Nomikos, 2006). Mice deficient in PACAP (Hashimoto et al., 2001) and PACAP type 1 receptors (Otto et al., 2001) exhibit less anxiety. These results together support the idea that PLC/PKC signals modulate anxiety (see below).
Neuronal NMDA receptors include the synaptic and extrasynaptic populations and recent studies suggest that activation of these two pools of NMDA receptors exerts distinct functions (Gladding and Raymond, 2011; Hardingham and Bading, 2010). Our result that CCK facilitates NMDA EPSCs evoked by stimulation of the perforant path suggests that CCK targets synaptic NMDA receptors. Moreover, NMDA receptors on the acutely dissociated granule cells should be extrasynaptic because almost all the spines on the dendrites of the neurons where synaptic NMDA receptors reside are lost after dissociation. Exogenous application of NMDA still enhances NMDA currents recorded from the dissociated granule cells thereby supporting that CCK targets the extrasynaptic NMDA receptors as well. There is compelling evidence indicating that activation of PKC enhances NMDA receptor function via activation of Src tyrosine kinase (Grosshans and Browning, 2001; Lu et al., 1999; Macdonald et al., 2005; MacDonald et al., 2001) or increases membrane trafficking of NMDA receptors (Carroll and Zukin, 2002; Ferreira et al., 2011; Fong et al., 2002; Lan et al., 2001a; Lan et al., 2001b; Lau et al., 2010). Further studies will determine the roles of Src and membrane trafficking in CCK-mediated augmentation of NMDA currents in dentate gyrus granule cells.
Whereas our results demonstrate that CCK enhances NMDA currents recorded from dentate granule cells, there is a study showing that application of CCK to cultured hippocampal neurons induces < 20% of inhibition in a non-concentration and non-competition manner (Wei et al., 2009). However, this study did not test whether vehicles used to dissolve CCK mediate the response because CCK induces the same level of inhibition from 0.01 to 1 μM suggesting a non-biological effect of CCK receptors. Furthermore, it is unknown whether CCK receptors are involved in CCK-mediated inhibition of NMDA currents in cultured hippocampal neurons. Without sufficient information, it is really difficult to compare our results with the effects of CCK on NMDA currents in cultured hippocampal neurons. Another factor contributing to the difference is the density of CCK receptors in cultured hippocampal neurons because CCK receptors are most concentrated in the dentate granule cell layer with only moderate to light expression in other regions including CA3, CA2 and CA1 regions (Kritzer et al., 1988) and cultured hippocampal neurons likely include many hippocampal cells other than granule cells.
Whereas CCK has long been known to generate anxiogenic effects in both humans and animal models (Bradwejn and Koszycki, 1994; Bradwejn et al., 1990; Crawley and Corwin, 1994; Noble and Roques, 1999; Rehfeld, 2000; Rodgers and Johnson, 1995), the underlying mechanisms are elusive. Compelling evidence indicates that elevation of glutamatergic function increases anxiety (Bergink et al., 2004; Gorman, 2003; Kent et al., 2002). CCK exerts powerful up-regulation of glutamatergic function in the hippocampus. First, CCK increases glutamate concentration in the perfusate of hippocampal slices (Migaud et al., 1994) and hippocampal synaptosomes (Breukel et al., 1997). Second, CCK facilitates presynaptic glutamate release in the hippocampus especially at the synapses formed between the perforant pathway and dentate gyrus granule cells (Deng et al., 2010). Third, the present study indicates that CCK facilitates NMDA receptor functions in the dentate gyrus granule cells. Because CCK-mediated facilitations of both glutamate release and NMDA receptor function require the functions of PLC and PKC, we focus on the roles of this pathway in CCK-mediated increases in anxiety-like behavior. With both pharmacological as well as genetic approaches, we have demonstrated that microinjection of CCK into the dentate gyrus of the hippocampal formation concentration-dependently facilitates the level of anxiety-like behavior via activation of CCK-2 receptors, PLCβ1 and PKCγ. Consistent with our results, deletion of the genes for PLCβ1 (McOmish et al., 2008), PKCγ (Bowers et al., 2000) and PKCε (Van Kolen et al., 2008) has been shown to inhibit anxiety. Furthermore, there is increasing evidence demonstrating that the activity of PKC exerts close control for emotional responses (Bowers et al., 2000; DiazGranados and Zarate, 2008; Szabo et al., 2009; Varadarajulu et al., 2011).
In addition to facilitating glutamate release, CCK also transiently increases (Deng and Lei, 2006; Földy et al., 2007; Karson et al., 2008; Miller et al., 1997) followed by a persistent reduction in GABA release (Deng and Lei, 2006). Theoretically, CCK-mediated transient increase in GABA release should result in anxiolytic effect whereas CCK-induced persistent reduction in GABA release should lead to anxiogenesis. However, we have further shown that CCK-mediated modulation of GABAergic transmission is overwhelmed by its strong facilitation of glutamatergic transmission when both GABAergic and glutamatergic functions are active (Deng et al., 2010). Furthermore, we have shown previously that CCK-mediated modulation of GABA release is independent of PLC and PKC (Deng and Lei, 2006) whereas the functions of PLC and PKC are required for CCK-induced facilitations of both glutamate release and NMDA receptor function. These results together indicate that it is unlikely that CCK enhances anxiety-like behavior via modulation of GABAergic transmission. In agreement with our results, injection of bicuculline intracerebroventricularly (Biro et al., 1997) or into the hippocampus CA1 region (Rezayat et al., 2005) failed to affect CCK-induced anxiogenesis although there are conflict results as to whether application of bicuculline by itself alters the level of anxiety (Dalvi and Rodgers, 1996; Sanders and Shekhar, 1995; Zarrindast et al., 2001) possibly depending on the injection sites (Rezayat et al., 2005). Collectively, our results demonstrate that activation of CCK-2 receptors increase the functions of PLC and PKC to enhance anxiety-like behavior.
Acknowledgments
Grant Sponsor: NIH
Grant Number: R01MH082881 (S.L.)
REFERENCES
- Beinfeld MC. An introduction to neuronal cholecystokinin. Peptides. 2001;22(8):1197–200. doi: 10.1016/s0196-9781(01)00442-9. [DOI] [PubMed] [Google Scholar]
- Beinfeld MC, Meyer DK, Eskay RL, Jensen RT, Brownstein MJ. The distribution of cholecystokinin immunoreactivity in the central nervous system of the rat as determined by radioimmunoassay. Brain Res. 1981;212(1):51–7. doi: 10.1016/0006-8993(81)90031-7. [DOI] [PubMed] [Google Scholar]
- Bergink V, van Megen HJ, Westenberg HG. Glutamate and anxiety. Eur Neuropsychopharmacol. 2004;14(3):175–83. doi: 10.1016/S0924-977X(03)00100-7. [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ, Guimaraes FS, Zangrossi H., Jr. Lack of interaction between NMDA and cholecystokinin-2 receptor-mediated neurotransmission in the dorsolateral periaqueductal gray in the regulation of rat defensive behaviors. Life Sci. 2006;79(23):2238–44. doi: 10.1016/j.lfs.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ, Zangrossi H., Jr. Involvement of dorsolateral periaqueductal gray cholecystokinin-2 receptors in the regulation of a panic-related behavior in rats. Brain Res. 2005;1059(1):46–51. doi: 10.1016/j.brainres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry. 2009;15(6):574–88. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro E, Penke B, Telegdy G. Role of different neurotransmitter systems in the cholecystokinin octapeptide-induced anxiogenic response in rats. Neuropeptides. 1997;31(3):281–5. doi: 10.1016/s0143-4179(97)90060-3. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49(4):589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Bowers BJ, Collins AC, Tritto T, Wehner JM. Mice lacking PKC gamma exhibit decreased anxiety. Behav Genet. 2000;30(2):111–21. doi: 10.1023/a:1001951104208. [DOI] [PubMed] [Google Scholar]
- Bradwejn J, Koszycki D. The cholecystokinin hypothesis of anxiety and panic disorder. Ann N Y Acad Sci. 1994;713:273–82. doi: 10.1111/j.1749-6632.1994.tb44075.x. [DOI] [PubMed] [Google Scholar]
- Bradwejn J, Koszycki D, Meterissian G. Cholecystokinin-tetrapeptide induces panic attacks in patients with panic disorder. Can J Psychiatry. 1990;35(1):83–5. doi: 10.1177/070674379003500115. [DOI] [PubMed] [Google Scholar]
- Breukel AI, Lopes da Silva FH, Ghijsen WE. Cholecystokinin (CCK-8) modulates vesicular release of excitatory amino acids in rat hippocampal nerve endings. Neurosci Lett. 1997;234(1):67–70. doi: 10.1016/s0304-3940(97)00678-2. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25(11):571–7. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- Chen Q, Nakajima A, Meacham C, Tang YP. Elevated cholecystokininergic tone constitutes an important molecular/neuronal mechanism for the expression of anxiety in the mouse. Proc Natl Acad Sci U S A. 2006;103(10):3881–6. doi: 10.1073/pnas.0505407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JY, Chang WT, Cherng CG, Kao GS, Yu L. Repeated co-administrations of alcohol- and methamphetamine-produced anxiogenic effect could be associated with the neurotoxicity in the dentate gyrus. J Neural Transm. 2011 doi: 10.1007/s00702-011-0645-2. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Kotler M. Inhibition of anxiety in rats by antisense to cholecystokinin precursor protein. Biol Psychiatry. 1998;44(9):915–7. doi: 10.1016/s0006-3223(98)00010-9. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Corwin RL. Biological actions of cholecystokinin. Peptides. 1994;15(4):731–55. doi: 10.1016/0196-9781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Dalvi A, Rodgers RJ. GABAergic influences on plus-maze behaviour in mice. Psychopharmacology (Berl) 1996;128(4):380–97. doi: 10.1007/s002130050148. [DOI] [PubMed] [Google Scholar]
- Degroot A, Nomikos GG. Genetic deletion of muscarinic M4 receptors is anxiolytic in the shock-probe burying model. Eur J Pharmacol. 2006;531(1-3):183–6. doi: 10.1016/j.ejphar.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Dekeyne A, Brocco M, Adhumeau A, Gobert A, Millan MJ. The selective serotonin (5-HT)1A receptor ligand, S15535, displays anxiolytic-like effects in the social interaction and Vogel models and suppresses dialysate levels of 5-HT in the dorsal hippocampus of freely-moving rats. A comparison with other anxiolytic agents. Psychopharmacology (Berl) 2000;152(1):55–66. doi: 10.1007/s002130000449. [DOI] [PubMed] [Google Scholar]
- Deng PY, Lei S. Bidirectional modulation of GABAergic transmission by cholecystokinin in hippocampal dentate gyrus granule cells of juvenile rats. J Physiol. 2006;572(Pt 2):425–42. doi: 10.1113/jphysiol.2005.104463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Lei S. Long-term depression in identified stellate neurons of juvenile rat entorhinal cortex. J Neurophysiol. 2007;97(1):727–37. doi: 10.1152/jn.01089.2006. [DOI] [PubMed] [Google Scholar]
- Deng PY, Porter JE, Shin HS, Lei S. Thyrotropin-releasing hormone increases GABA release in rat hippocampus. J Physiol. 2006;577(Pt 2):497–511. doi: 10.1113/jphysiol.2006.118141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Xiao Z, Jha A, Ramonet D, Matsui T, Leitges M, Shin HS, Porter JE, Geiger JD, Lei S. Cholecystokinin facilitates glutamate release by increasing the number of readily releasable vesicles and releasing probability. J Neurosci. 2010;30(15):5136–48. doi: 10.1523/JNEUROSCI.5711-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Xiao Z, Yang C, Rojanathammanee L, Grisanti L, Watt J, Geiger JD, Liu R, Porter JE, Lei S. GABA(B) receptor activation inhibits neuronal excitability and spatial learning in the entorhinal cortex by activating TREK-2 K+ channels. Neuron. 2009;63(2):230–43. doi: 10.1016/j.neuron.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N, Zarate CA., Jr. A review of the preclinical and clinical evidence for protein kinase C as a target for drug development for bipolar disorder. Curr Psychiatry Rep. 2008;10(6):510–9. doi: 10.1007/s11920-008-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- Ferreira JS, Rooyakkers A, She K, Ribeiro L, Carvalho AL, Craig AM. Activity and Protein Kinase C Regulate Synaptic Accumulation of N-Methyl-D-aspartate (NMDA) Receptors Independently of GluN1 Splice Variant. J Biol Chem. 2011;286(32):28331–42. doi: 10.1074/jbc.M111.222539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink H, Rex A, Voits M, Voigt JP. Major biological actions of CCK--a critical evaluation of research findings. Exp Brain Res. 1998;123(1-2):77–83. doi: 10.1007/s002210050546. [DOI] [PubMed] [Google Scholar]
- Földy C, Lee SY, Szabadics J, Neu A, Soltesz I. Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat Neurosci. 2007;10(9):1128–30. doi: 10.1038/nn1952. [DOI] [PubMed] [Google Scholar]
- Fong DK, Rao A, Crump FT, Craig AM. Rapid synaptic remodeling by protein kinase C: reciprocal translocation of NMDA receptors and calcium/calmodulin-dependent kinase II. J Neurosci. 2002;22(6):2153–64. doi: 10.1523/JNEUROSCI.22-06-02153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladding CM, Raymond LA. Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol Cell Neurosci. 2011 doi: 10.1016/j.mcn.2011.05.001. (in press) [DOI] [PubMed] [Google Scholar]
- Gorman JM. New molecular targets for antianxiety interventions. J Clin Psychiatry. 2003;64(Suppl 3):28–35. [PubMed] [Google Scholar]
- Greenwood RS, Godar SE, Reaves TA, Jr., Hayward JN. Cholecystokinin in hippocampal pathways. J Comp Neurol. 1981;203(3):335–50. doi: 10.1002/cne.902030303. [DOI] [PubMed] [Google Scholar]
- Grosshans DR, Browning MD. Protein kinase C activation induces tyrosine phosphorylation of the NR2A and NR2B subunits of the NMDA receptor. J Neurochem. 2001;76(3):737–44. doi: 10.1046/j.1471-4159.2001.00034.x. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11(10):682–96. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Tanaka K, Mori W, Hirose M, Matsuda T, Sakaue M, Miyazaki J, Niwa H, Tashiro F. Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP). Proc Natl Acad Sci U S A. 2001;98(23):13355–60. doi: 10.1073/pnas.231094498. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120–43. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8(10):1319–28. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- Hill DR, Campbell NJ, Shaw TM, Woodruff GN. Autoradiographic localization and biochemical characterization of peripheral type CCK receptors in rat CNS using highly selective nonpeptide CCK antagonists. J Neurosci. 1987;7(9):2967–76. doi: 10.1523/JNEUROSCI.07-09-02967.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DR, Shaw TM, Graham W, Woodruff GN. Autoradiographical detection of cholecystokinin-A receptors in primate brain using 125I-Bolton Hunter CCK-8 and 3H-MK-329. J Neurosci. 1990;10(4):1070–81. doi: 10.1523/JNEUROSCI.10-04-01070.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hughes J, Boden P, Costall B, Domeney A, Kelly E, Horwell DC, Hunter JC, Pinnock RD, Woodruff GN. Development of a class of selective cholecystokinin type B receptor antagonists having potent anxiolytic activity. Proc Natl Acad Sci U S A. 1990;87(17):6728–32. doi: 10.1073/pnas.87.17.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AR, Seckl JR, Dutia MB. Role of the flocculus in mediating vestibular nucleus neuron plasticity during vestibular compensation in the rat. J Physiol. 2002;545(Pt 3):903–11. doi: 10.1113/jphysiol.2002.024281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karson MA, Whittington KC, Alger BE. Cholecystokinin inhibits endocannabinoid-sensitive hippocampal IPSPs and stimulates others. Neuropharmacology. 2008;54(1):117–28. doi: 10.1016/j.neuropharm.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent JM, Mathew SJ, Gorman JM. Molecular targets in the treatment of anxiety. Biol Psychiatry. 2002;52(10):1008–30. doi: 10.1016/s0006-3223(02)01672-4. [DOI] [PubMed] [Google Scholar]
- Kim D, Jun KS, Lee SB, Kang NG, Min DS, Kim YH, Ryu SH, Suh PG, Shin HS. Phospholipase C isozymes selectively couple to specific neurotransmitter receptors. Nature. 1997;389(6648):290–3. doi: 10.1038/38508. [DOI] [PubMed] [Google Scholar]
- Kohler C, Chan-Palay V. The distribution of cholecystokinin-like immunoreactive neurons and nerve terminals in the retrohippocampal region in the rat and guinea pig. J Comp Neurol. 1982;210(2):136–46. doi: 10.1002/cne.902100204. [DOI] [PubMed] [Google Scholar]
- Kohler C, Chan-Palay V. Cholecystokinin-octapeptide (CCK-8) receptors in the hippocampal region: a comparative in vitro autoradiographic study in the rat, monkey and the postmortem human brain. Neurosci Lett. 1988;90(1-2):51–6. doi: 10.1016/0304-3940(88)90785-9. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Innis RB, Goldman-Rakic PS. Regional distribution of cholecystokinin receptors in macaque medial temporal lobe determined by in vitro receptor autoradiography. J Comp Neurol. 1988;276(2):219–30. doi: 10.1002/cne.902760206. [DOI] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MV, Zukin RS. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci. 2001a;4(4):382–90. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Zheng X, Bennett MV, Zukin RS. Activation of metabotropic glutamate receptor 1 accelerates NMDA receptor trafficking. J Neurosci. 2001b;21(16):6058–68. doi: 10.1523/JNEUROSCI.21-16-06058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Takayasu Y, Rodenas-Ruano A, Paternain AV, Lerma J, Bennett MV, Zukin RS. SNAP-25 is a target of protein kinase C phosphorylation critical to NMDA receptor trafficking. J Neurosci. 2010;30(1):242–54. doi: 10.1523/JNEUROSCI.4933-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, Czerwinska E, Czerwinski W, Walsh MP, MacDonald JF. Regulation of NMDA receptor activity by F-actin and myosin light chain kinase. J Neurosci. 2001;21(21):8464–72. doi: 10.1523/JNEUROSCI.21-21-08464.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, Deng PY, Porter JE, Shin HS. Adrenergic facilitation of GABAergic transmission in rat entorhinal cortex. J Neurophysiol. 2007;98(5):2868–77. doi: 10.1152/jn.00679.2007. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ. Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology (Berl) 2010;212(2):251–65. doi: 10.1007/s00213-010-1948-y. [DOI] [PubMed] [Google Scholar]
- Lieberman DN, Mody I. Substance P enhances NMDA channel function in hippocampal dentate gyrus granule cells. J Neurophysiol. 1998;80(1):113–9. doi: 10.1152/jn.1998.80.1.113. [DOI] [PubMed] [Google Scholar]
- Lima VC, Molchanov ML, Aguiar DC, Campos AC, Guimaraes FS. Modulation of defensive responses and anxiety-like behaviors by group I metabotropic glutamate receptors located in the dorsolateral periaqueductal gray. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(1):178–85. doi: 10.1016/j.pnpbp.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Lotstra F, Vanderhaeghen JJ. High concentration of cholecystokinin neurons in the newborn human entorhinal cortex. Neurosci Lett. 1987;80(2):191–6. doi: 10.1016/0304-3940(87)90652-5. [DOI] [PubMed] [Google Scholar]
- Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD, MacDonald JF. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci. 1999;2(4):331–8. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- Macdonald DS, Weerapura M, Beazely MA, Martin L, Czerwinski W, Roder JC, Orser BA, MacDonald JF. Modulation of NMDA receptors by pituitary adenylate cyclase activating peptide in CA1 neurons requires G alpha q, protein kinase C, and activation of Src. J Neurosci. 2005;25(49):11374–84. doi: 10.1523/JNEUROSCI.3871-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JF, Kotecha SA, Lu WY, Jackson MF. Convergence of PKC-dependent kinase signal cascades on NMDA receptors. Curr Drug Targets. 2001;2(3):299–312. doi: 10.2174/1389450013348452. [DOI] [PubMed] [Google Scholar]
- McOmish CE, Burrows EL, Howard M, Hannan AJ. PLC-beta1 knockout mice as a model of disrupted cortical development and plasticity: behavioral endophenotypes and dysregulation of RGS4 gene expression. Hippocampus. 2008;18(8):824–34. doi: 10.1002/hipo.20443. [DOI] [PubMed] [Google Scholar]
- Migaud M, Roques BP, Durieux C. Effects of cholecystokinin octapeptide and BC 264, a potent and selective CCK-B agonist on aspartate and glutamate release from rat hippocampal slices. Neuropharmacology. 1994;33(6):737–43. doi: 10.1016/0028-3908(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Miller KK, Hoffer A, Svoboda KR, Lupica CR. Cholecystokinin increases GABA release by inhibiting a resting K+ conductance in hippocampal interneurons. J Neurosci. 1997;17(13):4994–5003. doi: 10.1523/JNEUROSCI.17-13-04994.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TH, Robinson PH, Goldrich MS, McHugh PR. Two brain cholecystokinin receptors: implications for behavioral actions. Brain Res. 1986;362(1):175–9. doi: 10.1016/0006-8993(86)91413-7. [DOI] [PubMed] [Google Scholar]
- Nagata A, Ito M, Iwata N, Kuno J, Takano H, Minowa O, Chihara K, Matsui T, Noda T. G protein-coupled cholecystokinin-B/gastrin receptors are responsible for physiological cell growth of the stomach mucosa in vivo. Proc Natl Acad Sci U S A. 1996;93(21):11825–30. doi: 10.1073/pnas.93.21.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble F, Roques BP. CCK-B receptor: chemistry, molecular biology, biochemistry and pharmacology. Prog Neurobiol. 1999;58(4):349–79. doi: 10.1016/s0301-0082(98)00090-2. [DOI] [PubMed] [Google Scholar]
- Otto C, Martin M, Wolfer DP, Lipp HP, Maldonado R, Schutz G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res Mol Brain Res. 2001;92(1-2):78–84. doi: 10.1016/s0169-328x(01)00153-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 2009. [Google Scholar]
- Pittaluga A, Feligioni M, Longordo F, Arvigo M, Raiteri M. Somatostatin-induced activation and up-regulation of N-methyl-D-aspartate receptor function: mediation through calmodulin-dependent protein kinase II, phospholipase C, protein kinase C, and tyrosine kinase in hippocampal noradrenergic nerve endings. J Pharmacol Exp Ther. 2005;313(1):242–9. doi: 10.1124/jpet.104.079590. [DOI] [PubMed] [Google Scholar]
- Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci. 2008;29(10):493–8. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Rehfeld JF. Cholecystokinin and panic disorder--three unsettled questions. Regul Pept. 2000;93(1-3):79–83. doi: 10.1016/s0167-0115(00)00179-8. [DOI] [PubMed] [Google Scholar]
- Rezayat M, Roohbakhsh A, Zarrindast MR, Massoudi R, Djahanguiri B. Cholecystokinin and GABA interaction in the dorsal hippocampus of rats in the elevated plus-maze test of anxiety. Physiol Behav. 2005;84(5):775–82. doi: 10.1016/j.physbeh.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Johnson NJ. Cholecystokinin and anxiety: promises and pitfalls. Crit Rev Neurobiol. 1995;9(4):345–69. [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52(4):701–6. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Sebret A, Lena I, Crete D, Matsui T, Roques BP, Dauge V. Rat hippocampal neurons are critically involved in physiological improvement of memory processes induced by cholecystokinin-B receptor stimulation. J Neurosci. 1999;19(16):7230–7. doi: 10.1523/JNEUROSCI.19-16-07230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg PH. The TINS/TiPS Lecture. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993;16(9):359–65. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- Shigeyoshi Y, Okamura H, Inatomi T, Matsui T, Ito M, Kaji H, Abe H, Nakata H, Chiba T, Chihara K. Distribution of mRNA for CCK-B receptor in the brain of Mastomys natalensis: abundant expression in telencephalic neurons. Brain Res. 1994;640(1-2):81–92. doi: 10.1016/0006-8993(94)91859-7. [DOI] [PubMed] [Google Scholar]
- Singh L, Lewis AS, Field MJ, Hughes J, Woodruff GN. Evidence for an involvement of the brain cholecystokinin B receptor in anxiety. Proc Natl Acad Sci U S A. 1991;88(4):1130–3. doi: 10.1073/pnas.88.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeberdis VA, Lan J, Opitz T, Zheng X, Bennett MV, Zukin RS. mGluR1-mediated potentiation of NMDA receptors involves a rise in intracellular calcium and activation of protein kinase C. Neuropharmacology. 2001;40(7):856–65. doi: 10.1016/s0028-3908(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Spolidorio PC, Echeverry MB, Iyomasa M, Guimaraes FS, Del Bel EA. Anxiolytic effects induced by inhibition of the nitric oxide-cGMP pathway in the rat dorsal hippocampus. Psychopharmacology (Berl) 2007;195(2):183–92. doi: 10.1007/s00213-007-0890-0. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Machado-Vieira R, Yuan P, Wang Y, Wei Y, Falke C, Cirelli C, Tononi G, Manji HK, Du J. Glutamate receptors as targets of protein kinase C in the pathophysiology and treatment of animal models of mania. Neuropharmacology. 2009;56(1):47–55. doi: 10.1016/j.neuropharm.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsenis T, Ma XH, Lo Iacono L, Beck SG, Gross C. Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat Neurosci. 2007;10(7):896–902. doi: 10.1038/nn1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39(3):401–7. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Van der Zee EA, Palm IF, O'Connor M, Maizels ET, Hunzicker-Dunn M, Disterhoft JF. Aging-related alterations in the distribution of Ca2+-dependent PKC isoforms in rabbit hippocampus. Hippocampus. 2004;14(7):849–60. doi: 10.1002/hipo.20000. [DOI] [PubMed] [Google Scholar]
- Van Dijk A, Richards JG, Trzeciak A, Gillessen D, Mohler H. Cholecystokinin receptors: biochemical demonstration and autoradiographical localization in rat brain and pancreas using [3H] cholecystokinin 8 as radioligand. J Neurosci. 1984;4(4):1021–33. doi: 10.1523/JNEUROSCI.04-04-01021.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kolen K, Pullan S, Neefs JM, Dautzenberg FM. Nociceptive and behavioural sensitisation by protein kinase Cepsilon signalling in the CNS. J Neurochem. 2008;104(1):1–13. doi: 10.1111/j.1471-4159.2007.04986.x. [DOI] [PubMed] [Google Scholar]
- Varadarajulu J, Lebar M, Krishnamoorthy G, Habelt S, Lu J, Bernard Weinstein I, Li H, Holsboer F, Turck CW, Touma C. Increased anxiety-related behaviour in Hint1 knockout mice. Behav Brain Res. 2011;220(2):305–11. doi: 10.1016/j.bbr.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Wang H, Wong PT, Spiess J, Zhu YZ. Cholecystokinin-2 (CCK2) receptor-mediated anxiety-like behaviors in rats. Neurosci Biobehav Rev. 2005;29(8):1361–73. doi: 10.1016/j.neubiorev.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Wank SA. Cholecystokinin receptors. Am J Physiol. 1995;269(5 Pt 1):G628–46. doi: 10.1152/ajpgi.1995.269.5.G628. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Nakamura M, Sato K, Kano M, Simon MI, Inoue Y. Patterns of expression for the mRNA corresponding to the four isoforms of phospholipase Cbeta in mouse brain. Eur J Neurosci. 1998;10(6):2016–25. doi: 10.1046/j.1460-9568.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- Wei X, Zhang Z, Zhao L, Si J. CCK-8S inhibited the NMDA-activated current of cultured hippocampal neuron under normal and ethanol exposure conditions. Neurosci Lett. 2009;449(1):34–7. doi: 10.1016/j.neulet.2008.10.078. [DOI] [PubMed] [Google Scholar]
- Yang K, Lei G, Jackson MF, Macdonald JF. The involvement of PACAP/VIP system in the synaptic transmission in the hippocampus. J Mol Neurosci. 2010;42(3):319–26. doi: 10.1007/s12031-010-9372-7. [DOI] [PubMed] [Google Scholar]
- Zarrindast M, Rostami P, Sadeghi-Hariri M. GABA(A) but not GABA(B) receptor stimulation induces antianxiety profile in rats. Pharmacol Biochem Behav. 2001;69(1-2):9–15. doi: 10.1016/s0091-3057(01)00518-4. [DOI] [PubMed] [Google Scholar]