Abstract
Background and Purpose
Argatroban is a direct thrombin inhibitor that safely augments recanalization achieved by tPA in animal stroke models. The Argatroban tPA Stroke Study was an open-label, pilot safety study of tPA plus Argatroban in patients with ischemic stroke due to proximal intracranial occlusion.
Methods
During standard dose IV tPA, a 100μg/kg bolus of Argatroban and infusion for 48 hours was adjusted to a target partial thromboplastin time of 1.75 times baseline. The primary outcome was incidence of significant intracerebral hemorrhage defined as either symptomatic intracerebral hemorrhage (sICH) or parenchymal hemorrhage-type 2 (PH-2). Recanalization was measured at 2 and 24 hours by transcranial Doppler (TCD) or CT angiography.
Results
Sixty-five patients were enrolled (45% men, mean age 63±14 years, median NIHSS = 13). The median (IQR) time tPA to Argatroban bolus was 51 (38, 60) minutes. Target anticoagulation was reached at a median (IQR) of 3 (2, 7) hours. Significant intracerebral hemorrhage occurred in 4 patients (6.2%, 95% CI 1.7–15.0). Of these, 3 were symptomatic (4.6%, 95% CI 0.9–12.9). Seven patients (10%) died in the first 7 days. Within the 2 hour monitoring period, TCD recanalization (n=47) occurred in 29 (61%) patients: complete in 19 (40%) and partial in another 10 (21%).
Conclusion
The combination of Argatroban and IV tPA is potentially safe in patients with moderate neurological deficits due to proximal intracranial arterial occlusions, and may produce more complete recanalization than tPA alone. Continued evaluation of this treatment combination is warranted.
Keywords: Anticoagulation, acute stroke, thrombolysis, Argatroban, thrombin-inhibition
INTRODUCTION
The thrombin inhibitor Argatroban (GlaxoSmithKline, Philadelphia, PA), selectively inhibits free and clot-associated thrombin.1, 2 Safety has been demonstrated with and without thrombolytics or with aspirin in patients with acute myocardial infarction.3–5 In a randomized trial of Argatroban versus heparin in combination with intravenous thrombolysis for acute myocardial infarction, complete coronary reperfusion was significantly more frequent with Argatroban compared to heparin.3 In animal stroke models, Argatroban safely augments the benefit of recombinant tissue plasminogen activator (tPA) by improving microcirculatory flow, increasing speed and completeness of recanalization, and preventing reocclusion.6–10 Argatroban monotherapy (in a double-blind, randomized Phase II trial) in 60 stroke patients within 48 hours of onset improved neurological outcome compared with placebo.11 The Argatroban Anticoagulation in Patients with Acute Ischemic Stroke (ARGIS-1) study showed that Argatroban (mean doses of 1.2 and 2.7 μg/kg per minute) monotherapy given within 12 hours of ischemic stroke was safe but no clinical benefit was observed.12
Fifty-seven percent of patients in the National Institute of Neurological Disorders and Stroke (NINDS) rt-PA Stroke Study and 58% in the Second European-Australian Cooperative Acute Stroke Study (ECASS-II) failed to show a favorable clinical response to IV rt-PA monotherapy.13–15 Benefit of tPA in acute stroke is linked to the speed and degree of clot lysis and artery recanalization.16–18 However, only 20% to 30% of patients will have complete recanalization on transcranial Doppler imaging (TCD) within 2 hours of IV-tPA therapy, 60% will have only partial recanalization, and 34% of those with any recanalization will experience reocclusion.19, 20 Because of its short half-life, allowing careful titration of the anticoagulant effect, we hypothesized that Argatroban might be safely added to full-dose IV-tPA. Further, we hypothesized that the addition of Argatroban to tPA would improve recanalization rates. The first 15 of the current study (total = 65) patients were published previously21 and the current manuscript focuses on the whole cohort.
PURPOSE
The primary purpose of this study was to assess the safety of combined Argatroban and tPA in ischemic stroke as measured by the incidence of significant ICH. The secondary objective was to evaluate drug activity by determining the speed and completeness of arterial recanalization and reocclusion.
METHODS
DESIGN
The Argatroban tPA Stroke Study was a prospective multicenter, single-arm, open-label, uncontrolled study, which included careful monitoring by an independent physician medical monitor. Because this was the first ever exposure of patients with acute stroke to the combination of tPA and Argatroban, a prespecified group of 15 patients was treated in phase 1 to obtain a preliminary assessment of safety. If safe, then continued enrollment would occur. Safety was defined as a rate of sICH or PH-2 intracranial hemorrhage not exceeding 10%. We hypothesized that a hemorrhage rate of 10% might be acceptable only in the setting of significant increases in arterial recanalization which is highly associated with improved clinical outcomes.
PATIENT SELECTION
Inclusion criteria were: (1) age 18 to 85; (2) symptom onset within 3 hours (beginning in September 2009, after 27 enrollments, patients receiving tPA up to 4.5 hours were included according to each study site’s treatment protocol)22; (3) clot causing complete or partial occlusion by TCD (Thrombolysis in Brain Ischemia [TIBI] flow grades of 0, 1, 2, or 3) or CTA (Thrombolysis in Myocardial Infarction [TIMI] flow grade of 0 or 1) before Argatroban infusion in the middle cerebral artery (MCA, M1 [45- to 65-mm depth] or M2 [<45-mm]), terminal internal carotid artery, posterior cerebral artery (PCA, P1 or proximal P2), distal vertebral or basilar artery. In all cases, the depth of worst TIBI signals on TCD findings were used as the target occlusion; and (4) being eligible by NINDS criteria for IV-tPA treatment.
Exclusions were (1) National Institutes of Health Stroke Scale (NIHSS) level-of-consciousness score ≥ 2; (2) baseline NIHSS score < 5; (3) baseline NIHSS score >17 (modified to >15 after the first 15 patients) for right hemisphere and >22 (modified to >20) for left hemisphere strokes; (4) preexisting modified Rankin Scale (mRS) score ≥ 2; (5) history of ICH or significant bleeding within 3 months; (6) hypoattenuation ≥ ½ of the MCA territory on CT; (7) coagulopathy or clinically significant bleeding; (8) INR > 1.5; (9) major surgery within 6 weeks or anticipated surgery within the next 7 days; (10) significant liver disease; (11) brain tumor; (12) severe organic brain disorder; (13) stroke, myocardial infarction, pericarditis, intracranial surgery, or significant head trauma within 3 months; (14) pre-stroke life expectancy <3 months; (15) need for concomitant use of anticoagulants other than Argatroban.
After informed consent from the patient, family, or legal representative and before starting Argatroban, all patients had routine admission laboratory tests, TCD (CTA in patients without temporal windows or lack of TCD availability), NIHSS and mRS. All patients received intravenous tPA (0.9 mg/kg). There was no delay in starting IV-tPA as a result of participation in this study. Informed consent and other qualifying activities for the study took place after the IV rt-PA bolus was given. Argatroban 100-μg/kg bolus over 3 to 5 minutes was administered intravenously within 1 hour of tPA bolus, followed by a continuous Argatroban infusion of 1.0 μg/kg per minute for 48 hours, adjusted to a target aPTT of 1.75 times baseline (±10%). Argatroban infusion rate was adjusted in response to the aPTT according to a dosing algorithm 2, 6, 12, and 24 hours after initiation of Argatroban; at the end of Argatroban infusion; within 2 to 4 hours of any dose adjustment; and in the event of a major bleed in which case the infusion was terminated immediately.
Concomitant anticoagulants or antiplatelet agents were not permitted during Argatroban. CT was performed at baseline, 48 hours after tPA bolus, and for any increase in NIHSS score of ≥2 points more than baseline. CT scans demonstrating any ICH were reviewed (along with a clinical summary) by an independent physician safety monitor. NIHSS scores were measured at 2, 24, and 48 hours after tPA bolus and for clinical worsening. mRS, Barthel index, and Glasgow Outcome Scale scores were obtained 48 hours and 7 days after tPA bolus. 90 day mRS scores were obtained if part of routine follow-up care.
Vessel Imaging
Patients were eligible for enrollment through either a TCD or CTA pathway. The TCD pathway mandated a diagnostic TCD to confirm vessel occlusion. Repeat assessments (<1 minute of ultrasound exposure) were performed at the start of Argatroban infusion, and at 30, 60, 90, and 120 minutes and 24 and 48 hours after tPA bolus. All TCD studies were carried out by a certified technologist. Arterial recanalization was graded by the previously validated TIBI system.17 TCD criteria had been previously validated against CTA.23 TCD and CTA studies were reviewed and adjudicated by central readers (ZG, RM and AMD) who were unaware of the patients’ clinical course. In MCA strokes, recanalization and reocclusion were determined from the most proximal portion of the MCA with the lowest qualifying TIBI score. If flow was absent in a segment, the immediately proximal segment was used. If TIBI was 3 in all segments, the most distal segment was used. Recanalization was defined as an increase in TIBI by ≥ 1 grade compared with baseline and an overall TIBI ≥ 2. Partial recanalization was improvement to grade 2 or 3, and complete recanalization was improvement to grade 4 or 5. Reocclusion was worsening of TIBI by ≥ 1 grade (whether or not recanalization had occurred), with the following exceptions: TIBI 2 or 3 had to decrease to TIBI grade ≤ 1 and TIBI 4 or 5 had to decrease to TIBI ≤ 3.
Patients enrolled via the CTA pathway underwent a pre-treatment CTA utilizing each site’s standard helical CT scanner technology. Post-processing of the images into 2-D and 3-D projections was obtained per local protocols. Intracranial arterial occlusions of the proximal vessels were eligible (TIMI ≤ 1). Follow-up CTAs were mandated at 24 hours; partial recanalization was improvement to TIMI 2, and complete to TIMI 3.
Safety monitoring and statistical considerations
This study was approved by each participating institution’s Committee for the Protection of Human Subjects and an independent data and safety monitoring committee provided safety oversight. The primary outcome, significant ICH, was defined as either symptomatic or parenchymal hemorrhage type 2 (PH-2). Symptomatic intracerebral hemorrhage (sICH) was defined as ICH present on cerebral computed tomography (CT) temporally related to a decline in neurological status in the judgment of the clinical investigator. PH-2 was confluent bleeding occupying more than 30% of the infarct volume and causing significant mass effect.24
Major systemic bleeding was any bleeding associated with a fall in hemoglobin of ≥ 2 g/dL, or resulting in a blood transfusion. A prospective stopping rule mandated enrollment cessation if more than 2 significant hemorrhages occurred in the first 15 patients. The results of the first 15 patients were compiled, analyzed, and sent to the FDA.21 At their request, because of two sICH in the first 15 patients, the sample size was increased to 65, and we agreed upon the following stopping rules: Using asymmetric group sequential method, stopping rules were calculated for every additional group of 5 patients that mandated enrollment cessation if the lower limit of 80% confidence interval for significant hemorrhage rate exceeded 10% or the upper limit of 90% confidence interval was below 10%. Analyses were conducted using SAS version 9.2, Cary, North Carolina.
RESULTS
From May 2003 through August 2010, 65 patients (29 men) were enrolled. Although it was originally intended to complete the study over 5 years, regulatory issues prior to recruitment and during interim safety reviews meant that recruitment did not occur between years 2 and 3; additionally, time-limited funding further slowed the recruitment rate. Ninety-percent of patients had MCA occlusions (66% M1, 34% M2). Median baseline NIHSS score was 13 (range, 3–25) (Table 1). Median time from symptom onset to tPA bolus was 128 (IQR 94,170) minutes. Table 2 and figure 1 describe the anticoagulation response as well as Argatroban infusion results. Fifty-eight patients (89%) started the Argatroban before the end of the tPA infusion, while 7 (11%) experienced a delay of 14 ± 13 minutes. One patient received only the Argatroban bolus without the infusion, due to suspected (but not subsequently confirmed) hemorrhagic transformation. Thirty-one (48%) of 65 patients reached or exceeded target aPTT within 2 hours.
Table 1.
Baseline characteristics.
| Age, mean ± SD | 63 ± 14 |
|
| |
| Male, n (%) | 29 (45) |
| Ethnicity, n (%) | |
| Caucasian | 37 (57) |
| African-American | 21 (32) |
| Hispanic | 4 (6) |
| Asian | 3 (5) |
|
| |
| NIHSS, median (range) | |
| Pre-tPA | 13 (3–25) |
| % of patients with ≥ 10 | 80 |
| Pre-Argatroban | 13 (3–22) |
| % of patients with ≥ 10 | 76 |
|
| |
| Past Medical History, n (%) | |
| Hypertension | 45 (69) |
| Hyperlipidemia | 20 (31) |
| Atrial Fibrillation | 20 (31) |
| Diabetes Mellitus | 19 (29) |
| Coronary Artery Disease | 16 (25) |
| Prior stroke/TIA | 10 (15) |
| Congestive Heart Failure | 9 (14) |
|
| |
| Antithrombotic medications at presentation, n (%) | |
| Aspirin | 18 (28) |
| Clopidogrel | 3 (5) |
| Warfarin | 10 (15) |
| Total | 30 (46) |
|
| |
| Occluded Vessel, n (%) | |
| MCA | 59 (90) |
| Proximal | 39 (66) |
| Distal | 20 (34) |
| Terminal ICA | 5 (8) |
| Vertebral | 1 (2) |
|
| |
| Vessel Imaging Modality, n (%) | |
| Transcranial Doppler | 48 (74) |
| CT-Angiogram | 17 (26) |
|
| |
| Baseline TIBI score (TCD patients only), mean ± SD | 2 ± 1 |
|
| |
| ASPECTS score, median (range) | 10 (4–10) |
|
| |
| Median (IQR) time from symptom onset to tPA bolus (min) | 128 (94,170) |
Table 2.
PTT and Argatroban infusion results, median (IQR).
| Minutes from tPA bolus to Argatroban bolus | 51 (38, 60) |
| Minutes of tPA-Argatroban overlap | 17 (4, 26) |
| Hours to (or above) target aPTT | 3 (2, 7) |
| Hours of Argatroban infusion | 48 (7–48) |
| Hours at (or above) target aPTT | 25 (19, 33) |
| # of Argatroban infusion adjustments | 6 (5,8) |
| First aPTT after Argatroban bolus range | 45, 41–72 (25–149) |
Figure 1.
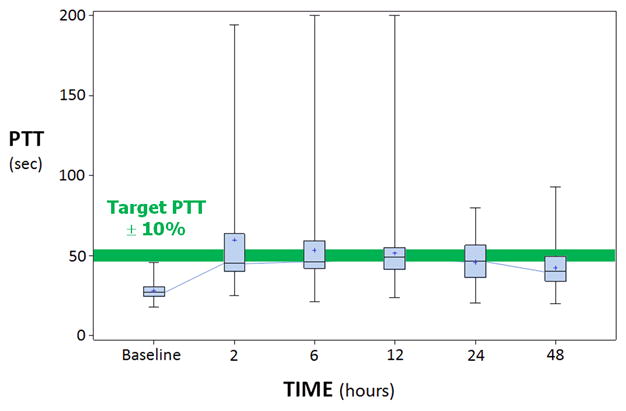
PTT values: baseline (pre-tPA), 2, 6, 12, 24 and 48 hours. Target aPTT is displayed as the horizontal bar.
Safety Outcomes
Table 3 details the safety results. Significant intracerebral hemorrhage occurred in 4 (6.2%) of 65 patients (95% CI, 1.7–15). Of these, 3 were symptomatic (4.6%, 95% CI, 0.9–12.9). Two PH-2 hematomas occurred (3.1%, 95% CI 0.4–10.7) - one patient experienced a PH-2 that was also symptomatic. All cases of significant ICH occurred in the setting of a large territorial infarction.
Table 3.
Safety and bleeding results.
| Significant Intracranial Hemorrhage, n (%, 95% CI) | |
| Total | 4 (6.2, 1.7–15) |
| Symptomatic ICH | 3 (4.6, 0.9–12.9) |
| † Parenchymal Hematoma type-2 | 2 (3.1, 0.4–10.7) |
|
| |
| Asymptomatic Intracranial Hemorrhage, n (%, 95% CI) | |
| Parenchymal Hematoma type-1 | 3 (4.6, 1–12.9) |
| Hemorrhagic Transformation type-2 | 4 (6.2, 1.7–15.0) |
| Hemorrhagic Transformation type-1 | 8 (12, 5.5–22.8) |
| Total | 15 (23, 13.5–35.2) |
|
| |
| Other bleeding serious adverse events, n | 0 |
|
| |
| Adverse Events, n | 187 |
|
| |
| Serious Adverse Events, total | 32 |
| Death (brain herniation or respiratory failure), n (%) | 7 (21.9) |
| Stroke Progression / Neurological worsening, n (%) | 5 (15.6) |
| Significant ICH, n (%) | 4 (12.5) |
| Cerebral Edema, n (%) | 4 (12.5) |
| Pneumonia / Respiratory failure, n (%) | 3 (9.3) |
| Cardiac Arrest, n (%) | 3 (9.3) |
| Asymptomatic ICH, n (%) | 2 (6.3) |
| * Significant laboratory abnormality, n (%) | 2 (6.3) |
| Angioedema, n (%) | 1 (3.1) |
| Seizure, n (%) | 1 (3.1) |
| Definitely related to treatment | 0 |
| Probably related to treatment | 3 |
|
| |
| Mortality (at discharge or Day 7), n (%) | 7 (10.8) |
One patient had a PH-2 that was also symptomatic.
1 patient had elevation in creatinine and liver function tests due to decreased cardiac output and 1 patient had severe hypokalemia.
The first two significant hemorrhages occurred with NIHSS scores of 15 and 21 (both right MCA strokes), prompting the data and safety monitoring board to reduce the upper limit of the NIHSS score to 15 (right hemisphere) and 20 (left hemisphere). The 4 hemorrhages occurred 26, 19, 3.5 and 23 hours after tPA treatment. The highest PTT values for these patients were 61.3, 43.5, 59.7 and 32.7. A total of 32 serious adverse events occurred in 22 patients (see Table 3).
Clinical and Recanalization Outcomes
At 7 days, there was a median decrease (improvement) in NIHSS scores from baseline by 8 points (P<0.001). Also at day 7 or discharge, 29% had a mRS of 0 or 1, the median Barthel Index was 55 (IQR 17.5, 92.5) and the median Glasgow Outcome Scale was 4 (IQR 3, 5). Upon discharge, 76% of patients went either home or to acute rehabilitation, and 7 patients died (10.8%). Five of the 7 deaths resulted from large hemispheric infarction with herniation while the other 2 died from respiratory failure. Six of the 7 deaths occurred after family requested withdraw of care. At 90 days, data was available from 50 patients: 18 patients (36%) experienced a mRS of 0 or 1; 5 were mRS of 2 (10%); 4 were mRS of 3 (8%); 13 were mRS of 4 (26%), 2 were mRS of 5 (4%) and 8 patients were dead (16%).
The distribution of qualifying lesions on TCD and CTA are in Table 1. Six non-MCA cases were included (5 terminal ICA and 1 vertebral artery occlusion). Of the 59 MCA occlusions, 39 (66%) were M1 and 20 (34%) M2-segments. Recanalization adjudication was performed on all patients except for 5 cases where images were unavailable. In these five, the local assessment of recanalization was used for analysis (1 complete recanalization, 2 partial recanalizations and 2 without recanalization). Of the 60 adjudicated cases, there was agreement in 57 (95%) between the local interpretation and the blinded rater. All TCD cases were in agreement. Three CTA cases underwent additional review and agreement was obtained after further discussion between the PI and the blinded assessor. Within the 2 hour monitoring period, TCD recanalization occurred in 29 of 47 (61%) patients: complete in 19 (40%) and partial in 10 (21%) (Table 4). Six (12.7%) patients reoccluded after earlier complete or partial recanalization. 60 patients had vessel imaging at 24 hours demonstrating complete recanalization in 63% (n=38) and partial in 15% (n=15) – total 78% any recanalization.
Table 4.
Recanalization results.
| 2-HOURS (n=47) | n (%) |
|---|---|
| Any recanalization within 2-hours | |
| Complete | 19 (40) |
| Partial | 10 (21) |
| Total | 29 (61) |
| Sustained recanalization at 2-hours | |
| Complete | 14 (30) |
| Partial | 12 (25) |
| Total | 26 (55) |
| Reocclusion | 6 (12.7) |
|
| |
| 24-HOURS (n=60) | n (%) |
|
| |
| Complete recanalization | 38 (63) |
| Partial recanalization | 9 (15) |
| Total | 47 (78) |
Of all patients who had TCD data at 2-hours or CTA patients who had 24-hour recanalization data available (n=63), more patients who had either complete or partial recanalization experienced a mRS of 0 or 1 at day 7 compared with non-recanalizers (44% versus 30%, p=0.336). Proximal clot location experienced higher rates of complete or partial recanalization compared with distally located thrombi (69% versus 38%, p=0.019). However, of the 5 terminal ICA occlusions, 3 failed to recanalize and 2 partially recanalized.
DISCUSSION
Current guidelines do not allow antiplatelets, antithrombotics or anticoagulants until after 24 hours from thrombolysis. Despite limited sample size, our study provides preliminary evidence that anticoagulation with Argatroban during this time frame appears safe. The 95% C.I. for sICH was 0.9–12.9, for PH-2 was 0.4–10.7, and for either was 1.7–15.0. At no time during the study were we 80% or more certain that the true rate of either sICH or PH-2 exceeded 10%. These rates of bleeding are of the same order of magnitude seen with IV rt-PA alone and therefore low enough to justify further evaluation in more patients in order to arrive at a more confident assessment of the true risks of bleeding. Argatroban was chosen because it has been shown to increase the speed and completeness of recanalization while improving flow in the microcirculation.1, 2 In addition, safety has been demonstrated in combination with tPA in both experimental models and clinical cardiac trials.3, 5, 6, 8 Platelet glycoprotein IIb/IIIa antagonists or other antithrombotic agents might also be used advantageously in combination with tPA.25 An advantage of Argatroban is its short half-life, which allows rapid offset of action in case of bleeding, and the ease of monitoring its antithrombotic effect by the aPTT. Thrombin inhibition also prevents injury to vascular endothelium, thus facilitating endogenous plasminogen activator production.26 We chose the Argatroban dose based on 2 considerations. We wanted to give standard dose tPA so that patients would not be deprived of proven effective therapy. We started with a low dose algorithm of Argatroban because in previous trials it was safe.11, 12 Higher doses of Argatroban might also be safe and even more effective, but this will require careful evaluation.
The 48 hour duration of treatment was chosen because it was used in previous studies and we wanted to be sure to treat long enough to prevent any reocclusion.12 However, 3 of our 4 significant hemorrhages occurred more than 18 hours into the infusion. A 12–18 hour infusion might produce even safer and equally effective results. The rate of symptomatic ICH and PH-2 in this study approximates a comparable cohort of patients and historical data.13, 27 Because the NIHSS score predicts bleeding risk from tPA, we put a ceiling on admission NIHSS score after our first two hemorrhages. Subsequent studies might explore if such limits are necessary.
Although an overall effective treatment for ischemic stroke, intravenous tPA is less efficacious for larger, more proximal occlusions. Only 4.4% of distal ICA and 30–32% of MCA clots recanalize.28 In addition, reocclusion causes neurological deterioration, higher inhospital mortality and is likely related to the short treatment effect of tPA.20 There exists an urgent need for safe amplification of tPA reperfusion that can be universally available and performed in any emergency department setting. Our goal was to safely achieve immediate and more complete recanalization quickly after tPA treatment. Based on our previous experience27–29, we think that our high rate of complete or partial recanalization (55%) at 2 hours after tPA treatment may be better than tPA alone, and as useful as any other intervention currently available, particularly considering the potential widespread applicability of this combined pharmacologic approach.
Limitations
Study limitations include possible selection bias and investigators unblinded to treatment. However, these design characteristics are typical of pilot safety analyses evaluating first human exposure to a particular treatment that may have high risks. Furthermore, by appointing an independent monitor to adjudicate all hemorrhagic events, we have minimized these limitations. In addition, although 60/65 patients had 24 hour recanalization data, only 47 were available at 2 hours.
CONCLUSION
In conclusion, Argatroban in combination with IV-tPA appears potentially safe in patients with moderate neurological deficits due to proximal intracranial arterial occlusions and may produce more complete recanalization compared to tPA alone. Further study of this treatment combination appears warranted.
Acknowledgments
ARTSS Clinical Sites and Investigators (in order of recruitment):
University of Texas-Houston (39 cases): A. D. Barreto, N. R. Gonzales, S. I. Savitz, R. Sugg, T. Wu, J. C. Grotta, H. Hallevi, G. Lopez, J. K. Pary, K. Uchino, A. M. Khaja, K. C. Albright, H. M. Shaltoni, R. Mikulik, Z. Garami, S. G. Shaw, D. E. Meyer, T. Bui, E. Fulep, O. Y. Chernyshev, K. Illoh
University of Alabama-Birmingham (21 cases): A. V. Alexandrov, A. Sisson, C. Balucani, L. Zhao, K. Barlinn
Cedars Sinai Medical Center (3 cases): P. Lyden, D. Palestrant, C. Miller
University of Texas-Southwestern (2 cases): J. Lee, C. Hall, M. Johnson
Data Safety and Monitoring Board:
David Chiu, MD, Debra del Junco, PhD, S. Chris Pappas, MD, Igor Cherches, MD, Thomas A. Kent, MD
Independent Physician Safety Monitor:
Pitchaiah Mandava, MD
Sources of Funding
This study was supported by grant P50NS044227 from the National Institute of Neurological Disorders and Stroke (NINDS) and supplement from the American Recovery and Revitalization Act to the Argatroban tPA Stroke Study; training grant T32NS07412 from the NIH to the University of Texas–Houston Medical School Stroke Program; and grants 1K23NS02229-01 and 1P50NS044227 from the NINDS to the CLOTBUST trial. This work was also supported by the Center for Clinical and Translational Sciences, which is funded by NIH Clinical and Translational Award UL1 RR024148 [TL1 RR024147 for the T32 program; KL2 RR0224149 for the K12 program] from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the NIH. Glaxo-Smith-Kline (GSK) provided study medication and support for clinical monitoring expenses. GSK was not involved in the study design, analysis or manuscript preparation.
Footnotes
Conflict of Interest
Clinical Trial Registration: URL:http://www.clinicaltrials.gov. Unique identifier: NCT00268762
Financial Disclosure:
During the first two years of this study, Dr. James C. Grotta received grant support from Texas Biotechnology Corporation. Other officials of The University of Texas Health Science Center at Houston not connected with this study, had equity interest in Texas Biotechnology Corporation.
References
- 1.Walenga JM. An overview of the direct thrombin inhibitor argatroban. Pathophysiol Haemost Thromb. 2002;32 (Suppl 3):9–14. doi: 10.1159/000069103. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka KA, Szlam F, Katori N, Sato N, Vega JD, Levy JH. The effects of argatroban on thrombin generation and hemostatic activation in vitro. Anesth Analg. 2004;99:1283–1289. doi: 10.1213/01.ANE.0000134685.75813.EB. [DOI] [PubMed] [Google Scholar]
- 3.Jang IK, Brown DF, Giugliano RP, Anderson HV, Losordo D, Nicolau JC, et al. A multicenter, randomized study of argatroban versus heparin as adjunct to tissue plasminogen activator (tpa) in acute myocardial infarction: Myocardial infarction with novastan and tpa (MINT) study. J Am Coll Cardiol. 1999;33:1879–1885. doi: 10.1016/s0735-1097(99)00107-2. [DOI] [PubMed] [Google Scholar]
- 4.Moledina M, Chakir M, Gandhi PJ. A synopsis of the clinical uses of argatroban. J Thromb Thrombolysis. 2001;12:141–149. doi: 10.1023/a:1012919404290. [DOI] [PubMed] [Google Scholar]
- 5.Wykrzykowska JJ, Kathiresan S, Jang IK. Clinician update: Direct thrombin inhibitors in acute coronary syndromes. J Thromb Thrombolysis. 2003;15:47–57. doi: 10.1023/a:1026144518686. [DOI] [PubMed] [Google Scholar]
- 6.Jang IK, Gold HK, Leinbach RC, Fallon JT, Collen D. In vivo thrombin inhibition enhances and sustains arterial recanalization with recombinant tissue-type plasminogen activator. Circ Res. 1990;67:1552–1561. doi: 10.1161/01.res.67.6.1552. [DOI] [PubMed] [Google Scholar]
- 7.Kawai H, Umemura K, Nakashima M. Effect of argatroban on microthrombi formation and brain damage in the rat middle cerebral artery thrombosis model. Jpn J Pharmacol. 1995;69:143–148. doi: 10.1254/jjp.69.143. [DOI] [PubMed] [Google Scholar]
- 8.Morris DC, Zhang L, Zhang ZG, Lu M, Berens KL, Brown PM, Chopp M. Extension of the therapeutic window for recombinant tissue plasminogen activator with argatroban in a rat model of embolic stroke. Stroke. 2001;32:2635–2640. doi: 10.1161/hs1101.097390. [DOI] [PubMed] [Google Scholar]
- 9.Tamao Y, Kikumoto R. Effect of argatroban, a selective thrombin inhibitor, on animal models of cerebral thrombosis. Semin Thromb Hemost. 1997;23:523–530. doi: 10.1055/s-2007-996130. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka YS. Therapeutic effect of argatroban (MD-805), antithrombotic agent, in the acute stage of cerebral thrombosis. J Clin Ther Med. 1987;3:133–142. [Google Scholar]
- 11.Kobayashi S, Tazaki Y. Effect of the thrombin inhibitor argatroban in acute cerebral thrombosis. Semin Thromb Hemost. 1997;23:531–534. doi: 10.1055/s-2007-996131. [DOI] [PubMed] [Google Scholar]
- 12.LaMonte MP, Nash ML, Wang DZ, Woolfenden AR, Schultz J, Hursting MJ, et al. Argatroban anticoagulation in patients with acute ischemic stroke (ARGIS-1): A randomized, placebo-controlled safety study. Stroke. 2004;35:1677–1682. doi: 10.1161/01.STR.0000131549.20581.ba. [DOI] [PubMed] [Google Scholar]
- 13.Tissue plasminogen activator for acute ischemic stroke. The national institute of neurological disorders and stroke rt-pa stroke study group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 14.Hacke W, Brott T, Caplan L, Meier D, Fieschi C, von Kummer R, et al. Thrombolysis in acute ischemic stroke: Controlled trials and clinical experience. Neurology. 1999;53:S3–14. [PubMed] [Google Scholar]
- 15.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second european-australasian acute stroke study investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 16.Alexandrov AV, Burgin WS, Demchuk AM, El-Mitwalli A, Grotta JC. Speed of intracranial clot lysis with intravenous tissue plasminogen activator therapy: Sonographic classification and short-term improvement. Circulation. 2001;103:2897–2902. doi: 10.1161/01.cir.103.24.2897. [DOI] [PubMed] [Google Scholar]
- 17.Demchuk AM, Burgin WS, Christou I, Felberg RA, Barber PA, Hill MD, et al. Thrombolysis in brain ischemia (TIBI) transcranial doppler flow grades predict clinical severity, early recovery, and mortality in patients treated with intravenous tissue plasminogen activator. Stroke. 2001;32:89–93. doi: 10.1161/01.str.32.1.89. [DOI] [PubMed] [Google Scholar]
- 18.Labiche LA, Al-Senani F, Wojner AW, Grotta JC, Malkoff M, Alexandrov AV. Is the benefit of early recanalization sustained at 3 months? A prospective cohort study. Stroke. 2003;34:695–698. doi: 10.1161/01.STR.0000055940.00316.6B. [DOI] [PubMed] [Google Scholar]
- 19.Alexandrov AV, Demchuk AM, Burgin WS, Robinson DJ, Grotta JC. Ultrasound-enhanced thrombolysis for acute ischemic stroke: Phase I. Findings of the clotbust trial. J Neuroimaging. 2004;14:113–117. [PubMed] [Google Scholar]
- 20.Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59:862–867. doi: 10.1212/wnl.59.6.862. [DOI] [PubMed] [Google Scholar]
- 21.Sugg RM, Pary JK, Uchino K, Baraniuk S, Shaltoni HM, Gonzales NR, et al. Argatroban tPA stroke study: Study design and results in the first treated cohort. Arch Neurol. 2006;63:1057–1062. doi: 10.1001/archneur.63.8.1057. [DOI] [PubMed] [Google Scholar]
- 22.Del Zoppo GJ, Saver JL, Jauch EC, Adams HP., Jr Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator. A science advisory from the american heart association/american stroke association. Stroke. 2009;40:2945–2948. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsivgoulis G, Sharma VK, Lao AY, Malkoff MD, Alexandrov AV. Validation of transcranial doppler with computed tomography angiography in acute cerebral ischemia. Stroke. 2007;38:1245–1249. doi: 10.1161/01.STR.0000259712.64772.85. [DOI] [PubMed] [Google Scholar]
- 24.Fiorelli M, Bastianello S, von Kummer R, del Zoppo GJ, Larrue V, Lesaffre E, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: Relationships with early clinical deterioration and 3-month outcome in the european cooperative acute stroke study I (ECASS I) cohort. Stroke. 1999;30:2280–2284. doi: 10.1161/01.str.30.11.2280. [DOI] [PubMed] [Google Scholar]
- 25.Ciccone A, Abraha I, Santilli I. Glycoprotein IIb-IIIa inhibitors for acute ischemic stroke. Stroke. 2007;38:1113–1114. doi: 10.1002/14651858.CD005208.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Ueshima S, Fukao H, Okada K, Matsuo O. The effect of argatroban on injured endothelial cells by thrombin. Blood Coagul Fibrinolysis. 2000;11:631–639. doi: 10.1097/00001721-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 28.Saqqur M, Uchino K, Demchuk AM, Molina CA, Garami Z, Calleja S, et al. Site of arterial occlusion identified by transcranial doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38:948–954. doi: 10.1161/01.STR.0000257304.21967.ba. [DOI] [PubMed] [Google Scholar]
- 29.Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: Real-world experience and a call for action. Stroke. 2010;41:2254–2258. doi: 10.1161/STROKEAHA.110.592535. [DOI] [PubMed] [Google Scholar]


