Abstract
Depth and orientational dependencies of microscopic MRI T2 and T1ρ sensitivities were studied in native and trypsin-degraded articular cartilage, before and after being soaked in 1 mM Gd-DTPA2− solution. When the cartilage surface was perpendicular to B0, a typical laminar appearance was visible in T2 weighted images but not in T1ρ weighted images, especially when the spin-lock field was high (2 kHz). At the magic angle (55°) orientation, neither T2 nor T1ρ weighted image had a laminar appearance. Trypsin degradation caused a depth and orientational dependent T2 increase (4–64%) and a more uniform T1ρ increase at a sufficiently high spin-lock field (55–81%). The presence of the Gd ions caused both T2 and T1ρ to decrease significantly in the degraded tissue (6–38% and 44–49% respectively) but less notably in the native tissue (5–10% and 16–28% respectively). A quantity Sensitivity was introduced that combined both the percentage change and the absolute change in the relaxation analysis. An MRI experimental protocol based on two T1ρ measurements (without and with the presence of the Gd ions) was proposed to be a new imaging marker for cartilage degradation.
Keywords: T2, T1ρ, MRI, cartilage, proteoglycan, magic angle, anisotropy, spin lock
Introduction
Articular cartilage, which is a thin layer of soft tissue covering the ends of bone in joint to distribute external loading, consists mainly of structured collagen fibrils, negatively charged proteoglycans (PG) and water molecules in its extracellular matrix (1,2). Since the orientation of the collagen fibrils in articular cartilage is depth dependent, the total depth (thickness) of articular cartilage is commonly subdivided based on the local fibril orientation into multiple structural zones, such as the superficial zone at the top surface (SZ), the transitional zone (TZ) in the middle, and the radial zone (RZ) that interfaces with the underlining bone. The role of negatively charged PG molecules is to generate a volumetric tension inside the tissue by absorbing water. A reduction of PG in cartilage is an early event in the gradual process of tissue degradation, which will eventually lead to clinical diseases such as osteoarthritis (OA) that affects over 20 million people in the United States (3).
Magnetic resonance imaging (MRI) is a sensitive diagnostic technique suitable for the noninvasive assessment of soft tissue such as articular cartilage. Conventional (i.e., intensity based) MRI, however, does not have the sensitivity towards the subtle changes associated with the early stage of cartilage lesions (4–6). A number of quantitative techniques have been developed in MRI to better evaluate the structural and concentrational changes associated with the tissue degradation, including three MRI techniques that utilize the relaxation time measurement: the value and anisotropy of T2 relaxation (7–9), the gadolinium-enhanced T1 relaxation (dGEMRIC) (10–14), and T1ρ relaxation (spin-lattice relaxation in the rotating frame) (15–18).
The transverse relaxation time T2 is sensitive to the anisotropic motion of water molecules mediated by the collagen orientation via dipolar interaction (7). This T2 anisotropy can cause articular cartilage to have a depth-dependent laminar appearance in MRI when the normal axis of cartilage surface is in parallel with the external magnetic field B0 (7,19–21). When the normal axis is about 55° to B0, the minimization of the dipolar interaction causes the disappearing of the laminar appearance and the increased cartilage intensity, an effect known as the “magic-angle effect” in the literature of cartilage MRI (8,9,21).
The longitudinal relaxation time T1, in contrast, is insensitive to the anisotropic motion of water molecules mediated by the collagen orientation (7). Hence T1 in articular cartilage is nearly isotropic and largely depth-independent (7). The usefulness of T1 measurement in cartilage MRI was pioneered by the development of the clinical procedure (dGEMRIC, delayed Gadolinium Enhanced Magnetic Resonance Imaging of Cartilage) (10,22), which, in principle, images patient/specimen twice before/after it is injected with or immersed in a solution of a paramagnetic contrast agent, Gd-DTPA2−. Since gadolinium ions shorten the T1 relaxation and distribute in an inverse relation to the concentration of the negatively charged PG molecules in cartilage, both before and after injection/immersion images can be used to calculate the PG concentration in cartilage.
The spin-lattice relaxation time in the rotating frame T1ρ is also sensitive to the slow motional interactions between water and macromolecules (proteins) in biological tissues and has been used in recent years in cartilage imaging (16,23–25). Since T1ρ is less sensitive to the local fibril orientation, T1ρ has less anisotropy in articular cartilage MRI, which is a welcome feature in human MRI where the specimen orientation cannot be adjusted easily. A unique feature of T1ρ is the dependency of its values on the strength of the spin-lock field (the rf field that locks the magnetization in the transverse plane), a phenomenon termed T1ρ dispersion (26).
This microscopic imaging project concerns the depth and orientational dependencies of both T2 and T1ρ sensitivities in cartilage MRI, in the absence and presence of the contrast agent Gd-DTPA2− in the tissue. The dependency of T2 in the presence of (gadolinium) Gd has been discussed in several studies (27–30), but rarely the dependency of T1ρ in the presence of Gd ions. In addition, the influence of the specimen orientation in the magnetic field on T2 and T1ρ measurements has not been adequately investigated. In this high-resolution imaging project, the sensitivities of T2 and T1ρ were studied at microscopic resolution (13 µm transverse resolution) in native and trypsin-degraded articular cartilage without and with the presence of Gd ions in the tissue. The influence of the specimen orientation in the magnet and the dependency of T1ρ on the strength of the spin-lock field were both being considered.
Materials and Methods
Cartilage Specimens
Canine humeral heads were harvested shortly after the sacrifice of three mature (1–2 years old) and musculoskeletally healthy dogs that were used for unrelated biomedical research (ongoing for more than the last 10 years). For each of the three dogs, two adjacent cartilage-bone blocks (about 1.8×1.5×6 mm3) were harvested from the central part of one humeral head. The specimens were first imaged by the same µMRI protocols as their own controls. After the initial MRI, one specimen was immersed in 1 mM Gd-DTPA2− (Magnevist, Berlex, NJ) solution in saline with 1% protease inhibitor (Sigma, Missouri) for more than 8 hours before the second MRI experiment (13). The other specimen in the pair was first soaked in 0.1 mg/ml trypsin (Sigma, Missouri) solution for more than 8 hours to deplete proteoglycans (31) and then soaked in fresh saline with 1% protease inhibitor to remove excess trypsin before repeating the MRI. After repeating the MRI, this PG-depleted specimen was immersed in the Gd-DTPA2− saline and subsequently imaged using the same protocol for the third time. These experiments were repeated with four more cartilage blocks from two other dogs; the results were nearly identical (within the error range).
Microscopic MRI (µMRI) Protocols
All µMRI experiments were performed at room temperature on a Bruker AVANCE–300 NMR spectrometer equipped with a 7 Tesla/89 mm vertical-bore superconducting magnet and microimaging accessory (Bruker Instrument, Billerica, MA). A homemade 3 mm coil was used in the µMRI experiments, which had a 90° hard pulse of 5 µs. Quantitative T2 experiments were performed using a CPMG magnetization-prepared T2 imaging sequence (32). The T1ρ imaging sequence preceded with a 90° hard pulse followed by a spin-lock pulse. The strengths of the spin-lock field were 0.5 kHz and 2 kHz, which were calibrated by the strength of the 90° pulse.
The µMRI experiments were carried out with an acquisition matrix of 256×128 (13×26 µm pixel resolution) and a slice thickness of 1 mm. The repetition time TR was 2 s for the specimens without Gd-DTPA2− immersion and 0.8 s for the specimens soaked in Gd-DTPA2− solution. The echo spacing in the CPMG T2-weighting segment was 1 ms and the number of echoes were 2, 4, 10, 30, 60 when the cartilage surface was perpendicular to the static magnetic field and 2, 14, 36, 60, 120 at the magic angle, respectively, which resulted in five T2-weighted images for each tissue orientation (at 0°: the effective contrast TEs = 2, 4, 10, 30, 60ms; at 55°: the effective contrast TEs = 2, 14, 36, 60, 120 ms). The lengths of the spin-locking pulse were 2, 6, 12, 40, 80 ms when the cartilage surface was perpendicular to the static magnetic field and 2, 18, 40, 80, 140 ms at the magic angle, respectively.
The 2D T1ρ images were calculated pixel-by-pixel by an expression: Sig(TSL) = Sig0exp(−TSL/T1ρ) + K, where Sig was the signal intensity of the observed signal, Sig0 was the thermal equilibrium magnetization, TSL was the time of spin-lock pulse, and K was the constant offset. The 2D T2 images were calculated based on a similar expression: Sig(TE) = Sig0exp(−TE/T2)+K, where TE was the echo time. Other experimental details have been described elsewhere (13,32).
Results
Images and depth-dependent profiles of T2 and T1ρ
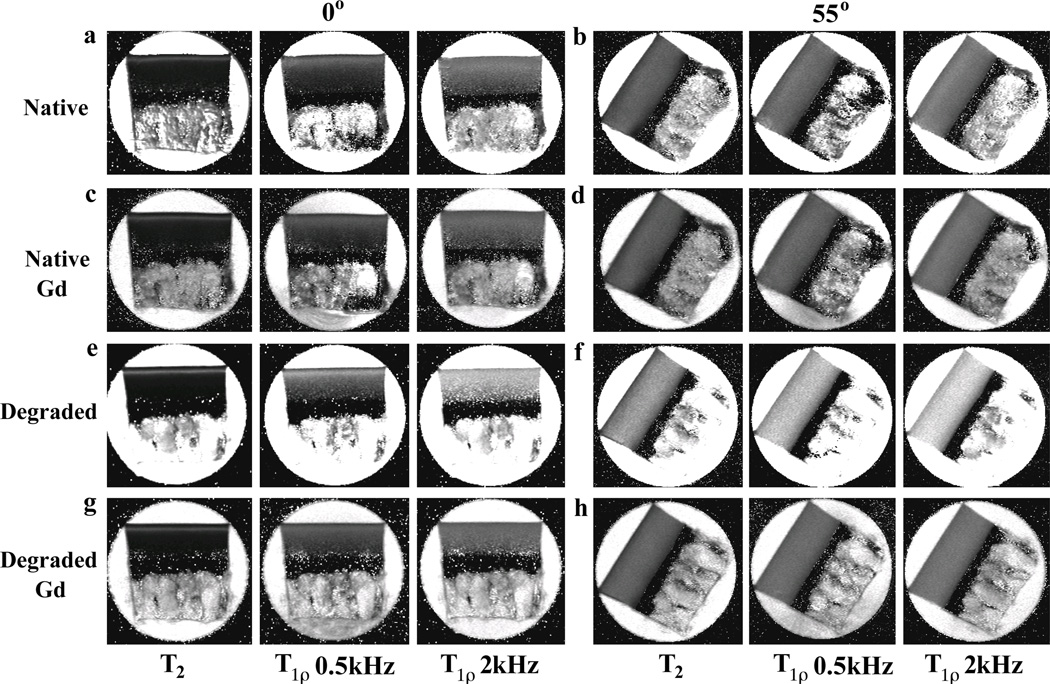
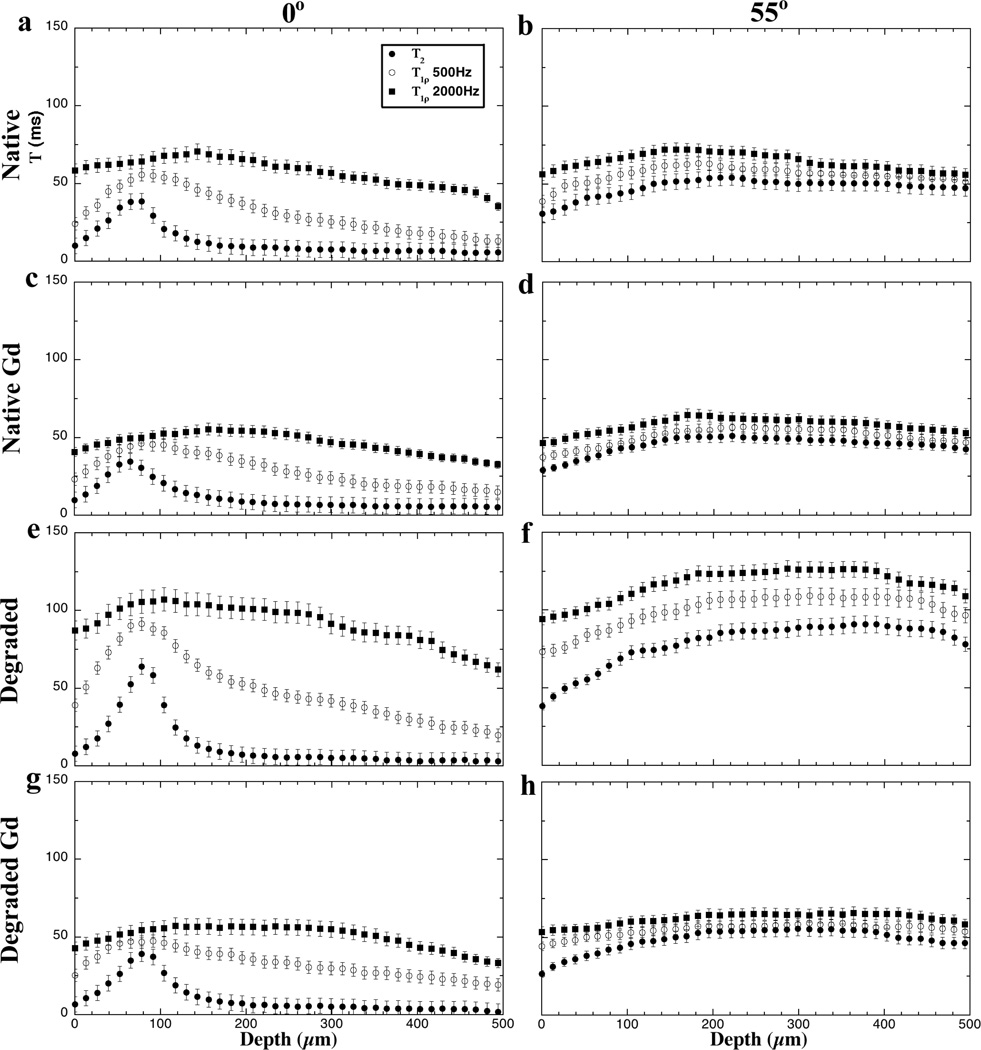
Fig 1 and Fig 2 summarize the quantitative images and their depth-dependent profiles for four different types of specimens: native tissue (i.e., untreated), native tissue soaked in the Gd solution, trypsin-degraded tissue, and trypsin-degraded tissue soaked in the Gd solution. Several distinct characteristics can be identified in these complex T2 and T1ρ results at two different orientations (0° and 55° with respect to B0). First, the tissue has clear laminar appearance in the T2 and low spin-lock (0.5 kHz) T1ρ images at 0°, likely due to the strong influence of the dipolar interaction at 0° (the left column in Fig 2). Second, both T2 and T1ρ at 55° are practically uniform (the right column in Fig 2), which is to be expected due to the minimization of the dipolar interaction at the magic angle. Third, even at 0°, the T1ρ images at 2 kHz are considerably uniform and have higher value, which signifies the minimization of the dipolar interaction by the high spin-lock field. Forth, regardless of the specimen orientation, T2 at any tissue location always has the lowest value while T1ρ at higher spin-lock (2 kHz) always has the highest values (Fig 2). Among the T1ρ results, the larger the spin-lock field, the longer the T1ρ relaxation values, which is known as T1ρ dispersion.
Figure 1.
T2 and T1ρ images at four sets of experimental conditions: (a–b) native specimens, (c–d) native specimens immersed in Gd-DTPA2−, (e–f) trypsin-degraded specimens, (g–h) trypsin-degraded specimens immersed in Gd-DTPA2−. All images were plotted with the same intensity limits (0 – 200 ms). The angles 0° (left) and 55° (right) refer to the orientation between the normal axis of the surface and the magnetic field (vertically up).
Figure 2.
T2 and T1ρ profiles of articular cartilage at four experimental conditions, at 0° (left) and 55° (right). All profiles were plotted with the same intensity limits (0 – 150 ms).
The effects of trypsin digestion and Gd immersion
Two interesting features can be identified when the tissue is digested in trypsin or immersed in the Gd-DTPA2− solution. First, the trypsin digestion causes the increase of T2 and T1ρ over the entire tissue depth, regardless of whether the specimen is at 0° (Fig 2a vs Fig 2e) or 55° (Fig 2b vs Fig 2f). Second, for the native tissue, the soaking of specimen in the Gd solution does not change T2 or T1ρ significantly (Fig 2a vs Fig 2c, and Fig 2b vs Fig 2d). However, the soaking of the degraded specimen in the Gd solution significantly reduces both T2 and T1ρ (Fig 2e vs Fig 2g, and Fig 2f vs Fig 2h).
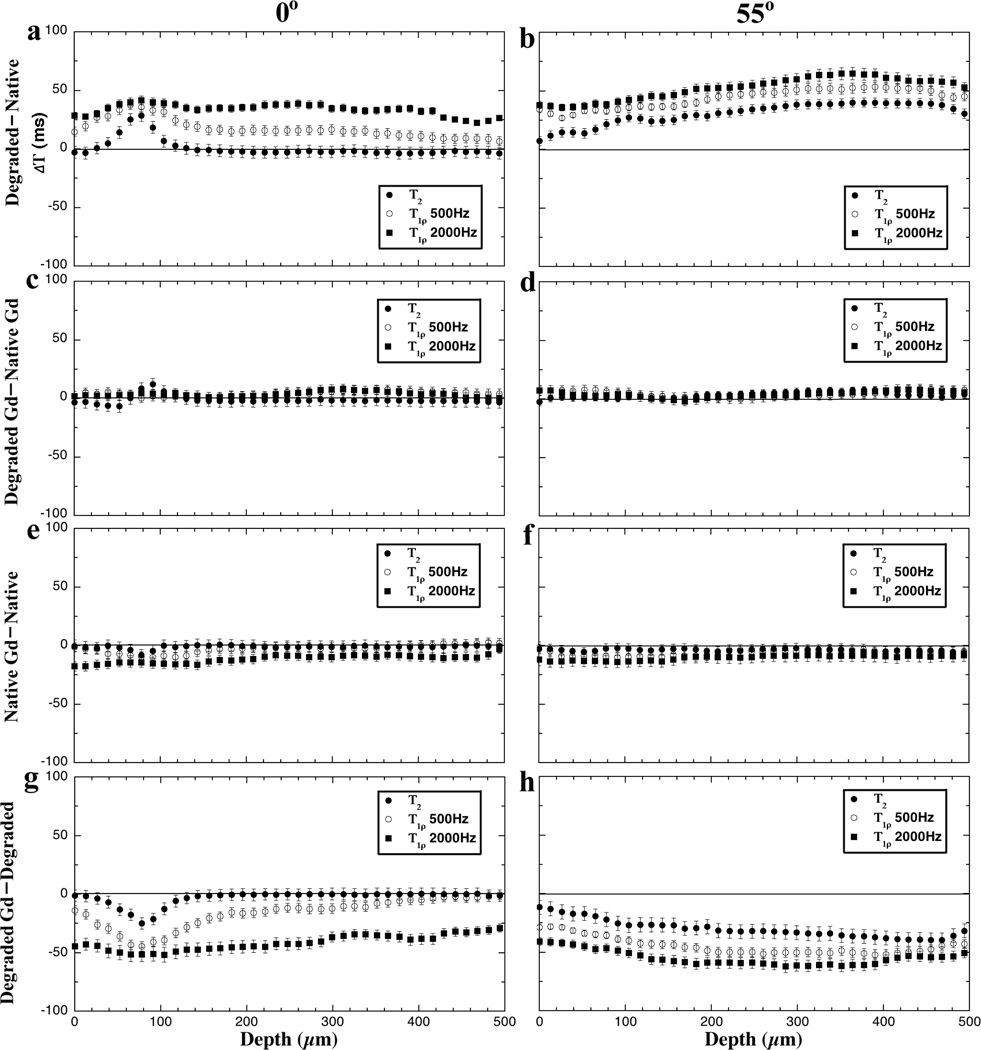
These variations in the T2 and T1ρ profiles are quantified as the differences between the paired measurements in Fig 3, which have three depth-dependent characteristics. First, the T2 difference is strongly depth-dependent at the 0° orientation - being mostly sensitive in the transitional zone (TZ) than at any other depth towards (1) the trypsin digestion (Fig 3a) and (2) the immersion of degraded tissue in Gd (Fig 3g). However, this T2 difference in the transitional zone is significantly reduced when (1) both the native and degraded tissues have been immersed in Gd (Fig 3c) and (2) the native tissue is immersed in Gd (Fig 3e). Second, the weakly spin-locked T1ρ data (0.5 kHz) appears to have the similar characteristics as T2, on a lesser scale. When the spin-lock filed is increased to 2 kHz, there is no obvious depth-dependence in T1ρ profiles. Third, both T2 and T1ρ differences become somewhat depth-independent at the magic angle. In fact, all differences at the magic angle show a larger difference in the deeper part of the tissue than the surface part of the tissue (Fig 3b and 3h).
Figure 3.
The difference profiles of T2 and T1ρ at 0° (left) and 55° (right) at four sets of comparisons.
The zonal sensitivity of T2 and T1ρ toward tissue degradation
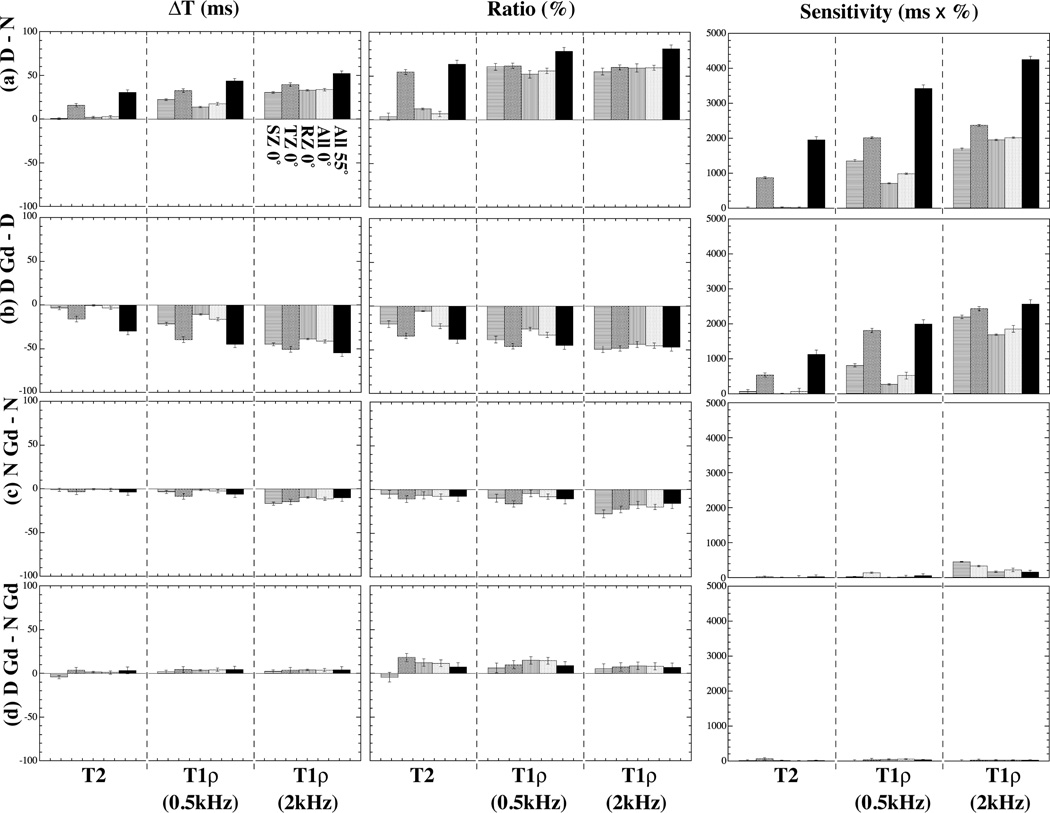
Since most MRI studies of articular cartilage cannot achieve the fine spatial resolution as in this work (13–26 µm), the differences between the four paired experiments are averaged in each histological zone and plotted in two different ways (Fig 4): the percentage ratio and the absolute change. The division of zones was based on our knowledge of the tissue characteristics from the previous experiments – the nearly identical type of tissue has been studied in our lab for over 17 years by multidisciplinary imaging techniques at microscopic resolutions (33,34). In this project, the thicknesses of three structural zones were determined as, the superficial zone = 52 µm, the transitional zone = 78 µm, and the radial zone = 500 µm.
Figure 4.
The zonal averaged differences of T2 and T1ρ at 0° and 55°. The left column: the difference of relaxation measurement (ΔT in ms); the middle column: the percentage ratio of relaxation measurement (%); the right column: the sensitivity of relaxation measurement (ms × %). (a) – (d) correspond to the four sets of comparisons shown in Fig 3.
It was noticed that neither the percentage ratio nor the absolute change was a reliable indicator for the sensitivity of the measurement, due to two practical factors in the relaxation measurement, the influence of the experimental noises and the unreliability of low value relaxation measurement. For example, when a T2 changed from 2 ms to 4 ms, it would be a 100% change. However, this 100% change at a low relaxation value of several milliseconds could contain a large error from the influence of experimental noises and the intrinsic unreliability of determining short relaxation times when the echo-time (TE) of an imaging experiment is at least several milliseconds or longer. To better determine the sensitivity of the relaxation measurement, we define a term Sensitivity, which is given by,
| (1), |
, where the Percentage-Ratio is (Tafter – Tbefore)/Tbefore in percentages, and the Absolute-Change is (Tafter – Tbefore) in ms in this project. This quantity has the form of an equilateral hyperbola and can be plotted as a set of 2D contours, where each hyperbola equals a constant Sensitivity.
Fig 4 summarizes the zonal averaged Absolute-Change (ΔT), Percentage-Ratio (%) and Sensitivity (ms × %) in four sets of experimental conditions: (Fig 4a) degraded – native, (Fig 4b) degraded with Gd – degraded, (Fig 4c) native with Gd – native, and (Fig 4d) degraded with Gd – native with Gd. Several distinct features can be clearly identified. First, T2 has sufficient sensitivity in all zones at 55° but only in TZ at 0° (Fig 4a) for the first and second sets of experimental comparisons. Second, T1ρ has sufficient sensitivity in all zones at both 0° and 55° for the first and second sets of experimental comparisons (Fig 4b). Neither T2 nor T1ρ has sufficient sensitivity for the later two sets of experimental comparisons.
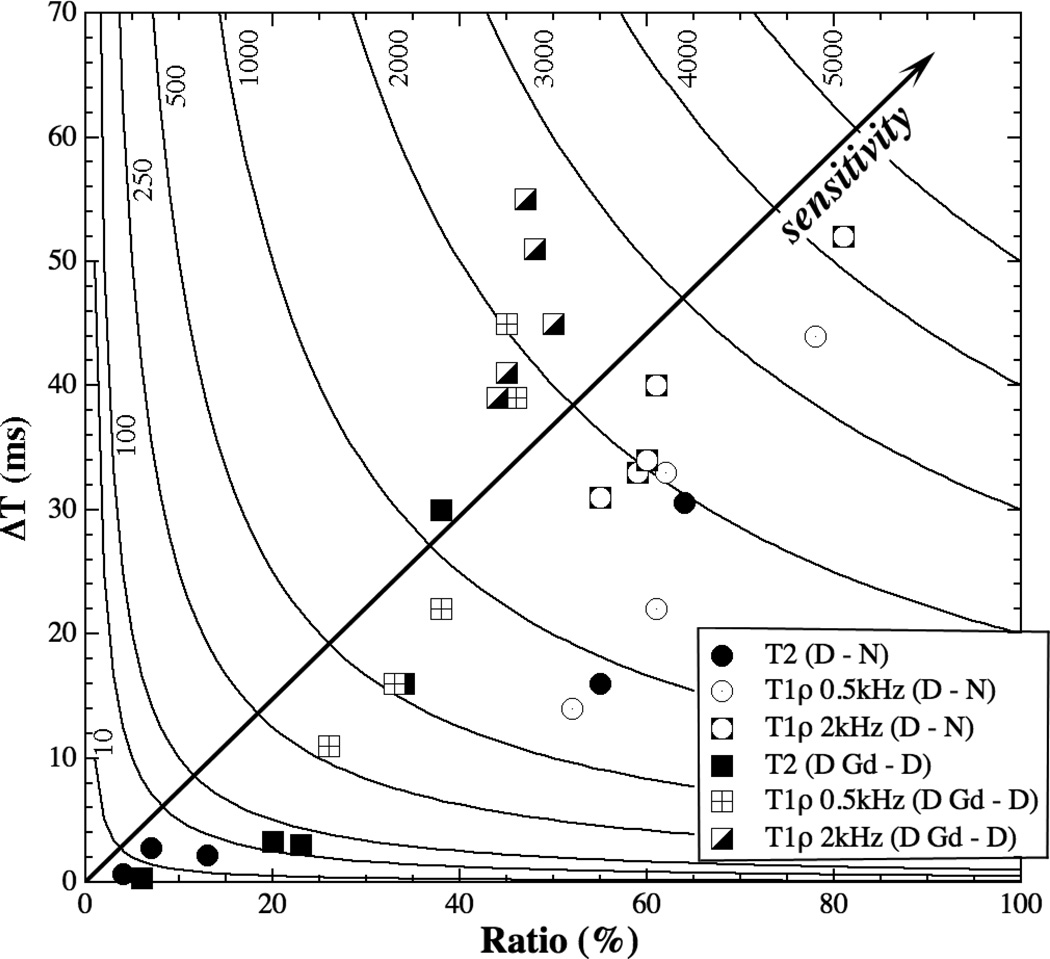
The trends of the individual sensitivities in the first and second sets of experimental comparisons can be visualized more clearly in a hyperbola contour plot (Fig 5), where the diagonal line points to an increasing Sensitivity. Any group of data points that are close together implies less variability in Sensitivity. Any data point (i.e., experimental condition) that is not near one of the axes or the origin is sensitive in the relaxation measurement. (For this reason, the data in Fig 4c and 4d are not plotted in Fig 5 since they all congregate near the origin.)
Figure 5.
The sensitivities in the first and second sets of comparisons in a hyperbola contour, where the diagonal arrow line points to an increasing Sensitivity.
Discussion
In this study, high-resolution T2 and T1ρ MRI experiments were carried out to study native and trypsin-degraded canine articular cartilage, at both the 0° and the magic angle. Several issues related to the measurement sensitivities of T2 and T1ρ relaxation times towards cartilage degradation were investigated. These issues include (1) the characteristics of T1ρ dispersion (the value of T1ρ as the function of the spin-lock field), (2) the orientations of the specimen in the magnetic field B0, (3) the PG content in tissue, and (4) the absence and presence of Gd ions in tissue. To better determine the usefulness of T2 and T1ρ measurements in MRI diagnostics, a term Sensitivity is introduced in the data analysis, which includes the contributions from both the percentage changes and the absolute changes. We analyze the data in four different cases of experimental conditions: (Case a) the degradation of tissue without Gd ions (Fig 4a), (Case b) the degraded tissue without and with Gd ions (Fig 4b), (Case c) the native tissue without and with Gd ions (Fig 4c), and (Case d) the native and degraded tissues both in the presence of Gd ions (Fig 4d).
(Case a) Sensitivities of T2 and T1ρ towards tissue degradation without the presence of Gd
T2 is known to be sensitive to PG concentration and has been used in cartilage MRI (7,34–40). This report confirms the sensitivity of T2 towards tissue degradation – T2 increases when the PG content is reduced (Fig 4a). However, the sensitivity of MRI T2 detection of cartilage lesion is both depth dependent and orientational dependent (Fig 4a), which is influenced by the dipolar interaction due to the anisotropic motion of water molecules associated with the collagen fibrils (7). This T2 anisotropy can therefore be used to explore the collagen structure in cartilage non-invasively. Since human joints never have any simple geometric shape, however, it is impossible to orient human in the magnet in such a way that the dipolar interaction has a uniform influence over the entire joint cartilage. Consequently, although T2 is a reliable marker in the research labs to study ex vivo tissue blocks (34), its sensitivity in human in vivo MRI depends on not only by the healthy status of cartilage but also by the orientation of cartilage in the MRI magnet. In comparison, the T1ρ sensitivity to tissue digestion is nearly uniform and isotropic, especially when the dipolar interaction is sufficiently minimized at the high spin-lock field (2 kHz). This makes the T1ρ protocol better suited for human MRI. However, a high spin-lock field increases the rf power deposition on the tissue, which is a distinct disadvantage in the T1ρ protocol when comparing with the T2 protocol.
(Case b) Sensitivities of T2 and T1ρ in degraded tissue without and with the presence of Gd ions
Gd ions diffuse into cartilage inversely to the local PG content in tissue: more into the PG-poor tissue region (e.g., SZ) and less into to PG-rich tissue region (e.g., RZ) (13). It is interesting to note that both T2 and T1ρ are still very sensitive to the immersion of Gd ions in the degraded tissue (Fig 4b), which results in a set of sensitivity features similar to the degradation process in the absence of Gd ions (Fig 4a), including the strong depth-dependent T2 sensitivity, the slightly depth-dependent T1ρ sensitivity at low spin-lock field, and the uniformly high T1ρ sensitivity at high spin lock field. The fact that T2 at 0° is still depth-dependent demonstrates that the dipolar interaction still plays an important role in the relaxation process in the degraded tissue. The fact that T2 at 55° is reduced (instead of increased) remarkably suggests the influx of additional paramagnetic Gd ions into the tissue, which dominates the relaxation process when the dipolar interaction is minimized (either at the magic angle or under the high spin-lock field). Our results are consistent with a human tissue MRI work by Taylor et al (30), with some interesting differences. For example, Taylor et al found that the addition of 1 mM Gd contrast agent shortened T1 and T1ρ values significantly, but could not affect T2 values in articular cartilage. Additional investigation would be beneficial to identify the molecular mechanisms of these discrepancies.
(Case c) Sensitivities of T2 and T1ρ in native tissue without and with the presence of Gd ions
When the native tissue is soaked in the Gd solution, however, both T2 and T1ρ reduce slightly (Fig 4c). This result indicates that neither T2 nor T1ρ has sufficient sensitivity to the presence of Gd ions in the native (i.e., healthy tissue with normal PG concentrations) tissue. This notable difference in the sensitivities of T2 and T1ρ between the native tissue (Case c) with the degraded tissue (Case b) is a direct consequence of more PG in the native tissue, which limits the amount of Gd ions that can be diffused into the tissue.
(Case d) Sensitivities of T2 and T1ρ in native and degraded tissue both in the presence of Gd ions
In the presence of the Gd ions, there are no significant differences in either T2 or T1ρ between the native tissue and the degraded tissue (Fig 4d). This result reveals that the Gd ions largely eliminate the sensitivities of T2 and T1ρ to PG degradation; in other words, the value of T2 or T1ρ in the presence of Gd ions cannot be used as an indicator of a tissue’s healthy status.
Sensitivities of T2 and T1ρ at the magic angle
Since the profiles of T2 and T1ρ at 55° are nearly depth-independent, these profiles were averaged over the entire tissue depth and presented in Fig 4 as one averaged value. However, the T2 and T1ρ profiles at 55° clearly show that both T2 and T1ρ also have some weak depth-dependent sensitivities at 55°, bout are more sensitive towards the deeper tissue (Fig 3a and 3h). This trend is likely caused by the non-uniform PG concentration in articular cartilage – more PG at the deeper tissue (13). Hence there would be more PG loss at the deep tissue after the trypsin treatment, which results in more free water in deeper tissue and consequently a bigger difference in both T2 and T1ρ.
A potential clinical MRI protocol for tissue degradation by T1ρ
It’s interesting to note that T1ρ is more sensitive to the presence of Gd-DTPA2− in PG-degraded specimens than in native specimens, i.e., the T1ρ-native – T1ρ-native-Gd (Fig 4c) are much smaller than the T1ρ-degraded – T1ρ-degraded-Gd (Fig 4b). This significant T1ρ difference between native specimens and trypsin-degraded specimens with and without the presence of Gd ions suggests a possible clinical protocol that can be very sensitive to the tissue degradation, that is, instead of examining the T1ρ values directly, one could do MRI T1ρ experiments, twice, with the second time in the presence of Gd ions. This double T1ρ procedure would be very similar to the current dGEMRIC procedure where T1 of cartilage is imaged twice, with the second time in the presence of Gd ions. If there were little difference between the two T1ρ scans, the tissue would be healthy. More PG loss will result in a larger difference. Please note that the authors are not promoting a frequent use of the Gd ions in human MRI. This new protocol merely suggests that if Gd ions are being administrated into patients (like in the dGEMRIC protocol), then a double T1ρ protocol is more advantageous than a proper dGEMRIC protocol which requires two T1 scans, since T1ρ-before has its own clinical significance (15,41) but T1-before does not (7). (Note that it is common in dGEMRIC to assume a constant T1-before and to acquire only the T1-after scan (42). However, considering the recent observation that T1-before and T1-after are both strain dependent (43), the assumption of a constant T1-before might be problematic if we need to consider the loading or loading history of the patient – the osteoarthritic cartilage is soft and, hence, easier to deform.)
It should be pointed that the high-resolution experiments in this project were performed at room temperature and a 7 Tesla magnet, which are somewhat different from the clinical conditions (1.5–3 Tesla and body temperature). Despite of its limitations in magnet size and other factors, µMRI is a perfect tool in the study of articular cartilage because of its ability to resolve fine tissue structures (tens of microns transversely) and its sensitivities to the delicate molecular environment. The developments at both microscopic and clinical resolutions should follow the resolution ‘scaling law’ in cartilage MRI (44), eventually leading to the successful diagnostics and management of cartilage lesion in human at its early stages.
Conclusions
In this microscopic imaging project, both T2 and T1ρ relaxation times were studied in native and trypsin-degraded articular cartilage specimens, without and with the presence of 1 mM Gd-DTPA2− and at both 0° and the magic angle. T1ρ values were very sensitive to PG loss regardless of the specimen orientation in the magnet field. The sensitivity of T2 measurements to PG loss, by comparison, depended on the fibril orientation (more sensitive at the magic angle than at 0°) and the depth of cartilage (at 0°, more sensitive at TZ than SZ and RZ; at 55°, nearly uniform sensitivity). Compared to a slight decrement between native tissue with and without Gd solution, there was a more significant change between degraded tissue before/after soaked in Gd solution. The presence of 1 mM Gd-DTPA2− reduced the sensitivity of both T2 and T1ρ to cartilage degradation. However, a compound parameter, T1ρ without and with the presence of Gd-DTPA2−, might become a sensitive parameter to detect the cartilage disease.
Acknowledgment
Yang Xia is grateful to the National Institutes of Health for the R01 grants (AR 045172 and AR 052353). The authors thank Drs. Mengjun Wang and Kefei Zhang in the Labs of Drs. C Les and H Sabbah (Henry Ford Hospital, Detroit) for helping the harvest of canine specimens, Ms Janelle Spann (Michigan Resonance Imaging, Rochester Hills, Michigan) for providing the contrast agent, and Ms Carol Searight (Oakland University) for editorial comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maroudas A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheol. 1975;12(3–4):233–248. doi: 10.3233/bir-1975-123-416. [DOI] [PubMed] [Google Scholar]
- 2.Venn M, Maroudas A. Chemical composition and swelling of normal and osteoarthritic femoral head cartilage. I. Chemical composition. Ann Rheum Dis. 1977;36(2):121. doi: 10.1136/ard.36.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instructional Course Lectures. 2005;54:465. [PubMed] [Google Scholar]
- 4.Kim DK, Ceckler TL, Hascall VC, Calabro A, Balaban RS. Analysis of water-macromolecule proton magnetization transfer in articular cartilage. Magn Reson Med. 1993;29(2):211–215. doi: 10.1002/mrm.1910290209. [DOI] [PubMed] [Google Scholar]
- 5.Bacic G, Liu KJ, Goda F, Hoopes PJ, Rosen GM, Swartz HM. MRI contrast enhanced study of cartilage proteoglycan degradation in the rabbit knee. Magn Reson Med. 1997;37(5):764–768. doi: 10.1002/mrm.1910370520. [DOI] [PubMed] [Google Scholar]
- 6.Gray ML, Burstein D, Xia Y. Biochemical (and functional) imaging of articular cartilage. Semin Musculoskelet Radiol. 2001;5:329–344. doi: 10.1055/s-2001-19043. [DOI] [PubMed] [Google Scholar]
- 7.Xia Y. Relaxation anisotropy in cartilage by NMR microscopy (µMRI) at 14µm resolution. Magn Reson Med. 1998;39(6):941–949. doi: 10.1002/mrm.1910390612. [DOI] [PubMed] [Google Scholar]
- 8.Xia Y. Magic-angle effect in magnetic resonance imaging of articular cartilage: A review. Investigative Radiol. 2000;35(10):602–621. doi: 10.1097/00004424-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Mlynarik V, Mosher TJ, Smith HE, Dardzinski B. Magic angle effect in articular cartilage. Am J Roentgenol. 2002;178(5):1287–1288. doi: 10.2214/ajr.178.5.1781287. [DOI] [PubMed] [Google Scholar]
- 10.Bashir A, Gray ML, Burstein D. Gd-DTPA2− as a measure of cartilage degradation. Magn Reson Med. 1996;36(5):665–673. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 11.Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, Boutin RD, Gray ML. Protocol issues for delayed Gd-DTPA2− enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45(1):36–41. doi: 10.1002/1522-2594(200101)45:1<36::aid-mrm1006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 12.Tiderius CJ, Olsson LE, Leander P, Ekberg O, Dahlberg L. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn Reson Med. 2003;49(3):488–492. doi: 10.1002/mrm.10389. [DOI] [PubMed] [Google Scholar]
- 13.Xia Y, Zheng SK, Bidthanapally A. Depth dependent profiles of glycosaminoglycans in articular cartilage by MRI and histochemistry. J Magn Reson Imaging. 2008;28(1):151–157. doi: 10.1002/jmri.21392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng SK, Xia Y. The impact of the relaxivity definition on the quantitative measurement of glycosaminoglycans in cartilage by the MRI dGEMRIC method. Magn Reson Med. 2010;63(1):25–32. doi: 10.1002/mrm.22169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duvvuri U, Kudchodkar S, Reddy R, Leigh JS. T1ρ relaxation can assess longitudinal proteoglycan loss from articular cartilage in vitro. Osteoarthritis Cartilage. 2002;10(11):838–844. doi: 10.1053/joca.2002.0826. [DOI] [PubMed] [Google Scholar]
- 16.Regatte RR, Akella SVS, Lonner JH, Kneeland JB, Reddy R. T1ρ relaxation mapping in human osteoarthritis (OA) cartilage: Comparison of T1ρ with T2. J Magn Reson Imaging. 2006;23(4):547–553. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 17.Bolbos RI, Link TM, Benjamin Ma C, Majumdar S, Li X. T1ρ relaxation time of the meniscus and its relationship with T1ρ of adjacent cartilage in knees with acute ACL injuries at 3 T. Osteoarthritis cartilage. 2009;17(1):12–18. doi: 10.1016/j.joca.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link T, Ma B, Ries M, Majumdar S. Spatial distribution and relationship of T1ρ and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med. 2009;61(6):1310–1318. doi: 10.1002/mrm.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fullerton G, Cameron I, Ord V. Orientation of tendons in the magnetic field and its effect on T2 relaxation times. Radiol. 1985;155(2):433–435. doi: 10.1148/radiology.155.2.3983395. [DOI] [PubMed] [Google Scholar]
- 20.Peto S, Gillis P, Henri VP. Structure and dynamics of water in tendon from NMR relaxation measurements. Biophys J. 1990;57(1):71–84. doi: 10.1016/S0006-3495(90)82508-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia Y, Farquhar T, Burton-Wurster N, Lust G. Origin of cartilage laminae in MRI. J Magn Reson Imaging. 1997;7(5):887–894. doi: 10.1002/jmri.1880070518. [DOI] [PubMed] [Google Scholar]
- 22.Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41(5):857–865. doi: 10.1002/(sici)1522-2594(199905)41:5<857::aid-mrm1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Virta A, Komu M, Kormano M. T1ρ of protein solutions at very low fields: dependence on molecular weight, concentration, and structure. Magn Reson Med. 1997;37(1):53–57. doi: 10.1002/mrm.1910370109. [DOI] [PubMed] [Google Scholar]
- 24.Duvvuri U, Goldberg AD, Kranz JK, Hoang L, Reddy R, Wehrli FW, Wand AJ, Englander S, Leigh JS. Water magnetic relaxation dispersion in biological systems: the contribution of proton exchange and implications for the noninvasive detection of cartilage degradation. Proceedings Nat Acad Sci USA. 2001;98(22):12479–12484. doi: 10.1073/pnas.221471898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Han ET, Busse RF, Majumdar S. In vivo T1ρ mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS) Magn Reson Med. 2008;59(2):298–307. doi: 10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akella SVS, Regatte RR, Wheaton AJ, Borthakur A, Reddy R. Reduction of residual dipolar interaction in cartilage by spin-lock technique. Magn Reson Med. 2004;52(5):1103–1109. doi: 10.1002/mrm.20241. [DOI] [PubMed] [Google Scholar]
- 27.Stanisz GJ, Henkelman RM. Gd-DTPA relaxivity depends on macromolecular content. Magn Reson Med. 2000;44(5):665–667. doi: 10.1002/1522-2594(200011)44:5<665::aid-mrm1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 28.Henkelman RM, Stanisz GJ, Menezes N, Burstein D. Can MTR be used to assess cartilage in the presence of Gd-DTPA2−? Magn Reson Med. 2002;48(6):1081–1084. doi: 10.1002/mrm.10322. [DOI] [PubMed] [Google Scholar]
- 29.Nieminen MT, Menezes NM, Williams A, Burstein D. T2 of articular cartilage in the presence of Gd-DTPA2−. Magn Reson Med. 2004;51(6):1147–1152. doi: 10.1002/mrm.20083. [DOI] [PubMed] [Google Scholar]
- 30.Taylor C, Carballido-Gamio J, Majumdar S, Li X. Comparison of quantitative imaging of cartilage for osteoarthritis: T2, T1ρ, dGEMRIC and contrast-enhanced computed tomography. Magn Reson Imaging. 2009;27(6):779–784. doi: 10.1016/j.mri.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia Y, Farquhar T, Burton-Wurster N, Vernier-Singer M, Lust G, Jelinski L. Self-diffusion monitors degraded cartilage. Arch Biochem Biophys. 1995;323(2):323–328. doi: 10.1006/abbi.1995.9958. [DOI] [PubMed] [Google Scholar]
- 32.Zheng SK, Xia Y, Bidthanapally A, Badar F, Ilsar I, Duvoisin N. Damages to the extracellular matrix in articular cartilage due to cryopreservation by microscopic magnetic resonance imaging and biochemistry. Magn Reson Imaging. 2009;27(5):648–655. doi: 10.1016/j.mri.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia Y, Moody J, Burton-Wurster N, Lust G. Quantitative In Situ Correlation Between Microscopic MRI and Polarized Light Microscopy Studies of Articular Cartilage. Osteoarthritis Cartilage. 2001;9(5):393–406. doi: 10.1053/joca.2000.0405. [DOI] [PubMed] [Google Scholar]
- 34.Alhadlaq H, Xia Y, Moody JB, Matyas J. Detecting Structural Changes in Early Experimental Osteoarthritis of Tibial Cartilage by Microscopic MRI and Polarized Light Microscopy. Ann Rheum Dis. 2004;63(6):709–717. doi: 10.1136/ard.2003.011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosher TJ, Smith H, Dardzinski BJ, Schmithorst VJ, Smith MB. MR imaging and T2 mapping of femoral cartilage: in vivo determination of the magic angle effect. Am J Roentgenol. 2001;177(3):665–669. doi: 10.2214/ajr.177.3.1770665. [DOI] [PubMed] [Google Scholar]
- 36.Menezes NM, Gray ML, Hartke JR, Burstein D. T2 and T1ρ MRI in articular cartilage systems. Magn Reson Med. 2004;51(3):503–509. doi: 10.1002/mrm.10710. [DOI] [PubMed] [Google Scholar]
- 37.Trattnig S, Millington SA, Szomolanyi P, Marlovits S. MR imaging of osteochondral grafts and autologous chondrocyte implantation. Eur Radiol. 2007;17(1):103–118. doi: 10.1007/s00330-006-0333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe A, Boesch C, Siebenrock K, Obata T, Anderson SE. T2 mapping of hip articular cartilage in healthy volunteers at 3T: a study of topographic variation. J Magn Reson Imaging. 2007;26(1):165–171. doi: 10.1002/jmri.21014. [DOI] [PubMed] [Google Scholar]
- 39.Nissi MJ, Rieppo J, Toyras J, Laasanen MS, Kiviranta I, Nieminen MT, Jurvelin JS. Estimation of mechanical properties of articular cartilage with MRI - dGEMRIC, T2 and T1 imaging in different species with variable stages of maturation. Osteoarthritis Cartilage. 2007;15(10):1141–1148. doi: 10.1016/j.joca.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 40.Zheng S, Xia Y. Multi-components of T2 relaxation in ex vivo cartilage and tendon. J Magn Reson. 2009;198(2):188–196. doi: 10.1016/j.jmr.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Regatte RR, Akella SVS, Borthakur A, Kneeland JB, Reddy R. Proteoglycan Depletion-Induced Changes in Transverse Relaxation Maps of Cartilage: Comparison of T2 and T1ρ. Acad Radiol. 2002;9(12):1388–1394. doi: 10.1016/s1076-6332(03)80666-9. [DOI] [PubMed] [Google Scholar]
- 42.Kim YJ, Jaramillo D, Millis MB, Gray ML, Burstein D. Assessment of early osteoarthritis in hip dysplasia with delayed gadolinium-enhanced magnetic resonance imaging of cartilage. J Bone Joint Surg. 2003;85A(10):1987–1992. doi: 10.2106/00004623-200310000-00019. [DOI] [PubMed] [Google Scholar]
- 43.Xia Y, Wang N, Lee J, Badar F. Strain-dependent T1 relaxation profiles in articular cartilage by MRI at microscopic resolutions. Magn Reson Med. 2011;65:1733–1737. doi: 10.1002/mrm.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia Y. Resolution 'scaling law' in MRI of articular cartilage. Osteoarthritis Cartilage. 2007;15(4):363–365. doi: 10.1016/j.joca.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]