Abstract
Recurrent apnea with intermittent hypoxia is a major clinical problem in preterm infants. Recent studies, although limited, showed that adults who were born preterm exhibit increased incidence of sleep-disordered breathing and hypertension, suggesting that apnea of prematurity predisposes to autonomic dysfunction in adulthood. Here, we demonstrate that adult rats that were exposed to intermittent hypoxia as neonates exhibit exaggerated responses to hypoxia by the carotid body and adrenal chromaffin cells, which regulate cardio-respiratory function, resulting in irregular breathing with apneas and hypertension. The enhanced hypoxic sensitivity was associated with elevated oxidative stress, decreased expression of genes encoding antioxidant enzymes, and increased expression of pro-oxidant enzymes. Decreased expression of the Sod2 gene, which encodes the antioxidant enzyme superoxide dismutase 2, was associated with DNA hypermethylation of a single CpG dinucleotide close to the transcription start site. Treating neonatal rats with decitabine, an inhibitor of DNA methylation, during intermittent hypoxia exposure prevented oxidative stress, enhanced hypoxic sensitivity, and autonomic dysfunction. These findings implicate a hitherto uncharacterized role for DNA methylation in mediating neonatal programming of hypoxic sensitivity and the ensuing autonomic dysfunction in adulthood.
Keywords: blood pressure, developmental programming, norepinephrine
In preterm infants, respiratory disorders with recurrent apnea and the associated intermittent hypoxemia (IH) are major clinical problems (1). Infants with recurrent apnea exhibit an enhanced hypoxic ventilatory response (2), an effect that was attributed to a heightened chemo-reflex arising from the carotid body, which is a sensory organ that detects changes in arterial blood O2 levels (3). Carotid body sensitivity to hypoxia is reset after birth and this effect is modulated by chronic hypoxia (4–6). Neonatal rats exposed to IH showed exaggerated carotid body and ventilatory responses to hypoxia (7, 8). Catecholamine secretion from the adrenal medulla is another important homeostatic mechanism that preserves cardiovascular function under hypoxia (9, 10). In neonates, hypoxia facilitates catecholamine secretion by directly affecting the excitability of adrenal chromaffin cells (11), a response that is markedly augmented in neonatal rats subjected to IH (12, 13). Exaggerated hypoxic sensing of carotid body and adrenal chromaffin cells in neonates by IH is attributed to increased oxidative stress (12, 14). IH also augments hypoxic responses of the carotid body and adrenal medulla in adult rats (15, 16), which is completely reversed following reoxygenation (15). In striking contrast, in neonates the augmented hypoxic sensitivity that is induced by IH persists into adulthood (8, 12, 14). The molecular mechanisms underlying the long-lasting effects of neonatal IH on hypoxic sensing and its physiological consequences in adult life are not known.
It is being increasingly recognized that environmental factors during prenatal and early postnatal periods influence developmental programming of homeostatic mechanisms that profoundly impact on susceptibility to diseases, such as hypertension in adulthood (17–19). Emerging evidence suggests that aberrant epigenetic regulation is an underlying mechanism for neonatal basis of diseases in adulthood (20–24). Epigenetic mechanisms are complex and include changes in small interfering RNAs, DNA methylation, and histone modifications. Of these mechanisms, DNA methylation appears critical for mediating neonatal effects on adult diseases (25, 26). We tested the hypothesis that the long-term effects of neonatal IH on hypoxic sensing are a result of changes in DNA methylation in the carotid body and adrenal medulla. Given that carotid body reflexes regulate breathing and that catecholamine secretion from the adrenal medulla contributes to cardiovascular homeostasis, we further determined whether neonatal IH affects cardio-respiratory functions in adulthood.
Results
Effects of Neonatal IH on the Expression of DNA Methyltransferases.
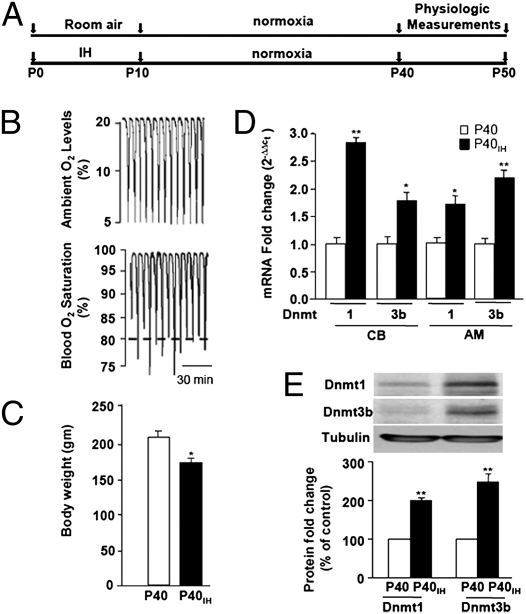
Rat pups and their dams were subjected to IH (alternating cycles of 5% O2 for 15 s and 21% O2 for 5 min), 8 h per day for 10 d, as described in Materials and Methods. Control studies were performed on age-matched rats treated with alternating cycles of room air. Following 10 d of IH, rats were reared in room air until postnatal day 40 (P40) and all measurements were made between P40 and P50 (Fig. 1A). Arterial blood O2 saturations were determined during IH exposure in a subset of rat pups at age P5, as described in Materials and Methods. An example illustrating the blood O2 saturations during IH exposure in a P5 rat is shown in Fig. 1B. During each episode of IH, blood O2 saturation decreased from 98 ± 2 to 82 ± 4% (mean ± SEM; n = 7). Adult rats exposed to neonatal IH (P40IH) exhibited a modest but significant mean decrease in body weight compared with controls (P40) (Fig. 1C).
Fig. 1.
Effects of neonatal IH on DNA methyltransferase expression in adult rats. (A) Schematic presentation of experimental protocols. (B) Ambient O2 levels and blood O2 saturation during exposure to IH in a P5 conscious rat. (C) Body weights of P40 rats that were exposed to either room air (P40) or neonatal IH (P40IH) from ages P0 to P10. Data shown are mean ± SEM from n = 10 rats from three different litters for each group. (D) RT-qPCR analysis of Dnmt1 and Dnmt3b mRNA levels in carotid body (CB) and adrenal medulla (AM) from P40 and P40IH rats. Data are expressed relative to 18S rRNA and are presented as mean ± SEM from four independent experiments. (E) Immunoblot assays of Dnmt1 and Dnmt3b protein in adrenal medulla from P40 and P40IH rats. Densitometry data are presented as mean ± SEM from five experiments. *P < 0.05 and **P < 0.01 compared with control normoxic P40 rats.
DNA methylation is catalyzed by the DNA methyltransferases Dnmt1, Dnmt3a, and Dnmt3b. Whereas Dnmt1 is responsible for maintenance of methylation, Dnmt3a and Dnmt3b are de novo methylases (20). To investigate the role of DNA methylation, we first determined whether expression of Dnmts is altered in adult rats that were subjected to neonatal IH. Dnmt mRNA levels were determined by quantitative real-time reverse-transcription PCR (RT-qPCR) in both carotid body and adrenal medulla and the corresponding proteins were analyzed by immunoblot in adrenal medulla only because of the small size of the carotid body (∼50 μg wet weight). The levels of Dnmt1 and Dnmt3b mRNA and protein were significantly increased in rats exposed to neonatal IH (P40IH) compared with controls (P40) (Fig. 1 D and E). In contrast, Dnmt3a mRNA expression was unchanged.
Neonatal IH Increases DNA Methylation.
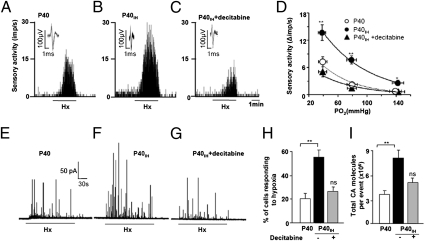
We hypothesized that if DNA methylation mediates exaggerated hypoxic sensing, then administration of a DNA hypomethylating agent should prevent this response. To test this hypothesis, neonatal rats received intraperitoneal injections of decitabine, an inhibitor of DNA methylation, at a dose of 1 mg/kg of body weight every third day during the 10-d exposure to IH. Following decitabine treatment, rat pups were reared in room air until age P40. Subsequently, carotid body activity and catecholamine secretion from chromaffin cells were determined. The carotid body response to hypoxia significantly increased in adult rats exposed to neonatal IH and this effect was eliminated by decitabine (Fig. 2 A–D). In contrast, decitabine had no significant effect on carotid body excitation by CO2, another physiological stimulus to the carotid body, nor did it alter carotid body morphology (Fig. S1 A–C). In control rats, decitabine had no significant effect on carotid body response to hypoxia (Fig. S1D).
Fig. 2.
Effect of neonatal IH on carotid body and adrenal chromaffin cell responses to hypoxia in adult rats. (A–C) Isolated superfused carotid body sensory responses to hypoxia (Hx; ∼40 mmHg) in P40 to P50 rats that were exposed to normoxia (P40; A) or neonatal IH (P40IH; B), or treated with decitabine during neonatal IH (P40IH + decitabine; C). Black bar represents the duration of the hypoxic challenge. Integrated carotid body sensory activity presented as impulses per second (imp/s). Superimposed action potential from the “single” fiber from which the data are derived is presented (Inset). (D) Mean carotid body responses to graded hypoxia from P40, P40IH, and P40IH + decitabine-treated rats shown as the difference in response between baseline and hypoxia (Δimp/s). Data presented are mean ± SEM of n = 18 (P40), 20 (P40IH), and 21 (P40IH + decitabine) fibers from 10 to 11 rats each. (E–G) Hypoxia-evoked catecholamine secretion from chromaffin cells isolated from P40 rats exposed to normoxia (P40; E) or neonatal IH (P40IH; F), or treated with decitabine during exposure to neonatal IH (P40IH + decitabine; G). Hx = PO2 ∼30 mmHg. Black bar represents the duration of the hypoxic challenge. (H) Mean number of cells responding to hypoxia and (I) total catecholamine (CA) secreted during the hypoxic challenge (number of secretory events × CA molecules secreted per event). Data shown are mean ± SEM from n = 14 control cells (P40; open bar), 18 cells from neonatal IH (P40IH; closed bar), and 9 cells from P40IH + decitabine-treated rats (gray bar) from three different litters in each group. **P < 0.01 compared with P40 normoxic control rats. n.s., P > 0.05 for P40 vs. P40IH + decitabine.
Catecholamine secretion from single adrenal chromaffin cells was determined by carbon-fiber amperometry. The number of chromaffin cells responding to hypoxia and the magnitude of low pO2-evoked catecholamine secretion were significantly greater in rats exposed to neonatal IH, whereas decitabine treatment prevented this exaggerated sensitivity to hypoxia (Fig. 2 E–I). In contrast, catecholamine secretion evoked by K+, a nonselective depolarizing agent, was unaffected by decitabine treatment (Fig. S1 E and F).
Gene Targets of DNA Methylation in Response to Neonatal IH.
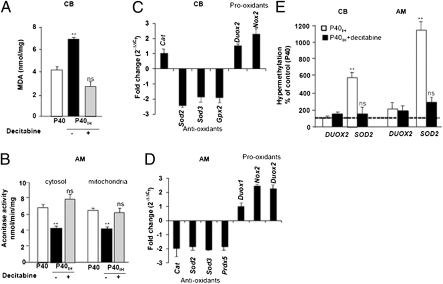
Previous studies have documented that oxidative stress mediates the effects of neonatal IH on the carotid body and adrenal medulla (12, 14). To determine whether adult rats that were subjected to neonatal IH exhibit oxidative stress, we measured malondialdehyde levels (in the carotid body) and aconitase activity (in the adrenal medulla), which are two established markers of oxidative stress (27, 28). Carotid body as well as adrenal medulla from adult rats exposed to neonatal IH exhibited significantly increased oxidative stress and decitabine treatment prevented this effect (Fig. 3 A and B). Decitabine had no significant effect on aconitase activity in adrenal medulla in control P40 rats (Fig. S1G). We hypothesized that increased oxidative stress is caused by alterations in the expression of genes encoding pro-oxidant and antioxidant enzymes, which regulate the redox state. RT-qPCR analysis revealed increased expression of genes encoding pro-oxidant enzymes and decreased expression of genes encoding antioxidant enzymes in carotid body and adrenal medulla from rats treated with neonatal IH compared with controls (Fig. 3 C and D).
Fig. 3.
Effects of neonatal IH on oxidative stress, mRNA levels of pro-oxidant and antioxidant enzymes, and DNA methylation of the Sod2 gene. (A and B) Increased oxidative stress evidenced by elevated malondialdehyde (MDA) levels in the carotid body (A) and decreased cytosolic and mitochondrial aconitase activity in adrenal medulla (B) of P40 rats that were exposed to IH as neonates (P40IH) but not in P40IH + decitabine-treated rats. Data shown are mean ± SEM from four independent experiments each. (C and D) Expression of mRNAs encoding pro-oxidant and antioxidant enzymes in the carotid body (C) and adrenal medulla (D) from P40 rats exposed to neonatal IH, as analyzed by quantitative real-time RT-PCR: catalase (Cat), superoxide dismutase (Sod1, 2, and 3), glutathione peroxidase 2 (Gpx2), peroxiredoxin 5 (Prdx5), dual oxidase 1 and 2 (Duox 1, -2), and NADPH oxidase 2 (Nox2). Mean ± SEM (n = 4) data for the fold-change in rats exposed to neonatal IH relative to control rats are shown. (E) Hypermethylation of Sod2 but not Duox2 in carotid body and adrenal medulla of P40 rats exposed to neonatal IH (P40IH; open bars) and absence of this response following decitabine treatment (P40IH + decitabine; closed bars). Data shown are mean ± SEM from five independent experiments each. **P < 0.01 compared with normoxic P40 controls; n.s., P > 0.05 for P40 vs. P40IH + decitabine.
Because DNA methylation represses gene transcription (20), we reasoned that the down-regulation of genes encoding antioxidant enzymes could be because of DNA hypermethylation. To test this possibility, genomic DNA was isolated from carotid bodies and adrenal medullae and the DNA methylation status of the Sod2 (encoding manganese superoxide dismutase) and Duox2 (encoding dual oxidase 2) genes representing antioxidant and pro-oxidant enzymes, respectively, was determined. Epitect Methyl qPCR primer assays were designed for the analysis of DNA methylation status of predicted CpG islands in the rat Sod2 (Chr. 1: 41869781–41870602) and Duox2 (Chr. 3: 109080491–109080755) genes (29). DNA methylation of the Sod2 gene increased 6- and 12-fold in carotid body and adrenal medulla, respectively, of rats exposed to neonatal IH, whereas DNA methylation of the Duox2 gene was unchanged (Fig. 3E). Decitabine treatment during neonatal IH exposure prevented DNA methylation of the Sod2 gene in both carotid body and adrenal medulla (Fig. 3E).
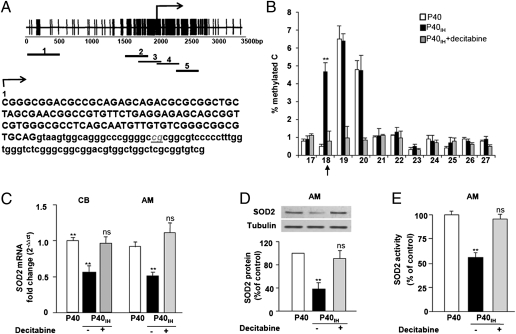
Methylation of CpG Dinucleotides in the Sod2 Gene in Response to Neonatal IH.
To identify the specific CpG dinucleotides that were affected by neonatal IH within the CpG island that overlaps the Sod2 promoter, DNA methylation was analyzed by bisulfite sequencing, using primers that spanned from −2 kb to +2 kb relative to the transcription start site (Fig. 4A). These experiments were performed on adrenal medullary tissue because of its larger mass relative to carotid body. Bisulfite sequencing revealed that a single CpG dinucleotide in the first intron (+128 bp from the transcription start site) was methylated specifically in samples from rats exposed to neonatal IH, whereas the adjacent two CpG dinucleotides showed constitutive methylation and neonatal IH had no further effect (Fig. 4B). Decitabine eliminated the neonatal IH-induced methylated CpG dinucleotide, as well as the constitutively methylated CpG dinucleotides. DNA methylation of the Sod2 gene in response to neonatal IH was associated with down-regulation of Sod2 mRNA, protein, and enzyme activity and these effects were absent in decitabine-treated samples (Fig. 4 C–E). Decitabine treatment, however, had no effect on Sod2 mRNA in carotid bodies and adrenal medullae from control P40 rats (Fig. S1H).
Fig. 4.
Analysis of cytosine methylation in the Sod2 gene in adrenal medullae from P40 rats exposed to neonatal IH (P40IH). (A) (Upper) Schematic representation of CpG islands in the Sod2 gene. Each vertical line represents a single CpG dinucleotide. Bent arrow denotes the transcription start site. For PCR amplification and sequence analysis of bisulfite modified DNA, the region was divided into five subregions represented as short solid lines (numbered 1–5). (Lower) Nucleotide sequence of the Sod2 gene starting from the transcription start site. Nucleotides in upper and lowercase represent exon 1 and intron 1 sequences, respectively. The CpG dinucleotide in intron 1 that is hypermethylated in response to neonatal IH is underscored. (B) Quantitative analysis of percent methylated cytosine (C) represented on y axis calculated from the formula [meC/(C + meC)] × 100, where meC and C represent methylated and nonmethylated cytosine, respectively. The x-axis represents CpG dinucleotides numbered from the transcription start site. CpG dinucleotide at position 18 (arrow) is methylated in neonatal IH-exposed (P40IH) samples, whereas CpG dinucleotides at 19 and 20 were constitutively methylated. Decitabine treatment (P40IH+decitabine) eliminated both the IH-induced and constitutively methylated CpG dinucleotides. Data presented are mean values obtained from three independent experiments performed on adrenal medulla harvested from eight rats in each group. (C–E) Analysis of Sod2 mRNA in carotid body and adrenal medulla (C), and analysis of SOD2 protein (D), and enzyme activity (E) in adrenal medulla from P40 rats exposed to neonatal IH (P40IH) in the presence (+) or absence (−) of decitabine. Data shown are mean ± SEM from three to six independent experiments. **P < 0.01 compared with normoxic P40 controls; n.s., P > 0.05 for P40 vs. P40IH + decitabine.
Autonomic Dysfunction in Adult Rats Exposed to Neonatal IH.
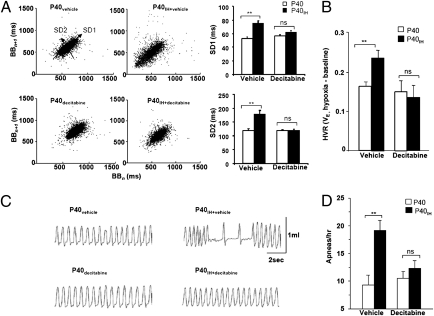
Reflexes arising from the carotid body and catecholamine secretion from adrenal medulla play key roles in the maintenance of cardio-respiratory homeostasis. To assess whether the exaggerated carotid body and adrenal chromaffin cell responses to hypoxia affect cardio-respiratory function, breathing and blood pressure were determined in conscious rats by whole-body plethysmography and tail-cuff methods, respectively. Breathing patterns were analyzed while rats were quiet, without body movements, as judged visually. Sniffs and sighs were excluded in the analysis. Breathing variability was assessed by measuring the breath-to-breath (BBn) interval versus the subsequent BB interval (BBn+1) for 500 breaths while breathing room air. The data are presented as Poincaré plots (Fig. 5A). Variability of BB intervals was greater in rats exposed to neonatal IH compared with control rats, as indicated by the data scatter (Fig. 5A). Analysis of the SD of BB intervals (30) showed that SD1 (SD of data points from the ascending 45° lines) (Fig. 5A, Left) and SD2 (SD of data points from the line orthogonal to SD1) were significantly greater in rats exposed to neonatal IH than age-matched controls, indicative of irregular breathing (Fig. 5A, Right). The magnitude of hypoxic ventilatory response was significantly greater in rats exposed to neonatal IH (Fig. 5B). Furthermore, the number of spontaneous apneas (defined as cessation of breathing for more than three breaths) was significantly greater in rats exposed to neonatal IH than controls (Fig. 5 C and D). Remarkably, decitabine treatment prevented irregular breathing, apneas, and the enhanced hypoxic ventilatory response in neonatal IH exposed rats, whereas it had no effect on respiratory variables in control P40 rats (Fig. 5).
Fig. 5.
Effects of neonatal IH on breathing in adult rats. (A) (Left and Center) Poincarè plots of breath-to-breath (BBn; x axis) versus next BB interval (BBn+1; y axis) for 500 breaths analyzed in vehicle or decitabine-treated control (P40), neonatal IH-exposed (P40IH) rats. (Right) SD1, SD of data points from the ascending 45° line, and SD2, SD of data points from the line orthogonal to SD1, are presented as mean ± SEM from 10 rats in each group. (B) Hypoxic ventilatory response (HVR) in control (P40) and neonatal IH (P40IH) rats treated with vehicle or decitabine. Data presented are mean ± SEM of change in minute ventilation (VE) [i.e., respiratory rate (breaths/min) × tidal volume (normalized to per gram of body weight) upon shift from 21% O2 to 12% O2 (n = 8 each)]. (C) Representative examples of breathing in adult rats exposed to normoxia (P40) or neonatal IH (P40IH) treated with vehicle or decitabine. Note periods of apnea (cessation of breathing for more than three breaths) in tracing at upper right. (D) Mean ± SEM number of apneas per hour from 10 rats in each group. **P < 0.01; n.s., P > 0.05.
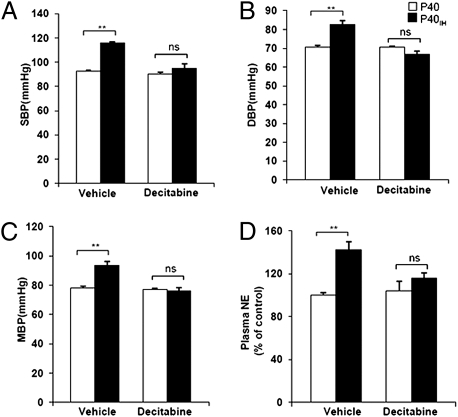
Mean blood pressures were significantly higher in adult rats exposed to neonatal IH, which was because of a significant increase in systolic and diastolic pressures (Fig. 6 A–C). The elevated blood pressures were associated with increased plasma norepinephrine levels (Fig. 6D). Decitabine treatment prevented hypertension and elevated plasma norepinephrine levels in neonatal IH-exposed rats, whereas similar treatment with decitabine in control rats exposed only to room air in the neonatal period had no significant effect on blood pressure and plasma norepinephrine levels (Fig. 6).
Fig. 6.
Effects of neonatal IH on blood pressure and plasma norepinephrine levels in adult rats. Systolic (SBP), diastolic (DBP), and mean (MBP) blood pressure (A–C) and plasma norepinephrine (NE) levels (D) in rats exposed to normoxia (P40) or neonatal IH (P40IH) treated with vehicle or decitabine. Data presented as mean ± SEM from 10 rats in each group. **P < 0.01. n.s., P > 0.05.
Discussion
The present study demonstrates that: (i) perturbations in O2 homeostasis because of IH during neonatal life predispose to enhanced hypoxic sensing in adulthood; and (ii) this effect involves DNA hypermethylation of the Sod2 gene, which encodes the principal mitochondrial antioxidant enzyme, leading to disruption of redox homeostasis and oxidative stress. Remarkably, neonatal IH-evoked hypermethylation involves a single CpG dinucleotide within the Sod2 gene close to the transcription initiation site and was associated with reduced Sod2 mRNA, protein, and enzyme activity. Although CpG dinucleotide analysis was performed only in adrenal glands, it is likely that similar changes also occur in the carotid body, because both these organs exhibit similar sensitivity to hypoxia. Decitabine prevented Sod2 DNA hypermethylation, as well as oxidative stress, and eliminated hypersensitivity of the carotid body and adrenal chromaffin cells to hypoxia. The effects of decitabine are unlikely to be the result of nonselective actions because: (i) decitabine had no effect on responses of the carotid body and adrenal medulla to stimuli other than hypoxia in adult rats exposed to neonatal IH; and (ii) in control rats, neonatal decitabine treatment had no impact on physiological responses to hypoxia, redox status, or Sod2 mRNA expression. The expression of genes encoding other antioxidant enzymes was also down-regulated in rats exposed to neonatal IH. Whether these effects are mediated by DNA methylation remains to be studied. Taken together, these findings demonstrate a hitherto uncharacterized role for epigenetic modulation of hypoxic sensing via DNA methylation.
Although rats were exposed to IH for only 10 d in the neonatal period, they exhibited remarkable cardio-respiratory abnormalities in adulthood, manifested as irregular breathing, spontaneous apneas, and hypertension. Irregular breathing with apneas represents instability of the ventilatory control system and hyperactive carotid body function has been implicated in triggering ventilatory instability (31, 32). Given that hypoxic sensitivity of the carotid body is exaggerated in these rats, irregular breathing is likely caused by heightened carotid body reflex function. Supporting this possibility is the finding that rats exposed to neonatal IH displayed an enhanced hypoxic ventilarory response, a hallmark reflex initiated by the carotid body. The transcriptional activators hypoxia-inducible factor 1 (HIF-1) and HIF-2 have been implicated in developmental maturity of hypoxia sensing by the carotid body (33). Whether epigenetic changes induced by neonatal IH impact transcriptional regulation by HIF-1 and HIF-2 remains to be studied.
Circulating catecholamines play an important role in the regulation of blood pressure. In addition to its effect on breathing, carotid body stimulation by hypoxia causes reflex activation of the sympathetic nervous system. Indeed, rats exposed to neonatal IH manifested a hyperactive sympathetic nervous system, as evidenced by increased plasma norepinephrine levels. The hypertension observed in rats exposed to neonatal IH is likely a result of the combined effects of heightened carotid body reflex and enhanced catecholamine secretion from adrenal chromaffin cells. Decitabine treatment eliminated cardio-respiratory abnormalities. The site of action of decitabine is likely the carotid body and adrenal chromaffin cells, with secondary effects on brainstem neurons associated with reflex regulation of cardio-respiratory function by the carotid body. Together, these findings demonstrate that exaggerated hypoxic sensing caused by exposure of neonatal rats to IH predisposes to autonomic dysfunction in adult life.
The current findings are of considerable relevance for understanding the early onset of autonomic dysfunction in adults who were born preterm. Recent studies suggest that young adults who were born preterm exhibit increased incidence of sleep-disordered breathing with apneas (34–36). Furthermore, adults (30 y of age) who had been born preterm showed an increased incidence of hypertension (37). Altered programming of homeostatic mechanisms via epigenetic modulation during perinatal development has been proposed to be the cause of susceptibility to diseases in adulthood (20–24). Consistent with this idea, our data suggest that the early onset of autonomic dysfunction in adult individuals who had been born preterm may be because of exaggerated hypoxic sensing caused by epigenetic modifications involving DNA hypermethylation.
Materials and Methods
Preparation of Rats.
Experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Chicago. Experiments were performed on Sprague-Dawley rats of both sexes.
Exposure to IH.
Rat pups and their dams were exposed to IH from ages P1 to P10 between 9:00 AM and 5:00 PM, as described previously (8, 12, 38). Control experiments were performed on age-matched rats exposed to alternating cycles of room air instead of hypoxia. Experiments were performed on freely mobile rats fed ad libitum. Blood O2 saturation levels were monitored during IH in unsedated and unrestrained rat pups at age P5 using a small animal pulse oxymeter probe placed around the neck (MouseQx Plus; Starr Life Sciences).
Measurements of Breathing, Blood Pressure, and Plasma Catecholamines.
Breathing was monitored by whole-body plethysmography in conscious rats. Baseline breathing was recorded for 2 h while the rats breathed room air. Sniffs and movement artifacts were excluded in the analysis of breathing variability and apnea. Blood pressures were determined by tail cuff method in conscious rats using a noninvasive BP system (AD Instruments), as previously described (27, 39). Breathing and blood pressures were measured between 9:00 and 11:00 AM at ambient temperatures of 25 ± 1 °C. Plasma norepinephrine levels were determined as described previously (39).
Measurements of Carotid Body Sensory Activity and Catecholamine Secretion from Adrenal Chromaffin Cells by Amperometry.
Carotid bodies and adrenal glands were harvested from anesthetized rats (urethane, 1.2 g/kg, i.p.). Electrical activity of the sensory nerve was recorded from carotid bodies ex vivo, as described previously (7). The data were presented as absolute values or change in impulses per second (i.e., hypoxia − baseline). Adrenal chromaffin cells were enzymatically dissociated and plated on coverslips coated with type VII collagen (Sigma). Catecholamine secretion from single chromaffin cells was monitored by amperometry using carbon fiber electrodes, as described previously (12).
Real-Time RT-qPCR Assay.
Total RNA purified from rat carotid body and adrenal gland was used for cDNA synthesis. Aliquots of cDNA were used as template for qPCR, as described previously (39).
DNA Methylation Assays.
Genomic DNA was isolated from rat carotid body and adrenal medulla. DNA methylation-sensitive and methylation-independent restriction enzymes were used to selectively digest unmethylated or methylated DNA, respectively. The remaining DNA after digestion was quantified by qPCR using primers that flanked the predicted CpG islands. The relative concentrations of methylated and unmethylated DNA were determined by comparing the amount in each digest with that of a mock digest. Data were expressed as percent methylation relative to controls. Methylation status of the Sod2 gene was analyzed in adrenal medulla by bisulfate sequencing. Genomic DNA was isolated and incubated with 40% sodium bisulfate in 10 mM hydroquinone for 18 h at 55 °C, which converted nonmethylated cytosine to uracil. Primers chosen based on the region of interest (−2 kb to +2 kb from the transcription start site) were used to amplify the bisulfate-treated DNA segments, which were purified and sequenced. See Table S1 for the nucleotide sequence of primers used for the analysis of cytosine in the Sod2 gene.
Measurement of Malondialdehyde Levels, Aconitase, and Sod2 Enzyme Activities.
Malondialdehyde levels in the carotid body were determined as described previously (39). Mitochondrial and cytosolic fractions were isolated from adrenal medulla by differential centrifugation and Sod2 and aconitase activities were determined as described previously (39). Protein concentrations were determined and the data were expressed as nanomoles per milligram of protein.
Immunoblot Assays.
Tissue extracts were fractionated by 6% polyacrylamide-SDS gel electrophoresis and immunoblot assays were performed with antibodies against Sod2 (Millipore; 1:3,000 dilution), Dnmt1 (Novus Biologicals; 1:2,000 dilution), and Dnmt3b (Cell Signaling; 1:2,000 dilution).
Statistical Analysis.
The data are expressed as mean ± SEM. Statistical analysis was performed by ANOVA and P values < 0.05 were considered significant.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health Grants HL-76537, HL-90554, and HL-86493 (to N.R.P.) and by the Johns Hopkins Institute for Cell Engineering (G.L.S.). G.L.S. is the C. Michael Armstrong Professor at The Johns Hopkins University School of Medicine and N.R.P. is the Harold H. Hines, Jr., Professor at the University of Chicago, Pritzker School of Medicine.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120600109/-/DCSupplemental.
See Commentary on page 2192.
References
- 1.Abu-Shaweesh JM, Martin RJ. Neonatal apnea: What's new? Pediatr Pulmonol. 2008;43:937–944. doi: 10.1002/ppul.20832. [DOI] [PubMed] [Google Scholar]
- 2.Nock ML, Difiore JM, Arko MK, Martin RJ. Relationship of the ventilatory response to hypoxia with neonatal apnea in preterm infants. J Pediatr. 2004;144:291–295. doi: 10.1016/j.jpeds.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 3.Prabhakar NR. Oxygen sensing by the carotid body chemoreceptors. J Appl Physiol. 2000;88:2287–2295. doi: 10.1152/jappl.2000.88.6.2287. [DOI] [PubMed] [Google Scholar]
- 4.Blanco CE, Dawes GS, Hanson MA, McCooke HB. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. J Physiol. 1984;351:25–37. doi: 10.1113/jphysiol.1984.sp015229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hertzberg T, Hellström S, Holgert H, Lagercrantz H, Pequignot JM. Ventilatory response to hyperoxia in newborn rats born in hypoxia—Possible relationship to carotid body dopamine. J Physiol. 1992;456:645–654. doi: 10.1113/jphysiol.1992.sp019358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz-Salamon M, Jonsson B, Lagercrantz H. Blunted peripheral chemoreceptor response to hyperoxia in a group of infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 1995;20:101–106. doi: 10.1002/ppul.1950200209. [DOI] [PubMed] [Google Scholar]
- 7.Peng YJ, Rennison J, Prabhakar NR. Intermittent hypoxia augments carotid body and ventilatory response to hypoxia in neonatal rat pups. J Appl Physiol. 2004;97:2020–2025. doi: 10.1152/japplphysiol.00876.2003. [DOI] [PubMed] [Google Scholar]
- 8.Pawar A, Peng YJ, Jacono FJ, Prabhakar NR. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J Appl Physiol. 2008;104:1287–1294. doi: 10.1152/japplphysiol.00644.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagercrantz H, Bistoletti P. Catecholamine release in the newborn infant at birth. Pediatr Res. 1977;11:889–893. doi: 10.1203/00006450-197708000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Seidler FJ, Slotkin TA. Adrenomedullary function in the neonatal rat: Responses to acute hypoxia. J Physiol. 1985;358:1–16. doi: 10.1113/jphysiol.1985.sp015536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson RJ, Jackson A, Nurse CA. Developmental loss of hypoxic chemosensitivity in rat adrenomedullary chromaffin cells. J Physiol. 1997;498:503–510. doi: 10.1113/jphysiol.1997.sp021876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Souvannakitti D, Kumar GK, Fox A, Prabhakar NR. Neonatal intermittent hypoxia leads to long-lasting facilitation of acute hypoxia-evoked catecholamine secretion from rat chromaffin cells. J Neurophysiol. 2009;101:2837–2846. doi: 10.1152/jn.00036.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souvannakitti D, et al. NADPH oxidase-dependent regulation of T-type Ca2+ channels and ryanodine receptors mediate the augmented exocytosis of catecholamines from intermittent hypoxia-treated neonatal rat chromaffin cells. J Neurosci. 2010;30:10763–10772. doi: 10.1523/JNEUROSCI.2307-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pawar A, et al. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol. 2009;296:R735–R742. doi: 10.1152/ajpregu.90490.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: Implications for recurrent apneas. Proc Natl Acad Sci USA. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar GK, et al. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006;575:229–239. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen G, Jeffery H, Lagercrantz H, Katz-Salamon M. Long-term reprogramming of cardiovascular function in infants of active smokers. Hypertension. 2010;55:722–728. doi: 10.1161/HYPERTENSIONAHA.109.142695. [DOI] [PubMed] [Google Scholar]
- 18.Barker DJ, Osmond C, Simmonds SJ, Wield GA. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ. 1993;306:422–426. doi: 10.1136/bmj.306.6875.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gluckman PD, Cutfield W, Hofman P, Hanson MA. The fetal, neonatal, and infant environments-the long-term consequences for disease risk. Early Hum Dev. 2005;81:51–59. doi: 10.1016/j.earlhumdev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 21.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation: Linking early developmental environment to adult disease. Reprod Toxicol. 2007;23:297–307. doi: 10.1016/j.reprotox.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos F, Dean W. Epigenetic reprogramming during early development in mammals. Reproduction. 2004;127:643–651. doi: 10.1530/rep.1.00221. [DOI] [PubMed] [Google Scholar]
- 25.Niculescu MD, Craciunescu CN, Zeisel SH. Gene expression profiling of choline-deprived neural precursor cells isolated from mouse brain. Brain Res Mol Brain Res. 2005;134:309–322. doi: 10.1016/j.molbrainres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Waterland RA, Jirtle RL. Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng YJ, et al. Heterozygous HIF-1α deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner PR, Nguyen DD, White CW. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc Natl Acad Sci USA. 1994;91:12248–12252. doi: 10.1073/pnas.91.25.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ceccarelli V, et al. Eicosapentaenoic acid demethylates a single CpG that mediates expression of tumor suppressor CCAAT/enhancer-binding protein delta in U937 leukemia cells. J Biol Chem. 2011;286:27092–27102. doi: 10.1074/jbc.M111.253609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brennan M, Palaniswami M, Kamen P. Do existing measures of Poincaré plot geometry reflect nonlinear features of heart rate variability? IEEE Trans Biomed Eng. 2001;48:1342–1347. doi: 10.1109/10.959330. [DOI] [PubMed] [Google Scholar]
- 31.Dunai J, Kleiman J, Trinder J. Ventilatory instability during sleep onset in individuals with high peripheral chemosensitivity. J Appl Physiol. 1999;87:661–672. doi: 10.1152/jappl.1999.87.2.661. [DOI] [PubMed] [Google Scholar]
- 32.Cherniack NS, Longobardo GS. Mathematical models of periodic breathing and their usefulness in understanding cardiovascular and respiratory disorders. Exp Physiol. 2006;91:295–305. doi: 10.1113/expphysiol.2005.032268. [DOI] [PubMed] [Google Scholar]
- 33.Roux JC, Brismar H, Aperia A, Lagercrantz H. Developmental changes in HIF transcription factor in carotid body: Relevance for O2 sensing by chemoreceptors. Pediatr Res. 2005;58:53–57. doi: 10.1203/01.PDR.0000163390.78239.EA. [DOI] [PubMed] [Google Scholar]
- 34.Rosen CL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: Association with race and prematurity. J Pediatr. 2003;142:383–389. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 35.Paavonen EJ, et al. Very low birth weight increases risk for sleep-disordered breathing in young adulthood: The Helsinki Study of Very Low Birth Weight Adults. Pediatrics. 2007;120:778–784. doi: 10.1542/peds.2007-0540. [DOI] [PubMed] [Google Scholar]
- 36.Hibbs AM, et al. Prenatal and neonatal risk factors for sleep disordered breathing in school-aged children born preterm. J Pediatr. 2008;153:176–182. doi: 10.1016/j.jpeds.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalziel SR, Parag V, Rodgers A, Harding JE. Cardiovascular risk factors at age 30 following pre-term birth. Int J Epidemiol. 2007;36:907–915. doi: 10.1093/ije/dym067. [DOI] [PubMed] [Google Scholar]
- 38.Souvannakitti D, et al. Neonatal intermittent hypoxia impairs neuronal nicotinic receptor expression and function in adrenal chromaffin cells. Am J Physiol Cell Physiol. 2010;299:C381–C388. doi: 10.1152/ajpcell.00530.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng YJ, et al. Hypoxia-inducible factor 2α (HIF-2α) heterozygous-null mice exhibit exaggerated carotid body sensitivity to hypoxia, breathing instability, and hypertension. Proc Natl Acad Sci USA. 2011;108:3065–3070. doi: 10.1073/pnas.1100064108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.