Abstract
Abdominal aortic aneurysm (AAA) is a complex inflammatory vascular disease. There are currently limited treatment options for AAA when surgery is inapplicable. Therefore, insights into molecular mechanisms underlying AAA pathogenesis may reveal therapeutic targets that could be manipulated pharmacologically or biologically to halt disease progression. Using an elastase-induced AAA mouse model, we previously established that the complement alternative pathway (AP) plays a critical role in the development of AAA. However, the mechanism by which complement AP is initiated remains undefined. The complement protein properdin, traditionally viewed as a positive regulator of the AP, may also initiate complement activation by binding directly to target surfaces. In this study, we sought to determine whether properdin serves as a focal point for the initiation of the AP complement activation in AAA. Using a properdin loss of function mutation in mice and a mutant form of the complement factor B protein that produces a stable, properdin-free AP C3 convertase, we show that properdin is required for the development of elastase-induced AAA in its primary role as a convertase stabilizer. Unexpectedly, we find that, in AAA, natural IgG antibodies direct AP-mediated complement activation. The absence of IgG abrogates C3 deposition in elastase-perfused aortic wall and protects animals from AAA development. We also determine that blockade of properdin activity prevents aneurysm formation. These results indicate that an innate immune response to self-antigens activates the complement system and initiates the inflammatory cascade in AAA. Moreover, the study suggests that properdin-targeting strategies may halt aneurysmal growth.
Abdominal aortic aneurysm (AAA) is among the top 20 leading causes of mortality in the United States. It accounts for ∼15,000 deaths/y. AAA is a complex disease associated with male sex, advanced age, hypertension, hypercholesterolemia, coronary artery disease, atherosclerosis, and cigarette smoking (1–3). Presently, open surgical repair of large AAA (greater than 5.5 cm in diameter) is an effective option in preventing death from rupture. However, surgical treatment is associated with high postoperative mortality (up to 6%) (4). Endovascular repair is associated with lower postoperative mortality, but it offers no significant difference in overall long-term survival rate and has a higher incidence of reintervention (5–8). Given the high complication rate and morbidities associated with surgical treatment, medical therapy designed to slow progression of AAA would be desirable. Although the causes of AAA are not completely understood, it is apparent that the immune system is a major contributor to pathogenesis. Therefore, a better understanding of the underlying immune-mediated pathways involved in AAA may identify new targets that could be manipulated pharmacologically or biologically to halt disease progression.
The complement system is a branch of immunity that can rapidly respond to foreign intruders or pathogens without prior sensitization or exposure. It plays a key role in the identification and removal of injured tissue and debris (9), modulates the antibody repertoire (10), and participates in the activation of T-cell populations (11). Complement activation is mediated by the classical pathway (CP), which is commonly initiated by antigen–antibody complexes, the lectin pathway (LP), which is initiated by LP-specific carbohydrate recognition complexes, and the alternative pathway (AP), which is constitutively active at low level because of continuous hydrolysis of the complement protein C3. The three pathways converge on a central enzyme, the C3 convertase. This enzyme complex activity generates opsonins that promote target clearance, leads to the assembly of the membrane attack complex, and releases anaphylatoxins (C3a and C5a) that activate and recruit inflammatory cells (9).
We have shown that the complement system is a critical mediator of elastase-induced AAA (12). Using mice deficient in factor B (fB; an essential component of the AP) and C4 (a component of both the LP and CP), we established that the AP plays a major role in AAA development, whereas the CP and LP seem dispensable. Unlike the CP and LP, which are initiated by specific target structures, the AP is continuously activated in the fluid phase and on cell surfaces. It is limited to safe steady-state levels through the action of fluid-phase and surface-bound regulatory proteins. Several AP-dependent diseases, including age-related macular degeneration and atypical hemolytic uremic syndrome (aHUS), have been shown to be, in part, the result of inadequate regulation because of genetic variants in the complement regulator genes (13).
The AP C3 convertase is formed from three components: fB, a zymogen that carries the convertase catalytic site, C3b, which anchors the convertase to the target surface, and properdin, which stabilizes the complex by 5- to 10-fold (14). More recently, evidence has emerged that properdin may act as an initiator of the AP, binding directly to target cell surfaces and providing a platform for the AP C3 convertase assembly (15, 16). We previously obtained compelling histochemical evidence for the presence of properdin on the luminal surface of human AAA specimens (12), and this finding led us to hypothesize that properdin may serve as a focal point for the initiation of the AP in AAA. Here, we show that properdin plays a critical role in the pathogenesis of elastase-induced AAA, suggesting that properdin-targeting strategies merit additional investigation. We assessed the role of properdin in AAA as a stabilizer vs. initiator of AP convertase by using a mutant form of the fB protein that produces a stable properdin-free AP C3 convertase. Our findings indicate that the primary role of properdin in elastase-induced AAA is as a convertase stabilizer. However, we found that complement activation in AAA is dependent on the presence of natural antibodies, lending support to previous reports suggesting that recognition of self-antigen(s) by antibodies initiates the inflammatory cascade in AAA (17).
Results
Complement AP Protein Properdin Is Required for Elastase-Induced AAA.
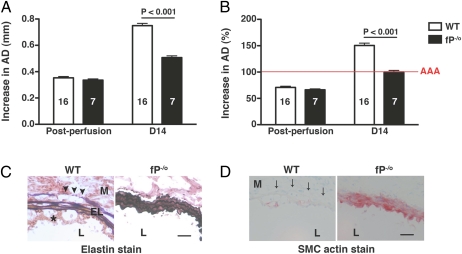
To examine the role of properdin, we monitored AAA development in hemizygous properdin-deficient male mice (fP−/o). WT and fP−/o mice were transiently perfused with elastase on day 0. Immediately after elastase perfusion, there was mild aortic dilatation (∼70%), which did not differ between WT and fP−/o mice (Fig. 1 A and B). This aortic diameter (AD) remained relatively stable up to day 7, after which there was rapid and significant increase in AD in WT mice (12, 18). AAA is typically defined on day 14 as an increase in AD of 100% or greater than the diameter measured before elastase perfusion (12, 18). We have previously shown that fB−/− mice are largely resistant to the development of AAA (increase in AD of 105 ± 4%) (12). We now find that the absence of properdin protected mice from elastase-induced AAA to the same extent as fB deficiency (increase in AD of 0.50 ± 0.02 mm or 100 ± 3% in fP−/o mice compared with increase in AD of 0.75 ± 0.02 mm or 151 ± 5% in WT animals, P < 0.001) (Fig. 1 A and B). Histological analysis of day 14 aortas from fP−/o mice showed well-preserved elastic fibers (elastin degradation score of 2.01 ± 0.10 in fP−/o aortas compared with 3.15 ± 0.15 in WT aortas, P < 0.001) (Fig. 1C) and smooth muscle cell (SMC) actin content (SMC actin score of 2.39 ± 0.19 in fP−/o aortas compared with 3.92 ± 0.08 in WT aortas, P < 0.001) (Fig. 1D), whereas analysis of WT aortas revealed pronounced destruction of the elastic lamellae and complete depletion of SMC actin content. Taken together, these results confirm that properdin is required for the complement-mediated acute inflammatory response in elastase-induced AAA.
Fig. 1.
Properdin is essential for AAA development. WT and fP−/o mice were transiently perfused with elastase on day 0, and their AD was measured immediately postperfusion and on day 14. Increase in AD was expressed in millimeters (A) or percentage (B). AAA (red line in B) is defined by an increase in AD of 100% or greater than the diameter measured before elastase perfusion. Values represent mean ± SEM. The number of animals per genotype is indicated. Immunohistochemistry revealed intact elastic fibers (C) and preserved SMC actin content (D, red) in properdin-deficient (fP−/o) aortas, whereas WT aortas showed elastic fiber fragmentation (arrowheads) and depletion of SMC actin (arrows). EL, elastic lamella; L, lumen; M, media. *Intraluminal thrombus. Photomicrographs are representative of n = 5 aortas per genotype. (Scale bar: 50 μm.)
Systemic Properdin Is Sufficient for Complement-Mediated AAA Development.
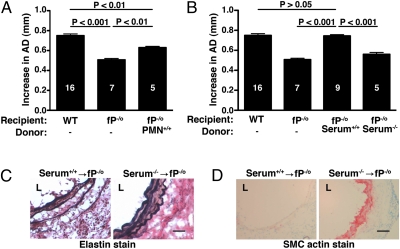
Properdin is in the plasma and also stored in neutrophil granules, where it is rapidly released during cell stimulation (15, 19). To determine whether locally secreted properdin (derived from infiltrating neutrophils) can drive the AAA phenotype, we reconstituted fP−/o mice with purified bone marrow-derived neutrophils using a regimen that we had previously used to successfully restore AAA phenotype in the dipeptidyl peptidase I-deficient mice (18). WT neutrophils (1e7) were injected i.v. into fP−/o mice on days 0 and 1 after elastase perfusion. We found that adoptive transfer of WT neutrophils induced small AAA in fP−/o mice by day 14 (AD = 0.63 ± 0.01, P < 0.01 compared with WT) (Fig. 2A). However, transfer of WT serum (200 μL each on days 0 and 1) restored AAA to WT level in fP−/o mice (AD = 0.74 ± 0.01, P > 0.05 compared with WT) (Fig. 2B). Administration of properdin-deficient serum at equivalent dose did not restore the WT AAA phenotype in fP−/o mice (AD = 0.56 ± 0.02, P < 0.001 compared with WT). Restoration of the AAA phenotype in fP−/o mice was accompanied by fragmentation of the elastic fibers (Fig. 2C) and depletion of SMC actin content (Fig. 2D). These results provide strong evidence that, although neutrophils can deliver the properdin needed to drive the disease phenotype, a systemic source of properdin (i.e., serum properdin) is sufficient to facilitate local AP activation, complement-mediated aortic wall injury, and subsequent AAA formation.
Fig. 2.
Serum properdin is sufficient for the promotion of AP-dependent AAA development. Properdin-deficient (fP−/o) mice were reconstituted with WT neutrophils (PMN+/+; A) or WT serum (Serum+/+; B). Pooled serum from properdin-deficient mice (Serum−/−) served as a control (B). Values represent mean ± SEM. The number of animals per genotype per treatment type is indicated. Reconstitution with Serum+/+ led to fragmentation of elastic fibers (C) and loss of SMC actin (D) in fP−/o aortas. L, lumen. Photomicrographs are representative of n = 5 aortas per treatment type. (Scale bar: 50 μm.)
Properdin Serves as a Stabilizer of AP C3 Convertase.
Properdin has the potential to facilitate AP activity through two distinct roles: as convertase stabilizer and convertase initiator. To differentiate these two functions in the development of AAA, we turned to a human fB gain of function mutant, fB D254G (also known as fB D279G; the substituted amino acid is 254 in the plasma protein and 279 in the unprocessed polypeptide precursor) (20). This single amino acid substitution is located at the Mg2+-dependent C3b binding site of the Bb subunit (21) and results in a stabilized properdin-free convertase, C3bBb (20, 22). We reasoned that, if the essential role of properdin in AAA development is to stabilize the convertase, then the introduction of the stabilizing fB mutant protein into fP−/o animals would compensate for the properdin deficiency and pathology would be restored. If, however, the essential role of properdin is to initiate convertase assembly, then introduction of the stabilizing fB mutant protein in fP−/o animals would fail to compensate for properdin deficiency and AAA would not be restored.
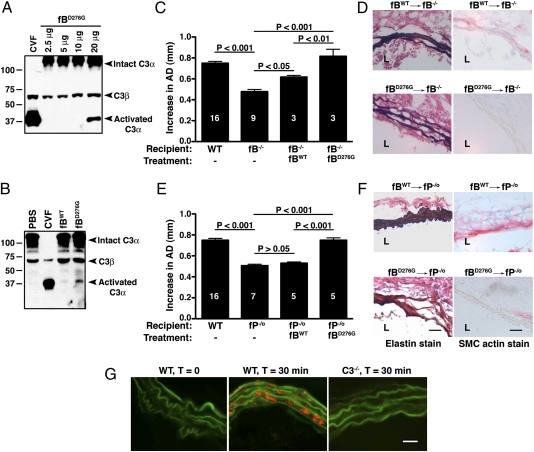
In a separate study, transgenic mice were generated that express the D279G-equivalent mouse fB mutant protein, D276G. As expected from the properties of the human fB D279G mutant protein (20, 22), the murine fB D276G mutant protein forms a much more stable AP C3 convertase than fB WT, which results in a substantial consumption of C3 in the plasma of these transgenic mice. For this investigation, we purified WT and mutant factor B proteins from plasma derived from the transgenic mice and their WT controls by affinity chromatography using an anti-mouse fB monoclonal antibody. Of note, the transgenic mice express both the mutant and WT fB proteins. Because the purification method does not resolve the two forms, the D276G preparation is actually a mixture of both WT and D276G fB proteins (Fig. S1). We found that i.v. treatment of WT mice with the D276G mutant fB protein preparation (but not the fB from WT control mice) promoted C3 turnover (Fig. 3 A and B), consistent with the subnormal C3 levels observed in the plasma of D276G mutant fB transgenic mice and aHUS patients heterozygous for the factor B D279G mutation (23). Next, we reconstituted fB−/− mice with different doses of D276G mutant protein preparation and determined that 20 μg D276G preparation were sufficient to restore AAA phenotype in these mice (AD = 0.82 ± 0.07, P < 0.001 compared with fB−/− mice) (Fig. 3C) along with fragmentation of elastic fibers and SMC depletion (Fig. 3D). An equivalent dose (20 μg) of purified WT fB protein also restored aneurysm formation in fB−/− mice, albeit to a smaller extent (AD = 0.62 ± 0.02, P < 0.05 compared with fB−/− mice) (Fig. 3C). Finally, we administered WT and D276G mutant fB proteins to fP−/o mice. We found that 20 μg D276G mutant fB preparation fully restored AAA phenotype in fP−/o mice, whereas the same amount of WT fB protein failed to do so (AD = 0.75 ± 0.02 in mice injected with D276G mutant fB compared with AD = 0.53 ± 0.01 in mice injected with WT fB, P < 0.001) (Fig. 3E). D276G mutant fB-induced AAA was accompanied by degeneration of the elastic lamellae and SMC depletion (Fig. 3F). Taken together, these results suggest that the essential function of properdin in elastase-induced AAA is to serve as a stabilizer of the AP C3 convertase. Consistent with these results, we also found that the presence of C3 (and the AP C3 convertase) is required for the local binding of properdin (to the aortic wall), because the absence of C3 completely abolished properdin staining in elastase-perfused aortic wall (Fig. 3G).
Fig. 3.
Properdin stabilizes the AP C3 convertase. (A) WT mice were injected i.v. with the indicated dose of fB mutant protein (fBD276G) or cobra venom factor (two units), and serum was obtained after 30 min. C3 activation is detected on Western blots by the appearance of a C3α chain split product at 37 kDa when probed with an anti-mouse C3 antibody. (B) Injection of the WT fB protein (fBWT) did not lead to significant complement activation. Cobra venom factor, a strong complement activator, completely degraded intact C3α chain within 30 min of injection and served as a positive control, whereas injection of PBS served as a negative control. (C) A single injection of 20 μg fBD276G reconstituted the aneurysmal phenotype in fB−/− mice, whereas an equivalent dose of fBWT promoted smaller aneurysms in fB−/− mice. Aortic diameter of nonreconstituted fB−/− mice was previously published in ref. 12. Aneurysm formation was accompanied by elastic fiber fragmentation and SMC depletion in fB−/− aortas (D). A single injection of 20 μg fBD276G fully reconstituted the aneurysmal phenotype in fP−/o mice, whereas the equivalent dose of fBWT did not (E). Values represent mean ± SEM. The number of animals per genotype per treatment type is indicated. Injection of fBD276G led to degradation of the elastic lamellae and loss of SMC in fP−/o aortas (F). L, lumen. Photomicrographs are representative of at least five aortas studied per treatment type. (Scale bar: 50 μm.) (G) Binding of properdin to elastase-perfused aortic wall is dependent on C3. Mice were perfused with elastase, and the aortas were harvested immediately or after 30 min. Red, properdin; green, autofluorescence of elastic lamellae. (Scale bar: 25 μm.) Photomicrographs are representative of serial aortic sections derived from two independent experiments.
Complement Activation in AAA Is Initiated by Antibodies.
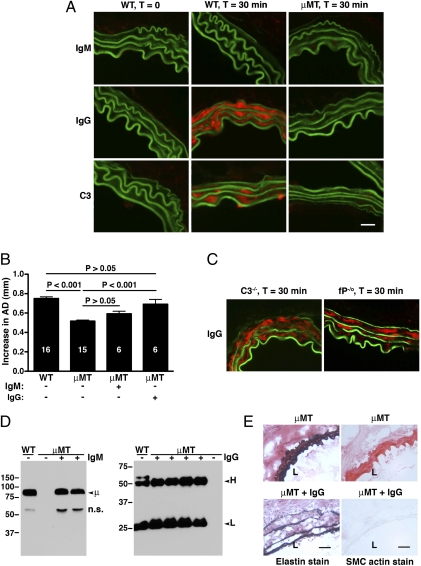
Evidence above shows that activation of the AP is an essential component of the pathophysiology of AAA. It seems, however, that it is not properdin that initiates and directs AP activation by binding to the target tissue, and it, therefore, remains to be clarified what targets initiate AP activation in the pathophysiology of AAA. Because previous in vitro and in vivo work has clearly shown that the AP can be activated on antibody-sensitized targets and by circulating immune complexes (24–26), we investigated the possible involvement of antibody in AAA. In a first approach, we examined aortas obtained from WT mice 30 min after elastase perfusion. We found abundant IgG, but not IgM, deposition in the aortic wall of elastase-perfused animals (Fig. 4A). Next, we induced disease in a cohort of mice deficient in B cells (μMT mice) and hence, deficient in all Igs (27). We found that the absence of Igs abrogated C3 deposition in elastase-perfused aortic wall (Fig. 4A) and protected μMT mice from AAA development (AD = 0.51 ± 0.01, P < 0.001 compared with WT) (Fig. 4B). In contrast, we observed that IgG deposition in elastase-perfused C3−/− and fP−/o aortas proceeded normally, suggesting that antibody binding to aortic wall is independent of complement activation (Fig. 4C). Next, we asked whether reconstitution with mouse natural antibodies purified from pooled sera of WT mice would restore susceptibility to AAA in μMT mice. We found that reconstitution with pooled mouse IgM to WT level (Fig. 4D) did not significantly increase the susceptibility of μMT mice to aneurysm formation (AD = 0.59 ± 0.02 in IgM-reconstituted μMT mice, P > 0.05 compared with untreated μMT mice). In contrast, reconstitution with pooled mouse IgG to the WT level rendered μMT mice susceptible to AAA formation (AD = 0.69 ± 0.05, P < 0.01 compared with untreated μMT mice and P > 0.05 compared with WT) (Fig. 4B). Histology confirmed that the AAA phenotype in IgG-reconstituted mice was accompanied by elastic fiber fragmentation and SMC depletion (Fig. 4E). These results suggest that natural IgG antibodies recognize an exposed antigen in the elastase-perfused aortic wall and their presence supports C3 convertase assembly and function.
Fig. 4.
Complement activation is initiated by antibodies in elastase-induced AAA. (A) WT and B cell-deficient (μMT) mice were perfused with elastase, and the aortas were harvested immediately or 30 min after perfusion. Presence of antibodies (red) was detected with a goat anti-mouse IgM (Top) or IgG (Middle) antibody. C3 (red) was detected with a goat anti-mouse C3 antibody (Bottom). Green, autofluorescence of elastic lamellae. (Scale bar: 25 μm.) (B) μMT mice were reconstituted with pooled mouse IgM (1 mg per mouse) or IgG (5 mg per mouse) immediately after elastase perfusion. Reconstitution with mouse IgG restored the AAA phenotype in μMT mice. Values represent mean ± SEM. The number of animals per treatment type is indicated. (C) C3−/− and fP−/o aortas were harvested 30 min after elastase perfusion and stained for presence of IgG (red) as above. (D) Serum was obtained for Western blot analysis of IgM or IgG after reconstitution with the indicated Ig (+). Untreated μMT mice (−) have no detectable IgM or IgG. H, IgG heavy chain; L, IgG light chain; μ, IgM μ-chain; n.s., nonspecific cross-reactive band. (E) Immunohistochemistry revealed fragmentation of elastic fibers and loss of SMC actin content in the aortas of μMT mice reconstituted with IgG. L, lumen. Photomicrographs are representative of n = 5 aortas per treatment type. (Scale bar: 50 μm.)
Blockade of Properdin Activity Protects Against AAA Development.
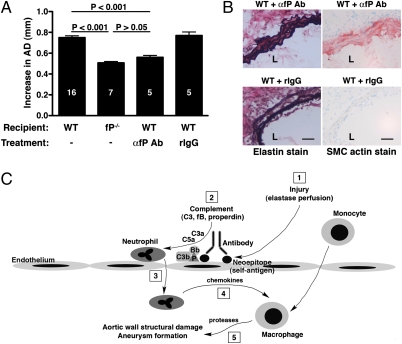
We have previously presented evidence that complement activation occurs in human AAA (12). Whether complement activation in human AAA is a cause or consequence of aortic wall tissue injury and damage requires additional studies. The demonstration that anticomplement therapy will halt the progression of AAA in an animal model would help to justify pursuing complement-targeting strategies in humans. To this end, we administered a rabbit anti-mouse properdin antibody that has previously been shown to block properdin activity in vivo (28). Treatment with properdin-blocking antibody prevented the development of AAA to a large extent (AD = 0.56 ± 0.02, P < 0.001 compared with WT) (Fig. 5A). This protection was reflected in relatively well-preserved elastic fibers [elastin degradation score of 2.42 ± 0.16 in WT mice treated with properdin-blocking antibody compared with 3.03 ± 0.14 in WT mice treated with nonimmune rabbit IgG (P < 0.05) and 3.15 ± 0.15 in untreated WT mice (P < 0.001)] and intact SMC actin content [loss of SMC actin score of 2.46 ± 0.26 in WT mice treated with properdin-blocking antibody compared with 3.54 ± 0.13 in WT mice treated with nonimmune rabbit IgG (P < 0.001) and 3.92 ± 0.08 in untreated WT mice (P < 0.001)] (Fig. 5B). In contrast, mice that received nonimmune rabbit IgG remained fully susceptible to AAA development (AD = 0.77 ± 0.03, P > 0.05 compared with WT) (Fig. 5A). These results suggest that blockade of properdin activity protects against complement-mediated injury after elastase perfusion.
Fig. 5.
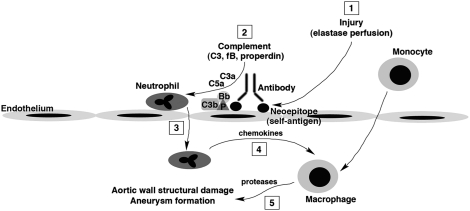
Blockade of properdin activity protects against AAA development. WT mice were injected i.v. with 1 mg polyclonal rabbit anti-mouse properdin (αfP Ab) 60 min before, immediately after elastase perfusion, and on day 1 postsurgery. Injection of nonimmune pooled rabbit IgG (rIgG) at the same dose served as control. Values represent mean ± SEM. The number of animals per treatment type is indicated. Treatment with properdin-blocking Ab prevented the AAA phenotype (A) and elastic fiber degradation and SMC depletion (B). L, lumen. Photomicrographs are representative of n = 5 aortas per treatment type. (Scale bar: 50 μm.) (C) Proposed mechanism of AP complement activation and order of events in elastase-induced AAA. (1) Elastase perfusion unmasks hidden neoepitopes (self-antigens) that are recognized by IgG antibody, providing a platform for properdin-stabilized AP C3 convertase (C3bBbP) assembly. (2) Complement activation leads to the release of anaphylatoxins (C3a and C5a). (3) These anaphylatoxins act as chemoattractants in the recruitment of neutrophils. (4) Activated neutrophils release chemokines that attract other inflammatory leukocytes, including monocytes/macrophages. (5) Macrophages release proteases and other inflammatory mediators that cause structural damage, weakening of the aortic wall, and eventual characteristic aneurysmal changes.
Discussion
AAA is a multifactorial disease with genetic and environmental influences and immune-mediated features involving the innate and adaptive immune responses in the initiation and propagation of the inflammatory process (17). Using an established elastase-induced model of AAA in mice that recapitulates several of the most important features of human AAA (29), we previously showed that neutrophil recruitment and the development of disease in an elastase-induced AAA model system is critically dependent on the complement system AP (12). Moreover, we found that disease phenotype is mediated by the anaphylatoxins C3a and C5a, although unlike other complement-dependent inflammatory disease models in mouse, C5a was not obligatory (12). Here, we show that properdin, a major component of the complement AP, is critical for elastase-induced AAA, and we provide strong evidence that complement initiation requires the presence of antibodies.
The AP convertases are assembled on target surfaces where they direct opsonization, cell lysis, and generation of C3a and C5a. Properdin, a component of the AP C3 and C5 convertases, has long been known to stabilize these otherwise short-lived complexes. Recently, evidence has accumulated that properdin can bind certain cellular and microbial surfaces and provide a platform for convertase assembly and function (16). This study was undertaken to determine whether a properdin-dependent target recognition mechanism might be involved in the initiation of complement activation in AAA. Using both properdin-deficient animals and function-blocking antiproperdin antibody, we established that properdin is required for elastase-induced AAA. We showed that, although local neutrophil-derived properdin can promote aneurysm formation to some extent, plasma-derived properdin alone was completely sufficient to promote disease development. Next, we used the mouse factor B D276G reagent, which confers convertase stability in the absence of properdin, to obtain strong evidence that the properdin activity essential to disease phenotype is convertase stabilization and not convertase initiation. Finally, we showed that disease phenotype is dependent on natural IgG antibody. In the absence of IgG (as in μMT mice), we saw no C3 deposition in elastase-perfused aortic wall, thus essentially ruling out an activation mechanism initiated by properdin binding directly to target tissues. This observation, coupled with the previous finding that C4-deficient animals are susceptible to AAA formation (12), indicates that CP activity is not required for AP activation in this case. We conclude that, in elastase-induced AAA, exposed/damaged aortic tissue is first recognized by IgG, and the resulting tissue-antibody complexes likely provide a protected site for direct AP activation (Fig. 5C) (24).
Previously, we showed the presence of the AP proteins factor B and properdin as well as the CP/LP protein C4 in human AAA tissue samples. Recently, Hinterseher et al. (30) conducted a genome-wide microarray expression profile of human AAA tissue samples vs. normal tissue and found increased expression of the genes encoding the CP/LP protein C2 and the CP protein C1 (C1qA and C1qC) as well as the anaphylatoxin receptors C3aR and C5aR (30). Moreover, the work by Hinterseher et al. (30) also showed increased C2 in the disease tissue. Although our results in the elastase-induced AAA model indicate that AP activity may be the only activation pathway essential for the disease phenotype (12), it remains possible that the LP can still play a role in AAA development. A C2-dependent C4-bypass LP was recently described (31), and the contribution of this pathway to AAA formation in this model is currently being evaluated. Alternatively, the prominent contribution of the AP in our model system may be explained by the fact that we focused on the early initiating events of aneurysm development, whereas the human tissue samples were obtained at late-stage disease. In contrast to our results, the study by Hinterseher et al. (30) did not detect the presence of factor B in the AAA tissue samples (30, 32). This discrepancy could possibly result from the different detection antibodies used. Our polyclonal anti-fB antibody would bind to free factor B, the Bb fragment, and the C3bBbP complex.
The AP mediates numerous diseases and injury states (13). Age-related macular degeneration, aHUS, and dense deposit disease (membranoproliferative glomerulonephritis type II) are caused by dysregulation of the AP and associated with alleles that encode impaired forms of the AP regulator proteins factor H, factor I, and CD46 or gain of function forms of the AP convertase components fB and C3 (13). This finding does not seem to be the major cause of AAA, because in the works by both Hinterseher et al. (30) and Bradley et al. (32), consistent associations between AAA disease and AP genetic polymorphisms were not found (30, 32). Instead, we found that antibody binding to the injured aortic wall (in this case, damaged by elastase perfusion) leads to complement activation, thereby initiating the inflammatory cascade that eventually culminates in AAA formation. Indeed, studies of human AAA tissues suggest that an immune response to autologous components of the aortic wall triggers the inflammatory process in this disease, which was evidenced by large amounts of Igs and B lymphocytes in AAA tissues in germinal center-like microstructures (33), similar to those structures found in other autoimmune disorders such as rheumatoid arthritis. The works by Xia et al. (34) and Hirose et al. (35) have shown that Igs extracted from AAA tissues exhibit immunoreactivity against aortic wall proteins (34, 35). One of these proteins, termed AAA protein, shares sequence homology with vitronectin and fibrinogen (34, 35). Moreover, antibodies against vitronectin and fibrinogen react with adventitial matrix proteins in the abdominal aorta (35). Whether this dysregulated immune response against autologous aortic wall proteins is a cause or consequence of the inflammatory process in human AAA remains undefined. However, our results herein suggest a causative role for Igs in elastase-induced AAA. In fact, low levels of natural antibodies against phosphorylcholine, a constituent of oxidized forms of LDL, have been hypothesized as a potential pathogenic mechanism in atherosclerotic disease (36). Antibody also plays a critical role in several other AP-dependent disease models in the mouse, including the K/BxN serum transfer model of arthritis (26).
The AP is continuously activated because of the low-level spontaneous hydrolysis of C3, resulting in the generation of C3 fragments in the fluid phase and on self-tissue that are rapidly inactivated by endogenous complement regulatory proteins. Antibody:antigen reactions can direct AP activity independently of the CP or LP (the antibody-dependent AP) (24–26). In particular, antibodies bound to self-tissue can provide sites for C3 deposition that are protected from the inhibitory activities of the complement regulators and thus, confer AP-activating capacity. Although natural antibodies are usually of the IgM isotype, in some cases, normal mouse IgG has also been shown to possess polyreactive autoantibody function (37). Indeed, we found a high density of IgG binding to autologous antigens exposed by elastase perfusion in the aortic wall tissues. This finding is consistent with a previous observation that IgG can activate the AP, whereas IgM preferentially activates the CP (38). From these results, we propose that, in the AAA mouse model, elastase treatment unmasks hidden epitopes (neoepitopes) that are recognized by natural IgG antibodies and the resulting surface-bound antibodies provide protected sites for the assembly and activation of properdin-stabilized AP C3 convertase, with the ensuing release of anaphylatoxins C3a and C5a. These anaphylatoxins activate and recruit neutrophils and other leukocytes to the site of injury, where they initiate an inflammatory cascade that causes profound aortic wall structural damage and aneurysm formation (Fig. 5C). It should be noted that previous studies indicate that mouse CP C5 convertase is relatively ineffective compared with the human enzyme (39). Because of this issue, mouse models that are dependent only on C5a for pathogenesis may overvalue the role of the AP compared with the corresponding human disease. However, because we have shown in this mouse model that either C3a or C5a can drive aneurysm development (12), an active C3 convertase from either activation pathway would be sufficient. Taken together, these observations strengthen the applicability of the findings in this mouse model of AAA to the human condition.
In summary, we have shown that properdin plays a critical role in AAA development and that properdin-targeting strategies may halt aneurysmal growth and expansion. Strategies designed for the clinical inhibition of the complement AP have largely overlooked properdin as a therapeutic target, likely because properdin is not absolutely required for the in vitro assembly and function of the AP convertases. Until recently, the significance of properdin in vivo was revealed only by the extreme vulnerability of properdin-deficient individuals to meningococcal disease (40, 41). The advent of properdin-deficient animals has provided new tools for the assessment of properdin function and the possibilities of properdin-based anticomplement therapy in AP-dependent diseases. The work by Kimura et al. (28) has shown the importance of properdin activity for the K/BxN model of arthritis, and here, we show that properdin antagonism also prevented AAA formation. Properdin is a potentially attractive therapeutic target given that it is unique to the AP and present at a relatively low concentration in the plasma (42). In addition, its stoichiometric mode of action may make properdin amenable to inhibition. Lastly, identification of more proximal molecular events in the pathogenesis of AAA, such as the detection of pathogenic autoantibodies and autologous antigens, merits additional investigation, because this identification will potentially yield novel therapeutic approaches for this common disorder. As with many immune diseases, the specific self-antigen(s) recognized by natural antibody in elastase-induced AAA is unknown. The search for this self-antigen(s) is ongoing in our laboratory.
Materials and Methods
Animals.
WT C57BL/6 and B cell-deficient (B6.129S2.Igh-6tm1Cgn/J) mice were obtained from the Jackson Laboratory. C3−/− and fB−/− mice in C57BL/6 strain were previously described (43, 44). Heterozygous embryos carrying loss of function mutation in properdin (Cfptm1Cmst; fully backcrossed to C57BL/6 background) were obtained from the Transgenic Unit of the Division of Biomedical Services at the University of Leicester, Leicester, United Kingdom (45) and transferred to pseudopregnant Swiss Webster mice. Pups were screened for the presence of the targeted properdin gene. Intercrossing resulted in properdin-deficient hemizygous males (fP−/o) because of X-chromosome linkage of the properdin gene. All mice were kept in a pathogen-free condition at Washington University Specialized Research Facility, and all of the experiments were performed according to protocols approved by the Division of Comparative Medicine at Washington University School of Medicine.
Elastase Perfusion Model of AAA.
AAA was induced in 8- to 12-wk-old male mice as previously described (12, 18). Briefly, mice were anesthetized with 55–60 mg/kg i.p. sodium pentobarbital. A laparotomy was performed under sterile conditions. The abdominal aorta was isolated, and the preperfused AD was measured with a calibrated ocular grid. Temporary 7–0 silk ligatures were placed around the proximal and distal aorta to interrupt proximal flow. An aortotomy was created at the inferior ligature using the tip of a 30-gauge needle, and the aortic lumen was perfused for 5 min at 100 mm Hg with a solution containing 0.145 U/mL type 1 porcine pancreatic elastase (Sigma). After removal of the catheter, the aortotomy was repaired without constriction of the lumen to restore the flow. At day 14, a second laparotomy was performed, and the perfused segment of the abdominal aorta was reexposed and measured in situ before euthanasia and tissue procurement. AD was assessed on day 14 unless otherwise indicated. In some experiments, mice were injected i.v. with 1e7 purified bone marrow-derived neutrophils (18), 200 μL pooled sera (12), or indicated dose of WT or D276G mutant fB in 200 μL PBS at the indicated times. For properdin activity blockade, WT mice were injected i.p. with 1 mg polyclonal rabbit anti-mouse properdin (28) in 1 mL PBS at the indicated times; purified rabbit IgG (Jackson ImmunoResearch Laboratories) served as a negative control. For Ig reconstitution, B cell-deficient mice were injected i.p. with 5 mg pooled mouse IgG (Jackson ImmunoResearch Laboratories) or 1 mg pooled mouse IgM (Rockland Immunochemicals) in 1 mL PBS immediately after elastase perfusion.
Immunohistochemistry.
Immunohistochemistry was performed as previously described (12). Briefly, mouse abdominal aorta was dissected, snap-frozen in optimal cutting temperature (OCT) compound, and sectioned at 5 mm. Elastin was stained with Verhoeff–van Gieson using an Accustain Elastic Stain Kit (Sigma). Elastin degradation was graded on a scale of one to four: 1, less than 25% degradation; 2, 25–50% degradation; 3, 50–75% degradation; 4, greater than 75% degradation. SMC content was evaluated using an alkaline phosphatase-conjugated antibody to α-smooth muscle actin (1:200 dilution; Sigma). Color was visualized using an alkaline phosphatase substrate kit (Vector Laboratories). SMC actin content was graded on a scale of one to four: 1, less than 25% loss; 2, 25–50% loss; 3, 50–75% loss; 4, greater than 75% loss. Histological grading was performed on six to nine serial cross-sections per aorta and five aortas per genotype/treatment type by a blinded observer.
Immunofluorescence.
Frozen cross-sections of aortic tissues (5 μm) were fixed in methanol, blocked in 3% (wt/vol) dry milk in PBS, and incubated with rabbit anti-mouse properdin antibody (2μg/mL final concentration) (28) or goat anti-mouse C3 (1:5,000 dilution; MP Biomedicals) for 1 h at room temperature, washed, and then incubated with the appropriate rhodamine-conjugated secondary antibody (Jackson ImmunoResearch Laboratories). Some sections were stained with rhodamine-conjugated goat anti-mouse IgM or IgG (1:200 dilution) (Jackson ImmunoResearch Laboratories). All images were visualized on a Nikon Eclipse microscope and acquired with QCapture software.
Purification of Murine fB.
Fresh EDTA plasma was collected from normal FVB and FVB transgenic mice that express the D276G fB mutant protein. An anti-human/mouse fB monoclonal antibody (SIM307.28.4.2; in house) was coupled to a cyanogen bromide-activated Sepharose 4B column following the manufacturer's instructions (GE Healthcare). Mouse plasma was applied, and bound fB was eluted with 0.1 M glycine/HCl, pH 2.5, and immediately neutralized with 2 M Tris, pH 8.6. Aggregated materials and minor contaminants were removed by gel filtration on Superdex 200. Purity of the proteins was verified by SDS/PAGE (Fig. S1).
Western Blot Analysis.
Serum samples (15 μL 1:100 dilution) were fractionated by SDS/PAGE under reducing conditions and blotted with (i) goat anti-mouse C3 antibody (MP Biomedicals) followed by incubation with HRP-conjugated donkey anti-goat IgG (Jackson ImmunoResearch Laboratories); (ii) HRP-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories); or (iii) goat anti-mouse IgM (μ-chain–specific; Jackson ImmunoResearch Laboratories) followed by incubation with HRP-conjugated donkey anti-goat IgG. Bands were visualized using a SuperSignal Western Blotting Kit (Pierce).
Statistics.
Comparisons between groups were made by one-way ANOVA followed by Bonferroni's posttest to compare all groups of data. Data are presented as the mean ± SEM. P values less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Ying Hu and Jennifer Huang for excellent technical assistance. The properdin-deficient mice were rederived from frozen embryos by the Pulmonary Mouse Genetic and Smoking Core at Washington University, which is supported by National Institutes of Health Grants HL029594, HL084922, and AI070489. This work was supported by British Heart Foundation Grant PG/09/053/27836 (to C.M.S.); the Ciber de Enfermedades Raras and the Fundación Renal Iñigo Alvarez de Toledo (T.M.F. and S.R.d.C.); National Institutes of Health Grants AI049261 (To C.T.N.P.), AI049261-08S1 (To C.T.N.P.), AI085596 (to W.-C.S.), HL56701 (to R.W.T.), AI041592 (to J.P.A.), AI051436 (to D.E.H.), and AI049261-08S2 (to C.T.N.P.); and Spanish Ministerio de Ciencia e Innovacion Grant SAF2008-00226 (to S.R.d.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 2202.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119000109/-/DCSupplemental.
References
- 1.Alcorn HG, Wolfson SK, Jr, Sutton-Tyrrell K, Kuller LH, O'Leary D. Risk factors for abdominal aortic aneurysms in older adults enrolled in The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1996;16:963–970. doi: 10.1161/01.atv.16.8.963. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard JF, Armenian HK, Friesen PP. Risk factors for abdominal aortic aneurysm: Results of a case-control study. Am J Epidemiol. 2000;151:575–583. doi: 10.1093/oxfordjournals.aje.a010245. [DOI] [PubMed] [Google Scholar]
- 3.Lederle FA, Nelson DB, Joseph AM. Smokers’ relative risk for aortic aneurysm compared with other smoking-related diseases: A systematic review. J Vasc Surg. 2003;38:329–334. doi: 10.1016/s0741-5214(03)00136-8. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. The UK Small Aneurysm Trial Participants. Lancet. 1998;352:1649–1655. [PubMed] [Google Scholar]
- 5.Lederle FA, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437–1444. doi: 10.1056/NEJMoa012573. [DOI] [PubMed] [Google Scholar]
- 6.Lederle FA, Kane RL, MacDonald R, Wilt TJ. Systematic review: Repair of unruptured abdominal aortic aneurysm. Ann Intern Med. 2007;146:735–741. doi: 10.7326/0003-4819-146-10-200705150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Thompson RW. Detection and management of small aortic aneurysms. N Engl J Med. 2002;346:1484–1486. doi: 10.1056/NEJM200205093461910. [DOI] [PubMed] [Google Scholar]
- 8.Becquemin JP, et al. A randomized controlled trial of endovascular aneurysm repair versus open surgery for abdominal aortic aneurysms in low- to moderate-risk patients. J Vasc Surg. 2011;53:1167–1173. doi: 10.1016/j.jvs.2010.10.124. [DOI] [PubMed] [Google Scholar]
- 9.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: A key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 11.Kemper C, Atkinson JP. T-cell regulation: With complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 12.Pagano MB, et al. Complement-dependent neutrophil recruitment is critical for the development of elastase-induced abdominal aortic aneurysm. Circulation. 2009;119:1805–1813. doi: 10.1161/CIRCULATIONAHA.108.832972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holers VM. The spectrum of complement alternative pathway-mediated diseases. Immunol Rev. 2008;223:300–316. doi: 10.1111/j.1600-065X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 14.Fearon DT, Austen KF. Properdin: Binding to C3b and stabilization of the C3b-dependent C3 convertase. J Exp Med. 1975;142:856–863. doi: 10.1084/jem.142.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemper C, Mitchell LM, Zhang L, Hourcade DE. The complement protein properdin binds apoptotic T cells and promotes complement activation and phagocytosis. Proc Natl Acad Sci USA. 2008;105:9023–9028. doi: 10.1073/pnas.0801015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kemper C, Hourcade DE. Properdin: New roles in pattern recognition and target clearance. Mol Immunol. 2008;45:4048–4056. doi: 10.1016/j.molimm.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagadesham VP, Scott DJ, Carding SR. Abdominal aortic aneurysms: An autoimmune disease? Trends Mol Med. 2008;14:522–529. doi: 10.1016/j.molmed.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Pagano MB, et al. Critical role of dipeptidyl peptidase I in neutrophil recruitment during the development of experimental abdominal aortic aneurysms. Proc Natl Acad Sci USA. 2007;104:2855–2860. doi: 10.1073/pnas.0606091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wirthmueller U, et al. Properdin, a positive regulator of complement activation, is released from secondary granules of stimulated peripheral blood neutrophils. J Immunol. 1997;158:4444–4451. [PubMed] [Google Scholar]
- 20.Hourcade DE, Mitchell LM, Oglesby TJ. Mutations of the type A domain of complement factor B that promote high-affinity C3b-binding. J Immunol. 1999;162:2906–2911. [PubMed] [Google Scholar]
- 21.Rooijakkers SH, et al. Structural and functional implications of the alternative complement pathway C3 convertase stabilized by a staphylococcal inhibitor. Nat Immunol. 2009;10:721–727. doi: 10.1038/ni.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goicoechea de Jorge E, et al. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci USA. 2007;104:240–245. doi: 10.1073/pnas.0603420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roumenina LT, et al. Hyperfunctional C3 convertase leads to complement deposition on endothelial cells and contributes to atypical hemolytic uremic syndrome. Blood. 2009;114:2837–2845. doi: 10.1182/blood-2009-01-197640. [DOI] [PubMed] [Google Scholar]
- 24.Ratnoff WD, Fearon DT, Austen KF. The role of antibody in the activation of the alternative complement pathway. Springer Semin Immunopathol. 1983;6:361–371. doi: 10.1007/BF02116280. [DOI] [PubMed] [Google Scholar]
- 25.Banda NK, et al. Initiation of the alternative pathway of murine complement by immune complexes is dependent on N-glycans in IgG antibodies. Arthritis Rheum. 2008;58:3081–3089. doi: 10.1002/art.23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji H, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura D, Roes J, Kühn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 28.Kimura Y, Zhou L, Miwa T, Song WC. Genetic and therapeutic targeting of properdin in mice prevents complement-mediated tissue injury. J Clin Invest. 2010;120:3545–3554. doi: 10.1172/JCI41782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson RW, Geraghty PJ, Lee JK. Abdominal aortic aneurysms: Basic mechanisms and clinical implications. Curr Probl Surg. 2002;39:110–230. doi: 10.1067/msg.2002.121421. [DOI] [PubMed] [Google Scholar]
- 30.Hinterseher I, et al. Role of complement cascade in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2011;31:1653–1660. doi: 10.1161/ATVBAHA.111.227652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwaeble WJ, et al. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc Natl Acad Sci USA. 2011;108:7523–7528. doi: 10.1073/pnas.1101748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradley DT, Badger SA, Bown MJ, Sayers RD, Hughes AE. Coding polymorphisms in the genes of the alternative complement pathway and abdominal aortic aneurysm. Int J Immunogenet. 2011;38:243–248. doi: 10.1111/j.1744-313X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 33.Bobryshev YV, Lord RS. Vascular-associated lymphoid tissue (VALT) involvement in aortic aneurysm. Atherosclerosis. 2001;154:15–21. doi: 10.1016/s0021-9150(00)00441-x. [DOI] [PubMed] [Google Scholar]
- 34.Xia S, Ozsvath K, Hirose H, Tilson MD. Partial amino acid sequence of a novel 40-kDa human aortic protein, with vitronectin-like, fibrinogen-like, and calcium binding domains: Aortic aneurysm-associated protein-40 (AAAP-40) [human MAGP-3, proposed] Biochem Biophys Res Commun. 1996;219:36–39. doi: 10.1006/bbrc.1996.0177. [DOI] [PubMed] [Google Scholar]
- 35.Hirose H, Ozsvath KJ, Xia S, Gaetz HP, Tilson MD. Immunoreactivity of adventitial matrix fibrils of normal and aneurysmal abdominal aorta with antibodies against vitronectin and fibrinogen. Pathobiology. 1998;66:1–4. doi: 10.1159/000027988. [DOI] [PubMed] [Google Scholar]
- 36.Frostegård J. Low level natural antibodies against phosphorylcholine: A novel risk marker and potential mechanism in atherosclerosis and cardiovascular disease. Clin Immunol. 2010;134:47–54. doi: 10.1016/j.clim.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Berneman A, Ternynck T, Avrameas S. Natural mouse IgG reacts with self antigens including molecules involved in the immune response. Eur J Immunol. 1992;22:625–633. doi: 10.1002/eji.1830220303. [DOI] [PubMed] [Google Scholar]
- 38.Lucisano Valim YM, Lachmann PJ. The effect of antibody isotype and antigenic epitope density on the complement-fixing activity of immune complexes: A systematic study using chimaeric anti-NIP antibodies with human Fc regions. Clin Exp Immunol. 1991;84:1–8. doi: 10.1111/j.1365-2249.1991.tb08115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebanks RO, Isenman DE. Mouse complement component C4 is devoid of classical pathway C5 convertase subunit activity. Mol Immunol. 1996;33:297–309. doi: 10.1016/0161-5890(95)00135-2. [DOI] [PubMed] [Google Scholar]
- 40.Braconier JH, Sjöholm AG, Söderström C. Fulminant meningococcal infections in a family with inherited deficiency of properdin. Scand J Infect Dis. 1983;15:339–345. doi: 10.3109/inf.1983.15.issue-4.04. [DOI] [PubMed] [Google Scholar]
- 41.Densen P, Weiler JM, Griffiss JM, Hoffmann LG. Familial properdin deficiency and fatal meningococcemia. Correction of the bactericidal defect by vaccination. N Engl J Med. 1987;316:922–926. doi: 10.1056/NEJM198704093161506. [DOI] [PubMed] [Google Scholar]
- 42.Schwaeble WJ, Reid KB. Does properdin crosslink the cellular and the humoral immune response? Immunol Today. 1999;20:17–21. doi: 10.1016/s0167-5699(98)01376-0. [DOI] [PubMed] [Google Scholar]
- 43.Circolo A, et al. Genetic disruption of the murine complement C3 promoter region generates deficient mice with extrahepatic expression of C3 mRNA. Immunopharmacology. 1999;42:135–149. doi: 10.1016/s0162-3109(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto M, et al. Abrogation of the alternative complement pathway by targeted deletion of murine factor B. Proc Natl Acad Sci USA. 1997;94:8720–8725. doi: 10.1073/pnas.94.16.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stover CM, et al. Properdin plays a protective role in polymicrobial septic peritonitis. J Immunol. 2008;180:3313–3318. doi: 10.4049/jimmunol.180.5.3313. [DOI] [PubMed] [Google Scholar]