Abstract
The mechanisms underlying cognitive and neurobehavioral abnormalities associated with childhood exposure to manganese (Mn) are not well understood but may be influenced by neuroinflammatory activation of microglia and astrocytes that results in nitrosative stress due to expression of inducible nitric oxide synthase (iNOS/NOS2). We therefore postulated that gene deletion of NOS2 would protect against the neurotoxic effects of Mn in vivo and in vitro. Juvenile NOS2 knockout (NOS2−/−) mice were orally exposed to 50 mg/kg of MnCl2 by intragastric gavage from days 21 to 34 postnatal. Results indicate that NOS2−/− mice exposed to Mn were protected against neurobehavioral alterations, despite histopathological activation of astrocytes and microglia in Mn-treated mice in both genotypes. NOS2−/− mice had decreased Mn-induced formation of 3-nitrotyrosine protein adducts within neurons in the basal ganglia that correlated with protection against Mn-induced neurobehavioral defects. Primary striatal astrocytes from wildtype mice caused apoptosis in cocultured striatal neurons following treatment with MnCl2 and tumor necrosis factor-α, whereas NOS2−/− astrocytes failed to cause any increase in markers of apoptosis in striatal neurons. Additionally, scavenging nitric oxide (NO) with 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) prevented the ability of Mn- and cytokine-treated wildtype astrocytes to cause apoptosis in cocultured striatal neurons. These data demonstrate that NO plays a crucial role in Mn-induced neurological dysfunction in juvenile mice and that NOS2 expression in activated glia is an important mediator of neuroinflammatory injury during Mn exposure.
Keywords: astrocyte, microglia, 3-nitrotyrosine, inducible nitric oxide synthase, manganese
Manganese (Mn) is an essential element that functions as a cofactor for numerous homeostatic and trophic enzymes in the central nervous system (CNS) but at abnormally high intake levels Mn accumulates in the brain and causes neurotoxicity (Aschner et al., 2009). Excessive environmental or dietary exposure leads to neuroinflammation in the CNS, particularly in the striatal-pallidum and substantia nigra pars reticulata, resulting in loss of striatal dopamine (DA) and motor features resembling, but distinct from, Parkinson’s disease (Calne et al., 1994; Newland et al., 1989; Perl and Olanow, 2007). Patients suffering from Mn neurotoxicity often experience neuropsychological effects such as cognitive deficiencies, irritability, and anxiety, which have been extensively documented (Bouchard et al., 2008; Bowler et al., 2006; Wasserman et al., 2006; Woolf et al., 2002). Epidemiological studies also indicate adverse neurological affects such as hyperactivity, learning, and cognitive disabilities in children consuming water with levels of Mn exceeding 300 μg/l (Bouchard et al., 2007, 2011; Wasserman et al., 2006; Zhang et al., 1995). Animal studies describing neurological and behavioral effects of early developmental Mn exposure suggest that there is a sensitive developmental period during which damage to the dopaminergic system can lead to lasting impacts into adulthood (Dorman et al., 2000; Kern et al., 2010; Moreno et al., 2009b). Studies of Mn exposure in rodents have generally focused on adult animals, and there is subsequently much less information regarding the effects of Mn exposure during juvenile development.
Neuropathology in manganism is associated with robust astrogliosis in the basal ganglia (Olanow, 2004), and studies conducted in our laboratory and others suggest that glial-derived inflammatory cytokines and nitric oxide (NO) influence the progression of neuronal injury (Filipov et al., 2005; Liu et al., 2005, 2006; Moreno et al., 2009a,b; Verina et al., 2011). Increased expression of NOS2 by activated glial cells in response to Mn results in nitrosative stress throughout the basal ganglia (Moreno et al., 2009a) and enhanced apoptosis within selected populations of neurons in the globus pallidus and striatum (Liu et al., 2006). NOS2 is exclusively expressed in glia and produces high levels of NO, which forms highly reactive peroxynitrite anion (ONOO−) upon combining with superoxide (O2−), resulting in electrophilic nitration of cellular proteins that damages neurons (Ischiropoulos, 2003). Peroxynitrite-mediated nitrosative stress is implicated in a number of neurological disorders, including Alzheimer’s disease (Smith et al., 1997), Parkinson’s disease (Jenner, 2003), and Mn neurotoxicity (Moreno et al., 2009a).
NOS2 is predominantly regulated by the Rel-family transcription factor, nuclear factor kappa B (NF-κB) (Xie et al., 1993), and previous studies indicate that expression of NOS2 in response to Mn both in vitro and in vivo requires activation of NF-κB (Filipov et al., 2005; Moreno et al., 2008, 2011). Recent studies from our laboratory found that Mn exposure in juvenile transgenic mice (NF-κB-driven EGFP reporter) caused increased neuronal protein nitration and astrocytic expression of NOS2 and NF-κB-EGFP that was inhibited in vivo by estrogen (Moreno et al., 2011). In studies using the dopaminergic neurotoxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), pharmacological or genetic inhibition of NOS2 expression in mice prevented MPTP-mediated neuronal injury (Du et al., 2001; Liberatore et al., 1999; Wu et al., 2002). However, it is unknown whether ablation of NOS2 expression is sufficient to prevent Mn-induced neuroinflammation and neuronal injury in developing mice.
To directly test the role of glial-derived NO in Mn-induced neurological dysfunction, we used NOS2 knockout mice (Laubach et al., 1995; Liberatore et al., 1999; Wu et al., 2002) to address the question of whether this isoform is directly involved in the glial inflammatory response leading to neuronal nitration and behavioral dysfunction in juvenile mice orally exposed to Mn. We postulated that mice lacking NOS2 (NOS2−/−) would be protected against the neurotoxic effects of Mn. Our results indicate that loss of NOS2 attenuates Mn-related peroxynitrite adduct formation in the striatal-pallidum and substantia nigra pars reticulata associated with alterations in neurobehavioral function and neurochemistry in vivo and also prevents astrocyte-mediated neuronal apoptosis in vitro. Collectively, these data implicate Mn-induced production of NO by glial cells in the mechanism of neurotoxicity and behavioral alterations in developing mice.
MATERIALS AND METHODS
Animal model.
Three-week-old male wildtype (C57Bl/6) and NOS2-deficient mice (NOS2−/−; B6.129P2-Nos2tm1Lau/J, bred to congenicity onto a C57Bl/6 background) were obtained from the Jackson Laboratory (Bar Harbor, Maine), and littermates were exposed to 0.9% saline (control) or 50 mg/kg MnCl2 by daily intragastric gavage. Only male mice were used, given their increased sensitivity relative to females, as we previously reported (Moreno et al., 2009b). The dose of Mn was adjusted for the molar stoichiometry in the tetrahydrate form and administered at 50 mg/kg based upon daily weighing of each animal. The dosing regimen was based upon previous data from our group, and others demonstrating that juvenile mice and rats exposed to similar doses (30–50 mg/kg/oral) displayed behavioral alterations and increased expression of neuroinflammatory biomarkers (Kern and Smith, 2010; Moreno et al., 2009a). Mice (n= 7–10 animals per group) were treated for 2 weeks (days 20–34 postnatal) prior to termination for neurochemical and immunohistochemical analysis. Animals were kept on a 12-h light/dark cycle and provided water and standard laboratory chow ad libitum (2018 Harlan-Teklad Global 18% Protein Rodent Diet). This diet contains low levels of essential trace elements, including 118 μg/g Mn and 225 μg/g Fe, yielding a baseline consumption of approximately 0.2–0.3 mg Mn per day for juvenile mice (juvenile mice consume 2–3 g chow per day), in addition to the delivered dose of Mn, which was 0.3–0.75 mg Mn per day for mice ranging from 10 to 25 g during this period of juvenile development. All procedures were performed under a protocol approved by the Institutional Animal Care and Use Committee at Colorado State University under the care of veterinary staff at the Laboratory Animal Resources Facility.
Neurobehavioral analysis.
Mice were preconditioned 1 day prior to open field activity parameters using Versamax behavior chambers with an infrared beam grid detection array to assess animals movements in X, Y, and Z planes. Multiple behavioral parameters pertaining to basal ganglia function were collected and analyzed using VersaDat software (Accuscan Instruments, Inc., Columbus, OH), including total distance traveled, number of movements, rearing activity, and margin time as previously studied in our laboratory (Liu et al., 2006). Activity measured juvenile exposure every other day for 2 weeks during the period of oral gavage. Anxiety behavior was determined using the elevated plus maze for mice according to Walf and Frye (2007) that was conducted prior to termination on day 34. Briefly, mice were individually assessed in a 30 × 5 × 15 cm open/closed arm chamber for 5 min and recorded by video for data collection, analysis recorded observed time spent in open arms versus closed arms. Catecholamines and monoamines were determined by high-performance liquid chromatography with electrochemical detection (Vanderbilt University, Center for Molecular Neuroscience core facility). Levels of Mn and other trace metals were determined by inductively coupled plasma mass spectrometry as we previously reported (Moreno et al., 2009b).
Immunohistochemistry and immunofluorescence.
Mice were anesthetized by isofluorane inhalation and perfused intracardially as published previously by our laboratory (Moreno et al., 2009a). Briefly, brains were collected and fixed in 4% paraformaldehyde for 4–6 h and stored in graded sucrose and 0.1M NaKPO4 buffer at 4°C. Brains were embedded in optimal cutting temperature compound and cut in 10μM coronal serial sections for examination for immunohistochemistry. For gliosis, primary antibodies to glial fibrillary acidic protein (GFAP) (1:500; Sigma, St Louis, MO) and IBA1 (1:500; Wako, Osaka, Japan) were used. The substantia nigra pars reticulata (SN) and striatum-pallidum (ST) regions were evaluated due to their known susceptibility to Mn neurotoxicity (Moreno et al., 2009a; Olanow, 2004; Verina et al., 2011). Sections were developed using horseradish peroxidase–conjugated secondary antibodies and diaminobenzidine reagents from the Vectastain ABC Kit. Bright field images were collected from each region and scored blinded by a veterinary pathologist for glial morphology and pathological scoring as previously detailed (Moreno et al., 2009a). For coimmunofluorescence studies, sections from the ST and SN were incubated with Anti-neuronal nuclear antigen (NeuN) (1:500; Millipore, Bedford, MA) and 3-nitrotyrosine (3NT) (1:100; Millipore) antibodies in combination to assess levels of protein nitration in neurons. Specific protein epitopes were visualized with secondary antibodies labeled with Alexa Fluor 488 or 647 (Molecular Probes, Eugene, OR), and slides were mounted in media containing 4′,6-diamidino-2-phenylindole (DAPI) to identify cell nuclei. Serial sections were systematically imaged using common anatomical landmarks, and fields were selected based upon unbiased acquisition of fields using DAPI. Fluorescence intensity was quantified by masking the sum intensity per field of 3NT fluorescence in areas expressing NeuN. Images were acquired using a Zeiss 20× or 40× Air PlanApochromat objective with six microscopic fields examined per region from three serial sections for a total of 18 microscopic fields that were averaged for each 4–6 animals per group.
Astrocyte-neuron coculture.
Neurons were isolated from the striatum of 1-day-old C57Bl/6 mice as previously described (Carbone et al., 2009) and were grown for 4–6 days on poly-D-lysine-coated glass coverslips to permit axonal development and phenotypic maturation. Neurons were maintained in neurobasal media supplemented with B27, L-Glutamine and 1% 50 units/ml penicillin, 50 ng/ml streptomycin, and 100 ng/ml neomycin (PSN). Primary striatal astrocytes were isolated from 1- to 3-day-old C57Bl/6 and NOS2−/− mice as described by Aschner and Kimelberg (1991), modified according to our previous studies (Carbone et al., 2008; Moreno et al., 2008) and grown 18 days to maturity before use in experiments. Astrocytes were maintained in minimum essential medium with l-glutamine, supplemented with 10% heat-inactivated fetal bovine serum, 1% PSN at 37°C with 5% CO2. After reaching confluence, astrocytes were subcultured onto permeable cell culture inserts (pore size = 0.4μM) at 1 × 104/well approximately 7 days before treatments. Astrocytes were exposed for 24 h prior to coculture with saline or 30μM MnCl2 and 10 pg/ml tumor necrosis factor-α (TNFα), washed with PBS (pH 7.4), and then inserted over neurons in the presence or absence of 100μM 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) for 6 h. Thereafter, inserts containing astrocytes were removed, and neurons were assessed for multiple indices of apoptosis by live-cell fluorescence imaging. Caspase activity was determined using 100μM rhodamine-110 bis-(l-aspartic acid amide) (488 nm excitation), phosphatidylserine appearance on the cell surface was determined with 50nM annexin V-Alexa Fluor 647 conjugate, and nuclei were identified using the cell-permeable DNA stain, Hoechst 33342 (2μM final concentration in imaging medium). Using a 20× Plan Apochromat air objective, 10–12 fields per treatment were blindly captured by differential interference contrast and analyzed by sum intensity for fluorescent signal intensity, which was normalized to the number of cells in each field. Data were acquired and analyzed using SlideBook v 5.0 (Intelligent Imaging Innovations, Denver, CO).
Statistical analysis.
Differences between treatment and genotype were accessed by two-way ANOVA. When interactions were significant, pair-wise comparisons were analyzed by Student’s t-test. Histopathological scoring of glial pathology was analyzed by one-way ANOVA followed by Kruskal-Wallis post hoc test. In vitro studies, comparing three or more means were performed using one-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison post hoc test using Prism software (v4.0c, GraphPad Software, Inc., San Diego, CA). Results are expressed as the mean ± SEM from a minimum of three independent studies and for all experiments, p < 0.05 was considered significant.
RESULTS
NOS2−/− Mice Are Protected Against Mn-Induced Behavioral Disinhibition
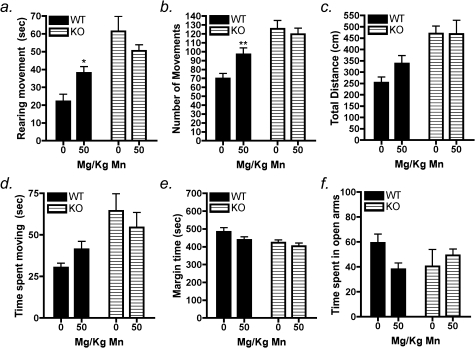
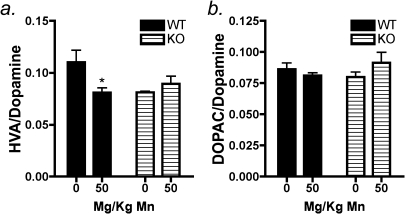
Neurobehavioral parameters were evaluated in order to determine if subchronic Mn exposure led to differential alterations in neuromotor function between wildtype and NOS2−/− mice. Our previous studies in juvenile C57Bl/6 mice reported elevated novelty seeking and hyperactive behaviors following oral exposure to 30 mg/kg Mn from days 21 to 34 postnatal that correlated with increased levels of Mn in the ST and SN of these mice (Moreno et al., 2009b). In the present studies, behavioral assessments in open-field activity chambers indicated that 50 mg/kg Mn in wildtype mice increased rearing movements after 14 days of exposure (Fig. 1a; p < 0.03). There was also a significant increase in the total number of movements (Fig. 1b; p < 0.01) with Mn treatment compared with wildtype controls as well as a trend toward an increase in the total distance traveled. No changes were detected in the time spent in the chamber margins in either genotype. In NOS2−/− mice, no changes were detected between control and treated animals in any behavioral parameter (Fig. 1a–f). We assessed anxiety-related behavior by the elevated plus maze assay, which indicated a trend toward decreased time spent in the open arms of the chamber in Mn-treated wildtype mice; however, these changes were not statistically significant (Fig. 1f). In parallel with behavioral assessments, levels of catecholamine and monoamine neurotransmitters were determined in the striatum, including DA, 3,4-dihydroxyphenylacetic acid (DOPAC), 3-methoxytyramine (3MT), homovanillic acid (HVA), serotonin, 5HIAA, noradrenaline, and adrenaline. No differences were detected between any neurotransmitters or metabolites in wildtype or knockout mice (Table 1). However, the HVA/DA ratio was decreased in wildtype mice exposed to Mn (p < 0.01) (Fig. 2a), but no change was detected in the DOPAC/DA ratio (Fig. 2b). In knockout mice, no significant changes in the HVA/DA or DOPAC/DA ratios were detected between control- and Mn-treated groups. However, the HVA/DA ratio in both control- and Mn-treated knockout mice was similar to values observed in Mn-treated wildtype mice. No change was observed the ratio of 3MT/DA in either genotype (data not shown). Levels of Mn were increased in the basal ganglia in treated mice in both genotypes but no changes were detected in iron or copper in any brain region evaluated (Supplementary table 1).
FIG. 1.
NOS2−/− mice are protected against Mn-induced behavioral disinhibition. Treatment with 50 mg/kg Mn by oral gavage for 14 days significantly increased rearing movements in Mn-treated wildtype mice compared with wildtype control (a). A significant increase was observed in total number of movements in Mn-treated wildtype mice in addition to a trend toward increase in the total distance traveled compared with wildtype controls (b and c). There was no change seen in margin time (e). There was no detectible change in any behavioral parameter in Mn-treated NOS2−/− mice (a–f). In the elevated plus maze assay, Mn-treated wildtype mice did not spend significantly less time in the open arms compared with controls. NOS2−/− mice had no measurable difference between control and Mn-treated groups (f). *p < 0.05, **p < 0.01.
TABLE 1.
Striatal Catecholamine and Monoamine Levels
| Wild type |
NOS2−/− |
|||
| Treatment | 0 | 50 mg/kg | 0 | 50 mg/kg |
| DA | 98.09 ± 11.8 | 97.24 ± 5.9 | 113.96 ± 7.9 | 92.87 ± 12.5 |
| DOPAC | 8.36 ± 0.75 | 7.97 ± 0.64 | 9.15 ± 1.06 | 8.48 ± 1.49 |
| 3MT | 3.74 ± 0.86 | 3.31 ± 0.22 | 3.43 ± 0.18 | 2.94 ± 0.45 |
| HVA | 10.63 ± 1.21 | 7.88 ± 0.70 | 9.27 ± 0.74 | 8.08 ± 0.28 |
| 5HT | 8.89 ± 1.4 | 7.46 ± 0.78 | 7.80 ± 0.57 | 8.21 ± 0.28 |
| 5HIAA | 3.30 ± 0.61 | 2.51 ± 0.24 | 3.33 ± 0.14 | 2.68 ± 0.14 |
| NE | 3.31 ± 0.68 | 2.95 ± 0.72 | 2.9 ± 0.94 | 3.55 ± 0.23 |
| E | 2.57 ± 0.08 | 2.52 ± 0.20 | 2.85 ± 0.27 | 3.12 ± 0.29 |
Notes. Concentrations expressed as nanogram of neurotransmitter per milligram of protein. Data represented are mean ± SEM (n = 3–4 animals per group).
FIG. 2.
Mn-induced changes in striatal HVA/DA ratios are prevented in NOS2−/− mice. (a) Wildtype mice treated with Mn had a significantly decreased ratio of HVA/DA compared with saline-treated controls, and NOS2−/− had no change in the HVA/DA ratio between genotype. (b) DOPAC/DA ratios also indicated no measurable change in wildtype or NOS2−/− mice. *p < 0.05.
Mn-Induced Glial Activation Is Not Directly Regulated by NOS2
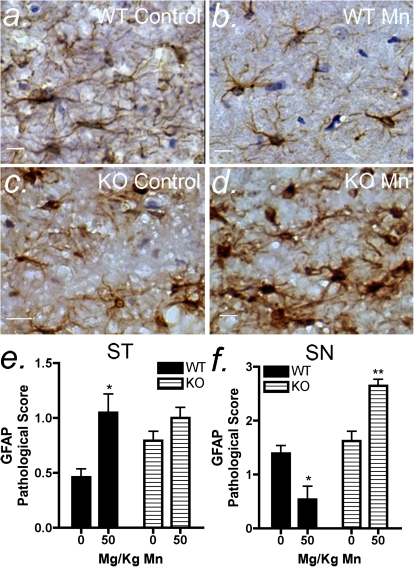
Activation of astrocytes and microglia was determined using blinded pathological scoring by a veterinary pathologist based upon immunohistochemical staining for the astrocyte marker, GFAP (Fig. 3), or the microglial marker, ionized calcium binding adaptor molecule (IBA1) (Fig. 4), in the ST and SN. Morphologically, Mn treatment in wildtype mice caused a decrease in the apparent intensity of GFAP staining as well as a decrease in the abundance of astrocytes with an activated phenotype in the SN (Figs. 3a and b), whereas astrocyte activation was increased in Mn-treated knockout mice in the SN (Figs. 3c and d). In the ST of wildtype mice, Mn treatment caused an increase in the level of astrocyte activation (Fig. 3e). Mn treatment in NOS2−/− mice did not cause a significant change in astrogliosis in the ST but did cause an increase in astroglial activation in the SN compared with control NOS2−/− mice (Fig. 3f). Pathological scoring for astrocytic hyperplasia and hypertrophy indicated that astrocyte activation occurred in the SN of Mn-treated knockout mice despite lack of expression of NOS2 (Figs. 3c and d).
FIG. 3.
Mn-induced astrocyte activation in the SN is differentially regulated between wildtype and NOS2−/− mice. Mice were exposed to 50 mg/kg Mn by oral gavage for 14 days, and tissue assessed by immunohistochemistry for GFAP intensity. Representative images of the SN are depicted for control and Mn treated mice for wildtype (a and b) and NOS2−/− (c and d) groups. Analysis of pathological scoring data in the ST (e) and SN (f) of wildtype and NOS2−/− mice was performed by one-way ANOVA with Kruskal-Wallis posttest. Scale bar = 10 μm. *p < 0.05 and **p < 0.01.
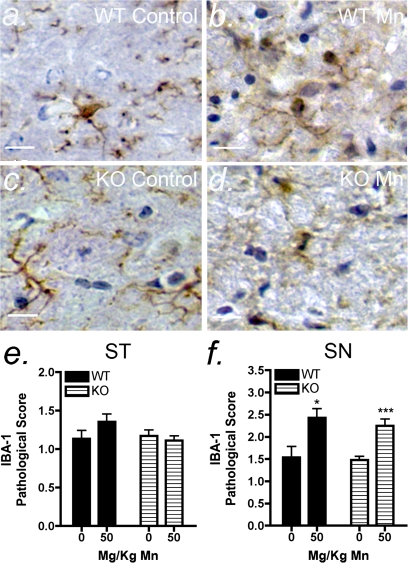
FIG. 4.
Mn-induced microglial activation is not directly regulated by NOS2. Mice were exposed to 50 mg/kg Mn by oral gavage for 14 days, and tissue assessed by immunohistochemistry for IBA1 staining for wildtype (a–b) and NOS2−/− (c–d) groups. Analysis of pathological scoring data in the ST (e) and SN (f) of wildtype and NOS2−/− mice was performed by one-way ANOVA with Kruskal-Wallis posttest. Scale bar = 10 μm. *p < 0.05 and ***p < 0.001.
Activation of microglia was also determined by pathological scoring based on immunohistochemical staining for IBA1, which is upregulated during inflammatory activation of microglia and macrophages. Sections were scored using morphological criteria according the methods used to analyze activation of astrocytes. In mice exposed to 50 mg/kg Mn, there was an increase in the number of activated microglia in the SN (Figs. 4a, b, and f). The same trend was observed in the SN in NOS2−/− mice (Figs. 4a, b, and f), whereas no change in microglial activation was detected in the ST following Mn treatment (Fig. 4e).
Mn-Induced Neuronal Protein Nitration Is Attenuated in NOS2−/− Mice
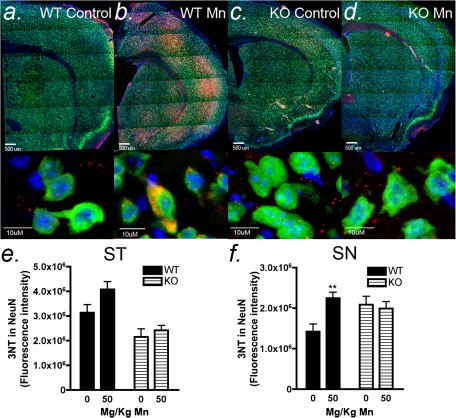
Levels of neuronal 3NT protein adducts were assessed by immunofluorescence in wildtype and NOS2−/− mice to determine the extent of oxidative/nitrosative stress resulting from Mn-induced production of NO/peroxynitrite (Fig. 5). Overproduction of NO during conditions of oxidative stress can modulate the activity of dopaminergic synapses by inhibiting DA release, whereas under physiological conditions, NO facilitates DA neurotransmission (Trabace and Kendrick, 2000). Representative low power images of 3NT immunofluorescence reveal extensive areas of peroxynitrite adduct formation throughout the striatum and cortex. Colocalization of 3NT adducts within neuronal soma was determined in the ST and SN by immunofluorescence (Figs. 5a–d). Sections were masked on NeuN (green, fluorescein isothiocyanate), and the intensity of neuron-specific 3NT fluorescence (red, CY5) in colocalized images was determined in saline- and Mn-treated mice for each genotype. In wildtype mice, a trend toward increased neuronal 3NT adducts was observed in NeuN-positive soma in the ST, although this increase was not significant (Fig. 5e). In the SN of wildtype mice, a significant increase in neuronal 3NT adducts was observed in Mn-treated mice that was prevented in NOS2−/− animals (Fig. 6f).
FIG. 5.
Mn-induced increases in neuronal protein nitration are attenuated in NOS2−/− mice. NO readily combines with superoxide to form the powerful nitrating agent, peroxynitrite, which can be detected by immunofluorescence with antibodies against 3NT protein adducts. Serial sections through the ST and SN from control and Mn-treated wildtype (a–b) and NOS2−/− (c–d) mice were stained for NeuN (green, fluorescein isothiocyanate) and 3NT (red, CY5) and counter stained with 4′,6-diamidino-2-phenylindole (DAPI, blue) to visualize cell nuclei. Representative images of 3NT-modified proteins from the striatum indicate co-localization within neuronal soma. Quantitation analysis of fluorescence intensity in wildtype and NOS2−/− mice indicates a significant increase in Mn-related 3NT-modified proteins in the SN in wildtype mice that is prevented in NOS2−/− animals. Scale bar = 10 μm. ***p < 0.001.
FIG. 6.
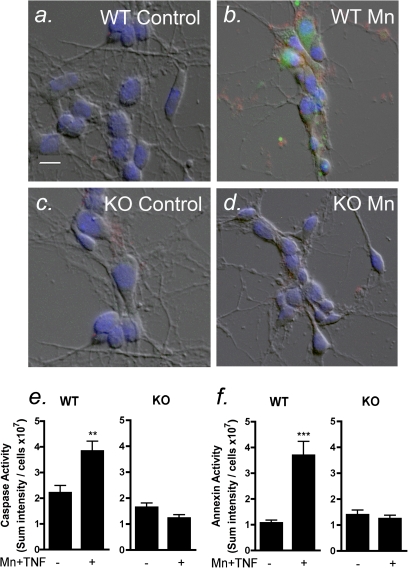
Astrocyte-derived NO mediates neuronal apoptosis. Apoptosis was determined in primary striatal neurons by live-cell fluorescence imaging of phosphatidylserine (annexin V, red) and caspase-3 (green) after 6 h of coculture with astrocytes that had been treated for 24 h with 30μM with Mn and 10 pg/ml TNFα. Representative images are depicted of neurons cocultured with control and Mn-treated astrocytes cultured from wildtype (a–b) and NOS2−/− (c–d) mice. Mn-treated wildtype astrocytes cocultured with neurons caused an increase in caspase activity (e) and annexin V staining (f). Mn-treated NOS2−/− astrocytes had no change in levels of caspase activity or annexin V staining. Scale bar = 10 μm. **p < 0.01 and ***p < 0.001.
Astrocyte-Derived NO Mediates Neuronal Apoptosis
In previous studies, we demonstrated that astrocytes exposed to low levels of Mn and inflammatory cytokines induce apoptosis in cocultured neurons (Liu et al., 2005; Moreno et al., 2011). We therefore exposed primary striatal astrocytes isolated from NOS2−/− mice to 30μM MnCl2 and 10 pg/ml TNFα (Mn + TNFα) to determine whether loss of NOS2 expression in astrocytes would protect primary striatal neurons from apoptosis (Fig. 6). Wildtype astrocytes were treated with saline or Mn + TNFα, after which the treatment medium was changed to fresh culture medium, and astrocytes were cocultured with neurons for 6 h. Caspase-3 activation and annexin V staining were used as indicators of neuronal apoptosis and determined by live-cell fluorescence imaging. Wildtype astrocytes treated with Mn + TNFα caused an increase in caspase activity and annexin V staining in striatal neurons (Figs. 6e and f) that was completely prevented when neurons were cocultured with similarly treated astrocytes isolated from NOS2−/− mice (Figs. 6e and f). Astrocytes exposed to Mn + TNFα displayed an activated phenotype that was characterized by stress fiber formation and hypertrophy of cytoplasmic processes, based upon immunofluorescence staining for GFAP and the high-affinity glutamate transporter, GLAST (Supplementary figs. 1a and b). This activated phenotype was not associated with astrocytic cell death, as determined using the live/dead viability assay for propidium iodide labeling of cell nuclei (Supplementary figs. 1c and d).
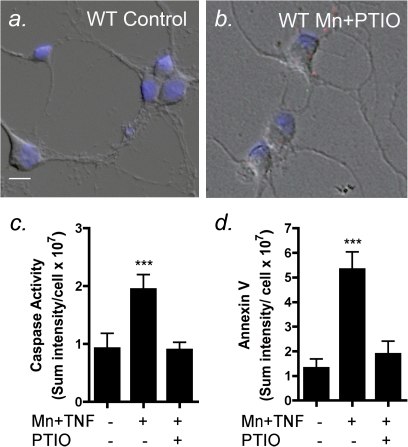
To further determine the etiological role of astrocyte-derived NO in Mn-dependent apoptosis in striatal neurons, wildtype astrocytes were exposed to Mn + TNFα and then incubated with striatal neurons in the presence or absence of the NO scavenger, 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO) (Fig. 7). The addition of 100μM PTIO during the coculture period completely attenuated caspase-3 and annexin V staining in striatal neurons following incubation with Mn-treated wildtype astrocytes, whereas wildtype astrocytes exposed to Mn + TNFα in the presence of vehicle control (dimethyl sulfoxide) caused large increase in the number of apoptotic neurons (Figs. 7c and d).
FIG. 7.
Chemically scavenging NO prevents apoptosis in striatal neurons co-cultured with wildtype astrocytes exposed to Mn and TNFα. Striatal astrocytes cultured from wildtype mice were exposed to 30 μM with Mn and 10 pg/ml TNFα for 24 h and incubated with striatal neurons in the presence or absence of the NO scavenger, 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO). Representative images are shown from neurons cocultured with control (a) and Mn-treated (b) wildtype astrocytes in the presence of PTIO, which prevented both caspase activation (c) and annexin V staining (d). Scale bar = 10 μm. ***p < 0.001.
DISCUSSION
Nitrosative stress is implicated in neuronal injury during Mn intoxication (Aschner et al., 2009) and aberrant expression of NOS2 by activated glia may be critical to this response by enhancing oxidative damage to neurons and promoting a cycle of neuroinflammation that ultimately leads to synaptic dysfunction, loss of neurons, and deprecations in neurological function (Liu et al., 2006; Moreno et al., 2009a; Verina et al., 2011). The present studies support this model because neurobehavioral changes observed in juvenile C57Bl/6 wildtype mice exposed to Mn were absent in NOS2−/− mice (Fig. 1). These results are consistent with previous studies that identify juvenile development as a particularly vulnerable time to Mn-induced behavioral dysfunction, associated with increased spontaneous movement, hyperactivity, and behavioral disinhibition (Brenneman et al., 1999; Kern et al., 2010; Moreno et al., 2009b). Likewise, clinical studies associate childhood exposure to Mn with hyperactivity and behavioral disinhibition (Bouchard et al., 2007; Ericson et al., 2007). The increased number of total movements and rearing movements observed in Mn-treated wildtype mice is consistent with Mn-induced hyperactivity and with the observed decrease in the HVA/DA ratio in these animals. Protection against these Mn-induced behavioral changes in NOS2−/− mice suggests that overproduction of NO by activated glial cells plays a direct role in the observed alterations in neurobehavioral function in Mn-treated mice. NOS2-deficient mice have a distinct behavioral phenotype characterized by increased susceptibility to stress, anxiety, and elevated corticosterone levels (Abu-Ghanem et al., 2008; Buskila et al., 2007), which likely explains their baseline differences in locomotor activity compared with congenic wildtype controls. The glia in these mice have previously been shown to have significantly elevated levels of NOS activity in the neocortex through calcium-dependent isoforms (NOS1 and NOS3) (Buskila et al., 2007), which also likely contributes to baseline changes in neurobehavioral function.
Although there were changes in locomotor function and neurobehavioral activity in Mn-treated wildtype mice, neurotransmitter levels were unaltered by exposure in either genotype (Table 1). However, there was a significant decrease in the HVA/DA ratio (Fig. 3), suggesting either a decrease in DA catabolism or an increase in DA release. We previously reported that mice orally exposed to 30 mg/kg Mn during this period of juvenile development had increased striatal DA levels (Moreno et al., 2009b), whereas adult mice orally exposed to 100 mg/kg Mn for 8 weeks displayed loss of striatal DA (Liu et al., 2006), suggesting a biphasic response where low-dose or short duration exposure to Mn causes an increase in synaptic release that causes increased DA turnover and neuronal injury over longer duration exposures. These earlier studies also reported that juvenile mice exposed to 30 mg/kg Mn had a decrease in the DOPAC/DA ratio in the striatum that was not observed in adult animals exposed to the same dose of Mn for 8 weeks (Moreno et al., 2009b), suggesting that DA catabolism or synthesis is more vulnerable in juveniles than adults. Similarly, in the present studies, we observed a decrease in the HVA/DA ratio in Mn-treated wildtype mice that was prevented in NOS2−/− mice, which could explain the locomotor differences between the two genotypes. Additionally, Mn can directly increase the activity of tyrosine hydroxylase (TH) (Zhang et al., 2011), which could also explain the decrease in the HVA/DA we observed in Mn-treated wildtype mice. Excess NO that occurs during neuroinflammation alters dopaminergic neurotransmission and regulation of locomotor activity in the striatum (Del Bel et al., 2005; West et al., 2002), possibly through inhibition of DA reuptake or synthesis (Kiss and Vizi, 2001; West et al., 2002). These data suggest that age and duration of exposure to Mn are important factors in modulating behavioral outcomes and neurotransmitter metabolism in the basal ganglia, in part through modulating the extent of neuroinflammatory production of NO by activated glia.
Gliosis was determined by immunohistopathological examination of GFAP and IBA1 protein expression in the basal ganglia. In wildtype mice, 50 mg/kg Mn caused a significant increase in activation of GFAP-positive astrocytes in the striatum and decreased astrocyte activation in the SN (Fig. 3). We previously observed a similar trend toward a decrease in GFAP staining in the SN at 30 mg/kg Mn in juvenile mice (Moreno et al., 2009a), suggesting the basal level of proliferative activity of astrocytes is inhibited by Mn exposure during postweaning juvenile development, a period during which a high level of astrocyte proliferation occurs (Rice and Barone, 2000). These findings indicate that the effects of juvenile exposure to Mn could result in lasting dysfunction in the development of the SN that could promote greater susceptibility to secondary neurotoxicant exposures during adulthood, as we previously reported for Mn (Moreno et al., 2009a,b). Excessive NO has cytostatic effects on neuronal growth, and it is possible that proliferating astrocytes respond similarly with a reduction in growth due to Mn-induced NOS2 activity that could have a lasting impact on affected brain regions (Peunova and Enikolopov, 1995). In NOS2−/− mice exposed to Mn, there was a significant increase in GFAP activation in both the ST and SN, indicating that astrocyte activation still occurred despite loss of function of NOS2. This suggests that glial-derived NO is critical to Mn-induced neurological dysfunction because alterations in both neurobehavioral function and neurochemistry were prevented by gene deletion of NOS2 despite the increase in astrocyte activation.
The progression of Mn neurotoxicity has also been associated with activation of microglia and subsequent release of inflammatory cytokines and NO (Verina et al., 2011). Notably, we observed an increase in abundance of IBA1-positive microglia in the SN with Mn treatment in both wildtype and NOS2−/− mice (Fig. 4). Microglia are particularly dense in this region (Lawson et al., 1990) and the observed activation of microglia following Mn exposure is consistent with earlier studies from our laboratory, which reported a similar increase in microglial activation in juvenile mice exposed to Mn at 30 mg/kg Mn (Moreno et al., 2009a). Collectively, these data indicate that gene deletion of NOS2 does not alter microglial activation in Mn-treated mice but rather differentially affects activation and proliferation of astrocytes in the basal ganglia. This finding supports a direct role for activated glia in neuroinflammatory injury and nitrosative stress following Mn exposure.
Mn-induced activation of astrocytes and microglia results in increased levels of NOS2 and NO (Bae et al., 2006; Moreno et al., 2009a; Verina et al., 2011). However, glial activation in NOS2−/− mice exposed to Mn did not increase neuronal protein nitration compared with saline controls (Fig. 5), indicating that NOS2 is responsible for elevated levels of nitrosative stress and peroxynitrite formation in Mn-treated mice. These results support that nitrosative stress in the basal ganglia is an important pathological feature of Mn neurotoxicity that influences neuronal injury and locomotor activity in exposed mice. Elevated baseline levels of 3NT adducts in NOS2−/− mice are likely due to compensatory increases in NOS1 and NOS3 activity, as previously reported for these mice (Buskila et al., 2007). Nitration of tyrosine residues inhibits the enzymatic activity of TH, which can alter dopaminergic neurotransmission in response to MPTP treatment (Ara et al., 1998). A similar mechanism may underlie the changes in neurobehavioral function observed in Mn-treated mice because ablating NOS2 prevented increases in NT-labeled proteins in Mn-exposed knockout mice and reversed the behavioral changes that occurred in Mn-treated wildtype animals.
Coculture studies using primary striatal astrocytes and neurons indicated that wildtype astrocytes exposed to Mn and TNFα caused apoptosis in striatal neurons (Fig. 6) that was inhibited by NOS2−/− astrocytes, indicating that NO is required for astrocyte-mediated neuronal injury. These findings confirm our previous observations that pharmacologic inhibition of NOS2 in Mn- and cytokine-treated astrocytes prevented apoptosis in PC12 neurons (Liu et al., 2005). Glial-derived NO causes neuronal injury in part by inhibiting mitochondrial Complex IV, resulting in loss of ATP and increased production of superoxide (Bal-Price and Brown, 2001; Brown and Neher, 2010). The present data suggest a similar mechanism because the NO scavenger, PTIO, prevented neuronal apoptosis following incubation with wildtype astrocytes stimulated with Mn and TNFα (Fig. 7). These data are consistent with our previous findings that Mn promotes NOS2 expression in astrocytes in orally exposed mice associated with neuronal apoptosis in striatal and pallidal interneurons expressing enkephalin, ChAT, and NOS1 (Liu et al., 2006).
In summary, these studies demonstrate that juvenile mice lacking functional NOS2 are protected from Mn-induced locomotor dysfunction and glial-mediated nitrosative stress, indicating that production of NO by activated glia contributes to neurotoxicity. Further studies are necessary to determine the temporal pattern of NOS2 expression in astrocytes and microglia as well as the cellular signaling mechanisms leading to inducible expression of NOS2. It will also be important to understand how the activation of NOS2 during juvenile Mn exposure may enhance susceptibility to secondary exposures and neurological disease during aging.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (ES012941 to R.B.T.).
Supplementary Material
References
- Abu-Ghanem Y, Cohen H, Buskila Y, Grauer E, Amitai Y. Enhanced stress reactivity in nitric oxide synthase type 2 mutant mice: Findings in support of astrocytic nitrosative modulation of behavior. Neuroscience. 2008;156:257–265. doi: 10.1016/j.neuroscience.2008.07.043. [DOI] [PubMed] [Google Scholar]
- Ara J, Przedborski S, Naini AB, Jackson-Lewis V, Trifiletti RR, Horwitz J, Ischiropoulos H. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Proc. Natl. Acad. Sci. U.S.A. 1998;95:7659–7663. doi: 10.1073/pnas.95.13.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Herrero Hernandez E, Tjalkens R. Manganese and its role in Parkinson's disease: From transport to neuropathology. Neuro. Mol. Med. 2009;11:252–266. doi: 10.1007/s12017-009-8083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Kimelberg HK. The use of astrocytes in culture as model systems for evaluating neurotoxic-induced-injury. Neurotoxicology. 1991;12:505–517. [PubMed] [Google Scholar]
- Bae JH, Jang BC, Suh SI, Ha E, Baik HH, Kim SS, Lee MY, Shin DH. Manganese induces inducible nitric oxide synthase (iNOS) expression via activation of both MAP kinase and PI3K/Akt pathways in BV2 microglial cells. Neurosci. Lett. 2006;398:151–154. doi: 10.1016/j.neulet.2005.12.067. [DOI] [PubMed] [Google Scholar]
- Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J. Neurosci. 2001;21:6480–6491. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: Pilot study of school-age children exposed through tap water. Environ. Health Perspect. 2007;115:122–127. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M, Mergler D, Baldwin ME, Panisset M. Manganese cumulative exposure and symptoms: A follow-up study of alloy workers. Neurotoxicology. 2008;29:577–583. doi: 10.1016/j.neuro.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Sauve S, Barbeau B, Legrand M, Brodeur ME, Bouffard T, Limoges E, Bellinger DC, Mergler D. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ. Health Perspect. 2011;119:138–143. doi: 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler RM, Koller W, Schulz PE. Parkinsonism due to manganism in a welder: Neurological and neuropsychological sequelae. Neurotoxicology. 2006;27:327–332. doi: 10.1016/j.neuro.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Brenneman KA, Cattley RC, Ali SF, Dorman DC. Manganese-induced developmental neurotoxicity in the CD rat: Is oxidative damage a mechanism of action? Neurotoxicology. 1999;20:477–487. [PubMed] [Google Scholar]
- Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol. Neurobiol. 2010;41:242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- Buskila Y, Abu-Ghanem Y, Levi Y, Moran A, Grauer E, Amitai Y. Enhanced astrocytic nitric oxide production and neuronal modifications in the neocortex of a NOS2 mutant mouse. PLoS One. 2007;2:e843. doi: 10.1371/journal.pone.0000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic Parkinsonism: Similarities and differences. Neurology. 1994;44:1583–1586. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Carbone DL, Moreno JA, Tjalkens RB. Nuclear factor kappa-B mediates selective induction of neuronal nitric oxide synthase in astrocytes during low-level inflammatory stimulation with MPTP. Brain Res. 2008;1217:1–9. doi: 10.1016/j.brainres.2008.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone DL, Popichak KA, Moreno JA, Safe S, Tjalkens RB. Suppression of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced nitric-oxide synthase 2 expression in astrocytes by a novel diindolylmethane analog protects striatal neurons against apoptosis. Mol. Pharmacol. 2009;75:35–43. doi: 10.1124/mol.108.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bel EA, Guimarães FS, Bermúdez-Echeverry M, Gomes MZ, Schiaveto-de-souza A, Padovan-Neto FE, Tumas V, Barion-Cavalcanti AP, Lazzarini M, Nucci-da-Silva LP, et al. Role of nitric oxide on motor behavior. Cell. Mol. Neurobiol. 2005;25:371–392. doi: 10.1007/s10571-005-3065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Vitarella D, Byerly FL, Goetz J, Miller R. Neurotoxicity of manganese chloride in neonatal and adult CD rats following subchronic (21-day) high-dose oral exposure. J. Appl. Toxicol. 2000;20:179–187. doi: 10.1002/(sici)1099-1263(200005/06)20:3<179::aid-jat631>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson JE, Crinella FM, Clarke-Stewart KA, Allhusen VD, Chan T, Robertson RT. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicol. Teratol. 2007;29:181–187. doi: 10.1016/j.ntt.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Filipov NM, Seegal RF, Lawrence DA. Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B-dependent mechanism. Toxicol. Sci. 2005;84:139–148. doi: 10.1093/toxsci/kfi055. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem. Biophys. Res. Commun. 2003;305:776–783. doi: 10.1016/s0006-291x(03)00814-3. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson's disease. Ann. Neurol. 2003;53(Suppl. 3):S26–S36. doi: 10.1002/ana.10483. ; discussion S36–S28. [DOI] [PubMed] [Google Scholar]
- Kern CH, Smith DR. Preweaning Mn exposure leads to prolonged astrocyte activation and lasting effects on the dopaminergic system in adult male rats. Synapse. 2010;64:363–378. doi: 10.1002/syn.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern CH, Stanwood GD, Smith DR. Preweaning manganese exposure causes hyperactivity, disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels. Synapse. 2010;64:363–378. doi: 10.1002/syn.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JP, Vizi ES. Nitric oxide: A novel link between synaptic and nonsynaptic transmission. Trends Neurosci. 2001;24:211–215. doi: 10.1016/s0166-2236(00)01745-8. [DOI] [PubMed] [Google Scholar]
- Laubach VE, Shesely EG, Smithies O, Sherman PA. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc. Natl. Acad. Sci. U.S.A. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Liu X, Buffington JA, Tjalkens RB. NF-kappaB-dependent production of nitric oxide by astrocytes mediates apoptosis in differentiated PC12 neurons following exposure to manganese and cytokines. Brain Res. Mol. Brain Res. 2005;141:39–47. doi: 10.1016/j.molbrainres.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Liu X, Sullivan KA, Madl JE, Legare M, Tjalkens RB. Manganese-induced neurotoxicity: The role of astroglial-derived nitric oxide in striatal interneuron degeneration. Toxicol. Sci. 2006;91:521–531. doi: 10.1093/toxsci/kfj150. [DOI] [PubMed] [Google Scholar]
- Moreno JA, Streifel KM, Sullivan KA, Hanneman WH, Tjalkens RB. Manganese-induced NF-{kappa}B activation and nitrosative stress is decreased by estrogen in juvenile mice. Toxicol. Sci. 2011;122:121–133. doi: 10.1093/toxsci/kfr091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Streifel KM, Sullivan KA, Legare ME, Tjalkens RB. Developmental exposure to manganese increases adult susceptibility to inflammatory activation of glia and neuronal protein nitration. Toxicol. Sci. 2009a;112:405–415. doi: 10.1093/toxsci/kfp221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Sullivan KA, Carbone DL, Hanneman WH, Tjalkens RB. Manganese potentiates nuclear factor-kappaB-dependent expression of nitric oxide synthase 2 in astrocytes by activating soluble guanylate cyclase and extracellular responsive kinase signaling pathways. J. Neurosci. Res. 2008;86:2028–2038. doi: 10.1002/jnr.21640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Yeomans EC, Streifel KM, Brattin BL, Taylor RJ, Tjalkens RB. Age-dependent susceptibility to manganese-induced neurological dysfunction. Toxicol. Sci. 2009b;112:394–404. doi: 10.1093/toxsci/kfp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland MC, Ceckler TL, Kordower JH, Weiss B. Visualizing manganese in the primate basal ganglia with magnetic resonance imaging. Exp. Neurol. 1989;106:251–258. doi: 10.1016/0014-4886(89)90157-x. [DOI] [PubMed] [Google Scholar]
- Olanow CW. Manganese-induced parkinsonism and Parkinson's disease. Ann. N. Y. Acad. Sci. 2004;1012:209–223. doi: 10.1196/annals.1306.018. [DOI] [PubMed] [Google Scholar]
- Perl DP, Olanow CW. The neuropathology of manganese-induced Parkinsonism. J. Neuropathol. Exp. Neurol. 2007;66:675–682. doi: 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- Peunova N, Enikolopov G. Nitric oxide triggers a switch to growth arrest during differentiation of neuronal cells. Nature. 1995;375:68–73. doi: 10.1038/375068a0. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J. Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabace L, Kendrick KM. Nitric oxide can differentially modulate striatal neurotransmitter concentrations via soluble guanylate cyclase and peroxynitrite formation. J. Neurochem. 2000;75:1664–1674. doi: 10.1046/j.1471-4159.2000.0751664.x. [DOI] [PubMed] [Google Scholar]
- Verina T, Kiihl SF, Schneider JS, Guilarte TR. Manganese exposure induces microglia activation and dystrophy in the substantia nigra of non-human primates. Neurotoxicology. 2011;32:215–226. doi: 10.1016/j.neuro.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, LoIacono NJ, et al. Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Environ. Health Perspect. 2006;114:124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AR, Galloway MP, Grace AA. Regulation of striatal dopamine neurotransmission by nitric oxide: Effector pathways and signaling mechanisms. Synapse. 2002;44:227–245. doi: 10.1002/syn.10076. [DOI] [PubMed] [Google Scholar]
- Woolf A, Wright R, Amarasiriwardena C, Bellinger D. A child with chronic manganese exposure from drinking water. Environ. Health Perspect. 2002;110:613–616. doi: 10.1289/ehp.02110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J. Exp. Med. 1993;177:1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Kanthasamy A, Anantharam V. Effects of manganese on tyrosine hydroxylase (TH) activity and TH-phosphorylation in a dopaminergic neural cell line. Toxicol. Appl. Pharmacol. 2011;254:54–71. doi: 10.1016/j.taap.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Liu D, He P. [Effects of manganese on learning abilities in school children] Zhonghua Yu Fang Yi Xue Za Zhi. 1995;29:156–158. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.