Abstract
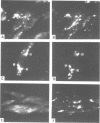
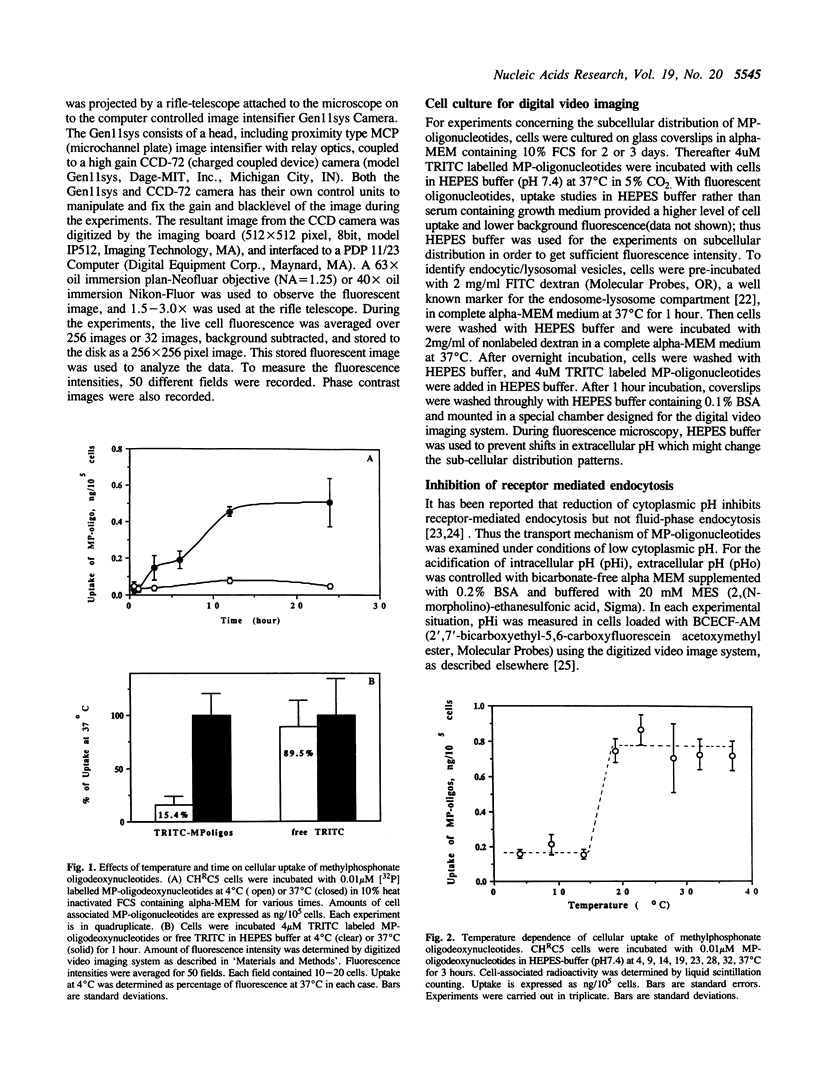
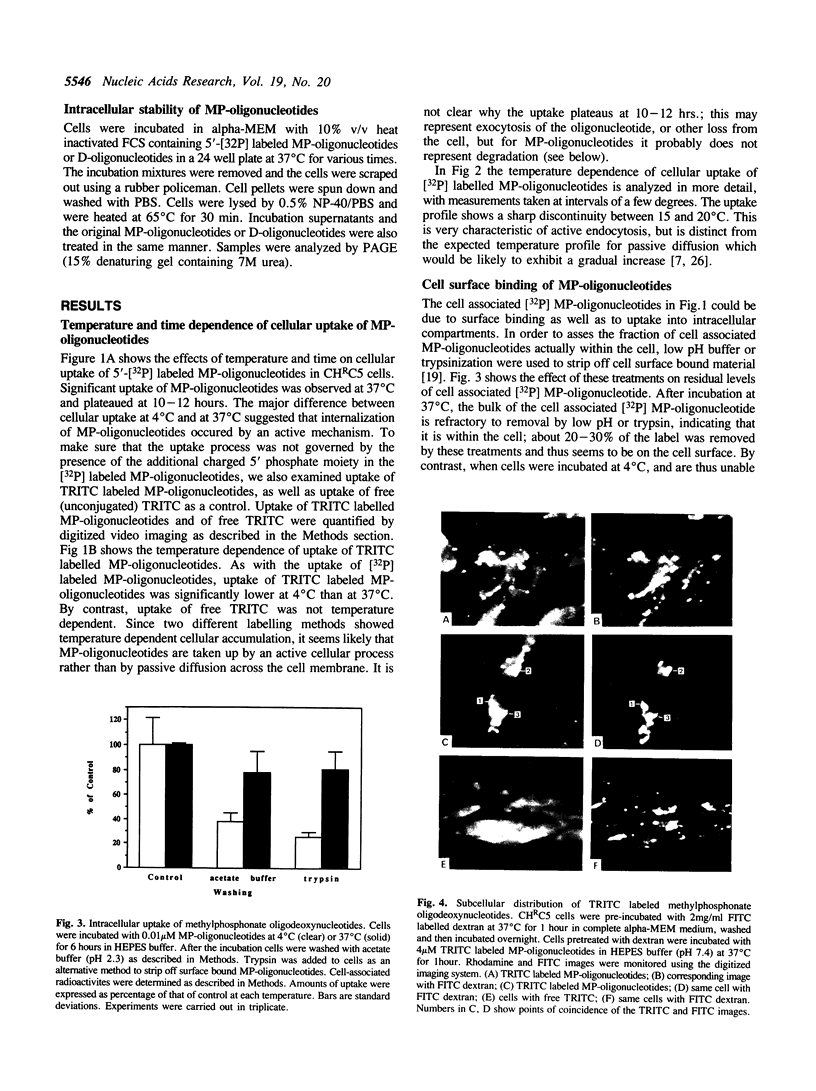
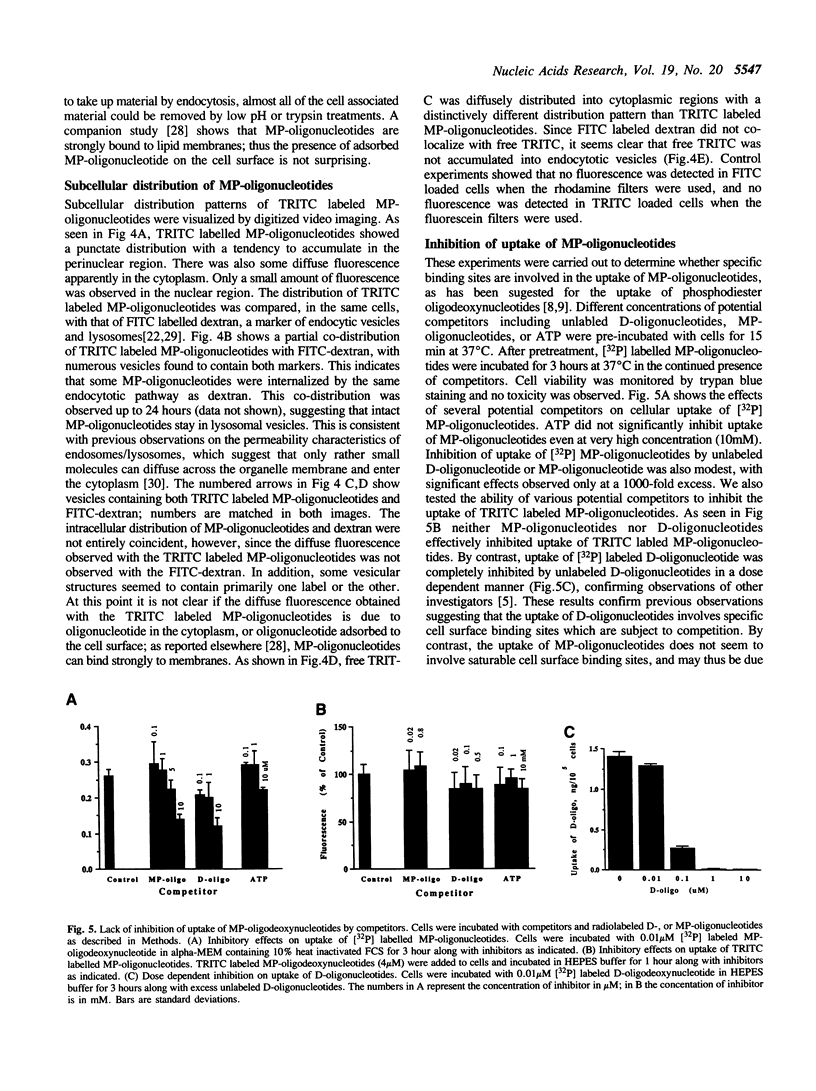
The cellular uptake and intracellular distribution of methylphosphonate oligonucleotides (15 mers) has been examined using both 32P labeled and fluorescent labeled oligonucleotides. The cellular uptake process for methylphosphonate oligonucleotides is highly temperature dependent, with a major increase in uptake occurring between 15 and 20 degrees C. Most of the label which becomes cell associated at 37 degrees C cannot be removed by acid washing or trypsinization and thus seems to be within the cell. Visualization of rhodamine labeled methylphosphonate oligonucleotides using digital imaging fluorescence microscopy reveals a vesicular subcellular distribution suggestive of an endosomal localization. There was extensive co-localization of rhodamine labeled methylphosphonate oligonucleotides with fluorescein-dextran, an endosomal/lysosomal marker substance. The apparent endocytotic uptake of labeled methylphosphonate oligonucleotides could not be blocked by competition with unlabeled methylphosphonate or phosphodiester oligonucleotides, nor by ATP. This contrasts with the situation for radiolabeled phosphodiester oligonucleotides whose uptake can be completely blocked with unlabeled competitor. Uptake of phosphodiester oligonucleotides, but not of methylphosphonate oligonucleotides, could be blocked by acidification of the cytosol. These observations suggest that the pathway of cellular uptake of methylphosphonate oligonucleotides involves fluid phase or adsorbtive endocytosis, and is distinct from the uptake pathway for phosphodiester oligonucleotides.
Full text
PDF

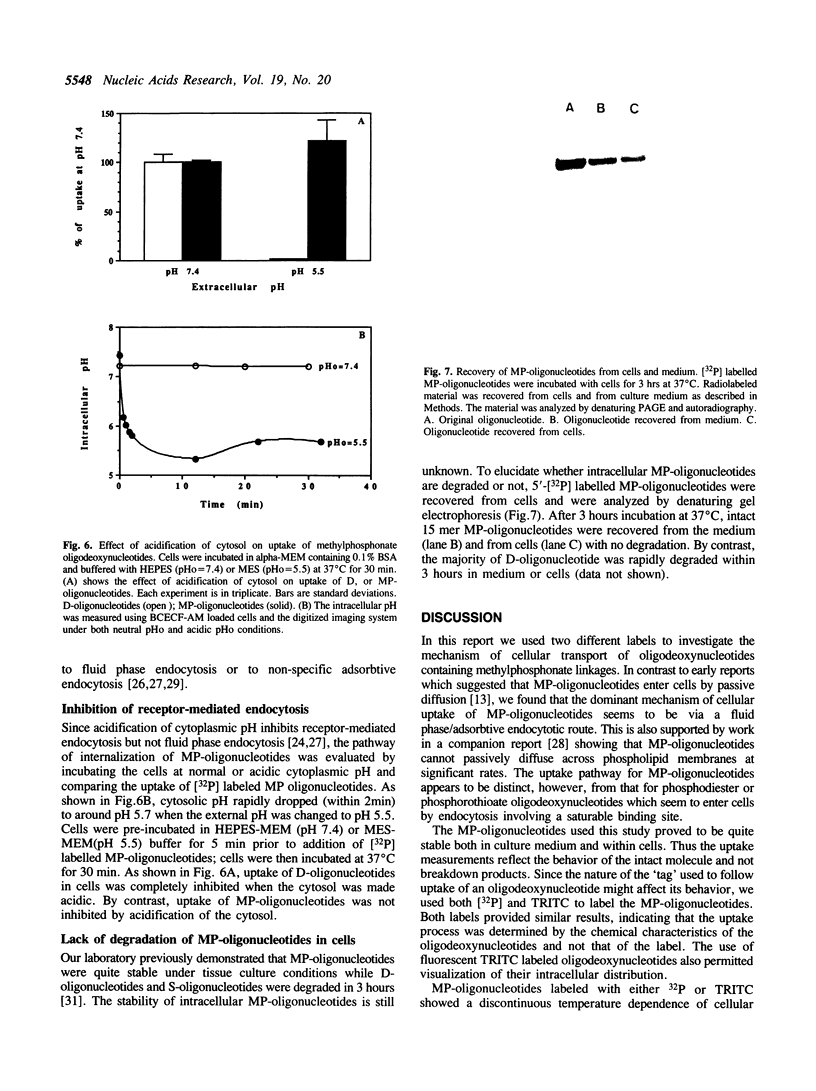


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Ikeuchi T., Sun D., Sarin P. S., Konopka A., Maizel J., Zamecnik P. C. Inhibition of human immunodeficiency virus in early infected and chronically infected cells by antisense oligodeoxynucleotides and their phosphorothioate analogues. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7790–7794. doi: 10.1073/pnas.86.20.7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley G., Juranka P. F., Ling V. Mechanism of multidrug resistance. Biochim Biophys Acta. 1988 Aug 3;948(1):87–128. doi: 10.1016/0304-419x(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Chin D. J., Green G. A., Zon G., Szoka F. C., Jr, Straubinger R. M. Rapid nuclear accumulation of injected oligodeoxyribonucleotides. New Biol. 1990 Dec;2(12):1091–1100. [PubMed] [Google Scholar]
- Davoust J., Gruenberg J., Howell K. E. Two threshold values of low pH block endocytosis at different stages. EMBO J. 1987 Dec 1;6(12):3601–3609. doi: 10.1002/j.1460-2075.1987.tb02691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich B. A., Cohn Z. A. The fate of peptides pinocytosed by macrophages in vitro. J Exp Med. 1969 Jan 1;129(1):227–245. doi: 10.1084/jem.129.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gores G. J., Nieminen A. L., Wray B. E., Herman B., Lemasters J. J. Intracellular pH during "chemical hypoxia" in cultured rat hepatocytes. Protection by intracellular acidosis against the onset of cell death. J Clin Invest. 1989 Feb;83(2):386–396. doi: 10.1172/JCI113896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Lemasters J. J., DiGuiseppi J., Nieminen A. L., Herman B. Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature. 1987 Jan 1;325(6099):78–81. doi: 10.1038/325078a0. [DOI] [PubMed] [Google Scholar]
- Leonetti J. P., Mechti N., Degols G., Gagnor C., Lebleu B. Intracellular distribution of microinjected antisense oligonucleotides. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2702–2706. doi: 10.1073/pnas.88.7.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke S. L., Stein C. A., Zhang X. H., Mori K., Nakanishi M., Subasinghe C., Cohen J. S., Neckers L. M. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., McParland K. B., Jayaraman K., Ts'o P. O. Biochemical and biological effects of nonionic nucleic acid methylphosphonates. Biochemistry. 1981 Mar 31;20(7):1874–1880. doi: 10.1021/bi00510a024. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Petersen O. W., van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987 Aug;105(2):679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sczekan M. M., Juliano R. L. Internalization of the fibronectin receptor is a constitutive process. J Cell Physiol. 1990 Mar;142(3):574–580. doi: 10.1002/jcp.1041420317. [DOI] [PubMed] [Google Scholar]
- Smith C. C., Aurelian L., Reddy M. P., Miller P. S., Ts'o P. O. Antiviral effect of an oligo(nucleoside methylphosphonate) complementary to the splice junction of herpes simplex virus type 1 immediate early pre-mRNAs 4 and 5. Proc Natl Acad Sci U S A. 1986 May;83(9):2787–2791. doi: 10.1073/pnas.83.9.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom E. Oligodeoxynucleotide stability in subcellular extracts and culture media. J Biochem Biophys Methods. 1986 Sep;13(2):97–102. doi: 10.1016/0165-022x(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Wolosin J. M., Ginsburg H., Lieb W. R., Stein W. D. Diffusion within egg lecithin bilayers resembles that within soft polymers. J Gen Physiol. 1978 Jan;71(1):93–100. doi: 10.1085/jgp.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakubov L. A., Deeva E. A., Zarytova V. F., Ivanova E. M., Ryte A. S., Yurchenko L. V., Vlassov V. V. Mechanism of oligonucleotide uptake by cells: involvement of specific receptors? Proc Natl Acad Sci U S A. 1989 Sep;86(17):6454–6458. doi: 10.1073/pnas.86.17.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro D. J., Maxfield F. R. Kinetics of endosome acidification in mutant and wild-type Chinese hamster ovary cells. J Cell Biol. 1987 Dec;105(6 Pt 1):2713–2721. doi: 10.1083/jcb.105.6.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon G. Oligonucleotide analogues as potential chemotherapeutic agents. Pharm Res. 1988 Sep;5(9):539–549. doi: 10.1023/a:1015985728434. [DOI] [PubMed] [Google Scholar]
- van Deurs B., Petersen O. W., Olsnes S., Sandvig K. The ways of endocytosis. Int Rev Cytol. 1989;117:131–177. doi: 10.1016/s0074-7696(08)61336-4. [DOI] [PubMed] [Google Scholar]