Abstract
The goal of this study was to examine whether growth delay can serve as an index of allostatic load during early development, as it is well known that the activity of stress-mediating systems inhibits growth. The participants were children adopted internationally from institutional care (n = 36), children adopted internationally from foster care (n = 6), and nonadopted children (n = 35). For the adopted children, height-for-age and weight-for-height were assessed at adoption; for all children, disinhibited social approach (DSA; termed elsewhere as “indiscriminate friendliness”) and diurnal cortisol were assessed at 6–8 years (M = 6.9 years). For internationally adopted children in general, and postinstitutionalized children specifically, linear growth delay assessed at the time of adoption was associated with more dysregulated behavior in response to an unfamiliar adult (i.e., greater DSA) and a more dysregulated diurnal cortisol rhythm (i.e., higher late-afternoon and evening values). Further, among the most growth-delayed children, higher cortisol levels later in the day were correlated with DSA. The potential for using growth delay as an allostatic load indicator and the possible problems and limitations in its use in child populations are discussed.
Keywords: allostatic load, psychosocial growth delay, indiscriminate friendliness, disinhibited social approach, salivary cortisol
Allostasis, a concept introduced by Sterling and Eyer (1988), describes the maintenance of stability or homeostasis through change. McEwen and Stellar (1993) applied allostasis to explain the relations between stress and disease. Psychological and physical threats to homeostasis (i.e., stressors) activate a network of allostatic mediators that are involved in stress responses (i.e., cortisol, dehydroepiandrosterone, sympathetic and parasympathetic activity, pro- and anti-inflammatory cytokines, and oxidative stress). These allostatic mediators regulate each other within a nonlinear network (McEwen, 2006). Thus, allostasis is the active process of adapting to maintain homeostasis.
Activation of stress responses, however, produces wear and tear on the brain and body. The allostatic load model proposes a cascade of multisystemic physiological dysregulations resulting from accumulated overactivation of stress-mediating systems (Seeman et al., 2010). The resulting multisystem dysregulation contributes to a wide range of disorders (McEwen & Gianaros, 2010; Seeman, Singer, Rowe, Horwitz, & McEwen, 1997). Although physical disorders and cognitive aging have been the focus of much of the research on allostatic load (see Juster, McEwen, & Lupien, 2010), cumulative overactivation of stress-mediating systems also should affect psychological health. Ganzel, Morris, and Wethington (2010) argued that the brain regions critical for emotional functioning constitute the primary mediator of the relations between stress and disease and the neural focus of wear and tear due to ongoing adaptation. Indeed, stress-mediating systems impact the structure and function of the hippocampus, amygdala, and prefrontal cortex (Arnsten, 2009; Juster et al., 2010; McEwen, 2006).
Although the allostatic load model has been applied primarily to adults, there is increased interest in applying it to children (e.g., the articles in this volume; Repetti, Taylor, & Seeman, 2002; Thompson & Levitt, 2010; Turner-Cobb, 2005). From a developmental psychopathology perspective, early experiences exert long-term impacts by shaping the development of biological and psychological systems. Maladaptation is seen as having its roots in the accumulation of multisystemic organizational deficiencies, which impair an individual’s capacity to cope with ongoing stress and adversity (Cicchetti & Gunnar, 2008; Cicchetti & Tucker, 1994). The allostatic load model, with its emphasis on multisystemic biological dysregulation following chronic stress, is compatible with a developmental psychopathology framework. Indeed, a core principle of developmental psychopathology is that the aggregation of multiple risk factors is particularly detrimental to development (Cicchetti & Sroufe, 2000; Sameroff, 1989); the allostatic load model offers one set of interactive pathways through which cumulative risk increases the probability of maladaptive physical and mental health outcomes.
Applying the allostatic load model to children is attractive but poses a number of questions (Ganzel et al., 2010). First, there are developmental changes in children’s stress-mediating systems, which likely result in nonlinear relations among these systems. How these changes might influence application of the allostatic load model to infants, children, and adolescents is unclear. Second, the long-term consequences of allostatic load might vary depending on the developmental timing and duration of overactivation of stress-mediating systems. This may be especially critical to differentiating long-term effects of allostatic load in developing versus mature organisms. Third, we need to consider how the allostatic load model relates to models of the early origins of adult disease, which are based on constructs like programming and predictive adaptation during sensitive periods rather than simply cumulative wear and tear (Barker, 2007; Rutter & O’Connor, 2004). Finally, it is unclear whether we can use the same indices to assess allostatic load in children as in adults (Ganzel et al., 2010).
To assess allostatic load in adulthood, panels of indices have been developed. These include systolic and diastolic blood pressure, high-density and low-density lipoprotein cholesterol, insulin resistance, inflammatory cytokines, heart rate variability, and activity of the hypothalamic-pituitary-adrenocortical (HPA) axis (e.g., the cortisol awakening response and diurnal cortisol slope; Seeman et al., 2010). By young adulthood, statistical models of these measures are consistent with a multisystem allostatic load model. Although all of these measures can be taken in young children, it is unlikely that the wear and tear of allostatic mediators in the first few years of life can accumulate sufficiently to lead to elevated blood pressure, increased cholesterol levels, and insulin resistance. There is evidence of dysregulation of the HPA axis and relations between cardiovascular measures and behavior dysregulation in young children exposed to adverse experiences (Gunnar & Quevedo, 2007a). However, the allostatic load model argues that multiple systems need to be assessed and that it is their interacting pattern of dysregulation that indexes allostatic load (Seeman et al., 2010). Related to this, from a developmental psychopathology perspective, Cicchetti and Toth (2009) contend that a multilevel analysis approach is critical to understanding the bidirectional processes underlying development. Thus, we need to identify additional systems that are dysregulated by allostatic mediators and that can be detected early in development to track the development of allostatic load.
Stress and Physical Growth
Energy (i.e., metabolism) and its regulation are central to the concept of allostasis. Periods of threat and challenge are energy demanding (McEwen & Wingfield, 2010; Romero, Dickens, & Cyr, 2009), and prolonged or chronic stress can involve times when metabolic demands outstrip readily available resources, thus threatening survival (McEwen & Wingfield, 2010). One function of allostatic mediators at these times is to alter behavior and shift physiological activity to reduce metabolic demands or increase energy intake. Age and developmental stage are important in this equation as they relate to daily energy needs (McEwen & Wingfield, 2010; Romero et al., 2009). Physical growth is metabolically demanding, and one impact of stress, mediated in part by the HPA axis, is to inhibit growth (Chrousos, 2009; Romero et al., 2009). Cortisol releasing hormone (CRH) operates through CRH-2 receptors to reduce appetite (Dautzenberg, Kilpatrick, Hauger, & Moreau, 2001) and stimulate somatostatin, which inhibits growth hormone release (Rivier & Vale, 1985). Cortisol, in contrast, stimulates appetite, but reduces the liver’s production of insulin-like growth factor (IGF-1), which is needed for bone growth and health (Olney, 2009). In mature organisms, a hallmark of chronic stress is weight reduction that rebounds after the stressor has been alleviated (Awerman & Romero, 2010). In developing organisms, chronic stress induced by lack of supportive care impairs bone growth (i.e., height and head circumference) and body weight, which both rebound once conditions improve (Johnson et al., 2010). Thus, growth delay might be an index of allostatic load that is particularly relevant to children.
Growth and Conditions of Early Psychosocial Deprivation
Children reared in institutional care experience an early environment with too many children sharing too few resources; this not only deprives children of physical and stimulatory needs but also greatly reduces the opportunity to build a consistent relationship with a caregiver. Growth failure among institutionalized infants and young children has long been recognized, with estimates of the loss of 1 month of linear growth for every 2 to 3 months in institutional care (Miller & Hendrie, 2000). However, children adopted out of institutions into advantaged and stable homes provide a natural experiment of the plasticity of neurobehavioral systems, including the growth system. In a recent meta-analysis of internationally adopted children, Van IJzendoorn, Bakermans-Kranenburg, and Juffer (2007) confirmed significant stunting in linear growth and weight at adoption that was related to the duration of institutional care, with marked rebound in growth parameters following adoption. Height and weight continued to approach population norms throughout childhood; following puberty, though, the children who were growth delayed at adoption failed to achieve population norms and were significantly smaller than peers who had not experienced early deprivation. The pattern of growth delay and recovery described by Van IJzendoorn and colleagues has been replicated in recent studies of postinstitutionalized children (Johnson et al., 2010; Loman, Wiik, Frenn, Pollak, & Gunnar, 2009; Sonuga-Barke, Schlotz, & Rutter, 2010; Van den Dries, Juffer, Van IJzendoorn, & Bakermans-Kranenburg, 2010).
Debates regarding the basis for growth delay among institutionalized children often have pitted malnutrition against psychosocial deprivation. Arguments for psychosocial deprivation include the well-established phenomenon of psychosocial short stature, a syndrome associated with neglect and abuse (Blizzard & Bulatovic, 1996). Although there are a number of subtypes based on age of onset and factors like response to growth hormone, the subtypes share two common features: otherwise unexplained growth failure occurring in association with socially stressful conditions and significant catch-up growth when the environment is improved (Gohlke, Khadilkar, Skuse, & Stanhope, 1998). Arguments for subnutrition include the fact that, unlike older children with psychosocial short stature for whom height-for-age is reduced and weight-for-height is normal (Himes et al., 2008), infants who experience adverse care display general growth failure that encompasses height, weight, and head circumference (Johnson et al., 2010). Suppression of weight-for-height is believed to index caloric restriction, and the data from a recent study showed that as many as 30% of infants in Romanian institutions were more than 2 SDs below the mean on weight-for-height, suggesting considerable subnutrition (Johnson et al., 2010). The greater involvement of subnutrition in growth failure among infants might be secondary to lack of supportive psychosocial care. Infants are dependent upon adults for their nutrition, and behavioral withdrawal induced by lack of supportive care (e.g., hospitalism; Spitz, 1945) might further compromise nutrient intake. Additionally, in a recent study of the impact of growth hormone deficiency in infancy, Mehta et al. (2005) challenged the subnutrition argument; when experienced in infancy, deficiencies in growth hormone were found to produce global growth failure (i.e., height, weight, and head circumference) despite adequate caloric intake. Further, caregiver quality, independent of diet, was a significant predictor of catch-up in height and weight through age 54 months in children randomized to receive foster care versus continued institutional care in the Bucharest Early Intervention Project (Johnson et al., 2010).
Despite evidence that subnutrition is not required for growth delay among institutionalized children, examining the associations between delayed growth and outcomes and controlling for weight-for-height in analyses increases the likelihood that psychosocial deprivation is being indexed. It should be noted, however, that the need to make allostatic adjustments to conserve energy in response to subnutrition also fits into the allostatic load framework (McEwen & Gianaros, 2010; Romero et al., 2009). There is evidence that prolonged periods of subnutrition prior to age two years produce a chronic stress signature in activity of the HPA axis long after adequate nutrition has been restored (Fernald & Grantham-McGregor, 1998). Thus, based on the allostatic load model, whether the growth failure among institutionalized children is mediated by subnutrition or not is somewhat immaterial, although this differentiation has critical policy implications.
Activity of the HPA Axis and Conditions of Early Psychosocial Deprivation
In rodent models of adverse early care, researchers have observed evidence of epigenetic programming of the HPA axis (Meaney & Szyf, 2005) and evidence that extremely adverse care produces temporary stunting of physical growth (Rice, Sandman, Lenjavi, & Baram, 2008). In nonhuman primate models of adverse early care, researchers also have noted dysregulation of the normal diurnal cortisol rhythm (Sánchez et al., 2005). Among children, there is mounting evidence that early adverse care has lasting effects on HPA axis regulation, although these effects appear to depend on the duration, timing, and type of adversity and individual physical and emotional vulnerabilities (Gunnar & Quevedo, 2007b; Turner-Cobb, 2005).
Pertinent to the present study, during institutional care or soon after adoption, children tend to exhibit lower-than-expected early-morning cortisol levels and higher-than-expected late-afternoon and evening levels, resulting in a relatively flat pattern across the day (Carlson & Earls, 1997; Gunnar, 2000; cf., Dobrova-Krol, Van IJzendoorn, Bakermans-Kranenburg, Cyr, & Juffer, 2008). There is little evidence that early institutional care, per se, has persistent effects on HPA axis regulation after children have spent several years in their adoptive families (Kertes, Gunnar, Madsen, & Long, 2008). However, elevated set points for diurnal cortisol have been noted among children who experienced the type of severe privation associated with significant growth delays (Gunnar, Morison, Chisholm, & Schuder, 2001; Wismer Fries, Shirtcliff, & Pollak, 2008). Most notably, among children adopted from institutions, those with significant linear (i.e., height-for-age) growth delay at adoption tend to have higher cortisol set points several years after adoption relative to those who were not severely growth delayed (Gunnar, Frenn, Wewerka, & Van Ryzin, 2009; Kertes et al., 2008). This is consistent with evidence that, among children still living in institutions, those who are significantly growth delayed exhibit elevated levels of cortisol (Dobrova-Krol et al., 2008). Thus, for at least some children, deprivation-induced growth delay might be associated with elevations in cortisol and might serve as an index of allostatic load predicting long-term altered HPA axis activity.
Present Study
In this study, we examined whether growth delay might serve as an index of allostatic load during early development. We focused on internationally adopted children who had spent most of their preadoption lives in institutional care. Height-for-age at adoption, controlling for weight-for-height at adoption, was used to predict behavioral and neuroendocrine dysregulation several years postadoption.
We examined disinhibited social approach (DSA, termed elsewhere as “indiscriminate friendliness”; Bruce, Tarullo, & Gunnar, 2009) as a measure of behavioral dysregulation. This behavioral pattern includes an inappropriate (and often intrusive) approach to and physical contact with unfamiliar adults, a lack of normal wariness toward unfamiliar adults, and a willingness to accompany unfamiliar adults (Chisholm, 1998; Kumsta et al., 2010; Rutter, Kreppner, & Sonuga-Barke, 2009). This pattern of behavior has been noted for decades among children reared in institutions (Provence & Lipton, 1962; Tizard, 1977) and has been described as one of the patterns of dysregulation specific to deprived early care (Kumsta et al., 2010). DSA tends to persist for many years after adoption (Hodges & Tizard, 1989; Rutter et al., 2007) and, along with quasi-autistic behavior, appears to partially mediate psychopathology in adolescents who have been adopted from conditions of severe privation (Sonuga-Barke, Schlotz, & Kreppner, 2010). In a previous report on our sample, the postinstitutionalized children demonstrated increased DSA compared to the nonadopted children, whereas the children adopted from foster care exhibited DSA levels that fell between these two groups. DSA was also negatively correlated with inhibitory control (Bruce et al., 2009). Thus, this behavior might reflect a deprivation-induced dysregulation of behavior and neural functioning.
The neuroendocrine measures in our sample were salivary cortisol samples obtained thrice daily at home: wake-up, late afternoon, and near bedtime. As noted above, linear growth delay at adoption predicts HPA axis activity years following adoption (Gunnar et al., 2009; Kertes et al., 2008). To further these findings, we tested whether growth delay could predict diurnal cortisol patterns suggestive of HPA axis dysregulation several years after removal from adverse care. Consistent with the previous studies, we predicted an overall increase in diurnal cortisol levels (i.e., increase in the set point of the axis) and/or difficulty in bringing cortisol production to low levels by late afternoon and evening. We also examined whether the measures of DSA and altered HPA axis activity, which were hypothesized to reflect problems of dysregulation associated with allostatic load, were correlated, especially among children with severe growth delay at adoption.
Methods
Participants
The participants included 120 children (M age = 6.85 years, SD = 0.56 years) divided equally into three groups (each with 30 girls and 10 boys) based on early care experiences. The postinstitutionalized (PI) children had been internationally adopted at 12–36 months of age (M = 18.60 months, SD = 6.8 months) after spending at least 70% of their lives in institutional care. The foster care (FC) children had been internationally adopted at 2–14 months of age (M = 8.00 months, SD = 5.19 months) after spending at least 70% of their lives in foster care. The nonadopted (NA) children were born and raised in their families in the United States. The FC and NA groups were matched on age and sex to the PI group to serve as an adoption comparison group and as a nonadopted, socioeconomic status (SES) comparison group, respectively.
All children included in the present analyses had home cortisol data (95%, N = 113) and no medical condition that would affect HPA axis activity (1 NA child was removed). Both groups of internationally adopted (IA) children included in the present analyses had data on height and weight at adoption (PI = 95%, n = 36; FC = 67%, n = 29). The resulting groups (PI n = 36; FC n = 26; NA n = 35) included 72–74% girls, which did not differ significantly from the original sample (75% girls). Estimated IQ based on the vocabulary and block design subtests of the Wechsler Intelligence Scale for Children, 3rd Edition (Wechsler, 1991) differed by group, F(2, 93) = 9.28, p < .001 (PI M = 102.58, SD = 16.82; FC M = 111.72, SD = 13.41; NA M = 118.48, SD = 15.75). However, in all three groups, 90% of the children had estimated IQs broadly within the normal range (i.e., > 85). All of the mothers reported their race/ethnicity as White, non-Hispanic; two of the fathers in the NA group were reported as being of another race/ethnicity. The PI children were from China (n = 15), Russia (n = 6), Romania (n = 5), Ukraine (n = 3), Bulgaria (n = 3), India (n = 3), and Peru (n = 1). The FC children were from Korea (n = 22), Guatemala (n = 3), and Chile (n = 1).
Procedure
The IA children were recruited through a registry at the University of Minnesota of parents of internationally adopted children who were interested in participating in research. The NA children were recruited from a similar registry of birth families interested in developmental research. The children visited the laboratory with a parent and completed a laboratory assessment that typically lasted 2.5 hours.
Measures
Preadoption care
Each adoptive parent completed a questionnaire about the child’s postnatal care before adoption. In addition to information on the type (i.e., parent/relative, institution, foster care, and unknown) and duration of care, the adoptive parents rated early care risk factors: physical neglect, social neglect, physical and sexual abuse, number of placement transitions, and discrimination due to ethnicity. Each parent’s responses were summed to yield an index of preadoption care adversity.
Growth parameters at testing and adoption
At the laboratory assessment, height and weight were assessed for all children. Body mass index (BMI) was calculated based on height and weight, and z scores of height-for-age, weight-for-age, and BMI were calculated based on the 2000 CDC growth charts at adoption. Each PI and FC parent was asked to review the child’s baby books and medical records and report on the child’s age, height, and weight at the first physician visit following adoption. Any parent who also was recruited from the registry for subsequent studies completed release of information forms allowing contact with the child’s physicians to obtain adoption height and weight information and indicated that this growth information could be used for similar studies. Slightly different inclusion criteria (e.g., adoption under age 8 months) were used to select the FC group in subsequent studies; therefore, only 50% of the FC sample in the present study was recruited for subsequent studies. In contrast, nearly all of the PI children in the present study were recruited for subsequent studies. Thus, the difference in the percentages of FC children (67%) and PI children (95%) with adoption growth data reflects the greater opportunity to collect adoption growth data in subsequent studies.
Diurnal salivary cortisol
At the laboratory assessment, each parent was trained to collect saliva samples from the child and was given a saliva collection kit that included all of the needed materials. Each parent was asked to collect the samples on two typical school days. School days were targeted because child daily routines tend to be more consistent on these days than on weekend days. Each parent was also instructed to avoid sampling when the child was ill and to refrain from giving the child dairy or caffeinated products prior to sampling. The samples were collected three times over the course of the day: within 30 min of waking (morning; M = 7:58 a.m., SD = 57 min), between 4 and 5 p.m. (afternoon; M = 4:22 p.m., SD = 69 min), and within 30 min of bedtime (evening; M = 8:40 p.m., SD = 25 min). The sampling times did not significantly differ across the three groups of children. For the saliva collection, the child chewed a piece of Trident™ Original sugarless gum to stimulate salivation. This gum has been shown to have little effect on cortisol levels (Schwartz, Granger, Susman, Gunnar, & Laird, 1998). The child then used a straw to expel the saliva into a prelabeled vial. The samples were stored in the refrigerator until all of the samples had been collected and then were stored at −20° C in our laboratory until mailed to the Biochemical Laboratory at the University of Trier for analysis. Prior research has shown that conditions experienced during mailing do not influence salivary cortisol concentrations (Clements & Parker, 1998).
The samples were assayed for cortisol using a time-resolved fluorescence immunoassay (DELFIA). All of the samples from each child were included in the same assay batch, and the assay batches were balanced by group and sex. The samples were assayed in duplicate and were averaged. Duplicates varying by more than 15% were re-assayed. The inter-assay and intra-assay coefficients of variance were 5.4% and 8.1%, respectively. Cortisol levels were normally distributed and thus were not transformed prior to analysis.
DSA
A composite measure of DSA was created by combining an observational measure and a parent-report measure. The observational measure, which was adapted from procedures used by Tizard and Rees (1975), was employed at the beginning of the laboratory assessment. According to protocol, we examined each child’s response to an unfamiliar adult through a graded series of steps from the adult sitting quietly in a chair to the adult actively interacting and playing with the child. The parent was present throughout but was instructed to complete questionnaires quietly. The interaction was videotaped and coded for latency to first vocalization to the unfamiliar adult and frequency of vocalizations. Two coders reviewed 20% of the audiotapes to calculate interrater reliability. For latency, the coders were within 3 s of each other 83% of the time; for frequency, the Cohen kappa was .86. Latency and frequency were highly correlated, r = −.68, p < .001, and were standardized and combined to create an observational measure of DSA. The parent-report measure was based on a semistructured interview developed by O’Connor, Bredenkamp, and Rutter (1999) for use in their study of postinstitutionalized Romanian children. Three questions were used to assess DSA: Is the child too eager to approach unfamiliar adults? Does she/he make personal comments to unfamiliar adults? Does she/he initiate physical contact with unfamiliar adults? If any of the questions were endorsed, specific examples were requested. Each interview was audiotaped and coded by trained coders who were blind to group membership. Two coders reviewed 20% of the audiotapes to calculate interrater reliability (κ = 1.00–0.88). Questions were scored on a 3-point scale, with the highest score indicating persistent DSA (α = .65, item–total correlations ≥ .44, factor loadings ≥ .75). The observational and parent-report measures were significantly correlated, r = .40, p < .001, and were standardized and averaged to create a composite measure of DSA.
Missing data analyses
As noted above, 19 of the 120 children were missing data for the present analyses. The results from t-tests across groups and within groups revealed no significant differences between excluded and included children on the following variables: diurnal cortisol levels, DSA, height, weight, BMI, and IQ at the laboratory assessment. However, because of adoption growth data, there were more missing cases in the FC group (35%) than in the PI (10%) and NA (12%) groups, χ2(2) = 9.78, p < .01. Therefore, analyses examining adoption growth delay were first computed by including the FC children and then recomputed to only compare the PI and NA children.
Preliminary Analyses and Data Analysis Plan
The larger percentage of girls reflected the percentage of girls adopted from institutions at the time of the present study. Preliminary analyses were computed to examine the main effects of Sex and Sex × Group on all of the variables used in the present analyses. None of these analyses was significant, thus sex was not considered further.
For the IA children, Pearson correlations were computed examining height-for-age at the laboratory assessment, using height-for-age at adoption and the four outcome measures (DSA and three cortisol sample levels), to determine whether current height needed to be controlled statistically in the analyses. The results indicated that height-for-age at the laboratory assessment was significantly correlated with height-for-age at adoption, r = .30, N = 60, p < .05. Among the outcome measures, height-for-age at the laboratory assessment was only associated with evening cortisol levels, r = −.26, N = 60, p < .05. However, in the repeated measures within general linear models (GLM-RM) analysis of cortisol over the day, entering height-for-age at the laboratory assessment as a covariate did not yield a significant covariate effect. Thus, it was not included as a covariate in the analyses reported below.
The analysis plan first involved examining differences in height-for-age at adoption and at the laboratory assessment in the PI and FC groups. Because caloric restriction tends to affect weight-for-height more than height, especially beyond infancy, weight-for-height at adoption was examined as an index of caloric restriction. BMI at the laboratory assessment also was examined. Preadoption care adversity and age-at-adoption were then examined in relation to these indices and the difference between FC and PI children was re-examined controlling for preadoption care adversity and age-at-adoption to explore the extent to which the degree and duration of adversity influenced group differences in growth parameters.
The relations between the adoption growth parameters and the outcome measures were examined using the correlation coefficients of the IA children combined and then for only the PI children. To examine whether delayed growth at adoption influenced differences in DSA and cortisol between the IA children and the NA children, we could not enter adoption growth parameters as covariates because they were not available for the NA children. Thus, height-for-age at adoption was used to create two groups of IA children: those with severe growth delay at adoption and those with less growth delay at adoption. While -2 SDs below the mean would be the more expected cut-point for “stunted” growth, the cut-point was placed at 1.5 SD below the mean to increase the number of children in the “stunted” category and to reduce the imbalance of cell sizes. Stunted and nonstunted IA children were then compared to the NA group. Because there were few FC children in the stunted group (see details below), the analyses were repeated with only the PI and NA groups in the analyses. Following an examination of the adoption growth parameters and DSA and cortisol measures, correlations were computed within the stunted IA, nonstunted IA, and NA groups to determine whether growth stunting influenced the associations between DSA and diurnal cortisol. If growth stunting was an index of allostatic load that predicted regulatory functioning, we would expect the associations between DSA and cortisol to emerge or to be the strongest among the stunted IA children.
Results
Growth Parameters
At adoption
A multivariate ANOVA examining height-for-age, weight-for-age, and weight-for-height at adoption as a function of group (PI versus FC) yielded a significant multivariate effect, Hotelling’s F(3, 55) = 9.48, p < .001, with follow-up univariate effects being significant for height-for-age, F(1, 57) = 14.48, p < .001, and weight-for-age, F(1, 57) = 28.54, p < .001, but not weight-for-height, F(1, 57) = 2.55, ns (see Table 1). The PI children were significantly shorter and lighter but were not thinner for their age at adoption. However, 16–17% of the PI and FC children were below 2 SD of the mean on weight-for-height, suggesting significant caloric restriction. To ensure that ethnic differences did not account for the differences between the PI and FC children, the analysis was repeated with Asian Countries versus Other Countries as a factor. This factor was not significant and did not reduce the effect of group. To examine the extent to which linear growth delay might have been related to caloric restriction, we examined the correlation between height-for-age and weight-for-height within the PI and FC groups. This association was not significant among the PI children, r = −.20, N = 36, ns, but was significant among the FC children, r = −.61, N = 26, p < .01, and the two correlations were marginally different, z = 1.86, p = .06. Parent-reported preadoption care adversity was significantly associated with height-for-age, r = −.50, N = 60, p < .001, but not with weight-for-height, r = −.002, N = 60, ns. Age at adoption correlated with height-for-age within the PI group, r = −.50, N = 36, p < .01, but not within the FC group, r = −.15, N = 26, ns. In neither group was age at adoption associated with weight-for-height (rs < −.10, ns). When group differences between the PI and FC children for the three growth parameters were analyzed using age at adoption and preadoption care adversity as covariates, the effect of the covariates was significant, F(2, 57) = 8.48, p < .001, but the effect of group was reduced to nonsignificance, F(1, 57) = 0.51, ns.
Table 1.
Growth Parameters at Adoption and the Laboratory Assessment
| Growth parameter | Postinstitutionalized children (n = 36) | Foster care children (n = 26) | Nonadopted children (n = 35) |
|---|---|---|---|
| M (SD) | M (SD) | M (SD) | |
| At adoption | |||
| Heighta | −1.54 (1.49) | −0.03 (1.46) | |
| Weighta | −2.04 (1.25) | −0.23 (1.09) | |
| Weight-for-heighta | −0.94 (1.30) | −0.35 (1.50) | |
| At assessment | |||
| Heighta | −0.45 (1.17) | −0.47 (0.97) | 0.79 (1.07) |
| Weighta | −0.33 (1.08) | −0.04 (1.14) | 0.50 (0.78) |
| BMI | 15.64 (1.39) | 16.51 (2.06) | 15.95 (1.81) |
z-score for age CDC 2000 norms.
In the PI group, 30% of the children were at least 2 SD below the mean on height-for-age, and 50% of the children were at least 1.5 SD below the mean on height-for-age. In contrast, in the FC group, these percentages were 8% and 12.4%, respectively. When grouped by height-for-age into those above (nonstunted) and below (stunted) z = −1.5, the PI children were more likely to be stunted than the FC children, χ2(1) = 9.97, p < .001. The lack of stunted children in the FC group precluded examining the impact of growth stunting within that group. Thus, subsequent analyses based on stunted group membership were conducted by examining the IA group (PI and FC combined) or the PI group.
At the laboratory assessment
There were no differences in height-for-age, weight-for-age, or BMI at the laboratory assessment between the PI and FC groups, Hotellings F(3, 57) = 1.5, ns (see Table 1). However, compared to the NA children, both groups were smaller, Hotelling’s F(3, 91) = 10.02, p < .001 (i.e., shorter and lighter but not thinner). Neither age at adoption nor preadoption care adversity score correlated with height-for-age at the laboratory assessment within the PI or FC group (rs < .15, ns).
Adoption Growth Delay and DSA
A partial correlation was computed between height-for-age at adoption and DSA, controlling for weight-for-height at adoption: IA group partial r = −.28, N = 56, p < .05; PI group partial r = −.33, N = 36, p = .06. To further examine this association, stunted and nonstunted IA children were compared to the NA children. The analyses were computed first using all children and then removing the FC children. The resulting GLM ANOVA was significant, F(2, 94) = 5.12, p < .008, η2 = .10, with Bonferroni post-hoc tests indicating that only the stunted IA children differed significantly from the NA children on DSA; specifically, the stunted IA group exhibited higher levels of DSA than the NA group. Excluding the FC children, the results held, F(2, 68) = 5.81, p < .005, η2 = .15. The results using the PI and NA children are shown in Figure 1.
Figure 1.
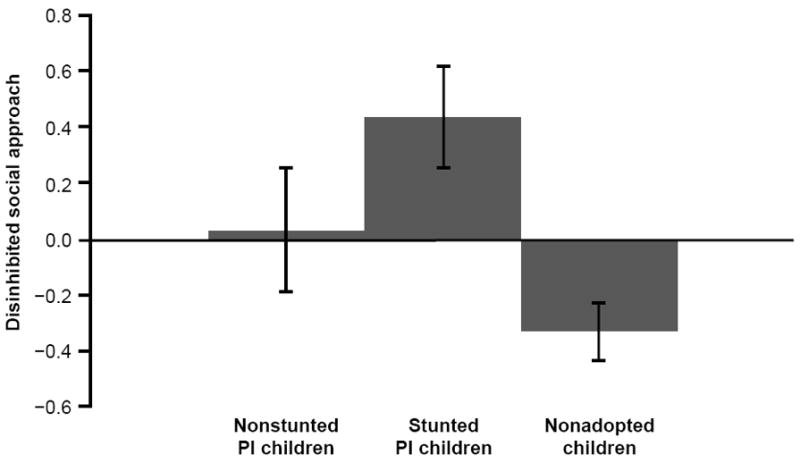
Scores on disinhibited social approach among nonstunted postinstitutionalized (PI) children, stunted PI children, and nonadopted children. The stunted PI group exhibited significantly higher levels of DSA than the NA group. Note. M (SEM) for nonstunted PI (n = 18) DSA = .03 (.22); stunted PI (n = 18) DSA = .43 (.19); nonadopted (n = 35) DSA = -.33 (.11).
Adoption Growth Delay and Diurnal Cortisol Levels
Partial correlations were computed between height-for-age at adoption and morning, afternoon, and evening cortisol levels, controlling for weight-for-height at adoption. For the IA group, the correlations were r = −.03, N = 56, ns, r = −.26, N = 56, p = .05, and r = −.45, N = 56, p < .001, respectively. For the PI group, the correlations were r = .02, N = 36, ns, r = −.26, N = 36, p = .13, and r = −.51, N = 36, p < .01, respectively. Again, the stunted and nonstunted groups were compared to the NA children using a GLM-RM ANOVA. There was a significant interaction of stunted group by time-of-day, with Greenhouse-Geisser correction, F(2.46, 115.75) = 2.93, p = .046, η2 = .06. Despite a reduction in degrees of freedom, the interaction remained significant when the PI and NA children were used in the analysis, F(2.46, 83.85) = 2.92, p = .05, η2 = .08. The slope for the stunted PI children was flatter, with slightly lower AM cortisol and higher afternoon and evening cortisol concentrations (see Figure 2).
Figure 2.
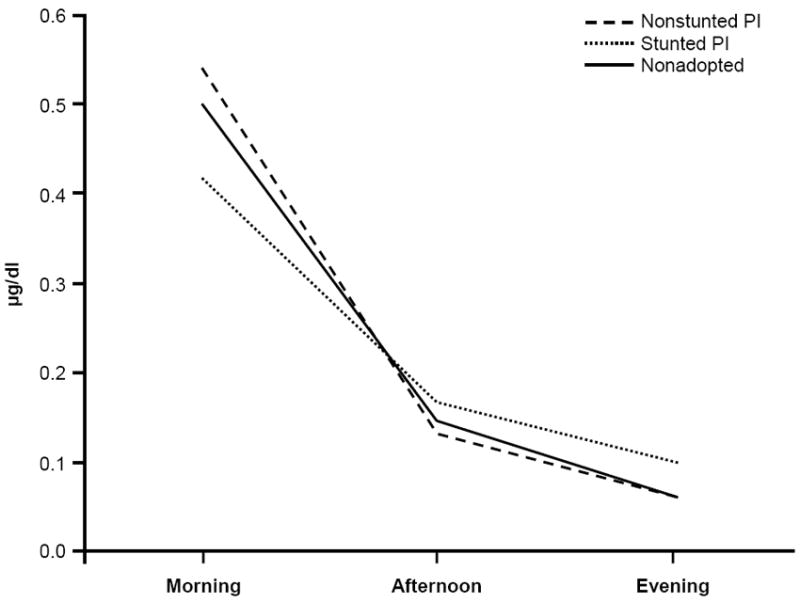
Salivary cortisol (μg/dl; morning, afternoon, and evening) for nonstunted and stunted postinstitutionalized (PI) children and nonadopted children. The slope for the stunted PI group was flatter, with slightly lower AM cortisol and higher afternoon and evening cortisol levels. Note. M (SEM) for nonstunted PI (n = 18) morning = .54 (.04), afternoon = . 14 (.02), evening = .06 (.02); stunted PI (n = 18) morning = .42 (.04), afternoon = .16 (.02), evening = .10 (.02); nonadopted (n = 35) morning = .50 (.03), afternoon = .14 (.01), evening = .07 (.01).
Associations between Diurnal Cortisol Levels and DSA within Stunted and Nonstunted Groups
We examined the correlations between diurnal cortisol levels and DSA within the stunted and nonstunted groups. First, the simple correlations between DSA and morning, afternoon, and evening cortisol levels for the IA group were r = −.07, N = 62, ns, r = .34, N = 62, p < .01, and r = .43, N = 62, p < .001, respectively. Within the stunted IA group, no association was noted with morning cortisol. For the nonstunted IA group, evening levels were positively correlated with DSA, r = .33, N = 41, p = .04, but this correlation was no longer significant when only the nonstunted PI children were examined. For the stunted IA children, afternoon, r = .46, N = 21, p < .05, and evening cortisol levels, r = .52, N = 21, p < .02, were positively correlated with DSA scores; this remained significant when only the stunted PI children were examined. The correlations for the stunted PI children, nonstunted PI children, and NA children are shown in Table 2. We found that none of the correlation coefficients differed between the stunted and nonstunted PI children, but there was a significant difference between the stunted PI children and the NA children for the association between DSA and evening cortisol, z = 1.97, p < .05. Thus, among the stunted PI children, greater DSA was associated with higher afternoon and evening cortisol levels, and this correlation differed significantly from that of the NA children in the evening.
Table 2.
Correlation Coefficients for Disinhibited Social Approach With Home Cortisol Levels for Stunted and Nonstunted Postinstitutionalized Children Versus Nonadopted Children
| Group | Morning cortisol | Afternoon cortisol | Evening cortisol |
|---|---|---|---|
| Stunted postinstitutionalized (n = 18) | .23 | .46* | .52* |
| Nonstunted postinstitutionalized (n = 18) | −.17 | .30 | .31 |
| Nonadopted (n = 35) | −.06 | .13 | −.04 |
p < .05
Discussion
Stress has long been known to inhibit growth (Chrousos, 2009; Romero et al., 2009), with CRH and cortisol mediating these effects through their interaction with the growth hormone–insulin growth factor axis. The impact of stress on growth is highly consistent with the concept of allostasis as energy is preserved for more immediate survival functions by inhibiting growth. Indeed, in current models of allostasis, energy and its regulation is recognized as central to the construct (McEwen & Wingfield, 2010; Romero et al., 2009). Our results provide support for considering growth delay as an index of allostatic load in young children who would be growing rapidly in the absence of adverse care. We found that, for internationally adopted children in general and for postinstitutionalized children specifically, linear growth delay at adoption was associated with alterations in diurnal activity of the HPA axis and a common behavioral problem among postinstitutionalized children: DSA. In addition, particularly among the most growth-delayed children, higher levels of cortisol later in the day, when cortisol levels should be decreasing to their lowest levels, were correlated with DSA. The potential for using growth delay as an allostatic load indicator and possible problems and limitations in its use in child populations are discussed below.
As was expected given the existing literature on internationally adopted children (e.g., Van IJzendoorn et al., 2007), postinstitutionalized children were more delayed in linear growth and weight at adoption compared to children adopted from foster care. There was little evidence that these children differed, however, in subnutrition: approximately 16% of the postinstitutionalized children and foster care children exhibited weight-for-height that was more than 2 SD below norms. Furthermore, height-for-age was not correlated with weight-for-height among the postinstitutionalized children but was correlated with parent reports of quality of care prior to adoption and age at adoption. When quality of care and adoption age were controlled statistically, the difference in height and weight at adoption between the postinstitutionalized and foster care groups was no longer significant. Notably, there was no evidence that the children adopted from Asian orphanages were any smaller at adoption than the children adopted from elsewhere. Thus, the adoption growth data supports a model of growth delay among postinstitutionalized children as an expression of adverse early care. By early childhood, the growth difference between the postinstitutionalized and foster care children was no longer significant, and the growth measure results were close to age norms for both groups. The postinstitutionalized and foster care children were, however, shorter and lighter than the nonadopted children. Thus, on average, the postinstitutionalized children suffered significant growth delay while in institutional care and experienced a rebound in growth following adoption.
For the internationally adopted children (specifically the postinstitutionalized children), linear growth delay at adoption predicted overly friendly behavior in early childhood. Bruce and colleagues (2009) reported that postinstitutionalized children express more overly friendly behavior than nonadopted children, with foster care children scoring midway between. When we examined the effects of growth stunting, we found that internationally adopted children who were extremely short for their age at adoption differed from the nonadopted children in overly friendly behavior. Because there were too few foster care children who met this criterion, we could only examine the postinstitutionalized children in comparison to the nonadopted children. Again, the stunted postinstitutionalized children exhibited more overly friendly behavior towards unfamiliar adults than the nonadopted children.
In a previous study, disinhibited attachment disorder, a diagnosis heavily determined by indiscriminately friendly behavior, was unrelated to growth parameters at adoption (Sonuga-Barke et al., 2008). There may be several reasons for the discrepancy between these results and the results of the present study. First, although DSA was a core component of their index of attachment disorder, their attachment disorder measure also included other behaviors. Second, their analyses focused on weight-for-age, not height-for-age. Although these measures are correlated, the association is not perfect. They also treated attachment disorder as a categorical variable; perhaps this was a less powerful method of detecting relations with growth delay at adoption. Finally, their sample consisted of children adopted from Romanian institutions that were extremely impoverished at the time of their study. Thus, it is possible that we were able to examine a broader range of preadoption care and detect associations between DSA and adoption growth delay.
Our results also yielded evidence of associations between linear growth delays at adoption and diurnal cortisol levels for internationally adopted children (specifically for postinstitutionalized children). In addition, we observed slightly, but not significantly, lower morning cortisol levels and significantly higher afternoon and evening cortisol levels for the stunted postinstitutionalized children. Overall, the shape of the diurnal cortisol rhythm from morning to bedtime was flatter for the stunted postinstitutionalized children than for the nonstunted postinstitutionalized children or the nonadopted children. This atypical diurnal pattern is consistent with the pattern of diurnal cortisol used to index allostatic load in adults (Seeman et al., 2010). The alterations in the afternoon and evening cortisol levels are also consistent with earlier findings on children adopted from the extremely impoverished Romanian institutions (Gunnar et al., 2001).
Notably, however, our findings were inconsistent with the findings of Kertes et al. (2008), who reported that height-for-age at adoption is positively associated with morning levels but not with evening levels. In the previous study, the parents were asked to sample on days when the children were home in the afternoon and evening hours because evening activities have been shown to elevate cortisol levels for some children (Kertes & Gunnar, 2004). This was not done in the present study. Although that difference might explain the effects later in the day in the present study, it does not explain why we failed to see higher morning levels in children who were more growth delayed at adoption.
Our results also differed from those of Gunnar et al. (2009), who noted that the children who were not stunted at adoption had lower cortisol levels than nonadopted children when tested in the laboratory in the late afternoon and that the children who were stunted at adoption and the nonadopted children did not differ (although the means were in the same direction as the present study). The difference in findings between these two studies could be the difference in testing context: home versus laboratory.
In sum, although the results of the present study are consistent with the results of previous studies in finding associations between cortisol levels years after adoption and linear growth delays at adoption, the specifics about time-of-day when effects were noted and patterns relative to nonadopted and nonstunted postinstitutionalized children differed across these studies. This raises the possibility that other factors might affect the association between growth delays at adoption and later activity of the HPA axis.
We also found evidence of an association between DSA and cortisol levels among the stunted postinstitutionalized children. When we examined the postinstitutionalized and foster care children, we noted a significant positive correlation between evening cortisol and DSA among the nonstunted group. However, this correlation did not remain significant when only the postinstitutionalized children were examined. Associations between DSA and afternoon and evening cortisol levels were noted among the stunted postinstitutionalized children. However, it is important to note that while the associations between DSA and cortisol levels were not significant for the nonstunted postinstitutionalized children, the correlations were in the same direction and did not differ significantly from those observed for the stunted postinstitutionalized children. Thus, although the association might be stronger for the stunted postinstitutionalized children, this finding needs replication with a larger group of children.
Our findings raise several issues in regard to indexing allostatic load during early development. First, the allostatic load model focuses on the organism’s life history of cumulative stress (McEwen & Wingfield, 2010). There is nothing in the current model that addresses whether there are sensitive periods during which the action of allostatic mediators might produce more significant long-term impacts. Although the current model incorporates the contributions of age, sex, and social status, the role of age presumably reflects the different energy demands of different stages of the life cycle (see McEwen & Wingfield, 2010).
Researchers studying early life stress, including stress during fetal development, have noted that there might be programming effects of early care experiences. With regards to growth restriction, Barker (2007) argued that growth restriction in utero due to poor nutrition and/or stress programs the organism to deal with a harsh postuterine environment. If such an environment is not encountered (and instead the environment is supportive and rich in resources), the organism is susceptible to increased risk of metabolic syndrome and cardiovascular disease through many of the same mechanisms assessed as indices of allostatic load (e.g., waist–hip ratio, elevated blood pressure, increased inflammatory activity, and elevated cortisol). Likewise, Meaney and Szyf (2005) argued that maternal programming of HPA axis regulation through methylation of the glucocorticoid receptor gene in the hippocampus is one of a number of mechanisms found in nature whereby early experiences program the organism to deal with the environment that can be anticipated throughout life (i.e., predictive adaptation). Risk to health and functioning become particularly apparent when the later environment does not match the environment to which the organism has been programmed; its behavioral and physiological responses are not necessary for survival; thus, the risks outweigh their survival benefit.
In early life stress models, early experiences have long term consequences because they occur as the organism is developing and are not easily reprogrammed by later experiences. These are not cumulative wear and tear models, but early experience/sensitive period models. Accordingly, allostatic load during these periods is not equivalent to similar allostatic load once the organism has matured. Nor would we expect that the neurobiological impact of allostatic mediators is the same on the developing organism as on the mature organism. Indeed, it may be more parsimonious to view early experiences as toning the systems that regulate the allostatic mediators rather than simply applying the allostatic load model directly, without developmental consideration, to our understanding of adverse early life conditions. It will be critical to integrate the allostatic load model with a developmental psychopathology framework emphasizing the importance of developmental timing and the processes through which early experiences influence future adaptation (Cicchetti & Toth, 2009). Viewed this way, allostatic load in early life is particularly important as it adds to the cumulative wear and tear produced by allostatic mediators and affects how those systems function later in life.
In regard to growth delay during periods of adverse early care, we need to determine whether the processes leading to growth restriction or the processes involved in growth rebound are critical in predicting later outcomes. In terms of metabolic disorder and fetal growth restriction, Barker’s (1997) hypothesis identifies the rapid increase in weight during postnatal development to be as critical to the development of metabolic disorder as the fetal restriction in growth. We do not know if this is the case for postnatal growth restriction due to privation in care, although it will be important to examine precursors of metabolic disease in children who experience growth stunting and marked increases in growth following adoption. It is also possible that the rebound in growth will be as or more predictive of the degree of behavioral and neuroendocrine dysregulation than measures such as height-for-age at adoption. For example, growth rebound might serve as a better index of growth stunting following a period of adverse care. That is, two children might be similarly short for age, but growth rebound might index a greater degree of growth delay for a child who is genetically capable of great stature than for one who is not. The former child should rebound more once the environment has improved. Alternatively, the rate of growth after conditions have improved might strain the child’s resources. For example, there is evidence of iron deficiency among postinstitutionalized children who display rapid growth following adoption because they begin to outstrip their available iron as they grow. This deficiency could serve to compromise their neurobiological development (Fuglestad et al., 2008). There is also evidence that parental care among postinstitutionalized children influences the rate of recovery in height and weight. After controlling for growth delay and age at placement, more sensitive and responsive care is associated with a more complete return to normal growth parameters among preschool-aged children (Johnson et al., 2010). Thus, although we used a simple height-for-age measure at adoption as an index of allostatic load in the present study, a more dynamic analysis of growth rates during and following periods of allostatic load should be used in future studies. Such a measure should also address issues of genetic influences on growth.
Finally, it should be noted that, unlike other indices of allostatic load, growth delay is expected to be a transient measure. That is, growth is expected to rebound once conditions improve. Thus, although growth delay might reflect the cumulative impact of allostatic mediators, these mediators might continue to exert impacts on child functioning long after growth has rebounded. Of course, if growth delay due to adverse care affects mechanisms determining the child’s ultimate height (see Van IJzendoorn et al., 2007), perhaps growth delay is not transient when experienced early in life; if so, we will need information on growth delay during periods of adverse care and growth at maturity to fully use growth as an index of allostatic load. Nonetheless, it seems likely that its utility as an index of allostatic load will be limited to periods during and surrounding times of allostatic load. Our measures of height and weight did not differ between the foster care and postinstitutionalized children several years after adoption, but we have every indication that they experienced differing degrees and durations of allostatic load prior to adoption.
There are a number of limitations to the present study. First, we did not have information on each child’s genetic potential for growth (e.g., mid-parental height). Estimates of mid-parental height would have refined our measure of growth delay and would have allowed us to understand whether the processes leading to growth restriction and/or growth rebound are more critical in predicting later outcomes. Second, we were not able to identify all conditions that contributed to poor growth at the time of adoption. Although we controlled for weight-for-height to partially account for subnutrition, subnutrition impacts all dimensions of growth during early development: we cannot rule out subnutrition as a component of the observed effects. Notably, the allostatic load model would not exclude subnutrition as a mechanism, because it is central to energy regulation. In fact, growth delay in the context of subnutrition might reduce allostatic load by decreasing the energy demands that growth places on the body. Third, it is certainly likely that some of the internationally adopted children were growth delayed at adoption in part because of adverse prenatal conditions. We did not have access to measures of birth weight or gestational age and thus could not examine the contribution of fetal growth restriction to the adoption growth parameters. Notably, however, if fetal growth restriction were the predominant influence on adoption growth parameters, we might have expected similar poor growth among the postinstitutionalized and foster care children as both groups of children were born to women who were unable to care for them. Fourth, some of the children did not collect the home cortisol samples, and several children in the foster group did not have adoption growth measures. Thus, these children were not included in the present analyses. However, missing data was of most concern for the foster care group (35%) versus the postinstitutionalized and nonadopted groups (10% and 13%, respectively). Furthermore, our findings were stable or stronger when analyses were conducted after removing the foster care group. Fifth, although we included indices of the preadoption environment and adoption growth measures, DSA and diurnal cortisol were only assessed at one age. In future studies, the development of DSA and HPA axis function should be examined at several points postadoption to provide a richer developmental analysis. Sixth, our findings cannot generalize to other aspects of functioning that might be associated with growth delay under conditions of adversity (see Johnson et al., 2010; Sonuga-Barke et al., 2008). Finally, not all children who shared similar adverse early experiences showed similar growth delays, and our findings cannot explain such differential impacts of experience on growth. The role of potential mediators, such as genes involved in growth or growth delay stress mediators, should be examined in future studies.
Despite these limitations, our results support the argument that growth delay at adoption predicts later functioning of the HPA axis and extend this finding to evidence that growth delay predicts DSA, an impairment noted for many postinstitutionalized children. The mediators of allostatic load have long been known to inhibit growth; thus, periods of stress-induced growth slowing might serve as one useful index of allostatic load in studies of young children. The predictive value of growth delay for behavioral and neuroendocrine dysregulation, if replicated in future studies, has implications for social policy and prevention/intervention work: if children with stunted growth are at particular risk of maladaptive outcomes, they should be targeted for early psychosocial intervention. Given that allostatic load leads to multisystemic dysregulation and in keeping with the multilevel approach used in the present study, it will be important for such interventions to employ biological and behavioral measures to evaluate their effectiveness (Cicchetti & Gunnar, 2008).
Acknowledgments
The authors wish to thank the parents and children who participated this study and Matthew Rabel for editorial assistance. Support for this research was provided by MH059848, MH078105, and MH018264, NIMH, U.S. PHS; HD007151, NICHD, U.S. PHS; and a University of Minnesota Graduate School Grant.
Contributor Information
Anna E. Johnson, University of Minnesota
Jacqueline Bruce, Oregon Social Learning Center and Center for Research to Practice.
Amanda R. Tarullo, Columbia University
Megan R. Gunnar, University of Minnesota
References
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Review Neuroscience. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awerman JL, Romero LM. Chronic psychological stress alters body weight and blood chemistry in European starlings (Sturnus vulgaris) Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology. 2010;156:136–142. doi: 10.1016/j.cbpa.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997;13:807–813. doi: 10.1016/s0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The origins of the developmental origins theory. Journal of Internal Medicine. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Blizzard RM, Bulatovic A. Syndromes of psychosocial short stature. In: Lifshitz F, editor. Pediatric endocrinology. 3. New York, NY: Marcel Dekker; 1996. pp. 83–93. [Google Scholar]
- Bruce J, Tarullo AR, Gunnar MR. Disinhibited social behavior among internationally adopted children. Development and Psychopathology. 2009;21:151–171. doi: 10.1017/S0954579409000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Chisholm K. A three year follow-up of attachment and indiscriminate friendliness in children adopted from Romanian orphanages. Child Development. 1998;69:1092–1106. [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nature Reviews Endocrinology. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Gunnar MR, editors. Development and Psychopathology. Vol. 20. 2008. Integrating biological processes into the design and evaluation of preventive interventions; pp. 737–1022. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Sroufe LA. The past as prologue to the future: The times, they’ve been a changin’. Development and Psychopathology. 2000;12:255–264. doi: 10.1017/s0954579400003011. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. The past achievements and future promises of developmental psychopathology: The coming of age of a discipline. Journal of Child Psychology and Psychiatry. 2009;50:16–25. doi: 10.1111/j.1469-7610.2008.01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Tucker D. Development and self-regulatory structures of the mind. Development and Psychopathology. 1994;6:533–549. [Google Scholar]
- Clements AD, Parker RC. The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology. 1998;23:613–616. doi: 10.1016/s0306-4530(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Kilpatrick GJ, Hauger RL, Moreau J. Molecular biology of the CRH receptors—in the mood. Peptides. 2001;22:753–760. doi: 10.1016/s0196-9781(01)00388-6. [DOI] [PubMed] [Google Scholar]
- Dobrova-Krol NA, Van IJzendoorn MH, Bakermans-Kranenburg MJ, Cyr C, Juffer F. Physical growth delays and stress dysregulation in stunted and non-stunted Ukrainian institution-reared children. Infant Behavior and Development. 2008;31:539–553. doi: 10.1016/j.infbeh.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Fernald LC, Grantham-McGregor SM. Stress response in school-age children who have been growth retarded since early childhood. American Journal of Clinical Nutrition. 1998;68:691–698. doi: 10.1093/ajcn/68.3.691. [DOI] [PubMed] [Google Scholar]
- Fuglestad AJ, Lehmann AE, Kroupina MG, Petryk A, Miller BS, Iverson SL, et al. Iron deficiency in international adoptees from Eastern Europe. Journal of Pediatrics. 2008;153:272–277. doi: 10.1016/j.jpeds.2008.02.048. [DOI] [PubMed] [Google Scholar]
- Ganzel BL, Morris PA, Wethington E. Allostasis and the human brain: Integrating models of stress from the social and life sciences. Psychological Review. 2010;117:134–174. doi: 10.1037/a0017773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohlke BD, Khadilkar VV, Skuse D, Stanhope R. Recognition of children with psychosocial short stature. Journal of Pediatric Endocrinology and Metabolism. 1998;11:509–517. doi: 10.1515/jpem.1998.11.4.509. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Early adversity and the development of stress reactivity and regulation. In: Nelson CA, editor. The effects of adversity on neurobehavioral development. The Minnesota Symposia on Child Psychology. Vol. 31. Mahwah, NJ: Erlbaum; 2000. pp. 163–200. [Google Scholar]
- Gunnar MR, Frenn K, Wewerka S, Van Ryzin MJ. Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10- to 12-year-old children. Psychoneuoendocrinology. 2009;34:62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Development and Psychopathology. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo KM. The neurobiology of stress and development. Annual Review of Psychology. 2007a;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo KM. Early care experiences and HPA axis regulation in children: A mechanism for later trauma vulnerability. Progress in Brain Research. 2007b;167:137–149. doi: 10.1016/S0079-6123(07)67010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes JH, Park K, Iverson SL, Mason P, Federici R, Johnson DE. Physical growth and sexual maturation of severely deprived children reared in Romanian orphanages. In: Ashizawa K, Cameron N, editors. Advances in the study of human growth and development. London, UK: Smith-Gordon; 2008. pp. 93–98. [Google Scholar]
- Hodges J, Tizard B. IQ and behavioural adjustment of ex-institutional adolescents. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1989;30:53–75. doi: 10.1111/j.1469-7610.1989.tb00769.x. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Guthrie D, Smyke AT, Koga SF, Fox NA, Zeanah CH, et al. Growth and associations between auxology, caregiving environment, and cognition in socially deprived Romanian children randomized to foster vs ongoing institutional care. Archives of Pediatric and Adolescent Medicine. 2010;164:507–516. doi: 10.1001/archpediatrics.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Gunnar MR. Evening activities as a potential confound in research on the adrenocortical system in children. Child Development. 2004;75:193–204. doi: 10.1111/j.1467-8624.2004.00663.x. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Gunnar MR, Madsen NJ, Long J. Early deprivation and home basal cortisol levels: A study of internationally-adopted children. Development and Psychopathology. 2008;20:473–491. doi: 10.1017/S0954579408000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumsta R, Kreppner J, Rutter M, Beckett C, Castle J, Stevens S, et al. III. Deprivation-specific psychological patterns. Monographs of the Society for Research in Child. 2010;75:48–78. doi: 10.1111/j.1540-5834.2010.00550.x. [DOI] [PubMed] [Google Scholar]
- Loman MM, Wiik KL, Frenn KA, Pollak SD, Gunnar MR. Postinstitutionalized children’s development: Growth, cognitive, and language outcomes. Developmental and Behavioral Pediatrics. 2009;30:426–434. doi: 10.1097/DBP.0b013e3181b1fd08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: Central role of the brain. Dialogues in Clinical Neuroscience. 2006;8:367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Science. 2010;118:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. What is in a name? Integrating homeostasis, allostasis and stress. Hormones and Behavior. 2010;57:105–111. doi: 10.1016/j.yhbeh.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A, Hindmarsh PC, Stanhope RG, Turton JP, Cole TJ, Preece MA, et al. The role of growth hormone in determining birth size and early postnatal growth, using congenital growth hormone deficiency (GHD) as a model. Clinical Endocrinology. 2005;63:223–231. doi: 10.1111/j.1365-2265.2005.02330.x. [DOI] [PubMed] [Google Scholar]
- Miller LC, Hendrie NW. Health of children adopted from China. Pediatrics. 2000;105(6):1–6. doi: 10.1542/peds.105.6.e76. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Bredenkamp D, Rutter M. Attachment disturbances and disorders in children exposed to early severe deprivation. Infant Mental Health Journal. 1999;20:10–29. [Google Scholar]
- Olney RC. Mechanisms of impaired growth: effect of steroids on bone and cartilage. Hormone Research. 2009;72(Suppl. 1):30–35. doi: 10.1159/000229761. [DOI] [PubMed] [Google Scholar]
- Provence S, Lipton RC. Infants in institutions: A comparison of their development with family reared infants during the first year of life. New York, NY: International Universities Press; 1962. [Google Scholar]
- Repetti R, Taylor SE, Seeman T. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Vale W. Involvement of corticotropin-releasing factor and somatostatin in stress-induced inhibition of growth hormone secretion in the rat. Endocrinology. 1985;117:2478–2482. doi: 10.1210/endo-117-6-2478. [DOI] [PubMed] [Google Scholar]
- Romero LM, Dickens MJ, Cyr NE. The reactive scope model: A new model integrating homeostasis, allostasis, and stress. Hormones and Behavior. 2009;55:375–389. doi: 10.1016/j.yhbeh.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Rutter M, Colvert E, Kreppner J, Beckett C, Castle J, Groothues C, et al. Early adolescent outcomes for institutionally-deprived and non-deprived adoptees. I: disinhibited attachment. Journal of Child Psychology and Psychiatry. 2007;48:17–30. doi: 10.1111/j.1469-7610.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Kreppner J, Sonuga-Barke E. Emanuel Miller Lecture: Attachment insecurity, disinhibited attachment, and attachment disorders: Where do research findings leave the concepts? Journal of Child Psychology and Psychiatry. 2009;50:529–543. doi: 10.1111/j.1469-7610.2009.02042.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, O’Connor TG. Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees. Developmental Psychology. 2004;40:81–94. doi: 10.1037/0012-1649.40.1.81. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ. Models of developmental regulation: The environtype. In: Cicchetti D, editor. Rochester Symposium on Developmental Psychopathology: The emergence of a discipline. Vol. 1. Hillsdale, NJ: Erlbaum; 1989. pp. 41–68. [Google Scholar]
- Sánchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, et al. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biological Psychiatry. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Development. 1998;69:1503–1513. [PubMed] [Google Scholar]
- Seeman T, Gruenewald T, Karlamangla A, Sidney S, Liu K, McEwen B, et al. Modeling multisystem biological risk in young adults: The Coronary Artery Risk Development in Young Adults Study. American Journal of Human Biology. 2010;22:463–472. doi: 10.1002/ajhb.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T, Singer BH, Rowe J, Horwitz RI, McEwen B. Price of adaptation - allostatic load and its health consequences. Archives of Internal Medicine. 1997;157:2259–2268. [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Beckett C, Kreppner J, Castle J, Colvert E, Stevens S, et al. Is sub-nutrition necessary for a poor outcome following early institutional deprivation? Developmental Medicine and Child Neurology. 2008;50:604–671. doi: 10.1111/j.1469-8749.2008.03065.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Schlotz W, Kreppner J. V. Differentiating developmental trajectories for conduct, emotion, and peer problems following early deprivation. Monographs of the Society for Research in Child Development. 2010;75:102–124. doi: 10.1111/j.1540-5834.2010.00552.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Schlotz W, Rutter M. VII. Physical growth and maturation following early severe institutional deprivation: Do they mediate specific psychopathological effects? Monographs of the Society for Research in Child Development. 2010;75:143–166. doi: 10.1111/j.1540-5834.2010.00554.x. [DOI] [PubMed] [Google Scholar]
- Spitz R. Hospitalism: An inquiry into the genesis of psychiatric conditions in early childhood. In: Freud A, Hartmann H, Kris E, editors. The psychoanalytic study of the child. Vol. 1. New York, NY: International Universities Press; 1945. pp. 53–74. [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of life stress, cognition and health. New York, NY: Wiley; 1988. pp. 629–649. [Google Scholar]
- Thompson BL, Levitt P. Now you see it, now you don’t—closing in on allostasis and developmental basis of psychiatric disorders. Neuron. 2010;65:437–439. doi: 10.1016/j.neuron.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Tizard B. Adoption: A second chance. London, UK: Open Books; 1977. [Google Scholar]
- Tizard B, Rees J. The effect of early institutional rearing on the behavioural problems and affectional relationships of four-year-old children. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1975;16:61–73. doi: 10.1111/j.1469-7610.1975.tb01872.x. [DOI] [PubMed] [Google Scholar]
- Turner-Cobb JM. Psychological and stress hormone correlates in early life: A key to HPA-axis dysregulation and normalisation. Stress. 2005;8:47–57. doi: 10.1080/10253890500095200. [DOI] [PubMed] [Google Scholar]
- Van den Dries L, Juffer F, Van IJzendoorn MH, Bakermans-Kranenburg MJ. Infants’ physical and cognitive development after international adoption from foster care or institutions in China. Journal of Developmental & Behavioral Pediatrics. 2010;31:114–150. doi: 10.1097/DBP.0b013e3181cdaa3a. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Bakermans-Kranenburg MJ, Juffer F. Plasticity of growth in height, weight, and head circumference: Meta-analytic evidence of massive catch-up after international adoption. Journal of Developmental and Behavioral Pediatrics. 2007;28:334–343. doi: 10.1097/DBP.0b013e31811320aa. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- Wismer Fries AB, Shirtcliff EA, Pollak SD. Neuroendocrine dysregulation following early social deprivation in children. Developmental Psychobiology. 2008;50:588–599. doi: 10.1002/dev.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]


