Abstract
Individuals with haemophilia A exhibit bleeding tendencies that are not always predicted by their factor (f)VIII level. It has been suggested that bleeding in haemophilia is due not only to defective prothrombin activation but also aberrant fibrinolysis. Thrombin activatable fibrinolysis inhibitor (TAFI) activation was measured in tissue factor (Tf)-initiated blood coagulation in blood samples of 28 haemophiliacs and 5 controls. Reactions were quenched over time with FPRck and citrate and assayed for TAFIa and thrombin-antithrombin (TAT). The TAFIa potential (TP), TAFI activation rate and the TAFIa level at 20 minutes (TAFIa20min) was extracted from the TAFI activation progress curve. In general, the time course of TAFI activation follows thrombin generation regardless of fVIII activity and as expected the rate of TAFI activation and TP decreases as fVIII decreases. The magnitude of TP was similar among the control subjects and subjects with < 11% fVIII. In severe subjects with < 1% fVIII at the time of blood collection, the TAFIa20min was inversely and significantly correlated with hemarthrosis (-0.77, p=0.03) and total bleeds (-0.75, p=0.03). In all cases, TAFIa20min was more strongly correlated with bleeding than TAT levels at 20 minutes. Overall, this study shows that TAFI activation in whole blood can be quantified and related to the clinical bleeding phenotype. Measuring TAFIa along with thrombin generation can potentially be useful to evaluate the differential bleeding phenotype in haemophilia A.
Keywords: TAFIa, haemophilia A, factor VIII, bleeding score, thrombin generation, carboxypeptidase U
Introduction
Haemophilia A is an extensively studied bleeding disorder in which mutations of the factor (f)VIII gene result in impaired fVIII activity and a heterogeneous phenotype (1-3). FVIII:C does not adequately predict the bleeding phenotype in haemophilia, especially when fVIII:C is <1% (4). Previous studies have shown that fVIII inhibitors (5), discrepancies in fVIII:C using the one-stage compared to two-stage assay (6) or the presence of fV Leiden (7) can help explain some of the discrepancy between fVIII activity and the bleeding phenotype.
The molecular hallmark of haemophilia A is dampened fX activation (8) which has a negative downstream effect on thrombin generation, and therefore, clot formation (9). While most of the literature on haemophilia A focuses on diminished thrombin-dependent procoagulant activity, relatively little work has been conducted on the anti-fibrinolytic potential that thrombin affords through the activation of thrombin activatable fibrinolysis inhibitor (TAFI), also known as procarboxypeptidase U, plasma procarboxypeptidase B or procarboxypeptidase R (10, 11). It has been suggested that bleeding in haemophilia A is due in part to enhanced fibrinolysis (12-14) and consequently, enzymes that inhibit or prolong fibrinolysis, such as TAFIa, might influence bleeding in a positive way in haemophilia A.
TAFIa-dependent prolongation of the clot lysis time has been qualitatively demonstrated in haemophilia plasma by showing that a TAFIa inhibitor produces a small but significant reduction in the clot lysis time (12, 13, 15-17). The total TAFI antigen (18, 19) and TAFI zymogen (18) antigen present in haemophilia A and healthy control plasmas has been measured by ELISA at a single time point. Conflicting results have been reported regarding the level of TAFI antigen in haemophilia A versus normal plasma. One study showed that both total TAFI antigen and TAFI zymogen antigen were decreased (18), whereas Guo et al. showed that there was no significant difference in total TAFI antigen in haemophilia versus normal plasma (19). Only one study exists where TAFIa was quantified at intervals over the course of clotting and fibrinolysis (14). This study, while informative, was conducted in plasma immuno-depleted of fVIII.
In this current study, phlebotomy blood, maintained in the presence of corn trypsin inhibitor (CTI) was obtained from haemophiliacs and healthy individuals and induced to clot with relipidated tissue factor (TF). TAFI activation was quantified in the whole blood of subjects with haemophilia A over time and compared to thrombin generation. New measures of fibrinolysis potential, termed TAFIa potential (14) and TAFIa20min, were extracted from the time course of TAFI activation to describe the potential cumulative effect of TAFIa on the clot over the course of the experiment.
Subjects, Materials and Methods
Subjects
Individuals with haemophilia A and healthy controls were recruited and advised according to a protocol approved by the Institutional Review Board at the University of Vermont Human Studies Committee and the Centre Hospitalier Universitaire Sainte-Justine (Montreal, Canada). Informed written consent was obtained from 28 subjects with haemophilia A (6 mild (>5% fVIII at the time of original diagnosis), 1 moderate (1-5% fVIII) and 21 severe (<1% fVIII)) and 5 healthy controls. All haemophiliacs that required prophylaxis used fVIII replacement therapy and did not have any known inhibitors. All individuals that were included in the study were told not to withhold replacement therapy and did not need to self infuse with fVIII from 6 hours to 4 days prior to the blood draw. Subjects within the severe population were on different prophylaxis programs. Therefore, at the time of the blood draw, fVIII levels varied within the severe population from 0.07 to 22% (Table 1). Fibrinogen, platelets, and PT were in the normal range for all subjects.
Table 1.
Clinical bleeding phenotype and fVIII level at the time of blood collection.
| Subject | fVIII (%) |
Hemarthrosis Score Mean (SD) |
Soft Tissue Score Mean (SD) |
FVIII Replacement Score Mean (SD) |
Total Score Mean (SD) |
|---|---|---|---|---|---|
| Mild 1 | 21 | 0 | 2 | 0 | 2 |
| Mild 2 | 16 | 0 | 2 | 1.5 | 3.5 |
| Mild 3 | 51 | 0 | 0 | 0 | 0 |
| Mild 4 | 35 | 0.0 | 0.3 (0.3) | 0.5 (0.5) | 0.8 |
| Mild 5 | 6 | ND | ND | ND | ND |
| Mild 6 | 5 | ND | ND | ND | ND |
| Mod 1 | 4 | 0.4 (0.4) | 0.3 (0.3) | 0.9 (0.6) | 1.6 |
| S1 | 16 | 12.0 | 6.4 (1) | 12.0 | 30.4 (1) |
| S2 | 10 | 3.0 | 8.0 | 5.4 (0.6) | 16.4 (0.6) |
| S3 | 22 | 2.5 (0.9) | 0 | 9 (1.3) | 11.5 (0.9) |
| S4 | 10 | 9.6 (1.1) | 4.8 (0.8) | 6 | 20.4 (1.3) |
| S5 | 11 | 6 (1.8) | 3.3 (1.5) | 6.0 | 15.3 (3) |
| S6 | 2.4 | 1.2 (1.2) | 2.8 (0.5) | 3.0 | 7 (1.5) |
| S7 | 1.65 | 6 (3) | 3.3 (2.4) | 6.0 | 15.3 (5.4) |
| S8 | 1.2 | 8 (1) | 8.0 | 6.0 | 22 (1) |
| S9 | 1 | 5.4 (0.6) | 2.4 (0.4) | 6 | 13.8 (0.8) |
| S10 | 4 | 2 (0.6) | 1.7 (0.6) | 6.0 | 9.7 (0.8) |
| S11 | 1 | 10.8 (0.7) | 2.8 (0.8) | 6 | 19.6 (1.3) |
| S12 | 3 | 7 (1.3) | 2.7 (1) | 4.5 (0.7) | 14.2 (2.4) |
| S13 | 0.42 | 7.8 (1.2) | 3.2 (0.8) | 6.0 | 17 (0.6) |
| S14 | 0.42 | 0.6 (0.6) | 1.6 (0.7) | 6.0 | 8.2 (1.3) |
| S15 | 0.24 | 9.6 (0.6) | 3.2 (1) | 3.0 | 15.8 (1.3) |
| S16 | 0.16 | 3 (1.7) | 1.3 (1.3) | 12.0 | 16.3 (2.2) |
| S17 | 0.18 | 8.4 (1.1) | 5.2 (1) | 12.0 | 25.6 (2.1) |
| S18 | 0.42 | 2 (1) | 3.3 (1.3) | 6.0 | 11.3 (0.3) |
| S19 | 0.07 | 1.5 (0.9) | 0.0 | 7.5 (1.5) | 9 (2.1) |
| S20 | 0.1 | 3.6 (0.6) | 4 (0.6) | 9.6 (1.5) | 17.2 (1.6) |
| S21 | 0.16 | 4.8 (1.4) | 7.2 (0.9) | 5.4 (0.4) | 17.4 (0.8) |
ND: Not determined, Mild=Mild, Mod=Moderate and S=Severe by original diagnosis.
Bleeding phenotype
Various degrees of clinical bleeding phenotypes were present in our haemophilia population. Briefly, the bleeding score as previously described (20) includes the number of hemarthrosis, soft tissue hematomas and annual fVIII unit/kg usage (Table 1). Bleeding score points were allocated as follows: hemarthrosis, 1–3/y 3, 4–6/y 6, 7–12/y 9, > 12/y 12; soft tissue hematoma, 1–3/y 2, 4–6/y 4, 7–12/y 6, > 8/y 8; annual fVIII unit/kg usage, 0/y 0, < 1000/y 3, 1000–3000/y 6, > 3000/y 12. Surgery, dental extractions and major accidents were excluded for calculation of annual fVIII unit usage. Scores are reported as means of annual scores for a 5-year observation period for all haemophilia A subjects with available bleeding score phenotype. The possible range of bleeding scores is from 0-32.
Materials
Whole blood assay
HEPES, Tris-HCl, citrate, TFA, 1-palmitoyl-2-oleoyl-phosphatidyl serine (PS), 1-palmitoyl-2-oleoyl-phosphatidyl choline (PC) were purchased from Sigma Chemical Co. (St. Louis, MO). Recombinant TF was provided by Drs. Lundblad and Liu (Hyland division, Baxter Healthcare Corp, Duarte, CA) and was relipidated in PCPS (75% PC:25% PS) vesicles by a previously described protocol (21). CTI was prepared as described (22). D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (FPRck) was provided by Dr. Richard Jenny (Haematologic Technologies, Essex Junction, VT) or purchased from Calbiochem (San Diego, CA, USA).
TAFIa assay
Thrombin and fibrinogen were prepared as previously described (23) with one exception: for the fibrinogen preparation, the solution was made to 1.2% PEG-8000 instead of 2% PEG-8000 by the addition of 40% (w/v) PEG-8000 in water, subsequent to β-alanine precipitation. Fibrin degradation products labeled with the quencher QSY9 C5-maleimide (QSY-FDP), TAFIa standards, TAFI-deficient plasma (TDP), recombinant human Pg (S741C) and the fluorescein derivative (5IAF-Pg) were prepared as described (24-26). QSY9 C5-maleimide and 5-iodoamidofluorescein were purchased from Invitrogen Canada Inc. (Burlington, ON, Canada). Plasmin was purchased from Haematologic Technologies Inc. (Essex Junction, VT, USA). Solulin was a generous gift from Dr. Achim Schuttler, Paion, GmbH (Aachen, Germany). The plasmin inhibitor D-Val-Phe-Lys chloromethyl ketone was purchased from Calbiochem (San Diego, CA, USA). All other reagents were of analytical quality.
Methods
Whole blood coagulation assay
Experiments were performed as previously described (27, 28) at 37°C on a rocking platform. Briefly, venous blood was obtained by antecubital phlebotomy and added (1mL) to tubes preloaded with CTI (100μg/mL) and relipidated TF (5pM, PCPS 1:2000 protein:lipid). A time course was set up from either 0-20 minutes (18 subjects) or 0-30 minutes (15 subjects) depending on the amount of whole blood obtained from each individual. Samples were quenched with FPRck (50μM) and citrate (9 parts blood mixed with 1 part sodium citrate, 3.2%; final concentration 10.8mM), promptly placed on ice to prevent thermal inactivation of TAFIa, and stored at -80°C until assayed. A second set of samples from contemporaneous experiments were quenched (EDTA:25mM final, benzamidine-HCl:10mM and FPRck:50μM, pH 7.4) and assayed for the TAT complex (Behring, Westwood, MA, USA) (29).
TAFIa assay
Samples were thawed by moving the samples between 4°C and ambient temperature to prevent thermal inactivation of TAFIa while ensuring solubility of all plasma proteins. All samples were diluted serially by 5-fold using TAFI deficient plasma and assayed for TAFIa as previously described (24). The small residual rate in TAFI-deficient plasma, presumably due to CPN is accounted for in the standard curve and is therefore subtracted from each sample.
Determination of the TAFIa potential
The TAFIa concentration was plotted and the TAFIa potential (TP) was determined by calculating the area under the curve over the interval of 0-20 minutes.
Statistics
Between group comparisons were conducted using one way ANOVA. Data were presented as mean ± standard error of the mean. To correlate the TP or TAFIa20min to the clinical bleeding score Spearman rank correlation analysis was used. For all statistical analysis p<0.05 was considered statistically significant.
Results
The relationship between TAFI activation and thrombin generation in whole blood
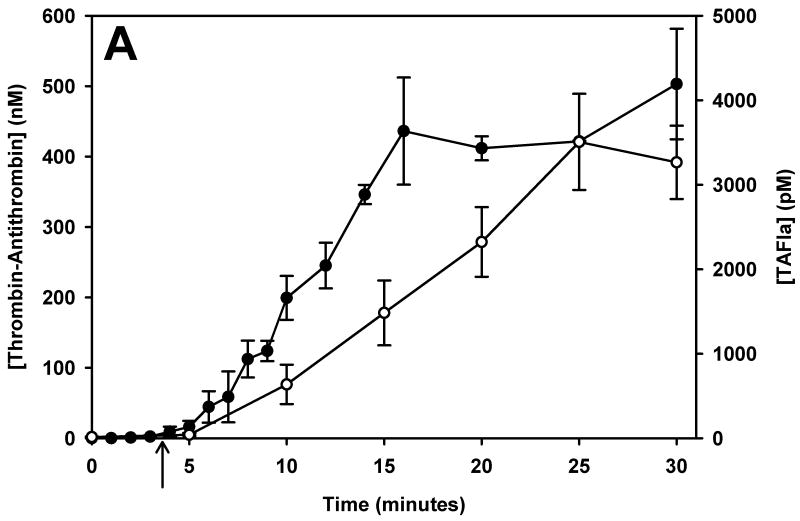
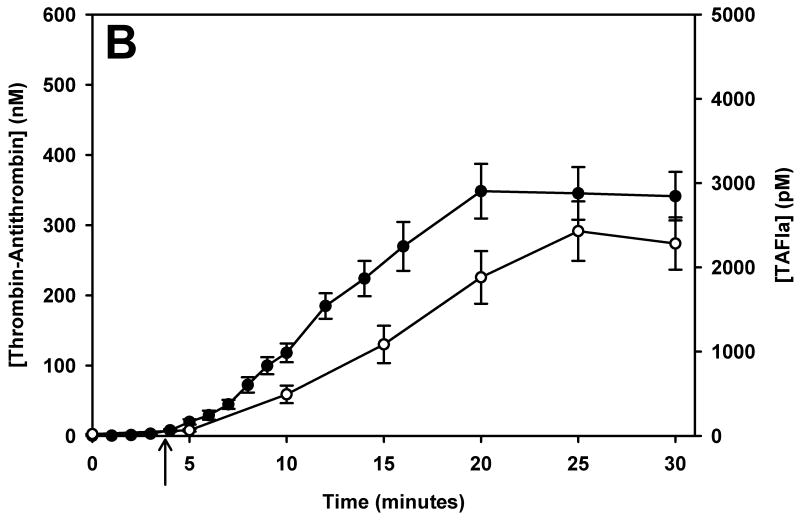
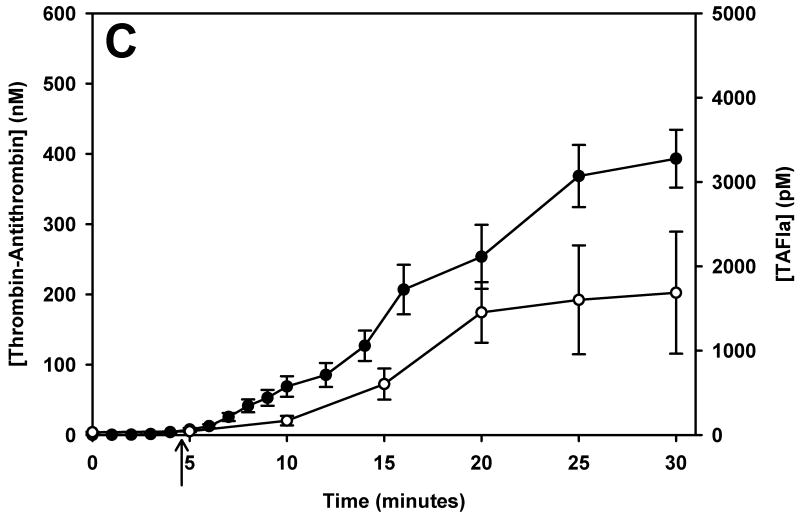
TAFI activation was monitored over time in phlebotomy blood of 5 healthy control subjects and 28 mild, moderate and severe haemophiliacs. Since haemophilia subjects were not asked to withhold their treatment for this study, many subjects had residual factor VIII levels at the time of blood collection. To account for the short washout period (sometimes as short as 6 hours) we reclassified subjects by their fVIII level at the time of the blood draw. TAT formation and TAFI activation is presented in Figure 1 for control subjects (panel A) and the haemophilia population (panels B – D, grouped by their fVIII level at the time of the blood collection [> 5% fVIII, 1-5% fVIII and < 1% fVIII]). Average clot times (CT) are shown with an arrow below the respective curves.
Figure 1.
The temporal relationship of thrombin generation and TAFI activation in subjects grouped by their fVIII level at the time of blood collection. Thrombin-antithrombin (●) and TAFIa (○) were assayed over time in whole blood from controls (panel A), and haemophilic subjects with > 5% fVIII (B), 1-5% fVIII (C) and < 1% fVIII (D). The maximal concentration of thrombin-antithrombin and the rate of TAT formation decreased as the fVIII concentration decreased. Similarly, both the rate and maximal concentration of TAFIa decreased as the fVIII concentration decreased. In all groups, TAFI activation was slightly delayed compared to thrombin generation. In each panel the clot time is denoted by an arrow. Data are presented as the mean ± standard error.
On average, TAFI activation closely mirrors TAT generation, with TAFI activation lagging behind TAT formation. In healthy controls (Figure 1, panel A), TF-initiated whole blood clotted at 3.7 ± 0.35 min directly followed by TAT formation and TAFI activation. In the haemophilia population, the CT increased slightly as the fVIII level decreased. The haemophilic group with > 5% fVIII had a clot time of 3.84 ± 0.22 min while the groups with 1-5% fVIII and <1% fVIII had significantly increased clot times of 4.7 ± 0.73 min and 4.7 ± 0.68 min, respectively (p=0.037 and p=0.023, respectively). The rate of TAT formation decreases as the fVIII concentration decreases; control (36.7 ± 2.3 nM/min), >5% fVIII (21.9 ± 2.6nM/min), 1-5% fVIII (16.4 ± 3.0nM/min) and <1% fVIII (10.7 ± 2.6 nM/min). The rate of TAT formation was significantly reduced in both the group with 1-5% fVIII (p=0.012) and the group with <1% fVIII (p=0.003) compared to controls.
Regardless of the fVIII level, there is a temporal relationship between thrombin generation (TAT) and TAFI activation. When the haemophilia subjects are separated by fVIII level at the time of blood collection, the total amount of thrombin generated (peak TAT) is similar in all groups with greater than 1% fVIII (Figure 1, panels B and C), with the maximum level of TAT generated being 356.7 ± 29.2 nM. The group with <1% fVIII (Figure 1D) had maximal TAT levels of 166.7 ± 27.1 nM at 20 minutes. In these subjects the TAT levels do appear to be still increasing at the end of the time course, therefore, the discrepancy between subjects with <1% fVIII and the haemophiliacs with greater fVIII levels remains unknown at the 30 minute time point.
Data presented in Figure 1 shows that thrombin generation has essentially ceased after 20 minutes in controls and in most haemophiliacs, suggesting that the propagation phase has ended. Since most TAFI activation occurs during the propagation phase, 20 minutes is a reasonable interval for measuring the TAFIa potential. In the interval after thrombin generation has ended and, therefore, TAFI activation has ceased, TAFIa levels can be predicted using the rate constant for spontaneous decay of TAFIa (30). In subjects who have not reached a plateau in TAT by 20 minutes, the TAFIa potential is not a reliable measure since it often only incorporates a single measurement above baseline. For these subjects, the TAFIa level at 20 minutes (TAFIa20min) can be used to describe each individual's propensity to activate TAFI.
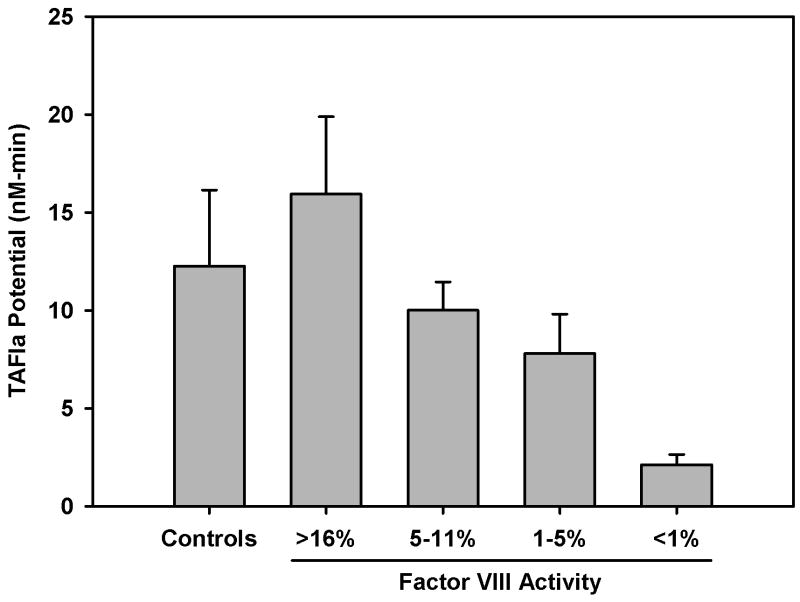
TAFIa potential in haemophilia A
The time courses of TAFI activation (those used to construct Figure 1, individual profiles not shown) were used to extract the TAFIa potential as described in Methods. The average TAFIa potential in subjects with >16% fVIII was slightly higher (15.95 ± 3.95) than controls (12.25±3.88 nM-min). In haemophilia subjects with 5 – 11% or 1 – 5% fVIII the TAFIa potential was reduced to 10.00 ± 1.45 and 7.80 ± 2.00 nM-min, respectively. Although TAFIa potential is not a reliable measure in subjects with < 1% fVIII, the TAFIa potential was 2.13 ± 0.53 nM-min. There is an obvious trend toward reduced TAFIa potential at lower fVIII levels, however, there was no significant difference in TAFIa potential when comparing controls, and haemophilic subjects with 1 – 5%, 5 – 11% or >16% fVIII.
Correlations of TAFI activation or thrombin generation to bleeding phenotype
Clinically severe subjects with < 1% fVIII at the time of the blood draw were evaluated for correlations between measures of thrombin generation (TAT20min) and TAFI activation (TAFIa20min) to bleeding phenotype. Of the 28 haemophilia subjects, 9 met these criteria (S13 – S21). In these 9 subjects, the TAFIa20min was significantly correlated with the hemarthrosis (-0.77, p=0.03) and total bleeds (-0.75, p=0.03). TAT20min did not reach the level of statistical significance in any bleeding category. Correlations between TAFIa20min and bleeding were always stronger than the corresponding values for TAT20min. A summary of the correlations of both TAT20min and TAFIa20min to bleeding phenotype are presented in Table 2.
Table 2.
Spearman correlation of the clinical bleeding phenotype to TAFIa and thrombin-antithrombin levels at 20 minutes. Total bleeds represent the sum of hemarthrosis and soft tissue scores.
| r | p | ||
|---|---|---|---|
| Hemarthrosis | TAT20min | -0.52 | 0.14 |
| TAFIa20min | -0.77 | 0.03 | |
| Soft tissue | TAT20min | -0.30 | 0.40 |
| TAFIa20min | -0.43 | 0.23 | |
| Total Bleeds | TAT20min | -0.45 | 0.20 |
| TAFIa20min | -0.75 | 0.03 | |
| FVIII Replacement | TAT20min | 0 | 0.95 |
| TAFIa20min | 0.13 | 0.67 | |
| Total Score | TAT20min | -0.65 | 0.07 |
| TAFIa20min | -0.63 | 0.07 | |
Discussion
In this study, we evaluated TF-initiated coagulation in contact pathway suppressed whole blood in haemophiliacs with varying fVIII levels and compared them to healthy controls. Our results show that TAFI activation is temporally related to thrombin generation regardless of fVIII level. Our data show that haemophilic individuals with higher fVIII had a TAFIa potential similar to controls. Finally, in this small study, TAFI activation (TAFIa20min) was more strongly correlated with the clinical bleeding phenotype in haemophilia than thrombin generation (TAT20min).
Only a relatively small percentage (<1%) of available TAFI is activated in plasma when clotting is initiated with low levels of thrombin (31). As recently reported, the percentage of fVIII in plasma greatly influences TAFI activation over the interval between clot formation and tPA induced clot lysis (14) especially in the range of 0-10% fVIII. We expanded upon this previous study to gain insight into the extent of TAFI activation in the whole blood of haemophilia A subjects. The correction of TAFIa potential in subjects with > 5% fVIII to control levels suggests that as little as 5-11% fVIII:C is required to normalize TAFI activation in whole blood, whereas up to 50% fVIII is required to correct TAFI activation in plasma (14). In both plasma (14), and whole blood (Figure 1) a very small percentage of TAFI is activated to TAFIa and regardless of the system (plasma or blood), both the rate and extent of TAFI activation appears to be influenced by the fVIII level. Previously, Butenas et al. demonstrated that platelets increase both the rate and amount of thrombin generated in the presence of physiologically normal levels of fVIII (32). Since platelets provide a procoagulant surface for many reactions in coagulation (33), a platelet-dependent increase in thrombin generation would not be entirely dependent on fVIII. Therefore, at lower fVIII levels, platelets would still enhance thrombin generation thus leading to the correction of TAFI activation at lower concentrations of fVIII.
The hypothesis that bleeding in haemophilia is partially due to enhanced fibrinolysis was first suggested by Broze and Higuchi in 1996 (12). The potential mechanisms for aberrant fibrinolysis in haemophilia include impaired cross-linking (9), lower TAFI levels (22) and impaired TAFI activation (14). Fibrinolysis is an often overlooked component of haemostasis and the TAFIa20min may prove useful in giving a more comprehensive, global assessment of haemostasis in pathological bleeding since it accurately reflects thrombin generation but also is a measure of the clot's resistance to fibrinolysis. TAFIa20min is more strongly correlated with the clinical bleeding phenotype than TAT20min and consequently, the variable bleeding tendency among individuals with severe haemophilia might be better explained and predicted by the TAFIa20min. The data presented here are consistent with the hypothesis that variable TAFI activation contributes to variable bleeding in haemophilia. In this small study, we show that the TAFIa20min is more strongly correlated with the clinical bleeding phenotype than TAT20min. The fact that TAFIa20min is significantly correlated with aspects of the clinical bleeding phenotype in such a small population is very promising; however, larger studies should be conducted to confirm these finding and determine if impaired TAFI activation directly contributes to the bleeding in haemophilia.
Currently, patients with haemophilia A are treated as required or by prophylaxis with factors that correct or bypass the defective tenase complex (5). It is unclear if these treatment strategies also fully correct secondary thrombin-dependent events such as TAFI activation. In this small study, we have determined that the TAFIa20min but not TAT20min is significantly correlated with bleeding in severe haemophilia and could prove to be a useful global measure of haemostasis in predicting the bleeding phenotype of individuals with severe haemophilia.
Figure 2.
Average TAFIa potential in haemophilia A and control subjects. Subjects were grouped by their fVIII level at the time of blood collection (Table 1). Since, in most cases, the TAFIa potential of the group with < 1% fVIII was only a reflection of one point above baseline this group was not included in the statistical test but is shown for relative comparison. No statistically significant difference in the TAFIa potential was found among the controls, >16%, 5-11% or 1-5% fVIII groups. Between group comparisons were conducted using one way ANOVA and the data are presented as mean ± standard error of the mean.
Acknowledgments
We would like to thank Matthew Whelihan for his technical assistance and Francine Derome for her invaluable help as a clinical nurse in working with us on the haemophilia individuals. This work was supported by grants from The National Institutes of Health, USA [HL46703, MEN and KBZ], the Heart and Stroke Foundation of Ontario (T5575) (MEN) and a Heart and Stroke Foundation of Canada Doctoral Fellowship (JHF).
References
- 1.Kitchens CS. Occult hemophilia. Johns Hopkins Med J. 1980;146:255–9. [PubMed] [Google Scholar]
- 2.Hoyer LW. Molecular pathology and immunology of factor VIII (hemophilia A and factor VIII inhibitors) Hum Pathol. 1987;18:153–61. doi: 10.1016/s0046-8177(87)80333-7. [DOI] [PubMed] [Google Scholar]
- 3.Shetty S, Bhave M, Ghosh K. Challenges of multiple mutations in individual patients with haemophilia. Eur J Haematol. 2011;86:185–90. doi: 10.1111/j.1600-0609.2010.01564.x. [DOI] [PubMed] [Google Scholar]
- 4.Aledort LM, Haschmeyer RH, Pettersson H. A longitudinal study of orthopaedic outcomes for severe factor-VIII-deficient haemophiliacs. The Orthopaedic Outcome Study Group. J Intern Med. 1994;236:391–9. doi: 10.1111/j.1365-2796.1994.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 5.Mannucci PM, Tuddenham EG. The hemophilias--from royal genes to gene therapy. N Engl J Med. 2001;344:1773–9. doi: 10.1056/NEJM200106073442307. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh K, Shetty S, Mohanty D. Milder clinical presentation of haemophilia A with severe deficiency of factor VIII as measured by one-stage assay. Haemophilia. 2001;7:9–12. doi: 10.1046/j.1365-2516.2001.00455.x. [DOI] [PubMed] [Google Scholar]
- 7.Franchini M, Lippi G. Factor V Leiden and hemophilia. Thromb Res. 2010;125:119–23. doi: 10.1016/j.thromres.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Butenas S, van't Veer C, Mann KG. Evalulation of the Initiation Phase of Blood Coagulation Using Ultrasensitive Assays for Serine Proteases. J Biol Chem. 1997;272:21527–33. doi: 10.1074/jbc.272.34.21527. [DOI] [PubMed] [Google Scholar]
- 9.Brummel-Ziedins KE, Branda RF, Butenas S, Mann KG. Discordant fibrin formation in hemophilia. J Thromb Haemost. 2009;7:825–32. doi: 10.1111/j.1538-7836.2009.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bajzar L, Manuel R, Nesheim ME. Purification and Characterization of TAFI, a Thrombin Activatable Fibrinolysis Inhibitor. J Biol Chem. 1995;270:14477–84. doi: 10.1074/jbc.270.24.14477. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Boffa MB, Bajzar L, Walker JB, Nesheim ME. A Study of the Mechanism of Inhibition of Fibrinolysis by Activated Thrombin-activable Fibrinolysis Inhibitor. J Biol Chem. 1998;273:27176–81. doi: 10.1074/jbc.273.42.27176. [DOI] [PubMed] [Google Scholar]
- 12.Broze GJ, Jr, Higuchi DA. Coagulation-Dependent Inhibition of Fibrinolysis: Role of Carboxypeptidase-U and the Premature Lysis of Clots From Hemophilic Plasma. Blood. 1996;88:3815–23. [PubMed] [Google Scholar]
- 13.Mosnier LO, Lisman T, Van den Berg HM, Nieuwenhuis HK, Meijers JCM, Bouma BN. The defective down regulation of fibrinolysis in haemophilia A can be restored by increasing the TAFI plasma concentration. Thromb Haemost. 2001;86:1035–9. [PubMed] [Google Scholar]
- 14.Foley JH, Nesheim ME. Soluble thrombomodulin partially corrects the premature lysis defect in FVIII-deficient plasma by stimulating the activation of thrombin activatable fibrinolysis inhibitor. J Thromb Haemost. 2009;7:453–9. doi: 10.1111/j.1538-7836.2008.03261.x. [DOI] [PubMed] [Google Scholar]
- 15.Lisman T, de Groot PG, Lambert T, Rojkjaer R, Persson E. Enhanced in vitro procoagulant and antifibrinolytic potential of superactive variants of recombinant factor VIIa in severe hemophilia A. J Thromb Haemost. 2003;1:2175–8. doi: 10.1046/j.1538-7836.2003.00444.x. [DOI] [PubMed] [Google Scholar]
- 16.Antovic JP, Antovic A, He S, Tengborn L, Blomback M. Overall haemostatic potential can be used for estimation of thrombin-activatable fibrinolysis inhibitor-dependent fibrinolysis in vivo and for possible follow-up of recombinant factor VIIa treatment in patients with inhibitors to factor VIII. Haemophilia. 2002;8:781–6. doi: 10.1046/j.1365-2516.2002.00689.x. [DOI] [PubMed] [Google Scholar]
- 17.Lisman T, Mosnier LO, Lambert T, Mauser-Bunschoten EP, Meijers JCM, Nieuwenhuis HK, et al. Inhibition of fibrinolysis by recombinant factor VIIA in plasma from patients with severe hemophilia A. Blood. 2002;99:175–9. doi: 10.1182/blood.v99.1.175. [DOI] [PubMed] [Google Scholar]
- 18.Antovic J, Schulman S, Eelde A, Blomback M. Total thrombin-activatable fibrinolysis inhibitor (TAFI) antigen and pro-TAFI in patients with haemophilia A. Haemophilia. 2001;7:557–60. doi: 10.1046/j.1365-2516.2001.00571.x. [DOI] [PubMed] [Google Scholar]
- 19.Guo XY, Okada N, Okada H. CPR-total (TAFI and activated TAFI) levels in plasma/serum of hemophiliacs. Microbiol Immunol. 2000;44:77–8. doi: 10.1111/j.1348-0421.2000.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 20.Brummel-Ziedins KE, Whelihan MF, Gissel M, Mann KG, Rivard GE. Thrombin generation and bleeding in haemophilia A. Haemophilia. 2009;15:1118–25. doi: 10.1111/j.1365-2516.2009.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawson JH, Krishnaswamy S, Butenas S, Mann KG. Extrinsic pathway proteolytic activity. Methods Enzymol. 1993;222:177–95. doi: 10.1016/0076-6879(93)22013-6. [DOI] [PubMed] [Google Scholar]
- 22.Hojima Y, Pierce JV, Pisano JJ. Hageman Factor Fragment Inhibitor in Corn Seeds: Purification and Characterization. Thromb Res. 1980;20:149–62. doi: 10.1016/0049-3848(80)90381-3. [DOI] [PubMed] [Google Scholar]
- 23.Walker JB, Nesheim ME. The Molecular Weights, Mass Distribution, Chain Composition, and Structure of Soluble Fibrin Degradation Products Released from a Fibrin Clot Perfused with Plasmin. J Biol Chem. 1999;274:5201–12. doi: 10.1074/jbc.274.8.5201. [DOI] [PubMed] [Google Scholar]
- 24.Kim PY, Foley J, Hsu G, Kim PY, Nesheim ME. An assay for measuring functional activated thrombin-activatable fibrinolysis inhibitor in plasma. Anal Biochem. 2008;372:32–40. doi: 10.1016/j.ab.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 25.Neill EK, Stewart RJ, Schneider MM, Nesheim ME. A functional assay for measuring activated thrombin-activatable fibrinolysis inhibitor in plasma. Anal Biochem. 2004;330:332–41. doi: 10.1016/j.ab.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Horrevoets AJG, Pannekoek H, Nesheim ME. Production and Characterization of Recombinant Human Plasminogen (S741C-Fluorescein): A Novel Approach to Study Zymogen Activation Without Generation of Active Protease. J Biol Chem. 1997;272:2176–82. doi: 10.1074/jbc.272.4.2176. [DOI] [PubMed] [Google Scholar]
- 27.Rand MD, Lock JB, van't Veer C, Gaffney DP, Mann KG. Blood clotting in minimally altered whole blood. Blood. 1996;88:3432–45. [PubMed] [Google Scholar]
- 28.Brummel KE, Paradis SG, Butenas S, Mann KG. Thrombin functions during tissue factor-induced blood coagulation. Blood. 2002;100:148–52. doi: 10.1182/blood.v100.1.148. [DOI] [PubMed] [Google Scholar]
- 29.Cawthern KM, van't Veer C, Lock JB, DiLorenzo ME, Branda RF, Mann KG. Bood Coagulation in Hemophilia A and Hemophilia C. Blood. 1998;91:4581–92. [PubMed] [Google Scholar]
- 30.Boffa MB, Bell R, Stevens WK, Nesheim ME. Roles of Thermal Instability and Proteolytic Cleavage in Regulation of Activated Thrombin Activable Fibrinolysis Inhibitor. J Biol Chem. 2000;275:12868–78. doi: 10.1074/jbc.275.17.12868. [DOI] [PubMed] [Google Scholar]
- 31.Foley JH, Kim PY, Nesheim ME. TAFI zymogen does not play a significant role in the attenuation of fibrinolysis. J Biol Chem. 2008;283:8863–7. doi: 10.1074/jbc.M800127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butenas S, Branda RF, van't Veer C, Cawthern KM, Mann KG. Platelets and phospholipids in tissue factor-initiated thrombin generation. Thromb Haemost. 2001;86:660–7. [PubMed] [Google Scholar]
- 33.Mann KG, Nesheim ME, Church WR, Haley P, Krishnaswamy S. Surface-Dependent Reactions of the Vitamin K-Dependent Enzyme Complexes. Blood. 1990;76:1–16. [PubMed] [Google Scholar]