Abstract
Cognitive reserve, broadly conceived, encompasses aspects of brain structure and function that optimize individual performance in the presence of injury or pathology. Reserve is defined as a feature of brain structure and/or function that modifies the relationship between injury or pathology and performance on neuropsychological tasks or clinical outcomes. Reserve is challenging to study for two reasons. The first is: reserve is a hypothetical construct, and direct measures of reserve are not available. Proxy variables and latent variable models are used to attempt to operationalize reserve. The second is: in vivo measures of neuronal pathology are not widely available. It is challenging to develop and test models involving a risk factor (injury or pathology), a moderator (reserve) and an outcome (performance or clinical status) when neither the risk factor nor the moderator are measured directly. We discuss approaches for quantifying reserve with latent variable models, with emphasis on their application in the analysis of data from observational studies. Increasingly latent variable models are used to generate composites of cognitive reserve based on multiple proxies. We review the theoretical and ontological status of latent variable modeling approaches to cognitive reserve, and suggest research strategies for advancing the field.
Keywords: Cognitive reserve, Brain reserve, Latent variable, Aging, Cognition, Neuronal plasticity, Multivariate Analysis, Environment, Social Environment, Education, Social Class, Intelligence
WHAT IS RESERVE?
Reserve is a hypothetical construct believed to explain individual differences in the relationship between neuropathology and cognitive performance (Satz, Cole, Hardy, & Rassovsky, 2010; Stern, 2009; Whalley, Deary, Appleton, & Starr, 2004). The concept of reserve has been cited as a theoretical framework for explaining individual differences in risk for, and patterns of, cognitive impairment associated with neuropathological changes in dementing disorders (Satz, 1993; Stern, 2002, 2003), brain injury (Fay et al., 2010), or medical illness (Satz, 1993). The vast majority of research on reserve concerns Alzheimer’s disease (AD) or nonspecific cognitive impairment and decline. The reserve concept, which is applicable to a broad array of clinical disorders (Sachdev & Valenzuela, 2009), describes variability across persons in the relationship of pathologic changes with clinical expression of disease (Satz, 1993; Studenski et al., 2006). In his most recent review, Stern (2009) articulates two models of reserve pertaining to cognitive functioning: brain and cognitive. Brain reserve refers to structural aspects of the brain, and cognitive reserve relates to how cognitive tasks are initiated and coordinated, involving access to complex cognitive networks. Brain reserve is conceptualized as a passive process and evokes a threshold model (a critical level of brain capacity, or brain reserve capacity) for the adequate performance of cognitive tasks in the presence of pathology or depletion. Cognitive reserve is conceptualized as an active process by which pathology or depletion are met with greater efficiencies in pre-existing cognitive processes (neural reserve) or the enlistment of alternative processes (neural compensation) to successfully complete cognitive tasks (Stern, 2009).
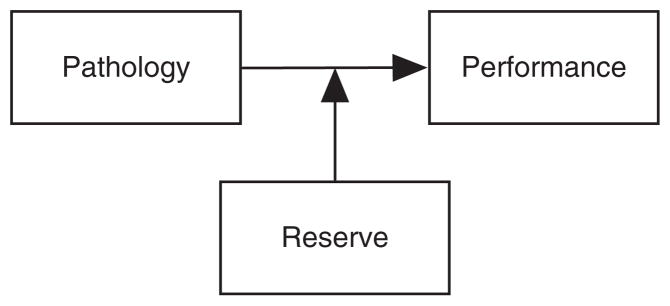
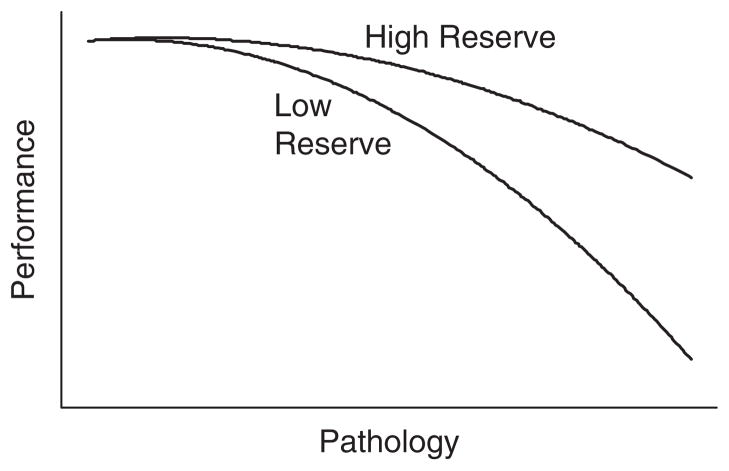
Notions of brain and cognitive reserve developed from observations that some individuals demonstrate less cognitive impairment than others with comparable brain injury or neuropathology (Blessed, Tomlinson, & Roth, 1968; Katzman, 1993; Rothschild & Trainor, 1937; Satz, 1993). Higher functioning individuals were postulated to possess a reserve factor that acted to delay or ameliorate the impairments of cognitive functioning accompanying neurodegeneration. Figure 1 shows an illustration of the basic notion of reserve using conventions of path diagram notation, where arrows represent regression relationships and boxes represent variables. An alternative but equivalent representation is shown in Figure 2, which shows a hypothetical line plot describing a different relationship between performance and pathology at different levels of reserve.1
Fig. 1.
Reserve modifies the relationship between pathology and performance.
Fig. 2.
The relationship between pathology and performance is attenuated in high relative to low reserve.
The reserve model suggested in Figure 1 may be an oversimplification. There are omitted relationships, and a temporal orientation is not represented. For example, depending upon how reserve is operationalized, there may be direct and/or bidirectional relationships between pathology and reserve and performance and reserve. The point of Figure 1 is to illustrate a basic theoretical definition of the reserve concept, and the oversimplification suffices in this context.
The concept of reserve is common in medicine (Sachdev & Valenzuela, 2009). Particularly so in geriatric medicine, where it is encompassed by the concept of “homeostenosis,” a gradual decline in physiological reserve that begins in young adults across organ systems independently and is moderated by environmental, host, and genetic factors (Resnick & Marcantonio, 1997). With regard to the brain, reserve is the capacity to withstand external stresses, revealed by individual differences in the functional and behavioral responses to neuronal disease or injury. Reserve theory (Stern, 2009) is consistent with a general theory of Cognitive Plasticity of cognitive aging (Willis, Schaie, & Martin, 2009), which posits that continual adaption to the environment at the neuronal level (e.g., remodulation of neurons, synaptic connections, neurogenesis) and cognitive level (acquisition of new skills) represents normal brain functioning (Draganski & May, 2008; Mercado, 2008; Pascual-Leone, Amedi, Fregni, & Merabet, 2005). Individual differences in plasticity can be influenced by psychosocial characteristics and manifest as cognitive or brain reserve (Willis et al., 2009).
The construct validity of the reserve concept was recently addressed in a review paper by Satz and his colleagues (2010). This review addresses, in greater detail than we provide here, a summary of different conceptualizations of reserve and their application in research. Satz et al. conclude that the concept of reserve is left “wanting as an explanatory construct” because a multitude of indicators (e.g., education, intracranial volume) are used as reserve indicators and there has been little theoretical work articulating an organizational structure for groups of such indicators. Our study echoes this theme and offers additional theoretical orientation for conceptualizing, developing, and ultimately testing latent variable models for reserve.
OPERATIONAL DEFINITIONS OF RESERVE IN OBSERVATIONAL RESEARCH
A critical first step in studying cognitive reserve is to identify indicators that accurately measure and quantify the key concepts: performance, pathology, and reserve. For the purposes of this study, we assume that the measurement of cognitive performance, such as with a neuropsychological assessment battery, is noncontroversial. If reserve is broadly defined as a discrepancy between observed and expected impairment associated with a given degree of neuropathology (c.f., Figure 1), then operationalizing reserve requires measures of both performance and neuropathology.
In vivo measures of neuropathology in Alzheimer’s disease, such as amyloid imaging, have only recently been identified and are not widely available (Ikonomovic et al., 2008; Rentz et al., 2010). Without a measure of neuropathology, the presence and severity of disease are approximated with age and/or time from clinical diagnosis and measures of function. Other measures of neuropathology include brain volume, white matter hyperintensity burden, fractional anisotropy from diffusion tensor imaging, cerebral blood flow patterns, and resting blood oxygen level dependent network activation patterns. Sumowski, Wylie, DeLuca and Chiaravalloti (2010) have presented innovative applications of such measures in the study of reserve in multiple sclerosis.
As a hypothetical construct, reserve is not measured directly. Proposed proxy indicators of cognitive reserve include educational attainment, occupational achievement, and intelligence (Stern, 2002). In a recent pair of meta-analyses of reserve in dementia risk and cognitive decline, Valenzuela and Sachdev (2006a, 2006b) identify the most frequently used proxy variables for cognitive reserve as educational attainment, occupation, mental activities, and premorbid IQ. Proposed indicators of brain reserve under a passive or threshold model include structural features such as intracranial volume, head circumference, and brain size, which are reflective of neuronal number and synapse density (Stern, 2002).
Among the indicators of reserve, educational attainment may be the most widely studied, and it is not uncommon to find research where education alone stands in for the concept of cognitive reserve (Valenzuela & Sachdev, 2006a, 2006b). The theoretical rationale for using an indicator such as education as a proxy for reserve seems compelling: education may increase brain reserve by promoting synaptic growth (Katzman, 1993) and may foster cognitive reserve by generating new cognitive strategies (Manly, Byrd, Touradji, Sanchez, & Stern, 2004; Stern, 2002). The strong and robust association of educational attainment with risk for dementia has led some investigators to claim that education may be the most important risk factor for dementia (Mortimer & Graves, 1993). To date, a handful of studies have confirmed that education modifies the association between a direct measure of neuropathology or neurodegeneration and neuropsychological test performance (Bennett et al., 2003; Dufouil, Alperovitch, & Tzourio, 2003; Rentz et al., 2010).
Other socioeconomic status (SES) indicators of reserve include occupational status. Valenzuela and Sachdev (Valenzuela & Sachdev, 2006b) found that “high” (contrasting unskilled, semi-skilled, trade, and clerical workers versus managerial, technical, and professional occupations) occupational status was associated with approximately a 50% reduction in the risk for dementia.
CRITIQUE OF PROXY MEASURES OF COGNITIVE RESERVE
An important limitation of most measures of cognitive reserve is that they may be linked to neuropsychological test performance via many alternative paths, not only via the hypothesized “reserve” mechanisms. Distinguishing between these alternatives is crucial both to understand the etiology of cognitive aging, and to assess whether the pathways can be modified via public health interventions. We focus on the example of educational attainment as the operationalization of reserve. Education may predict neuropsychological test performance in elderly populations because it confers cognitive reserve, or due to confounding by age (i.e., age may affect both education and neuropsychological test performance); confounding by childhood IQ or cognitive skills that themselves influence educational attainment; direct pathways by which education influences the development of neuropathology or neurodegeneration; or non-linearities in the rate of cognitive decline.
Because of the tremendous increase in average educational attainment and quality of educational opportunities over the course of the twentieth century, there are large age cohort differences in the average level of educational attainment in US samples (Goldin, 1998; Schaie, Willis, & Pennak, 2005). It is, therefore, possible that the association of education with cognitive impairment reflects, in whole or in part, a mis-specified model for the age–cognitive impairment relationship.
Historical and enduring differences across major population subgroups (especially gender and race) exist in the quality, access to, and social pressures for remaining in school (Jones, 2003) as well as for opportunities for achievement of certain occupational categories. Such challenges can be addressed in design and analysis. For example Manly, Jacobs, Touradji, Small, and Stern (2002) used a literacy test as an indicator of educational quality, on the grounds that the ideal indicator of school quality is what the student learned from school. However, premorbid IQ is also likely to influence literacy level (Valenzuela & Sachdev, 2006a). The association between literacy and neuropsychological test performance may thus reflect either the effects of school quality on cognitive skills or the association between premorbid IQ and performance on any neuropsychological test. Even in highly disadvantaged populations, in which access to education is severely restricted, attained education is probably partially influenced by cognitive skills that enable a child to excel at school. We know that early life cognitive measures predict dementia in old age (Whalley et al., 2000), and thus it is difficult to disentangle the influence of education from the influence of pre-school cognitive skills.
Cognitive reserve researchers must also recognize that educational attainment and occupation are principle facets of formal definitions of SES (Oakes & Rossi, 2003). Socioeconomic status differences in health and disease noted in the nineteenth century have persisted into the twentieth century (Link & Phelan, 1995). Link and Phelan have argued that SES is a fundamental cause of disease. That is to say, SES influences access to resources necessary to maintain good health, and affects multiple health outcomes through putatively different mechanisms (1995). Educational attainment is a main ingredient in socioeconomic status and may directly affect the progression of age-related neurodegeneration. For example, education may influence exposure to environmental hazards such as lead, promote engagement in leisure-time physical activity, or affect the ability to prevent or manage major chronic conditions that accelerate cognitive aging, such as diabetes and hypertension. Since educational attainment is a main component in summary variables intended to capture socioeconomic status, why education would be afforded a direct causal role in brain diseases and an indirect causal role in other somatic illnesses must be addressed. To this, Gottfredson takes the argument to the logical extreme, and argues that it is general intellectual ability (g), that explains the SES gradient and the appropriate fundamental cause of health outcomes in general (Gottfredson, 2004; Gottfredson & Deary, 2004).
It is this ambiguity—the possibility that any single measure of cognitive reserve may predict cognitive aging for reasons other than protection from expression of pathology—that has partially motivated the interest in multiple-indicator methods for measuring reserve. A multiple indicator model, in which the shared variance between several admittedly imperfect indicators of cognitive reserve is used to infer the latent variable “reserve,” may have several advantages. First, it may avoid some of the bias from non-reserve pathways, to the extent that those pathways are relevant for only one of the multiple indicators (e.g., education). Second, it may provide a more precise measure of reserve than could be obtained with any single indicator. Finally, it allows us to summarize the relationship between reserve and function with a single coefficient, instead of presenting several coefficients across different scales.
With this background, there is a danger that the ideas behind the reserve concept will degenerate into an insoluble struggle between social epidemiologists and cognitive reserve protagonists. To emerge from this, we return to Figure 1. The concept of reserve is not intrinsically tied to any proposed proxy measures of reserve, and indifferent to inadequate or even biased proxy measures of reserve. Reserve is recognized to be a hypothetical factor, meaning it is unseen, not directly measured: it is a latent construct (Stern, 2006; Whalley et al., 2004). Until a direct measure of reserve is identified, it is, therefore, appropriate to consider latent variable data analysis approaches that might help test theories regarding the putative role of cognitive reserve.
CRITIQUE OF LATENT VARIABLE MODELS OF RESERVE
Composite and latent variable approaches to reserve have appeared in the scientific literature. One of the earliest of these is the work of Scarmeas and colleagues, who used a principal component of performance on two vocabulary tests and years of completed education to define a cognitive reserve variable (Scarmeas et al., 2004). More recently Siedlecki and colleagues (2009) proposed a latent variable measurement model for cognitive reserve using data from the Northern Manhattan Study. The Siedlecki cognitive reserve model postulated a latent factor that was causally responsible for shared covariation among measures of educational attainment and performance on two vocabulary tests.
This work is significant for several reasons. The authors attempt to move the field of cognitive reserve beyond simple demographic proxies for reserve (Valenzuela & Sachdev, 2006a, 2006b). However, this work poses a theoretical and methodological challenge to cognitive reserve theory. This challenge hinges on the match between the underlying theory and the formulation of a latent variable measurement model. The idea behind using a latent variable measurement model to quantify cognitive reserve, i.e., using educational attainment and estimates of crystallized ability [the Wide Range Achievement Test (Wilkinson, 1993) and Peabody Picture Vocabulary Test (Dunn & Dunn, 1965)] is that “each of these variables presumably reflects life experiences, above and beyond that of age, that have the potential to provide protection against clinical manifestation of disease in the brain and has been used in the literature as a proxy for cognitive reserve” (Siedlecki et al., 2009, p. 560). This conceptualization is non-controversial, but the latent factor measurement model is incompatible with this theory, as discussed below.
This critique requires an explanation of two different kinds of latent variable measurement models: formative and reflective. The distinction is highlighted in Figures 3 and 4 [see also Borsboom, Mellenbergh, & van Heerden (2003) and Diamantopoulos & Siguaw (2006)]. In both Figures 3 and 4, we have illustrated a latent variable (drawn with circles labeled ξ) and manifest or observed variables (drawn with squares and labeled x1–x3). In psychosocial research typical latent variables include IQ and depression. Latent variables such as these are well measured by reflective indicators (Figure 4). For example, a reflective indicator of intelligence may be performance on a particular question presumed to measure innate intelligence, and a reflective indicator of depression might be a person’s response to a question about their experience of sad mood or loss of interest in usual activities. The main idea of reflective measurement models is that the observed data (x1–x3) are caused by the unobserved variable. A high level of depression causes people to respond positively to questions regarding feeling sad or blue.
Fig. 3.
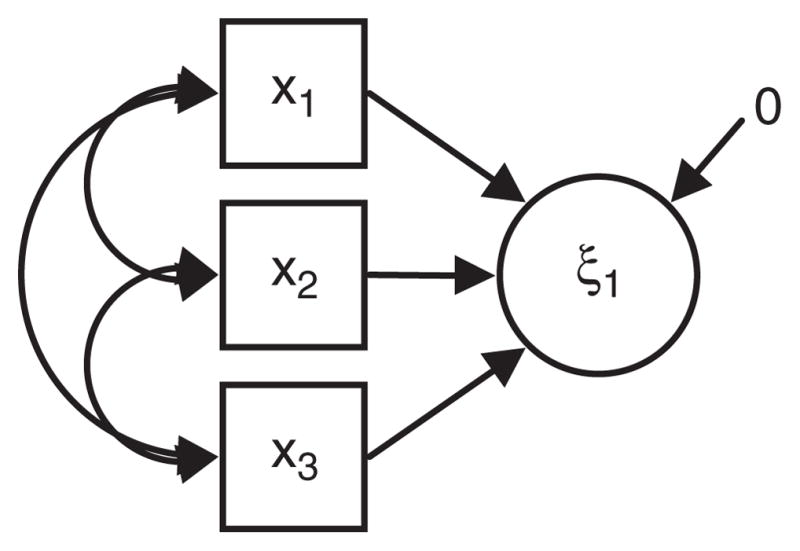
A formative measurement model.
Fig. 4.
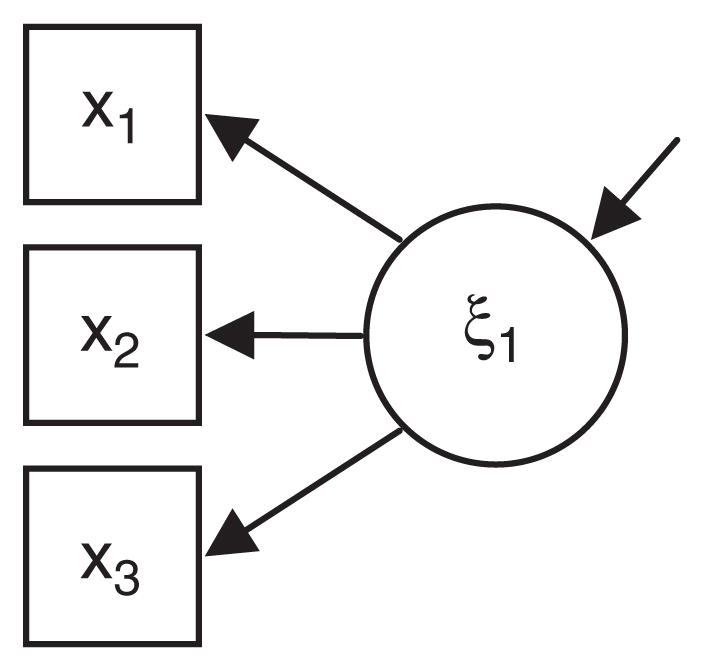
A reflective measurement model.
Other types of latent variables are well measured by formative indicators (Figure 3). An example of a latent variable that fits a formative indicator model is SES. In formative measurement the latent variable are the consequent causes of their formative indicators. For example, people do not have a certain level of education because they have a high underlying level of socioeconomic status. Instead, a high level of education leads to a cascade of life events that raise SES. In fact, as the example of education indicates, many commonly used indicators of reserve may be partially consistent with both reflective and formative models. For example, there may be characteristics such as cognitive flexibility that influence an individual to pursue extensive education and also provide reserve to reduce the expression of neuropathological disease in old age. This problem is almost unavoidable because the reserve measures at any point in time reflect the accumulation of a lifetime of experiences.
Inattention to the formative vs. reflective nature of latent variables and their measures is an enduring problem in psychosocial research (see for example, Mueller & Parcel, 1981). Since proxy indicators of cognitive reserve and socioeconomic status overlap to such a degree, researchers in reserve must give careful thought to the theoretical causal connection between their proxy measures and the underlying theoretical causal agent that is of primary research interest.
It seems reasonable to conclude that the appropriate causal ordering of cognitive reserve proxy variables such as education and vocabulary performance is at least partially characterized by a formative measurement model. Cognitive reserve does not cause high educational attainment and good performance on measures of crystallized ability. Instead, “each of these variables presumably reflects life experiences, above and beyond that of age, that have the potential to provide protection against clinical manifestation of disease in the brain” (Siedlecki et al., 2009, p. 560). The causal ordering holds that more education and associated life experience creates a cognitive reserve in late life. A reflective measurement model interpretation of proxy measures of cognitive reserve (i.e., a hypothesis regarding an unobserved factor that causes observed covariation among education and vocabulary skills) requires that the latent factor be re-conceptualized as a factor antecedent to education and vocabulary development. Such a factor might be aspects of the genome or early life envirome that predispose to high educational achievement and vocabulary development. However, this interpretation of the latent variable underlying cognitive reserve is not the model that has been articulated among cognitive reserve researchers, but does appear in the fields of social epidemiology (Link & Phelan, 1995).
However, simply re-specifying latent variable measurement models using formative rather than reflective measurement relationships will not solve the challenges of cognitive reserve research using latent variable data analysis techniques. The reason is that formative measurement models are uninformative about latent variables (Borsboom, 2005). This point is illustrated in Figures 5 and 6. Figure 5 is similar to Figure 3, but an observed outcome variable to the model (y1, e.g., cognitive decline). In Figure 6 a path diagram for a multiple regression relationship relating the observed variables is shown. Given conditions of model identification (Brown, 2006) the models illustrated in Figure 5 and Figure 6 are statistically identical. This means that there can be no empirical basis for choosing one over the other, the identification of the correct model must be driven by substantive knowledge and theoretical orientation.
Fig. 5.
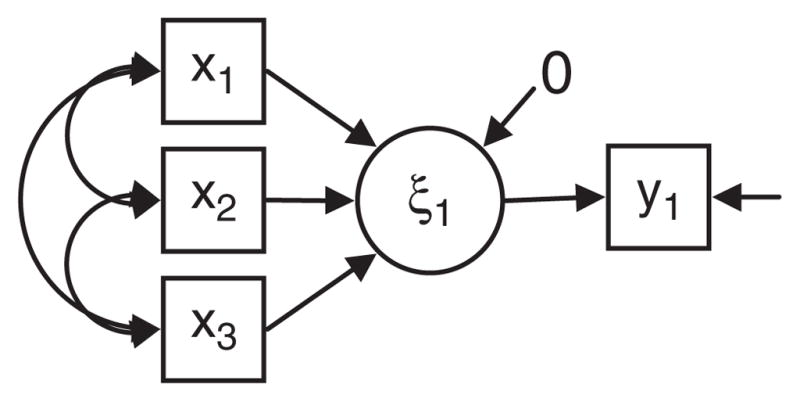
A formative measurement with an outcome.
Fig. 6.
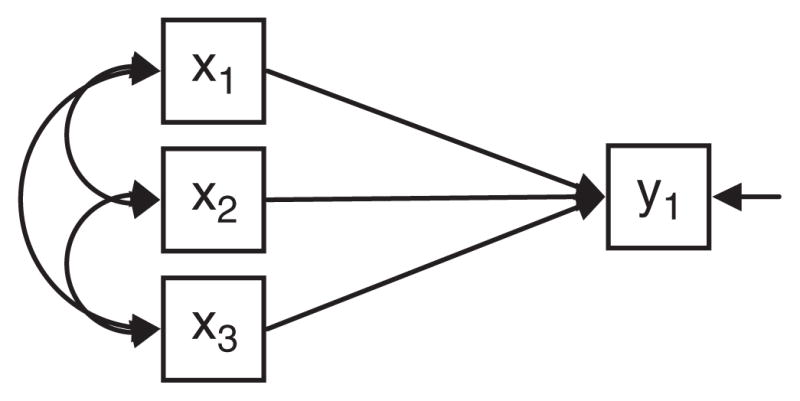
A multiple regression model.
Since formative measurement models are statistically equivalent to multiple regression models, the specification and evaluation of models based exclusively on formative measurement provides no insight into the validity of the hypothesized latent variable. Thus theory development and testing regarding latent variables requires reflective measures of the latent variable. Latent variable theory is most productive when the underlying conceptual framework recognizes and tests the causal relationships suggested by the reflective measurement model (Borsboom, 2005).
The same reflective vs. formative measurement model critique could be leveled against latent variable data analyses of the brain reserve concept. For example, Brickman and colleagues (2009) have proposed a latent variable measurement model for brain reserve using data from the Washington Heights/Hamilton Heights Inwood Columbia Aging Project. The latent variable pursued by Brickman and colleagues is one that captures “the brain’s structural integrity” and those that “may be most important in providing reserve or may promote resistance against the development of brain pathology” (p 2/11). The observables that are used to measure this latent variable were “physical variables and incorporated anthropometric estimates of development that might directly reflect the quality of physical development” (p 3/11) operationalized with height, total cranial volume and cranial length. Here the articulated causal ordering is one where the observables are arguably antecedent to the latent construct.
It is reasonable to ask what practical consequence results from mis-specifying the causal ordering of observable and latent variables as outlined above. After all, as the eminent psychometrician Frederic Lord (1953) famously pointed out, empirical questions can be answered even if a data analysis approach violates statistical and measurement theory assumptions. The answer depends upon the view of the ontological status of the latent variables. The conceptual framework for latent variables offered by Borsboom (2005) is useful for organizing a response to this question. Borsboom describes two broad classes of theories about latent variables: realist and anti-realist. Anti-realist interpretations of latent variables include constructivist (the concept is a construction of the human mind, a socially agreed upon concept negotiated by scientists) and operationalist/instrumentalist (the concept is nothing more than a mathematical abstraction) stances. Anti-realist positions share an ontological perspective on latent variables: they have no independent existence apart from our measurements. The realist position starts from an assumption that the latent variables do exist independent of our measurements. Borsboom articulates a cogent argument concluding that the only position that results in a logically consistent philosophy of science is the realist. If our concern is construct validity, i.e., a concern with the fundamental existence of reserve or the validity of our measurements of reserve, then a realist ontological stance is suggested and the causal ordering of latent variables, their indicators, and their causes is of fundamental importance. An appropriately specified measurement model represents a formal test of the validity of the latent variable under consideration (e.g., reserve). On the other hand, perhaps our goal is to perform a statistical adjustment for multiple correlated factors in a regression model. With no concern as to whether the covariance of the multiple factors reflects the action of any characteristic of persons with existence apart from the individual measurements, an anti-realist approach is theoretically sufficient and statistically efficient. However, antirealist positions and models do not advance knowledge of the latent variable under study.
The formative/reflective distinction comes into play at various levels. As a practical example of the possible implications of mis-specifying the causal ordering of putative reserve markers, consider the example of cognitive training interventions. Cognitive training interventions have been shown to improve trained cognitive abilities (Ball et al., 2002), and some—but not all—interventions show transfer to non-trained cognitive abilities and functional outcomes (Valenzuela & Sachdev, 2009; Willis et al., 2006). If cognitive activity is a reflective reserve indicator, then an intervention that improves cognitive performance through training and practice would not be expected to boost cognitive reserve. This is because cognitive activity is downstream of cognitive reserve. Analogously, we would not expect that lowering body temperature would treat an infection in an ill patient. If on the other hand cognitive activity is a formative indicator of reserve, then a cognitive training or mental exercise intervention that improves performance would be expected to boost reserve. Consequently, if a reflective measurement model is presumed then embarking upon a cognitive training intervention would be illogical. Thus the theory informs the kinds of questions that are suitable for being addressed with experimental studies. In the presence of results of intervention studies, regardless of the orientation of the generating theoretical framework, evidence for transfer effects can be informative of the reflective/formative distinction. In the ACTIVE cognitive intervention trial, only the logical reasoning intervention demonstrated transfer effects to functional outcomes (Willis et al., 2006). This suggests that the activity indicator represented by random assignment to logical reasoning exercise may be formative with respect to reserve.
REFLECTIVE MEASUREMENT MODELS ARE NEEDED FOR TESTING RESERVE THEORIES
Figure 1 clearly suggests a reflective measurement model for reserve. The reflective measures are the regressions of performance on pathology. While regression relationships are not typical reflective indicators of latent constructs, new data analysis techniques that permit generality and allow for both random effects and latent variables in a single analytic framework will make such model development and theory testing possible (Muthén & Muthén, 1998–2010)—provided appropriate in vivo measures of neuropathology are available. Recent work toward this goal includes that of Reed and his colleagues (Reed et al., 2010). This group has proposed the use of structural MRI to define cognitive reserve, that is, defining reserve as the residual of the regression using memory performance on normalized brain volume. They have shown that this formulation of reserve predicts cognitive decline and incident dementia (Reed et al., 2010).
CONCLUSION
To advance the theory of cognitive reserve using latent variable models and observational research designs, good reflective measures of reserve are needed. In their recent review, Satz and colleagues offer distinct latent variable models for studying reserve, models that incorporate demographic characteristics such as age and education, anthropometric characteristics such as head circumference and total intracranial volume, neuropsychological measures including measures of processing resources (divided attention, processing speed, working memory) as well as measures of executive function and crystallized ability, and measures of cerebral activation and metabolism (Satz et al., 2010). Our treatment has been more circumscribed. However, if Borsboom’s (2005) challenge that construct validation requires a realist ontological position, measurement models provide information about latent variables when they include reflective indicators, and that the causal ordering of latent and observable variables is of fundamental importance, then each of three hypothesized models of Satz et al. requires some reformulation. We urge that investigators give careful thought and cogent justification for considering demographic measures such as age and anthropometric variables such as brain volume on equal ontological footing in measurement models of “brain reserve capacity”, and occupation and working memory on equal ontological footing in measurement models of “cognitive reserve.”
Given a general model of reserve as illustrated in Figure 1, in vivo measures of brain pathology that can be included in prospective observational research studies are essential and need to be bolstered by assessments of life course developmental pathways. Approaches such as natural (Glymour, Kawachi, Jenks, & Berkman, 2008) and designed (Jobe et al., 2001) experiments are also promising approaches. Another approach might be to compare how a “random shock” that typically causes neurologic damage affects cognitive decline in people with high or low “reserve.” For example, a study might examine the change in performance pre–post myocardial infarction, stroke, head injury, bypass surgery, or as a consequence of an acute cognitive disorder such as delirium (Jones et al., 2010, 2006). Additionally, Sumowski and his colleagues use functional neuroimaging to characterize patterns of neural activation that provide insight into how reserve may operate to preserve cognitive performance in neurologic disease (2010). Findings from this research move beyond trying to define what reserve is to characterizing how reserve may operate (Stern, 2009).
Given the challenges in operationalizing and measuring the construct of reserve, it may be worthwhile to consider the future utility of “reserve” as a construct in cognitive aging. It may be more informative and accurate to avoid reification and describe variables as they are measured. Such an approach has the added benefit of facilitating drawing more direct connections between the growing evidence base from animal models of enriched environments and brain structure, function, and aging in animals (Cracchiolo et al., 2007; Hu et al., 2010) and analogous observational studies in humans (Valenzuela & Sachdev, 2006a, 2006b). Latent variables are often used as a tool for addressing collinearity among correlated predictor variables. Alternative methods of multivariate data analysis that do not rely upon postulated latent variables, such as path analysis or principle components analysis, may be better suited than methods of factor analysis for describing patterns of dependency and handling shared covariation (see for example Richards & Deary, 2005, and Jefferson et al. in this issue). However, it may be premature to abandon the concept of reserve altogether. There are many examples in the history of science of postulated entities that were conceived of before the development of when precise measures were developed. Our main point, to recapitulate that of Satz and his colleagues (2010), is only that the measurement of reserve requires continual refinement and construct validation.
Finally, there is emerging evidence that reserve may be a potentially modifiable characteristic, for example through mental or physical exercise. To the extent that putative proxy measures of reserve are good measures, and if the true causal pathway is reasonably strong and in a supportive direction, reserve may be promoted at a societal level through compulsory education, physical education, and cultural norms. Valenzuela and Sachdev (2006b), in their meta analysis found that participating in mentally stimulating activities was the most robust reserve measure (relative to education, occupation, and premorbid IQ). Scarmeas, Levy, Tang, Manly, and Stern (2001) have found that incident dementia was related to intellectual, physical, and social activity participation. Experimental evidence in humans suggests that even older adults can benefit from training in basic cognitive abilities (Ball et al., 2002; Valenzuela & Sachdev, 2009; Willis et al., 2006; Wolinsky, Unverzagt, Smith, Jones, Stoddard, et al., 2006; Wolinsky, Unverzagt, Smith, Jones, Wright, et al., 2006). Such evidence is exciting because it suggests that older people can modify their risk for cognitive decline and maintain independence through mental activity.
Acknowledgments
Collaborative work on this project by the co-authors was partially supported by a conference grant on Advanced Psychometric Methods in Cognitive Aging Research (R13AG030995). Dr. Jones was also supported by NIH/NIA grant P01AG031720.
Footnotes
Parenthetically, it is worth pointing out that, if a non-linear relationship between pathology and performance exists as suggested in Figure 2, any factor associated with performance at baseline would also be related to the pace of decline. Consequently, a sufficient quantity of data across the range of pathology under consideration and careful data analysis are required to avoid spurious inference regarding the influence of baseline factors.
The authors have no conflict of interest.
References
- Ball K, Berch D, Helmers K, Jobe J, Leveck M, Marsiske M, Willis S. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes De Leon CF, Arnold SE, Bienias JL. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60(12):1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson B, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. British Journal of Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Borsboom D. Measuring the mind: Conceptual issues in contemporary psychometrics. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Borsboom D, Mellenbergh GJ, van Heerden J. The theoretical status of latent variables. Psychological Review. 2003;110(2):203–218. doi: 10.1037/0033-295X.110.2.203. [DOI] [PubMed] [Google Scholar]
- Brickman A, Siedlecki K, Muraskin J, Manly J, Luchsinger J, Yeung L, Stern Y. White matter hyperintensities and cognition: Testing the reserve hypothesis. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.10.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA. Confirmatory factor analysis for applied research. New York: Guilford Publications; 2006. [Google Scholar]
- Cracchiolo J, Mori T, Nazian S, Tan J, Potter H, Arendash G. Enhanced cognitive activity–over and above social or physical activity–is required to protect Alzheimer’s mice against cognitive impairment, reduce A [beta] deposition, and increase synaptic immunoreactivity. Neurobiology of Learning and Memory. 2007;88(3):277–294. doi: 10.1016/j.nlm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantopoulos A, Siguaw JA. Formative versus reflective indicators in organizational measure development: A comparison and empirical illustration. British Journal of Management. 2006;17:263–282. [Google Scholar]
- Draganski B, May A. Training-induced structural changes in the adult human brain. Behavioural Brain Research. 2008;192(1):137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Dufouil C, Alperovitch A, Tzourio C. Influence of education on the relationship between white matter lesions and cognition. Neurology. 2003;60(5):831–836. doi: 10.1212/01.wnl.0000049456.33231.96. [DOI] [PubMed] [Google Scholar]
- Dunn L, Dunn L. Peabody picture vocabulary test. Circle Pines, MN: American Guidance Service; 1965. [Google Scholar]
- Fay TB, Yeates KO, Taylor HG, Bangert B, Dietrich A, Nuss KE, Wright M. Cognitive reserve as a moderator of postconcussive symptoms in children with complicated and uncomplicated mild traumatic brain injury. Journal of the International Neuropsychological Society. 2010;16(01):94–105. doi: 10.1017/S1355617709991007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour M, Kawachi I, Jenks C, Berkman LF. Does childhood schooling affect old age memory or mental status? Using state schooling laws as natural experiments. Journal of Epidemiology and Community Health. 2008;62:532–537. doi: 10.1136/jech.2006.059469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin C. America’s graduation from high school: The evolution and spread of secondary schooling in the twentieth century. Journal of Economic History. 1998;58(2):345–374. [Google Scholar]
- Gottfredson LS. Intelligence: Is it the epidemiologists’ elusive “fundamental cause” of social class inequalities in health. Journal of Personality and Social Psychology. 2004;86(1):174–199. doi: 10.1037/0022-3514.86.1.174. [DOI] [PubMed] [Google Scholar]
- Gottfredson LS, Deary IJ. Intelligence predicts health and longevity, but why? Current Directions in Psychological Science. 2004;13(1):1–4. [Google Scholar]
- Hu Y, Xu P, Pigino G, Brady S, Larson J, Lazarov O. Complex environment experience rescues impaired neurogenesis, enhances synaptic plasticity, and attenuates neuropathology in familial Alzheimer’s disease-linked APPswe/PS1 {Delta} E9 mice. The FASEB Journal. 2010;24(6):1667. doi: 10.1096/fj.09-136945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, DeKosky ST. Postmortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131(6):1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe J, Smith D, Ball K, Tennstedt S, Marsiske M, Willis S, Kleinman K. ACTIVE: A cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials. 2001;22(4):453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. Racial bias in the assessment of cognitive functioning of older adults. Aging & Mental Health. 2003;7(2):83–102. doi: 10.1080/1360786031000045872. [DOI] [PubMed] [Google Scholar]
- Jones R, Fong TG, Metzger E, Tulebaev S, Yang FM, Alsop DC, Inouye SK. Aging, brain disease, and reserve: Implications for delirium. American Journal of Geriatric Psychiatry. 2010;18(2):117–127. doi: 10.1097/JGP.0b013e3181b972e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R, Yang FM, Zhang Y, Kiely DK, Marcantonio ER, Inouye SK. Does educational attainment contribute to risk for delirium? A potential role for cognitive reserve. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2006;61A(12):1307–1311. doi: 10.1093/gerona/61.12.1307. [DOI] [PubMed] [Google Scholar]
- Katzman R. Education and the prevalence of dementia and Alzheimer’s disease. Neurology. 1993;43:13–20. doi: 10.1212/wnl.43.1_part_1.13. [DOI] [PubMed] [Google Scholar]
- Link BG, Phelan J. Social conditions as fundamental causes of disease. Journal of Health and Social Behavior. 1995;35(extra issue):80–94. [PubMed] [Google Scholar]
- Lord F. On the statistical treatement of football numbers. American Psychologist. 1953;8:750–751. [Google Scholar]
- Manly JJ, Byrd D, Touradji P, Sanchez D, Stern Y. Literacy and cognitive change among ethnically diverse elders. International Journal of Psychology. 2004;39(1):47–60. [Google Scholar]
- Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y. Reading level attenuates differences in neuropsychological test performance between African American and White elders. Journal of the International Neuropsychological Society. 2002;8(3):341–348. doi: 10.1017/s1355617702813157. [DOI] [PubMed] [Google Scholar]
- Mercado E. Neural and cognitive plasticity: From maps to minds. Psychological Bulletin. 2008;134(1):109. doi: 10.1037/0033-2909.134.1.109. [DOI] [PubMed] [Google Scholar]
- Mortimer J, Graves A. Education and other socioeconomic determinants of dementia and Alzheimer’s disease. Neurology. 1993;43(S4):S39–S44. [Google Scholar]
- Mueller CW, Parcel TL. Measures of socioeconomic status: Alternatives and recommendations. Child Development. 1981;52(1):13–30. [Google Scholar]
- Muthén L, Muthén B. Mplus users guide. 6. Los Angeles, CA: Muthén & Muthén; 1998–2010. [Google Scholar]
- Oakes JM, Rossi PH. The measurement of SES in health research: Current practice and steps toward a new approach. Social Science and Medicine. 2003;56(4):769–784. doi: 10.1016/s0277-9536(02)00073-4. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet L. The plastic human brain cortex. Annual Review Neuroscience. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Reed B, Mungas D, Farias S, Harvey D, Beckett L, Widaman K, DeCarli C. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain. 2010;133(Pt 8):2196–2209. doi: 10.1093/brain/awq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz D, Locascio J, Becker J, Moran E, Eng E, Buckner R, Johnson K. Cognition, reserve, and amyloid deposition in normal aging. Annals of Neurology. 2010;67(3):353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick NM, Marcantonio ER. How should clinical care of the aged differ? Lancet. 1997;350(9085):1157. doi: 10.1016/S0140-6736(05)63817-2. [DOI] [PubMed] [Google Scholar]
- Richards M, Deary IJ. A life course approach to cognitive reserve: A model for cognitive aging and development? Annals of Neurology. 2005;58(4):617–622. doi: 10.1002/ana.20637. [DOI] [PubMed] [Google Scholar]
- Rothschild D, Trainor MA. Pathologic changes in senile psychoses and their psychobiologic significance. American Journal of Psychiatry. 1937;93(4):757–788. [Google Scholar]
- Sachdev PS, Valenzuela M. Brain and cognitive reserve. American Journal of Geriatric Psychiatry. 2009;17(3):175. doi: 10.1097/JGP.0b013e318196a661. [DOI] [PubMed] [Google Scholar]
- Satz P. Brain reserve capacity on symptom onset after brain injury: A formulation and review of evidence for threshold theory. Neuropsychology. 1993;7(3):273–295. [Google Scholar]
- Satz P, Cole M, Hardy D, Rassovsky Y. Brain and cognitive reserve: Mediator(s) and construct validity, a critique. Journal of Clinical and Experimental Neuropsychology. 2010 doi: 10.1080/13803395.2010.493151. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Levy G, Tang M, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Zarahn E, Anderson KE, Honig LS, Park A, Hilton J, Stern Y. Cognitive reserve-mediated modulation of positron emission tomographic activations during memory tasks in Alzheimer disease. Archives of Neurology. 2004;61(1):73–78. doi: 10.1001/archneur.61.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie K, Willis S, Pennak S. An historical framework for cohort differences in intelligence. Research in Human Development. 2005;2(1&2):43–67. doi: 10.1080/15427609.2005.9683344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedlecki K, Stern Y, Reuben A, Sacco R, Elkind M, Wright C. Construct validity of cognitive reserve in a multiethnic cohort: The Northern Manhattan Study. Journal of the International Neuropsychological Society. 2009;15(4):558–569. doi: 10.1017/S1355617709090857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8(3):448–460. [PubMed] [Google Scholar]
- Stern Y. The concept of cognitive reserve: A catalyst for research. Journal of Clinical and Experimental Neuropsychology. 2003;25(5):589–593. doi: 10.1076/jcen.25.5.589.14571. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Disease and Associated Disorders. 2006;20(2):112–117. doi: 10.1097/01.wad.0000213815.20177.19. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studenski S, Carlson MC, Fillit H, Greenough WT, Kramer A, Rebok GW. From bedside to bench: Does mental and physical activity promote cognitive vitality in late life? Science of Aging Knowledge Environment. 2006;2006(10):pe21. doi: 10.1126/sageke.2006.10.pe21. [DOI] [PubMed] [Google Scholar]
- Sumowski J, Wylie G, DeLuca J, Chiaravalloti N. Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: Functional magnetic resonance imaging evidence for cognitive reserve. Brain. 2010;133(2):362–374. doi: 10.1093/brain/awp307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela M, Sachdev P. Brain reserve and cognitive decline: A non-parametric systematic review. Psychological Medicine. 2006a;36(8):1065–1073. doi: 10.1017/S0033291706007744. [DOI] [PubMed] [Google Scholar]
- Valenzuela M, Sachdev P. Brain reserve and dementia: A systematic review. Psychological Medicine. 2006b;36(4):441–454. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- Valenzuela M, Sachdev P. Can cognitive exercise prevent the onset of dementia? Systematic review of randomized clinical trials with longitudinal follow-up. American Journal of Geriatric Psychiatry. 2009;17(3):179. doi: 10.1097/JGP.0b013e3181953b57. [DOI] [PubMed] [Google Scholar]
- Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing Research Reviews. 2004;3(4):369–382. doi: 10.1016/j.arr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Whalley LJ, Starr JM, Athawes R, Hunter D, Pattie A, Deary IJ. Childhood mental ability and dementia. Neurology. 2000;55(10):1455–1459. doi: 10.1212/wnl.55.10.1455. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. Wide range achievement test. Wilmington, DE: Wide Range, Inc; 1993. [Google Scholar]
- Willis S, Schaie K, Martin M. Cognitive plasticity. In: Bengtson VL, Gans D, Putney N, Silverstein M, editors. Handbook of theories of aging. New York: Springer Publishing Company; 2009. pp. 295–322. [Google Scholar]
- Willis S, Tennstedt S, Marsiske M, Ball K, Elias J, Koepke K for the Active Study Group. Long-term effects of cognitive training on everyday functional outcomes in older adults. Journal of the American Medical Association. 2006;296(23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Stoddard A, Tennstedt SL. The ACTIVE cognitive training trial and health-related quality of life: Protection that lasts for 5 years. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006;61(12):1324–1329. doi: 10.1093/gerona/61.12.1324. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Wright E, Tennstedt SL. The effects of the ACTIVE cognitive training trial on clinically relevant declines in health-related quality of life. The Journals of Gerontology: Psychological Sciences and Social Sciences. 2006;61(5):S281–S287. doi: 10.1093/geronb/61.5.s281. [DOI] [PubMed] [Google Scholar]