Abstract
Rationale
Considerable evidence indicates that amphetamine derivatives can deplete brain monoaminergic neurotransmitters. However, the behavioral and cognitive consequences of neurochemical depletions induced by amphetamines are not well established.
Objectives
In this study, mice were exposed to dosing regimens of 3,4-methylenedioxymethamphetamine (MDMA), methamphetamine (METH), or para-chloroamphetamine (PCA) known to deplete the monoamine neurotransmitters dopamine and serotonin, and the effects of these dosing regimens on learning and memory were assessed.
Methods
In the same animals, we determined deficits in learning and memory via passive avoidance (PA) behavior and changes in tissue content of monoamine neurotransmitters and their primary metabolites in the striatum, frontal cortex, cingulate, hippocampus, and amygdala via ex vivo high pressure liquid chromatography.
Results
Consistent with previous studies, significant reductions in tissue content of dopamine and serotonin were readily apparent. In addition, exposure to METH and PCA impaired PA performance and resulted in significant depletions of dopamine, serotonin, and their metabolites in several brain regions. Multiple linear regression analysis revealed that the tissue concentration of dopamine in the anterior striatum was the strongest predictor of PA performance, with an additional significant contribution by the tissue concentration of the serotonin metabolite 5-hydroxyindoleacetic acid in the cingulate. In contrast to the effects of METH and PCA, exposure to MDMA did not deplete anterior striatal dopamine levels or cingulate levels of 5-hydroxyindoleacetic acid, and it did not impair PA performance.
Conclusions
These studies demonstrate that certain amphetamines impair PA performance in mice and that these impairments may be attributable to specific neurochemical depletions.
Keywords: Drug abuse, amphetamine, neurotoxicity, monoamine, mouse, dopamine, learning and memory, behavior, methamphetamine, HPLC
Introduction
Abuse of amphetamines has been associated with neural changes such as decreases in monoamine neurotransmitters (Kish et al. 2000; Wilson et al. 1996), neurotransmitter regulating proteins (McCann et al. 1998; Reneman et al. 2001), or basal brain metabolism (Buchert et al. 2001; Obrocki et al. 2002). Although these neural alterations may lead to behavioral and cognitive deficits, studies of the relationship between amphetamine derivative abuse and cognitive performance have yielded mixed results (Bolla et al. 1998; Hanson and Luciana 2004; McCann et al. 2007; McCardle et al. 2004). Nevertheless, recent meta-analytic reviews of the neuropsychiatric effects of methamphetamine (METH) (Scott et al. 2007) or 3,4-methylenedioxymethamphetamine (MDMA) (Kalechstein et al. 2007) abuse found a consistent spectrum of “frontal-subcortical” deficits, including learning and memory deficits. As a whole, this work supports the supposition that abuse of amphetamine derivatives is an important public health concern because it may lead to a cognitively impaired patient population.
In the first pre-clinical studies of the persistent effects of amphetamine derivatives, Pletscher and coworkers (1963; 1964) reported a pronounced and a sustained decrease in serotonin in rats exposed to para-chloromethamphetamine. This work was extended by the finding that, among several chlorinated amphetamines, para-chloroamphetamine (PCA) was the most potent serotonin depletor (Fuller et al. 1977; Fuller et al. 1965). Subsequent work showed that exposure to PCA also depleted markers for (Itzhak et al. 2004) and tissue concentrations of (Saadat et al. 2006a) dopamine. Similar studies have examined the sustained neurochemical effects of MDMA and METH. In the rat, METH exposure decreased tissue levels of serotonin and dopamine (Gibb et al. 1990) whereas MDMA was selective for serotonin (Ricaurte 1989; Schmidt et al. 1987). In murine subjects, both METH and MDMA elicited more pronounced dopaminergic depletions than serotonergic depletions (Ali et al. 1994; Colado et al. 2004; Kita et al. 2003; O'Callaghan and Miller 1994; Stone et al. 1987). Furthermore, these dopamine depletions occurred within 72 hours of exposure and could last up to 8 weeks, without any recovery (Ali et al. 1994; Colado et al. 2004; Kita et al. 2003; O'Callaghan and Miller 1994; Stone et al. 1987). As a whole, these findings demonstrate complex but well-supported sustained neurochemical effects of these amphetamine derivatives.
Despite these profound and sustained neurochemical effects, it has been difficult to establish the existence and nature of consequential behavioral and cognitive deficits. This, in part, has led some to question the use of the appellation “neurotoxicity” for the persistent neurochemical effects of amphetamines, and to speculate that these changes may in fact not be true toxicity, but rather represent a form of neuroadaptation. In particular, the ongoing debate as to the nature and relevance of these persistent neurochemical effects has been fueled by the mixed evidence provided by the few pre-clinical studies to examine the behavioral and cognitive consequences of these neurochemical changes. In this regard, exposure to amphetamines has been shown to result in deficits in locomotor activity (Timar et al. 2003) and appetitive and aversive Pavlovian learning (Achat-Mendes et al. 2005; Achat-Mendes et al. 2007) but did not alter impulsivity (Saadat et al. 2006b) or repeated-acquisition performance (Winsauer et al. 2002). Other researchers have shown that cognitive deficits in mice are highly dependent on the dosing regimen used (Belcher et al. 2008) or that deficits in locomotor activity only occur after neurochemical depletions have recovered (Krasnova et al. 2009). Although these studies have not provided unambiguous support for cognitive or behavioral impairments induced by amphetamines, as previously noted, a recent meta-analytic review found a consistent spectrum of “frontal-subcortical” deficits, including learning and memory deficits, in human METH (Scott et al. 2007) and MDMA (Kalechstein et al. 2007) abusers. Accordingly, further preclinical studies of the behavioral and cognitive consequences of exposure to amphetamine derivatives are warranted.
In the present study, we examined the effects of MDMA, METH, and PCA on passive avoidance (PA) behavior in mice using dosing regimens that have been shown to engender robust neurochemical depletions. The PA assay was chosen for a number of reasons. First, it is a simple one-trial test of learning and memory. Second, age-related cognitive decline in mice has been shown to be more likely to lead to deficits in PA, which requires sustained retention of information, rather than cue or discrimination-based learning and memory paradigms (Gower and Lamberty 1993). Third, PA may be mediated via dopaminergic mechanisms as direct dopamine receptor agonists facilitate whereas antagonists impair one-trial inhibitory avoidance behavior (Adriani et al. 1998) and depletion of dopamine by 6-hydroxydopamine lesions impairs PA performance (Taghzouti et al. 1985). Fourth, PA may be independent of serotonergic mechanisms as depletion of serotonin by PCA (Santucci et al. 1996), 3,4-methyeledioxyethamphetamine (Barrionuevo et al. 2000) or dosal raphe lesions (Santucci et al. 1996) in the rat did not impair PA behavior (for a review see Myhrer 2003). Finally, we predicted that, in the mouse, each of the derivatives tested would deplete tissue content of dopamine. Using this simple yet possibly selective assay, we compared the effects of the drugs of abuse MDMA and METH to PCA because PCA is considered by some to be a more definitive neurotoxin than either MDMA or METH. Given the ambiguity of previous attempts to determine cognitive impairments following administration of amphetamine derivatives, an effect scaling procedure was utilized, wherein the unit dose of each derivative was increased until greater than 10% lethality was achieved (Fantegrossi et al. 2008; Wang et al. 2004). Finally, correlation analysis was undertaken to determine whether any significant changes in the tissue concentrations of the monoamine neurotransmitters and their primary metabolites predicted deficits in PA performance. The specific hypothesis tested was whether exposure to these amphetamine derivatives would deplete dopamine levels and concomitantly impair PA performance.
Materials and Methods
Animals
Male Swiss Webster mice (Charles River Laboratories, Inc., Wilmington, MA) aged 6 to 10 weeks served as subjects. Animals were housed 2 or 3 per cage in a temperature-controlled room. Animals had access to food (Laboratory Rodent Diet #5001, PMI Feeds, Inc., St. Louis, MO, USA) and water ad libitum. All studies were carried out in accordance with the Guide for Care and Use of Laboratory animals as adopted and promulgated by the National Institutes of Health, and experimental protocols were approved by the Institutional Animal Care and Use Committee at Emory University.
Drugs
S,R(+/-)-MDMA and S,R(+/-)-METH were supplied by the National Institute on Drug Abuse (Research Technology Branch, Research Triangle Park, NC, USA). Chemicals and reagents used for in vitro assays and PCA were commercially purchased (Sigma, St. Louis, MO, USA or ESA Biosciences, Chelmsford, MA, USA). All injections were administered intraperitoneally at a volume of 1 ml/100 g.
Procedure
Dosing regimen
All drugs were administered 4 times with 2 h separating each administration. Amphetamine derivatives have been previously shown to induce persistent neurochemical depletions and terminal degenerations in the mouse when administered using this dosing regimen (Miller and O'Callaghan 1995; O'Callaghan and Miller 1994). All treatments are described as the unit dose per administration. To achieve near maximal toxicity and to achieve equivalence of physiological effects across the three different drugs, we increased the unit dose of each drug until a dosing regimen that produced greater than 10% lethality was found in the subjects that underwent PA testing and whose brains were extracted for neurochemical analysis. The unit doses were 10 (n = 5) or 20 (n = 7) mg/kg/inj for MDMA, 10 (n = 7) or 20 (n = 7) mg/kg/inj for METH, and 5 (n = 5), 7.5 (n = 5), or 10 (n = 7) mg/kg/inj for PCA, and these dosing regimens were compared to an identically conducted saline regimen (n = 10). In separate subjects, we verified that MDMA (n = 7), METH (n = 7), and PCA (n = 8) were eliciting equivalent physiological effects at their respective maximal dosing regimens by comparing their effects on body weight and rectal temperature to one another and to saline (n = 8). Equilibrating drug dosing based on physiological effects has been previously described to be an effective means to correct for potency and pharmacokinetic differences between drugs when comparing drug-induced changes in brain chemistry or behavior (Fantegrossi et al. 2008; Wang et al. 2004).
Passive avoidance
Passive avoidance procedures were carried out in a custom built step-through inhibitory avoidance apparatus. The “light” compartment had an open top, a textured floor, and the walls were constructed from clear Lexan. The “dark” compartment had a closed top, the walls and floor were constructed from opaque Lexan, and the floor was covered by a crossbeam section of metal rods spaced 0.5 cm apart. The metal crossbeams were connected in series to an ENV-410B shock generator (Med Associates, St. Albans, VT, USA) that was set to produce a current of 0.3 mA. The two compartments of the chamber were separated by a sliding guillotine door. Two days after receiving a dosing regimen, the subjects underwent a PA training session. On the training day, the subject was placed directly into the light compartment and confined to this side for 30 seconds. Next, the dividing door was raised and the animal was allowed 600 seconds to enter the dark compartment. A dark compartment entry (i.e. a step-through) was operationally defined as placing all four paws on the metal crossbeams. Immediately following a step-through, the subject was confined to the dark compartment. Thirty seconds later, a series of 3 square wave electrical stimuli were applied through the metal crossbeams at an amplitude of 0.3 mA for a 2 second duration, with each stimulus separated by 15 seconds. Fifteen seconds after the termination of the final stimulus in the series, each subject was returned to the box in which it was housed. The time from the opening of the guillotine door to the entry of the mouse into the dark compartment was recorded as the step-through latency of that subject. Two days later and 4 d after its dosing regimen, PA retention was determined in each subject by recording its step-through latency. In this retention session, each subject was returned to the box in which it was housed 600 seconds after the door opened, regardless of whether it crossed into the dark compartment or not. The next day, each mouse was euthanized for neurochemical analysis following the procedures described below. The times chosen for PA training and testing were based on previous work examining the persistent neurochemical effects of these derivatives (Ali et al. 1994; Colado et al. 2004; Kita et al. 2003; O'Callaghan and Miller 1994; Stone et al. 1987), and the effectiveness of these procedures were initially established in preliminary experiments with untreated animals.
Brain dissection
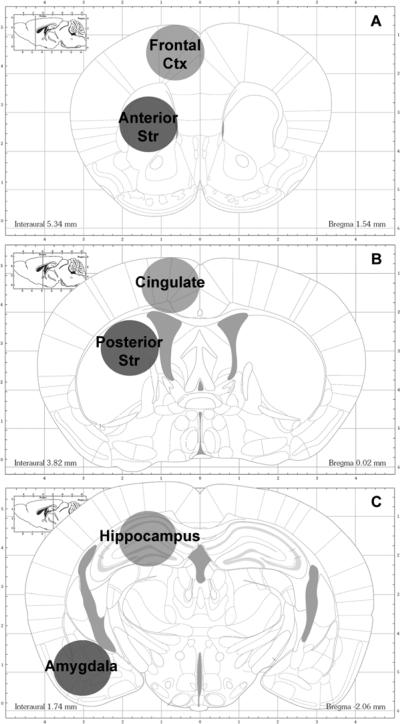
Five days after completion of the dosing regimen and 1 day after completion of the PA retention test, mice were euthanized by cervical dislocation and decapitation. Brains were rapidly removed on ice and cryogenically stored at -80°C for subsequent analysis. Whole frozen brains were shipped on dry ice (solid CO2) to Wayne State University School of Medicine for neurochemical analysis. To obtain region specific tissue samples, brains were thawed at 4°C and placed in an ice-cold mouse brain matrix. Brains were sliced into 2 mm thick coronal sections, and these slices were placed flat on a block of dry ice. Using a 1.5 mm diameter tissue biopsy-punch, tissue samples were taken from individual slices containing regions of interest. An illustration of the anatomical localization of the regions examined (anterior and posterior striatum, frontal and cingulate cortex, hippocampus, and amygdala) overlaid on sections reproduced from Paxinos and Franklin (2001) is presented in Figure 1.
Figure 1.
An illustration of the approximate anatomical localization of regions removed for neurochemical analysis overlaid on coronal sections reproduced from Paxinos and Franklin (2001). A. Coronal section at 1.54 mm anterior to Bregma showing the localization of the frontal cortex (light gray circle) and anterior striatum (dark gray circle). B. Coronal section at 0.02 mm anterior to Bregma showing the localization of the cingulate (light gray circle) and posterior striatum (dark gray circle). C. Coronal section at 2.06 mm posterior to Bregma showing the localization of the hippocampus (light gray circle) and amygdala (dark gray circle).
Neurochemical measurements
Frozen tissues were weighed, sonically disrupted in 200 μl of 200 mM HClO4 and centrifuged for 5 minutes at 4°C to remove cellular debris. A 100 μl aliquot of the supernatant was placed in an ESA 542 auto injector maintained at 4°C and 10 μl injected onto a C18-RP column (30°C) with ESA MD-TM mobile phase running at a flow rate of 0.6 ml/min. Coulometric detection was accomplished with an ESA 5011A dual electrode cell and the signal analyzed on an EZ Chrome Elite data processing platform. Absolute tissue concentrations (ng/mg) for the monoamine neurotransmitters dopamine, serotonin, and norepinephrine were determined by comparison with external standard curves and corrected for tissue weight. Identical procedures were used to quantify the tissue concentrations of the primary metabolites of dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), and the primary metabolite of serotonin, 5-hydroxyindoleacetic acid (5-HIAA).
Body weight and temperature
In separate subjects, the physiological equivalence of each drug at its maximum dosing regimen was verified by determining their effects on body weight and rectal temperature. Each metric was recorded immediately prior to each injection. Temperature was measured by inserting a lubricated probe 1.5 cm into the rectum and recording the readout from a connected TH-8 Thermalert temperature monitor (Physitemp Instruments, Clifton, NJ, USA) after the signal reached steady state.
Data analysis
All graphical data presentations were created using GraphPad Prism 4 (GraphPad Software Inc., La Jolla, CA, USA). Pearson correlation analysis determined the relationship, within subject, between significant changes in neurochemistry and behavior. Stepwise multiple linear regression analysis (SPSS 17; SPSS Inc., Chicago, Il, USA) determined the set of neurochemical changes that was the strongest predictor of PA performance. A Bonferroni method was used to correct for multiple comparisons by dividing the alpha value by the number of correlations. Treatment effect data were assessed via a one-way analysis of variance (ANOVA) with correction for multiple comparisons utilizing appropriate post-hoc analyses (SigmaStat 3; Systat Software, San Jose, CA, USA) to maintain the probability of making a type 1 error at 5%.
Results
Passive avoidance
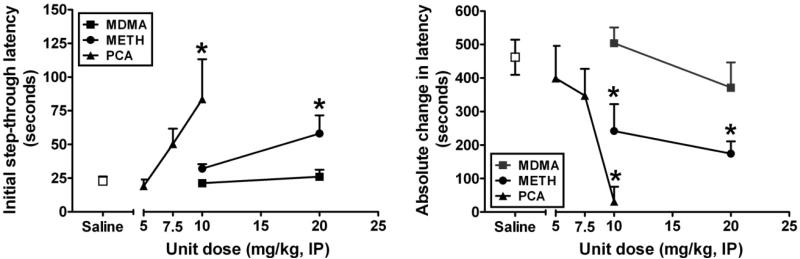
There was a significant main effect of treatment (F45,7 = 3.101; p < 0.009) on baseline step-through latencies (Figure 2, left). The power of this test was 0.738 and the effect size (eta-squared; η2) was 0.325. Post-hoc analysis revealed that PCA (q = 3.590) at a unit dose of 10 mg/kg/injection and METH (q = 2.168) at a unit dose of 20 mg/kg/injection were significantly different from saline. Because there were significant differences in baseline step-through latencies, the test session retention data were normalized for each subject to its own baseline latency by calculating the absolute change in step-through latency from the training day to the test day (post training) (Figure 2, right). There was a significant main effect of treatment (F45,7 = 4.041; p = 0.002) on this measure. The power of this test was 0.905 and the effect size was 0.386. Post-hoc analysis revealed that PCA was significantly different from saline at a unit dose of 10 mg/kg/injection (q = 4.296) whereas METH was significantly different at both 10 (q = 2.543) and 20 mg/kg/injection (q = 3.040). Exposure to MDMA had no significant effect on baseline or post-training step-through latencies.
Figure 2.
Effects of MDMA (closed squares), METH (closed circles), and PCA (closed triangles) in comparison to saline (open squares) on passive avoidance behavior. All points represent the mean ± SEM. Abscissae: Unit dose of each dosing regimen expressed as milligrams of drug / kilogram of body weight and plotted on a linear scale. Ordinates: Initial step-through latency (left) or the absolute change in step-though latency from the training session to the test session (right) expressed in seconds and plotted on a linear scale. An * indicates a significant difference (p < 0.05) from saline treatment assessed via a one-way repeated measures analysis of variance with post-hoc analysis carried out using Dunnett's test.
Neurochemistry
The results of the neurochemical analysis for the monoamine neurotransmitters are summarized in Table 1. The main effect of treatment was assessed via a one-way ANOVA. Post-hoc analysis was used to determine which unit doses of a given drug were significantly different from saline treatment via Dunnett's method. In this way, each treatment was assessed for 18 different main effects (3 neurotransmitters × 6 brain regions), and Bonferroni correction for multiple comparisons therefore required that each individual main effect was greater than p < 0.003 to achieve significance. Under the procedures employed, tissue concentrations of dopamine were significantly decreased in the anterior striatum (F44,7 = 10.052; p < 0.001) and posterior striatum (F42,7 = 13.250; p < 0.001). The power of each of these tests was 1.000 and their effects sizes were 0.615 and 0.688, respectively. In the anterior striatum, post-hoc analysis revealed that there was a significant difference (p < 0.050) compared to saline treatment of METH at both 10 (q = 4.444) and 20 (q = 4.612) mg/kg/injection and PCA at 10 (q = 4.632) mg/kg/injection. Neither dosing regimen of MDMA was significantly different from saline. In the posterior striatum, post-hoc analysis revealed that there was a significant difference compared to saline treatment of MDMA at 20 (q = 3.880), METH at both 10 (q = 6.423) and 20 (q = 7.230), and PCA at 10 (q = 5.586) mg/kg/injection. Tissue concentrations of dopamine are not reported in any other brain region as they were below the limit of detection in the saline control group.
Table 1.
Tissue concentrations of dopamine, serotonin, and norepinephrine in the anterior striatum and posterior striatum and tissue concentrations of serotonin and norepinephrine in the frontal cortex, cingulate, hippocampus, and amygdala.
| Anterior str | Posterior str | Frontal ctx | Cing | HC | Amyg | |
|---|---|---|---|---|---|---|
| Dopamine | ||||||
| Saline | 7.17 (0.98) | 7.98 (0.52) | ||||
| MDMA 10 | 5.03 (0.77) | 7.38 (2.80) | ||||
| MDMA 20 | 5.46 (1.53) | 3.80 (0.75)* | ||||
| METH 10 | 1.57 (0.59)* | 1.07 (0.30)* | ||||
| METH 20 | 1.15 (0.29)* | 0.83 (0.18)* | ||||
| PCA 5 | 9.52 (1.51) | 6.56 (0.62) | ||||
| PCA 7.5 | 7.34 (1.32) | 4.92 (0.84) | ||||
| PCA 10 | 0.84 (0.11)* | 1.60 (0.91)* | ||||
| Serotonin | ||||||
| Saline | 0.51 (0.02) | 0.56 (0.06) | 0.41 (0.03) | 0.31 (0.02) | 0.44 (0.06) | 0.79 (0.06) |
| MDMA 10 | 0.47 (0.07) | 0.43 (0.10) | 0.30 (0.09) | 0.27 (0.07) | 0.58 (0.05) | 0.57 (0.05) |
| MDMA 20 | 0.48 (0.02) | 0.33 (0.04)* | 0.34 (0.05) | 0.21 (0.02)* | 0.38 (0.04) | 0.59 (0.12) |
| METH 10 | 0.44 (0.03) | 0.39 (0.02) | 0.33 (0.02) | 0.24 (0.02) | 0.50 (0.04) | 0.66 (0.09) |
| METH 20 | 0.49 (0.05) | 0.38 (0.03)* | 0.35 (0.04) | 0.17 (0.02)* | 0.36 (0.02) | 0.66 (0.09) |
| PCA 5 | 0.42 (0.05) | 0.47 (0.05) | 0.32 (0.03) | 0.16 (0.01)* | 0.30 (0.02) | 0.66 (0.02) |
| PCA 7.5 | 0.24 (0.01)* | 0.33 (0.07)* | 0.10 (0.01)* | 0.22 (0.02) | ND | 0.29 (0.06)* |
| PCA 10 | 0.17 (0.02)* | 0.13 (0.04)* | 0.08 (0.03)* | 0.14 (0.01)* | 0.24 (0.03)* | 0.13 (0.01)* |
| Norepinephrine | ||||||
| Saline | 0.25 (0.02) | 0.26 (0.06) | 0.40 (0.05) | 0.36 (0.01) | 0.44 (0.03) | 0.38 (0.03) |
| MDMA 10 | 0.21 (0.04) | 0.19 (0.02) | 0.38 (0.02) | 0.23 (0.04)* | 0.45 (0.06) | ND |
| MDMA 20 | 0.33 (0.04) | 0.20 (0.03) | 0.48 (0.04) | 0.42 (0.03) | 0.57 (0.04) | 0.42 (0.02) |
| METH 10 | 0.19 (0.02) | 0.27 (0.03) | 0.71 (0.22) | 0.36 (0.02) | 0.59 (0.05) | 0.33 (0.06) |
| METH 20 | 0.33 (0.02) | 0.32 (0.04) | 0.49 (0.02) | 0.43 (0.03) | 0.56 (0.02) | 0.43 (0.02) |
| PCA 5 | 0.28 (0.06) | 0.35 (0.06) | 0.47 (0.04) | 0.46 (0.03)* | 0.56 (0.03) | ND |
| PCA 7.5 | 0.16 (0.02) | 0.25 (0.03) | 0.20 (0.03) | 0.29 (0.02) | ND | ND |
| PCA 10 | 0.21 (0.04) | 0.32 (0.03) | 0.39 (0.03) | 0.35 (0.02) | 0.55 (0.02) | ND |
Dopamine concentrations in regions outside the striatum are not reported as they were below the limit of detection in the saline control group. Concentrations are expressed as nanograms neurochemical / milligram tissue weight (ng/mg). Each value presented in boldface and followed by an * represents a significant difference (p < 0.05) compared to saline treatment.
Anterior str = anterior striatum; Posterior str = posterior striatum; Frontal ctx = frontal cortex; Cing = cingulate; HC= hippocampus; Amyg = amygdala; ND = data not determined.
Tissue concentrations of serotonin were significantly decreased in the anterior striatum (F44,7 = 12.942; p < 0.001), posterior striatum (F42,7 = 5.162; p < 0.001), frontal cortex (F39,7 = 8.507; p < 0.001), cingulate (F44,7 = 5.324; p < 0.001), hippocampus (F40,7 = 7.146; p < 0.001), and amygdala (F41,7 = 8.024; p < 0.001). The power of each of these tests was 1.000 and their effects sizes were respectively 0.673, 0.462, 0.604, 0.458, 0.517, and 0.517. Post-hoc analysis revealed that MDMA significantly decreased serotonin concentrations in the posterior striatum (q = 3.167) and cingulate (q = 2.867) at 20 mg/kg/injection. METH also significantly decreased serotonin concentrations in the posterior striatum (q = 2.787) and cingulate (q = 3.783) at 20 mg/kg/injection. PCA significantly decreased serotonin concentrations in the anterior striatum at 7.5 (q = 5.804) and 10 (q = 7.334), in the posterior striatum at 7.5 (q = 3.063) and 10 (q = 5.599), in the frontal cortex at 7.5 (q = 5.836) and 10 (q = 5.671), in the cingulate at 5 (q = 4.205) and 10 (q = 4.957), in the hippocampus at 10 (q = 3.526), and in the amygdala at 7.5 (q = 5.061) and 10 (q = 6.240) mg/kg/injection.
Tissue concentrations of norepinephrine were only significantly altered in the cingulate (F44,7 = 8.761; p < 0.001). The power of this test was 1.000 and the effect size was 0.584. In this region, post-hoc analysis showed that MDMA significantly decreased norepinephrine at 10 mg/kg/injection (q = 4.095) whereas PCA significantly increased the concentration of norepinephrine at 5 mg/kg/injection (q = 3.129).
The results of the neurochemical analysis for the metabolites examined are summarized in Table 2. The main effect of treatment was assessed via a one-way ANOVA. Post-hoc analysis was used to determine which unit doses of a given drug were significantly different from saline treatment via Dunnett's method. In this way, each treatment was assessed for 18 different main effects (3 metabolites × 6 brain regions), and therefore Bonferroni correction for multiple comparisons required that each individual main effect was greater than p < 0.003 to achieve significance. Under the procedures employed, tissue concentrations of DOPAC were significantly decreased in the anterior striatum (F44,7 = 8.776; p < 0.001) and posterior striatum (F42,7 = 18.196; p < 0.001). The power of each of these tests was 1.000 and their effect sizes were 0.583 and 0.752, respectively. In the anterior striatum, post-hoc analysis revealed that there was a significant difference (p < 0.050) compared to saline treatment of METH at both 10 (q = 3.740) and 20 (q = 4.041) mg/kg/injection and PCA at 10 (q = 4.133) mg/kg/injection. Neither dosing regimen of MDMA was significantly different from saline. In the posterior striatum, post-hoc analysis revealed that there was a significant difference compared to saline treatment of MDMA at 20 (q = 3.125), METH at both 10 (q = 6.948) and 20 (q = 7.333), and PCA at 10 (q = 5.325) mg/kg/injection. Tissue concentrations of DOPAC are not reported in any other brain region as they were below the limit of detection in the saline control group.
Table 2.
Tissue concentrations of DOPAC, HVA, and 5-HIAA in the anterior striatum and posterior striatum and tissue concentrations of 5-HIAA in the frontal cortex, cingulate, hippocampus, and amygdala.
| Anterior str | Posterior str | Frontal ctx | Cing | HC | Amyg | |
|---|---|---|---|---|---|---|
| DOPAC | ||||||
| Saline | 0.93 (0.10) | 0.83 (0.04) | ||||
| MDMA 10 | 0.76 (0.13) | 0.94 (0.08) | ||||
| MDMA 20 | 0.79 (0.19) | 0.53 (0.08)* | ||||
| METH 10 | 0.39 (0.10)* | 0.17 (0.02)* | ||||
| METH 20 | 0.32 (0.05)* | 0.19 (0.03)* | ||||
| PCA 5 | 1.33 (0.15) | 0.89 (0.03) | ||||
| PCA 7.5 | 0.87 (0.11) | 0.88 (0.02) | ||||
| PCA 10 | 0.84 (0.11)* | 0.29 (0.07)* | ||||
| HVA | ||||||
| Saline | 0.58 (0.08) | 0.65 (0.04) | ||||
| MDMA 10 | 0.46 (0.06) | 0.68 (0.20) | ||||
| MDMA 20 | 0.72 (0.17) | 0.57 (0.05) | ||||
| METH 10 | 0.38 (0.04) | 0.24 (0.03)* | ||||
| METH 20 | 0.48 (0.06) | 0.26 (0.05)* | ||||
| PCA 5 | 1.07 (0.14) | 0.77 (0.05) | ||||
| PCA 7.5 | 0.62 (0.08) | 0.56 (0.05) | ||||
| PCA 10 | 0.30 (0.04) | 0.34 (0.08)* | ||||
| 5-HIAA | ||||||
| Saline | 0.35 (0.02) | 0.41 (0.02) | 0.31 (0.02) | 0.24 (0.02) | 0.41 (0.06) | 0.36 (0.02) |
| MDMA 10 | 0.30 (0.03) | 0.36 (0.06) | 0.25 (0.06) | 0.20 (0.05) | 0.47 (0.04) | 0.32 (0.01) |
| MDMA 20 | 0.40 (0.03) | 0.35 (0.02) | 0.24 (0.02) | 0.17 (0.02) | 0.43 (0.10) | 0.38 (0.06) |
| METH 10 | 0.37 (0.04) | 0.40 (0.04) | 0.23 (0.03) | 0.19 (0.01) | 0.50 (0.03) | 0.37 (0.02) |
| METH 20 | 0.39 (0.02) | 0.32 (0.02) | 0.19 (0.01)* | 0.13 (0.01)* | 0.37 (0.02) | 0.29 (0.02) |
| PCA 5 | 0.41 (0.04) | 0.44 (0.02) | 0.20 (0.01)* | 0.15 (0.01)* | 0.43 (0.04) | 0.42 (0.02) |
| PCA 7.5 | 0.20 (0.01)* | 0.38 (0.08) | 0.13 (0.02)* | 0.13 (0.02)* | ND | 0.22 (0.03)* |
| PCA 10 | 0.14 (0.01)* | 0.20 (0.02)* | 0.08 (0.02)* | 0.08 (0.01)* | 0.22 (0.02) | 0.08 (0.01)* |
Concentrations of DOPAC and HVA in regions outside the striatum are not reported as they were below the limit of detection in the saline control group. Concentrations are expressed as nanograms neurochemical / milligram tissue weight (ng/mg). Each value presented in boldface and followed by an * represents a significant difference (p < 0.05) compared to saline treatment.
Anterior str = anterior striatum; Posterior str = posterior striatum; Frontal ctx = frontal cortex; Cing = cingulate; HC= hippocampus; Amyg = amygdala; DOPAC = 3,4-Dihydroxyphenylacetic acid; HVA = Homovanillic acid; 5-HIAA = 5-Hydroxyindoleacetic acid; ND = data not determined.
Tissue concentrations of HVA were significantly decreased in the posterior striatum (F42,7 = 9.856; p < 0.001). The power of this test was 1.000 and its effect size was 0.622. Post-hoc analysis revealed that METH at 10 (q = 4.832) and 20 (q = 5.060) mg/kg/injection and PCA at 10 (q = 3.534) mg/kg/injection significantly decreased HVA concentrations in the posterior striatum. In contrast, there was no significant main effect of the treatments on HVA levels in the anterior striatum. Tissue concentrations of HVA are not reported in any other brain region as they were below the limit of detection in the saline control group.
Tissue concentrations of 5-HIAA were significantly decreased in the anterior striatum (F44,7 = 12.462; p < 0.001), posterior striatum (F42,7 = 4.155; p < 0.001), frontal cortex (F39,7 = 7.212; p < 0.001), cingulate (F44,7 = 7.147; p < 0.001), and amygdala (F41,7 = 13.361; p < 0.001). The powers of these tests were respectively 1.000, 0.913, 0.999, 0.999, and 1.000, and their effect sizes were respectively 0.664, 0.410, 0.415, 0.532, and 0.517. Post-hoc analysis revealed that METH significantly decreased 5-HIAA concentrations in the frontal cortex (q = 3.407) and cingulate (q = 3.932) at 20 mg/kg/injection. PCA significantly decreased 5-HIAA concentrations in the anterior striatum at 7.5 (q = 4.054) and 10 (q = 5.656), in the posterior striatum at 10 (q = 4.434), in the frontal cortex at 5 (q = 3.216), 7.5 (q = 4.948) and 10 (q = 6.172), in the cingulate at 5 (q = 3.215), 7.5 (q = 3.739) and 10 (q = 6.435), and in the amygdala at 7.5 (q = 3.887) and 10 (q = 7.530) mg/kg/injection. Post-hoc analysis did not find any significant effects of treatment with MDMA on 5-HIAA concentrations in the brain areas examined.
Effects on body weight and temperature
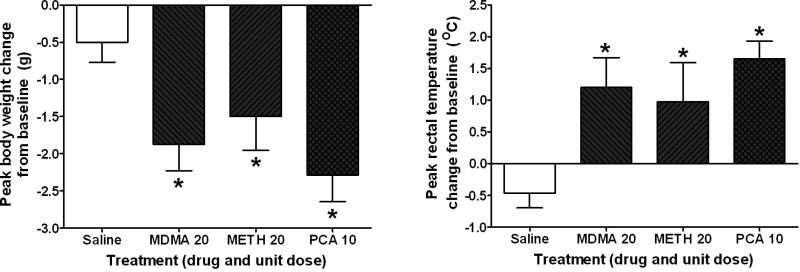
To determine the equivalence of each derivative using the employed effect scaling procedure, changes in body weight (Figure 3, left) and rectal temperature (Figure 3, right) at the maximum dosing regimen of each derivative were recorded and analyzed in separate groups of animals to those that underwent PA and whose brains were collected for neurochemical analysis. The peak absolute change on body weight (Figure 3, left) and rectal temperature (Figure 3, right) engendered by each drug was determined by averaging the maximum change from baseline in each subject across the entire dosing regimen, regardless of the time point at which the maximum change occurred. Under the procedures employed, there were significant main effects of treatment on body weight (F29,3 = 5.161; p = 0.006) and rectal temperature (F26,3 = 5.082; p = 0.007). The powers of these tests were 0.805 and 0.789, respectively, and their effect sizes were 0.348 and 0.340, respectively. Post-hoc analysis showed that treatment with MDMA (q = 4.113; p = 0.018), METH (q = 2.992; p = 0.043), or PCA (q = 5.142; p = 0.006) significantly decreased body weight compared to saline. None of these treatments was significantly different from one another. Furthermore, the peak change in rectal temperature was significantly different from saline for MDMA (q = 4.604; p = 0.009), METH (q = 3.241; p = 0.030), and PCA (q = 4.841; p = 0.011) and none of these treatments was significantly different from one another. Mean basal body weight, prior to the first injection, for the groups treated with saline, MDMA, METH, and PCA were 26.80 ± 0.49, 28.25 ± 0.51, 27.63 ± 0.64, and 27.43 ± 1.07 grams, respectively. Mean basal rectal temperature for the groups treated with saline, MDMA, METH, and PCA were 37.83 ± 0.26, 37.31 ± 0.17, 37.80 ± 0.17, and 37.91 ± 0.16 °C, respectively. The time courses of drug-induced changes in body weight were similar across all three drugs. Exposure to PCA resulted in a slower onset to peak rectal temperature change than exposure to METH or MDMA (Supplementary Figure 1). However, as described, the peak change was not significantly different between the three amphetamine derivatives.
Figure 3.
Peak absolute change in body weight (left) or rectal temperature (right) measured over the six hour dosing regimen at the maximum unit dosage used for each drug, regardless of the time point at which the peak change occurred. Abscissae: Drug treatment and the unit dose of the dosing regimen for that treatment. Ordinates: Peak absolute change in body weight (left) or rectal temperature (right) measured in grams or degrees Celsius, respectively, and plotted on a linear scale. Values are normalized to the baseline value for each subject. An * indicates a significant difference (p < 0.05) from saline treatment.
Correlation analysis
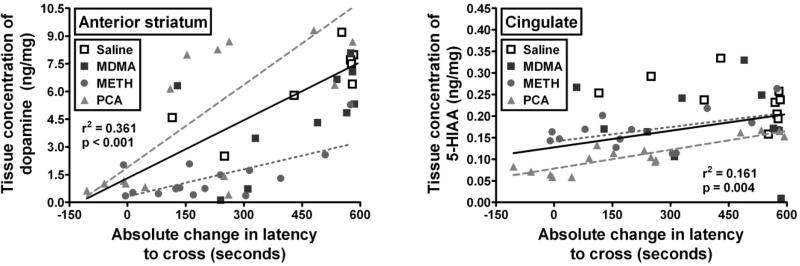
Correlation analysis was used to determine the relationship between changes in neurochemistry and PA behavior. Correlations were determined between PA behavior and the concentrations of any monoamine neurotransmitter within a brain region that was significantly depleted by both METH and PCA treatments. Therefore, 4 correlations were calculated between neurotransmitter depletions (anterior striatum dopamine, posterior striatum dopamine, posterior striatum serotonin, and cingulate serotonin) and both the step-through latencies of the mice in the training session and the absolute changes in step-through latency from the training to the test sessions. Since 4 correlations were calculated for each behavioral endpoint, Bonferroni correction for multiple comparisons required the alpha for each correlation to be greater than p < 0.013 to achieve significance. Using these procedures, a significant relationship across all treatments was found (r2 = 0.361; p < 0.001) between tissue concentration of dopamine in the anterior striatum and the absolute changes in step-through latency from the training to the test sessions (Figure 4, left). Furthermore, the correlations within just the PCA (r2 = 0.608; p < 0.001) and METH (r2 = 0.457; p = 0.006) treated groups were also significant, whereas the correlations were not significant within the saline (r2 = 0.484; p = 0.037) and MDMA (r2 = 0.001; p = 0.921) treated groups. There was no significant relationship across treatment between this measure and tissue concentration of dopamine in the posterior striatum, serotonin in the posterior striatum, or serotonin in the cingulate. No significant relationship was found between these neurotransmitter concentrations and the latency to cross in the training session (data not shown).
Figure 4.
Correlation between the absolute change in step-through latency from the training session to the test session in the passive avoidance assay and tissue concentration of dopamine in the anterior striatum (left) or 5-HIAA in the cingulate (right) for MDMA (black closed squares), METH (gray closed circles), PCA (light gray closed triangles), and saline (open squares) treated subjects. Each data point represents values from a single subject. Best fit regression lines are overlaid for all subjects (black solid line) and subjects treated with a dosing regimen of PCA (gray dashed line) or METH (gray dotted line). Abscissae: The absolute change in step-though latency from the training session to the test session expressed in seconds and plotted on a linear scale. Ordinates: Tissue concentration of dopamine (left) or 5-HIAA (right) expressed as nanograms neurochemical / milligram tissue weight (ng/mg) and plotted on a linear scale.
Correlations were also determined between PA behavior and the concentrations of any metabolite within a brain region that was significantly depleted by both METH and PCA treatments. Therefore, 5 correlations were calculated between metabolite depletions (anterior striatum DOPAC, posterior striatum DOPAC, posterior striatum HVA, frontal cortex 5-HIAA, and cingulate 5-HIAA) and both the step-through latencies of the mice in the training session and the absolute changes in step-through latency from the training to the test sessions. Since 5 correlations were calculated for each behavioral endpoint, Bonferroni correction for multiple comparisons required the alpha for each correlation to be greater than p < 0.010 to achieve significance. Using these procedures, a significant relationship across all treatments was found (r2 = 0.347; p < 0.001) between the tissue concentrations of DOPAC in the anterior striatum and the absolute changes in step-through latency from the training to the test sessions. Furthermore, the correlations within just the PCA (r2 = 0.591; p < 0.001) and METH (r2 = 0.525; p = 0.005) treated groups were also significant, whereas the correlations were not significant within the saline (r2 = 0.139; p = 0.291) and MDMA (r2 = 0.210; p = 0.183) treated groups. There was a significant correlation across all of the subjects between this measure and the tissue concentrations of DOPAC (r2 = 0.324; p < 0.001) and HVA (r2 = 0.228; p < 0.001) in the posterior striatum, but none of the correlations within any of groups treated with one of the drugs or saline was significant. A significant relationship across all treatments was found (r2 = 0.2145; p = 0.001) between tissue concentrations of 5-HIAA in the frontal cortex and the absolute changes in step-through latency from the training to the test sessions. The correlations within the PCA (r2 = 0.439; p = 0.005) and METH (r2 = 0.505; p = 0.006) treated groups but not the saline (r2 = 0.325; p = 0.181) or MDMA (r2 = 0.007; p = 0.822) treated groups were significant. A significant relationship across all treatments was found (r2 = 0.161; p = 0.004) between tissue concentration of 5-HIAA in the cingulate and the absolute changes in step-through latency from the training to the test sessions (Figure 4, right). The correlation within the PCA (r2 = 0.774; p < 0.001) treated group was significant, but the correlations within the METH (r2 = 0.246; p = 0.085), saline (r2 = 0.216; p = 0.176), and MDMA (r2 = 0.048; p = 0.519) treated groups were not significant. No significant relationship was found between these metabolite concentrations and the latencies of the mice to cross in the training session (data not shown).
Stepwise multiple regression analysis showed that a model that included the tissue concentration of dopamine in the anterior striatum and the tissue concentration of 5-HIAA in the cingulate as coefficients significantly predicted PA performance as indexed by the absolute changes in step-through latency from the training to the test sessions (r2 = 0.421; r2adj = 0.400; F56,2 = 20.019; p <0.001). Moreover, while the tissue concentration of dopamine in the anterior striatum was the strongest predictor of PA performance (β = 0.476; p < 0.001), the addition of the tissue concentrations of 5-HIAA in the cingulate significantly increased (F Change55,1 = 9.089; p = 0.004). In contrast, addition of the values for the tissue concentrations of dopamine in the posterior striatum, serotonin in the posterior striatum and cingulate, DOPAC in the anterior and posterior striatum, HVA in the posterior striatum, and 5-HIAA in the frontal cortex did not increase how well the multiple regression model predicted PA performance (Table 3).
Table 3.
Summary, coefficients, and excluded variables for the multiple regression analysis model.
| Model summary | R2 | Adjusted R2 | F | p |
|---|---|---|---|---|
| .421 | .400 | 20.019 | <0.001 |
| Coefficients | β | t | B | p |
|---|---|---|---|---|
| DA – Anterior striatum | 0.476 | 4.437 | 33.642 | <0.001 |
| 5-HIAA – Cingulate | 0.323 | 3.015 | 28.063 | 0.004 |
| Excluded variables | β | t | Tolerance | p |
|---|---|---|---|---|
| DA - Posterior striatum | 0.155 | 1.164 | 0.592 | 0.249 |
| 5-HT - Posterior striatum | 0.103 | 0.864 | 0.750 | 0.392 |
| 5-HT - Cingulate | 0.039 | 0.243 | 0.425 | 0.809 |
| DOPAC - Anterior striatum | 0.165 | 0.409 | 0.066 | 0.684 |
| DOPAC - Posterior striatum | 0.195 | 1.484 | 0.599 | 0.144 |
| HVA - Posterior striatum | 0.096 | 0.753 | 0.646 | 0.455 |
| 5-HIAA – Frontal cortex | 0.126 | 1.099 | 0.796 | 0.277 |
Discussion
In the present study, all three amphetamines tested effectively depleted tissue concentrations of both dopamine and serotonin. Some previous studies in the mouse indicate that PCA selectively induces depletions of serotonin (Sanders-Bush et al. 1975; Steranka et al. 1977; Steranka and Sanders-Bush 1980). However, more recent studies indicate that the hyperthermic effects of PCA are mediated by dopaminergic action (Sugimoto et al. 2001), and dosing regimens of PCA can lead to depletion of markers for (Itzhak et al. 2004) and tissue concentrations of (Saadat et al. 2006a) both serotonin and dopamine. Studies of the persistent effects of MDMA and METH in the mouse have mostly shown selective dopaminergic effects (O'Callaghan and Miller 1994; Stone et al. 1987); however, serotonergic effects have also been reported (Hirata et al. 1995; Renoir et al. 2008). It is unclear why these discrepancies across studies arise but they may be related to experimental conditions such as environmental factors, drug dosage, dose scheduling, or strain differences. Nevertheless, the consistent pattern across these previous studies and the present report is that, although each derivative can produce selective effects, there are conditions under which all three derivatives can affect either neurotransmitter in the mouse.
Although all three amphetamines were capable of depleting dopamine and serotonin levels in some of the brain regions examined, their effects were not identical, and they exhibited different patterns of effects on the regional concentrations of the major metabolites of these neurotransmitters. In this regard, PCA appears to have a more robust capacity for depleting serotonin than either MDMA or METH as it significantly decreased serotonin levels in all 6 brain regions examined, whereas MDMA and METH significantly decreased serotonin levels in only the posterior striatum and the cingulate. The dopamine depleting effects of these compounds also exhibited some specificity. Specifically, although all three derivates significantly decreased dopamine levels in the posterior striatum, only METH and PCA significantly decreased dopamine levels in the anterior striatum. Moreover, while all three derivatives engendered qualitatively similar depletions of the dopamine metabolite DOPAC in the posterior striatum, only METH and PCA significantly depleted DOPAC levels in the anterior striatum or HVA levels in the posterior striatum. Similarly, only METH and PCA significantly depleted tissue levels of the serotonin metabolite 5-HIAA in the frontal cortex or in the cingulate. As relatively little is known about the pharmacodynamic effects of PCA, it is difficult to know what pharmacological targets are mediating these differences in neurochemical effects. Nevertheless, the differential effects on neurochemistry of the amphetamines utilized in this study do not appear to be related to potency or pharmacokinetic differences as an effect scaling procedure (Fantegrossi et al. 2008; Wang et al. 2004) was used to control for these variables. As such, we propose that it is possible that potency differences at the monoamine transporters are not responsible for the differential neurochemical effects of these amphetamines, and determining the pharmacological effects of these compounds at other targets may help to explain their differential effects on neurochemistry.
The differential capacities of MDMA, METH, and PCA to impair PA performance may be related to their differential effects on neurochemistry. In this regard, exposure to METH and PCA impaired PA performance and engendered a specific set of neurochemical depletions, whereas exposure to MDMA neither engendered some of these neurochemical depletions nor impaired PA performance. For example, while MDMA depleted tissue content of dopamine in only the posterior striatum, METH and PCA depleted tissue content of dopamine in both the anterior and the posterior striatum. Importantly, not only did within subject correlation analysis show that anterior striatal dopamine levels predict PA performance, but stepwise multiple linear regression analysis showed that the level of dopamine in the anterior striatum was the strongest predictor of PA performance among all of the regional neurochemicals that were selectively depleted by both METH and PCA. Consistent with previous reports that PA behavior is independent of tissue levels of serotonin (Barrionuevo et al. 2000; Myhrer 2003; Santucci et al. 1996), serotonin levels were not selectively depleted by METH and PCA in any brain region. However, the tissue concentrations of the serotonin metabolite 5-HIAA in the cingulate were depleted by only METH and PCA, and addition of the tissue concentrations of 5-HIAA in the cingulate significantly enhanced the capacity of the multi regression model to predict PA performance. This work supports previous findings that PA may be mediated via dopaminergic mechanisms (Adriani et al. 1998), extends those findings by showing that there may be subregional specificity in the striatum underlying this learning and memory process, and indicates that other dopamine dependent behaviors may be impaired by amphetamine derivative exposure. Moreover, this work indicates that serotonergic systems may also have a modulatory role in PA behavior and that PA behavior may be influenced by interactions between the cingulate and the striatum.
The finding that exposure to METH and PCA impairs PA performance is consistent with the notion that exposure to these drugs impairs learning and memory. However, because we administered each amphetamine derivative prior to PA training and testing, some alternative interpretations of our results are that the METH- and PCA-treated animals were differentially sensitive to the training stimulus or that METH and PCA exposure engendered anxiogenic- or anxiolytic-like effects. Indeed, exposure to METH and PCA did alter the initial latencies of the mice to cross prior to training. Moreover, this design allowed us to potentially assess the effects of the drugs examined on overall learning and memory, but it did not allow us to determine whether any impairments observed represent selective effects on learning, selective effects on memory, or combined effects on both. Future studies should be designed to compare the effects of METH and PCA treatments when they are administered before the training session, shortly after the training session, or before the retention test because these experiments would begin to elucidate whether METH- and PCA-induced behavioral and cognitive deficits represent selective deficits in learning and memory.
Although it is difficult to make direct comparisons between cognitive processes in humans and laboratory animals, the results of this study appear to have relevance for human addicts of amphetamines. Some of the cognitive deficits in METH addicts that have the strongest support are deficits in information processing speed, attention, learning, memory, reaction times, and executive functions (Kalechstein et al. 2003; Simon et al. 2010). Indeed, a meta-analysis of the literature showed that the largest effects sizes in METH addicts were for deficits in executive functions, learning, and memory (Scott et al. 2007). Although PA may have little relevance for deficits in executive functions, it provides preclinical assessments of deficits in learning and memory. Given the consistency between the effects reported in this study and the deficits that have been described in human METH addicts, the continued use of the PA assay may allow us to determine the factors that influence some of the specific deficits that occur in this clinical population and allow us to study treatments that may reverse these deficits. It is important to note, however, that deficits in learning and memory have also been reported in MDMA abusers (Kalechstein et al. 2007), and the results of the present study are not consistent with those findings. This lack of consistency may be a result of the complexity of the terms learning and memory, as these concepts encompass a broad range of processes, and the possibility that each amphetamine derivative may engender discrete deficits. In this regard, it has been show the MDMA does impair conditioned place aversion induced by lithium chloride (Achat-Mendes et al. 2005), a learning process that is similar but not identical to PA. Similar to the present study, future studies should continue to compare and contrast the discrete neurochemical and cognitive deficits engendered by different amphetamines as this may advance our understanding of the neurobiology of learning and memory and our understanding of the cognitive consequences of exposure to these amphetamines.
In summary, the present study demonstrates that changes in tissue concentrations of dopamine in the anterior striatum strongly predict deficits in PA behavior in the mouse. METH and PCA significantly decreased dopamine in this brain region and concomitantly impaired PA behavior, whereas MDMA did not. Similarly, only METH and PCA significantly decreased 5-HIAA concentrations in the cingulate and depletion of this metabolite of serotonin also was predictive of PA performance. Differences in potency or pharmacokinetics do not appear to account for the differences between the neurochemical and cognitive consequences of exposure to MDMA and exposure to either METH or PCA, as the dosing regimens utilized for each compound were effectively matched using an effect scaling procedure. These studies demonstrate that certain amphetamines impair PA performance in mice and that these impairments may be attributable to specific neurochemical depletions.
Supplementary Material
Supplementary figure 1. Time course of the absolute change in body weight (left) or rectal temperature (right) measured over the six hour dosing regimen at the maximum unit dosage of MDMA (closed squares), METH (closed circles), and PCA (closed triangles) in comparison to saline (open squares). Abscissae: Elapsed experimental time expressed in minutes and plotted on a linear scale Ordinates: Peak absolute change in body weight (left) or rectal temperature (right) measured in grams or degrees Celsius respectively and plotted on a linear scale. Values are normalized to the baseline value for each subject.
Acknowledgments
These studies were funded by the National Institutes of Health [DA024760 (SAP), DA000517 (LLH), DA020645 (WEF)] and by the Yerkes Base Grant [RR00165 (KSM; LLH; WEF)]. Preliminary findings from these experiments were previously presented at the 2009 scientific meetings of the College on Drug Dependence in Reno, NV by KSM and the Society for Neuroscience in Chicago, IL by SAP.
Footnotes
(BJF *Current address*) Department of Clinical Psychology, Uniformed Services University, Bethesda, MD, USA
All authors have no conflicts of interest regarding this work.
Contributor Information
Kevin Sean Murnane, Division of Neuropharmacology and Neurologic Diseases, Yerkes National Primate Research Center, Atlanta, GA USA.
Shane Alan Perrine, Department of Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, Detroit, MI, USA.
Brendan James Finton, Division of Neuropharmacology and Neurologic Diseases, Yerkes National Primate Research Center, Atlanta, GA USA.
Matthew Peter Galloway, Department of Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, Detroit, MI, USA.
Leonard Lee Howell, Division of Neuropharmacology and Neurologic Diseases, Yerkes National Primate Research Center, Atlanta, GA USA; Department of Psychiatry and Behavioral Sciences, Emory University, Atlanta, GA, USA.
William Edward Fantegrossi, Department of Pharmacology and Toxicology, College of Medicine, University of Arkansas for Medical Sciences, Little Rock, AR, USA.
References
- Achat-Mendes C, Ali SF, Itzhak Y. Differential effects of amphetamines-induced neurotoxicity on appetitive and aversive Pavlovian conditioning in mice. Neuropsychopharmacology. 2005;30:1128–37. doi: 10.1038/sj.npp.1300675. [DOI] [PubMed] [Google Scholar]
- Achat-Mendes C, Anderson KL, Itzhak Y. Impairment in consolidation of learned place preference following dopaminergic neurotoxicity in mice is ameliorated by N-acetylcysteine but not D1 and D2 dopamine receptor agonists. Neuropsychopharmacology. 2007;32:531–41. doi: 10.1038/sj.npp.1301119. [DOI] [PubMed] [Google Scholar]
- Adriani W, Felici A, Sargolini F, Roullet P, Usiello A, Oliverio A, Mele A. N-methyl-D-aspartate and dopamine receptor involvement in the modulation of locomotor activity and memory processes. Exp Brain Res. 1998;123:52–9. doi: 10.1007/s002210050544. [DOI] [PubMed] [Google Scholar]
- Ali SF, Newport GD, Holson RR, Slikker W, Jr., Bowyer JF. Low environmental temperatures or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res. 1994;658:33–8. doi: 10.1016/s0006-8993(09)90007-5. [DOI] [PubMed] [Google Scholar]
- Barrionuevo M, Aguirre N, Del Rio JD, Lasheras B. Serotonergic deficits and impaired passive-avoidance learning in rats by MDEA: a comparison with MDMA. Pharmacol Biochem Behav. 2000;65:233–40. doi: 10.1016/s0091-3057(99)00170-7. [DOI] [PubMed] [Google Scholar]
- Belcher AM, Feinstein EM, O'Dell SJ, Marshall JF. Methamphetamine influences on recognition memory: comparison of escalating and single-day dosing regimens. Neuropsychopharmacology. 2008;33:1453–63. doi: 10.1038/sj.npp.1301510. [DOI] [PubMed] [Google Scholar]
- Bolla KI, McCann UD, Ricaurte GA. Memory impairment in abstinent MDMA (“Ecstasy”) users. Neurology. 1998;51:1532–7. doi: 10.1212/wnl.51.6.1532. [DOI] [PubMed] [Google Scholar]
- Buchert R, Obrocki J, Thomasius R, Vaterlein O, Petersen K, Jenicke L, Bohuslavizki KH, Clausen M. Long-term effects of ‘ecstasy’ abuse on the human brain studied by FDG PET. Nucl Med Commun. 2001;22:889–97. doi: 10.1097/00006231-200108000-00007. [DOI] [PubMed] [Google Scholar]
- Colado MI, O'Shea E, Green AR. Acute and long-term effects of MDMA on cerebral dopamine biochemistry and function. Psychopharmacology (Berl) 2004;173:249–63. doi: 10.1007/s00213-004-1788-8. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Ciullo JR, Wakabayashi KT, De La Garza R, 2nd, Traynor JR, Woods JH. A comparison of the physiological, behavioral, neurochemical and microglial effects of methamphetamine and 3,4-methylenedioxymethamphetamine in the mouse. Neuroscience. 2008;151:533–43. doi: 10.1016/j.neuroscience.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RW, Baker JC, Molloy BB. Biological disposition of rigid analogs of amphetamine. J Pharm Sci. 1977;66:271–2. doi: 10.1002/jps.2600660235. [DOI] [PubMed] [Google Scholar]
- Fuller RW, Hines CW, Mills J. Lowering of Brain Serotonin Level by Chloramphetamines. Biochem Pharmacol. 1965;14:483–8. doi: 10.1016/0006-2952(65)90221-2. [DOI] [PubMed] [Google Scholar]
- Gibb JW, Johnson M, Stone D, Hanson GR. MDMA: historical perspectives. Ann N Y Acad Sci. 1990;600:601–11. doi: 10.1111/j.1749-6632.1990.tb16913.x. discussion 611-2. [DOI] [PubMed] [Google Scholar]
- Gower AJ, Lamberty Y. The aged mouse as a model of cognitive decline with special emphasis on studies in NMRI mice. Behav Brain Res. 1993;57:163–73. doi: 10.1016/0166-4328(93)90132-a. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Luciana M. Neurocognitive function in users of MDMA: the importance of clinically significant patterns of use. Psychol Med. 2004;34:229–46. doi: 10.1017/s0033291703001132. [DOI] [PubMed] [Google Scholar]
- Hirata H, Ladenheim B, Rothman RB, Epstein C, Cadet JL. Methamphetamine-induced serotonin neurotoxicity is mediated by superoxide radicals. Brain Res. 1995;677:345–7. doi: 10.1016/0006-8993(95)00218-f. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Achat-Mendes CN, Ali SF, Anderson KL. Long-lasting behavioral sensitization to psychostimulants following p-chloroamphetamine-induced neurotoxicity in mice. Neuropharmacology. 2004;46:74–84. doi: 10.1016/s0028-3908(03)00316-2. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, De La Garza R, 2nd, Mahoney JJ, 3rd, Fantegrossi WE, Newton TF. MDMA use and neurocognition: a meta-analytic review. Psychopharmacology (Berl) 2007;189:531–7. doi: 10.1007/s00213-006-0601-2. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. J Neuropsychiatry Clin Neurosci. 2003;15:215–20. doi: 10.1176/jnp.15.2.215. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Furukawa Y, Ang L, Vorce SP, Kalasinsky KS. Striatal serotonin is depleted in brain of a human MDMA (Ecstasy) user. Neurology. 2000;55:294–6. doi: 10.1212/wnl.55.2.294. [DOI] [PubMed] [Google Scholar]
- Kita T, Wagner GC, Nakashima T. Current research on methamphetamine-induced neurotoxicity: animal models of monoamine disruption. J Pharmacol Sci. 2003;92:178–95. doi: 10.1254/jphs.92.178. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Hodges AB, Ladenheim B, Rhoades R, Phillip CG, Cesena A, Ivanova E, Hohmann CF, Cadet JL. Methamphetamine treatment causes delayed decrease in novelty-induced locomotor activity in mice. Neurosci Res. 2009;65:160–5. doi: 10.1016/j.neures.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Peterson SC, Ricaurte GA. The effect of catecholamine depletion by alpha-methyl-para-tyrosine on measures of cognitive performance and sleep in abstinent MDMA users. Neuropsychopharmacology. 2007;32:1695–706. doi: 10.1038/sj.npp.1301302. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. Lancet. 1998;352:1433–7. doi: 10.1016/s0140-6736(98)04329-3. [DOI] [PubMed] [Google Scholar]
- McCardle K, Luebbers S, Carter JD, Croft RJ, Stough C. Chronic MDMA (ecstasy) use, cognition and mood. Psychopharmacology (Berl) 2004;173:434–9. doi: 10.1007/s00213-004-1791-0. [DOI] [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP. The role of temperature, stress, and other factors in the neurotoxicity of the substituted amphetamines 3,4-methylenedioxymethamphetamine and fenfluramine. Mol Neurobiol. 1995;11:177–92. doi: 10.1007/BF02740694. [DOI] [PubMed] [Google Scholar]
- Myhrer T. Neurotransmitter systems involved in learning and memory in the rat: a meta-analysis based on studies of four behavioral tasks. Brain Res Brain Res Rev. 2003;41:268–87. doi: 10.1016/s0165-0173(02)00268-0. [DOI] [PubMed] [Google Scholar]
- O'Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:741–51. [PubMed] [Google Scholar]
- Obrocki J, Schmoldt A, Buchert R, Andresen B, Petersen K, Thomasius R. Specific neurotoxicity of chronic use of ecstasy. Toxicol Lett. 2002;127:285–97. doi: 10.1016/s0378-4274(01)00511-2. [DOI] [PubMed] [Google Scholar]
- Pletscher A, Bartholini G, Bruderer H, Burkard WP, Gey KF. Chlorinated Arylalkylamines Affecting the Cerebral Metabolism of 5-Hydroxytryptamine. J Pharmacol Exp Ther. 1964;145:344–50. [PubMed] [Google Scholar]
- Pletscher A, Burkard WP, Bruderer H, Gey KF. Decrease of Cerebral 5-Hydroxytryptamine and 5-Hydroxyindolacetic Acid by an Arylalkylamine. Life Sci. 1963;11:828–33. doi: 10.1016/0024-3205(63)90094-8. [DOI] [PubMed] [Google Scholar]
- Reneman L, Booij J, de Bruin K, Reitsma JB, de Wolff FA, Gunning WB, den Heeten GJ, van den Brink W. Effects of dose, sex, and long-term abstention from use on toxic effects of MDMA (ecstasy) on brain serotonin neurons. Lancet. 2001;358:1864–9. doi: 10.1016/S0140-6736(01)06888-X. [DOI] [PubMed] [Google Scholar]
- Renoir T, Paizanis E, Yacoubi ME, Saurini F, Hanoun N, Melfort M, Lesch KP, Hamon M, Lanfumey L. Differential long-term effects of MDMA on the serotoninergic system and hippocampal cell proliferation in 5-HTT knock-out vs. wild-type mice. Int J Neuropsychopharmacol. 2008;11:1149–62. doi: 10.1017/S1461145708009048. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA. Studies of MDMA-induced neurotoxicity in nonhuman primates: a basis for evaluating long-term effects in humans. NIDA Res Monogr. 1989;94:306–22. [PubMed] [Google Scholar]
- Saadat KS, Elliott JM, Colado MI, Green AR. The acute and long-term neurotoxic effects of MDMA on marble burying behaviour in mice. J Psychopharmacol. 2006a;20:264–71. doi: 10.1177/0269881106058022. [DOI] [PubMed] [Google Scholar]
- Saadat KS, Elliott JM, Green AR, Moran PM. High-dose MDMA does not result in long-term changes in impulsivity in the rat. Psychopharmacology (Berl) 2006b;188:75–83. doi: 10.1007/s00213-006-0470-8. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E, Bushing JA, Sulser F. Long-term effects of p-chloroamphetamine and related drugs on central serotonergic mechanisms. J Pharmacol Exp Ther. 1975;192:33–41. [PubMed] [Google Scholar]
- Santucci AC, Knott PJ, Haroutunian V. Excessive serotonin release, not depletion, leads to memory impairments in rats. Eur J Pharmacol. 1996;295:7–17. doi: 10.1016/0014-2999(95)00629-x. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Levin JA, Lovenberg W. In vitro and in vivo neurochemical effects of methylenedioxymethamphetamine on striatal monoaminergic systems in the rat brain. Biochem Pharmacol. 1987;36:747–55. doi: 10.1016/0006-2952(87)90729-5. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–97. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Simon SL, Dean AC, Cordova X, Monterosso JR, London ED. Methamphetamine dependence and neuropsychological functioning: evaluating change during early abstinence. J Stud Alcohol Drugs. 2010;71:335–44. doi: 10.15288/jsad.2010.71.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steranka L, Bessent R, Sanders-Bush E. Reversible and irreversible effects of p-chloroamphetamine on brain serotonin in mice. Commun Psychopharmacol. 1977;1:447–54. [PubMed] [Google Scholar]
- Steranka LR, Sanders-Bush E. Long-term effects of continuous exposure to amphetamine on brain dopamine concentration and synaptosomal uptake in mice. Eur J Pharmacol. 1980;65:439–43. doi: 10.1016/0014-2999(80)90351-9. [DOI] [PubMed] [Google Scholar]
- Stone DM, Hanson GR, Gibb JW. Differences in the central serotonergic effects of methylenedioxymethamphetamine (MDMA) in mice and rats. Neuropharmacology. 1987;26:1657–61. doi: 10.1016/0028-3908(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Ohkura M, Inoue K, Yamada J. Involvement of serotonergic and dopaminergic mechanisms in hyperthermia induced by a serotonin-releasing drug, p-chloroamphetamine in mice. Eur J Pharmacol. 2001;430:265–8. doi: 10.1016/s0014-2999(01)01386-3. [DOI] [PubMed] [Google Scholar]
- Taghzouti K, Simon H, Louilot A, Herman JP, Le Moal M. Behavioral study after local injection of 6-hydroxydopamine into the nucleus accumbens in the rat. Brain Res. 1985;344:9–20. doi: 10.1016/0006-8993(85)91184-9. [DOI] [PubMed] [Google Scholar]
- Timar J, Gyarmati S, Szabo A, Furst S. Behavioural changes in rats treated with a neurotoxic dose regimen of dextrorotatory amphetamine derivatives. Behav Pharmacol. 2003;14:199–206. doi: 10.1097/00008877-200305000-00003. [DOI] [PubMed] [Google Scholar]
- Wang X, Baumann MH, Xu H, Rothman RB. 3,4-methylenedioxymethamphetamine (MDMA) administration to rats decreases brain tissue serotonin but not serotonin transporter protein and glial fibrillary acidic protein. Synapse. 2004;53:240–8. doi: 10.1002/syn.20058. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, McCann UD, Yuan J, Delatte MS, Stevenson MW, Ricaurte GA, Moerschbaecher JM. Effects of fenfluramine, m-CPP and triazolam on repeated-acquisition in squirrel monkeys before and after neurotoxic MDMA administration. Psychopharmacology (Berl) 2002;159:388–96. doi: 10.1007/s00213-001-0942-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. Time course of the absolute change in body weight (left) or rectal temperature (right) measured over the six hour dosing regimen at the maximum unit dosage of MDMA (closed squares), METH (closed circles), and PCA (closed triangles) in comparison to saline (open squares). Abscissae: Elapsed experimental time expressed in minutes and plotted on a linear scale Ordinates: Peak absolute change in body weight (left) or rectal temperature (right) measured in grams or degrees Celsius respectively and plotted on a linear scale. Values are normalized to the baseline value for each subject.