Abstract
Membrane voltage controls the passage of ions through voltage-gated K (Kv) channels, and many studies have demonstrated that this is accomplished by a physical gate located at the cytoplasmic end of the pore. Critical to this determination were the findings that quaternary ammonium ions and certain peptides have access to their internal pore-blocking sites only when the channel gates are open, and that large blocking ions interfere with channel closing. Although an intracellular location for the physical gate of Kv channels is well established, it is not clear if such a cytoplasmic gate exists in all K+ channels. Some studies on large-conductance, voltage- and Ca2+-activated K+ (BK) channels suggest a cytoplasmic location for the gate, but other findings question this conclusion and, instead, support the concept that BK channels are gated by the pore selectivity filter. If the BK channel is gated by the selectivity filter, the interactions between the blocking ions and channel gating should be influenced by the permeant ion. Thus, we tested tetrabutyl ammonium (TBA) and the Shaker “ball” peptide (BP) on BK channels with either K+ or Rb+ as the permeant ion. When tested in K+ solutions, both TBA and the BP acted as open-channel blockers of BK channels, and the BP interfered with channel closing. In contrast, when Rb+ replaced K+ as the permeant ion, TBA and the BP blocked both closed and open BK channels, and the BP no longer interfered with channel closing. We also tested the cytoplasmically gated Shaker K channels and found the opposite behavior: the interactions of TBA and the BP with these Kv channels were independent of the permeant ion. Our results add significantly to the evidence against a cytoplasmic gate in BK channels and represent a positive test for selectivity filter gating.
INTRODUCTION
Ion channels are membrane proteins that provide a pathway for the movement of ions into or out of all cells and are critical for all physiological processes. Some channels discriminate poorly among various ions and some are quite selective, allowing passage of predominantly only one or very few types of ions. In certain subtypes of ion channels, the ion pathway is constitutively available; in others it is “gated.” That is, the availability of the pathway is controlled by voltage or a ligand or, in some cases, by both. Over the last few decades, considerable effort has been directed toward identifying the part of the protein that forms the physical gate of the channel. Much of this work has been focused on voltage-gated K (Kv) channels, and a clear picture has evolved.
The pioneering work of Armstrong (1966, 1971) probing squid axon K channels with tetraethyl ammonium ions and other quaternary ammonium (QA) compounds led to the idea that the gate was located on the cytoplasmic side of the protein. A key finding was that these blocking ions have access to their binding site in the channel pore only from the intracellular end of the channel and only when the channel is open. Larger ions including the long-chain QA analogue decyltriethylammonium, C10, and peptides based on the Shaker inactivation ball peptide (BP) are also open-channel blockers and are trapped by or interfere with the closing of the activation gate, results that add further evidence that the physical location of the gate is toward the cytoplasmic end of Kv channels (Armstrong and Hille, 1972; Yeh and Armstrong, 1978; Zagotta et al., 1990; Demo and Yellen, 1991; Toro et al., 1992; Choi et al., 1993; Holmgren et al., 1997). Additional studies using the Shaker Kv channel as a model (Holmgren et al., 1997; Liu et al., 1997; del Camino et al., 2000; del Camino and Yellen, 2001) identified parts of the sixth membrane-spanning region (S6) that could be chemically modified whether the channels were open or closed and other regions in S6, more toward the extracellular end, that could be modified only if the channels were open. These deeper regions were formed predominantly by residues in the S4–S5 linker. A critical part of these studies was the finding that even small ions like Cd2+ and Ag+ were excluded from these deeper structures by the channels’ gates. Finally, support for this picture of a relatively large intracellular gate in Kv channels has come from the solution of the crystal structures of several types of K channels (Doyle et al., 1998; Jiang et al., 2002; Long et al., 2005).
Thus, the idea of an intracellular gate is well established for Kv channels, but do other K channels work the same way? Some studies indicated that the large-conductance, voltage- and Ca2+-activated K+ (BK) channel may also have a large intracellular gate because, as in Kv channels, both C10 and the BP are open-channel blockers of these channels, and the BP interferes with BK channel closing (Li and Aldrich, 2004, 2006). However, there are several other findings with BK channels that raise questions about this conclusion. For example, in contrast to the open-channel block of BK channels by C10 and the BP, a relatively large QA compound, N-(4-[benzoyl]benyl)-N,N,N-tributylammonium) (bbTBA), does not require open, conducting channels to block (Wilkens and Aldrich, 2006; Tang et al., 2009). In addition, Ba2+ ions cannot access their BK pore-blocking site from either the extracellular or intracellular solutions when the channel is closed (Miller, 1987), whereas closed Kv channels can be blocked by external but not by internal Ba2+ (Armstrong and Taylor, 1980).
Thus, there are significant differences between the behavior of BK and Kv channels that raise doubts that the gating of BK channels occurs through an intracellular physical gate like that in Kv channels. If BK channels do not open and close via movement of a large intracellular part of its protein, how is the flow of K ions regulated? One hypothesis is that the selectivity filter itself controls ion flow through the channel (Li and Aldrich, 2006; Piskorowski and Aldrich, 2006; Tang et al., 2009), and recent studies have provided evidence in support of this idea (Chen and Aldrich, 2011; Zhou et al., 2011). If BK channel gating occurs with structural changes in the selectivity filter, it might be expected that permeant ions would alter channel gating. Rb+ is very similar in size and hydration energy to K+ and is permeant in all K channels. Replacing K+ with Rb+ or adding Rb+ to K+ solutions shifts the voltage dependence of BK channel activation (Demo and Yellen, 1992; Piskorowski and Aldrich, 2006). However, these effects are relatively weak and equivalent to ∼0.4 kcal/mole. The pore-blocking ions Cs+ and Ba2+ have a somewhat larger effect in that they destabilize the BK channel closed state by ∼2.3 and 1.5 kcal/mole, respectively (Miller et al., 1987; Demo and Yellen, 1992). Finally, replacing K+ with the permeant Tl+ ion produces significantly larger effects on BK gating: a +38-mV shift of the voltage dependence of channel activation (Piskorowski and Aldrich, 2006). However, even though Tl+ permeates BK (and Kv) channels, its complex electronic structure may make it a less than ideal probe of K channel activity.
Although the actions of Rb+, Cs+, and Ba2+ on BK channel gating are consistent with the expectations of selectivity filter gating, the rather small effects and the fact that Rb+ and Cs+ ions affect the gating of Kv as well as BK channels (Swenson and Armstrong, 1981; Clay and Shlesinger, 1983) indicate that these results cannot be considered as successful tests of this hypothesis. Nevertheless, if the selectivity filter acts as the gate in BK but not Kv channels, there should be some distinct effects of permeant ions on some aspect of BK but not Kv channel gating. Because the interaction of blocking ions with the gates of Kv channels helped establish the intracellular location of the gate in these channels, a selective alteration of these interactions in BK channels by permeant ions would constitute a convincing test of the selectivity filter gating mechanism. Thus, we used small (tetrabutyl ammonium [TBA]) and large (BP) molecules shown previously to interact with the gates of Kv channels as probes of BK channel gating with K+ or Rb+ as the permeant ion. As reported previously (Li and Aldrich, 2004, 2006), we found that these two molecules exhibited open-channel block of BK channels with K+ as the permeant ion, and the BP interfered with channel closing. However, simply replacing Rb+ for K+ as the permeant ion produced a profound difference: these pore-blocking ions had access to their binding sites independent of the activation state of BK channels, and the BP no longer interfered with the closing of the activation gate. Moreover, this effect was specific for BK channels: these blocking ions retained their interactions with the gates of Shaker Kv channels, independent of the permeant ion.
MATERIALS AND METHODS
The BK channel construct used here was the mouse form of KCa1.1 (mSlo) (Butler et al., 1993). The Shaker K (ShB) channel was the inactivation-deleted form with amino acids 6–46 removed (Hoshi et al., 1990). Channel proteins were expressed by RNA injection into oocytes from frogs (Xenopus laevis). Xenopus ovarian lobes were obtained from Nasco, and individual oocytes were isolated by standard procedures. Channel currents were obtained with excised inside/out macropatches with an amplifier (Axopatch 200B; Molecular Devices). Patch pipettes were pulled from quartz glass (Garner Class Co.). A holding potential of −80 mV was used for experiments on BK channels, and −100 mV was used for ShB channels. In BK channel experiments with Rb+ as the permeant ion, a hyperpolarization to −100 mV preceded the activating depolarizations. The pipette (extracellular) solution for BK channel experiments consisted of (in mM): 140 X-glutamate, 2 MgCl2, and 10 HEPES, pH 7.2, where X was either K or Rb. The external solution for Shaker channel experiments also included 2 mM CaCl2. Most BK channel experiments were done with a bath (cytoplasmic) solution containing 100 µM free Ca2+ (in mM): 140 X-glutamate, 3 NTA, 1.11 CaCl2, and 10 HEPES, pH 7.2, but a 10-µM solution was also used: 140 X-glutamate, 5 HEDTA, 3.6 CaCl2, and 10 HEPES, pH 7.2. These solutions also contained 50 µM crown ether (Sigma-Aldrich) to chelate any Ba2+ contamination of the Ca2+ salts. The cytoplasmic solution for Shaker channel experiments was nominally Ca2+ free (in mM): 140 X-glutamate, 1 EGTA, and 10 HEPES, pH 7.2. TBA-Cl was obtained from Sigma-Aldrich, and the enhanced Shaker BP (E12KD13K) (Murrell-Lagnado and Aldrich, 1993) was prepared by Biopeptide Co.
The accuracy of channel block at negative potentials where the open probability is small can be compromised by leak currents. To minimize such possible errors, only patches with a high seal resistance (low leak) were used, which required minimal analogue leak subtraction. In a few experiments in K solutions, we found comparable TBA block of BK channels without and with a P/4 procedure (not depicted). We preferred not to routinely use P/4 leak subtraction because this procedure increases noise, necessitating the averaging of currents from several depolarizations, which is problematic for “use-dependent” blockers. The minimal contribution of leak to the analysis is indicated by the fact that channel current and activation level smoothly approached zero at negative potentials where leak currents would continue to increase. Also, because Rb+ permeates both Shaker and BK channels less well than K+ (Latorre and Miller, 1983; Eisenman et al., 1986; Yool and Schwarz, 1991), channel currents would be smaller in Rb+ than in K+ solutions and, therefore, leak would represent a larger fraction of total current. Such a situation would tend to minimize the measured block in Rb+ solutions, but our results actually show more block of BK channels at negative potentials than was seen in K+ solutions. In addition, we found no correlation of block levels with current levels with either K+ or Rb+ as the permeant ion.
The slow kinetics of block by the BP can make it difficult to determine steady-state current levels (Zagotta et al., 1990; Li and Aldrich, 2006; and see Fig. 5). To minimize this problem in some experiments with few expressed channels, we averaged multiple current records. For these, we used a 3-Hz cycle time that we determined did not produce accumulated “use-dependent” block (not depicted). We indicate in the figure legends which examples were derived from averaging multiple records.
Figure 5.
Time course of BP block of BK channels in K+ and Rb+ solutions. Raw currents at the indicated potentials in the absence (black) and presence (red) of 2 µM BP recorded in 100 µM Ca2+ in K+ (left; calibration: 1.2 nA, 50 msec) and Rb+ (right; calibration: 0.25 nA, 50 msec) solutions. The holding potential for the K+ solutions was −80 mV. For the Rb+ solutions, the voltage level before depolarization was −100 mV, and after the depolarization it was −80 mV. The tail currents upon repolarization have been truncated for clarity.
Channel activation at a particular voltage was determined by the magnitude of the “tail” current after repolarization to the holding potential. The voltage dependence of channel activation was fit by the Boltzmann equation Amplitude/(1 + exp[−z(Vm − Vh)F/RT]) and presented as normalized to the maximum value. Because of the large patch to patch variability in the half-activation voltage (Vh) of heterologously expressed Slo channels (Stefani et al., 1997; Horrigan et al., 1999), we followed the procedure of other authors (Horrigan et al., 1999; Orio and Latorre, 2005) of shifting the BK channel activation curves from multiple cells so that the half-activation voltage from each cell coincides with the overall mean value and, through an interpolation procedure, computing a mean voltage–activation relation. Estimates of the channel deactivation kinetics were obtained from fits of a single-exponential time function to the tail currents after a depolarization that fully activated the channels. Mean values of various parameters are reported with SEM limits.
RESULTS
As noted in Introduction, the Shaker BP is an open-state blocker of BK channels (Li and Aldrich, 2006). The data presented in Fig. 1 were collected to summarize the previously reported behavior of the BP on BK channels. The top insets show BK channel currents collected in 100 µM Ca2+ during depolarizations to the indicated membrane voltages in the absence (black traces) and presence (red traces) of 2 uM of the BP applied to the inner surface of BK channels in an inside-out patch. The peptide produced minimal block at negative potentials where the channel open probability is small and much larger block at more depolarized potentials where the channels are open. The main part of Fig. 1 shows the close correlation between channel activation (■) and the fraction of unblocked channels (○). This close correlation is not the result of a voltage-dependent block by the BP that just happens to occur over the gating voltage range, because shifting channel activation by a reduction in intracellular Ca2+ produces an equal shift in the voltage dependence of block by the BP (Li and Aldrich, 2006).
Figure 1.
Open-state block of BK channels by the BP in K+ solutions. (Top inset) Time course of raw BK channel currents recorded in 100 µM Ca2+ in the absence (black) and presence (red) of 2 µM BP at the potentials indicated. Calibration: 0.4 nA, 50 msec. Zero-current level indicated by dashed line. (Main) Voltage dependence of BK channel activation (■) and fraction of channels not blocked by the BP (○) in 100 µM Ca2+. (Inset) Raw current recorded at + 60 mV from a holding potential of −80 mV in the absence (black) and presence (red) of 2 µM BP. Calibration: 2 nA, 50 msec. Zero-current level indicated by dashed line. (Boxed inset) Magnified view of the currents after repolarization to −80 mV, with the peak current in the BP scaled to match that of the control record. Calibration: 1 nA, 25 msec.
Another property reflecting the open-channel block by the BP can be seen in the time dependence of currents recorded in the presence of the BP, for example, the red traces at −10 and +10 mV in the top insets and at +60 mV in the main inset (Fig. 1). At the beginning of the pulse, the channels are closed and unblocked and begin to open normally, and the current in the presence of the blocker superimposes with the control records (black traces). Then, as the channels open, they become increasingly blocked.
A final hallmark of this bulky peptide is that it interferes with BK channel closing (Li and Aldrich, 2006). This can be seen in the boxed inset, which is a magnified view of the tail currents after repolarization in the absence (Fig. 1, black trace) and presence (red trace) of the BP. The peak amplitude of the tail current in the BP was scaled to match that of the control record. The presence of the BP substantially slowed the tail current; the channels could not completely close until the peptide left its binding site in the permeation pathway. Although we have not analyzed this process in detail, Li and Aldrich (2006) did and found evidence that a small fraction of blocked channels might not pass back through the open state before closing. Nevertheless, their results and ours clearly show that the presence of the BP profoundly interferes with the ability of BK channels to close.
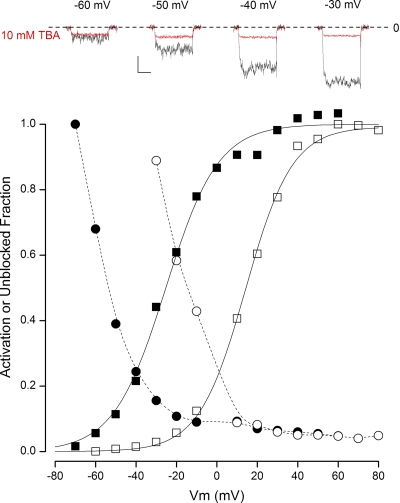
As described in Introduction, the QA compound C10 preferentially blocks open BK channels (Li and Aldrich, 2004), but less work has been done examining the properties of block by the smaller QA ion, TBA, on BK channels. As shown in Fig. 2, in K+ solutions, TBA appeared to predominantly block open BK channels. The top insets show BK channel currents collected in 100 µM Ca2+ during depolarizations to the indicated membrane voltages in the absence (black traces) and presence (red traces) of 10 mM of internally applied TBA. As with the BP, TBA produced little block of the channels at the most negative potentials where the channels have a low open probability, but there was a very large block at potentials where the channels are mostly open. This can be seen even more clearly in the main part of Fig. 2 where the voltage dependence of the fractional open probability and the fraction of unblocked channels in 100 µM Ca2+ are plotted as closed squares and closed circles, respectively. The reciprocal relationship between current inhibition and activation is a hallmark of open-channel block by this relatively small QA ion.
Figure 2.
Open-state block of BK channels by TBA in K+ solutions. (Top insets) Time course of raw BK channel currents recorded in 100 µM Ca2+ in the absence (black) and presence (red) of 10 mM TBA at the potentials indicated. Calibration: 0.4 nA, 50 msec. The zero-current level is indicated by the dashed line. (Main) Voltage dependence of BK channel activation in 100 µM Ca2+ (■) and in 10 µM Ca2+ (□). Also shown is the voltage dependence of the fraction of channels not blocked by 10 mM TBA in 100 µM Ca2+ (•) and in 10 µM Ca2+ (○). Solid lines, fits of the Boltzmann equation to the data; dashed lines, spline fits to the data.
Shifting the voltage dependence of channel activation to more positive potentials by reducing the intracellular Ca2+ level to 10 µM (Fig. 2, □) produced an equivalent shift in the voltage dependence of channel unblock (■), preserving the tight relationship between channel activation and channel block. It is also apparent from the raw current traces that TBA block was very fast, because the amount of block appears to be essentially independent of time during the depolarization. Although not shown in detail in Fig. 2, we found that TBA, unlike the BP, did not interfere with channel closing but slightly increased the rate of channel closing consistent with the findings of Li and Aldrich (2004).
The data in Fig. 1 summarized past published work with the BP (Li and Aldrich, 2006), showing that it is an open-state blocker of BK channels and that it interferes with BK channel closing. The results presented in Fig. 2 illustrated that TBA also preferentially blocked open BK channels. As with Kv channels, these types of findings lead naturally to a picture of BK channels with a Ca2+ and voltage-controlled intracellular gate that prevents even the relatively small TBA ion from blocking the channel in its closed conformation. In this view, the intracellular gate must first move to its open position before these ions can occupy their blocking sites in the intracellular end of the pore. The existence of such an intracellular gate can also account for how the large, slow BP interferes with channel closing. Although this picture of an intracellular gate is consistent with these data, we noted in Introduction that there are other findings with BK channels that call such a picture into question. If BK channel gating occurs with such an intracellular gate, changing the permeant ion from K+ to Rb+ should have little or no effect on the relationship between channel opening and block by TBA and the BP. Therefore, we examined the actions of TBA and BP on BK channels in Rb+ solutions, and an example of TBA block is illustrated in Fig. 3.
Figure 3.
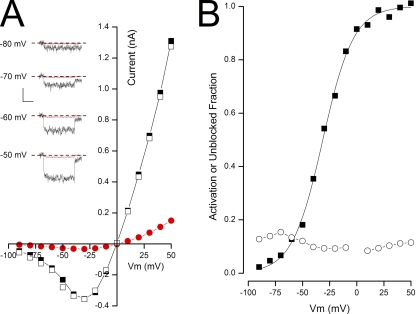
State-independent block of BK channels by TBA in Rb+ solutions. (A; insets) Time course of raw BK channel currents recorded in 100 µM Ca2+ in the absence (black) and presence (red) of 10 mM TBA at the potentials indicated. The voltage level before depolarization was −100 mV, and after the depolarization it was −80 mV (see Materials and methods). Calibration: 0.12 nA, 50 msec. Zero-current level indicated by dashed line. (Main) Steady-state current–voltage relation before (■), during (•), and after (□) the application of 10 mM TBA in Rb+ solutions. (B) Voltage dependence of BK channel activation (■) and fraction of channel not blocked by TBA (○) in 100 µM Ca2+.
The insets in Fig. 3 A contain raw BK channel currents recorded at the indicated voltages with Rb+ as the permeant ion in the absence (black traces) and presence (red traces) of 10 mM TBA. It is clear from these examples that TBA produces a substantial block of BK channel currents, even at very negative potentials where the channels have a low open probability. The main part of Fig. 3 A illustrates this same property over a larger voltage range and also shows that block by TBA is fully reversible in Rb+ solutions. The relationship between BK channel activation and block by TBA in this patch is illustrated in Fig. 3 B. Unlike the close correspondence between channel activation and TBA block seen in K+ solutions (Fig. 2), when Rb+ is the permeant ion, block was almost completely independent of voltage. This was a consistent finding as illustrated in Fig. 4, where mean values from several experiments are pooled together. It is clear that BK channel block by TBA in Rb+ was almost independent of membrane voltage and, significantly, there was substantial block of the channels even at very negative potentials where the open probability was quite small.
Figure 4.
State-independent block of BK channels by TBA in Rb+ solutions. Pooled data. Voltage dependence of BK channel activation (■) and fraction of channels not blocked by TBA (○) in 100 µM Ca2+. Mean data shown with standard error limits if larger than symbol. Data from six to nine experiments, except n = 3 at −90 mV.
Replacing K+ with Rb+ as the permeant ion also had a profound influence on BK channel block by the BP, as illustrated in Fig. 5. The left side of Fig. 5 shows examples of BK channel currents in K+ solutions in the absence (black) and presence (red) of 2 µM BP at the indicated potentials. As has been reported previously (Li and Aldrich, 2006) and can also be seen in Fig. 1, the current with K+ as the permeant ion in the presence of the peptide perfectly follows the control current at the beginning of the depolarization. Then only after the channels have been opened by the depolarization is the current inhibited. In contrast (Fig. 5, right), there was no such time-dependent change of current in the presence of the BP in Rb+ solutions: the current recorded with the BP is much smaller than control levels immediately upon depolarization and remains so throughout the pulse.
The data in Fig. 6 demonstrate that the lack of time dependence of BP block in Rb+ solutions seen in Fig. 5 was because, with Rb+ as the permeant ion, the BP had already blocked BK channels at the holding potential while they were closed. The insets in Fig. 6 A show BK channel currents recorded in Rb+ solutions in the absence (black traces) and presence (red traces) of 2 µM BP at the indicated potentials. It can be seen that the BP strongly blocked BK channels, even at negative potentials where the channel opening probability is low. The main part of Fig. 6 A illustrates this same observation over a larger voltage range and also illustrates the reversible nature of BP block in Rb+ solutions. Fig. 6 B shows the relationship between channel activation and BP block in this example patch. It is clear that with Rb+ as the permeant ion, the BP blocked closed channels essentially as well as it did open channels, in contrast to blocking only open channels when K+ was the permeant ion (Fig. 1).
Figure 6.
State-independent block of BK channels by BP in Rb+ solutions. (A; insets) Time course of raw BK channel currents recorded in 100 µM Ca2+ in the absence (black) and presence (red) of 2 µM BP at the potentials indicated. Calibration: 0.1 nA, 50 msec. (Main) Steady-state current–voltage relation before (■), during (•), and after (□) application of 2 µM BP in Rb+ solutions. (B) Voltage dependence of BK channel activation (■) and fraction of channels not blocked by BP (○) in 100 µM Ca2+. (Inset) BK currents in response to repolarization to −80 mV from +40 mV in the absence (black) and presence (red; average of four records) of 2 µM BP, with the peak current in the BP scaled to match that of the control record. Calibration: 0.2 nA, 10 msec.
The inset in Fig. 6 B shows BK channel tail currents recorded in Rb+ in the absence (black) and presence (red) of the BP, with the amplitude of the record in the presence of the BP scaled to match the control data. It is clear that the BP had no effect on the ability of the BK channels to close when Rb+ was the permeant ion, again in sharp contrast to the strong interference that occurs in K+ solutions (Fig. 1). These differences in the behavior of the BP with Rb+ as the permeant ion were consistent findings, as shown in Fig. 7. The main part of Fig. 7 establishes that, in Rb+ solutions, the BP blocked BK channels essentially independently of whether they were open or closed. The inset in Fig. 7 shows BK channel closing time constants over a range of potentials in the absence (■) and presence (○) of the BP. There was little or no affect of the BP on the channel closing rate with Rb+ as the permeant ion.
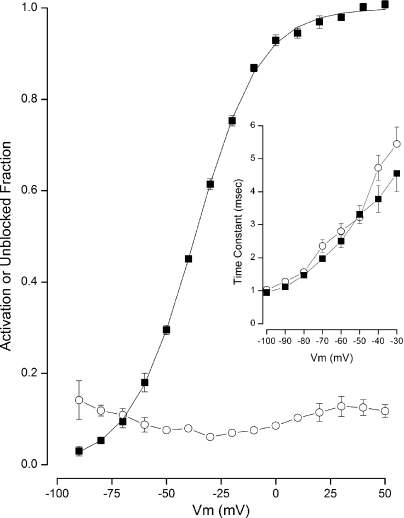
Figure 7.
State-independent block of BK channels by BP in Rb+ solutions. Pooled data. Voltage dependence of BK channel activation (■) and fraction of channels not blocked by 2 µM BP (○) in 100 µM Ca2+. Mean values from four experiments shown with standard error limits if larger than symbol. (Inset) Voltage dependence of the time constant of deactivation in the absence (■) and presence (○) of the BP. Mean values from three experiments shown with standard error limits if larger than symbol.
As described in Introduction, the overall weight of evidence strongly supports the existence of an intracellular gate for Kv channels. If BK channels are gated differently, for example within the selectivity filter, these two types of channels would likely differ in how permeant ions interact with state-dependent blocking ions. With K+ as the permeant ion, the BP is an open-channel blocker of Shaker K channels and interferes with the channel gate closing (Zagotta et al., 1990; Demo and Yellen, 1991). We tested if this remained true with Rb+ as the permeant ion, and the results are illustrated in Fig. 8.
Figure 8.
Open-state block of Shaker K channels by the BP in K+ and Rb+ solutions. (A) Block of Shaker channels by 2 µM BP in K+ solutions. (Top inset) Currents recorded at −40 mV from a holding potential of −100 mV in the absence (black) and presence (red) of 2 µM BP. Calibration: 0.2 nA, 100 msec. (Main) Voltage dependence of Shaker channel activation (■) and fraction of channels not blocked by 2 µM BP (○). (Inset) Shaker channel currents in response to repolarization to −100 mV (from +20 mV) in the absence (black) and presence (red) of 2 µM BP, with the peak current in the BP scaled to match that of the control record. Calibration: 0.75 nA, 20 msec. (B) Block of Shaker channels by 2 µM BP in Rb+ solutions. (Top inset) Currents recorded at −40 mV from a holding potential of −100 mV in the absence (black) and presence (red) of 2 µM BP. Calibration: 0.4 nA, 100 msec. (Main) Voltage dependence of Shaker channel activation (■) and fraction of channels not blocked by 0.5 µM BP (○). Mean values from three experiments shown with standard error limits if larger than symbol. (Inset) Shaker channel currents in response to repolarization to −120 mV (from +20 mV) in the absence (black) and presence (red) of 2 µM BP, with the peak current in the BP scaled to match that of the control record. Calibration: 1.2 nA, 20 msec. Dashed line in all insets represents the zero-current level.
The inset of Fig. 8 A shows time-dependent block of Shaker K channels in K+ solutions produced by 2 µM BP during a depolarization to −40 mV (red trace), and the main part of Fig. 8 A shows the open-state block characteristics of the BP. The inset contains tail currents recorded in the absence (black) and presence (red) of the BP, showing that the BP strongly interfered with channel closing in K+ solutions. Fig. 8 B shows that the open-channel block properties of Shaker K channels did not change when Rb+ replaced K+ as the permeant ion. The time-dependent block by the BP was preserved (Fig. 8 B, top), and the voltage dependence of the fraction of channels not blocked by the BP (○) mirrored channel activation (■) in Rb+ solutions just as in K+ solutions. The inset demonstrates that the BP continued to interfere with channel closing, even with Rb+ as the permeant ion, all in sharp contrast with the behavior of BK channels.
DISCUSSION
As summarized in Introduction, there is overwhelming evidence that Kv channels, including Shaker channels, contain a cytoplasmic gate that occludes the ion permeation pathway to prevent ion flow when the channels are in their closed, nonconducting state. Depolarization induces a conformational change that removes this intracellular restriction and allows permeant ion flow through the open channel. Significant contributions to this picture of the Kv channel gate have come from studies that showed that relatively small, intracellular QA compounds like TEA and TBA can reach their blocking sites in the pore only after the cytoplasmic gate opens upon channel activation (Armstrong, 1966; Armstrong and Hille, 1972; French and Shoukimas, 1981; Choi et al., 1993; del Camino et al., 2000; Zhou et al., 2001). Other important results confirming the intracellular location of the gate include the finding that large QA compounds and the BP are not only open-channel blockers of Kv channels but also interfere with the channel’s activation gate (Demo and Yellen, 1991; Choi et al., 1993).
As in Kv channels, C10 and the BP can only block open BK channels, and the BP interferes with BK channel closing (Li and Aldrich, 2004, 2006), which suggests that the BK channel may also be gated at the intracellular end of the pore in these channels. This view would seem to be supported by our data with TBA in K solutions showing that this small QA also required an open channel for block. However, there is strong evidence that a cytoplasmic gate does not guard the inner entrance to BK channels. A bulky TBA derivative, bbTBA, does not require open, conducting BK channels to block the channel pore (Wilkens and Aldrich, 2006; Tang et al., 2009), and deep pore sites near the selectivity filter can be modified by large methanethiosulfonate derivatives, even when the channels are closed (Zhou et al., 2011). Thus, the location of the physical gate of BK channels must lie elsewhere than at the cytoplasmic end of the protein.
If the physical gate of BK channels is not at the inner end of the pore as it is in Kv channels, where could it be? Considering the limited extent of the pore, there are only a few possible locations, and the selectivity filter has been suggested to be the leading candidate (Li and Aldrich, 2006; Piskorowski and Aldrich, 2006; Tang et al., 2009). The selectivity filter in BK channels is able to clearly distinguish between the very similar K+ and Rb+ ions, so if the selectivity filter is the actual physical gate, it would be expected that there would be large differences between the gating of K+ and Rb+ ions. As described in Introduction, there are differences, but these are rather small and, furthermore, also occur in Kv channels with their cytoplasmic gate. Thus, there is no definitive test that the BK channel gates within the selectivity filter. However, a recent study provided sufficient evidence to keep selectivity filter gating in the running (Chen and Aldrich, 2011). In this study, the authors found a strong coupling between the protonation of the side chain of a “deep pore” amino acid and BK channel gating. These results indicate that channel gating causes a conformational change that alters the physical environment (polar or nonpolar) of the side chain of this amino acid that is at the cytoplasmic entrance to the selectivity filter.
In spite of the small effect of permeant ions on BK channel gating, it still seems reasonable to expect that if the selectivity filter is the BK channel gate, there should be permeant ion–specific differences in some aspect of the gating of BK but not Kv channels. As reviewed here, there are strong interactions between Kv and BK channel gating and pore-blocking ions including TBA and the BP, at least in K+ solutions. There are also strong interactions between permeant ions and pore-blocking ions in a variety of K channels (Neyton and Miller, 1988a,b; Spassova and Lu, 1998; Immke et al., 1999; Immke and Korn, 2000; Guo and Lu, 2001; Thompson and Begenisich, 2001, 2003), and there are differences in permeant ion occupancy of the K channel selectivity filter (Doyle et al., 1998; Morais-Cabral et al., 2001; Zhou and MacKinnon, 2003). These properties of K channels lead us to consider that a sensitive test for selectivity filter gating in BK channels would be the role of permeant ions in the interaction between blocking ions like TBA and the BP and channel gating. In this view, a positive test would have two results: (1) a permeant ion–specific difference in how TBA and the BP interact with the activation gate of BK channels, and (2) no such difference in Shaker K channels. We found that TBA and the BP were open-channel blockers with K+ as the permeant ion but blocked closed and open channels when Rb+ replaced K+ as the permeant ion. In contrast, the state-dependent BP block of Shaker channels was largely insensitive to the nature of the permeant ion. Thus, we consider our findings to represent a positive test for selectivity filter gating in BK channels.
Our view is that a BK channel with a closed selectivity filter has a conformation that allows a K+ ion to bind near the TBA- and BP-blocking sites in the pore. These ions cannot bind to their blocking sites in the channel as long as K+ occupies its site, so they cannot block a closed channel. The blockers and the K+ ion need not bind at exactly the same site; they only need to compete for binding, either directly or electrostatically. In this view, when the BK channel opens, K+ ions will no longer significantly occupy its site in the selectivity filter, allowing TBA and the BP to block the open channel. If Rb+ ions do not significantly occupy the selectivity filter site, TBA and the BP will block both closed and open channels when Rb+ is the permeant ion. In this model, the interference of the BP with BK channel closing in K+ solutions is because the binding of K+ to the site contributes to the stability of the closed state. Thus, because occupancy of the site by the BP and K+ ions is mutually exclusive, the channel cannot be completely closed until BP vacates the site. BP exit from the channel takes much longer than normal deactivation, so closing will be slowed.
A selectivity filter gate has been identified in CNG channels (Contreras and Holmgren, 2006; Contreras et al., 2008), which are nonselective ion channels that are similar in structure to K+ channels, especially in the selectivity filter region. CNG channels are activated by the binding of cyclic nucleotides to the channel’s large intracellular domain (Kaupp and Seifert, 2002), analogous to the binding of Ca2+ to the large cytoplasmic domain of BK channels. It may be that coupling the binding of an intracellular ligand to the opening of the channel pore is best accomplished with a selectivity filter gate rather than by the intracellular parts of the sixth membrane-spanning domain, as occurs in Kv channels. In any case, our results demonstrate large differences in how blocking ions like TBA and the BP interact with the gating in Kv and BK channels. The simplest interpretation of our findings is that the activation gates in BK channels reside within the selectivity filter rather than in the intracellular end of the S6 domain, which forms the gating region of Kv channels.
Acknowledgments
We thank Robert Dirksen for discussions on this work.
This work was supported by the National Institutes of Health (grant DE016960).
Kenton J. Swartz served as editor.
Footnotes
Abbreviations used in this paper:
- BK
- large-conductance, voltage- and Ca2+-activated K+
- BP
- ball peptide
- Kv
- voltage-gated K
- QA
- quaternary ammonium
- TBA
- tetrabutyl ammonium
References
- Armstrong C.M. 1966. Time course of TEA+-induced anomalous rectification in squid giant axons. J. Gen. Physiol. 50:491–503 10.1085/jgp.50.2.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C.M. 1971. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J. Gen. Physiol. 58:413–437 10.1085/jgp.58.4.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C.M., Hille B. 1972. The inner quaternary ammonium ion receptor in potassium channels of the node of Ranvier. J. Gen. Physiol. 59:388–400 10.1085/jgp.59.4.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C.M., Taylor S.R. 1980. Interaction of barium ions with potassium channels in squid giant axons. Biophys. J. 30:473–488 10.1016/S0006-3495(80)85108-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A., Tsunoda S., McCobb D.P., Wei A., Salkoff L. 1993. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science. 261:221–224 10.1126/science.7687074 [DOI] [PubMed] [Google Scholar]
- Chen X., Aldrich R.W. 2011. Charge substitution for a deep-pore residue reveals structural dynamics during BK channel gating. J. Gen. Physiol. 138:137–154 10.1085/jgp.201110632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.L., Mossman C., Aubé J., Yellen G. 1993. The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron. 10:533–541 10.1016/0896-6273(93)90340-W [DOI] [PubMed] [Google Scholar]
- Clay J.R., Shlesinger M.F. 1983. Effects of external cesium and rubidium on outward potassium currents in squid axons. Biophys. J. 42:43–53 10.1016/S0006-3495(83)84367-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras J.E., Holmgren M. 2006. Access of quaternary ammonium blockers to the internal pore of cyclic nucleotide–gated channels: Implications for the location of the gate. J. Gen. Physiol. 127:481–494 10.1085/jgp.200509440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras J.E., Srikumar D., Holmgren M. 2008. Gating at the selectivity filter in cyclic nucleotide-gated channels. Proc. Natl. Acad. Sci. USA. 105:3310–3314 10.1073/pnas.0709809105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Camino D., Yellen G. 2001. Tight steric closure at the intracellular activation gate of a voltage-gated K(+) channel. Neuron. 32:649–656 10.1016/S0896-6273(01)00487-1 [DOI] [PubMed] [Google Scholar]
- del Camino D., Holmgren M., Liu Y., Yellen G. 2000. Blocker protection in the pore of a voltage-gated K+ channel and its structural implications. Nature. 403:321–325 10.1038/35002099 [DOI] [PubMed] [Google Scholar]
- Demo S.D., Yellen G. 1991. The inactivation gate of the Shaker K+ channel behaves like an open-channel blocker. Neuron. 7:743–753 10.1016/0896-6273(91)90277-7 [DOI] [PubMed] [Google Scholar]
- Demo S.D., Yellen G. 1992. Ion effects on gating of the Ca2+-activated K+ channel correlate with occupancy of the pore. Biophys. J. 61:639–648 10.1016/S0006-3495(92)81869-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle D.A., Morais Cabral J., Pfuetzner R.A., Kuo A., Gulbis J.M., Cohen S.L., Chait B.T., MacKinnon R. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77 10.1126/science.280.5360.69 [DOI] [PubMed] [Google Scholar]
- Eisenman G., Latorre R., Miller C. 1986. Multi-ion conduction and selectivity in the high-conductance Ca++-activated K+ channel from skeletal muscle. Biophys. J. 50:1025–1034 10.1016/S0006-3495(86)83546-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R.J., Shoukimas J.J. 1981. Blockage of squid axon potassium conductance by internal tetra-N-alkylammonium ions of various sizes. Biophys. J. 34:271–291 10.1016/S0006-3495(81)84849-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D., Lu Z. 2001. Kinetics of inward-rectifier K+ channel block by quaternary alkylammonium ions: Dimension and properties of the inner pore. J. Gen. Physiol. 117:395–406 10.1085/jgp.117.5.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren M., Smith P.L., Yellen G. 1997. Trapping of organic blockers by closing of voltage-dependent K+ channels: Evidence for a trap door mechanism of activation gating. J. Gen. Physiol. 109:527–535 10.1085/jgp.109.5.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan F.T., Cui J., Aldrich R.W. 1999. Allosteric voltage gating of potassium channels I. Mslo ionic currents in the absence of Ca2+. J. Gen. Physiol. 114:277–304 10.1085/jgp.114.2.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W.N., Aldrich R.W. 1990. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 250:533–538 10.1126/science.2122519 [DOI] [PubMed] [Google Scholar]
- Immke D., Korn S.J. 2000. Ion–ion interactions at the selectivity filter. Evidence from K+-dependent modulation of tetraethylammonium efficacy in Kv2.1 potassium channels. J. Gen. Physiol. 115:509–518 10.1085/jgp.115.4.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immke D., Wood M., Kiss L., Korn S.J. 1999. Potassium-dependent changes in the conformation of the Kv2.1 potassium channel pore. J. Gen. Physiol. 113:819–836 10.1085/jgp.113.6.819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Lee A., Chen J., Cadene M., Chait B.T., MacKinnon R. 2002. The open pore conformation of potassium channels. Nature. 417:523–526 10.1038/417523a [DOI] [PubMed] [Google Scholar]
- Kaupp U.B., Seifert R. 2002. Cyclic nucleotide-gated ion channels. Physiol. Rev. 82:769–824 [DOI] [PubMed] [Google Scholar]
- Latorre R., Miller C. 1983. Conduction and selectivity in potassium channels. J. Membr. Biol. 71:11–30 10.1007/BF01870671 [DOI] [PubMed] [Google Scholar]
- Li W., Aldrich R.W. 2004. Unique inner pore properties of BK channels revealed by quaternary ammonium block. J. Gen. Physiol. 124:43–57 10.1085/jgp.200409067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Aldrich R.W. 2006. State-dependent block of BK channels by synthesized Shaker ball peptides. J. Gen. Physiol. 128:423–441 10.1085/jgp.200609521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Holmgren M., Jurman M.E., Yellen G. 1997. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 19:175–184 10.1016/S0896-6273(00)80357-8 [DOI] [PubMed] [Google Scholar]
- Long S.B., Campbell E.B., Mackinnon R. 2005. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 309:897–903 10.1126/science.1116269 [DOI] [PubMed] [Google Scholar]
- Miller C. 1987. Trapping single ions inside single ion channels. Biophys. J. 52:123–126 10.1016/S0006-3495(87)83196-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., Latorre R., Reisin I. 1987. Coupling of voltage-dependent gating and Ba++ block in the high-conductance, Ca++-activated K+ channel. J. Gen. Physiol. 90:427–449 10.1085/jgp.90.3.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais-Cabral J.H., Zhou Y., MacKinnon R. 2001. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 414:37–42 10.1038/35102000 [DOI] [PubMed] [Google Scholar]
- Murrell-Lagnado R.D., Aldrich R.W. 1993. Interactions of amino terminal domains of Shaker K channels with a pore blocking site studied with synthetic peptides. J. Gen. Physiol. 102:949–975 10.1085/jgp.102.6.949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J., Miller C. 1988a. Potassium blocks barium permeation through a calcium-activated potassium channel. J. Gen. Physiol. 92:549–567 10.1085/jgp.92.5.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J., Miller C. 1988b. Discrete Ba2+ block as a probe of ion occupancy and pore structure in the high-conductance Ca2+-activated K+ channel. J. Gen. Physiol. 92:569–586 10.1085/jgp.92.5.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio P., Latorre R. 2005. Differential effects of β1 and β2 subunits on BK channel activity. J. Gen. Physiol. 125:395–411 10.1085/jgp.200409236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskorowski R.A., Aldrich R.W. 2006. Relationship between pore occupancy and gating in BK potassium channels. J. Gen. Physiol. 127:557–576 10.1085/jgp.200509482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova M., Lu Z. 1998. Coupled ion movement underlies rectification in an inward-rectifier K+ channel. J. Gen. Physiol. 112:211–221 10.1085/jgp.112.2.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani E., Ottolia M., Noceti F., Olcese R., Wallner M., Latorre R., Toro L. 1997. Voltage-controlled gating in a large conductance Ca2+-sensitive K+ channel (hslo). Proc. Natl. Acad. Sci. USA. 94:5427–5431 10.1073/pnas.94.10.5427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R.P., Jr, Armstrong C.M. 1981. K+ channels close more slowly in the presence of external K+ and Rb+. Nature. 291:427–429 10.1038/291427a0 [DOI] [PubMed] [Google Scholar]
- Tang Q.Y., Zeng X.H., Lingle C.J. 2009. Closed-channel block of BK potassium channels by bbTBA requires partial activation. J. Gen. Physiol. 134:409–436 10.1085/jgp.200910251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Begenisich T. 2001. Affinity and location of an internal K+ ion binding site in Shaker K channels. J. Gen. Physiol. 117:373–384 10.1085/jgp.117.5.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Begenisich T. 2003. Functional identification of ion binding sites at the internal end of the pore in Shaker K+ channels. J. Physiol. 549:107–120 10.1113/jphysiol.2002.038646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro L., Stefani E., Latorre R. 1992. Internal blockade of a Ca(2+)-activated K+ channel by Shaker B inactivating “ball” peptide. Neuron. 9:237–245 10.1016/0896-6273(92)90163-8 [DOI] [PubMed] [Google Scholar]
- Wilkens C.M., Aldrich R.W. 2006. State-independent block of BK channels by an intracellular quaternary ammonium. J. Gen. Physiol. 128:347–364 10.1085/jgp.200609579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J.Z., Armstrong C.M. 1978. Immobilisation of gating charge by a substance that simulates inactivation. Nature. 273:387–389 10.1038/273387a0 [DOI] [PubMed] [Google Scholar]
- Yool A.J., Schwarz T.L. 1991. Alteration of ionic selectivity of a K+ channel by mutation of the H5 region. Nature. 349:700–704 10.1038/349700a0 [DOI] [PubMed] [Google Scholar]
- Zagotta W.N., Hoshi T., Aldrich R.W. 1990. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 250:568–571 10.1126/science.2122520 [DOI] [PubMed] [Google Scholar]
- Zhou M., Morais-Cabral J.H., Mann S., MacKinnon R. 2001. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature. 411:657–661 10.1038/35079500 [DOI] [PubMed] [Google Scholar]
- Zhou Y., MacKinnon R. 2003. The occupancy of ions in the K+ selectivity filter: charge balance and coupling of ion binding to a protein conformational change underlie high conduction rates. J. Mol. Biol. 333:965–975 10.1016/j.jmb.2003.09.022 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Xia X.M., Lingle C.J. 2011. Cysteine scanning and modification reveal major differences between BK channels and Kv channels in the inner pore region. Proc. Natl. Acad. Sci. USA. 108:12161–12166 10.1073/pnas.1104150108 [DOI] [PMC free article] [PubMed] [Google Scholar]