Abstract
The hunt for a compound which inhibits the HIV-1 integrase had been painstakingly difficult. Integrase is essential for viral replication as it mediates the integration of the viral DNA genome into the host DNA resulting in the establishment of the permanent provirus. Persistent efforts have resulted in the discovery of Raltegravir (Isentress, MK-0518), the first integrase inhibitor approved by US Food and Drug Administration for the treatment in HIV-1 infected patients. Numerous clinical studies with raltegravir have found it to be safe and effective in treatment naïve as well as treatment experienced patients. Adverse events associated with raltegravir based therapy are milder compared to previously available regimens. Raltegravir is metabolized primarily via glucuronidation mediated by uridine diphosphate glucuronosyltransferase and has a favorable pharmacokinetics independent of age, gender, race, food, and drug-drug interactions. Within a short period of time of its introduction, raltegravir has been included as one of DHHS recommended preferred regimen for the treatment of HIV-1 infection in treatment naïve patients.
Keywords: raltegravir, HIV-1, integrase, antiretroviral therapy, treatment naïve
Introduction
With the approval of raltegravir for the treatment of HIV-1 in 2007, the HIV/AIDS patients have another drug in their armamentarium to increase their survival and improve the quality of life. According to the 2010 report of Joint United Nations Programme on HIV/AIDS (UNAIDS), there were nearly 33 million people infected with HIV-1 in 2009. This number is continuously increasing at a rate of ~ 7000 new infections everyday resulting in approximately 2.6 million new infections in 2009.1 Since the introduction of highly active antiretroviral therapy (HAART), HIV-1 infected patients have managed to maintain the quality of life and survive longer. However, HIV/AIDS remains incurable as none of these drugs eradicate the virus; rather just prevents viral replication. Due to the inevitable development of resistance to these drugs, search for inhibitors targeting novel viral or cellular targets has been an ongoing phenomenon. HIV-1 genome encodes for three viral enzymes, reverse transcriptase, protease, and integrase (IN), all essential for its replication. Several drugs have already been developed and approved for therapeutic use against reverse transcriptase and protease. Raltegravir belongs to a novel class of antiretroviral drugs as it inhibits the HIV-1 IN. Initially, raltegravir was approved for antiretroviral therapy (ART) in combination with other classes of drugs in treatment experienced patients. Later, its approved use was extended in ART naïve patients. Due to the absence of a IN homolog in human, inhibitors including raltegravir targeting IN possess lower toxicity and have better tolerability. Long term studies with raltegravir have proved it to be a safe and effective alternative to patients over the high toxicity associated with reverse transcriptase inhibitors and potential lipid profile changes in patients treated with protease inhibitors. This review discusses the findings from numerous clinical studies on the efficacy and safety of raltegravir primarily in treatment naïve patients.
Mechanism of Action
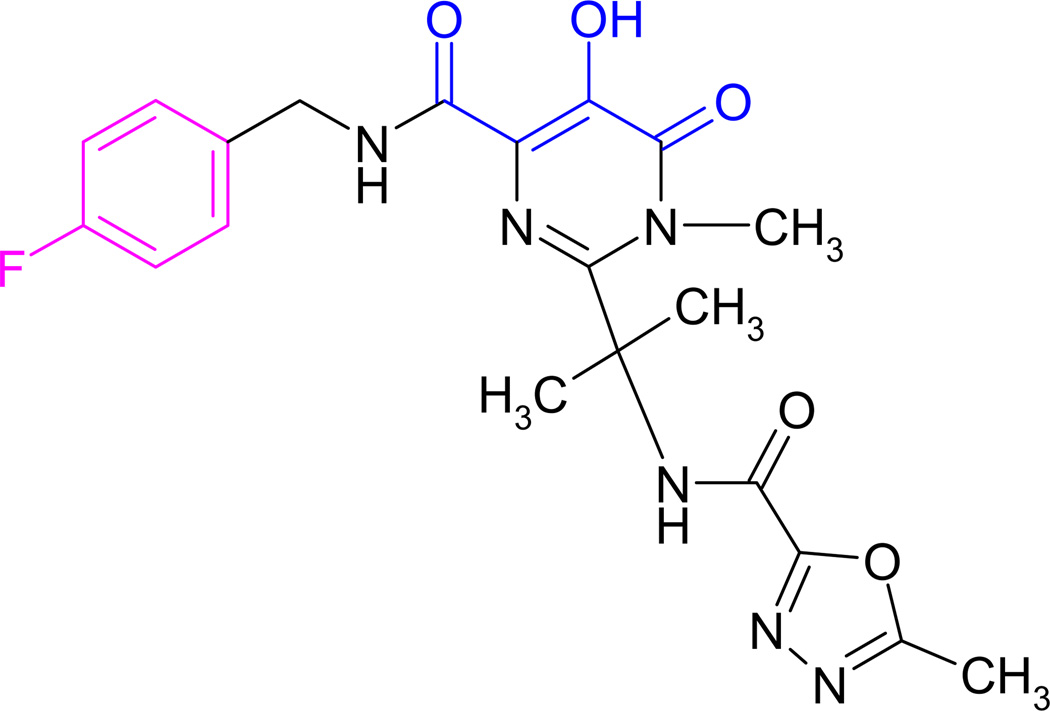
Raltegravir (Figure 1)2, developed by Merck & Co. is the first HIV-1 IN inhibitor approved by US Food and Drug Administration (FDA). Two other IN inhibitors, elvitegravir (GS-9137, JTK-303)3 and S/GSK 1349572 (dolutegravir)4,5 are in the late stages of clinical trials. IN is essential for viral replication as it is responsible for integration of viral DNA into the host DNA. IN is formed by the proteolytic processing of the gag-pro-pol precursor. HIV-1 IN contains 288 amino acids (aa) and is composed of three structural domains. The N-terminal domain (1–50 aa) contains a histidine-histidine-cysteine-cysteine (HHCC) motif that is involved in zinc binding and is associated with multimerization of IN.6 The catalytic core domain (50–212 aa) contains the catalytic triad of three acidic residues D-D-E motif comprising aspartic acid (D64, D116) and glutamic acid (E152) responsible for coordinating the metal ions and forming the IN active site.7,8 The C-terminal domain (213–288 aa), which adopts a SH3-like fold binds both the viral DNA and host DNA.9–11 During catalysis, IN first removes the terminal two nucleotides (GT) adjacent to the conserved CA motif from the 3’-end of the viral blunt-ended termini within the cytoplasmic preintegration complex in virus-infected cells leaving a recessed 3’-OH at both termini. In the next step, known as strand transfer, IN creates nicks on opposing strands of host DNA by a nucleophilic reaction using recessed 3’-OH ends and subsequent joining of the 3’-end of viral DNA into host DNA. Both of these reactions are separated temporally and occur in different cellular compartments. IN strand transfer inhibitors (STIs) including raltegravir inhibit the latter reaction and prevent the joining of viral DNA to the host DNA. STIs at the effective concentrations (IC50) have minimal effect on 3’-OH processing activity of IN.12,13
Figure 1.
Chemical structure of raltegravir. Diketo acid groups which chelate the Mg2+ from the active site are in blue. Halobenzyl group is in magenta color.
Chemical name of raltegravir is N-[(4-fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-2-[1-methyl-1-[[(5-methyl-1,3,4-oxadiazol-2yl)carbonyl]amino]ethyl]-6-oxo-4-pyrimidinecarboxamide.
Raltegravir blocks HIV-1 replication with a 95% inhibitory concentration (IC95) of 31 nM in 50% normal human serum in vivo.2,14 The biochemical mechanisms of raltegravir inhibitory effects have been investigated in vitro. STIs including raltegravir do not bind IN, rather they bind to the IN-DNA complex15,16, hence this class of inhibitors are also known as interfacial inhibitors.17 Binding of raltegravir to the IN-DNA complex is dependent on presence of divalent metal ions at the IN active site. Raltegravir chelate the metal ions through its diketo group in the active site within the IN-DNA complex (Figure 1). This complex, termed synaptic complex, is a transient intermediate in the concerted integration pathway in vitro. It is formed by the juxtaposition of two viral long terminal repeat (LTR) ends by IN and in presence of a target DNA forms the concerted integration products analogous to integration in vivo.18–20 Raltegravir and other STIs bind to and inactivate the synaptic complex, thereby preventing the binding of host target DNA thus inhibiting strand transfer.19–21 Raltegravir inhibits concerted integration activity with an IC50 value of ~21 nM in vitro.19 L-870,810, a sister compound of raltegravir which also contains an active diketo group has been shown to affect the structure of synaptic complex. L-870,810 binding within the synaptic complex moved the 5’-end of the non-transferred strand of U5 LTRs by more than 20 Å.18
Detailed mechanistic information about mode of action of STIs came from recent crystallization studies of IN from prototype foamy virus (PFV). Despite only ~20% sequence similarity between HIV-1 IN and PFV IN, the structures of individual domains in both INs are remarkably alike and share similar aa within the active site. PFV IN is also inhibited by HIV-1 IN STIs.22 The crystal structure of PFV IN complexed with its cognate substrate provided crucial insights into IN-DNA interactions.23,24 The crystal structure of PFV IN-DNA complex soaked with raltegravir and elvitegravir promoted an understanding of their inhibitory mechanism. Binding of raltegravir into the active site within the IN-DNA complex displaced the reactive 3’-OH group by more than 6 Å. The halobenzyl group localizes in the binding pocket created by the displacement of the 3’-OH group of terminal adenosine on the viral DNA.23 Inhibition by elvitegravir and other strand transfer inhibitors is similar to raltegravir.23,25 Attempts to crystallize the full-length HIV-1 IN alone or in complex with its DNA substrates have not yet been successful.
Metabolism and Pharmacokinetics
Raltegravir is metabolized via glucuronidation mediated primarily by uridine diphosphate glucuronosyltransferase (UGT)1A1 with minor contributions from UGT1A3 and UGT1A9.26 The major function of UGT1A1 and other related family enzymes are to metabolize drugs as well as a number of endogenous and exogenous substances including steroids, hormones, and bilirubin. Glucuronidation of raltegravir results in the formation of raltegravir glucuronide. Metabolism and disposition of raltegravir was determined using a single 200 mg dose of potassium salt of [14C]-raltegravir in healthy individuals. The 14C was monitored up to 240 h post-dose. Peak plasma concentration (Cmax 4.94 µM) and radioactivity was observed within 1 h of dosing. After 24 h of dosing, the raltegravir and radioactivity were undetectable suggesting a rapid clearance. Most of the radioactivity was eliminated through urine and feces at 32% and 51%, respectively, resulting in a total recovery of 83%. Of the total radioactivity recovered in urine, 95% of was released within first 8 h of dosing. LC-MS/MS analysis combined with radiochromatogram of urine and feces revealed two peaks correlating with raltegravir and its glucuronide derivative. In urine, approximately 8–11% of raltegravir was excreted unchanged and 23% of the dosage radioactivity was in form of raltegravir glucuronide.26,27 Raltegravir was the only peak observed in the fecal extracts. While in the plasma, 70% of the radioactivity was due to raltegravir and remaining in the form of its glucuronide derivative.
The pathway involved in raltegravir metabolism was established by in vitro studies. These studies utilized cDNAs expressing UGTs, human liver microsomes, and typical UGT1A1 substrates like bilirubin and β-estradiol acting as the inhibitors for raltegravir glucuronidation. Taken together, the in vitro studies confirmed that ~70% of raltegravir given in the dose was metabolized by UGT1A1.26
Metabolism of raltegravir is altered by the polymorphism present in UGT1A1. One of the common polymorphism associated with UGT1A1 contains TA repeat expansion (TA)7 in the TATAA box of the gene (Gilbert’s syndrome).28 Individuals homozygous for the (TA)7 allele, also known as UGTA1*28/*28 genotype, have higher serum bilirubin level because of the reduced UGT1A1 activity (~30%), compared to normal healthy individuals. This group of individuals having reduced UGT1A1 activity constitutes ~3–10% of the general population. However, in a study involving 30 subjects to analyze the pharmacokinetics of raltegravir (single 400 mg dose) in UGT1A1*28/*28 genotype, the reduced UGT1A1 activity did not result in clinically significant differences compared to the control subjects.29 The control arm of the study had 27 subjects containing normal length of TA repeats (TA)6 (UGT1A1*1/*1). The time to maximum concentration (Tmax) was remarkably similar (2.0 h) in both subject groups and the difference in half-life was not significant. However, reduced UGT1A1 activity in UGT1A1*28/*28 genotype subjects resulted in increased plasma raltegravir concentration (4.60 µmol/l) vs 3.20 µmol/l in subjects having normal genotype (UGT1A1*1/*1). Concentration at the 12 h time point (C12h) after administration of raltegravir was 91% higher in subjects with UGT1A1*28/*28 genotype. However, the higher raltegravir concentration did not result in any unexpected adverse effects. This finding was consistent with several other reports suggesting no dose-related toxicities with raltegravir.30,31
In comparison to other antiretroviral drugs, raltegravir has fewer drug-drug interactions. Interactions of raltegravir with other antiretroviral drugs have been described in detail elsewhere.31, 32 UGT1A1 expression is altered by a wide variety of drugs e.g. protease inhibitor atazanavir33 and non-nucleoside reverse transcriptase inhibitor efavirenz34 (Table 1). Rifampin is a broad potent inducer of UGT1A1 and other drug metabolizing enzymes like cytochrome P-450 (CYP) pathway. Rifampin reduces the effective raltegravir concentration. Rifampin is administered to HIV-1 infected individuals also diagnosed with tuberculosis (TB). TB is one of the leading causes of deaths in HIV-1 infected patients. Multiple studies have concluded that earlier initiation of ART in HIV-1 infected patients with tuberculosis leads to increased survival.35–37 Pharmacokinetics of raltegravir (400 mg single dose) in combination with rifampin (600 mg once a day) in healthy individuals showed that the area under the concentration curve (AUC0-∞) and Cmax for raltegravir was reduced 40% and 38%, respectively.38 In patients undergoing treatment with rifampin, the recommended dosage of raltegravir is 800 mg twice daily. The higher dose of raltegravir compensates the effect of rifampin on AUC0–12, however, the C12 remained largely unchanged probably due to the increased clearance caused by enhanced UGT1A1 activity resulting from rifampin exposure. In a clinical study involving four HIV-1 positive patients who had developed TB, raltegravir (800 mg twice daily) was given along with tenofovir/emtricitabine nearly two months after the beginning of TB treatment. At the end of TB treatment, all of the patients had undetectable level of HIV-1 RNA with no evidence of virological rebound. No significant adverse events due to the combined TB treatment and ART were reported. This study indicated that even though rifampin reduces the effective raltegravir concentration in plasma, raltegravir is still safe and effective in controlling HIV-1 replication in TB patients undergoing rifampin treatment.39 Pharmacokinetic study of raltegravir (800 mg twice a day) in two HIV-2 patients (one with HIV-1/HIV-2 dual infection) treated concurrently with rifampin for TB suggested that AUC value40 was as high as geometric mean value observed in phase II study with standard 400 mg twice a day dosage.30 A phase II clinical trial (NCT00822315) (Table 2) with treatment naïve HIV-1 infected patients receiving rifampin for TB, is underway to compare the efficacy and safety of two different doses of raltegravir (400 mg and 800 mg) and efavirenz in combination with tenofovir and lamivudine. The results from this study should help determine the appropriate dosage of raltegravir for patients undergoing rifampin treatment for TB.
Table 1.
FDA approved drugs for treatment in HIV-1 infected patients. Drugs are classified on the basis of their viral or cellular targets. Brand name of the drugs are in italics.
| Reverse Transcriptase | Protease | Integrase | Fusion | CCR5 | |
|---|---|---|---|---|---|
| NRTIs | NNRTIs | ||||
| Abacavir (ABC) Ziagen | Delavirdine (DLV) Rescriptor | Amprenavir (APV) Agenerase | Raltegravir (RAL) Isentress | Enfuvirtide (T20) Fuzeon | Maraviroc (MVC) Selzentry |
| Didanosine (ddI) Videx | Efavirenz (EFV) Sustiva | Atazanavir (ATV) Reyataz | |||
| Emtricitabine (FTC) Emtriva | Etravirine (ETR) Intelence | Darunavir (DRV) Prezista | |||
| Lamivudine (3TC) Epivir | Nevirapine (NVP) Viramune | Fosamprenavir (FPV) Lexiva | |||
| Stavudine (d4T) Zerit | Rilpivirine Edurant | Indinavir (IDV) Crixivan | |||
| Tenofovir (TDF, TFV) Viread | lopinavir/ritonavir (LPV/r) Kaletra | ||||
| Zidovudine (AZT, ZDV) Retrovir | Nelfinavir (NFV) Viracept | ||||
| Ritonavir (RTV) Norvir | |||||
| Combination Drugs | Saquinavir (SQV) Invirase | ||||
| Atripla (efavirenz/emtricitabine/tenofovir) | Tipranavir (TPV) Aptivus | ||||
| Combivir (lamivudine/zidovudine) | |||||
| Complera (emtricitabine/rilpivirine/tenofovir) | |||||
| Epzicom (abacavir/lamivudine) | |||||
| Trizivir (abacavir/lamivudine/zidovudine) | |||||
| Truvada (emtricitabine/tenofovir) | |||||
NRTIs, Nucleoside reverse transcriptase inhibitors; NNRTI Non-nucleoside reverse transcriptase inhibitors.
Table 2. A list of key clinical studies of raltegravir in antiretroviral treatment naïve patients.
This table was generated by compiling the information from Clinicaltrials.gov (accessed September, 2011) and published articles regarding use of raltegravir in treatment naïve patients.
| ClinicalTrials ID |
Study Description | Drugs | Ref |
|---|---|---|---|
| NCT0010004 (MK-0518-004) |
Multicenter, double-blind, randomized, dose ranging study to compare the safety and activity of MK-0518 with tenofovir and lamivudine versus efavirenz with tenofovir and lamivudine in ART-naive, HIV-infected patients (Phase II) | 100 mg monotherapy-10 days study, MK-0518 (100 mg b.id.) vs 200 mg monotherapy-10 days study, MK-0518 (200 mg b.id.) vs 400 mg monotherapy-10 days study, MK-0518 (400 mg b.id.) vs 600 mg monotherapy-10 days study, MK-0518 (600 mg b.id.) vs Placebo monotherapy, Placebo to MK-0518 (b.i.d) |
30,52–55 |
| Combination therapy 100 mg combination therapy, MK-0518 100 mg/tenofovir/ lamivudine vs 200 mg combination therapy, MK-0518 200 mg/tenofovir/ lamivudine vs 400 mg combination therapy, MK-0518 400 mg/tenofovir/ lamivudine vs 600 mg combination therapy, MK-0518 600 mg/tenofovir/ lamivudine vs EFV combination therapy, Efavirenz /tenofovir/lamivudine |
|||
|
NCT00369941 (STARTMRK, MK-0518-021) |
A multicenter, double-blind, randomized, active-controlled study to evaluate the safety and antiretroviral activity of MK-0518 (raltegravir) versus efavirenz in ART naive HIV-infected patients, each in combination with Truvada (Phase III) | Raltegravir (400 mg b.i.d.) and single tablet of Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) for up to 240 weeks vs Efavirenz tablet (600 mg q.h.s.) and single tablet of Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) for up to 240 weeks |
45–47 |
| NCT00632970 | A multi-center comparison of raltegravir to lopinavir/ritonavir, both in combination with Truvada, in ART naïve HIV-infected individuals (Phase IV) | Raltegravir (400 mg b.i.d.) vs Lopinavir/ritonavir 2 tablets twice a day |
|
| NCT01066065 | Metabolic profile and cardiovascular biomarker pattern compared in naïve patients initiating HAART with emtricitabine/tenofovir and raltegravir vs darunavir and ritonavir - a one year follow-up observational study | Raltegravir (400 mg b.i.d.) and single tablet of Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) vs Darunavir (800 mg q.d.) and Norvir (100 mg q.d.) with single tablet of Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) |
|
| NCT00745823 | A multicenter, double-blind, randomized, active comparator-controlled clinical trial to study the safety and efficacy of once daily raltegravir (MK-0518) versus twice daily raltegravir, each in combination with Truvada, in treatment-naïve HIV infected patients (Phase III) | Raltegravir (400 mg b.i.d.) and single tablet of Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) vs Raltegravir (800 mg q.d.) and single tablet of Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) Treatment period of 96 weeks. Study terminated |
49,50 |
|
NCT00660972 (A5248) |
Viral decay rates in treatment-naïve subjects initiating treatment with raltegravir and emtricitabine/tenofovir: a pilot study (Phase I) | Raltegravir (400 mg b.i.d.) and single tablet of Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) | |
| NCT00852618 | Intensive viral dynamics sub study of A5248 | Raltegravir (400 mg b.i.d.) vs Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) |
|
| NCT00752037 | Safety and efficacy of lopinavir/ritonavir in combination with raltegravir in HIV-infected patients (Phase IV) | Lopinavir/ritonavir (400 mg/100 mg b.i.d.) and raltegravir (400 mg b.i.d.) | |
|
NCT00811954 (ACTG5257) |
A comparative study of three non-nucleoside reverse transcriptase inhibitor (NNRTI)-sparing antiretroviral regimens for treatment-naïve HIV-1-infected volunteers (the ARDENT study: atazanavir, raltegravir, or darunavir with emtricitabine/tenofovir for naive treatment) (Phase III) | Emtricitabine/tenofovir, ritonavir, atazanavir vs Emtricitabine/tenofovir, raltegravir vs Emtricitabine/tenofovir, darunavir, ritonavir |
|
| NCT00851799 | Cardiovascular, anthropometric, and skeletal effects of ART initiation with emtricitabine/tenofovir and atazanavir/ritonavir (ATV/r), darunavir/ritonavir (DRV/r), or raltegravir: metabolic sub-study of A5257 | Atazanavir (ATV) and ritonavir (RTV) and Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) vs Raltegravir (400 mg b.i.d.) and single tablet of Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) vs Darunavir and ritonvair (DRV/r) in combination with Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) |
|
| NCT00711009 | A randomized, open-label study of lopinavir/ritonavir 400/100 mg tablet twice daily and co-formulated emtricitabine/tenofovir 200 mg/300 mg once daily versus lopinavir/ritonavir 400/100 mg tablet twice daily and raltegravir 400 mg twice daily in antiretroviral naive, HIV-1 infected subjects (Phase III) | Raltegravir (400 mg b.i.d.) and lopinavir/ritonavir (400/100 mg b.i.d.) vs Lopinavir/ritonavir (400/100 mg tablet b.i.d.) and Truvada (single tablet emtricitabine/tenofovir 200 mg/300 mg q.d.) |
|
| NCT01258439 | Post-prandial lipid effects of raltegravir vs ritonavir-boosted darunavir (DRV/r) in ART-naïve adults (Phase IV) | Raltegravir (400 mg b.i.d.) and single tablet of Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) for 24 weeks vs Ritonavir (100 mg), darunavir (800 mg), Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) for 24 weeks |
|
| NCT00677300 | Raltegravir and darunavir antiretroviral in antiretroviral naïve patients (RADAR) (Phase IV) | Raltegravir (400 mg b.i.d.), darunavir/ritonavir (800 mg/100 mg q.d.) vs Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) and darunavir/ritonavir (800 mg/100 mg q.d.) |
|
|
NCT00830804 (ACTG 5262) |
A pilot efficacy and safety trial of raltegravir plus darunavir/ritonavir for treatment-naive HIV-1-infected subjects (Phase IIb) | Raltegravir (400 mg b.i.d.), darunavir/ritonavir (800 mg/100 mg q.d.) | 51 |
| NCT00654147 | A pilot study to assess virologic suppression and immune recovery of raltegravir and lopinavir/ritonavir and raltegravir and emtricitabine/tenofovir in HIV-1 infected treatment-naïve subjects (Phase II) | Raltegravir (400 mg b.i.d.) and lopinavir/ritonavir (400 mg/100 mg b.i.d.) vs Raltegravir (400 mg b.i.d.) and single tablet of Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) |
|
| NCT01291459 | Pilot study of simplification to maraviroc - raltegravir dual therapy after 6 months of maraviroc - raltegravir - tenofovir - emtricitabine quadruple therapy in ART naïve HIV-1-infected patients with CCR5- virus (Phase II) | Maraviroc/raltegravir/emtricitabine/tenofovir for 24 weeks followed by maraviroc/raltegravir for 24 weeks | |
| NCT00822315 | Open-label randomized multicenter trial to compare the efficacy and safety of two different doses of raltegravir and efavirenz, all in combination with tenofovir and lamivudine, in naïve HIV-1-infected patients receiving rifampin for active tuberculosis (Phase II) | Tenofovir 245 mg/lamivudine 300 mg/efavirenz 600 mg vs Tenofovir 245 mg/lamivudine 300 mg/raltegravir 400 mg vs Tenofovir 245 mg/lamivudine 300 mg/raltegravir 800 mg |
|
| NCT00762892 | Randomized, prospective, single site trial evaluating raltegravir vs. atazanavir in combination with Truvada for the treatment of antiretroviral naïve HIV infected patients (Phase IV) | Raltegravir 400 mg b.i.d, Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) vs Atazanavir 300 mg q.d., Norvir 100 mg q.d. and Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) |
|
| NCT00740064 | A pilot study of highly active antiretroviral therapy using raltegravir and Epzicom (abacavir/lamivudine) in antiretroviral naïve HIV-infected subjects (Phase IV) | Raltegravir 400 mg b.i.d. and Epzicom (abacavir/lamivudine 600 mg/300 mg q.d.) | |
| NCT01204905 | Raltegravir and maraviroc in combination for the treatment of antiretroviral naïve HIV-1 infected patients | Raltegravir 400 mg b.i.d. Maraviroc 300 mg b.i.d. |
|
| NCT00808002 | Efficacy of treatment intensification with maraviroc on HIV-1 viral latency in recently infected HIV-1 naïve patients starting raltegravir plus tenofovir/emtricitabine. | Raltegravir 400 mg b.i.d., Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.), Maraviroc 300 mg b.i.d. vs Raltegravir 400 mg b.i.d., Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) |
|
| NCT01105611 | An open-label, randomized pilot study comparing the efficacy, safety and tolerability of raltegravir with protease inhibitor-based therapy in treatment-naïve, HIV/Hepatitis C co-infected injecting drug users receiving methadone (Phase IV) | Raltegravir 400 mg b.i.d., Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) vs Atazanavir 300 mg q.d., ritonavir 100 mg q.d. and Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) |
|
| NCT00525733 | A phase II, randomized trial of open-label Truvada with darunavir/ritonavir versus multiclass therapy with Truvada, darunavir/ritonavir, maraviroc and raltegravir in acutely HIV-1 infected antiretroviral-naïve subjects (Phase II) | Raltegravir 400 mg b.i.d., Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.), darunavir 800 mg/ritonavir 100 mg q.d., Maraviroc 150 mg b.i.d. vs Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.), darunavir 800 mg/ritonavir 100 mg q.d. |
|
| NCT01066962 | An open-label randomized two-year trial comparing two first-line regimens in HIV-infected antiretroviral naïve subjects: darunavir/r and tenofovir/emtricitabine vs. darunavir/r and raltegravir (ANRS143/NEAT 001) (Phase III) | Darunavir-two tablets of 400 mg q.d. Ritonavir-single capsule of 100 mg q.d. Raltegravir-single tablet of 400 mg b.i.d. vs Darunavir-two tablets of 400 mg q.d. Ritonavir-single capsule of 100 mg q.d. Tenofovir/emtricitabine 245 mg/200 mg, single tablet q.d. |
|
| NCT01042652 | A pilot study with randomized controlled open-label design to compare drug-drug interaction, antiretroviral efficacy and tolerability of raltegravir versus nevirapine as anchor drug in combination therapy for treatment-naïve HIV and Chinese injection drug users on methadone maintenance | Raltegravir (400 mg b.i.d.) vs Nevirapine |
|
| NCT00752856 | Nucleoside-sparing combination therapy with lopinavir/ritonavir and raltegravir vs. efavirenz and tenofovir/emtricitabine in antiretroviral-naïve patients (Phase II) | Raltegravir (single tablet 400 mg b.i.d.) and Kaletra (two tablets of lopinavir/ritonavir 400/100 mg b.i.d.) vs Atripla (single tablet containing efavirenz/tenofovir/emtricitabine 600/300/200 mg q.d.) |
|
| NCT01225705 | An open, prospective study to compare the safety and efficacy of raltegravir vs. atazanavir/ritonavir, both in combination with tenofovir and emtricitabine, in the treatment of HIV-infection in ART naïve subjects with HCV co-infection (Phase IV) | Raltegravir and Truvada (emtricitabine/tenofovir 200/300 mg) vs Atazanavir/ritonavir and Truvada (emtricitabine/tenofovir 200/300 mg) |
|
| NCT01227824 | A randomized, double blind study of the safety and efficacy of GSK1349572 (50 mg once daily) to raltegravir (400 mg twice daily) both administered with fixed-dose dual nucleoside reverse transcriptase inhibitor therapy over 96 weeks in HIV-1 infected antiretroviral therapy naïve adult subjects (Phase III) | GSK1349572 (n=394) GSK1349572 (50 mg q.d.)/ raltegravir placebo (b.i.d.)/NRTI background therapy (q.d.) vs Raltegravir (n=394) Raltegravir (400 mg b.i.d.)/GSK1349572 placebo (q.d.)/NRTI background therapy (q.d.) |
|
| NCT01059422 | Efficacy and safety of an initial regimen raltegravir and lamivudine/abacavir fixed-dose combination (3TC/ABC) for 48 weeks in ART-naïve, HIV/TB co-infected adult subjects receiving rifabutin-containing, first-line anti-TB therapy (Phase IIIb/IV) | Raltegravir (400 mg b.i.d.), Epzicom (abacavir/lamivudine 600 mg/300 mg q.d.) | |
| NCT00863668 | Decay kinetics of HIV with the integrase inhibitor raltegravir | Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.) vs Atripla (efavirenz/emtricitabine/tenofovir- 600/200 mg/300 mg) |
|
| NCT00667433 | CID 0706 - Safety, tolerability, pharmacokinetic, and metabolic features of raltegravir among African-American men and women with HIV infection (Phase IV) | Raltegravir (400 mg b.i.d.) and single tablet of Truvada (emtricitabine/tenofovir 200 mg/300 mg q.d.)- 104 weeks | |
| NCT00661960 | CCRC: A pilot project of virologic, pharmacologic and immunologic correlates of gastrointestinal-associated lymphoid tissue immune reconstitution following raltegravir therapy | HIV Negative individuals- No intervention vs HIV-Positive individuals-Raltegravir and two other NRTIs vs HIV-Positive individuals-efavirenz or any other NNRTI in combination with two other NRTIs |
q.d. once a day; b.i.d. twice a day; q.h.s. every night at bedtime.
ATP binding cassette (ABC) efflux transporter family and solute carrier uptake transporters have been implicated in transport of raltegravir as well as several other antiretroviral drugs.41 Polymorphism in ABC efflux transporter family ABCB1 gene (3435C-T) coding for a P-glycoprotein (a transporter protein) results in ~60% lower raltegravir plasma concentration. The median plasma raltegravir concentration in individuals with CT/TT genotype was 221 ng/ml [IQR 92–626] compared to 530 ng/ml [IQR 212–1066] in normal individuals with CC genotype.42 However, the viral response to raltegravir was not significantly affected by this polymorphism.
Pharmacokinetics of raltegravir in treatment naïve HIV-1 patients receiving different dosage of raltegravir (100, 200, 400 or 600 mg twice a day) suggested no apparent dose response with regard to its virological efficacy.30 Mean trough raltegravir concentration (C12h) in each dosage group was higher than the in vitro IC95 value of 33 nM. Mean values for Cmax and AUC0–12h increased up to the 400 mg dose but were similar for the 400 and 600 mg regimens.30 In a phase I multi-dose pharmacokinetic study in healthy individuals, trough levels (C12) were more than 33 nM for raltegravir dosage levels 100 mg or higher.27 Results from these two studies supports the recommended 400 mg twice a day dosage in patients as it will maintain the C12 higher than the IC95 value. AUC0-∞ was found to be similar in male and female subjects given a single 400 mg dose of raltegravir. AUC was considered to be the most appropriate pharmacokinetic parameter for raltegravir to determine its clinical significance. In multiple studies, raltegravir followed a biphasic pharmacokinetics. At the recommended raltegravir dose 400 mg twice a day, the terminal elimination half-life is ~9 h (7–12 h range) with shorter 1 h half-life in α-phase.27,31 Steady state is generally reached in 2 days. No major toxicities were reported with higher doses of raltegravir up to 1600 mg/day. Gender or the race (black vs non-black) of the patients had no significant effect on raltegravir pharmacokinetics.43
There have been no studies to determine the pharmacokinetics or efficacy of raltegravir in pediatric HIV-1 patients. Pediatric formulations in the form of oral granules in suspension and chewable ethyl cellulose tablets given to healthy adult individuals (n=12) had the similar pharmacokinetics as the marketed poloxamer tablet.44 The individuals were given a single 400 mg dose of raltegravir in different formulations. Both pediatric formulations were well tolerated and had moderately higher AUC0-∞ and Cmax compared to the tablet formulation. No serious clinical adverse events were reported in this study. The individuals who took raltegravir along with a high fat diet led to an increase in C12h, a reduction in Cmax and delay in Tmax, however, the AUC0-∞ was not changed.44 At present, raltegravir is not approved for pediatric use.
Safety and Efficacy in Clinical Studies
A list of key clinical studies with raltegravir in treatment naïve patients is given in Table 2. Monotherapy with multiple doses of raltegravir in treatment naïve HIV-1 infected individuals for 10 days showed it to contain superior antiretroviral activity compared to the placebo.30 The subject individuals had ≥5000 copies/ml viral RNA and CD4 cell count of greater than 100 cell/mm3. Raltegravir was administered twice a day at a fixed dose (100, 200 400 or 600 mg). Patients in each group treated with raltegravir showed at least 2-fold log reduction in HIV-1 RNA load. At least 50% of the subjects in each dosage group achieved viral RNA level <400 copies/ml.
STARTMRK is an ongoing phase III clinical trial (ClinicalTrials.gov identifier NCT00369941, Merck MK-0518 protocol 021) designed to compare the efficacy and safety of raltegravir in ART naïve HIV-1 patients. This study compared raltegravir against efavirenz, both in combination of tenofovir and emtricitabine (Table 1). Raltegravir at 400 mg twice a day or the placebo was given at 12 h interval. Tenofovir (300 mg) and emtricitabine (200 mg), nucleoside reverse transcriptase inhibitors, were administered as a single dose of Truvada in the morning with food. Patients in the efavirenz arm were advised to take 600 mg dose (or placebo) on empty stomach at the bed time. The enrolled patients were ≥18 years old, had >5000 copies/ml of HIV-1 RNA with no evidence of resistance to efavirenz, tenofovir or emtricitabine. Patients were from broad demographics belonging to five continents and hence infected with HIV-1 subtype B as well as non-B clades. Patients were sub grouped on the basis of baseline HIV-1 RNA level (less than or more than 50,000 copies/ml), their Hepatitis B and Hepatitis C status. Results obtained at 156 week of the study suggested that raltegravir exhibited durable antiretroviral response that was non-inferior to the efavirenz.45 Even though both of the treatment regimens (raltegravir and efavirenz) were well tolerated, the subjects in raltegravir arm experienced significantly fewer drug related adverse events. Considering all the non-completers as the failures (NC=F), 75.4% (212/281) patients on raltegravir regimen achieved viral RNA level <50 copies/ml. In comparison, among the patients receiving efavirenz, ~68% (192/282) of the individuals achieved viral RNA level <50 copies/ml. Similar results were reported at the 48 week and 96 week of the study duration. At 48 weeks, 86% of patients on raltegravir regimen compared to 82% receiving efavirenz had <50 copies/ml HIV-1 RNA.46 At 96 weeks, the viral suppression to <50 copies/ml was 81% and 79%, respectively, in two arms of study.47 In fact, raltegravir was superior to efavirenz (85% vs 79%) in reducing the viral load to less than 50 copies/ml, when the 156 week analysis was performed considering only the treatment related discontinuations as failure. The number of patients who achieved <400 copies/ml at 156 week of treatment was ~80% and 72%, in raltegravir and efavirenz study groups, respectively.45
Efficacy of raltegravir in patients infected with non-B subtype HIV-1 was evaluated in STARTMRK and BENCHMRK trials.48 Non-B subtype HIV-1 infections account for approximately 90% of infections world-wide and is prevalent in developing countries like India, South Africa, and countries in Southern America. Subtype C alone is responsible for nearly 50% of all HIV-1 infections. Subtype-B infections are prevalent in Europe and United States. After 96 week of raltegravir treatment in ART naïve patients, virologic, and immunologic response in non-B subtype patients was similar to subtype B patients with 95% and 89% of patients, respectively, achieved HIV-1 RNA level <50 copies/ml. Similar efficacy of raltegravir in non B subtype was observed in treatment experienced patients in BENCHMRK studies.48
A 96 week study to determine the efficacy and safety of 800 mg once-daily raltegravir dose compared to twice a day 400 mg raltegravir (ClinicalTrials.gov identifier NCT00745823) was carried out in treatment naïve patients.49 This phase III clinical trial was multicenter, double blinded and randomized. Patients in both arms (400 mg raltegravir twice a day vs 800 mg single dose) were also given a single dose of Truvada (200 mg of emtricitabine and 300 mg of tenofovir). In the patient group receiving 800 mg single dose, 83% (n=318/382) of patients achieved HIV-1 RNA level <50 copies/ml, compared to ~89% (n=343/386) in the group receiving 400 mg raltegravir twice a day. The differences between two dosage groups were more stark in patients with high viral load (>100,000 copies/ml of HIV-1 RNA). In these patients, only 74% (n=113/152) of individuals in once-daily dose (800 mg) group achieved viral suppression compared to 84% of individuals in twice-daily dose group. Even though these results with once-daily dosage seem promising, it did not meet the pre-defined statistical criteria for non-inferiority. 49,50 Based on these findings, Merck terminated the trial and the patients were switched to the approved twice-daily raltegravir dose.
Efficacy of raltegravir in combination with protease inhibitors darunavir and ritonavir (Table 1) in treatment naïve HIV-1 patients was determined in a phase 2b study.51 The study was carried out to determine if these two classes of inhibitors were sufficient to control the HIV-1 infection and hence the patients will be spared of the potential severe toxicity associated with reverse transcriptase inhibitors. The enrolled patients were ≥18 years of age, with HIV-1 viral RNA load ≥5000 copies/ml, and contained no more than one darunavir resistance mutation or known raltegravir associated major resistance mutation in IN. Patients (n=112) were given darunavir (two tablets of 400 mg), single 100 mg capsule of ritonavir, and the recommended twice a day dose (400 mg) of raltegravir. Darunavir/ritonavir in combination with raltegravir was well tolerated in patients. The primary end point was virologic failure at week 24 or earlier. The viral load was <50 copies/ml in 79% and <200 copies/ml in 93% of the patients at 24 week analysis using an intent to treat approach. At 24 weeks, 16% of patients had virologic failure and by week 48, virologic failure rate was 26%. The patients with a baseline viral load of more than 100,000 copies/ml and lower CD4 counts had a higher tendency to experience virologic failure (n=18/43). In summary, even though the virologic efficacy observed in this study with darunavir/ritonavir in combination with raltegravir met the required protocol definition of being acceptable at 24 weeks but at 48 weeks only 71% of patients had less than 50 copies/ml of HIV-1 RNA.51 The unacceptably high virologic failure associated with patients with baseline viral load of >100,000 copies/ml might be due to presence of low level raltegravir resistance mutations in patients.
Final analysis of Protocol 004 (Merck) showed durable efficacy and tolerability of raltegravir in combination with tenofovir/lamivudine (300/300 mg single dose a day) in treatment naïve HIV-1 infected patients.52 The patients enrolled in this study (ClinicalTrials.gov identifier- NCT00100048) had >5000 copies/ml of HIV-1 RNA, CD4 cells >100 cells/mm3, and no prior resistance to the drugs used in the study. During the first 48 weeks, patients in one group (n=160) were given different doses of raltegravir (100/200/400/600 mg twice a day) and the patients in another group (n=38) received efavirenz.53 All treatment groups showed significant rapid and durable improvement in their plasma HIV-1 RNA level with 83% to 88% of patients achieving <50 copies/ml. Patients receiving raltegravir achieved RNA levels below the detection much faster than the patients on efavirenz therapy. In this study, raltegravir was well tolerated at all the doses. After 48 weeks, all patients in the raltegravir group received 400 mg twice a day. Similar efficacy and safety in the raltegravir arm of the study was reported at 96 week54 and 192 week.55 At 96 week, 83% of the patients in the raltegravir group and 84% in the efavirenz group achieved <50 copies/ml HIV-1 RNA. At week 192, 74% of the patients in both groups (raltegravir vs efavirenz) maintained <50 copies/ml HIV-1 RNA.
Final analysis of this study at 240 week reported that patient group receiving raltegravir had a slightly higher percentage (69%) of people with <50 copies/ml compared to efavirenz group (63%). The number of CD4 T-cells increased continuously during this study period. Exploratory analysis revealed a strong correlation between the baseline CD4 counts and log HIV-1 RNA decline at 8 week with the progressive increase in the CD4 cells over the duration of treatment. This finding also underscores the need to start the treatment early in HIV-1 infected individuals to have a better prognosis. Raltegravir was generally well tolerated for 5 years and was associated with fewer adverse events related to the treatment compared to efavirenz in combination with tenofovir/lamivudine.52
The frequent clinical adverse events associated with raltegravir therapy included nausea, dizziness, headache, diarrhea, vertigo, and fatigue. Patients on raltegravir regimen had minimal effects on LDL-cholesterol and triglycerides. Frequency of immune reconstitution inflammatory response (IRIS) in raltegravir treated group was similar to as in groups receiving other antiretroviral drugs. A long term safety profile of raltegravir and associated adverse events have been reviewed earlier.56
Raltegravir is equally effective in a diverse cohort of patients. Safety and efficacy of raltegravir was determined in a clinical study which enrolled 74% of (156/209) HIV-1 infected black patients and 47% (98/209) females. Some of these individuals were ART naïve (n=22), ART experienced with failing previous treatment (n=98) and remaining were intolerant to current therapy (n=89). Results reported after 48 weeks of treatment with raltegravir in combination of other approved ART drugs showed that 68% (98/145) of black patients and 78% (39/50) of non-black patients achieved HIV-1 RNA levels below 50 copies/ml. Similarly, 68% (61/90) of female patients compared to 72% (76/105) male patients achieved HIV-1 RNA level below 50 copies/ml.43
Resistance to Raltegravir
Development of resistance to antiviral drugs seems to be an inevitable phenomenon due to high mutation rate in HIV-1 replication.57 Treatment with raltegravir results in the emergence of resistant viruses containing mutations in IN, specifically in proximity to the active site. Raltegravir has a low genetic barrier; even a single mutation in IN results in marked reduction in virus susceptibility. In BENCHMRK 1 and BENCHMRK 2 studies, genotyping of IN in the patients experiencing virologic failure suggested two predominantly pathways associated with the presence of N155H or Q148H/R/K.58 Often, the N155H pathway with the lower resistance is replaced by the Q148H/R/K pathway resulting in higher replication rate and significant reduction in the susceptibility to raltegravir.59,60 Substitution at Q148 resulted in more severe defect in replication61 and IN activity compared to the mutations at N155.19 In small populations, another pathway with Y143/C/H/R mutations was also observed.58,62 The level of resistance provided by mutations at Q148 and Y143 is always higher than N155 mutations.63 These primary mutations are often associated with secondary mutations (N155H-E92Q, Q95K, T97A, Y143R/H, V151I, L74M, G163R; Q148H/R/K-G140S/A, E138K; Y143C/H/R- L74M, T97A, E138A/D, G163R, S230R) which provide much higher level of resistance and replication rate of mutant viruses.64–69 Primary resistance mutations and majority of secondary mutations do not result from the natural polymorphism in the raltegravir naïve patients.69–71 The patients with higher baseline viral load were at greater risk of developing resistance to raltegravir. Several of these mutations provide cross-resistance to elvitegravir. It rules out the use of elvitegravir as salvage therapy in patients failing the raltegravir treatment. Fortunately, several of these mutant viruses are susceptible to dolutegravir (S/GSK134972), a second generation IN inhibitor.72,73 Moreover, these mutations in the active site of IN do not affect the susceptibility of the virus to other antiviral drugs like protease inhibitors, reverse transcriptase inhibitors and entry inhibitors (Table 1). Hence, a combination therapy with drugs targeting multiple viral targets is always recommended.
Till now, the primary resistance mutations were not observed in IN STI naïve therapy patients; hence the existing treatment guidelines did not recommend an IN genotype before starting the raltegravir based treatment.74 However, recently two reports have indicated the occurrence of transmitted resistance to raltegravir, one involving N155H mutation75 and another patient with Q148H and G140S.76 These mutations caused the raltegravir treatment failure in these patients. These cases illustrate the presence of multi-drug resistance even in treatment naïve patients and underscore the need to obtain the genotype present in individual and tailor the therapy accordingly.
Patient preference
By virtue of being a recently approved drug for HIV-1 treatment, there are few clinical studies which have deciphered the patient preference for raltegravir compared to other drugs. However, raltegravir therapy is generally well-tolerated with significantly less severe adverse events compared to other available treatments. Patients who were on a stable efavirenz treatment for more than three years reported a better quality of life when they switched to raltegravir.77 Adverse events associated with efavirenz include neuropsychiatric issues, depression, anxiety, and sleep problems. Patients who were switched to raltegravir reported significant improvements in anxiety and stress as well as their lipid profile.77 The patients switched to raltegravir from a tenofovir/emtricitabine and ritonavir boosted protease inhibitor treatment reported improvement in the renal function, increased glomerular filtration rate and a drop in the urine protein level.78 In patients with multidrug-resistant HIV-1 infection under control (viral RNA level <400 copies/ml), a switch from fusion inhibitor enfuvirtide based regimen to raltegravir (400 mg twice daily) in combination with same background regimen was well tolerated and had sustained antiviral efficacy for up to 48 weeks.79 Out of the total 85 patients who had switched to raltegravir based regimen, only one experienced virologic failure and none of the patients were lost in follow up or died.
In SWITCHMRK1 and 2 studies, patients on lopinavir/ritonavir (Kaletra) therapy who had HIV-1 replication in control and viral RNA level <75 copies/ml for three months were switched to raltegravir.81 Both groups also continued background therapy of at least two nucleoside or non-nucleoside reverse transcriptase inhibitors. Lopinavir/ritonavir (Kaletra) therapy can cause lipid abnormalities in patients.80 At 12 week analysis after switching to raltegravir, patients had greater reduction in baseline fasting cholesterol and triglycerides compared to the patients continuing the lopinavir/ritonavir (Kaletra) regimen. However, the virological efficacy analyses after 24 week of treatment did not establish the non-inferiority of raltegravir to lopinavir/ritonavir (Kaletra) and hence the study was stopped.81 In this study, patients were not tested for resistance because all the enrolled patients needed to meet undetectable viral load. It highlights the need to have the information about previous treatment and possible resistance mutations in the viral population. ART naïve patients receiving raltegravir in combination with lopinavir/ritonavir (Kaletra) had less severe effect on bone density compared to traditional antiretroviral regimen – emtricitabine/tenofovir (Truvada) and lopinavir/ritonavir (Kaletra). Both of these treatment regimens had similar antiviral efficacy over 96 weeks of therapy.82
At 48 weeks, patients switching from ritonavir boosted protease inhibitors to raltegravir (SPIRAL study) with combination therapy experienced similar efficacy, better lipid profile and lower total to HDL ratios than continuing the ritonavir boosted protease inhibitor.83 Ritonavir boosted protease inhibitor therapy is associated with an increased risk of myocardial infarcation caused by dyslipidemia. In HIV-1 uninfected individuals, raltegravir (400 mg twice a day) induced much less postprandial (after meal) lipid changes compared to the low dose of ritonavir (100 mg once a day).84 In summary, generally the patients switching to raltegravir based treatment from most other regimens observed similar potent antiviral efficacy with an added advantage of less severe adverse events.
Place in therapy
Guidelines issued by Department of Health and Human Services (DHHS) in 2011 recommends four preferred regimens for the treatment of HIV/AIDS in ART naïve patients.74,85 These guidelines advocate earlier initiation of ART to reduce both AIDS related and non-related morbidity and mortality and the prevention of sexual transmission of HIV-1. These regimens were proposed on the basis of randomized controlled trials, efficacy, and safety of drugs. A list of all of the FDA approved drugs for HIV-1 treatment is given in Table 1. Each of these regimens include drugs targeting (i) reverse transcriptase - (NRTIs, Nucleoside reverse transcriptase inhibitors; NNRTIs, Non-nucleoside reverse transcriptase inhibitors) – efavirenz/tenofovir/emtricitabine, (ii) protease - atazanavir/ritonavir (ATV/r) in combination with NRTIs, (iii) protease - darunavir/ritonavir (DRV/r) in combination with NRTIs tenofovir/emtricitabine, and (iv) IN - raltegravir in combination with NRTIs tenofovir/emtricitabine. Initially, raltegravir was approved to use in treatment experienced HIV-1 infected individuals with multidrug resistance and in patients as salvage therapy. In 2009, FDA approved raltegravir for use in treatment naïve patients. Raltegravir in combination with NRTIs, abacavir or zidovudine, and lamivudine is also an acceptable regimen for ART naïve patients. In a pilot study, raltegravir (400 mg twice a day) in combination with abacavir/lamivudine (600/300 mg once a day) was effective and well tolerated with no serious adverse events during the 48 week of the treatment regimen.86 Advantage of raltegravir in comparison to other drugs is its tolerability and fewer serious adverse events related to the drug. Since, raltegravir is not metabolized by liver CYP enzymes, but rather via UGT1A1 mediated glucuronidation, it has very few interactions with other antiretroviral drugs. In patients with mild to moderate hepatic or renal insufficiency, no dosage adjustment of raltegravir was required. Compared to other treatment plans, raltegravir based therapy has certain limitations; raltegravir must be administered twice a day. It would restrict its use as in a “QUAD like” drug having a combination of drugs and once a day dosing. A single-tablet once a day dose of combination drug “QUAD” containing IN inhibitor elvitegravir, CYP3A inhibitor and pharmacokinetic booster cobicistat, reverse transcriptase inhibitors emtricitabine and tenofovir showed non-inferiority to Atripla (efavirenz, emtricitabine and tenofovir) in treatment naïve patients (ClinicalTrials.gov identifier NCT01095796). A low genetic barrier to raltegravir resistance is another risk, a single mutation in IN can render raltegravir largely ineffective. Clinical studies involving raltegravir in combination with other classes of approved HIV-1 drugs are ongoing and should result in an increase in approved regimens utilizing raltegravir.
Patients on effective ART have residual plasma viremia which may be due to low-level replication or release of virus from long-lived HIV-1 infected cells. Efforts have been made to add another drug to existing effective regimens in patients to lower the residual viremia. Raltegravir intensification in several studies where patients were on optimized therapy with controlled viral replication did not further reduce the HIV-1 RNA level in plasma87–91 or in cerebrospinal fluid.92 Some studies have indicated that raltegravir intensification results in a specific and transient increase in episomal 2-LTR circles and immune activation which, however, normalized to the baseline level over longer (beyond 4 week) period of treatment93 and a decrease in unspliced HIV-1 RNA level in CD4 cells from ileum.90 The results from majority of studies suggest that low level plasma viremia is not due to ongoing viral replication and eradication of HIV-1 will require novel therapeutic approaches.
Conclusions
Raltegravir belongs to an important new class of antiretroviral drugs which inhibits IN. Raltegravir has proved to be safe and effective in treatment naïve as well as treatment experienced patients failing the existing antiretroviral regimens. However, raltegravir is not without its own drawbacks, foremost is its low genetic barrier resulting in development of resistance. Several IN inhibitors with a longer dissociation rate have shown promise in being effective against the emerging raltegravir resistant viruses.4,5 Efforts to target IN oligomerization with small molecule inhibitors, 3’-OH processing, and novel pharmacophores to inhibit the strand transfer are ongoing. The need to develop antiretroviral agents with novel mechanism of action persists for treatment in naïve and experienced patients. It is especially crucial to target and eliminate the viral reservoirs in HIV-1 latently infected cells to eradicate viral infection. Along with the better education to control the HIV-1 transmission and the continued improvement in the treatment regimens, we can hope a better outcome for HIV-1 patients. It may be interesting to identify novel targets for therapy or vaccines by taking cues from the “elite controllers”, HIV-1 infected people some of whom control their viremia to low levels and do not develop AIDS.94 Teaching the immune system to control the HIV-1 replication might be a better choice than life-long treatment with antiretroviral drugs.
Acknowledgements
Author is supported by National Institute of Allergy and Infectious Diseases Grant R21-AI081629 awarded to Dr. Duane Grandgenett. I would like to thank Dr. Duane Grandgenett and Dr. Sibes Bera for providing critical comments on the manuscript.
Abbreviations
- ART
antiretroviral therapy
- AUC
area under concentration curve
- C12
concentration after 12 h of dose administration
- Cmax
maximum concentration after a dose is given (peak plasma concentration)
- DHHS
Department of Health and Human Services
- FDA
food and drug administration
- HAART
highly active antiretroviral therapy
- HIV-1
human immunodeficiency virus type 1
- IN
integrase
- NNRTIs
non- nucleoside reverse transcriptase inhibitors
- NRTIs
nucleoside reverse transcriptase inhibitors
- STIs
strand transfer inhibitors
- TB
tuberculosis
- UNAIDS
Joint United Nations Programme on HIV/AIDS
Footnotes
Author Disclosure: Author declares no competing financial interest.
References
- 1.UNAIDS. UNAIDS report on the global AIDS epidemic. [Accessed September 2011];2011 http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf.
- 2.Summa V, Petrocchi A, Bonelli F, et al. Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhibitor for the treatment of HIV-AIDS infection. J Med Chem. 2008 Sep 25;51(18):5843–5855. doi: 10.1021/jm800245z. [DOI] [PubMed] [Google Scholar]
- 3.Shimura K, Kodama EN. Elvitegravir: a new HIV integrase inhibitor. Antivir Chem Chemother. 2009;20(2):79–85. doi: 10.3851/IMP1397. [DOI] [PubMed] [Google Scholar]
- 4.Hightower KE, Wang R, Deanda F, et al. Dolutegravir (S/GSK1349572) Exhibits Significantly Slower Dissociation than Raltegravir and Elvitegravir from Wild-Type and Integrase Inhibitor-Resistant HIV-1 Integrase-DNA Complexes. Antimicrob Agents Chemother. 2011 Oct;55(10):4552–4559. doi: 10.1128/AAC.00157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi M, Yoshinaga T, Seki T, et al. In Vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother. 2011 Feb;55(2):813–821. doi: 10.1128/AAC.01209-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng R, Jenkins TM, Craigie R. Zinc folds the N-terminal domain of HIV-1 integrase, promotes multimerization, and enhances catalytic activity. Proc Natl Acad Sci U S A. 1996 Nov 26;93(24):13659–13664. doi: 10.1073/pnas.93.24.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelman A, Craigie R. Identification of conserved amino acid residues critical for human immunodeficiency virus type 1 integrase function in vitro. J Virol. 1992 Nov;66(11):6361–6369. doi: 10.1128/jvi.66.11.6361-6369.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulkosky J, Jones KS, Katz RA, Mack JP, Skalka AM. Residues critical for retroviral integrative recombination in a region that is highly conserved among retroviral/retrotransposon integrases and bacterial insertion sequence transposases. Mol Cell Biol. 1992 May;12(5):2331–2338. doi: 10.1128/mcb.12.5.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelman A, Hickman AB, Craigie R. The core and carboxyl-terminal domains of the integrase protein of human immunodeficiency virus type 1 each contribute to nonspecific DNA binding. J Virol. 1994 Sep;68(9):5911–5917. doi: 10.1128/jvi.68.9.5911-5917.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutzke RA, Plasterk RH. Structure-based mutational analysis of the C-terminal DNA-binding domain of human immunodeficiency virus type 1 integrase: critical residues for protein oligomerization and DNA binding. J Virol. 1998 Jun;72(6):4841–4848. doi: 10.1128/jvi.72.6.4841-4848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eijkelenboom AP, Lutzke RA, Boelens R, Plasterk RH, Kaptein R, Hard K. The DNA-binding domain of HIV-1 integrase has an SH3-like fold. Nat Struct Biol. 1995 Sep;2(9):807–810. doi: 10.1038/nsb0995-807. [DOI] [PubMed] [Google Scholar]
- 12.Bera S, Pandey KK, Vora AC, Grandgenett DP. HIV-1 Integrase Strand Transfer Inhibitors Stabilize an Integrase-Single Blunt-Ended DNA Complex. J Mol Biol. 2011 Feb 3;410(5):831–836. doi: 10.1016/j.jmb.2011.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazuda DJ, Anthony NJ, Gomez RP, et al. A naphthyridine carboxamide provides evidence for discordant resistance between mechanistically identical inhibitors of HIV-1 integrase. Proc Natl Acad Sci U S A. 2004 Aug 3;101(31):11233–11238. doi: 10.1073/pnas.0402357101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merck Merck and Co. Inc. Isentress Prescribing Information. Whitehouse Station, NJ, USA: 2011. http://www.merck.com/product/usa/pi_circulars/i/isentress/isentress_pi.pdf. [Google Scholar]
- 15.Espeseth AS, Felock P, Wolfe A, et al. HIV-1 integrase inhibitors that compete with the target DNA substrate define a unique strand transfer conformation for integrase. Proc Natl Acad Sci U S A. 2000 Oct 10;97(21):11244–11249. doi: 10.1073/pnas.200139397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grobler JA, Stillmock K, Hu B, et al. Diketo acid inhibitor mechanism and HIV-1 integrase: implications for metal binding in the active site of phosphotransferase enzymes. Proc Natl Acad Sci U S A. 2002 May 14;99(10):6661–6666. doi: 10.1073/pnas.092056199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pommier Y, Johnson AA, Marchand C. Integrase inhibitors to treat HIV/AIDS. Nat Rev Drug Discov. 2005 Mar;4(3):236–248. doi: 10.1038/nrd1660. [DOI] [PubMed] [Google Scholar]
- 18.Bera S, Pandey KK, Vora AC, Grandgenett DP. Molecular Interactions between HIV-1 integrase and the two viral DNA ends within the synaptic complex that mediates concerted integration. J Mol Biol. 2009 May 29;389(1):183–198. doi: 10.1016/j.jmb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandey KK, Bera S, Vora AC, Grandgenett DP. Physical trapping of HIV-1 synaptic complex by different structural classes of integrase strand transfer inhibitors. Biochemistry. 2010 Sep 28;49(38):8376–8387. doi: 10.1021/bi100514s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandey KK, Bera S, Zahm J, et al. Inhibition of human immunodeficiency virus type 1 concerted integration by strand transfer inhibitors which recognize a transient structural intermediate. J Virol. 2007 Nov;81(22):12189–12199. doi: 10.1128/JVI.02863-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandey KK, Grandgenett DP. HIV-1 Integrase Strand Transfer Inhibitors: Novel Insights into their Mechanism of Action. Retrovirology. 2008 Nov 5;2:11–16. doi: 10.4137/rrt.s1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valkov E, Gupta SS, Hare S, et al. Functional and structural characterization of the integrase from the prototype foamy virus. Nucleic Acids Res. 2009 Jan;37(1):243–255. doi: 10.1093/nar/gkn938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hare S, Gupta SS, Valkov E, Engelman A, Cherepanov P. Retroviral intasome assembly and inhibition of DNA strand transfer. Nature. 2010 Jan 31;464(7286):232–236. doi: 10.1038/nature08784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cherepanov P, Maertens GN, Hare S. Structural insights into the retroviral DNA integration apparatus. Curr Opin Struct Biol. 2011 Apr;21(2):249–256. doi: 10.1016/j.sbi.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Hare S, Vos AM, Clayton RF, Thuring JW, Cummings MD, Cherepanov P. Molecular mechanisms of retroviral integrase inhibition and the evolution of viral resistance. Proc Natl Acad Sci U S A. 2010 Nov 16;107(46):20057–20062. doi: 10.1073/pnas.1010246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kassahun K, McIntosh I, Cui D, et al. Metabolism and disposition in humans of raltegravir (MK-0518), an anti-AIDS drug targeting the human immunodeficiency virus 1 integrase enzyme. Drug metabolism and disposition: the biological fate of chemicals. 2007 Sep;35(9):1657–1663. doi: 10.1124/dmd.107.016196. [DOI] [PubMed] [Google Scholar]
- 27.Iwamoto M, Wenning LA, Petry AS, et al. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin Pharmacol Ther. 2008 Feb;83(2):293–299. doi: 10.1038/sj.clpt.6100281. [DOI] [PubMed] [Google Scholar]
- 28.Bosma PJ, Chowdhury JR, Bakker C, et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert's syndrome. The New England journal of medicine. 1995 Nov 2;333(18):1171–1175. doi: 10.1056/NEJM199511023331802. [DOI] [PubMed] [Google Scholar]
- 29.Wenning LA, Petry AS, Kost JT, et al. Pharmacokinetics of raltegravir in individuals with UGT1A1 polymorphisms. Clin Pharmacol Ther. 2009 Jun;85(6):623–627. doi: 10.1038/clpt.2009.12. [DOI] [PubMed] [Google Scholar]
- 30.Markowitz M, Morales-Ramirez JO, Nguyen BY, et al. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2006 Dec 15;43(5):509–515. doi: 10.1097/QAI.0b013e31802b4956. [DOI] [PubMed] [Google Scholar]
- 31.Brainard DM, Wenning LA, Stone JA, Wagner JA, Iwamoto M. Clinical pharmacology profile of Raltegravir, an HIV-1 integrase strand transfer inhibitor. The Journal of Clinical Pharmacology. 2011 October 1;51(10):1376–1402. doi: 10.1177/0091270010387428. 2011. [DOI] [PubMed] [Google Scholar]
- 32.Brainard DM, Friedman EJ, Jin B, et al. Effect of low-, moderate-, and high-fat meals on raltegravir pharmacokinetics. J Clin Pharmacol. 2011 Mar;51(3):422–427. doi: 10.1177/0091270010367652. [DOI] [PubMed] [Google Scholar]
- 33.Zhang D, Chando TJ, Everett DW, Patten CJ, Dehal SS, Humphreys WG. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug metabolism and disposition: the biological fate of chemicals. 2005 Nov;33(11):1729–1739. doi: 10.1124/dmd.105.005447. [DOI] [PubMed] [Google Scholar]
- 34.Hariparsad N, Nallani SC, Sane RS, Buckley DJ, Buckley AR, Desai PB. Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and phenobarbital. J Clin Pharmacol. 2004 Nov;44(11):1273–1281. doi: 10.1177/0091270004269142. [DOI] [PubMed] [Google Scholar]
- 35.Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011 Oct 20;365(16):1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Havlir DV, Kendall MA, Ive P, et al. Timing of Antiretroviral Therapy for HIV-1 Infection and Tuberculosis. N Engl J Med. 2011 Oct 20;365(16):1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011 Oct 20;365(16):1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wenning LA, Hanley WD, Brainard DM, et al. Effect of rifampin, a potent inducer of drug-metabolizing enzymes, on the pharmacokinetics of raltegravir. Antimicrob Agents Chemother. 2009 Jul;53(7):2852–2856. doi: 10.1128/AAC.01468-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mena A, Vazquez P, Castro A, Lopez S, Bello L, Pedreira JD. Clinical experience of raltegravir-containing regimens in HIV-infected patients during rifampicin-containing treatment of tuberculosis. J Antimicrob Chemother. 2011 Apr;66(4):951–952. doi: 10.1093/jac/dkq540. [DOI] [PubMed] [Google Scholar]
- 40.Burger DM, Magis-Escurra C, van den Berk GE, Gelinck LB. Pharmacokinetics of double-dose raltegravir in two patients with HIV infection and tuberculosis. AIDS. 2010 Jan 16;24(2):328–330. doi: 10.1097/QAD.0b013e3283350f08. [DOI] [PubMed] [Google Scholar]
- 41.Kis O, Robillard K, Chan GN, Bendayan R. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharmacol Sci. 2010 Jan;31(1):22–35. doi: 10.1016/j.tips.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Alvarez E, Cuenca L, Morello J, et al. Polymorphisms in the ABCB1 gene (P-glycoprotein) influences Raltegravir concentration. Paper presented at: 6th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; July 17–20, 2011; Rome, Italy. [Google Scholar]
- 43.Squires K, Bekker L, Eron J, et al. Safety, tolerability, and efficacy of Raltegravir (RAL) in a diverse cohort of HIV-infected patients (pts): 48-week results from the REALMRK study. Paper presented at: 51st Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); September 17–20, 2011; Chicago, IL. [Google Scholar]
- 44.Brainard DM, Gendrano IN, Jin B, et al. A pharmacokinetic comparison of adult and pediatric formulations of Raltegravir (RAL) in healthy adults. Paper presented at: 17th Conference on Retroviruses and Opportunistic Infections; February 16–19, 2010; San Francisco, CA. [Google Scholar]
- 45.Rockstroh JK, Lennox JL, DeJesus E, et al. Long-term Treatment With Raltegravir or Efavirenz Combined With Tenofovir/Emtricitabine for Treatment-Naive Human Immunodeficiency Virus-1–Infected Patients: 156-Week Results From STARTMRK. Clin Infect Dis. 2011 October 15;53(8):807–816. doi: 10.1093/cid/cir510. 2011. [DOI] [PubMed] [Google Scholar]
- 46.Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009 Sep 5;374(9692):796–806. doi: 10.1016/S0140-6736(09)60918-1. [DOI] [PubMed] [Google Scholar]
- 47.Lennox JL, Dejesus E, Berger DS, et al. Raltegravir versus Efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr. 2010 Sep;55(1):39–48. doi: 10.1097/QAI.0b013e3181da1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rockstroh JK, Teppler H, Zhao J, et al. Clinical efficacy of raltegravir against B and non-B subtype HIV-1 in phase III clinical studies. AIDS. 2011 Jul 17;25(11):1365–1369. doi: 10.1097/QAD.0b013e328348065a. [DOI] [PubMed] [Google Scholar]
- 49.Eron JJ, Jr, Rockstroh JK, Reynes J, et al. Raltegravir once daily or twice daily in previously untreated patients with HIV-1: a randomised, active-controlled, phase 3 non-inferiority trial. Lancet Infect Dis. 2011 Sep 18;11(12):907–915. doi: 10.1016/S1473-3099(11)70196-7. [DOI] [PubMed] [Google Scholar]
- 50.Antiviral Brief. Initial results reported on raltegravir once-daily dosing. Aids Patient Care STDS. 2011 Feb;25(2):123. [PubMed] [Google Scholar]
- 51.Taiwo B, Zheng L, Gallien S, et al. Efficacy of a nucleoside-sparing regimen of darunavir/ritonavir plus raltegravir in treatment-naive HIV-1-infected patients (ACTG A5262) AIDS. 2011 Nov 13;25(17):2113–2122. doi: 10.1097/QAD.0b013e32834bbaa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gotuzzo E, Nguyen BY, Markowitz M, et al. Sustained efficacy and tolerability of raltegravir after 240 weeks of combination ART in treatment naive HIV-1-infected patients; final analysis of protocol 004. Paper presented at: 6th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; July 17–20, 2011; Rome, Italy. [Google Scholar]
- 53.Markowitz M, Nguyen BY, Gotuzzo E, et al. Rapid and durable antiretroviral effect of the HIV-1 Integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007 Oct 1;46(2):125–133. doi: 10.1097/QAI.0b013e318157131c. [DOI] [PubMed] [Google Scholar]
- 54.Markowitz M, Nguyen BY, Gotuzzo E, et al. Sustained antiretroviral effect of raltegravir after 96 weeks of combination therapy in treatment-naive patients with HIV-1 infection. J Acquir Immune Defic Syndr. 2009 Nov 1;52(3):350–356. doi: 10.1097/QAI.0b013e3181b064b0. [DOI] [PubMed] [Google Scholar]
- 55.Gotuzzo E, Nguyen BY, Markowitz M, et al. Sustained antiretroviral efficacy of Raltegravir after 192 weeks of combination ART in treatment-naive HIV-1 infected patients. Paper presented at: 17th Conference on Retroviruses and Opportunisitc Infections; February 16–19, 2010; San Francisco, CA. [Google Scholar]
- 56.Teppler H, Brown DD, Leavitt RY, et al. Long-term safety from the raltegravir clinical development program. Curr HIV Res. 2011 Jan 1;9(1):40–53. doi: 10.2174/157016211794582650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coffin JM. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995 Jan 27;267(5197):483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 58.Cooper DA, Steigbigel RT, Gatell JM, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med. 2008 Jul 24;359(4):355–365. doi: 10.1056/NEJMoa0708978. [DOI] [PubMed] [Google Scholar]
- 59.Mukherjee R, Jensen ST, Male F, et al. Switching between raltegravir resistance pathways analyzed by deep sequencing. AIDS. 2011 Oct 23;25(16):1951–1959. doi: 10.1097/QAD.0b013e32834b34de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fransen S, Karmochkine M, Huang W, Weiss L, Petropoulos CJ, Charpentier C. Longitudinal analysis of raltegravir susceptibility and integrase replication capacity of human immunodeficiency virus type 1 during virologic failure. Antimicrob Agents Chemother. 2009 Oct;53(10):4522–4524. doi: 10.1128/AAC.00651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quercia R, Dam E, Perez-Bercoff D, Clavel F. Selective-advantage profile of human immunodeficiency virus type 1 integrase mutants explains in vivo evolution of raltegravir resistance genotypes. J Virol. 2009 Oct;83(19):10245–10249. doi: 10.1128/JVI.00894-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delelis O, Thierry S, Subra F, et al. Impact of Y143 HIV-1 integrase mutations on resistance to raltegravir in vitro and in vivo. Antimicrob Agents Chemother. 2010 Jan;54(1):491–501. doi: 10.1128/AAC.01075-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hatano H, Lampiris H, Fransen S, et al. Evolution of integrase resistance during failure of integrase inhibitor-based antiretroviral therapy. J Acquir Immune Defic Syndr. 2010 Aug 1;54(4):389–393. doi: 10.1097/QAI.0b013e3181c42ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hazuda DJ. Resistance to inhibitors of the human immunodeficiency virus type 1 integration. Braz J Infect Dis. 2010 Sep–Oct;14(5):513–518. [PubMed] [Google Scholar]
- 65.Reigadas S, Anies G, Masquelier B, et al. The HIV-1 integrase mutations Y143C/R are an alternative pathway for resistance to Raltegravir and impact the enzyme functions. PLoS One. 2010;5(4):e10311. doi: 10.1371/journal.pone.0010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mbisa JL, Martin SA, Cane PA. Patterns of resistance development with integrase inhibitors in HIV. Infect Drug Resist. 2011;4:65–76. doi: 10.2147/IDR.S7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baldanti F, Paolucci S, Gulminetti R, Brandolini M, Barbarini G, Maserati R. Early emergence of raltegravir resistance mutations in patients receiving HAART salvage regimens. J Med Virol. 2010 Jan;82(1):116–122. doi: 10.1002/jmv.21651. [DOI] [PubMed] [Google Scholar]
- 68.Canducci F, Marinozzi MC, Sampaolo M, et al. Genotypic/phenotypic patterns of HIV-1 integrase resistance to raltegravir. J Antimicrob Chemother. 2010 Mar;65(3):425–433. doi: 10.1093/jac/dkp477. [DOI] [PubMed] [Google Scholar]
- 69.Sichtig N, Sierra S, Kaiser R, et al. Evolution of raltegravir resistance during therapy. J Antimicrob Chemother. 2009 Jul;64(1):25–32. doi: 10.1093/jac/dkp153. [DOI] [PubMed] [Google Scholar]
- 70.Ceccherini-Silberstein F, Malet I, D'Arrigo R, Antinori A, Marcelin AG, Perno CF. Characterization and structural analysis of HIV-1 integrase conservation. AIDS Rev. 2009 Jan–Mar;11(1):17–29. [PubMed] [Google Scholar]
- 71.Low A, Prada N, Topper M, et al. Natural polymorphisms of human immunodeficiency virus type 1 integrase and inherent susceptibilities to a panel of integrase inhibitors. Antimicrob Agents Chemother. 2009 Oct;53(10):4275–4282. doi: 10.1128/AAC.00397-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Canducci F, Ceresola ER, Boeri E, et al. Cross-resistance Profile of the Novel Integrase Inhibitor Dolutegravir (S/GSK1349572) Using Clonal Viral Variants Selected in Patients Failing Raltegravir. J Infect Dis. 2011 Dec;204(11):1811–1815. doi: 10.1093/infdis/jir636. [DOI] [PubMed] [Google Scholar]
- 73.Van Wesenbeeck L, Rondelez E, Feyaerts M, et al. Cross-resistance profile determination of two second-generation HIV-1 integrase inhibitors using a panel of recombinant viruses derived from raltegravir-treated clinical isolates. Antimicrob Agents Chemother. 2011 Jan;55(1):321–325. doi: 10.1128/AAC.01733-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.AIDSInfo. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [Accessed September 2011];2011 Jan 10; 2011: http://aidsinfonihgov/contentfiles/AdultandAdolescentGL.pdf.
- 75.Boyd SD, Maldarelli F, Sereti I, et al. Transmitted raltegravir resistance in an HIV-1 CRF_AG-infected patient. Antivir Ther. 2011;16(2):257–261. doi: 10.3851/IMP1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Young B, Fransen S, Greenberg KS, et al. Transmission of integrase strand-transfer inhibitor multidrug-resistant HIV-1: case report and response to raltegravir-containing antiretroviral therapy. Antivir Ther. 2011;16(2):253–256. doi: 10.3851/IMP1748. [DOI] [PubMed] [Google Scholar]
- 77.Nguyen A, Calmy A, Delhumeau C, et al. A randomized cross-over study to compare raltegravir and efavirenz (SWITCH-ER study) AIDS. 2011 Jul 31;25(12):1481–1487. doi: 10.1097/QAD.0b013e328348dab0. [DOI] [PubMed] [Google Scholar]
- 78.Bredeek F, Guadron R, Yolo R, Schneider S. A switch from TDF/FTC to raltegravir in patients on a boosted protease inhibitor is effective in reducing proteinuria and increasing GFR. Paper presented at: 51st Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); September 17–20, 2011; Chicago, IL. [Google Scholar]
- 79.Gallien S, Braun J, Delaugerre C, et al. Efficacy and safety of raltegravir in treatment-experienced HIV-1-infected patients switching from enfuvirtide-based regimens: 48 week results of the randomized EASIER ANRS 138 trial. J Antimicrob Chemother. 2011 Sep;66(9):2099–2106. doi: 10.1093/jac/dkr269. [DOI] [PubMed] [Google Scholar]
- 80.Croxtall JD, Perry CM. Lopinavir/Ritonavir: a review of its use in the management of HIV-1 infection. Drugs. 2010 Oct 1;70(14):1885–1915. doi: 10.2165/11204950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 81.Eron JJ, Young B, Cooper DA, et al. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trials. Lancet. 2010 Jan 30;375(9712):396–407. doi: 10.1016/S0140-6736(09)62041-9. [DOI] [PubMed] [Google Scholar]
- 82.Qaqish R, Trinh R, Tian M, et al. Bone mineral density (BMD) analysis in antiretroviral (ART)-naïve subjects taking lopinavir/ritonavir (LPV/r) combined with raltegravir (RAL) or tenofovir/emtricitabine (TDF/FTC) for 96 weeks in the PROGRESS study. Paper presented at: 6th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; July 17–20, 2011. [Google Scholar]
- 83.Martinez E, Larrousse M, Llibre JM, et al. Substitution of raltegravir for ritonavir-boosted protease inhibitors in HIV-infected patients: the SPIRAL study. AIDS. 2010 Jul 17;24(11):1697–1707. doi: 10.1097/QAD.0b013e32833a608a. [DOI] [PubMed] [Google Scholar]
- 84.Samaras K, Richardson R, Carr A. Postprandial lipid effects of low-dose ritonavir vs. raltegravir in HIV-uninfected adults. AIDS. 2010 Jul 17;24(11):1727–1731. doi: 10.1097/QAD.0b013e32833ac7be. [DOI] [PubMed] [Google Scholar]
- 85.Boyd SD. Management of HIV infection in treatment-naive patients: a review of the most current recommendations. Am J Health Syst Pharm. 2011 Jun 1;68(11):991–1001. doi: 10.2146/ajhp100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Young B, Vanig T, Dejesus E, et al. A pilot study of abacavir/lamivudine and raltegravir in antiretroviral-naive HIV-1-infected patients: 48-week results of the SHIELD trial. HIV Clin Trials. 2010 Sep–Oct;11(5):260–269. doi: 10.1310/hct1105-260. [DOI] [PubMed] [Google Scholar]
- 87.McMahon D, Jones J, Wiegand A, et al. Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin Infect Dis. 2010 Mar 15;50(6):912–919. doi: 10.1086/650749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7(8) doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gandhi RT, Coombs RW, Chan ES, et al. No Effect of Raltegravir Intensification on Viral Replication Markers in the Blood of HIV-1-infected Patients Receiving Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2011 Nov 11; doi: 10.1097/QAI.0b013e31823fd1f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yukl SA, Shergill AK, McQuaid K, et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. AIDS. 2010 Oct 23;24(16):2451–2460. doi: 10.1097/QAD.0b013e32833ef7bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hatano H, Hayes TL, Dahl V, et al. A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J Infect Dis. 2011 Apr 1;203(7):960–968. doi: 10.1093/infdis/jiq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dahl V, Lee E, Peterson J, et al. Raltegravir Treatment Intensification Does Not Alter Cerebrospinal Fluid HIV-1 Infection or Immunoactivation in Subjects on Suppressive Therapy. J Infect Dis. 2011 Dec;204(12):1936–1945. doi: 10.1093/infdis/jir667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Buzon MJ, Massanella M, Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010 Apr;16(4):460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 94.Autran B, Descours B, Avettand-Fenoel V, Rouzioux C. Elite controllers as a model of functional cure. Curr Opin HIV AIDS. 2011 May;6(3):181–187. doi: 10.1097/COH.0b013e328345a328. [DOI] [PubMed] [Google Scholar]