Abstract
Prolactin (PRL), synthesized and secreted from lactotrophs of the anterior pituitary gland, is tonically inhibited by hypothalamic dopamine (DA) throughout the female reproductive (estrous) cycle. Our laboratory has shown that DA hyperpolarizes these cells by activating G protein-coupled inwardly rectifying K+ (GIRK) channels; however, this response is only observed on proestrus. While the cellular mechanisms that allow for functional expression of this unique DA-signaling pathway are unclear, we hypothesized that activation of the DA-GIRK effector pathway is due to the rise in circulating estrogen (E2) during the preceding day of diestrus. Thus, we examined the effects of E2 on primary lactotrophs isolated from female rats. Treatment with a physiological concentration of E2 (40–80 pg/ml, in vivo or in vitro) induced a proestrous phenotype in diestrous lactotrophs. These cells exhibited a DA-induced membrane hyperpolarization, as well as a secretory rebound of PRL following DA withdrawal (characteristic of proestrous cells). Internal dialysis of GTPγS demonstrated that E2 exposure enabled functional expression of GIRK channels, and this regulation by E2 did not involve the D2R. The effect of E2 was blocked by the receptor antagonist, ICI 182,780, and by the protein synthesis inhibitor, cycloheximide. Single-cell analysis revealed increased mRNA expression of GIRK channel subunits in E2-treated lactotrophs. While E2 is known to have multiple actions on the lactotroph, the present findings illuminate a novel action of E2 in lactotrophs—regulation of the expression of a DA effector, the GIRK channel.
Keywords: prolactin, dopamine, single-cell RT-PCR, estrous cycle
prolactin (prl) is synthesized and secreted by lactotroph cells of the anterior pituitary (AP) gland. This peptide hormone, well known for its role in lactation, mammary gland development, fertility, and their functions (1, 14, 20, 31, 48, 57), exhibits a dynamic secretory pattern throughout the female reproductive cycle (5, 62). On any day of the reproductive cycle, PRL secretion (and synthesis) is inhibited by hypothalamic dopamine (DA) (2, 49). This principle inhibitory signal is transduced through the D2 type DA receptor (D2R) in the lactotroph membrane (6, 47), which is coupled to a pertussis toxin-sensitive G protein pathway and multiple effector proteins (9, 21, 59).
In female rodents, a precipitous drop in DA on the day of proestrus allows for the periovulatory surge in serum PRL (12). Our laboratory has shown that on this day DA activates an inwardly rectifying K+ current that rapidly hyperpolarizes the lactotroph membrane (25, 26), due to activation of G protein-coupled inwardly rectifying K+ channels (GIRKs, of the Kir 3.0 gene subfamily). The function of this DA-activated K+ channel (KDA) is not observed on any other day of the estrous cycle (22), indicating that DA signaling in the lactotroph switches on proestrus, and this change may contribute to the unique pattern of PRL secretion observed on proestrus. Indeed, a PRL secretory rebound elicited by the abrupt withdrawal of DA in vitro is characteristic only of proestrous lactotrophs and requires the membrane hyperpolarization activated during the presence of DA (22).
Because estrogen (E2) is a critical factor in producing the proestrous surge of PRL in vivo, we hypothesized that the “switch” in DA signaling is dependent on the rise in E2 during the preceding day of diestrus II and due to direct actions of E2 on the lactotroph. Therefore, we tested whether manipulation of E2, either in vivo or in vitro, could alter the lactotroph's electrophysiological and secretory responsiveness to DA. In the present study, we show that the proestrous phenotype, in which DA activates KDA and withdrawal of DA produces a PRL secretory rebound, is dependent upon E2 in vivo. In addition, E2 treatment of diestrous lactotrophs in vitro directly induces the functional expression of KDA and the secretory rebound following DA washout. This induction by E2 occurs at a target distal to the D2R and may be due to, at least in part, a significant increase in mRNA expression of GIRK channel subunits 1, 2, and 4.
MATERIALS AND METHODS
Animals and reagents.
Female Sprague-Dawley rats ages 2 to 5 mo (Harlan Laboratories; Frederick, MD) were maintained on a 14:10-h light-dark cycle (lights on at 6:00 AM). Only females demonstrating at least two consecutive 4-day estrous cycles (as determined by daily vaginal lavage) were used in this study. Food and water were available ad libitum. For in vivo manipulation of E2, bilateral ovariectomies were performed on the morning of diestrus II (prior to the rise in endogenous E2) through dorsolateral incisions with animals under isoflurane anesthesia. Seven days following surgery, animals received two subcutaneous Silastic implants (30 mm long; ID, 1.57 mm; OD, 3.18 mm), containing either estradiol-17β (150 μg/ml) or vehicle (oil), and AP glands were harvested 48 h later. All animal handling and procedures were carried out in a facility accredited by the American Association for the Accreditation of Laboratory Animal Care and were approved by the University of Cincinnati's Institutional Animal Care and Use Committee.
Horse serum was purchased from HyClone (Thermo Scientific, Logan, UT); tetrodotoxin and ICI 182,780 were purchased from Tocris Bioscience (Ellisville, MO). Ovine erythrocytes were obtained from Colorado Serum (Denver, CO). Anti-rat PRL (arPRL-86) for the reverse hemolytic plaque assay (RHPA) was generated in our laboratory and characterized, as previously described (30). Reagents for the rat PRL (rPRL) RIA were purchased from Dr. A. Parlow through the National Hormone and Peptide Program (University of California, Los Angeles). Primers were purchased from Integrated DNA Technologies (San Diego, CA). All other reagents and culture media, unless otherwise noted, were purchased from Sigma Chemicals (St. Louis, MO).
AP cell dissociation.
Animals were killed by rapid decapitation between 8:00 and 10:00 AM on specific days of the estrous cycle or 48 h after receiving Silastic implants, and AP cells were dissociated as previously described (26). In all experiments, a single AP gland was used for each cell preparation; therefore, each AP cell preparation represents an individual rat. Briefly, the AP gland (posterior pituitary removed) was minced and enzymatically dissociated in Hank's balanced salt solution (with Ca2+ and Mg2+) containing 0.20% trypsin for 15 min at 37°C. The digested tissue was washed in Hank's Ca2+, Mg2+-free medium (Hank's CMF), and triturated in Hank's CMF, containing deoxyribonuclease-I (0.075 mg/ml) and trypsin inhibitor (3.75 μg/ml). The final cell suspension was filtered through a sterile, 20-μm pore nylon mesh. This method routinely yielded 3–5 × 106 cells per AP gland, with viability in excess of 96%. Cells used for electrophysiology studies or single-cell RT-PCR were immediately subjected to the reverse hemolytic plaque assay. Cells for perifusion were transferred to 60-mm Petri dishes containing 7 ml of culture medium (DMEM with 10% heat-inactivated horse serum; DMES) and incubated on an orbital shaker (40 rpm) at 37°C in 5% CO2 for 1–3 days. For in vitro manipulation of E2, AP cells dissociated from each diestrous female rat were divided into two groups and treated overnight (∼18 h) or for 48 h in vitro with either vehicle (EtOH) or E2 (40–80 pg/ml; to mimic the rise in endogenous E2 that occurs during late diestrus II, see Ref. 5).
Perifusion.
Secretory activity of primary lactotrophs was monitored using a two-column perifusion system as previously described (27). Briefly, dissociated AP cells were mixed with preswollen polyacrylamide gel (Bio-Gel P2, 200–400 mesh; 3 × 106 cells/1 ml mesh) and then loaded into the chamber of the system. The perifusion medium was the same standard extracellular solution (SES) used in the electrophysiological experiments described below. Flow rate was 0.5 ml/min, and the effluents were collected in 2-min fractions into tubes containing 100 μl 2% BSA in PBS. Each perifusion experiment included a control and an E2-treated cell preparation perfused by the same solutions. Each preparation was derived from an individual rat. Protein levels of rPRL were determined on individual effluent samples by homologous double-antibody RIA. All samples from an individual perifusion experiment were included in a single assay.
RHPA.
The RHPA is a well-established method of identifying viable endocrine cell types based on antigen secretion (25, 28, 60). Briefly, ovine erythrocytes (oRBCs) were coated with Staphylococcus protein A in the presence of chromium chloride. The coated oRBCs [6% in DMEM/BSA (0.1%)] were mixed 1:1 with freshly dissociated AP cells (300,000 cells/ml). The mixture was infused into poly-l-lysine glass coverslip chambers (as described in Ref. 16), and cells were allowed to plate for 30 min at 37°C (5% CO2). The AP cell/oRBC mixture was treated as follows: 1) washed with DMEM/BSA (to remove nonadherent cells); 2) incubated with primary anti-rPRL antibody (arPRL-86; final dilution 1:200 in DMEM/BSA, 37°C, 5% CO2) for 90 min; 3) washed with DMEM/BSA (to remove unbound antibody); and 4) incubated with guinea pig serum complement (1:100 in DMEM/BSA, 37°C, 5% CO2) or autologous rat serum (1:120) until plaques were visible on the coverslip (∼15 min). Once plaques began to form, complement was rinsed out, and the chambers were dismantled. Coverslips, with cells attached, were incubated overnight in culture medium (DMES; 37°C; 5% CO2) without additives (proestrous cells) or in medium containing 17β-estradiol (E2; 40–80 pg/ml) or vehicle (EtOH) (diestrous cells). In some experiments, cells were also treated with the E2 receptor antagonist, ICI 182,780 (ICI; 40 ng/ml), or the protein synthesis inhibitor, cyclohexamide (Chx; 35 μg/ml).
Electrophysiology.
Membrane potentials and whole-cell currents of plaque-identified lactotrophs were recorded using the giga-ohm seal patch-clamp technique (29). Recording pipettes were filled with standard intracellular solution (SIS; 130 mM potassium aspartate, 20 mM KCl, 1 mM glucose, 10 mM HEPES) and, in some experiments, included 250 μM GTPγS. Cells were bathed in standard extracellular solution (SES; in mM): 145 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 glucose, and 10 HEPES. In the voltage-clamp experiments, K+ currents were isolated by including TTX (1 μM) to block voltage-dependent sodium channels and omitting CaCl2 (substituting MgCl2) to reduce calcium currents. All solutions were adjusted to pH 7.3–7.4 and 295–305 mOsmol.
Patch-clamp experiments were performed using either an Axopatch 1B or 1D amplifier with pClamp 8.0 software (Axon Instruments, Foster City, CA) and low-resistance (4–6 MΩ) glass microelectrodes. The recordings were carried out at room temperature in a Lexan recording chamber mounted on the stage of a Nikon Diaphot-inverted microscope. A coverslip, with plaque-identified lactotrophs attached, served as the floor of the recording chamber through which external solutions were continuously perfused during each experiment. DA solutions (made fresh immediately before use) and high [KCl] solutions (40 mM, substituted for NaCl) were applied via a U-tube apparatus that was positioned next to the cell during each recording. The positioning of the U-tube device allowed for rapid application and immediate withdrawal of the test solutions without mechanical disturbance of the cell being recorded. Whole cell voltage responses under current clamp were recorded wideband (∼50 kHz). Whole-cell K+ currents were recorded from single lactotrophs during a constant rate (0.44 V/s) ramp depolarization from −160 mV to +60 mV (−60 mV holding potential).
Analysis of mRNA expression by RT-PCR.
Whole tissue samples [rat AP, cerebellum (Cb) and cardiac atrium (C. Atr)] dissected from a female rat were flash frozen in liquid nitrogen and stored at −80°C until analyzed. Frozen tissues were homogenized in ice-cold TRIzol, and RNA was extracted according to standard protocol guidelines (Invitrogen, cat. no. 15596–018). RT of 0.5 μg of total RNA was completed in a final volume of 10 μl following the manufacturer's protocol (Qiagen, cat. no. 205310). Amplification of cDNA (1 μl, unquantitated) from each RT reaction was completed by PCR in a final reaction volume of 12.5 μl (Roche FastStart PCR, cat. no. 04 738 314 001). Following an initial hot start at 94°C for 2 min, reactions were sequentially cycled 30 times for 45-s durations at the following temperatures: 94°C (denaturing), Tm°C (annealing), and 72°C (extending). All reactions were incubated at 72°C for 5 min after the last cycle for final extension, and then maintained at 4°C. All PCR reactions for GIRK subunits 1–4 were completed at Tm = 58°C (primers sequences from Ref. 36).
For analysis of gene expression on a single-cell level, individual plaque-identified lactotrophs were collected in a heat-sterilized patch-pipette tip filled with sterile 1× PBS (pH 7.4). In this study, 10 plaque-identified lactotrophs from each treatment group (vehicle or E2) were collected from each of 5 rats. All samples were dispensed into a sterile 0.5-ml thin-walled PCR tube, and 6 μl of sterile dH2O was added immediately. Samples were frozen at −80°C until analyzed. All 6 μl of nonquantitated sample was reverse transcribed into cDNA following manufacturer's protocol (10 μl final reaction volume; Qiagen, cat. no. 205310). Amplification of each cDNA sample was completed using “nested primers” in two consecutive PCR reactions. In general, the initial PCR reaction (with “outside” primers) was completed using 1 μl of cDNA as the template in a final reaction volume of 12.5 μl. The nested reaction (with “inside” primers) was completed using 1 μl of the initial PCR reaction as a template in a final reaction volume of 12.5 μl. In general, PCR conditions were 94°C for 2 min, (94°C for 45 s, Tm°C for 45 s, and 72°C for 45 s) × n cycles, 72°C for 5 min, 4°C hold. Initial (“outer”) reactions were cycled with n = 40, and nested (“inner”) reactions were cycled with n = 35. Each sample was analyzed for expression of rPRL (gene accession no. NM_012629) using outer primers (forward 5′- GCAGGGACACTCCTCCTGCT-3′ and reverse 5′-ATGGGAGTTGTGACCAAACC-3′; Tm = 57°C; product = 517 bp) and inner primers (forward 5′-GCCCAGAAAGTCCCTCCGG-3′ and reverse 5′-CAATCCCTTCAAGAAGCCGC-3′; Tm = 59°C; product = 178 bp). Primer sequences and conditions for rGIRK subunits 1, 2, and 4 are described in Ref. 36. As another verification of cell phenotype, samples were also screened for the rat glycoprotein hormone alpha subunit (rGPHα; gene accession no. NM_053918) using the following outer primers (forward 5′-GCTGTCATTCTGGTCATGCT-3′ and reverse 5′-GCACTCCGTATGATTCTCCA-3′; Tm = 55°C; product = 306 bp) and inner primers (forward 5′-GCCCCCATCTACCAGTGTAT-3′ and reverse 5′-GCATTTCCCATTACTGTGGC-3′; Tm = 56°C; product = 155 bp).
Statistical analysis.
Membrane potential before and during DA treatment was compared using a Student's t-test. Inward K+ current data in the GTPγS experiments were subjected to one-way ANOVA followed by Tukey's multiple-comparison post hoc test. PRL release from AP cells without drug challenge remained stable throughout the period of perifusion (data not shown; see Ref. 27). Secretory responses of each treatment group to drug challenge were analyzed by comparison to extrapolated basal release using two-way ANOVA and Bonferroni post hoc analysis. Differences in secretory responses between vehicle- and E2-exposed cells were similarly analyzed. Differences between GIRK subunit expression data were also subjected to two-way ANOVA followed by Bonferroni's post hoc test. The overall percent expression of GIRK subunits in vehicle- vs. E2-treated lactotrophs was analyzed using the Mann-Whitney U-test. In all analyses, a P value of <0.05 was considered significant. Values are reported as means ± SE.
RESULTS
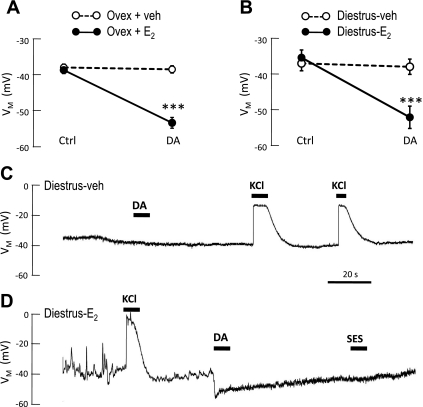
The results of the present experiments demonstrate that manipulation of E2, either in vivo or in vitro, can alter the lactotroph's electrophysiological and secretory responsiveness to DA. As shown in Figs. 1 and 2, both in vivo treatment and in vitro treatment with E2 produce lactotrophs with a proestrous phenotype. None of the lactotrophs (0 of 9) isolated from ovariectomized rats with vehicle implants demonstrated a DA-activated membrane hyperpolarization, even when a maximally effective concentration (with regard to inhibition of PRL release; see Refs. 6, 9, 17) was used. In contrast, the majority of lactotrophs (10 of 12) from E2-implanted rats responded to DA with a membrane hyperpolarization (Fig. 1A). The resting membrane potential (RMP) of vehicle- and E2-treated lactotrophs was not different (−37.8 ± 2.9 mV vs. −38.5 ± 3.3 mV, respectively).
Fig. 1.
Estrogen (E2) induces functional expression of the D2R-G protein-coupled inwardly rectifying K+ (GIRK) pathway in lactotrophs. A and B: mean membrane potentials of lactotrophs before (Ctrl) and during dopamine (DA; 1 μM) application. A: cells were derived from ovariectomized rats treated in vivo with vehicle [○; 9 cells from 2 independent preparations (2 rats)] or E2 (●; 12 cells, 2 rats). B: diestrous lactotrophs were treated in vitro with vehicle (○; 10 cells, 4 rats) or E2 (●; 13 cells, 4 rats). In both A and B, the membrane hyperpolarization (VM) of E2-exposed cells (but not vehicle-treated cells) was significantly hyperpolarized in the presence of DA (***P < 0.001 vs. Ctrl; Student's t-test). C and D: representative current-clamp recordings of diestrous lactotrophs treated overnight in vitro with vehicle (C) or E2 (D; 80 pg/ml), illustrating DA-induced hyperpolarization in E2-treated cells and absence of response in vehicle-treated cells. Control solution (standard extracellular solution, SES) did not alter the VM, demonstrating absence of mechanical disturbance by U-tube application, while 50 mM KCl caused membrane depolarization in all cells.
Fig. 2.
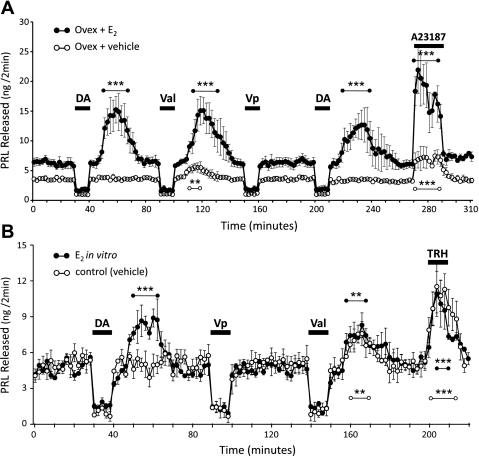
E2-treated anterior pituitary (AP) cells produce a secretory rebound of prolactin (PRL) following withdrawal of DA. Cells were challenged with 10-min exposures to DA (50 nM), verapamil (Vp; 20 μM), valinomycin (Val; 1 nM), A23187 (100 nM), or thyrotropin-releasing hormone (TRH; 100 nM). Cells were perifused with SES at the beginning of the experiment and between drug exposures. A: AP cells derived from ovariectomized rats treated in vivo with vehicle implants (○) or E2 implants (●). B: AP cells, derived from intact diestrous rats, treated in vitro with vehicle (○) or E2 (40 pg/ml, ●). In both A and B, only cells exposed to E2 produced a PRL secretory rebound following withdrawal of DA. Both vehicle- and E2-exposed cells responded with a significant increase in PRL secretion following Val withdrawal or upon stimulation by A23187 (A) or TRH (B). Only significant PRL release over baseline rate is indicated by asterisks (**P < 0.01; ***P < 0.001; 2-way ANOVA with Bonferroni post hoc analysis of drug-challenged release vs. basal release). PRL release was significantly reduced from baseline (P < 0.05) in all groups by DA, Vp, and Val (not indicated with asterisks). Symbols represent means ± SE from five (A) or three (B) independent perifusions, each representing an individual animal.
E2 has actions at multiple levels in the hypothalamic-pituitary axis. Therefore, we tested its effects directly on the lactotrophs by in vitro treatment of primary AP cells derived from diestrous rats. In agreement with previous findings (22), DA did not activate membrane hyperpolarization in control (vehicle-treated) diestrous lactotrophs (0 of 10; Fig. 1, B and C) In contrast, diestrous lactotrophs treated overnight with E2 did hyperpolarize in response to application of DA (11 out of 13) by an average of 17.0 ± 2.4 mV (Fig. 1, B and D). Again, RMP values of vehicle- vs. E2-treated lactotrophs were not significantly different (−35.9 ± 2.1 mV vs. −34.2 ± 2.2 mV, respectively).
Since the DA-induced hyperpolarization in proestrous lactotrophs is a critical mechanism in the secretory rebound of PRL that occurs subsequent to the withdrawal of DA (24, 27), we examined the secretory responses of lactotrophs whose exposure to E2 was manipulated in vivo or in vitro. In perifusion, lactotrophs from ovariectomized, E2-treated animals responded to application of a physiological concentration of DA (54) with decreased PRL release, followed by a significant elevation in secretory rate over basal PRL levels after DA withdrawal (P < 0.001; Fig. 2A). The enhanced release after DA removal was indeed a true “rebound secretion” and is not merely the release of PRL that had accumulated during the inhibitory phase. For example, the quantity of PRL released during the 10-min application of DA was a total of 22.4 ± 3.2 ng below baseline, whereas the amount of PRL released during the subsequent rebound was 91.9 ± 22.5 ng over baseline. In contrast, lactotrophs derived from ovariectomized, vehicle-treated rats, while still responsive to the inhibitory action of DA, did not produce a secretory rebound following washout of DA (Fig. 2A), but instead recovered to the pre-DA rate of PRL release. Cells from the vehicle-treated rats were inherently able to produce a PRL secretory rebound if directly hyperpolarized by the K+ ionophore, valinomycin (Val). The amount of PRL released over baseline in the period following Val withdrawal was significantly smaller in cells from vehicle-treated rats compared with the E2-treated group (18.2 ± 5.4 vs. 82.2 ± 15.9 ng PRL, respectively; P < 0.001). This was most likely due to the fact that these cells had been without the trophic effects of E2 for ∼9 days and, therefore, had lower intracellular stores of PRL. This interpretation is supported by the lower basal release from OVX + vehicle cells, which is ∼60% of that from the E2-treated cells (although not significantly different), and by the secretory response to the Ca2+ ionophore, A23187 (Fig. 2A). Directly increasing Ca2+ influx with A23187 significantly stimulated PRL release over baseline in both groups (P < 0.001), but the release from OVX + vehicle cells was again significantly less than that from OVX + E2 cells (32.4 ± 7.9 vs. 106.7 ± 20.9 ng PRL, respectively; P < 0.001). Regardless of the in vivo treatment, lactotrophs responded to the application of DA with decreased PRL release. Val and the Ca2+ channel blocker, verapamil (Vp), also inhibited PRL release during application. Vp directly blocks the influx of Ca2+ into the lactotrophs but does not change the membrane potential and, therefore, did not cause rebound secretion in either the vehicle-treated or E2-treated group (Fig. 2A).
We also observed similar changes in rebound PRL secretion following direct exposure of lactotrophs to E2. PRL release from diestrous lactotrophs treated overnight in vitro with either vehicle or E2 (40 pg/ml) was significantly inhibited below baseline in the presence of DA, Val, or Vp, (P < 0.01) and significantly increased by application of thyrotropin-releasing hormone (TRH; P < 0.001; Fig. 2B). However, only E2-treated cells responded to DA withdrawal with the anticipated rebound secretion of PRL (P < 0.001 vs. baseline PRL secretion), while PRL release from vehicle-treated cells returned only to basal levels (Fig. 2B). Again, direct hyperpolarization using Val produced a secretory rebound upon its removal that was not dependent upon E2 treatment and occurred in both groups of diestrous cells (P < 0.01; Fig. 2B). A significant difference in PRL release (during perifusion) was observed between the two in vitro treatment groups only during the period following DA washout (P < 0.01; minutes 50–68, Fig. 2B).
E2 action is distal to the D2R.
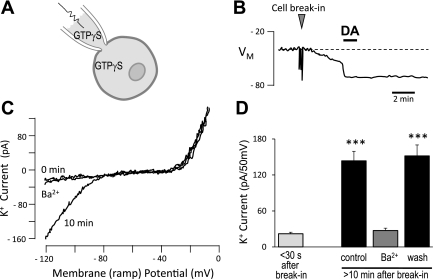
To test whether E2 may be acting on components of the signaling pathway other than the D2R, GTPγS was dialyzed by passive diffusion into patch-clamped lactotrophs via the recording pipette (Fig. 3A). Spontaneous GIRK channel openings, even in proestrous lactotrophs, are rare enough to be undetectable in the absence of DA (16). However, we had previously observed that internal dialysis of proestrous lactotrophs with GTPγS produced a slowly developing membrane hyperpolarization, as activated G proteins accumulated in the presence of this nonhydrolyzable GTP analog (Fig. 3B). This hyperpolarization was mediated by GIRK channels as determined by its Ba2+ sensitivity. DA application prior to the attainment of maximal hyperpolarization (∼EK) produced a rapid additional hyperpolarization that was not reversible (Fig. 3B). The GTPγS-induced hyperpolarization was found to reach a plateau by 8 to 9 min, and DA application after this time did not produce any additional change in membrane potential (data not shown). This indicated that the same GIRK channels activated by DA were activated by the intracellular GTPγS. Therefore, we used this approach to bypass the D2R in our current investigation of E2 action. Using voltage clamp to control for the driving force, K+ current was measured during a ramp depolarization. Fig. 3C illustrates the changing currents in a proestrous lactotroph immediately after whole-cell access (0 min) and after internal dialysis of GTPγS (10 min). An inwardly rectifying K+ current is present only after internal diffusion of the GTPγS, and this could be blocked by Ba2+. Note that outward K+ currents at more positive potentials are unchanged with time or Ba2+. Figure 3D summarizes the responses of 10 proestrous lactotrophs and shows a significant increase in inwardly rectifying K+ current following internal dialysis of GTPγS (P < 0.001 vs. break-in).
Fig. 3.
Internal dialysis of GTPγS causes membrane hyperpolarization of proestrous lactotrophs independent of D2R activation. A: plaque-identified, proestrous rat lactotrophs were dialyzed with GTPγS (250 μM) from the patch-clamp recording pipette. B: dialysis of GTPγS into the lactotroph after whole-cell access (“cell break-in”) resulted in a gradually developing hyperpolarization measured in current clamp. DA (300 nM) application during this time caused rapid hyperpolarization that was not reversible. C: K+ currents measured during ramp depolarization in a proestrous lactotroph, immediately after cell break-in (0 min), 10 min later, and then in the presence of 250 μM BaCl2 (Ba2+). D: summary of inwardly rectifying K+ currents in proestrous lactotrophs (10 cells, 2 rats) dialyzed with GTPγS. Currents at different times following whole-cell access were compared using one-way ANOVA with Tukey's multiple-comparison post hoc test (***P < 0.001).
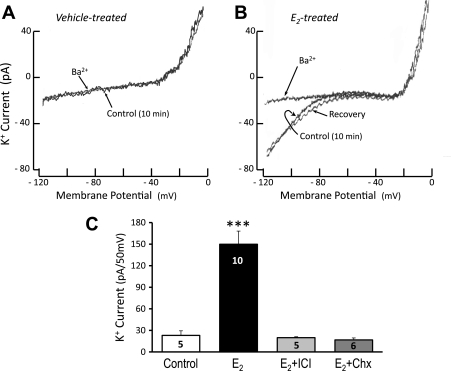
The activation of GIRK channels by GTPγS did not occur in control diestrous lactotrophs (Fig. 4A). However, in diestrous lactotrophs treated overnight with E2 (40 pg/ml), internal dialysis of GTPγS induced a Ba2+-sensitive inwardly rectifying K+ current similar to that observed in the proestrous cells (Fig. 4B). Again, the outward K+ current, activated at more positive potentials, was not altered. Fig. 4C presents summarized data from multiple diestrous lactotrophs, showing the robust activation of GIRK current by E2 treatment (P < 0.001 vs. vehicle-treated cells). This current was blocked when cells were treated with E2 in combination with the E2 receptor antagonist ICI 182,780 (40 nM) or the protein synthesis inhibitor, cycloheximide (Chx, 35 μM).
Fig. 4.
Barium-sensitive inwardly rectifying K+ (IRK) current is activated by GTPγS dialysis of diestrous lactotrophs treated in vitro with E2. A: internal dialysis of GTPγS (250 μM) does not activate IRK current in vehicle-treated, diestrous lactotrophs. B: diestrous cells treated overnight with E2 (40 pg/ml) develop Ba2+-sensitive IRK during internal dialysis with GTPγS. C: summary of IRK currents recorded from diestrous lactotrophs dialyzed with GTPγS. E2-treated lactotrophs have an increased IRK current over control diestrous lactotrophs. The effect of E2 is blocked by coincubation with ICI-182,780 (ICI, 40 nM) or cycloheximide (Chx, 35 μM). Numbers in bars indicate number of lactotroph cells tested in each treatment group. Cells were derived from four independent preparations (4 rats). Treatment groups were compared using one-way ANOVA with Tukey's multiple-comparison post hoc test (***P < 0.001).
E2 induces GIRK channel subunit expression in primary rat lactotrophs.
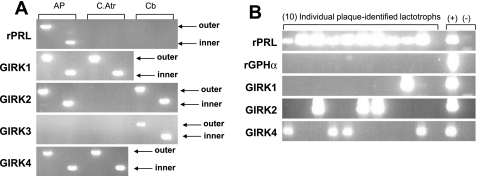
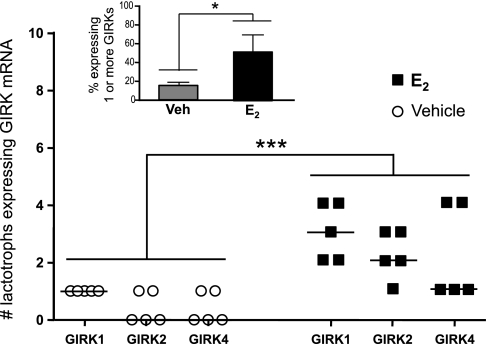
The ability of ICI 182,780 and Chx to block the effect of E2 indicated that the steroid was acting through its classical receptor to affect gene transcription. Therefore, we investigated the possibility that E2 might up-regulate the expression of the GIRK channel subunits themselves. Whole tissues were subjected to RT-PCR analysis to verify detectability of all four mammalian GIRK subunits (Fig. 5A). Consistent with the findings of other laboratories, C.Atr expressed GIRK 1 and 4 (10, 39); while Cb expressed GIRK 2 and GIRK 3 (35). Transcripts for GIRK channel subunits 1, 2, and 4 (but not GIRK 3) were detected in whole rat AP. Therefore, we analyzed the expression of these three GIRK subunits in individual, plaque-identified diestrous lactotrophs treated overnight with vehicle or E2 (80 pg/ml). All single-cell samples (100 lactotrophs, 5 rats) were screened for rPRL, rGPHα (common to the glycoprotein-secreting anterior pituitary cell types: gonadotrophs and thyrotrophs), and GIRK subunits 1, 2, and 4. A representative gel of 10 plaque-identified lactotrophs (Fig. 5B) illustrates that all single-cell samples yielded a strong signal for rPRL with no signal for rGPHα, indicating that lactotrophs (and not thyrotrophs or gonadotrophs) were isolated. Of the 50 cells analyzed per treatment group, E2-treated lactotrophs exhibit significantly greater expression of one or more GIRK subunit transcripts (50 ± 3% cells expressing detectable transcript) compared with vehicle-treated controls (16 ± 1% expressing detectable transcript; P < 0.05; Fig. 6). Also, in independent preparations of pituitary cells derived from five rats, a significant increase in the expression of all three GIRK subunits (1, 2, and 4) was observed in E2-treated lactotrophs (10 lactotrophs per treatment groups for each preparation; P < 0.001 vs. vehicle-treated cells; Fig. 6).
Fig. 5.
Transcripts for GIRK channel subunits 1, 2, and 4 (but not GIRK3) are expressed in the rat AP and are detectable in single identified lactotrophs. RNA isolated from tissues or cells was reverse transcribed, and the cDNA for each was amplified by PCR using nested (outer and inner) primers. A: to test the nested PCR primers, whole rat AP was used. Transcripts for GIRK channel subunits 1, 2, and 4 (but not 3) are detected in whole rat AP. Control tissues used were cardiac atrium (C. Atr for GIRK1 and GIRK4) and cerebellum (Cb for GIRK2 and GIRK3). B: representative gel of PCR products amplified from 10 single plaque-identified lactotrophs. Positive (+) control is RT reaction from whole rat AP (lane 12). Negative (−) control has RT reaction replaced with dH2O (lane 13).
Fig. 6.
E2 significantly increases GIRK channel subunit expression in primary rat lactotrophs. RNA was collected from single, plaque-identified lactotrophs and was reverse transcribed; the cDNA was then amplified by PCR using nested primers against GIRK channel subunits 1, 2, and 4. These data represent the number of lactotrophs expressing each GIRK channel subunit from five independent experiments (5 rats; 10 cells analyzed in each treatment group per rat). A significant, overall increase in GIRK channel subunit expression was observed after in vitro treatment with 80 pg/ml E2 compared with vehicle-treated cells (***P < 0.001; 2-way ANOVA with Bonferroni post hoc test). Inset: summary of all preparations (5 rats) shows that in vitro E2-treatment (80 pg/ml overnight) significantly increases the percent of lactotrophs expressing one or more GIRK channel subunits compared with vehicle-treated cells (*P < 0.05; Mann-Whitney U-test).
DISCUSSION
We have previously identified and characterized a GIRK channel as a physiological effector of DA action in pituitary lactotrophs (22, 26). Importantly, the functional expression of this DA-activated channel could be observed only in lactotrophs isolated from female rats in proestrus and not from animals on other days of the reproductive cycle (22). In the present paper, we demonstrate that it is E2 that controls the functional activation of the D2R-GIRK pathway. Moreover, this action of E2 involves components of this pathway that are distal to the D2R and includes the up-regulation of GIRK channel subunits. This represents a novel action of E2 on the pituitary lactotroph.
Pituitary PRL exhibits dynamic secretory patterns associated with reproduction in the mammal. These include bouts of high PRL secretion during the menstrual and estrous cycles, in pregnancy, in response to mating, and in response to suckling (20). The periovulatory surge of PRL on the afternoon of proestrus in the rodent has been extensively studied (5, 62, reviewed in Refs. 15 and 19), although the exact mechanisms contributing to this surge are far from settled. The primary regulatory input to pituitary lactotrophs is inhibitory, and this tonic inhibition is mediated by hypothalamic DA acting on D2Rs in the lactotroph membrane. In all mammalian species, removal of hypothalamic DA or blockade of the D2Rs results in an increase of PRL secretion. Throughout the estrous cycle, DA acts to inhibit PRL secretion from the AP. However, a precipitous drop in DA delivered to the anterior pituitary occurs midday on proestrus, just prior to and during the PRL surge (12, 38). In addition, a rapid decrease in the density of D2Rs in the AP occurs at the onset of the PRL surge (52). These two changes constitute a dramatic withdrawal of dopaminergic tone at the beginning of the proestrous PRL surge.
It is clear that the rise in circulating E2 on the preceding day (diestrus II) is the signal that initiates the hypothalamic-pituitary activities producing the surge of PRL on proestrus. Immunoneutralization of E2 on diestrus II, but not on proestrus, blocks the proestrus surge of PRL (51), and E2 administration to ovariectomized rats initiates daily afternoon surges of PRL that exhibit the same timing as the normal proestrous surge (50). E2 acts on both the DA neurons in the hypothalamus and the lactotrophs to promote PRL secretion. The synthesis and activity of tyrosine hydroxylase (the rate-limiting enzyme in DA synthesis) in TIDA neurons are inhibited by E2 (3, 53). These changes result in an E2-induced drop in DA in the hypophyseal portal vessels (8). At the lactotroph, E2 stimulates PRL synthesis through its classical receptor, directly activating PRL gene transcription (42). In addition, E2 can inhibit lactotroph responsiveness to DA (55), although the mechanisms underlying this reduced responsiveness have not been completely elucidated.
In the present study, we have demonstrated that one mechanism by which E2 alters lactotroph responsiveness to DA is by activating an additional transduction pathway, the GIRK channel. The electrophysiological phenotype of lactotrophs (specifically, whether DA can activate a GIRK current or not) is dependent upon the estrous status of the animal from which they are derived (22). In other words, lactotrophs prepared from proestrous rats (the day we know that DA input to the anterior pituitary falls) hyperpolarize in response to DA, while lactotrophs obtained on other days of the cycle do not. As we have previously reported (27), these phenotypes are observed immediately following dissociation (ex vivo) and remain stable in culture (in vitro) for as long as we have tested them (up to 4 days). Also coupled to this phenotype is the ability of the lactotrophs to produce a secretory rebound upon DA withdrawal (22). These data establish a temporal link between GIRK function and proestrus, when a reduction of dopaminergic tone underlies the PRL surge (see model in Fig. 7). Manipulations of E2, both in vivo and in vitro, that mimic the transition from diestrus II to proestrus resulted in a switch to the proestrous phenotype in lactotrophs.
Fig. 7.
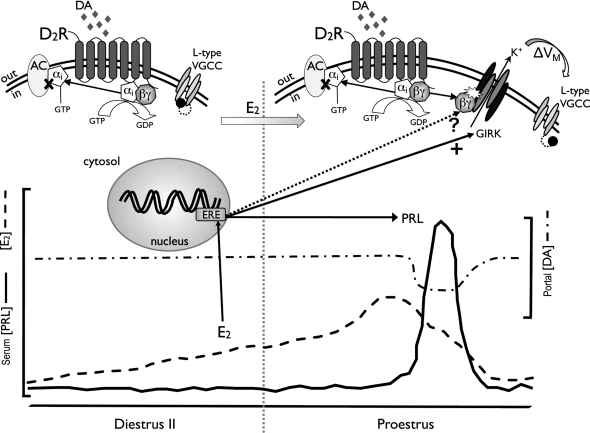
Model of E2 action on D2 receptor (D2R) signaling in the lactotroph. On diestrus II (left), the D2R is not coupled to the GIRK channel. Rising levels of circulating E2 (dashed line) during this time, however, result in a functional switch in DA signaling to include GIRK channel activation. This transduction pathway is complete by the morning of proestrus (right), when DA activates membrane hyperpolarization (ΔVM). The more negative membrane potential “primes” the lactotroph population by removing inactivation (illustrated as a ball-and-chain blocker) of voltage-gated Ca2+ channels (VGCC). When DA levels delivered to the AP via the portal vessels (dashed-dotted line) drop on the afternoon of proestrus, the primed lactotrophs depolarize, initiating increased Ca2+ influx through VGCC to support PRL release and to contribute to the PRL surge (solid line). E2 does not induce this functional switch in signaling by acting on the D2R, but by significantly increasing the expression of GIRK channel subunits (solid arrow). E2 may also regulate expression of the G protein βγ subunit isoforms (dotted arrow, ?), which are known to directly bind to and activate the GIRK channel.
E2-induction of the D2R-GIRK channel pathway, enabling DA to hyperpolarize the lactotroph membrane, appears to be through its classical receptor and attendant signaling pathways. The E2 receptor (ER) antagonist, ICI 182,780, blocked E2 induction of GTPγS-activated GIRK current. ICI is considered a “pure” antagonist (64) that competes with E2 for binding at both ERα and ERβ. E2 is a known transcription factor in pituitary lactotrophs, and we found that treatment with Chx also blocked the ability of E2 to induce GTPγS activation of GIRK current. Together, these data indicate a genomic action of E2 in the regulation of this signaling pathway (Fig. 7).
To investigate potential E2 targets in the lactotroph that could allow for the observed switch in DA signaling to a GIRK-dependent mechanism on proestrus, we considered the essential components of that pathway. The first element is the D2R, which couples to Gαi and thereby inhibits adenylate cyclase (11, 17). D2R density, without a change in affinity, has been shown to decrease in the afternoon of proestrus or with exposure to high concentrations of E2 (52). The present studies used E2 concentrations comparable to those of late diestrus II or early proestrus morning (5), which do not alter the density of D2Rs in the AP (54). Ligand binding parameters may not reveal altered intracellular interactions with transduction proteins, however. To definitively rule out E2-induced changes in D2R, we exploited our discovery that the GIRK channel current could be measured with intracellular dialysis of GTPγS into proestrous lactotrophs. Thus, in the absence of D2R activation, we found that E2 exposure, either in vivo or in vitro, could switch the diestrous phenotype of lactotrophs to a proestrous phenotype, with GTPγS activating the Ba2+-sensitive IRK current.
The second essential component of the D2R-GIRK pathway is the pertussis toxin-sensitive heterotrimeric G protein complex. While the Gαi subunit inhibits adenylate cyclase, the dissociated βγ complex directly binds to GIRK proteins to open the channel (33, 40, 44, 61, 65). E2 treatment of ovariectomized rats has been reported to decrease the percentage of lactotrophs with immunoreactive Gαi3 (43). Immunoneutralization of Gα subunits can increase the availability of free βγ complexes and, therefore, the activity of βγ signaling. Thus, without a concomitant decrease in βγ proteins, a reduction in Gαi could be one mechanism to increase the ability of DA to activate GIRK channels. The βγ subunit itself is another potential target of E2. Bayliss and colleagues have shown that different βγ isoforms have varying abilities to activate GIRK channels and, in fact, β5 acts as a dominant-negative inhibitor when coexpressed with other β-subunits (41). These different activities are associated only with the various isoforms of the β-subunit and do not change with different isoforms of the γ subunit. It is currently unknown which isoforms of these subunits are in lactotrophs, but an E2-induced change in their expression pattern could dramatically alter the ability of DA to activate GIRK.
The third essential component in this pathway is the GIRK channel itself. E2 has been shown to increase the density of another channel, the T-type Ca2+ channel, in the PRL-secreting GH3 cells (58), so we wanted to determine whether it could have a similar effect on GIRK subunits. Because GIRK transcripts and proteins are expressed at low levels (26), we chose to measure them using single-cell RT-PCR. The single-cell RT-PCR analysis technique has been utilized in several applications (4, 7, 34, 36), since it was first described by Eberwine and colleagues (13, 63). E2 significantly increased the percentage of lactotrophs with detectable levels of GIRK transcripts. This increase in mRNA expression was observed for all three GIRK channel subunits found in the rat AP gland: GIRK 1, GIRK 2, and GIRK 4.
Using patch clamp analysis, we found that ∼85% of proestrous lactotrophs respond to DA with a robust hyperpolarization (22). The electrophysiological data from E2-treated lactotrophs in the current experiments closely agree with this percentage (hyperpolarization was observed in 10 out of 12 cells, Fig. 1A and in 11 out of 13 cells, Fig. 1B). This is larger than the population in which we could detect GIRK expression. Since we know the channel functions in 85% of these cells (with GIRK identity verified by Ba2+ sensitivity), we assume that the levels of GIRK transcript in 30–35% of the lactotrophs are below detectable limits of single-cell RT-PCR. This discrepancy between electrophysiology and single-cell RT-PCR has been reported before for another Kir channel (66). Although the single-cell RT-PCR may underestimate the number of lactotrophs actually expressing GIRK subunits, our data demonstrate that estrogen treatment increases expression enough to significantly increase detection.
Our findings are consistent with several other reports that have demonstrated the ability of E2 to serve as a “first messenger,” modulating cell signaling pathways involving G protein-coupled receptors (GPCRs) in other cell types (reviewed in Ref. 37). These reports suggest several potential mechanisms by which E2 could modulate the coupling of GPCRs to GIRK channels, including the alteration of mRNA expression for transcripts that encode proteins involved in neurotransmission and neuropeptide secretion. To our knowledge, however, this is the first demonstration of E2 activation of a GPCR-GIRK signaling pathway in which the K+ channel has been identified by its inward rectification and by Ba2+ sensitivity: the two hallmarks of the Kir channel family. Moreover, these data show a direct up-regulation of the GIRK channel gene expression, indicating that in lactotrophs E2 action may be more direct. It is possible (and quite likely) that E2 alters several intracellular cascades that impinge upon the D2R-βγ-GIRK transduction pathway. GIRK1 subunits in heterologous expression systems can be phosphorylated, enabling βγ gating of the channel (46), and phosphatidylinositol 4,5-bisphosphate (PIP2) has been shown to directly activate inward rectifying K+ channels in cardiac myocytes (32). The convergence of multiple E2-regulated cellular processes may be required for the almost “all-or-nothing” switch in lactotroph GIRK function that we describe here. Nevertheless, our data clearly show that exposure to E2 directly alters the expression of this DA-signaling effector in lactotrophs and elicits a functional change in the secretory responses of these cells.
In the present studies, we found no E2-induced change in responsiveness to the inhibitory actions of DA. The majority (approximately two-thirds) of spontaneous PRL release is dependent upon the influx of Ca2+ through l-type voltage-gated Ca2+ channels (VGCCs). This is demonstrated by the response to verapamil in the present (Fig. 2) and previous studies (23, 30). In the absence of electrical or chemical stimulation, ∼30% of lactotrophs exhibit spontaneous depolarizations (“spiking activity”) of the membrane potential (24). The Ca2+ influx during these depolarizations support elevated cytosolic [Ca2+] that, in turn, supports a higher rate of PRL release from these cells than from electrically quiescent cells (30, 45). Hyperpolarization of the lactotroph membrane closes VGCCs, mediating the inhibitory effect of valinomycin on PRL release. In proestrous lactotrophs, the DA-induced hyperpolarization has the same effect. We and others have demonstrated that this is not a direct action of DA on the VGCCs, but an indirect, voltage-dependent closing of VGCCs (27, 56) (see Fig. 7). On other days of the estrous cycle, DA does not alter the lactotroph membrane potential, but it is still an effective inhibitor of PRL release. Clearly, there occurs a switch in the mechanism(s) by which DA causes this inhibition.
The qualitative, and perhaps most physiologically significant, change in PRL secretion induced by E2 treatment was the generation of a rebound release of PRL following DA withdrawal. We have previously shown that this secretory rebound is dependent upon the influx of Ca2+ through l-type VGCCs (24) and requires the preceding membrane hyperpolarization activated in the presence of DA (27). In the majority (∼ 67%) of quiescent lactotrophs, application and withdrawal of a hyperpolarizing agent (DA in proestrous cells or Val) result in initiation of spiking activity upon recovery of the RMP (24). This electrical activation of previously quiescent cells produces a prolonged rise in cytosolic Ca2+ concentration that correlates temporally with the rebound release of PRL and supports the hypothesis that a population of inactivated l-type VGCCs is recruited in response to the application and withdrawal of DA (30). Previous work by our laboratory shows that these channels are fully “reprimed” when the membrane potential is held at −60 mV (18, 27). In vivo, the activation of the D2R-GIRK pathway by the morning of proestrus would keep lactotroph membranes at the negative potentials needed for full recovery of VGCCs from inactivation. These channels would remain closed, but in a state capable of activation upon membrane depolarization (Fig. 7). Such depolarization would occur in response to the effective withdrawal of dopaminergic inhibition in the afternoon. The availability of all or most VGCCs would also make the lactotrophs more responsive to hypothalamic stimulatory factors, contributing to the surge potential of the lactotroph population.
Perspectives and Significance
The ability of DA to activate a hyperpolarizing signal transduction pathway in lactotrophs only on proestrus fits with the secretory requirements of the female reproductive cycle. PRL synergizes with luteinizing hormone (LH) as a luteotrophic hormone in rodents and is released in a surge coincident with the ovulatory surge of LH. As described above, the ability of the DA-induced hyperpolarization to recruit quiescent lactotrophs into active, highly secreting cells upon the loss of DA tone in the afternoon of proestrus supports this surge release. However, chronic elevations in circulating PRL can be antigonadotrophic, disrupting female fertility (19). The use of nonhyperpolarizing mechanisms of DA inhibition on the other days of the estrous cycle may be a safeguard to avoid large, surge-like releases of PRL during transient interruptions in DA (as seen during stress, for example) on those days. The present data demonstrate that E2 controls this “switch” in D2R signaling in rat lactotrophs to the proestrous phenotype. E2 induces the functional expression of the D2R-GIRK signaling pathway through a genomic mechanism that affects components distal to the D2R. Our data support the hypothesis that rising E2 levels during diestrus alter the mechanism of DA signaling in normal lactotrophs that contributes to the unique secretory profile of PRL secretion on proestrus, and here, we also identify a novel target of E2 action.
GRANTS
This work was supported by research grants from the National Institutes of Health (DK-40336 and DK-54966 to K. A. Gregerson). H. R. Christensen was a recipient of a predoctoral fellowship on NIH training grant HD-007463 (S. Handwerger, principal investigator).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Present address for Q. Zeng: Laboratory of Molecular and Cellular Physiology, School of Life Sciences, Northeast Normal University, Chanchun, Jilin, China.
REFERENCES
- 1. Banerjee MR, Menon RS. Synergistic actions of glucocorticoid and prolactin on milk protein gene expression. In: Actions of Prolactin on Molecular Processes, edited by Rillema JA. Boca Raton, FL: CRC Press, p. 121, 1987 [Google Scholar]
- 2. Ben-Jonathan N. Dopamine: a prolactin-inhibiting hormone. Endocr Rev 6: 564–589, 1985 [DOI] [PubMed] [Google Scholar]
- 3. Blum M, McEwen BS, Roberts JL. Transcriptional analysis of tyrosine hydroxylase gene expression in the tuberoinfundibular dopaminergic neurons of the rat arcuate nucleus after estrogen treatment. J Biol Chem 262: 817–821, 1987 [PubMed] [Google Scholar]
- 4. Bochet P, Audinat E, Lambolez B, Crepel F, Rossier J, Iino M, Tsuzuki K, Ozawa S. Subunit composition at the single-cell level explains functional properties of a glutamate-gated channel. Neuron 12: 383–388, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Butcher RL, Collins WE, Fugo NW. Plasma concentrations of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology 94: 1704–1708, 1974 [DOI] [PubMed] [Google Scholar]
- 6. Caron MG, Beaulieu M, Raymond V, Gagne B, Drouin J, Lefkowitz RJ, Labrie F. Dopaminergic receptors in the anterior pituitary gland: correlation of 3H-dihydroergocryptine binding with the dopaminergic control of prolactin release. J Biol Chem 253: 2244–2253, 1978 [PubMed] [Google Scholar]
- 7. Cornelison DDW, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol 191: 270–283, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Cramer CR, Parker OM, Porter JC. Estrogen inhibition of dopamine release into hypophysial portal blood. Endocrinology 104: 419–422, 1979 [DOI] [PubMed] [Google Scholar]
- 9. Cronin MJ, Myers GA, MacLeod RM, Hewlett EL. Pertussis toxin uncouples dopamine agonist inhibition of prolactin release. Am J Physiol Endocrinol Metab 244: E499–E504, 1983 [DOI] [PubMed] [Google Scholar]
- 10. Dascal N, Schreibmayer W, Lim NF, Wang W, Chavkin C, DiMagno L, Labarca C, Kieffer BL, Gaveriaux-Ruff C, Trollinger D, Lester HA, Davidson N. Atrial G protein-activated K+ channel: expression cloning and molecular properties. Proc Natl Acad Sci USA 90: 10235–10239, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeCamilli P, Macconi D, Spada A. Dopamine inhibits adenylate cyclase in human prolactin-secreting pituitary adenomas. Nature 278: 252–254, 1979 [DOI] [PubMed] [Google Scholar]
- 12. DeMaria JE, Livingstone JD, Freeman ME. Characterization of the dopaminergic input to the pituitary gland throughout the estrous cycle of the rat. Neuroendocrinology 67: 377–383, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Eberwine J, Yeh H, Miyashiro K, Cao Y, Nair S, Finnell R, Zettel M, Coleman P. Analysis of gene expression in single live neurons. Proc Natl Acad Sci USA 89: 3010–3014, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edwards CK, Yunger LM, Lorence RM, Dantzer R, Kelley KW. The pituitary gland is required for protection against lethal effects of Salmonella typhimurium. Proc Natl Acad Sci USA 88: 2274–2277, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Egli M, Leeners B, Kruger TH. Prolactin secretion patterns: basic mechanisms and clinical implications for reproduction. Reproduction 140: 643–654, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Einhorn LC, Gregerson KA, Oxford GS. D2 dopamine receptor activation of potassium channels in identified rat lactotrophs: whole-cell and single-channel recording. J Neurosci 11: 3727–3737, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Enjalbert A, Bockaert J. Pharmacological characterization of the D2 dopamine receptor negatively coupled with adenylate cyclase in the rat anterior pituitary. Mol Pharmacol 23: 576–584, 1983 [PubMed] [Google Scholar]
- 18. Fox AP, Nowycky MC, Tsien RW. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol 394: 149–172, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freeman ME. The ovarian cycle of the rat. In: The Physiology of Reproducion, edited by Knobil E, Neill JD. New York: Raven Press, p. 1893–1928, 1988 [Google Scholar]
- 20. Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev 80: 1523–1631, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Gonzalez-Iglesias AE, Murano T, Li S, Tomic M, Stojilkovic SS. Dopamine inhibits basal prolactin release in pituitary lactotrophs through pertussis toxin-sensitive and -insensitive signaling pathways. Endocrinology 149: 1470–1479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gregerson KA. Functional expression of the dopamine-activated K+ current in lactotrophs during the estrous cycle in female rats: correlation with prolactin secretory responses. Endocrine 20: 67–74, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Gregerson KA. Mechanisms of dopamine action on the lactotroph. In: Prolactin, edited by Horseman ND. Boston, MA: Kluwer Academic, p. 45, 2001 [Google Scholar]
- 24. Gregerson KA, Chuknyiska R, Golesorkhi N. Stimulation of prolactin release by dopamine withdrawal: role of calcium influx. Am J Physiol Endocrinol Metab 267: E789–E794, 1994 [DOI] [PubMed] [Google Scholar]
- 25. Gregerson KA, Einhorn L, Oxford GS. Modulation of potassium channels by dopamine in rat pituitary lactotrophs: a role in the regulation of prolactin secretion? In: Secretion and Its Control, edited by Oxford GS, Armstrong CM. New York: Rockefeller University Press, p. 123–141, 1989 [PubMed] [Google Scholar]
- 26. Gregerson KA, Flagg TP, O'Neill TJ, Anderson M, Lauring O, Horel JS, Welling PA. Identification of G protein-coupled inward rectifier potassium channel gene products from the rat anterior pituitary gland. Endocrinology 142: 2820–2832, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Gregerson KA, Golesorkhi N, Chuknyiska R. Stimulation of prolactin release by dopamine withdrawal: role of membrane hyperpolarization. Am J Physiol Endocrinol Metab 267: E781–E788, 1994 [DOI] [PubMed] [Google Scholar]
- 28. Gregerson KA, Oxford GS. Comparison of ionic currents in normal and reverse hemolytic plaque-identified rat pituitary cells under voltage clamp. Biophys J 51: 431a–438a, 1987 [Google Scholar]
- 29. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Eur J Physiol 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 30. Ho MY, Kao JP, Gregerson KA. Dopamine withdrawal elicits prolonged calcium rise to support prolactin rebound release. Endocrinology 137: 3513–3521, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J 16: 6926–6935, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature 391: 803–806, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Huang CL, Jan YN, Jan LY. Binding of the G protein beta-gamma subunit to multiple regions of G protein-gated inward-rectifying K+ channels. FEBS Lett 405: 291–298, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Johansen FF, Lambolez B, Audinat E, Bochet P, Rossier J. Single cell RT-PCR proceeds without the risk of genomic DNA amplification. Neurochem Int 26: 239–243, 1995 [DOI] [PubMed] [Google Scholar]
- 35. Karschin C, Dibmann E, Stuhmer W, Karschin A. IRK(1–3) and GIRK(1–4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci 16: 3559–3570, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kawano T, Zhao P, Nakajima S, Nakajima Y. Single-cell RT-PCR analysis of GIRK channels expressed in rat locus coeruleus and nucleus basalis neurons. Neurosci Lett 358: 63–67, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Kelly MJ, Qiu J, Ronnekleiv OK. Estrogen modulation of G protein-coupled receptor activation of potassium channels in the central nervous system. Ann New York Acad Sci 1007: 6–16, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Kerdelhue B, Bojda F, Lesieur P, Pasqualini C, el Abed A, Lenoir V, Douillet P, Chiueh MC, Palkovits M. Median eminence dopamine and serotonin neuronal activity. Neuroendocrinology 49: 176–180, 1989 [DOI] [PubMed] [Google Scholar]
- 39. Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature 374: 135–141, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Krapivinsky G, Kennedy ME, Nemec J, Medina I, Krapivinsky L, Clapham DE. Gβ binding to GIRK4 subunit is critical for G protein-gated K+ channel activation. J Biol Chem 273: 16946–16952, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Lei Q, Jones MB, Talley EM, Schrier AD, McIntire WE, Garrison JC, Bayliss DA. Activation and inhibition of G protein-coupled inwardly rectifying potassium (Kir3) channels by G protein beta gamma subunits. Proc Natl Acad Sci USA 97: 9771–9776, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lieberman ME, Maurer RA, Claude P, Wiklund J, Wertz N, Gorski J. Regulation of pituitary growth and prolactin gene expression by estrogen. Adv Exp Med Biol 138: 151–163, 1981 [DOI] [PubMed] [Google Scholar]
- 43. Livingston JD, Lerant A, Freeman ME. Ovarian steroids modulate responsiveness to dopamine and expression of G-proteins in lactotropes. Neuroendocrinology 68: 172–179, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 325: 321–326, 1987 [DOI] [PubMed] [Google Scholar]
- 45. Malgaroli A, Vallar L, Elahi FR, Pozzan T, Spada A, Meldolesi J. Dopamine inhibits cytosolic Ca2+ increases in rat lactotroph cells. Evidence of a dual mechanism of action. J Biol Chem 29: 13920–13927, 1987 [PubMed] [Google Scholar]
- 46. Medina I, Krapivinsky G, Arnold S, Kovoor P, Krapivinsky L, Clapham DE. A switch mechanism for Gβγ activation of IKACh. J Biol Chem 275: 29709–29716, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev 78: 189–225, 1998 [DOI] [PubMed] [Google Scholar]
- 48. Nagy E, Berczi I, Friesen HG. Regulation of immunity in rats by lactogenic and growth hormones. Acta Endocrinol 102: 351–357, 1983 [DOI] [PubMed] [Google Scholar]
- 49. Nansel DD, Gudelsky GA, Porter JC. Subcellular localization of dopamine in the anterior pituitary gland of the rat: apparent association of dopamine with prolactin secretory granules. Endocrinology 105: 1073–1077, 1979 [DOI] [PubMed] [Google Scholar]
- 50. Neill JD. Sexual differences in the hypothalamic regulation of prolactin secretion. Endocrinology 90: 1154–1159, 1972 [DOI] [PubMed] [Google Scholar]
- 51. Neill JD, Freeman ME, Tillson SA. Control of the proestrus surge of prolactin and luteinizing hormone secretion by estrogens in the rat. Endocrinology 89: 1448–1453, 1971 [DOI] [PubMed] [Google Scholar]
- 52. Pasqualini C, Lenoir V, el Abed A, Kerdolhue B. Anterior pituitary dopamine receptors during the rat estrous cycle. A detailed analysis of proestrus changes. Neuroendocrinology 38: 39–44, 1984 [DOI] [PubMed] [Google Scholar]
- 53. Pasqualini C, Leviel V, Guibert B, Faucon-Biguet N, Kerdelhue B. Inhibitory actions of acute estradiol treatment on the activity and quantity of tyrosine hydoxylase in the median eminence of ovariectomized rats. J Neuroendocrinol 3: 575–580, 1991 [DOI] [PubMed] [Google Scholar]
- 54. Pilotte NS, Burt DR, Barraclough CA. Ovarian steroids modulate the release of dopamine into hypophysial portal blood and the density of anterior pituitary [3H]spiperone-binding sites in ovariectomized rats. Endocrinology 114: 2306–2311, 1984 [DOI] [PubMed] [Google Scholar]
- 55. Raymond V, Beaulieu M, Labrie F, Boissier J. Potent antidopaminergic activity of estradiol at the pituitary level on prolactin release. Science 200: 1173–1175, 1978 [DOI] [PubMed] [Google Scholar]
- 56. Rendt J, Oxford GS. Absence of coupling between D2 dopamine receptors and calcium channels in lactotrophs from cycling female rats. Endocrinology 135: 501–508, 1994 [DOI] [PubMed] [Google Scholar]
- 57. Riddle O, Bates RW, Dykshorn SW. The preparation, identification and assay of prolactin—a hormone of the anterior pituitary. Am J Physiol 105: 191–216, 1933 [Google Scholar]
- 58. Ritchie AK. Estrogen increases low voltage-activated calcium current density in GH3 anterior pituitary cells. Endocrinology 132: 1621–1629, 1993 [DOI] [PubMed] [Google Scholar]
- 59. Senogles SE, Benovic JL, Amlaiky N, Unson C, Milligan G, Vinitsky R, Spiegel AM, Caron MG. The D2-dopamine receptor of anterior pituitary is functionally associated with a pertussis toxin-sensitive guanine nucleotide binding protein. J Biol Chem 262: 4860–4867, 1987 [PubMed] [Google Scholar]
- 60. Smith PF, Luque EH, Neill JD. Detection and measurement of secretion from individual neuroendocrine cells using a reverse hemolytic plaque assay. Methods Enzymol 124: 443–465, 1986 [DOI] [PubMed] [Google Scholar]
- 61. Takao K, Yoshii M, Kanda A, Kokubun S, Nukada T. A region of the muscarinic-gated atrial K+ channel critical for activation by G protein beta gamma subunits. Neuron 13: 747–755, 1994 [DOI] [PubMed] [Google Scholar]
- 62. Terry LC, Saunders A, Audet J, Willoughby JO, Brazeau P, Martin JB. Physiologic secretion of growth hormone and prolactin in male and female rats. Clin Endocrinol (Oxf) 6: 19S–28S, 1977 [DOI] [PubMed] [Google Scholar]
- 63. Van Gelder R, von Zastrow ME, Yool A, Dement WC, Barchas JD, Eberwine JH. Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc Natl Acad Sci USA 87: 1663–1667, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res 51: 3867–3873, 1991 [PubMed] [Google Scholar]
- 65. Yamada M, Inanobe A, Kurachi Y. G protein regulation of potassium ion channels. Pharmacol Rev 50: 723–760, 1998 [PubMed] [Google Scholar]
- 66. Zhang C, Bosch MA, Levine JE, Ronnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express KATP channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci 27: 10153–10164, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]