SUMMARY
Resistance to therapies develops rapidly for melanoma leading to more aggressive disease. Therefore, agents are needed that specifically inhibit proteins or pathways controlling the development of this disease, which can be combined, dependent on genes deregulated in a particular patient’s tumors. This study shows that elevated sphingosine-1-phosphate (S-1-P) levels resulting from increased activity of sphingosine kinase-1 (SPHK1) occur in advanced melanomas. Targeting SPHK1 using siRNA decreased anchorage dependent and independent growth as well as sensitized melanoma cells to apoptosis inducing agents. Pharmacological SPHK1 inhibitors SKI-I but not SKI-II decreased S-1-P content, elevated ceramide levels, caused a G2-M block and induced apoptotic cell death in melanomas. Targeting SPHK1 using siRNA or the pharmacological agent called SKI-I, decreased the levels of pAKT. Furthermore, SKI-I inhibited the expression of CYCLIN D1 protein and increased the activity of caspase-3/7, which in turn led to the degradation of PARP. In animals, SKI-I but not SKI-II retarded melanoma growth by 25-40%. Thus, targeting SPHK1 using siRNAs or SKI-I has therapeutic potential for melanoma treatment either alone or in combination with other targeted agents.
Keywords: melanoma, SKI-I, sphingosine kinase-1, S-1-P, AKT3, PRAS40, V600EB-RAF, apoptosis, proliferation, tumorigenesis
INTRODUCTION
Sphingosine kinases (SPHK1) are evolutionarily conserved lipid kinases associated with cell migration, differentiation, proliferation and survival (Melendez et al., 2000, Leclercq and Pitson, 2006, Sarkar et al., 2005). Two isoforms of SPHK have been identified, differing in sequence, catalytic properties, sub-cellular localization and function. SPHK1 has been implicated in the development of a variety of different cancers including melanoma (French et al., 2003, Xia et al., 2000, Vadas et al., 2008, Shida et al., 2008). However, the oncogenic role of SPHK2 in cancer development remains to be fully elucidated (Schnitzer et al., 2009, Nemoto et al., 2009). SPHK1 expression and activity are regulated by AKT and ERK1/2 in mammalian cells (Pitson et al., 2005, Leclercq and Pitson, 2006, Francy et al., 2007). Released S-1-P can modulate the expression of various mitogenic and apoptotic signaling proteins including AKT, ERK and CYCLINs to regulate cellular survival and death (Shin et al., 2007, Vadas et al., 2008, Song et al., 2011, Pitson, 2011). For example, inhibiting SPHK1 using siRNA and pharmacological agents decreased phosphorylated Akt and thereby sensitized glioma cells to apoptosis inducers (Guan et al., 2011). Furthermore, increasing the expression of SPHK1 elevated the levels of PI3K to upregulate the expression of phosphorylated Akt and downstream targets Bcl2, NFkB, cIAP-1 and cIAP-2 (Guan et al., 2011). SPHK1 converts pro-apoptotic ceramide derived sphingosine to pro-mitogenic sphingosine-1-phosphate (S-1-P) in mammalian cells (Edsall et al., 2001, Leclercq and Pitson, 2006, Huwiler and Zangemeister-Wittke, 2007, Spiegel and Milstien, 2003). A recent study has shown that SPHK1 increases autophagy to protect cells from nutrient starvation induced apoptosis (Lavieu et al., 2006). Studies have also shown that targeting SPHK1 using siRNA or chemotherapeutic agents can decrease the growth of breast, prostate and brain cancers (Sarkar et al., 2005, Edsall et al., 2001, Nava et al., 2002, Kapitonov et al., 2009). In contrast, overexpression of human SPHK1 in NIH3T3 fibroblasts led to cellular transformation and acquisition of a tumorigenic potential (Olivera et al., 1999, Xia et al., 2000). Cancer cells showing high levels of SPHK1 expression/activity, and normal cells transformed by SPHK1 overexpression exhibited increased resistance to chemotherapeutic agents such as anthracyclines, doxorubicin, and camptothecin (Pchejetski et al., 2008, Sarkar et al., 2005). High levels of SPHK1 activity counteracts ceramide-mediated cell death of human melanoma cells containing pro-survival protein BCL-2 (Bektas et al., 2005). Furthermore, liposomal ceramide inhibits melanoma cell growth in culture and in xenografted animal models (Tran et al., 2008b). Thus, therapeutically targeting oncogenic SPHK1 could be important for overcoming cancer chemoresistance.
Pharmacological agents SKI-I and SKI-II have been identified as selective SPHK inhibitors (SKIs) (French et al., 2003, French et al., 2006). Compared to lipid-based SphK inhibitors such as N,N’-dimethyl sphingosine, which are non-specific, these SKIs are small molecule compounds, inhibiting SphK activity to decrease cellular S-1-P levels (French et al., 2003). Recently, SKI-I has been shown to inhibit SPHK1 activity when tested in vitro. Furthermore, SKI-I is slightly more effective toward inhibiting SPHK1 compared to SPHK2 (Hengst et al., 2010b). In addition, SKI-I and SKI-II treatment inhibited the PI3 kinase signaling pathway thereby decreasing cell survival (French et al., 2003, French et al., 2006). In vivo, both SKI-I and II have been shown to decrease JC mammary tumor growth when administered either i.p. (50 mg/kg body weight at 3 day interval) or p.o. (SKI-II, 100 mg/kg every other day) (French et al., 2003).
Malignant melanoma is characterized by high metastatic potential and chemo-resistance (Robertson, 2005, Sinnberg et al., 2008, Hocker et al., 2008). The PI3 and MAP kinase pathways are key signaling cascades regulating survival, proliferation and metastasis of melanoma cells (Singh et al., 2008, Madhunapantula and Robertson, 2008, Madhunapantula and Robertson, 2009, Inamdar et al., 2010). Although elevated expression or activity of AKT3 in melanomas is known to decrease cellular apoptosis mediated through pPRAS40 to increase cell survival and resistance to apoptosis inducing agents (Stahl et al., 2004, Madhunapantula et al., 2007, Inamdar et al., 2010, Madhunapantula and Robertson, 2009); recent studies demonstrated that Akt2 is a downstream target of metabotropic glutamate receptor-1 (Grm1) and may also play a critical role in melanoma tumor development (Shin et al., 2010). In addition, phosphorylation of Akt2 has been associated with melanoma cell metastasis (Nogueira et al., 2010).
The most mutated gene in melanomas is an intermediate member of the MAP kinase signaling pathway called V600EB-RAF. Constitutively active V600EB-RAF triggers a series of consecutive phosphorylation events aiding melanoma cell proliferation (Madhunapantula and Robertson, 2008, Hingorani et al., 2003, Tuveson et al., 2003). SiRNAs or pharmacological agents vemurafenib (PLX-4032) inhibiting V600EB-RAF expression and activity, respectively, can retard melanoma tumor growth and lung metastasis development in animal models of this disease (Sharma et al., 2005, Sharma et al., 2006, Lee et al., 2010, Flaherty et al., 2010). Furthermore, AKT3 and V600EB-RAF cooperate to promote early-melanoma development and combined targeting of these two proteins, using nano-liposomal siRNA, leads to synergistically acting tumor inhibition (Cheung et al., 2008, Tran et al., 2008a). Although, it is feasible to target these two proteins in animals using siRNA molecules, it is not currently a viable treatment strategy for humans as there are no effective siRNA delivery vehicles available for human use. Moreover, pharmacological approaches using small molecule inhibitors such as perifosine to target PI3K-Akt pathway have failed in clinical trials due to poor efficacy at reducing tumor burden, off-target effects, and failure to extend disease free survival (Ernst et al., 2005). Furthermore, FDA approved Vemurafenib (PLX-4032) is effective only in 50% patients harboring V600EB-Raf (Flaherty et al., 2010, Inamdar et al., 2010) and recent studies demonstrated development of resistance to Vemurafenib in most patients initially responsive to the drug (Solit and Rosen., 2011, Inamdar et al., 2010, Aplin et al., 2011). Hence, identification of novel pharmacological agents that inhibit melanoma growth remains important. In this regard, recent evidence indicates that SPHK1 might be a novel target in melanoma. However, it is not known whether targeting SPHK1 using siRNA or non-lipid inhibitors SKI-I and SKI-II could reduce melanoma development. It is also not known which of these pharmacological agents might be potent and selective for inhibiting melanoma tumor growth in vitro and in animals.
In this study, the therapeutic effects of targeting SPHK1 in melanoma have been investigated. Melanoma cells are shown to exhibit elevated levels of SPHK1 activity compared to melanocytes and targeting SPHK1 using siRNA can retard melanoma cell growth by inhibiting cell proliferation and inducing G0/G1 cell cycle arrest as well as sensitize melanoma cells to staurosporine-induced apoptosis. In animals, intraperitoneal administration of SPHK1 inhibitor SKI-I but not SKI-II, decreased melanoma tumor growth. Similar to siRNA mediated SPHK1 inhibition, SKI-I treatment decreased cell viability and inhibited proliferation by arresting cells in the G2/M and S phases of cell cycle and increasing the rates of cellular apoptosis. Mechanistically, SKI-I treatment induced pro-apoptotic cellular ceramide levels and decreased pro-survival S-1-P content in melanomas. As a result, SKI-I treatment induced apoptotic cell death as indicated by elevated cleaved caspase-3 and PARP levels. Thus, targeting SPHK1 using SKI-I can decrease the tumorigenic potential of melanoma cells, and hence can be considered as a lead compound for developing novel therapeutic agents for treating this disease.
RESULTS
Sphingosine kinase activity is upregulated in metastatic melanomas
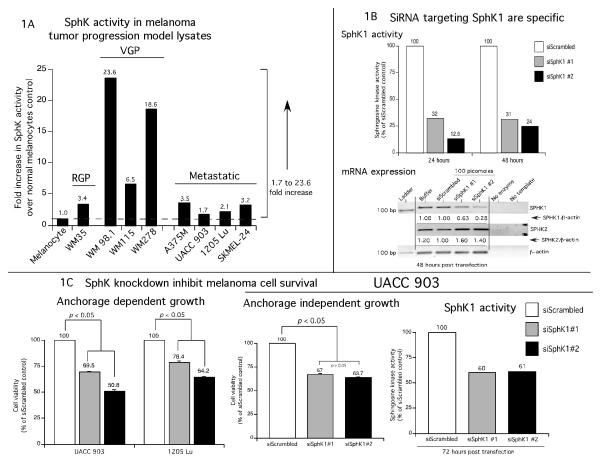
Compared to matched normal tissue, overexpression of SPHK1 has been reported for carcinomas of the breast, prostate, brain, colon, lung and kidney (French et al., 2003, Xia et al., 2000, Vadas et al., 2008, Bektas et al., 2005, Hait et al., 2006, Shida et al., 2008). However, the activity levels of SPHK1 in melanomas are unknown. Therefore, levels of SPHK1 activity in normal human melanocytes (NHEM) were compared to melanoma cell lines from the radial (WM35), vertical (WM98.1, WM115, WM278) and metastatic (A375M, UACC 903, 1205 Lu and SKMEL-24) stages of growth. These well-studied cell lines represent various growth stages of melanoma tumor progression. An increase of 1.7 - 23.6 fold in SphK activity was observed in all melanoma cell lines compared to normal human melanocytes (Fig. 1A, values are the averages of two independent experiments with three replicates in each experiment). Highest levels of SphK activity were observed in vertical growth phase cells ranging from 18 - 24 fold higher than occurred in melanocytes. Advanced stage metastatic cell lines had 1.7 - 3.5 fold higher SphK activity than that seen in melanocytes (Fig. 1A).
Figure 1. SPHK1 downregulation inhibits melanoma tumor development.
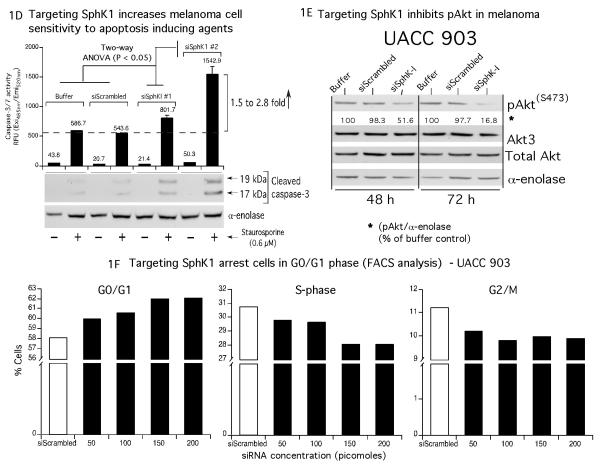
1A. SPHK1 activity is increased in melanoma cells compared to normal human epidermal melanocytes. Melanoma cell lines derived from radial, vertical and metastatic stages were harvested using SPHK1 lysis buffer and analyzed for SPHK1 activity. SPHK1 activity was elevated in early (2.9 – 3.4 fold in RGP; 6.5 – 23.6 fold in VGP) and late stage melanoma cell lines (1.7 – 3.5 fold). Bars indicate the averages from two independent experiments. 1B. siRNAs targeting SPHK1 decrease expression and activity. UACC 903 cells were nucleofected with two non-overlapping siRNAs (#1 and #2, 100 pmoles each) using Amaxa nucleofector program K17 and Kit-R. Cell lysates were harvested using the RP-20 Kit at 24 and 48 hours. SPHK1 mRNA and sphingosine kinase activity levels were inhibited at 24 and 48 hours. Cells nucleofected with siRNA reconstitution buffer or a scrambled siRNA served as controls. A slight increase in SPHK2 mRNA expression was noticed when cells were nucleofected with siSPHK1 #1 and siSPHK1 #2. Targeting SPHK1 using siSPHK1 #2 reduced >85% of SPHK activity indicating that SPHK1 accounted for SPHK activity. 1C. Targeting SPHK1 inhibits melanoma cell proliferation. UACC 903 and 1205 Lu cells were nucleofected with 200 pmoles siSPHK1 #1 and #2; and 24 hours later plated into a 96-well plate coated or uncoated with polyHEMA. Two days later, the number of viable cells determined using MTS. Cell lysates were collected from p60 plates to determine the SPHK1 activity and determine the mRNA levels (data not shown). Compared to cells transfected with controls siScrambled or DEPC water; siSPHK1 nucleofected cells decreased anchorage dependent independent growth of UACC 903 by 30 – 50 % and 1205 Lu by 22 - 36%. 1D, 1E and 1F. SiRNA mediated downregulation of SPHK1 decreased sensitized melanoma cells to therapeutic agents by decreasing pAkt levels and by inducing cell cycle arrest in G0/G1 phase. UACC 903 cells were transfected with 200 pmoles of siSPHK1 (#1 and #2), control siScrambled RNA or DEPC water and 48 hours later transfected cell were exposed to 0.6 μM staurosporine for 6 hours (Fig. 1D). Cells transfected with siSPHK1 had elevated levels of caspase-3/7 activity and cleaved caspase-3 fragments compared to controls (Fig. 1D, p < 0.05, One-way ANOVA). In addition, protein lysates were collected after 48 and 72 hours of siSPHK1 #2 siRNA transfection and analyzed by Western blotting for the expression of pAkt, Akt3, total Akt and α-enolase (Fig. 1E). Targeting SphK1 decreased pAkt levels in a time dependent manner (Fig. 1D). In addition, increasing amounts of (from 50 picomoles to 200 picomoles) siSPHK1 #2 siRNA was also introduced in to UACC 903 cells and 48 h later, stained with propidium iodide. The stained cells were analyzed by FACS. The data shows a dose dependent increase in the G0/G1 phase cell population (Fig. 1F). Results shown are from single experiment.
siRNA mediated downregulation of SPHK1 protein levels, inhibited anchorage dependent as well as independent growth and sensitized melanoma cells to the apoptosis inducing agent staurosporine by decreasing pAkt and arresting cells in G0/G1 phase of the cell cycle
To determine the contribution of SPHK1 isoform to the total SPHK activity and test whether inhibiting SPHK1 would retard melanoma cell growth, two independent siRNAs targeting SPHK1 were used to inhibit SphK protein levels in UACC 903 cells. At 24 and 48 h post transfection, both siRNAs reduced SphK activity by 68 to 76% for siSPHK1 #1 and 86 to 87% for siSPHK1 #2 compared to a scrambled siRNA control (Fig. 1B; values are the averages of two independent experiments with three replicates in each experiment). To demonstrate the specificity of SPHK1 siRNAs, levels of SPHK1 and SPHK2 mRNA were measured at 48 h post transfection. The data showed a 37 to 72% decrease in SPHK1 expression, with siSPHK1 #2 being more effective than siSPHK #1 when compared to control buffer or scrambled siRNA transfected cells (Fig. 1B).
To determine whether SPHK1 knockdown inhibited anchorage dependent growth of melanoma cells, siRNAs targeting SPHK1 were introduced into UACC 903 or 1205 Lu cell lines and cell viability measured. Compared to control scrambled siRNA transfected cells, a 22 to 50% decrease in cell viability was observed when SPHK1 protein expression was decreased (Fig. 1C, p < 0.05; One-way ANOVA). Next, to establish whether high SPHK1 activity in malignant melanoma cell lines provided cells with an anchorage independent growth advantage, UACC 903 cells transfected with scrambled siRNA or siRNAs targeting SPHK1 were plated into polyHEMA coated 96-well plates and cell growth measured by MTS assay. Compared to controls, a 33 to 37% decrease in the percentage of surviving cells was observed in SPHK1 knocked down cells (Fig. 1C, p < 0.05; One-way ANOVA). Knockdown of SPHK1 was confirmed in these cells by measuring sphingosine kinase activity, which was reduced by ~40% compared to controls transfected with a scrambled siRNA (Fig. 1C). Similar results were also observed when SPHK1 siRNAs were introduced in to 1205 Lu cells (data not shown). Next, to establish whether SPHK1 knockdown would sensitizes melanoma cells to apoptosis inducers such as staurosporine, UACC 903 cells were nucleofected with 200 pmoles of SPHK1 siRNA, scrambled siRNA or a buffer control and two days later, treated with 600 nM staurosporine or DMSO vehicle for 6 hours. Staurosporine treatment conditions, a well-studied apoptosis inducer, were chosen from a prior report (Madhunapantula et al., 2007). Expression levels of apoptosis markers cleaved caspase-3 in cell lysates, were measured using a fluorimetric Apo-ONE homogenous caspase-3/7 assay kit and by Western blotting, respectively (Fig. 1D). Cells nucleofected with SPHK1 siRNA showed 1.5 to 2.8 fold more caspase-3/7 activity with corresponding increases in cleaved caspase-3 fragments compared to buffer or scrambled siRNA nucleofected cells (Fig. 1D, p <0.05; One-way ANOVA).
Next, the mechanistic basis for SPHK1 knockdown mediated cell growth inhibition was investigated. Targeting SPHK1 using siRNAs reduced pAkt levels and induced cell cycle arrest in the G0/G1 phase of the cell cycle thereby decreasing melanoma cell viability (Figs. 1E and 1F). Experimentally, 200 picomoles of siRNA #2 was introduced into UACC 903 cells and protein lysates collected after 48 and 72h. Western blot analysis showed a time dependent decrease in pAkt expression only when SPHK1 siRNA was introduced into cells (Fig. 1E). No major changes in expression of pAkt were noticed in control buffer and scrambled siRNA transfected cells. Furthermore, expression of total Akt or Akt3 were unchanged by SPHK1 knockdown using siRNA (Fig. 1E). To measure the effect of SPHK1 inhibition on cell cycle progression, increasing concentrations of (50, 100, 150 and 200 picomoles) of SPHK1 siRNA #2 was introduced and two days later UACC 903 cells stained with propidium iodide for FACScan analysis. The data shows a dose dependent increase from 59.9% (in 50 picomoles) to 62.0% (in 200 picomoles) in the G0/G1 cell population with a simultaneous decrease in S-phase and G2/M phase cell percentages compared to control scrambled siRNA nucleofected cells (G0/G1% 58.0) (Fig. 1F). No significant increase in the sub-G0-G1 cell population, an indicator of apoptosis, was observed with the siRNA mediated SPHK1 inhibition (Fig. 1F).
Pharmacological SPHK1 inhibitor, SKI-I but not SKI-II inhibits the growth of melanoma cells without effecting normal human melanocyte or fibroblast cell proliferation
Using an MTS assay, efficacy and cancer cell selectivity of SKI-I and SKI-II inhibitors, whose structures are shown in Fig. 2A, were tested on FOM-103 melanocytes, FF2441 fibroblast cells, and melanoma cell lines WM35, WM115, WM278, A375M, UACC 903 and 1205 Lu following 24, 48, or 72 h treatment. Compared to FOM-103 melanocytes and FF2441 normal fibroblast cells, melanoma cells were more sensitive to SKI-I treatment following exposure (Table. 1). The average IC50 values of SKI-I for melanoma cells was 1.96, 5.3 and 8.4 fold lower than that observed for the average IC50 values melanocytes and fibroblast cells at 24, 48 and 72 h of exposure, respectively (Table. 1). Furthermore, SKI-I inhibited melanoma cell viability in a dose dependent manner with IC50 values ranging from 1.0 to 6.1 μM. In general, the average IC50 values for SKI-I and SKI-II decreased with increasing treatment time; however, SKI-I was ~10 fold more effective (Table. 1). For SKI-I treatment, the average IC50 values at 24, 48 and 72 h exposure were 20.6, 6.4, and 3.3 μM, respectively (Table. 1). In contrast, SKI-II was ineffective at comparable concentrations, suggesting it would be ineffective for melanoma treatment (Table. 1). Cell lines with relatively high SphK activity (WM35 and WM115) exhibited greater sensitivity to SKI-I and SKI-II compared to those with low SphK activity (UACC 903 and 1205 Lu) at 72 h of treatment (Fig. 1A and Table. 1). In contrast IC50 values of SKI-I for WM278 (with 18.6-fold higher activity than melanocytes) and A375M (with 3.3-fold higher SphK activity than melanocytes), respectively, are 6.1 and 1.0 μM at 72 h of treatment (Fig. 1A, Supplemental Fig. 1 and Table. 1). Although SphK activity is significantly higher in vertical growth phase WM115 and WM278, and exhibited sensitivity to SKI-I and SKI-II treatments, respectively, they failed to form tumors when injected sub-cutaneously into nude mice. Even though WM 98.1 form tumors, the latency period is >30 days when 10 million cells were injected sub-cutaneously in to nude mice (Schadendorf et al., 1996). Therefore, further studies have been performed using the tumor forming metastatic melanoma cell lines A375M (3.3-fold higher SphK activity than melanocytes), UACC 903 (1.7-fold higher SphK activity than melanocytes) and 1205 Lu (2.1-fold higher SphK activity than melanocytes).
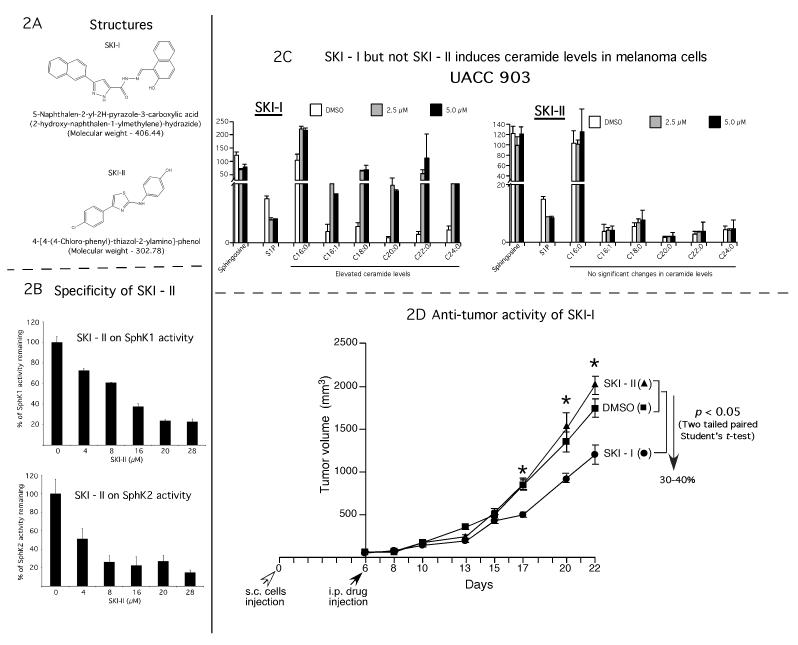
Figure 2. SKI-I inhibits growth of xenografted melanoma cells in animals.
2A. Structures of SPHK1 inhibitors. SKI-I and SKI-II are synthetic, non-lipid inhibitors identified from a chemical library screen using recombinant human SPHK1. 2B. SKI-II inhibited SPHK1 and SPHK2 activity in vitro. Insect cell produced, purified recombinant SPHK1 and SPHK2 proteins were preincubated for 15 minutes at room temperature with SKI-II in a 90 μL of assay buffer and the amount of S1-32P was extracted and measured by liquid scintillation counting. 2C. SKI-I but not SKI-II reduced cellular S-1-P and induced pro-apoptotic ceramide content in melanoma cells. 2 X 106 UACC 903 cells were plated in 100 mm culture dish and 24 hours later treated with SKI-I and SKI-II for 48 hours. Levels of D-erythro-sphingosine, sphingosine-1-phosphate, and ceramide were analyzed by LC/MS/MS (Wijesinghe et al., 2010). 2D. Intra peritoneal administration of SKI-I but not SKI-II retarded melanoma tumor growth. 5 X 106 UACC 903 cells were injected subcutaneously into left and right flanks near rib cages and six days later, mice were injected i. p. with 100 mg/kg body weight SKI-I or SKI-II on Monday, Wednesday and Friday for 3 weeks. Compared to SKI-II or DMSO vehicle, SKI-I significantly decreased tumor development beginning from day 17 (p < 0.05; Student’s t-test). At day 22, an ~40 % difference in tumor volume between vehicle control and SKI-I treatment group was observed. SKI-II had no effect on tumor growth.
Table 1. SKI-I selectively inhibits early and late stage melanoma cells in culture.
FF244.1 fibroblasts, FOM103 melanocytes and early and late stage melanoma cells were seeded in a 96-well plate and after 36 to 72 h, treated with increasing SKI-I or SKI-II for indicated time period. Number of viable cells determined using MTS and percentage decrease in viability calculated. IC50 values shown represent the averages of 2 independent experiments. SKI-I was more effective compared to SKI-II.
| Cell line | SKI-I Treatment time (h) |
SKI-II Treatment time (h) |
Melanoma stage cell line |
|||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| 24 | 48 | 72 | 24 | 48 | 72 | |||
|
|
||||||||
| Normal cells | FF2441 | 32.8 | 23.0 | 17.9 | >100 | >100 | >100 | |
| FOM103 | 47.9 | 44.9 | 37.2 | 53.5 | 55.7 | 64.1 | ||
|
|
||||||||
| Melanoma cells | WM35 | 37.0 | 6.1 | 1.9 | 37.0 | 10.3 | 6.6 | RGP |
| WM115 | 9.7 | 5.6 | 1.8 | 38.9 | 23.8 | 11.7 | VGP | |
| WM278 | 29.0 | 8.0 | 6.1 | 20.0 | 21.4 | 23.6 | ||
| A375M | 8.1 | 1.7 | 1.0 | >100 | 26.1 | 21.8 | Metastatic | |
| UACC 903 | 19.5 | 10.9 | 5.1 | >100 | 59.1 | 33.6 | ||
| 1205 Lu | 20.4 | 5.8 | 3.9 | >100 | 86.3 | 71.8 | ||
|
|
||||||||
| Average | 20.61 | 6.4 | 3.3 | 37.8 | 28.2 | |||
| Range | 8.1-37.0 | 1.7-10.9 | 1.0-5.1 | 20.0->100 | 10.3-86.3 | 6.6-71.8 | ||
SKI-I but not SKI-II effectively reduces cellular S-1-P and induced ceramide levels in melanoma cells
Although SKI-I inhibits SPHK1 and SPHK2, it more effectively inhibits SPHK1 compared to SPHK2 activity (Hengst et al., 2010b). Therefore, the specificity of SKI-II for inhibiting SPHK1 and SPHK2 using recombinant SPHK1 and SPHK2 was measured as described in previous reports (Hengst et al., 2010b, Hengst et al., 2010a). As reported, SKI-II was effective at inhibiting both SPHK1 and SPHK2, albeit, with a slightly enhanced selectivity to SPHK2 (Fig. 2B) (Antoon et al., 2011). Since SKI-I, reduced viability of melanoma cells more effectively compared to SKI-II, the effect of SKI-I and SKI-II on cellular sphingolipid metabolism was examined. SKI-I and SKI-II effectively reduced pro-survival S-1-P, however, only SKI-I induced pro-apoptotic cellular ceramide levels in UACC 903 cells (Fig. 2C). Similarly, SKI-I treatment reduced S-1-P and increased ceramide levels in 1205 Lu cells (Supplemental Fig. 2).
SKI-I is effective at inhibiting xenografted melanoma tumors
Since targeted inhibition of SPHK1 protein using siRNA reduced melanoma cell growth and sensitized cells to apoptosis inducing therapeutic agents, the effect of intraperitoneal administration of SKI-I and SKI-II on xenografted melanoma tumors development was examined next. 5 × 106 UACC 903 or 2.5 × 106 1205 Lu cells were subcutaneously injected into the flanks of 4-6 weeks old nude mice and tumor development let occur for 6 days. From day 6, mice were treated with SKI-I or SKI-II at 100 mg/kg body weight in 50 μl DMSO or DMSO vehicle control. Intra-peritoneal administration of SKI-I was effective at inhibiting xenografted UACC 903 and 1205 Lu melanoma tumors growth (Figs. 2D, p < 0.05; Student’s t-test, and Supplemental Fig. 3). In contrast, SKI-II administration did not effect tumor development (Fig. 2D).
SKI-I specifically inhibited melanoma cell proliferation and triggered apoptosis
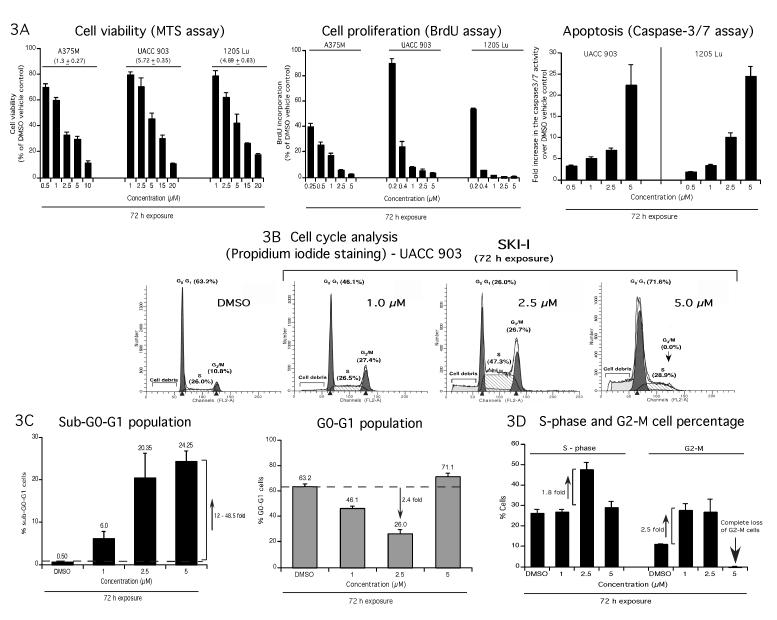
Since SKI-I but not SKI-II was potent at inhibiting cultured cell growth and tumor development in animals, effects of SKI-I treatment on melanoma cells viability (Fig. 3A, left), proliferation (Fig. 3A, middle) and apoptosis (Fig. 3A, right) were examined. A dose dependent decrease in cells incorporating BrdU compared to vehicle treated cells was observed upon incubation of melanoma cells A375M, UACC 903 and 1205 Lu with SKI-I (Fig. 3A, middle). Similarly, UACC 903 and 1205 Lu cell lines also showed a dose dependent increase in caspase-3/7 activity upon SKI-I treatment (Fig. 3A, right).
Figure 3. SKI-I inhibits melanoma cell growth by reducing cellular proliferation, triggering apoptosis and modulating cell cycle progression.
3A. SKI-I inhibits cultured melanoma cells growth by inhibiting proliferation and inducing apoptosis. A375M, UACC 903, and 1205 Lu cells were treated with increasing concentrations of SKI-I for 72 hours and cell viability, proliferation and apoptosis rates determined by MTS, BrdU incorporation or caspase-3/7 assays respectively. Data represent averages of at least 3 independent experiments; bars; S.E.M. 3B and 3C. SKI-I induces apoptotic sub G0/G1 cells in melanomas. UACC 903 cells were treated with 1, 2.5 and 5.0 μM SKI-I and vehicle DMSO control for 72 hours. Total floating and adherent cells were collected, and stained with propidium iodide to analyze the distribution of cells in different cell cycle stages using a FACScan analyzer. SKI-I treatment inhibited cell cycle progression by arresting cells in G2/M and S phases. Histograms represent a set of data from 3 independent experiments. 3D. SKI-I arrested cells in G2-M phase and increased the number of S phase population; bars, indicate values from 3 independent experiments ± S.E.M.
SKI-I inhibited melanoma cell cycle progression by inducing a G2/M blockade and increasing the sub-G0-G1 (apoptotic) cell population
Since SKI-I inhibited melanoma cell proliferation and triggered apoptosis, effect of SKI-I inhibition on cell cycle populations was examined. UACC 903 cells were treated with 1, 2.5 and 5.0 μM SKI or control DMSO vehicle for 72 h, stained with propidium iodide and cell cycle analysis undertaken. Unlike siRNA mediated SPHK1 inhibition, SKI-I treatment induced a 6.0 to 48.5 fold increase in the sub-G0/G1 population compared to control cells indicating increased rates of cellular apoptosis (Compare Fig. 1F and Figs. 3B and 3C). Furthermore, compared to DMSO treated cells, the number of cells in the G0/G1 cell population decreased by 2.4-fold (Fig. 3B and 3C), the S phase population decreased by 1.8-fold and the G2/M population increased by 2.5-fold when 2.5 μM of SKI-I was used (Fig. 3D). Compared to cells treated with 1-2.5 μM SKI-I, cells treated with 5 μM SKI-I had significantly more cells in the G0/G1 cell population and decreased the G2/M cell population to below detectable levels (Figs. 3B and 3D).
SKI-I decreased pAKT and CYCLIN D1 levels and induced markers of cellular apoptosis in melanoma cells
To determine the effect of SKI-I treatment on signaling pathways regulating survival, proliferation and apoptosis, WM278 that has high SPHK1 activity and metastatic melanoma cells, A375M, UACC 903 and 1205 Lu that have moderate to low SPHK1 activity, were exposed to increasing concentrations of SKI-I or DMSO vehicle for 48 or 72 h, and cellular lysates analyzed by western blotting (Fig. 4). Results showed a concentration dependent increase in caspase-3/7 activity with corresponding decreases in pAKT and downstream pPRAS40 levels in all cell lines (Fig. 4B and Supplemental Fig. 4). In addition, total AKT, AKT3 and total PRAS40 decreased in SKI-I treated advanced stage melanoma cells (Fig. 4A). A375M cells (IC50- 1.3 ± 0.27 μM) showed maximal caspase-3/7 activity at 2.5 μM SKI-I (Figs. 3A and Supplemental Fig. 4). Increasing SKI-I to 5 μM decreased caspase-3/7 activity due to fewer viable cells (Supplemental Fig. 4). In contrast, UACC 903 (IC50- 5.72 ± 0.35 μM) and 1205 Lu (IC50- 4.89 ± 0.63 μM) cells exhibited maximal caspase-3/7 activity at 5 μM SKI-I (Figs. 3A and Supplemental Fig. 4). Decreased pAKT correlated with elevated levels of pERK1/2 in all metastatic melanoma cells (Fig. 4B). No increase in pERK1/2 was observed when WM278 cells were treated with SKI-I (Fig. 4B, left panel).
Figure 4. SKI-I modulated key cell signaling pathways in melanoma cells. 4A. SKI-I inhibits Akt signaling in melanoma cells.
Vertical growth phase cell line WM278 containing high SPHK1 activity and metastatic melanoma cell lines A375M, UACC 903 and 1205 Lu containing moderately high SPHK1 activity were treated with increasing concentrations of SKI-I for 48 or 72 hours and cell lysates analyzed by Western blotting to measure the expression and activity levels of AKT signaling cascade proteins. Data shows SKI-I treatment induced the degradation of caspase-3 and PARP, inhibited pAKT and pPRAS40 levels in melanoma cells. Expression of total AKT and total PRAS40 levels decreased at higher concentrations of SKI-I. α-Enolase served as a control for equal protein loading. 4B and 4C. SKI-I induced pERK1/2 levels and inhibited cellular growth by decreasing CYCLIN D1 in melanomas. SKI-I treatment increased pERK1/2 levels (4B) and decreased the cyclin-D1 protein expression (4C) in melanomas. No changes in pERK1/2 were observed in vertical growth phase WM278 cell line with SKI-I treatment. A significant increase in p21 protein expression was also observed in UACC 903 and 1205 Lu cells with SKI-I treatment (4C). α-Enolase served as a control for equal protein loading.
Expression levels of cell proliferation marker CYCLIN D1 and cell cycle inhibitor p21 were compared next by Western blotting from cell lysates harvested after treatment with DMSO or SKI-I for 72 h (Fig. 4C). A dose dependent decrease in CYCLIN D1 was observed in all the four melanoma cell lines tested (Fig. 4C). For example, UACC 903 cells treated with 1 and 2.5 μM SKI-I showed 86.1 and 97.1% decrease in CYCLIN D1 expression compared to DMSO treated control cells. At 5 μM, CYCLIN D1 levels were almost undetectable (Fig. 4C). Similarly, a significant decrease in CYCLIN D1 levels was detected in WM278, A375M and 1205 Lu cells (Fig. 4C). However expression of p21 is not consistent among different melanoma cell lines tested. Whereas p21 levels elevated with SKI-I treatment in UACC 903 and 1205 Lu cell lines, a slight decrease was observed in WM278. No change in the expression of p21 was observed in A375M cells (Fig. 4C).
SKI-I inhibited melanoma cell survival and proliferation pathways
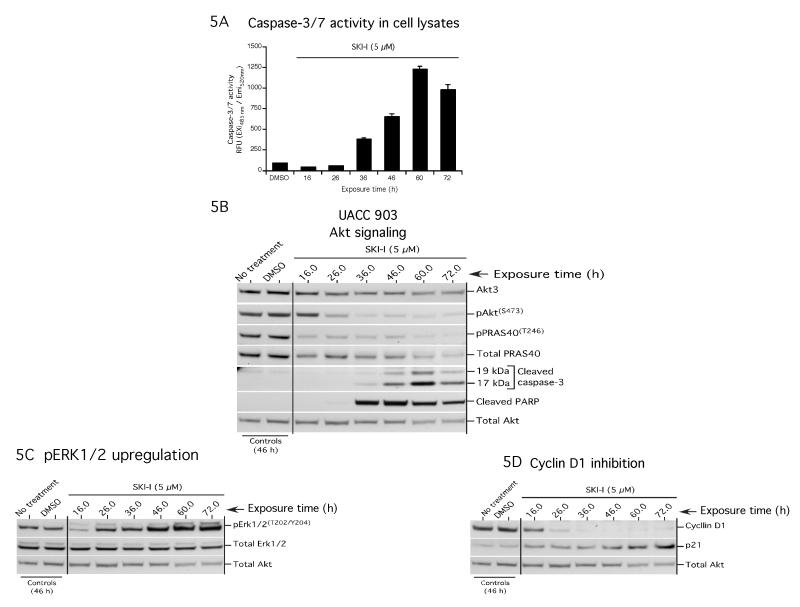
To delineate the early events leading to melanoma cell growth inhibition following SKI-I treatment; UACC 903 cells were treated with 5 μM SKI-I, which reduced cell viability by 50% at 72 hours (Table 1), and effect on apoptosis (caspase-3/7 activity, Fig. 5A), the expression and activity of proteins involved in cell survival (Fig. 5B) and proliferation (Figs. 5C and 5D) determined. Activity and expression of apoptosis markers caspase-3/7 and cleaved PARP increased progressively from 36 to 60 hours (Fig. 5A and 5B). 5 μM SKI-I treatment inhibited pAKT and pPRAS40 levels at 26 hours (Fig. 5B). At 72 h, a slight decrease in expression and activity of these proteins occurred indicating loss of viable cells. Decreased pAKT levels correlated with up-regulated MAP kinase activity as indicated by increased pERK1/2 levels, without altering total ERK1/2 protein expression (Fig. 5C). Furthermore, proliferation marker CYCLIN D1 decreased beginning at 16 h following SKI-I treatment and was not detectable after 36 h of treatment. Conversely, p21 levels progressively increased starting following 16 h of SKI-I treatment and reached maximal levels at 72 h (Fig. 5D).
Figure 5. SKI-I inhibits pAKT and decreases CYCLIN D1 levels.
5A and 5B. SKI-I triggers caspase activity. UACC 903 cells were treated with 5 μM SKI-I for 16, 26, 36, 46, 60 and 72 hours and protein lysates analyzed for caspase-3/7 activity (Fig. 5A) and expression of apoptosis regulator proteins by Western blotting. pAKT(S473) and pPRAS40(T246) levels decreased beginning at 24 hours (Fig. 5B). Cleaved caspase-3 and cleaved PARP increased from 36 hours. 5C and 5D. SKI-I induced MAP kinase activity and inhibited cellular growth by decreasing cyclinD1 and upregulating p21 expression. MAP kinase activity, in terms of pERK1/2, cyclin-D1 and p21expression was measured in UACC 903 cells following treatment with 5 μM SKI-I. Compared to vehicle DMSO control, levels of pERK1/2 increased following SKI-I treatment beginning 26 hours of exposure (5C). A significant decrease in pERK1/2 at 16 hours was noticed. Total AKT served as control for protein loading. CYCLIN D1 expression was inhibited following 16 hours of exposure and at 36 hours levels were not detectable (5D). Expression of p21 was maximal following 72 hours exposure (5D). Total AKT served as loading control.
DISCUSSION
Currently no therapeutic options are available for the long-term treatment of advanced stage melanoma (Hocker et al., 2008). Therefore, new agents targeting aberrant pathways deregulated in melanoma are needed (Inamdar et al., 2010). Recent evidence indicates that targeting members of PI3 and MAP kinase pathway alone may be insufficient to completely inhibit melanoma tumor development or growth (Smalley et al., 2006, Smalley and Herlyn, 2006). Hence, the current thought is that multiple pathways regulating melanoma development will need to be targeted in combination, to more effectively treat this disease.
One possible target is the lipid kinase, SPHK1, which has oncogenic properties in cancers of breast, brain, prostate, colon, lung, ovary and gastrointestinal tract (Ader et al., 2008, Lavieu et al., 2006, Sarkar et al., 2005, Bektas et al., 2005, Vadas et al., 2008, Xia et al., 2000, Guan et al., 2011). For example, elevated expression of SphK1, reported in gastric cancer cell lines and in gastric cancer lesions compared to normal controls, found to be associated with poor patient survival (Li et al., 2009). However, levels of SPHK1 activity as well as the impact of targeting this kinase in melanoma development in animal models had not been studied.
This study shows that activity of SPHK is elevated in radial, vertical and metastatic melanomas compared to normal human melanocyte. Furthermore, targeted inhibition of SPHK1 expression using siRNA not only reduced SPHK activity but also inhibited cell growth and enhanced sensitivity to therapeutic agents indicating that SPHK1 and not SPHK2 accounts for the SPHK activity observed in melanomas, suggesting that it could be a key therapeutic target in this disease. Mechanistically, targeting SPHK1 using siRNA decreased pAkt levels and arrested cells in G0/G1 phase to reduce melanoma cell proliferation. One of the possible explanations for the marginal changes observed in the cell cycle analysis when SphK1 expression was inhibited using siRNA is lack of complete inhibition of this protein. SPHK1 knockdown in breast cancer cells reduced EGF-and serum-stimulated growth by arresting cells in the G0/G1 phase of the cell cycle and thereby increased rates of cellular apoptosis (Sarkar et al., 2005). Furthermore, targeting SPHK1 enhanced sensitivity to doxorubicin by increasing caspase-7 activity and PARP cleavage (Sarkar et al., 2005). Inhibition of SPHK1 also sensitized prostate cancer cells to docataxel and campothecins in vitro and in vivo (Pchejetski et al., 2008). Pharmacological inhibition of SPHK1 along with campothecin treatment reduced prostate tumor growth synergistically in animals (Pchejetski et al., 2008).
SPHK1 promotes multi-drug resistance (MDR); angiogenesis, cell migration and can inhibit cellular apoptosis as well as autophagic cell death (French et al., 2003, Miller et al., 2008, Ader et al., 2008). Growth retardation following SPHK1 knockdown can be mediated by decreasing the S-1-P levels in cells thereby increasing the apoptotic ceramide concentration (Huwiler and Zangemeister-Wittke, 2007, Leclercq and Pitson, 2006), inhibiting AKT activity and triggering caspase-3/7 mediated apoptosis, leading to cell cycle arrest (French et al., 2003, French et al., 2006). A recent study has reported that SPHK1 counteracts ceramide mediated cell death in melanoma cells (Bektas et al., 2005). For example, whereas elevated SPHK1 expression reduced the sensitivity of melanoma cells to Fas- and ceramide-mediated apoptosis, siRNA-mediated down-regulation of SPHK1 decreased the resistance to apoptotic cell death (Bektas et al., 2005). In addition, overexpression of prosurvival BCL-2 markedly stimulated SPHK1 expression and activity, indicating that SPHK1 mediates resistance to chemotherapeutic agents in malignant melanomas (Bektas et al., 2005). SPHK1 has also been shown to regulate the expression of hypoxia inducible factor 1α via the PI3 kinase-AKT-GSK3β pathway in several human cancers including those of the prostate, brain, breast, kidney and lung (Ader et al., 2008). Targeting SPHK1 using siRNA or pharmacological agents inhibited pAKT(S473) to activate GSK3β, which in turn prevented accumulation of HIF-1α and its transcriptional activity (Ader et al., 2008., Guan et al., 2011). Similarly, in this study, we observed that treatment of metastatic melanoma cells with SphK inhibitors reduced pAKT(S473) and CYCLIN D1 levels as well as increased expression of p21 and protein markers of apoptosis cleaved caspase-3 and PARP.
SKI-I and SKI-II are non-lipid pharmacological agents identified from a screen of a Chembridge Corporation chemical library consisting of 16,000 compounds in an assay using recombinant human SphK (French et al., 2003). Both SKI-I and II inhibited purified and endogenous SPHK protein activity. Whereas SKI-I shows selectivity to SPHK1, SKI-II was found to inhibit SPHK2 more effectively than SPHK1 (Hengst et al., 2010b, Antoon et al., 2011). A recent study demonstrated that SKI-II does not inhibit SPHK1 directly, but rather induces lysosomal mediated degradation and it is this effect that led to its anti-tumor activity (Ren et al., 2010). Lack of efficacy of SKI-II for killing melanoma cells further supports this possibility.
In this study, the pre-clinical activity of non-lipid SPHK1 inhibitor SKI-I for inhibiting xenografted melanoma tumor is described. SKI-I but not SKI-II effectively decreased melanoma cells growth in vitro and in vivo. Both inhibitors have been shown to decrease proliferation and induce apoptosis in a panel of cancer cell lines (Leroux et al., 2007, French et al., 2006, French et al., 2003). SKIs have also been shown to be active against cells that express the drug transport proteins P-glycoprotein or MRP1 (French et al., 2003). Consistent with these observations, results in this study showed that SKI-I inhibited cellular proliferation in a dose and time dependent manner by inducing a G2/M block, down-regulating cyclin-D1 and increasing p21 expression levels. Mechanistically, SKI-I induced cell death in melanoma cells by decreasing cellular S-1-P levels while increasing concentrations of ceramides. Prior studies have shown that elevated ceramides can inhibit various survival kinases including AKT, induce apoptosis and decrease cell proliferation rates (Leroux et al., 2007, French et al., 2006, French et al., 2003). SKI-II inhibited SPHK1 and SPHK2 activities in in vitro kinase assays, but did not effect either S-1-P levels or cellular ceramides levels when melanoma cells were treated at 2.5 and 5 μM concentrations, explaining why this compound was ineffective at killing melanoma cells. This could be due to variations in the hydrophobicity of SKI-I and SKI-II. SKI-I has a cLog P of 6.54, which might be more likely to intercalate into cell membranes thereby inhibiting membrane localized SphKs more effectively than SKI-II having a cLog P value of 3.91. This study suggests that SKI-I treatment inhibit melanoma cell proliferation by decreasing cyclin-D1, cellular S-1-P and pAkt levels. It is currently unknown whether SKI-I is inhibiting additional targets in melanoma cells but this is a possibility since siRNA mediated inhibition caused a G0/G1 arrest rather than the G2/M block observed when cells were treated with SKI-I.
SKI-I also induced caspase-3/7 activity and PARP cleavage by inhibiting PI3K-AKT-PRAS40 signaling. Anti-proliferative activity and AKT signaling inhibitory properties of SKIs have been reported in mammary cancer cells (French et al., 2003, French et al., 2006). Targeting sphingosine kinase-1 using an isotype specific SPHK1 inhibitor SKI-I suppressed the growth of LN229 and U373 glioblastoma cells by reducing AKT phosphorylation (Kapitonov et al., 2009). For melanomas, AKT3 expression and activity are elevated in ~70% of tumors and inhibition using siRNA or pharmacological agents, such as ISC-4, induced apoptosis by decreasing cellular pPRAS40 and increasing expression of cleaved caspase-3 (Madhunapantula et al., 2007, Sharma et al., 2008, Stahl et al., 2004, Robertson, 2005). Furthermore, AKT inhibition has been shown to activate GSK3β and ERK1/2 to decrease cellular proliferation and induce cell senescence via CYCLIN D1 down regulation and p21 as well as p27 up-regulation (Cheung et al., 2008, Guo et al., 2008). Additionally, recent studies have documented that treatment of melanoma cells using PBISe or breast cancer cells with plumbagin inhibited AKT activity and led to a G2-M block (Madhunapantula et al., 2008). In conclusion, the results of this study suggest that siRNA mediated SPHK1 down-regulation, inhibits Akt activity, arrests cells in the G0/G1 of the cell cycle to reduce melanoma cell viability and sensitizes cells to apoptosis inducing therapeutic agents.. In addition, this report also demonstrates that sphingosine kinase inhibitor SKI-I decreases AKT activity to reduce cell proliferation, arrests cells in the G2/M phase of the cell cycle and triggers apoptosis in melanoma cells.
In addition, this study demonstrated that exposure of melanoma cells to SKI-I not only triggered apoptosis mediated by caspase-3/7 activation and PARP cleavage, but also induced expression of pERK1/2. Although prior reports showed that SKI-I inhibits the phosphorylation of MEK1/2, in melanomas, this study suggests that SKI-I induces phosphorylation of ERK1/2, which is probably due to elevated cellular ceramide levels. A prior report has demonstrated that C2-ceramide can transiently activate ERK in Malme-M3 melanoma cells (Han et al., 2002).
Intraperitoneal administration of SKI-I but not SKI-II at 100 mg/kg body weight, retarded melanoma tumor development compared to control DMSO vehicle treated animals resulting in a 21-40% decrease in tumor volume. Differences in the efficacy of SKI-I and SKI-II could be due to variations observed in the concentrations required to inhibit melanoma cell growth in vitro. The average IC50 of SKI-I for melanoma cells was 2.4 μM at 72 hours whereas for SKI-II it was >31.4 μM. Furthermore, variations in the therapeutic potential of SKI-I and SKI-II for inhibiting melanoma tumor growth in vivo could be due to differences in the concentration of these agents in the blood circulation as well as in the vicinity of the tumor. Prior studies have shown that the therapeutic efficacy of pharmacological agents for inhibiting tumor growth depends on the circulating concentrations as well as the amount of a drug in the vicinity of a tumor. The circulating concentrations of SKI-I (5.9 ± 3.3 μM) but not SKI-II (3.5 ± 4.9 μM), 1 hour after 50 mg/kg i.p. administration in BALB/c mice was significantly higher than the effective concentrations required to inhibit 50% of UACC 903 cells in vitro. This could provide an explanation for the efficacy of SKI-I for inhibiting xenografted melanoma tumor growth. No weight changes were observed following i.p. treatment with SKI-I or SKI-II, suggesting that the agents could be tolerated in animals with negligible toxicity.
In conclusion this study showed that SKI-I treatment reduced melanoma tumor development. However, further optimization is needed to increase drug potency or combining the agent with other therapeutics to completely inhibit tumor development. The two most important signaling pathways implicated in melanoma development, progression and metastasis are the MAP and PI3K signaling pathways. Targeting SPHK1 in combination with MAP kinase or PI3 kinase signaling pathway inhibitors such as BAY-439006, Vemurafenib, isoselenocyanates (AKT inhibitors) or nano-liposomal siRNA targeting these pathways might more effectively inhibit melanoma tumor development. For example, a recent study demonstrated that co-administration of sphingosine kinase inhibitors ABC294640 or ABC294735 with sorafenib synergistically inhibited human pancreatic adenocarcinoma and kidney carcinoma growth in vitro and in vivo (Beljanski, 2010). Thus, this study shows that SPHK1 activity is elevated in melanomas and inhibiting expression or activity of this protein using siRNA or pharmacological agents can reduce melanoma development.
MATERIALS AND METHODS
Cell lines and culture conditions
The human metastatic melanoma cell lines A375M, UACC 903, 1205 Lu, SK-MEL-24 and normal human fibroblast FF2441 cells were maintained in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (Hyclone, Logan, UT). Normal human epidermal melanocytes (FOM103), radial (WM35) and vertical (WM98.1, WM115, WM278) growth phase melanoma cell lines were cultured as described previously (Stahl et al., 2004).
Quantitation of sphingosine kinase mRNA levels
Relative SPHK1 mRNA levels were measured by extracting total RNA from melanoma cells using TRI reagent (Molecular Research Center, Cincinnati, OH) and treated with DNase (Ambion, Austin, TX) (French et al., 2006, French et al., 2003, Francy et al., 2007). cDNA was synthesized from equal quantities of RNA using the Superscript III Kit and Random Decamer Primers. Following determination of the linear range of DNA amplification, target primers were used to amplify PCR products for SPHK1 and SPHK2. As an internal control, 18S ribosomal RNA primers (QuantumRNA™ Classic 18S Internal Standard, Ambion, Austin, TX) were included in the PCR reaction mix with Promega GoTaq polymerase enzyme. Target and β-actin control products were quantified by normalizing to the 18S ribosomal RNA levels. Primer sequences for targets were as follows: SPHK1 (UniSTS RH47748); forward 5′-CCT TCC TCC TTC CCT AGG G-3′; reverse 5′-TAG AAG GCC TTA CAT AGG CAG C-3′. Primer sequences for SPHK2 were published previously (French et al., 2006, French et al., 2003, Francy et al., 2007).
Measurement of sphingosine kinase activity
Sphingosine kinase activity in cell lysates was measured as described previously (French et al., 2003). In brief, cell lysates were collected by addition of sphingosine kinase lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% v/v Triton X-100, 10 mM β- glycerophosphate, 50 mM sodium fluoride, 5 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 10 μM okadaic acid, and protease inhibitor cocktail for mammalian tissues). Supernatants were separated from cell debris by centrifugation and protein concentration determined using BCA assay (Thermo Fisher Scientific, Rockford, IL). Sphingosine kinase activity in the protein lysates was determined by combining 50 μM D-erythro-sphingosine (Avanti Polar Lipids, Alabaster, AL) and 200 μM ATP containing 2 μCi [γ32P] ATP (ICN Radiochemicals, Irvine, CA) in 100 μl SPHK1 activity assay buffer (50 mM HEPES, 50 mM MgCl2, 10 mM KCl, 10 mM NaF, 2 mM NaVO3, 500 μM 4-deoxypyridoxine, 0.05% Triton X-100, pH 7.4) with 10 μg protein lysate in a final reaction volume of 100 μl. The reaction mixture was agitated for 20 m at 37oC. After terminating the reaction using 6N HCl, labeled lipids were extracted by addition of 250 μl chloroform:methanol:HCl (100:200:1 v/v/v), 125 μl chloroform and 125 μl 1M KCl. Labeled S-1-P partitioned into the organic phase was collected and radioactivity measured in a scintillation counter.
Determination of SKI-I and SKI-II specificity
Specificity of SKI-I has been reported (Hengst et al., 2010b). To determine the specificity of SKI-II against SPHK1 and SPHK2, in vitro SphK activity assays were performed as described previously (Hengst et al., 2010b). Recombinant SPHK1 and SPHK2 proteins (Echelon, Salt Lake City, UT) were preincubated with increasing concentrations of SKI-II (dissolved in DMSO, 1% of total reaction volume) in 90 μL assay buffer for 15 min at room temp. Reactions were initiated by the addition of 10 μL of assay buffer containing 250 μM D-erythro-sphingosine, 10 mM MgCl2, 200 μM ATP and 1 μCi of γ-[32P]ATP. After 30 minutes incubation at 37°C, reactions were terminated by the addition of 10 μL 1 M HCL and S-1-32P was extracted and measured by liquid scintillation counting (Hengst et al., 2010b).
SKI-I and SKI-II treatments and estimation of cellular ceramide and S-1-P contents by LC-MS-MS
Sphingolipid levels in UACC 903 and 1205 Lu cells were measured by plating 2.0 x 106 cells, in triplicate, in a 100 mm culture dishes in DMEM containing 10% FBS. After 24 hours, cells were washed with PBS, and treated with vehicle DMSO control, 2.5 and 5.0 μM SKI-I or SKI-II in DMEM containing 10% FBS. Forty eight hours later, floating cells were collected by centrifugation at 500 x g for 5 minutes at 4°C, washed 3 times with ice-cold PBS. Adherent cells were also washed in ice-cold PBS, collected by scrapping and combined with floating cells. Combined cell fractions were extracted, and D-erythro-sphingosine, S-1-P, and various ceramides contents determined using LC-MS-MS as described previously (Wijesinghe et al., 2010).
SiRNA-mediated knockdown and determination of anchorage dependent as well as independent growth
Expression of SPHK1 was inhibited using siRNA (5′ GAG CUG CAA GGC CUU GCC CUU GCC G 3′) in melanoma cell lines UACC 903 and 1205 Lu as described previously (Sharma et al., 2005, Sharma et al., 2006). Specificity of siRNA for inhibiting SPHK1 but not SPHK2 was showed by a prior study (Bektas et al., 2005). In brief, 2X106 cells were nucleofected with 200 picomoles of siRNA using Amaxa nucleofector-1, K-17 program and Kit-R (Cheung et al., 2008). After 24 hours, 2X104 cells/well in 100 μl serum free media were trypsinized and plated into 96-well plastic plates or plates coated with polyHEMA (Cheung et al., 2008, Madhunapantula et al., 2007). Anchorage independent growth was measured after 2 days using a MTS (Promega, Madison, WI) assay. Results were represented as a percentage decrease in cell viability compared to scrambled siRNA transfected cells. Additionally, transfected cells were also plated in 60 mm culture dishes to measure SPHK1 mRNA expression and activity in whole cell lysates collected using RP1 buffer (USB Corporation, Cleveland, OH).
Staurosporine treatment of sphingosine kinase-1 siRNA transfected cells
1X106 UACC 903 cells transfected with 200 pmoles siRNA targeting SPHK1 were plated in a 60 mm culture plate and 2 days later, treated with vehicle control DMSO or 0.6 μM staurosporine (BioMol International, Plymouth meeting, PA) for 4 hours. Total adherent and floating cells were collected and protein lysates analyzed for the expression and activity of caspase-3 by Western blotting and Apo-ONE homogenous caspase-3/7 activity assay kit (Promega, Madison, WI) respectively (Madhunapantula et al., 2007). Cells transfected with buffer or a scrambled siRNA served as controls.
Western blot analysis
Cell lysates were harvested by the addition of lyses buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 10 mM EDTA, 10% glycerol, 1% Triton X-100, 1 mM sodium orthovanadate, 0.1 mM sodium molybdate, 1 mM phenylmethylsulfonyl fluoride, 20 μg/ml aprotinin, and 5 μg/ml leupeptin. Whole cell lysates were centrifuged (≥10,000 X g) for 10 minutes at 4 °C to remove cell debris. Protein concentrations were quantitated using the BCA assay from Pierce (Thermo Fisher Scientific, Rockford, IL), and 30 μg of lysate loaded per lane onto NuPAGE Gels (Life Technologies, Carlsbad, CA). Following electrophoresis, samples were transferred to polyvinylidene difluoride (PVDF) membrane (Pall Corporation, Pensacola, FL). Blots were probed with antibodies to total PRAS40 and phosphorylated PRAS40 (Thr246) from Invitrogen (Carlsbad, CA); antibodies to CYCLIN D1, p27, p21, α-enolase and secondary antibodies conjugated with horseradish peroxidase from Santa Cruz Biotechnology (Santa Cruz Biotechnology, Santa Cruz, CA); and antibodies to AKT3, phosphorylated-AKT (Ser473), total AKT, phosphorylated-Erk 1/2 (Thr202/Tyr204), total ERK1/2, caspase-3 and cleaved PARP from Cell Signaling Technology (Danvers, MA). Immunoblots were developed using the enhanced chemiluminescence (ECL) detection system (Thermo Fisher Scientific, Rockford, IL). Intensity of protein bands was quantitated using Image-J software and results represented as percent change compared to control DMSO treated cells.
Cell viability, proliferation, apoptosis and cell cycle analysis
Viability and IC50 of melanoma cells following treatment with SKI-I or SKI-II were measured using the MTS assay (Madhunapantula et al., 2008). 5 X 103 cells per well in 100 μL DMEM containing 10% FBS were grown in a 96-well plate for 36 or 72 hours, respectively for melanoma (WM35, WM115, WM278, A375M, UACC 903, and 1205 Lu) andhuman fibroblast (FF2441) and FOM melanocyte cell lines and treated with either DMSO vehicle or increasing concentrations (0.5-100 μM) of SKI-I or SKI-II for 24, 48 and 72 hours. Cellular viability compared to control treated cells was measured using the MTS assay. IC50 values of each compound for respective cell lines were determined from three independent experiments using GraphPad Prism version 4.01 (GraphPad Software, La Jolla, CA).
Cellular proliferation and apoptosis rates were measured by seeding 5X103 cells in 96-well plates, followed by treatment for 72 hours with each respective agent. In brief, 4 hours prior to ending of treatment, cells were either labeled with BrdU, for determining proliferation rates, or incubated with caspase-3/7 substrate Z-DEVD-R110 for measuring apoptosis rates. Numbers of proliferating and apoptotic cells were quantitated using a cell proliferation ELISA BrdU kit (Roche, Indianapolis, IN) or Apo-ONE Homogenous caspase-3/7 assay kit (Promega, Madison, WI), respectively. Percentage decrease in BrdU incorporation compared to control treated cells was calculated from 3 independent experiments and average values represented with SEM. Similarly, fold increase in caspase-3/7 activity compared to DMSO treated cells was represented as bar graph (Madhunapantula et al., 2008).
Cell cycle analysis was undertaken by (a) nucleofecting 50, 100, 150 and 200 picomoles SPHK1 siRNA in to 1 X 106 UACC 903 cells, followed by 48 h recovery in 100 mm Petri plates, and (b) growing 1.5X106 UACC 903 cells in 100-mm culture dishes followed by a treatment with SKI-I for 72 hours (Madhunapantula et al., 2008). Total floating and adherent cells were collected by trypsinization and stained using a propidium iodide staining solution (1 ml, 100 μg/ml; Sigma, St. Louis, MO), 20 μg/ml Ribonuclease A (Roche, Indianapolis, IN) 3 μg/mL Triton X-100 dissolved in 0.1% (W/V) sodium citrate) for 30 minutes at 4 °C. Stained cells were analyzed using the FACScan analyzer (Becton Dickinson, CA) and data processed utilizing ModFit LT software (Verity Software House, Topsham, ME).
Tumorigenicity assessments
Animal experimentation was performed according to protocols approved by the Institutional Animal Care and Use Committee at Penn State University (Madhunapantula et al., 2008, Sharma et al., 2008). Tumor kinetics were measured by subcutaneous injection of 5X106 UACC 903 or 2.5X106 1205 Lu cells in 0.2 ml of DMEM supplemented with 10% FBS above both left and right rib cages of 4-6 week old female nude mice (Harlan Sprague Dawley, Indianapolis, IN). Six days later, mice were randomly placed in control DMSO and experimental SKI-I or SKI-II groups (n=5 animals; 10 tumors total) followed by i.p. treatment (100 mg/kg body weight in 50 μl DMSO) on each Monday, Wednesday and Friday for 3 weeks. Dimensions (length, width and depth) of developing tumors and body weight measured using calipers or by weighing animals. A portion of vital organs (liver, heart, kidney, intestine pancreas and adrenal) from each animal was collected at the end of treatment, formalin fixed, paraffin-embedded and H&E stained to determine whether morphological changes in cell or organ morphology associated with toxicity had occurred.
Statistical analysis
Statistical analysis was undertaken using the One-way / Two-way ANOVA followed by the Tukey’s post hoc or by Student’s t-test with two-tailed paired comparison using GraphPad PRISM Version 4.01 software. Results were considered significant at a p-value of <0.05.
Supplementary Material
Supplemental figure 1: SKI-I inhibits vertical growth phase melanoma cells viability. 5 X 103 WM278.1 cells containing high SPHK1 activity were exposed to increasing concentrations of SKI-I for 24, 48, and 72 h and cell viability measured using MTS. Percentage decrease in the viability compared to control DMSO vehicle treated cells was determined and the data represented in bar diagram. A dose and time dependent decrease in WM278.1 cell viability with increasing SKI-I concentration was observed.
Supplemental figure 2: SKI-I but not SKI-II reduces cellular S-1-P and induced pro-apoptotic ceramide content in 1205 Lu melanoma cells. 2 X 106 1205 Lu cells were plated in 100 mm culture dish and 24 hours later treated with SKI-I and SKI-II for 48hours. Levels of D-erythro-sphingosine, sphingosine-1-phosphate, and ceramide were analyzed by LC/MS/MS (Wijesinghe et al., 2010). Although no significant change in sphingosine-1-phosphate and C16:0 ceramide levels was observed, C16:1 and longer chain ceramide levels were upregulated when 1205 Lu cells were exposed to SKI-I but not SKI-II.
Supplemental figure 3: Intra peritoneal administration of SKI-I but not SKI-II retarded melanoma tumor growth. 5 X 106 1205 Lu cells were injected subcutaneously into left and right flanks near rib cages and six days later, mice were injected i. p. with 100 mg/kg body weight SKI-I on Monday, Wednesday and Friday for 3 weeks. Compared to DMSO vehicle treated mice, SKI-I administered mice significantly decreased tumor development beginning from day 14 (Inset, p < 0.05; Student’s t-test). At day 21, an ~26 % difference in tumor volume between vehicle control and SKI-I treatment group was observed.
Supplemental figure 4: SKI-I elevates caspase-3/7 activity in melanoma cells. A375M, UACC 903 and 1205 Lu cells were treated with 1, 2.5, and 5.0 μM SKI-I for 72 hours and cell lysates analyzed for caspase-3/7 activity using Apo-ONE homogenous caspase-3/7 activity assay kit. An increase in the caspase-3/7 activity was observed with increasing SKI-I concentration.
SIGNIFICANCE.
Demonstration of the therapeutic efficacy of targeting SPHK1 in malignant melanoma using siRNA or non-lipid SPHK1 specific inhibitor SKI-I is the most significant aspect of this study. Since elevated SPHK1 activity can occur in melanomas, identification of pharmacological agent SKI-I that is effective for inhibiting activity of this protein in melanomas is a novel discovery. SKI-I inhibited SPHK1 activity in melanoma cells to decrease S-1-P production, which induced cellular ceramide as well as increased cleaved caspase-3 and PARP levels promoting an increase in cellular apoptosis.
ACKNOWLEDGEMENTS
We thank Arati Sharma and Elizabeth Conroy for technical assistance.
Grant support: NIH CA-127892-01A, The Foreman Foundation for Melanoma Research, Melanoma Research Foundation (SubbaRao V. Madhunapantula).
REFERENCES
- Ader I, Brizuela L, Bouquerel P, Malavaud B, Cuvillier O. Sphingosine kinase 1: a new modulator of hypoxia inducible factor 1alpha during hypoxia in human cancer cells. Cancer Res. 2008;68:8635–8642. doi: 10.1158/0008-5472.CAN-08-0917. [DOI] [PubMed] [Google Scholar]
- Antoon JW, Meacham WD, Bratton MR, Slaughter EM, Rhodes LV, Ashe HB, Wiese TE, Burow ME, Beckman BS. Pharmacological Inhibition of Sphingosine Kinase Isoforms Alters Estrogen Receptor Signaling in Human Breast Cancer. J Mol Endocrinol. 2011;28:205–216. doi: 10.1530/JME-10-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin AE, Kaplan FM, Shao Y. Mechanism of resistance to RAF inhibitors in melanoma. J. Invest. Dermatol. 2011;131:1817–1820. doi: 10.1038/jid.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bektas M, Jolly PS, Muller C, Eberle J, Spiegel S, Geilen CC. Sphingosine kinase activity counteracts ceramide-mediated cell death in human melanoma cells: role of Bcl-2 expression. Oncogene. 2005;24:178–187. doi: 10.1038/sj.onc.1208019. [DOI] [PubMed] [Google Scholar]
- Beljanski v., Knaak C, Zhuang Y, Smith CD. Combined anticancer effects of sphingosine kinase inhibitors and sorafenib. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9452-0. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M, Sharma A, Madhunapantula SV, Robertson GP. Akt3 and mutant V600E B-Raf cooperate to promote early melanoma development. Cancer Res. 2008;68:3429–3439. doi: 10.1158/0008-5472.CAN-07-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsall LC, Cuvillier O, Twitty S, Spiegel S, Milstien S. Sphingosine kinase expression regulates apoptosis and caspase activation in PC12 cells. J Neurochem. 2001;76:1573–1584. doi: 10.1046/j.1471-4159.2001.00164.x. [DOI] [PubMed] [Google Scholar]
- Ernst DS, Eisenhauer E, Walnman N, Davis M, Lohmann R, et al. Phase II study of perifosine in previously untreated patients with metastatic melanoma. Invest. New Drugs. 2005;23:569–575. doi: 10.1007/s10637-005-1157-4. [DOI] [PubMed] [Google Scholar]
- Flaherty KT, Puzanov K, Kim KB, Ribas A, McArthur GA, Sosman JS, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francy JM, Nag A, Conroy EJ, Hengst JA, Yun JK. Sphingosine kinase 1 expression is regulated by signaling through PI3K, AKT2, and mTOR in human coronary artery smooth muscle cells. Biochim Biophys Acta. 2007;1769:253–265. doi: 10.1016/j.bbaexp.2007.03.005. [DOI] [PubMed] [Google Scholar]
- French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, Yun JK, Smith CD. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–5969. [PubMed] [Google Scholar]
- French KJ, Upson JJ, Keller SN, Zhuang Y, Yun JK, Smith CD. Antitumor activity of sphingosine kinase inhibitors. J Pharmacol Exp Ther. 2006;318:596–603. doi: 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]
- Guan H, Song L, Cai J, Huang Y, Wu J, Yuan J, Li J, Li M. Sphingosine kinase 1 regulates the Akt/FOXO3a/Bim pathway and contributes to apoptosis resistance in glioma cells. PLoS One. 2011;6:e19946. doi: 10.1371/journal.pone.0019946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Ye J, Dai J, Li L, Chen F, Ma D, Ji C. Notch-1 regulates Akt signaling pathway and the expression of cell cycle regulatory proteins cyclin D1, CDK2 and p21 in T-ALL cell lines. Leuk Res. 2008;33:678–685. doi: 10.1016/j.leukres.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Han WS, Yoo JY, Youn SW, Kim DS, Park KC, Kim SY, Kim KH. Effects of C2-ceramide on the Malme-3M melanoma cell line. J Dermatol Sci. 2002;30:10–19. doi: 10.1016/s0923-1811(02)00044-0. [DOI] [PubMed] [Google Scholar]
- Hengst JA, Guilford JM, Conroy EJ, Wang X, Yun JK. Enhancement of sphingosine kinase 1 catalytic activity by deletion of 21 amino acids from the COOH-terminus. Arch Biochem Biophys. 2010a;494:23–31. doi: 10.1016/j.abb.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengst JA, Wang X, Sk UH, Sharma AK, Amin S, Yun JK. Development of a sphingosine kinase 1 specific small-molecule inhibitor. Bioorg Med Chem Lett. 2010b;20:7498–7502. doi: 10.1016/j.bmcl.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Jacobetz MA, Robertson GP, Herlyn M, Tuveson DA. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Res. 2003;63:5198–5202. [PubMed] [Google Scholar]
- Hocker TL, Singh MK, Tsao H. Melanoma genetics and therapeutic approaches in the 21st century: moving from the benchside to the bedside. J Invest Dermatol. 2008;128:2575–2595. doi: 10.1038/jid.2008.226. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Zangemeister-Wittke U. Targeting the conversion of ceramide to sphingosine 1-phosphate as a novel strategy for cancer therapy. Crit Rev Oncol Hematol. 2007;63:150–159. doi: 10.1016/j.critrevonc.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Inamdar GS, Madhunapantula SV, Robertson GP. Targeting the MAPK pathway in melanoma: why some approaches succeed and other fail. Biochem Pharmacol. 2010;80:624–637. doi: 10.1016/j.bcp.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitonov D, Allegood JC, Mitchell C, Hait NC, Almenara JA, Adams JK, Zipkin RE, Dent P, Kordula T, Milstien S, et al. Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Res. 2009;69:6915–6923. doi: 10.1158/0008-5472.CAN-09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavieu G, Scarlatti F, Sala G, Carpentier S, Levade T, Ghidoni R, Botti J, Codogno P. Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J Biol Chem. 2006;281:8518–8527. doi: 10.1074/jbc.M506182200. [DOI] [PubMed] [Google Scholar]
- Leclercq TM, Pitson SM. Cellular signalling by sphingosine kinase and sphingosine 1-phosphate. IUBMB Life. 2006;58:467–472. doi: 10.1080/15216540600871126. [DOI] [PubMed] [Google Scholar]
- Lee JT, Li L, Brafford PA, van den Eijnden M, Halloran MB, Sproesser K, Haass NK, Smalley KS, Tsai J, Bollag G, Herlyn M. PLX4032, a potent inhibitor of the B-Raf V600E oncogene, selectively inhibits V600E-positive melanomas. Pigment Cell Melanoma Res. 2010;23:820–827. doi: 10.1111/j.1755-148X.2010.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux ME, Auzenne E, Evans R, Hail N, Jr., Spohn W, Ghosh SC, Farquhar D, McDonnell T, Klostergaard J. Sphingolipids and the sphingosine kinase inhibitor, SKI II, induce BCL-2-independent apoptosis in human prostatic adenocarcinoma cells. Prostate. 2007;67:1699–1717. doi: 10.1002/pros.20645. [DOI] [PubMed] [Google Scholar]
- Li W, Yu CP, Xia JT, Zhang L, Weng GX, Zheng HQ, Kong QL, Hu LJ, Zeng MS, Zeng YX, Li M, Li J, Song LB. Sphingosine kinase 1 is associated with gastric cancer progression and poor survival of patients. Clin. Cancer Res. 2009;15:1393–1399. doi: 10.1158/1078-0432.CCR-08-1158. [DOI] [PubMed] [Google Scholar]
- Madhunapantula SV, Desai D, Sharma A, Huh SJ, Amin S, Robertson GP. PBISe, a novel selenium-containing drug for the treatment of malignant melanoma. Mol Cancer Ther. 2008;7:1297–1308. doi: 10.1158/1535-7163.MCT-07-2267. [DOI] [PubMed] [Google Scholar]
- Madhunapantula SV, Robertson GP. Is B-Raf a good therapeutic target for melanoma and other malignancies? Cancer Res. 2008;68:5–8. doi: 10.1158/0008-5472.CAN-07-2038. [DOI] [PubMed] [Google Scholar]
- Madhunapantula SV, Robertson GP. The PTEN-AKT3 signaling cascade as a therapeutic target in melanoma. Pigment Cell Melanoma Res. 2009;22:400–419. doi: 10.1111/j.1755-148X.2009.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhunapantula SV, Sharma A, Robertson GP. PRAS40 deregulates apoptosis in malignant melanoma. Cancer Res. 2007;67:3626–3636. doi: 10.1158/0008-5472.CAN-06-4234. [DOI] [PubMed] [Google Scholar]
- Melendez AJ, Carlos-Dias E, Gosink M, Allen JM, Takacs L. Human sphingosine kinase: molecular cloning, functional characterization and tissue distribution. Gene. 2000;251:19–26. doi: 10.1016/s0378-1119(00)00205-5. [DOI] [PubMed] [Google Scholar]
- Miller AV, Alvarez SE, Spiegel S, Lebman DA. Sphingosine kinases and sphingosine-1-phosphate are critical for transforming growth factor beta-induced extracellular signal-regulated kinase 1 and 2 activation and promotion of migration and invasion of esophageal cancer cells. Mol Cell Biol. 2008;28:4142–4151. doi: 10.1128/MCB.01465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava VE, Hobson JP, Murthy S, Milstien S, Spiegel S. Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Exp Cell Res. 2002;281:115–127. doi: 10.1006/excr.2002.5658. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Nakamura M, Osawa Y, Kono S, Itoh Y, Okano Y, Murate T, Hara A, Ueda H, Nozawa Y, et al. Sphingosine kinase isoforms regulate oxaliplatin sensitivity of human colon cancer cells through ceramide accumulation and Akt activation. J Biol Chem. 2009;284:10422–10432. doi: 10.1074/jbc.M900735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira C, Kim KH, Sung H, Paraiso KH, Dannenberg JH, Bosenberg M, Chin L, Kim M. Cooperative interactions of PTEN deficiency and RAS activation in melanoma metastasis. Oncogene. 2010;29:6222–6232. doi: 10.1038/onc.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, Spiegel S. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–558. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pchejetski D, Doumerc N, Golzio M, Naymark M, Teissie J, Kohama T, Waxman J, Malavaud B, Cuvillier O. Chemosensitizing effects of sphingosine kinase-1 inhibition in prostate cancer cell and animal models. Mol Cancer Ther. 2008;7:1836–1845. doi: 10.1158/1535-7163.MCT-07-2322. [DOI] [PubMed] [Google Scholar]
- Pitson SM. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem Sci. 2011;36:97–107. doi: 10.1016/j.tibs.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Pitson SM, Xia P, Leclercq TM, Moretti PA, Zebol JR, Lynn HE, Wattenberg BW, Vadas MA. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S, Xin C, Pfeilschifter J, Huwiler A. A novel mode of action of the putative sphingosine kinase inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl) thiazole (SKI II): induction of lysosomal sphingosine kinase 1 degradation. Cell Physiol Biochem. 2010;26:97–104. doi: 10.1159/000315110. [DOI] [PubMed] [Google Scholar]
- Robertson GP. Functional and therapeutic significance of Akt deregulation in malignant melanoma. Cancer Metastasis Rev. 2005;24:273–285. doi: 10.1007/s10555-005-1577-9. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Maceyka M, Hait NC, Paugh SW, Sankala H, Milstien S, Spiegel S. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett. 2005;579:5313–5317. doi: 10.1016/j.febslet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Schadendorf D, Flchtner I, Makki A, Alljagic S, Kupper M, Mrowletz U, Henz BM. Metastatic potential of human melanoma cells in nude mice-characterization of phenotype, cytokine-secretion and tumor-associated antigens. Br. J. Cancer. 1996;74:194–199. doi: 10.1038/bjc.1996.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer SE, Weigert A, Zhou J, Brune B. Hypoxia enhances sphingosine kinase 2 activity and provokes sphingosine-1-phosphate-mediated chemoresistance in A549 lung cancer cells. Mol Cancer Res. 2009;7:393–401. doi: 10.1158/1541-7786.MCR-08-0156. [DOI] [PubMed] [Google Scholar]
- Sharma A, Tran MA, Liang S, Sharma AK, Amin S, Smith CD, Dong C, Robertson GP. Targeting mitogen-activated protein kinase/extracellular signal-regulated kinase kinase in the mutant (V600E) B-Raf signaling cascade effectively inhibits melanoma lung metastases. Cancer Res. 2006;66:8200–8209. doi: 10.1158/0008-5472.CAN-06-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65:2412–2421. doi: 10.1158/0008-5472.CAN-04-2423. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Sharma A, Desai D, Madhunapantula SV, Huh SJ, Robertson GP, Amin S. Synthesis and anticancer activity comparison of phenylalkyl isoselenocyanates with corresponding naturally occurring and synthetic isothiocyanates. J Med Chem. 2008;51:7820–7826. doi: 10.1021/jm800993r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida D, Takabe K, Kapitonov D, Milstien S, Spiegel S. Targeting SphK1 as a new strategy against cancer. Curr Drug Targets. 2008;9:662–673. doi: 10.2174/138945008785132402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Choi GS, Kang WH, Myung KB. Sphingosine 1-phosphate triggers apoptotic signal for B16 melanoma cells via ERK and caspase activation. J Korean Med Sci. 2007;22:298–304. doi: 10.3346/jkms.2007.22.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SS, Wall BA, Goydos JS, Chen S. Akt2 is a downstream target of metabotropic glutamate receptor 1 (Grm1) Pigment Cell Melanoma Res. 2010;23:103–111. doi: 10.1111/j.1755-148X.2009.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Lin J, Hocker TL, Tsao H. Genetics of melanoma tumorigenesis. Br J Dermatol. 2008;158:15–21. doi: 10.1111/j.1365-2133.2007.08316.x. [DOI] [PubMed] [Google Scholar]
- Sinnberg T, Lasithiotakis K, Niessner H, Schittek B, Flaherty KT, Kulms D, Maczey E, Campos M, Gogel J, Garbe C, et al. Inhibition of PI3K-AKT-mTOR Signaling Sensitizes Melanoma Cells to Cisplatin and Temozolomide. J Invest Dermatol. 2009;129:1500–1515. doi: 10.1038/jid.2008.379. [DOI] [PubMed] [Google Scholar]
- Smalley KS, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–1144. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- Smalley KS, Herlyn M. Towards the targeted therapy of melanoma. Mini Rev Med Chem. 2006;6:387–393. doi: 10.2174/138955706776361402. [DOI] [PubMed] [Google Scholar]
- Solit DB, Rosen N. Resistance to BRAF inhibition in melanomas. N. Engl. J. Med. 2011;364:772–774. doi: 10.1056/NEJMcibr1013704. [DOI] [PubMed] [Google Scholar]
- Song L, Xiong H, Li M, Liao WT, Wang L, Wu J. Sphingosine kinase-1 Enhances Resistance to Apoptosis through Activation of PI3K/Akt/NF-{kappa}B Pathway in Human Non-small Cell Lung Cancer. Clin Cancer Res. 2011;17:1839–1849. doi: 10.1158/1078-0432.CCR-10-0720. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Stahl JM, Sharma A, Cheung M, Zimmerman M, Cheng JQ, Bosenberg MW, Kester M, Sandirasegarane L, Robertson GP. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–7010. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- Tran MA, Gowda R, Sharma A, Park EJ, Adair J, Kester M, Smith NB, Robertson GP. Targeting V600EB-Raf and Akt3 using nanoliposomal-small interfering RNA inhibits cutaneous melanocytic lesion development. Cancer Res. 2008a;68:7638–7649. doi: 10.1158/0008-5472.CAN-07-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran MA, Smith CD, Kester M, Robertson GP. Combining nanoliposomal ceramide with sorafenib synergistically inhibits melanoma and breast cancer cell survival to decrease tumor development. Clin Cancer Res. 2008b;14:3571–3581. doi: 10.1158/1078-0432.CCR-07-4881. [DOI] [PubMed] [Google Scholar]
- Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, Bremer R, Gillette S, Kong J, Haass NK, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuveson DA, Weber BL, Herlyn M. BRAF as a potential therapeutic target in melanoma and other malignancies. Cancer Cell. 2003;4:95–98. doi: 10.1016/s1535-6108(03)00189-2. [DOI] [PubMed] [Google Scholar]
- Vadas M, Xia P, McCaughan G, Gamble J. The role of sphingosine kinase 1 in cancer: oncogene or non-oncogene addiction? Biochim Biophys Acta. 2008;1781:442–447. doi: 10.1016/j.bbalip.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Wijesinghe DS, Allegood JC, Gentile LB, Fox TE, Kester M, Chalfant CE. Use of high performance liquid chromatography-electrospray ionization-tandem mass spectrometry for the analysis of ceramide-1-phosphate levels. J Lipid Res. 2010;51:641–651. doi: 10.1194/jlr.D000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Gamble JR, Wang L, Pitson SM, Moretti PA, Wattenberg BW, D’Andrea RJ, Vadas MA. An oncogenic role of sphingosine kinase. Curr Biol. 2000;10:1527–1530. doi: 10.1016/s0960-9822(00)00834-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1: SKI-I inhibits vertical growth phase melanoma cells viability. 5 X 103 WM278.1 cells containing high SPHK1 activity were exposed to increasing concentrations of SKI-I for 24, 48, and 72 h and cell viability measured using MTS. Percentage decrease in the viability compared to control DMSO vehicle treated cells was determined and the data represented in bar diagram. A dose and time dependent decrease in WM278.1 cell viability with increasing SKI-I concentration was observed.
Supplemental figure 2: SKI-I but not SKI-II reduces cellular S-1-P and induced pro-apoptotic ceramide content in 1205 Lu melanoma cells. 2 X 106 1205 Lu cells were plated in 100 mm culture dish and 24 hours later treated with SKI-I and SKI-II for 48hours. Levels of D-erythro-sphingosine, sphingosine-1-phosphate, and ceramide were analyzed by LC/MS/MS (Wijesinghe et al., 2010). Although no significant change in sphingosine-1-phosphate and C16:0 ceramide levels was observed, C16:1 and longer chain ceramide levels were upregulated when 1205 Lu cells were exposed to SKI-I but not SKI-II.
Supplemental figure 3: Intra peritoneal administration of SKI-I but not SKI-II retarded melanoma tumor growth. 5 X 106 1205 Lu cells were injected subcutaneously into left and right flanks near rib cages and six days later, mice were injected i. p. with 100 mg/kg body weight SKI-I on Monday, Wednesday and Friday for 3 weeks. Compared to DMSO vehicle treated mice, SKI-I administered mice significantly decreased tumor development beginning from day 14 (Inset, p < 0.05; Student’s t-test). At day 21, an ~26 % difference in tumor volume between vehicle control and SKI-I treatment group was observed.
Supplemental figure 4: SKI-I elevates caspase-3/7 activity in melanoma cells. A375M, UACC 903 and 1205 Lu cells were treated with 1, 2.5, and 5.0 μM SKI-I for 72 hours and cell lysates analyzed for caspase-3/7 activity using Apo-ONE homogenous caspase-3/7 activity assay kit. An increase in the caspase-3/7 activity was observed with increasing SKI-I concentration.