Abstract
Oligonucleoside phosphorodithioates 1 are modified DNA sequences with potential use as antisense oligonucleotides. The preparation of up to 20-mers containing all four bases by solid phase synthesis is described, with details on the preparation of the four monomer units (protected nucleoside thiophosphoramidites 2), the conditions used for the assembly of the strands with up to 19 phosphorodithioate linkages, and the purification and characterisation of the products. Full-length homogeneity of HPLC-purified all-phosphorodithioate products is demonstrated by PAGE, but 31P NMR discloses the presence of phosphorothioate impurities (typically 8-9%), the origin of which is discussed. Oligonucleoside phosphorodithioates are freely soluble in water at neutral or basic pH, and are very stable towards oxidation, hydrolysis, and nuclease cleavage. Their ability to hybridize to complementary DNA has been studied by UV melting point (Tm) measurements. The observed depression of Tm, 0.5-2 degrees C per phosphorodithioate linkage, is higher that the 0.4-0.6 degrees C found for phosphorothioates.
Full text
PDF
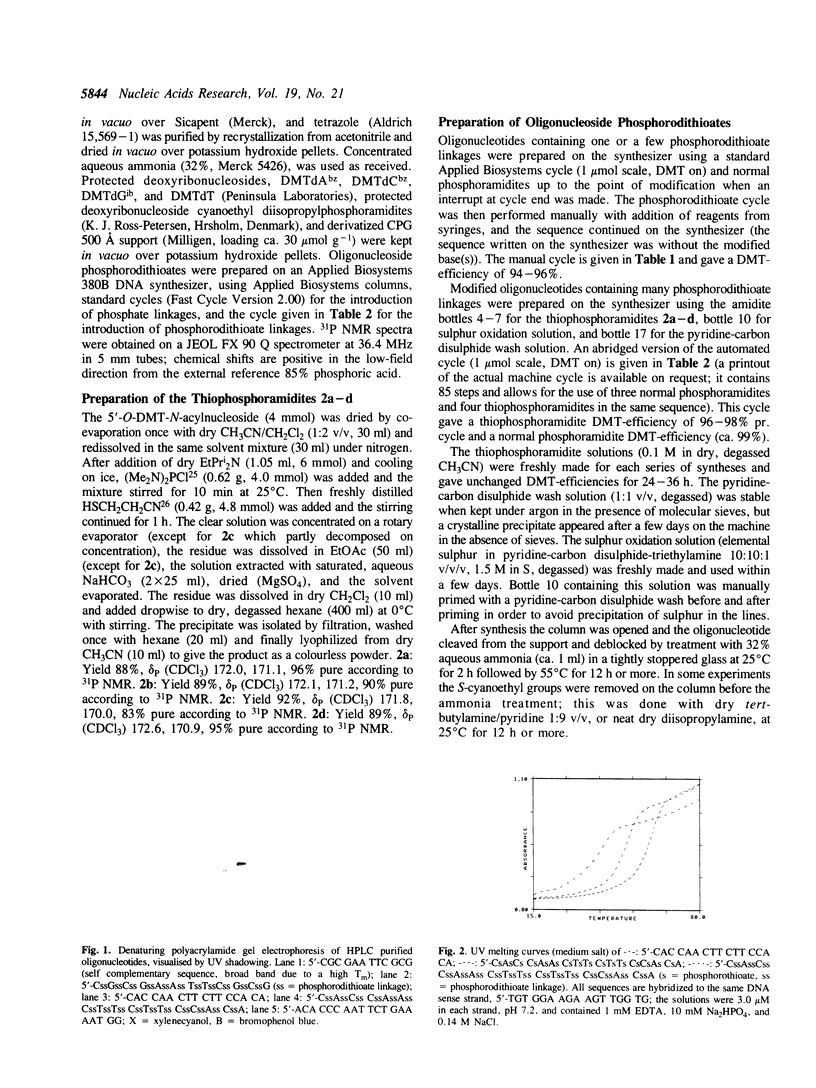
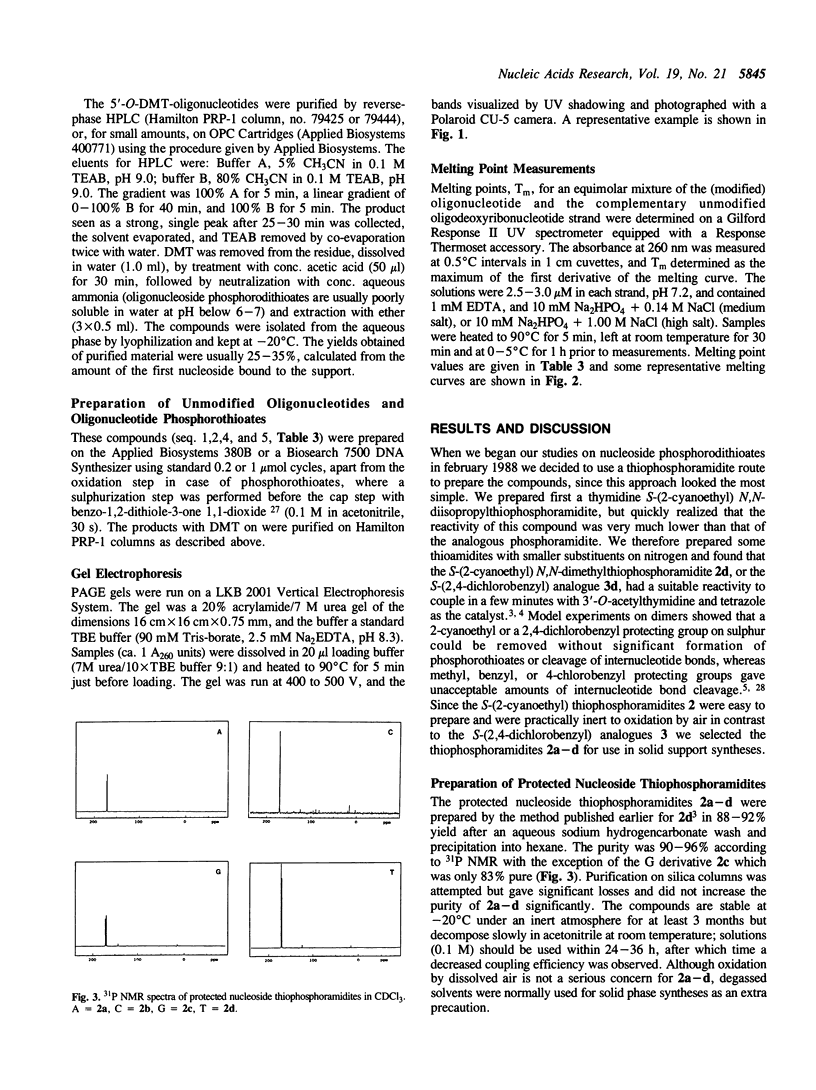
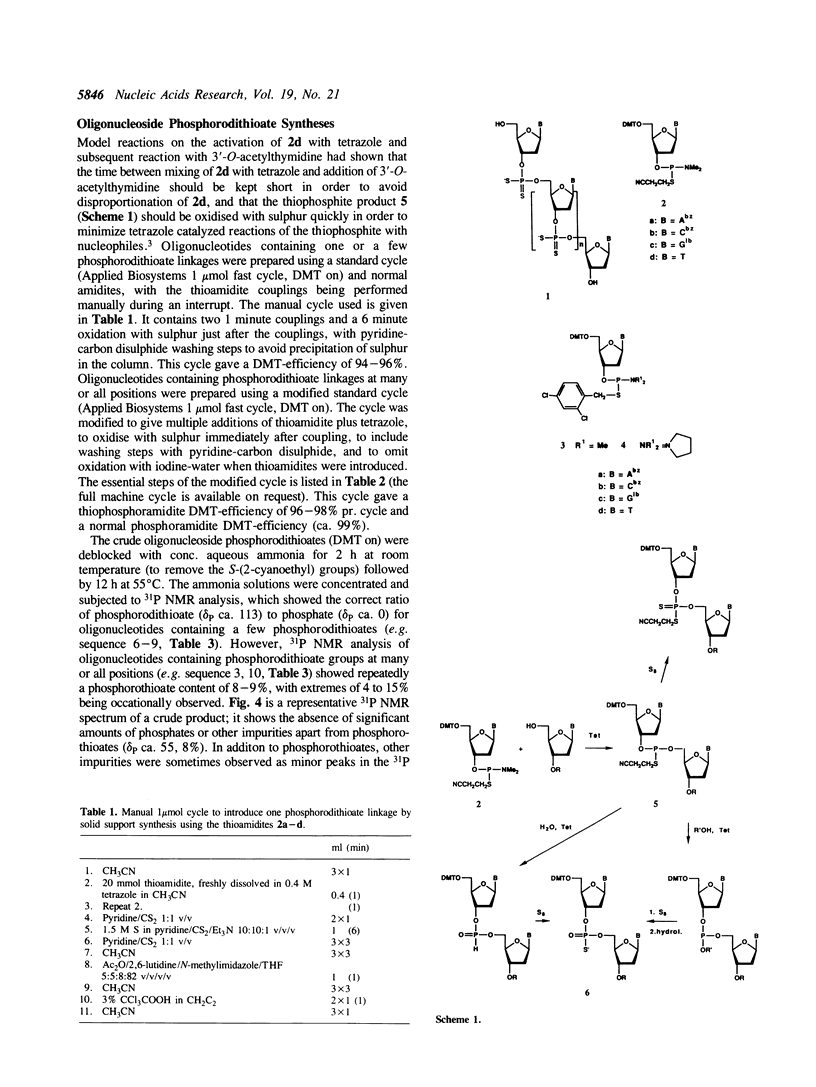


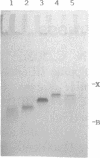
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alul R. H., Singman C. N., Zhang G. R., Letsinger R. L. Oxalyl-CPG: a labile support for synthesis of sensitive oligonucleotide derivatives. Nucleic Acids Res. 1991 Apr 11;19(7):1527–1532. doi: 10.1093/nar/19.7.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruthers M. H., Beaton G., Cummins L., Graff D., Ma Y. X., Marshall W. S., Sasmor H., Norris P., Yau E. K. Synthesis and biochemical studies of dithioate DNA. Ciba Found Symp. 1991;158:158–168. doi: 10.1002/9780470514085.ch11. [DOI] [PubMed] [Google Scholar]
- Cosstick R., Williams D. M. An approach to the stereoselective synthesis of Sp-dinucleoside phosphorothioates using phosphotriester chemistry. Nucleic Acids Res. 1987 Dec 10;15(23):9921–9932. doi: 10.1093/nar/15.23.9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T., Iwai S., Ohtsuka E. Synthesis and characterization of a substrate for T4 endonuclease V containing a phosphorodithioate linkage at the thymine dimer site. Nucleic Acids Res. 1990 Dec 25;18(24):7279–7286. doi: 10.1093/nar/18.24.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]