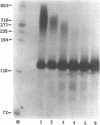
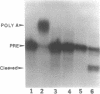
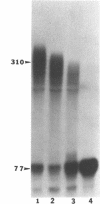
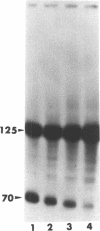
Abstract
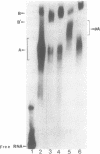
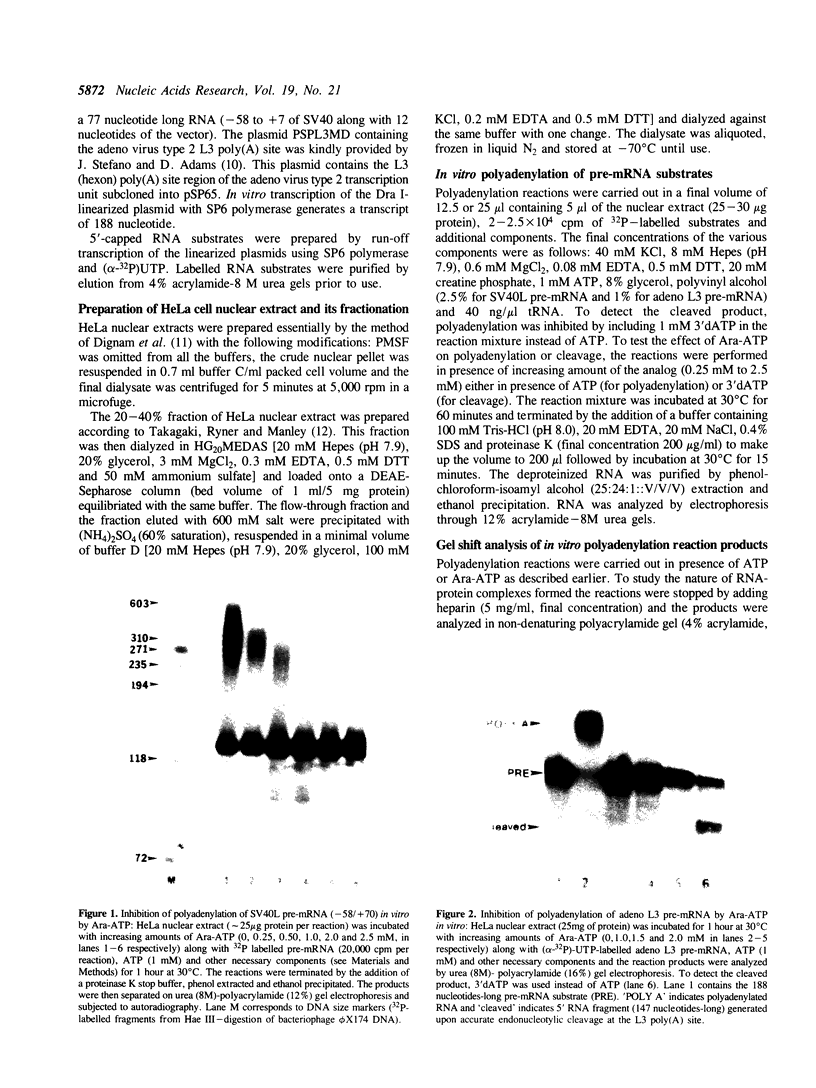
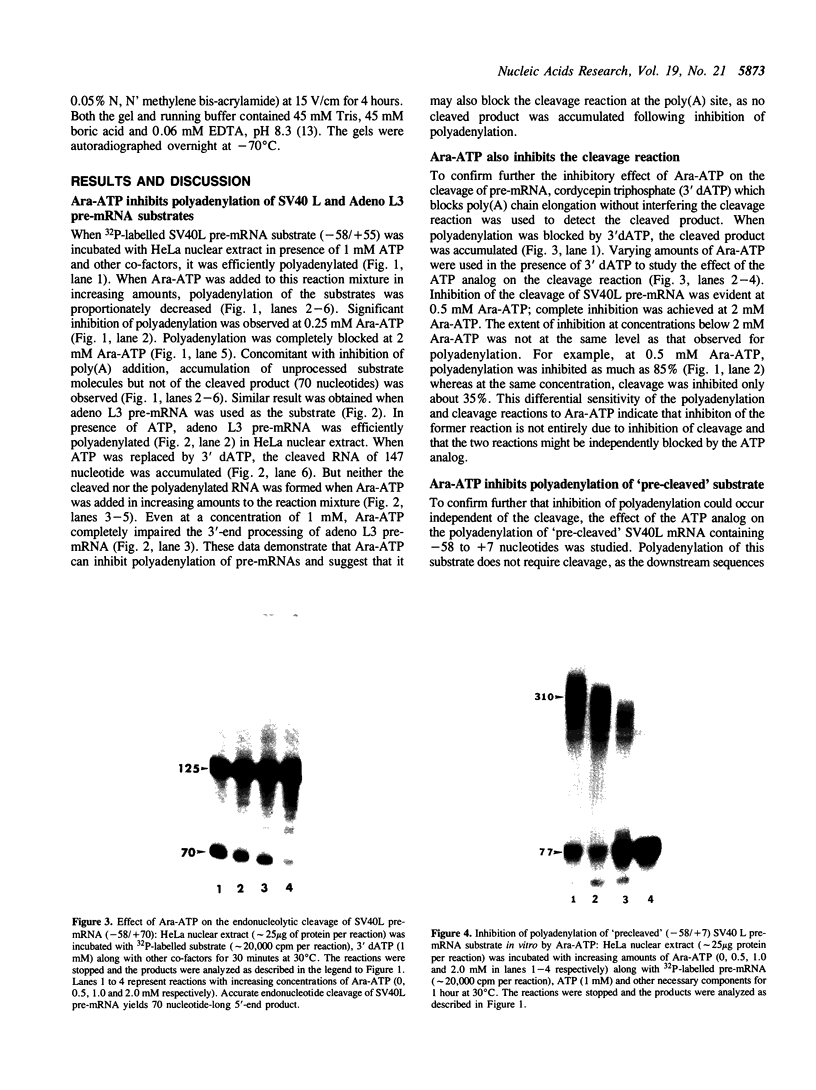
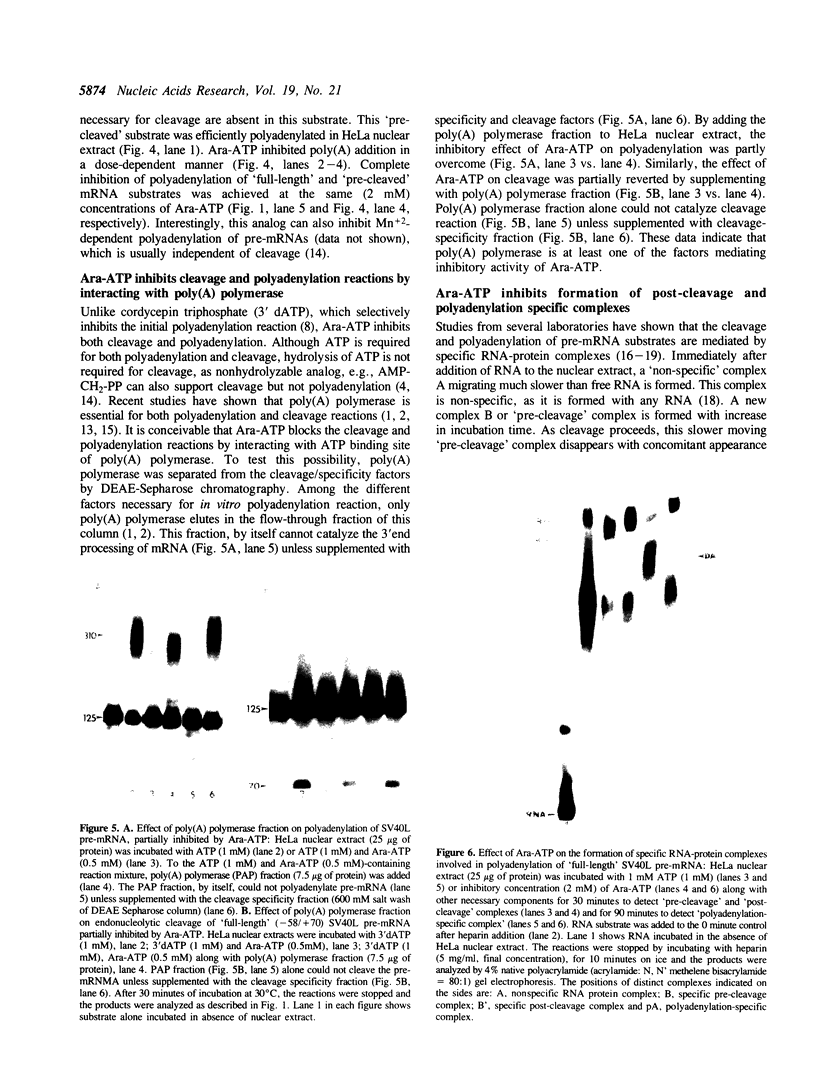
Previous studies have demonstrated that Ara-ATP can inhibit poly(A) polymerase activity by competing with ATP. To elucidate the mechanism of action of this compound, its effect on the cleavage and polyadenylation of two specific substrates, SV40L and adenovirus L3 pre-mRNAs, was studied in HeLa nuclear extracts. Unlike cordycepin 5' triphosphate, Ara-ATP inhibited both cleavage and poly(A) addition. Addition of poly(A) polymerase fraction devoid of any other factors required for the processing reactions overcame the inhibitory effect on cleavage as well as polyadenylation of pre-mRNAs. These data suggest that Ara-ATP inhibits both cleavage and polyadenylation reactions by interacting with the ATP-binding site on poly(A) polymerase, the activity of which is essential for the cleavage reaction. Ara-ATP also blocked formation of the post-cleavage and polyadenylation-specific complexes, which further confirmed the inhibitory effect of the ATP analog on the two tightly coupled 3'-end processing reactions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINK J. J., LEPAGE G. A. 9-BETA-D-ARABINOFURANOSYLADENINE AS AN INHIBITOR OF METABOLISM IN NORMAL AND NEOPLASTIC CELLS. Can J Biochem. 1965 Jan;43:1–15. doi: 10.1139/o65-001. [DOI] [PubMed] [Google Scholar]
- Bardwell V. J., Wickens M. Polyadenylation-specific complexes undergo a transition early in the polymerization of a poly(A) tail. Mol Cell Biol. 1990 Jan;10(1):295–302. doi: 10.1128/mcb.10.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofori G., Keller W. 3' cleavage and polyadenylation of mRNA precursors in vitro requires a poly(A) polymerase, a cleavage factor, and a snRNP. Cell. 1988 Sep 9;54(6):875–889. doi: 10.1016/s0092-8674(88)91263-9. [DOI] [PubMed] [Google Scholar]
- Christofori G., Keller W. Poly(A) polymerase purified from HeLa cell nuclear extract is required for both cleavage and polyadenylation of pre-mRNA in vitro. Mol Cell Biol. 1989 Jan;9(1):193–203. doi: 10.1128/mcb.9.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S. Introduction to the biochemistry of D-arabinosyl nucleosides. Prog Nucleic Acid Res Mol Biol. 1966;5:1–88. doi: 10.1016/s0079-6603(08)60231-7. [DOI] [PubMed] [Google Scholar]
- Dicioccio R. A., Srivastava B. I. Kinetics of inhibition of deoxynucleotide-polymerizing enzyme activities from normal and leukemic human cells by 9-beta-D-arabinofuranosyladenine 5'-triphosphate and 1-beta-D-arabinofuranosylcytosine 5'-triphosphate. Eur J Biochem. 1977 Oct 3;79(2):411–418. doi: 10.1111/j.1432-1033.1977.tb11823.x. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin G. M., Nevins J. R. An ordered pathway of assembly of components required for polyadenylation site recognition and processing. Genes Dev. 1989 Dec;3(12B):2180–2190. doi: 10.1101/gad.3.12b.2180. [DOI] [PubMed] [Google Scholar]
- Leonard T. B., Jacob S. T. Differential effects of cordycepin triphosphate and 9 beta-D-arabinofuranosyladenine triphosphate on tRNA and 5 S RNA synthesis in isolated nuclei. Biochim Biophys Acta. 1979 Jun 20;563(1):150–154. doi: 10.1016/0005-2787(79)90015-7. [DOI] [PubMed] [Google Scholar]
- Manley J. L. Polyadenylation of mRNA precursors. Biochim Biophys Acta. 1988 May 6;950(1):1–12. doi: 10.1016/0167-4781(88)90067-x. [DOI] [PubMed] [Google Scholar]
- Moore C. L., Sharp P. A. Accurate cleavage and polyadenylation of exogenous RNA substrate. Cell. 1985 Jul;41(3):845–855. doi: 10.1016/s0092-8674(85)80065-9. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Rohde H. J., Beyer R., Maidhof A., Lachmann M., Taschner H., Kahn R. K. Mode of action of 9-beta-D-arabinofuranosyladenine on the synthesis of DNA, RNA, and protein in vivo and in vitro. Cancer Res. 1975 Aug;35(8):2160–2168. [PubMed] [Google Scholar]
- NICHOLS W. W. IN VITRO CHROMOSOME BREAKAGE INDUCED BY ARABINOSYLADENINE IN HUMAN LEUKOCYTES. Cancer Res. 1964 Sep;24:1502–1505. [PubMed] [Google Scholar]
- Rose K. M., Bell L. E., Jacob S. T. Specific inhibition of chromatin-associated poly(A) synthesis in vitro by cordycepin 5'-triphosphate. Nature. 1977 May 12;267(5607):178–180. doi: 10.1038/267178a0. [DOI] [PubMed] [Google Scholar]
- Rose K. M., Jacob S. T. Selective inhibition of RNA polyadenylation by Ara-ATP in vitro: a possible mechanism for antiviral action of Ara-A. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1418–1424. doi: 10.1016/0006-291x(78)91294-9. [DOI] [PubMed] [Google Scholar]
- Sheets M. D., Ogg S. C., Wickens M. P. Point mutations in AAUAAA and the poly (A) addition site: effects on the accuracy and efficiency of cleavage and polyadenylation in vitro. Nucleic Acids Res. 1990 Oct 11;18(19):5799–5805. doi: 10.1093/nar/18.19.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siev M., Weinberg R., Penman S. The selective interruption of nucleolar RNA synthesis in HeLa cells by cordycepin. J Cell Biol. 1969 May;41(2):510–520. doi: 10.1083/jcb.41.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnik-David H., Moore C. L., Sharp P. A. Electrophoretic separation of polyadenylation-specific complexes. Genes Dev. 1987 Sep;1(7):672–682. doi: 10.1101/gad.1.7.672. [DOI] [PubMed] [Google Scholar]
- Stefano J. E., Adams D. E. Assembly of a polyadenylation-specific 25S ribonucleoprotein complex in vitro. Mol Cell Biol. 1988 May;8(5):2052–2062. doi: 10.1128/mcb.8.5.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagaki Y., Ryner L. C., Manley J. L. Four factors are required for 3'-end cleavage of pre-mRNAs. Genes Dev. 1989 Nov;3(11):1711–1724. doi: 10.1101/gad.3.11.1711. [DOI] [PubMed] [Google Scholar]
- Takagaki Y., Ryner L. C., Manley J. L. Separation and characterization of a poly(A) polymerase and a cleavage/specificity factor required for pre-mRNA polyadenylation. Cell. 1988 Mar 11;52(5):731–742. doi: 10.1016/0092-8674(88)90411-4. [DOI] [PubMed] [Google Scholar]
- Terns M. P., Jacob S. T. Role of poly(A) polymerase in the cleavage and polyadenylation of mRNA precursor. Mol Cell Biol. 1989 Apr;9(4):1435–1444. doi: 10.1128/mcb.9.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle E. Purification and characterization of a mammalian polyadenylate polymerase involved in the 3' end processing of messenger RNA precursors. J Biol Chem. 1991 Feb 15;266(5):3131–3139. [PubMed] [Google Scholar]
- Wickens M. How the messenger got its tail: addition of poly(A) in the nucleus. Trends Biochem Sci. 1990 Jul;15(7):277–281. doi: 10.1016/0968-0004(90)90054-f. [DOI] [PubMed] [Google Scholar]
- Zarkower D., Wickens M. Specific pre-cleavage and post-cleavage complexes involved in the formation of SV40 late mRNA 3' termini in vitro. EMBO J. 1987 Dec 20;6(13):4185–4192. doi: 10.1002/j.1460-2075.1987.tb02765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Cole C. N. Identification of a complex associated with processing and polyadenylation in vitro of herpes simplex virus type 1 thymidine kinase precursor RNA. Mol Cell Biol. 1987 Sep;7(9):3277–3286. doi: 10.1128/mcb.7.9.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]