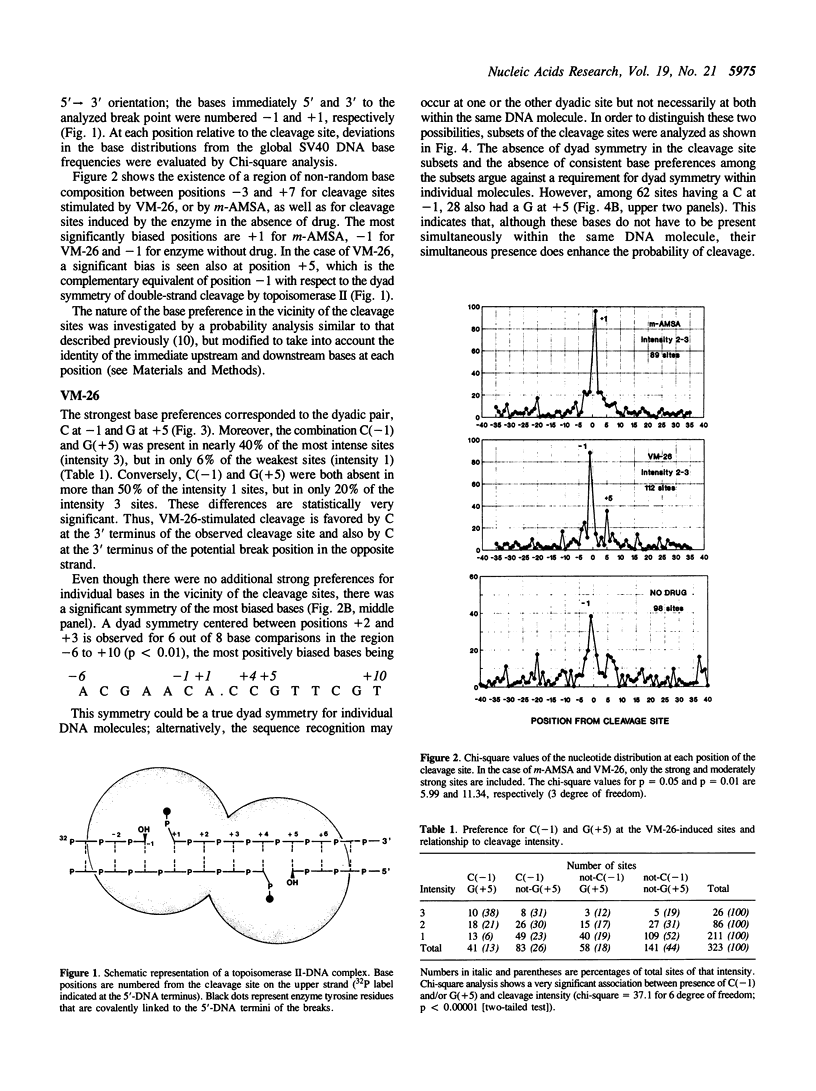
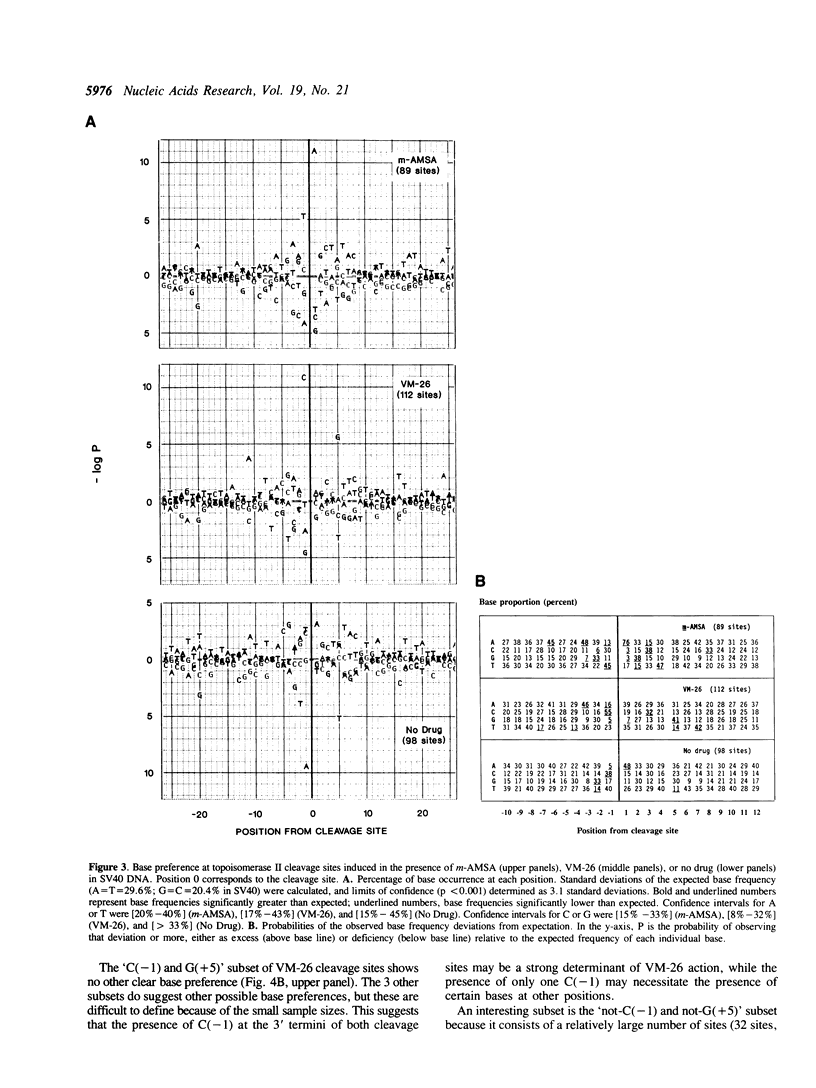
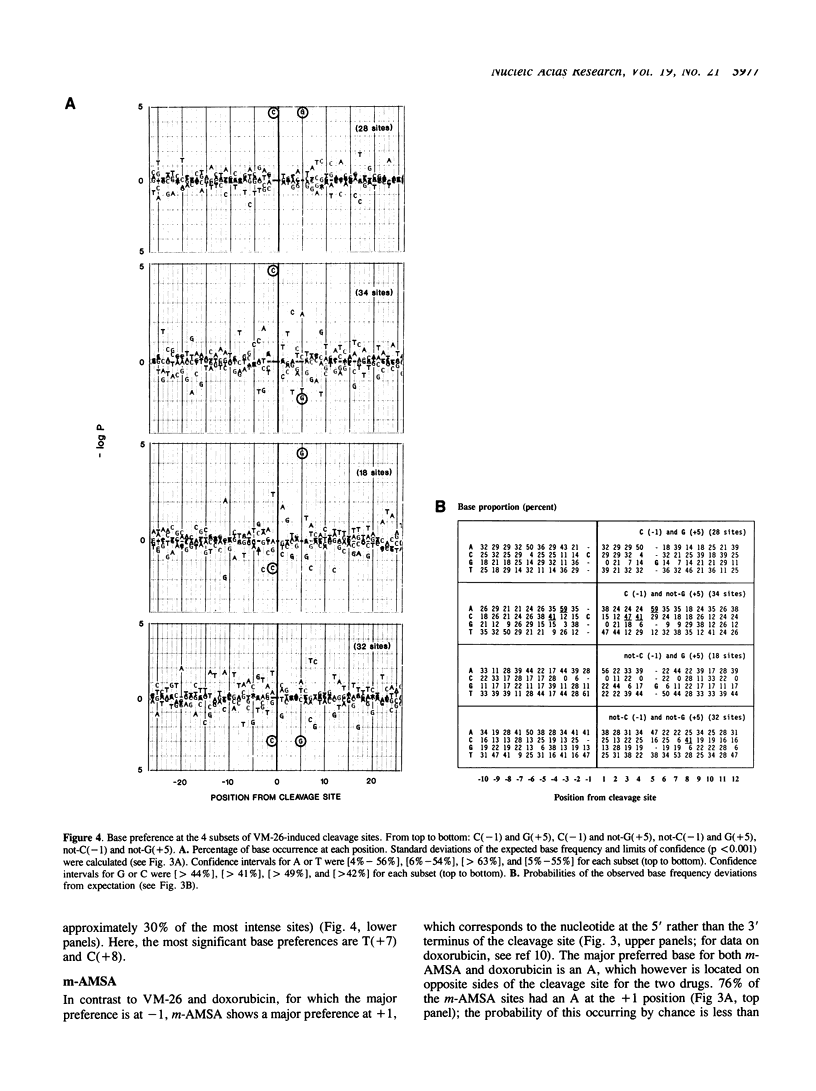
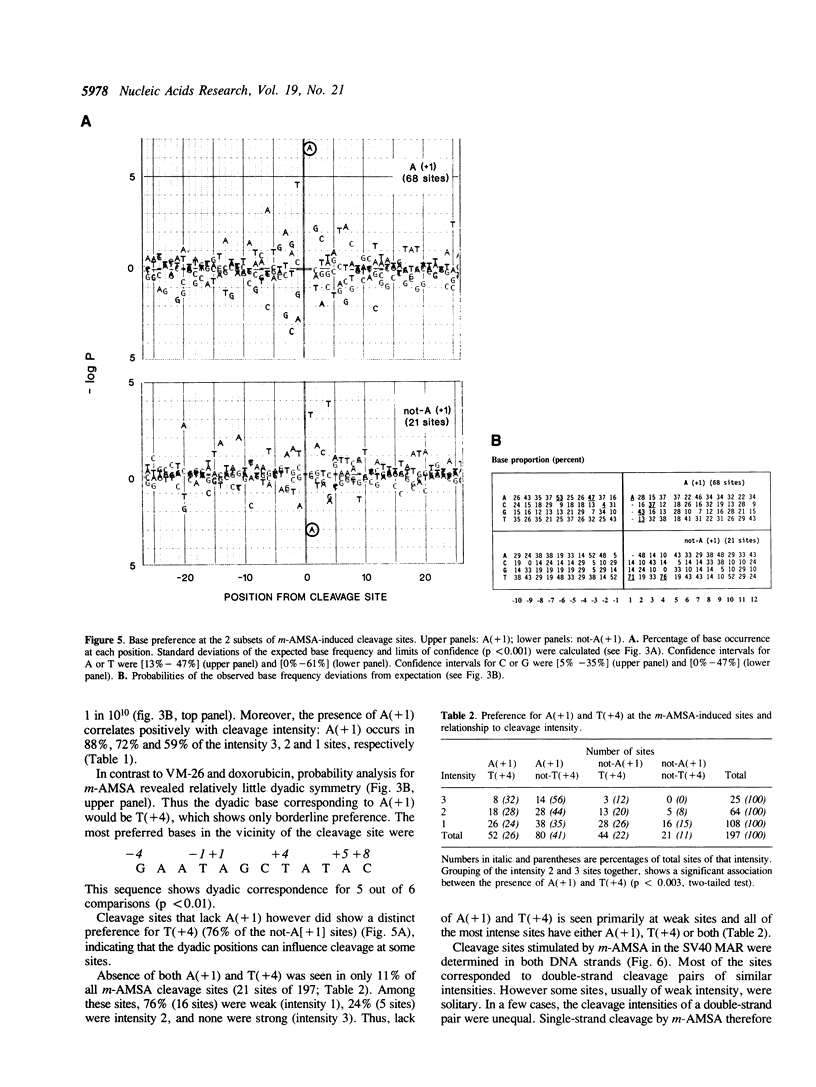
Abstract
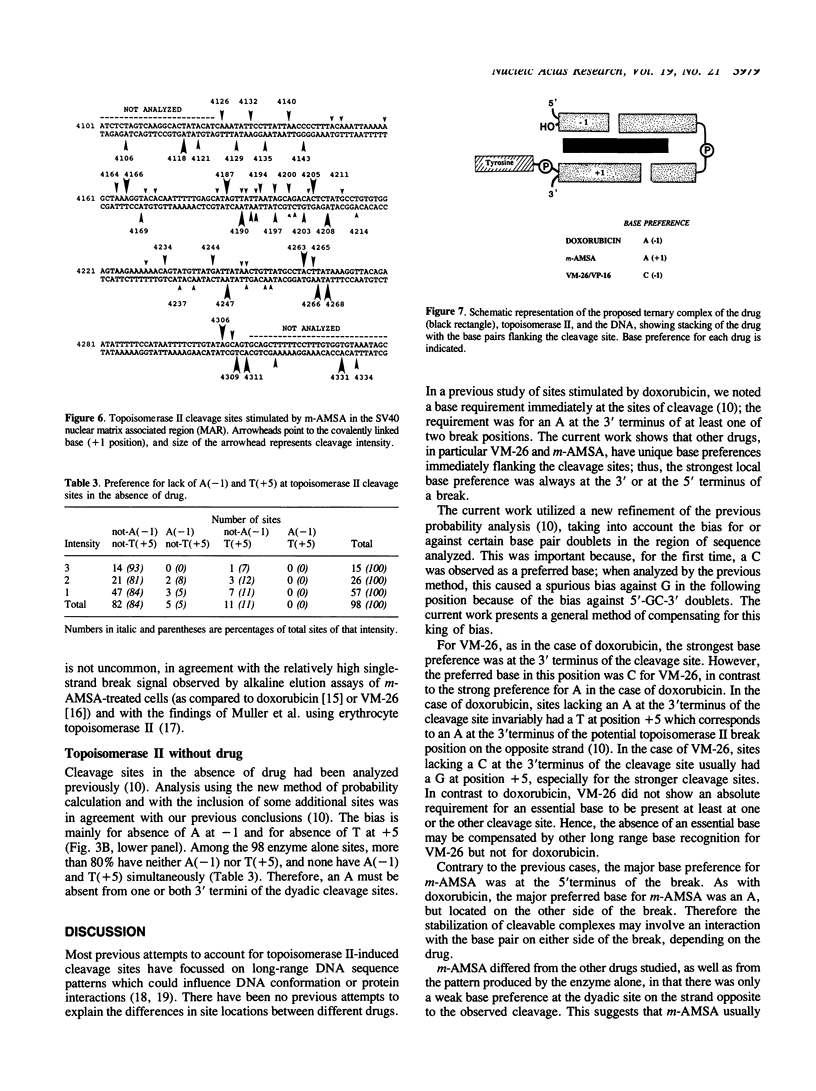
Several classes of antitumor drugs are known to stabilize topoisomerase complexes in which the enzyme is covalently bound to a terminus of a DNA strand break. The DNA cleavage sites generally are different for each class of drugs. We have determined the DNA sequence locations of a large number of drug-stimulated cleavage sites of topoisomerase II, and find that the results provide a clue to the possible structure of the complexes and the origin of the drug-specific differences. Cleavage enhancements by VM-26 and amsacrine (m-AMSA), which are representative of different classes of topoisomerase II inhibitors, have strong dependence on bases directly at the sites of cleavage. The preferred bases were C at the 3' terminus for VM-26 and A at the 5' terminus for m-AMSA. Also, a region of dyad symmetry of 12 to 16 base pairs was detected about the enzyme cleavage positions. These results are consistent with those obtained with doxorubicin, although in the case of doxorubicin, cleavage requires the presence of an A at the 3' terminus of at least one the pair of breaks that constitute a double-strand cleavage (Capranico et al., Nucleic Acids Res., 1990, 18: 6611). These findings suggest that topoisomerase II inhibitors may stack with one or the other base pair flanking the enzyme cleavage sites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capranico G., Jaxel C., Roberge M., Kohn K. W., Pommier Y. Nucleosome positioning as a critical determinant for the DNA cleavage sites of mammalian DNA topoisomerase II in reconstituted simian virus 40 chromatin. Nucleic Acids Res. 1990 Aug 11;18(15):4553–4559. doi: 10.1093/nar/18.15.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capranico G., Kohn K. W., Pommier Y. Local sequence requirements for DNA cleavage by mammalian topoisomerase II in the presence of doxorubicin. Nucleic Acids Res. 1990 Nov 25;18(22):6611–6619. doi: 10.1093/nar/18.22.6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capranico G., Zunino F., Kohn K. W., Pommier Y. Sequence-selective topoisomerase II inhibition by anthracycline derivatives in SV40 DNA: relationship with DNA binding affinity and cytotoxicity. Biochemistry. 1990 Jan 16;29(2):562–569. doi: 10.1021/bi00454a033. [DOI] [PubMed] [Google Scholar]
- Chow K. C., Macdonald T. L., Ross W. E. DNA binding by epipodophyllotoxins and N-acyl anthracyclines: implications for mechanism of topoisomerase II inhibition. Mol Pharmacol. 1988 Oct;34(4):467–473. [PubMed] [Google Scholar]
- Lee M. P., Sander M., Hsieh T. Nuclease protection by Drosophila DNA topoisomerase II. Enzyme/DNA contacts at the strong topoisomerase II cleavage sites. J Biol Chem. 1989 Dec 25;264(36):21779–21787. [PubMed] [Google Scholar]
- Liu L. F., Rowe T. C., Yang L., Tewey K. M., Chen G. L. Cleavage of DNA by mammalian DNA topoisomerase II. J Biol Chem. 1983 Dec 25;258(24):15365–15370. [PubMed] [Google Scholar]
- Long B. H., Musial S. T., Brattain M. G. Single- and double-strand DNA breakage and repair in human lung adenocarcinoma cells exposed to etoposide and teniposide. Cancer Res. 1985 Jul;45(7):3106–3112. [PubMed] [Google Scholar]
- Minford J., Pommier Y., Filipski J., Kohn K. W., Kerrigan D., Mattern M., Michaels S., Schwartz R., Zwelling L. A. Isolation of intercalator-dependent protein-linked DNA strand cleavage activity from cell nuclei and identification as topoisomerase II. Biochemistry. 1986 Jan 14;25(1):9–16. doi: 10.1021/bi00349a002. [DOI] [PubMed] [Google Scholar]
- Muller M. T., Spitzner J. R., DiDonato J. A., Mehta V. B., Tsutsui K., Tsutsui K. Single-strand DNA cleavages by eukaryotic topoisomerase II. Biochemistry. 1988 Nov 1;27(22):8369–8379. doi: 10.1021/bi00422a012. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Cockerill P. N., Kohn K. W., Garrard W. T. Identification within the simian virus 40 genome of a chromosomal loop attachment site that contains topoisomerase II cleavage sites. J Virol. 1990 Jan;64(1):419–423. doi: 10.1128/jvi.64.1.419-423.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y., Kerrigan D., Schwartz R. E., Swack J. A., McCurdy A. Altered DNA topoisomerase II activity in Chinese hamster cells resistant to topoisomerase II inhibitors. Cancer Res. 1986 Jun;46(6):3075–3081. [PubMed] [Google Scholar]
- Pommier Y., Kerrigan D., Schwartz R., Zwelling L. A. The formation and resealing of intercalator-induced DNA strand breaks in isolated L1210 cell nuclei. Biochem Biophys Res Commun. 1982 Jul 30;107(2):576–583. doi: 10.1016/0006-291x(82)91530-3. [DOI] [PubMed] [Google Scholar]
- Sander M., Hsieh T. S. Drosophila topoisomerase II double-strand DNA cleavage: analysis of DNA sequence homology at the cleavage site. Nucleic Acids Res. 1985 Feb 25;13(4):1057–1072. doi: 10.1093/nar/13.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M., Hsieh T. Double strand DNA cleavage by type II DNA topoisomerase from Drosophila melanogaster. J Biol Chem. 1983 Jul 10;258(13):8421–8428. [PubMed] [Google Scholar]
- Spitzner J. R., Muller M. T. A consensus sequence for cleavage by vertebrate DNA topoisomerase II. Nucleic Acids Res. 1988 Jun 24;16(12):5533–5556. doi: 10.1093/nar/16.12.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K., Schmid F. A., Brown G. F., González R. Chemotherapy of hamster tumors. Cancer Chemother Rep 2. 1971 Apr;2(1):141–175. [PubMed] [Google Scholar]
- Tewey K. M., Rowe T. C., Yang L., Halligan B. D., Liu L. F. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984 Oct 26;226(4673):466–468. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Recent studies of DNA topoisomerases. Biochim Biophys Acta. 1987 Jun 6;909(1):1–9. doi: 10.1016/0167-4781(87)90040-6. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Michaels S., Erickson L. C., Ungerleider R. S., Nichols M., Kohn K. W. Protein-associated deoxyribonucleic acid strand breaks in L1210 cells treated with the deoxyribonucleic acid intercalating agents 4'-(9-acridinylamino) methanesulfon-m-anisidide and adriamycin. Biochemistry. 1981 Nov 10;20(23):6553–6563. doi: 10.1021/bi00526a006. [DOI] [PubMed] [Google Scholar]


