Abstract
Vulvovaginal candidiasis (VVC), caused by Candida albicans, affects a significant number of women during their reproductive years. More than two decades of research have been focused on the mechanisms associated with susceptibility or resistance to symptomatic infection. Adaptive immunity by Th1-type CD4+ T cells and downstream cytokine responses are considered the predominant host defense mechanisms against mucosal Candida infections. However, numerous clinical and animal studies have indicated no or limited protective role of cells and cytokines of the Th1 or Th2 lineage against vaginal infection. The role for Th17 is only now begun to be investigated in-depth for VVC with results already showing significant controversy. On the other hand, a clinical live-challenge study and an established animal model have shown that a symptomatic condition is intimately associated with the vaginal infiltration of polymorphonuclear leukocytes (PMNs) but with no effect on vaginal fungal burden. Subsequent studies identified S100A8 and S100A9 Alarmins as key chemotactic mediators of the acute PMN response. These chemotactic danger signals appear to be secreted by vaginal epithelial cells upon interaction and early adherence of Candida. Thus, instead of a putative immunodeficiency against Candida involving classical immune cells and cytokines of the adaptive response, the pathological inflammation in VVC is now considered a consequence of a non-productive innate response initiated by non-classical immune mediators.
Keywords: Candida albicans, vaginitis, epithelial cells, inflammation, S100A8, S100A9
1. Introduction
Vulvovaginal candidiasis (VVC) is a prevalent infection caused by Candida species that affects approximately 75% of healthy women during their childbearing age [1]. Candida is a dimorphic fungal commensal organism of the gastrointestinal and genitourinary tracts where several Candida species colonize in healthy individuals [1]. Among those, C. albicans is the most common cause of diagnosed VVC cases (80-90%) [1]. Recurrent VVC (RVVC, three or more VVC episodes per year) is known to affect a separate population of 5-8% of menarchal women [2]. Both acute VVC and RVVC can be attributed to exogenous factors that may modulate host responses to Candida or may directly alter the growth of the organism as a consequence of environmental changes. Such exogenous factors include disturbance in reproductive hormone levels due to pregnancy, high-estrogen contraceptive usage or hormone replacement therapies, antibiotic usage, and uncontrolled diabetes [1]. In most acute VVC cases, the disease subsides once these predisposing factors are eliminated and/or antifungal therapy is used. However, for many women who suffer from RVVC, episodes are idiopathic and antifungal treatment does not prevent recurrence [2].
As a result of exposure to Candida early in life, most immunocompetent individuals have developed adaptive immunity toward Candida evidenced by serum/mucosal antibody production, and in vitro T-cell responses and related cytokine production (reviewed in [3]). These Candida-specific host immune responses are generally considered critical to generate protection at the mucosal-Candida interface and keep commensal Candida from converting into an opportunistic pathogen. In addition to VVC/RVVC, mucosal Candida infections can occur in other anatomical sites as seen in oropharyngeal candididiasis (OPC), esophageal and gastrointestinal candidiasis, or chronic mucocutaneous candidiasis (CMC). Unlike VVC/RVVC, these forms of disease predominantly occur in individuals with an immune deficiency (e.g. patients with HIV/AIDS, post-chemotherapy treatments) when CD4+ T cells and subsequent production of effector cytokines become reduced. Of those, it was originally hypothesized that Th1 cytokines, mainly IL-12 and IFN-γ, play an important role in mediating protective host defense against Candida at mucosal sites. IL-12 is a key cytokine produced by antigen-presenting cells (APCs) that initiates Th1-type cell-mediated immunity (CMI) hallmarked by IFN-γ-driven proinflammatory responses toward Candida [4, 5]. Thus, intact Th1 responses promote resistance to Candida in the immunocompetent host. In the event of Th2-skewed responses to Candida, however, the development of Th2 cells by IL-4 signaling and subsequent cytokine production (e.g. IL-10) are known to dampen the Th1-mediated protection, leading to susceptibility to Candida infection [4]. Although Candida can elicit an antibody response at systemic (IgG) and local (IgG and IgA) levels, no strong protective role for Th2-type humoral immunity by neutralizing Candida-specific antibodies has been demonstrated in humans or animal models [6, 7]. These findings indicate that the balance between the two arms of adaptive immunity is required for effective clearance of Candida from infected mucosal sites. The discovery of Th17 cells, however, has added a new perspective to the Th1-protective/Th2-nonprotective paradigm of mucosal immune responses to Candida. Th17 cells predominantly produce IL-17 and IL-22 that act on other innate immune cells such as epithelial and stromal cells to induce production of proinflammatory cytokines, chemokines and antimicrobial proteins [8]. Until recently, Th17 cells had been misinterpreted as Th1 cells due to the common subunit in their key cytokines for differentiation, IL-23 and IL-12, respectively. A protective role of IL-23/Th17 in OPC, rather than IL-12/Th1, was elegantly demonstrated by Conti et al. using IL-23p19−/− and IL-12p35−/− mice [9]. There is an increasing body of evidence showing a strong anti-Candida response by Th17 cells in OPC, CMC as well as disseminated candidiasis [9-11]. In gastrointestinal candidiasis, however, the activation of Th17 has been reported to lead to pathology of the disease by eliciting exacerbated inflammation and impaired immune resistance to Candida [12]. Furthermore, an epidemiological study on dectin-1 mutations found in a small group of individuals with familial recurrent candidiasis suggested a link between a defective Th17 response and susceptibility to mucocutaneous Candida infections, including RVVC, possibly due to the lack of dectin-1-mediated Th17 activation [13]. Yet, a protective role for Th17 immunity remains a subject of controversy as clinical evidence by others implicated otherwise [14].
In this review, we highlight current knowledge on cytokines and cells involved in host immune mechanisms against VVC and describe recent advances in identification of secretory immune mediators that may play a key role in the immunopathogenesis of VVC.
2. Review of studies evaluating the role of classical cytokines involved in adaptive immunity during vaginal candidiasis
2.1. Th1 responses
Early clinical reports implicated that mucosal candidiasis occurred predominantly in individuals with T cell immunodeficiency, and a strong role for CMI by Th1 cells against Candida was demonstrated by various experimental models [15-20]. Based on these observations, the original consensus was that resistance to mucosal infections, including VVC, was mediated by Th1-type CMI, while susceptibility was associated with Th2-type humoral responses [21]. However, highly variable results were obtained from clinical studies evaluating RVVC patients for systemic Candida-specific CMI where evidence for both normal and defective cellular immunity was reported [22-24]. These inconsistent results for a role of systemic CMI were further accompanied by additional clinical observations indicating that 1) women with RVVC did not exhibit increased susceptibility to other forms of mucosal candidiasis (e.g. OPC, CMC) and 2) incidence of RVVC in immunocompromised women (e.g. HIV+/AIDS) was equivalent to that in the healthy population [2, 25-27]. Together, a new hypothesis was proposed that mechanisms for resistance and susceptibility to infection lie within local immune dysfunctions at the vaginal mucosa rather than deficiency in systemic CMI.
Subsequent studies focused on local CMI by evaluating vaginal T cells and cytokines in vaginal secretions during infection. The majority of the studies conducted in humans and mice showed a sparse yet unique resident T cell composition in the vaginal mucosa and a lack of any T cell infiltration into the vaginal mucosa during infection [28-33]. These findings were further supported by low/undetectable levels of Th1 cytokines, namely IL-2, IL-12 and IFN-γ, in the vagina of inoculated mice [29]. In clinical studies, on the other hand, women exhibited constitutive Th1/Th0 cytokines in the vagina irrespective of the infection status [34, 35]. In both models, other proinflammatory cytokines and chemokines, such as MCP-1, IL-1α, TNF-α, MIP-1α, MCP-1, IL-8 (or MIP-2 in mice), were also unaffected during infection and had no influence on vaginal fungal burden [30, 34]. Nonetheless, the absence of Th1 responses to the vaginal presence of Candida became prominent although a few cases of contrary data have challenged this hypothesis. One is a rat vaginitis model in which an accumulation of vaginal CD4+ T cells in the lamina propria and epithelium occurred following inoculation [36]. These vaginal lymphocytes also showed in vitro proliferative activity in response to mannoprotein of C. albicans, and vaginal fluid of inoculated rats contained high levels of IL-12 during primary infection followed by the production of IL-2 and IFN-γ during the subsequent infections [37]. The other case is a reconstituted human vaginal epithelium (RHVE) model in which infection of the tissue with C.albicans induced strong proinflammatory cytokine and chemokine responses [38]. However, the conflicting results from the experimental rat vaginitis and the in vitro infection of RHVE with previous human studies may raise questions about the clinical relevance of the models.
As evidence for the lack of CMI against VVC accumulated, a series of animal studies were conducted to test the hypothesis that CMI at the vaginal mucosa is suppressed in the host while detectable Candida-specific Th1 immunity is present at the systemic level. Data from these studies showed constitutive expression of TGF-β in vaginal tissues of mice, which was further increased as a result of pseudoestrus and inoculation with C. albicans [29]. A constitutive elevation of TGF-β was also observed in humans irrespective of the infection status, while other Th1 and Th2 cytokines remained at low levels [34]. Thus, the involvement of TGF-β, a potent immunoregulatory cytokine, could explain the lack of CMI in the vagina despite the presence of systemic CMI against Candida. Further analyses of resident vaginal T cells revealed an increased number of γδ T cells in the vaginal mucosa of mice, and γδ T cell-deficiency showed increased resistance to vaginal infection [39]. This could represent another immunoregulatory mechanism involving γδ T cell-mediated induction of immune tolerance toward the vaginal presence of Candida. Subsequently, studies were designed to evaluate cell adhesion molecules on the vaginal epithelium and homing receptor expression on T cells within the draining lymph nodes. Although vaginal cell adhesion molecule expression was elevated following inoculation with Candida, T cells expressing the reciprocal homing receptors in the draining lymph nodes were reduced in inoculated mice, suggesting a means to limit Candida-specific T cell infiltration into the vagina [40]. Finally, in studies evaluating dendritic cells (DCs) in the vagina, the draining lymph nodes of mice inoculated with Candida showed the vast predominance of plasmacytoid DCs over myeloid DCs, characterized by the absence of cellular activation markers and lack of upregulation in co-stimulatory molecules [41]. This suggested a potential involvement of DCs in the initiation of immunoregulation toward Candida through the tolerogenic action of pDCs. Together, the lack of vaginal CMI against Candida has been postulated to be the result of a symbiotic interaction between the fungus and the vaginal tissue, which maintains tolerogenic responses to avoid a chronic inflammatory reaction toward commensal organisms at a reproductive site.
2.2. Th2 responses
Some of the pioneering studies conducted in an attempt to elucidate the factors associated with susceptibility to RVVC focused on putative deficiencies in Candida-specific antibody responses. However, no study could demonstrate any major difference in Candida-specific antibody levels in blood or vaginal secretions of those with RVVC compared to control women [6, 7]. Likewise, analyses of Th2 cytokines in clinical studies showed only a small amount of IL-4, IL-5 and IL-13, if detected, in vaginal fluid with no effect on fungal burden [34]. Contrary to these findings, Babula, et al. showed increased vaginal IL-4 with evidence of a polymorphism in IL-4 in women with RVVC, possibly resulting in reduced production of anticandidal compounds such as nitric oxide and mannose-binding lectin (MBL) [42]. A similar controversy has been shown in a mouse model where one study showed IL-4 and IL-10 to be extremely low in the vagina under uninoculated or inoculated conditions [29], while another study showed that increased IL-4 and IL-10 were associated with higher fungal burden and severity in pathology [43]. In the rat model, no IL-4 or IL-5 was detected in the vaginal fluid following inoculation [37], although a unique subset of B cells (CD5+) was identified following Candida antigenic stimulation [36]. Subsequent studies in rats showed that adoptive transfer of these B cells from Candida-immunized rats to naïve animals resulted in a significant reduction in vaginal fungal burden. In addition, in vitro production of antibodies specific to Candida-mannoprotein (MP), together with secretion of several inflammatory and anti-inflammatory cytokines by the B cells, suggested a protective role of anti-MP antibody producing B cells against vaginal infection [44]. Antibody-mediated protection against vaginitis was further evidenced through the protective immunization of naïve rats with vaginal fluid from immunized rats containing an anti-secreted aspartyl proteinase (SAP) antibody [45]. Although it would be interesting to investigate the presence of anti-MP or anti-SAP antibodies in humans, it is likely that the antibody response could be a species-specific outcome in rats based on the clinical data showing no antibody deficiency in RVVC women and the absence of protective Candida-specific antibody production in inoculated mice. On the other hand, studies by Han et al. showed that administration of isolated/purified anti-mannan IgM and IgG3 antibodies resulted in protection against vaginitis in mice [46]. This finding suggests that although such antibody-mediated protection may not naturally occur in mice or humans, protection could be elicited if protective monoclonal antibodies against a specific Candida immunogen could be identified and induced in abundance. This implication is further supported by the concept proposed by Casadevall regarding a pool of “protective”, “nonprotective” and “indifferent” antibodies induced by any one immunogen, with those present in the highest concentration predominating the overall immunological outcome (i.e. protection, pathology or no effect) against infection [47]. Thus, the lack of protective antibody responses in humans and mice could be explained by the low abundance of ‘protective’ antibodies whose concentration may not be high enough to elicit protection in the vaginal microenvironment, whereas such ‘protective’ antibodies are produced at high concentrations in rats. Designing vaccine strategies using specific Candida immunogens to promote ‘protective’ vaginal antibody production in experimental or clinical settings would be a great advancement in the development of potential immunotherapeutics for VVC/RVVC.
Another hypothesis previously proposed was that susceptibility to RVVC was associated with an allergic reaction triggered either by Candida-specific IgE production [48, 49] or through histamine-induced prostaglandin E2, resulting in the suppression of Th1-type CMI [50]. Limited clinical studies evaluating systemic and vaginal IgE showed that some women with acute VVC or cured VVC had elevated concentrations of IL-13 and IgE in the vaginal fluid suggestive of an allergic response in the pathogenesis of VVC [51]. Overall, it appears that only a small minority of RVVC cases are the consequence of vaginal IgE hypersensitivity [52]. In contrast, there appears to be some precedent for hyper IgE syndrome in OPC [53].
2.3. Th17 responses
Our understanding of adaptive immune responses to mucosal Candida infections advanced dramatically in the last few years, particularly by the addition of the Th17 axis of immunity into the conventional Th1/Th2 paradigm. The activation of Th17 cells and downstream cytokines, particularly IL-17 and IL-22, signals epithelial and stromal cells of infected mucosa to elicit proinflammatory responses [8]. Inflammatory mediators induced by IL-17 and/or IL-22 signaling include epithelial-derived antimicrobial proteins such as β-defensins and S100 proteins [54]. The resulting Th17-dependent inflammation via regulation of innate immune cells, mainly PMNs, has been shown to mediate rapid clearance of Candida in OPC and CMC, as well as disseminated candidiasis and Candida-induced skin abscesses [9-11, 55]. Despite the strong protective role in Candida infections, data from the gastrointestinal candidiasis model showed that a skewed Th17 response could result in tissue inflammatory pathology and exacerbated infection [12]. Therefore, Th17 immunity appears to have dichotomous effects in response to the same pathogen depending on the site of infection.
In the female genital tract, Th17 immunity has been shown to provide protection against Neisseria gonorrhoeae and Chlamydia muridarum infections in mice through the induction of PMN migration to the site of infection [56, 57]. In Candida vaginitis, there are only a few documented studies that evaluated systemic and local Th17 cytokine responses in women with RVVC. One was a study describing an association of dectin-1 deficiency with susceptibility to familial recurrent mucocutaneous candidiasis, including RVVC, reported by Ferwerda et al [13]. Data showed that PBMCs from dectin-1−/− individuals produced reduced IL-6 and IL-17 in response to a Candida β-glucan challenge, suggesting a protective role for a systemic Th17 response against Candida. However, these results are inconsistent with other clinical observations where no Th17 cytokine deficiency was found in blood or vaginal fluids of RVVC patients [13, 14]. In another study, Lev-Sagie et al. reported that IL-6, IL-12 and IL-23 were detected only in vaginal fluids from a small subset of RVVC women, implicating a lack of a major role for either Th1 or Th17 immunity [14]. Thus, this hypothesis requires additional studies under a more defined setting in order to properly investigate host immunological factors associated with RVVC. In animal models, Pietrella et al. recently demonstrated in mice that depletion of Th17 cells by halofuginone, an inhibitor of TGF-β signaling, significantly reduced IL-17 production by vaginal CD4+ T cells. The study also showed that the depletion of Th17 cells resulted in exacerbated vaginal infection, possibly due to reduced production of antimicrobial peptides β-defensin (BD)-2 and BD-3 by vaginal epithelial cells [58]. However, the study suffers somewhat from weak IL-17-mediated protection and only a partial effect of halofuginone on vaginal Th17 cells. In contrast to these results, recent data from our laboratory using IL-23p19−/−, IL-22−/− and IL-17 receptor A (IL-17RA)−/− mice showed little to no differences between each other or to wild-type mice in vaginal fungal burden or PMN infiltration following inoculation with Candida (Fidel, submitted). The animal study also revealed a negligible amount of IL-17 and IL-22, if detected, in the vaginal fluid of both wild-type and Th17-deficient mice irrespective of infection or inflammatory status. Thus, at the present time, the role of the Th17 axis in VVC remains controversial. A summary of the roles for various cytokines (Th1, Th2, Th17, proinflammatory, and regulatory) and chemokines investigated to date is illustrated in Table 1. Interestingly, note that no cytokine is reported unequivocally to play a role in protection.
Table 1.
Summary of vaginal cytokines and chemokines evaluated during vaginal candidiasis
| Cytokine | Reported outcome in VVC/RVVC |
References | |||
|---|---|---|---|---|---|
| Humans | Mice | Rats | |||
| Th1 | IL-2 | P− | P+ | P+ | [37, 43, 44, 52, 114] |
| IL-12 | P− | P− | P+ | [34, 37, 52, 114] | |
| IFN-γ | P−, S+ | P− | P+ | [34, 37, 51, 52] | |
| Th2 | IL-4 | P−, S+ | S+ | P− | [34, 37, 42, 43, 51, 115] |
| IL-5 | P− | P− | [37, 115] | ||
| IL-13 | S+ | [115] | |||
| Th17 | IL-23 | P− | P− | [14, 58](Fidel, submitted) | |
| IL-17 | P+/− | P+/− | [13, 58](Fidel, submitted) | ||
| IL-22 | P− | (Fidel, submitted) | |||
| Proinflammatory | IL-1α | P− | [34] | ||
| IL-6 | P− | P+ | [13, 34, 44, 114] | ||
| G-CSF | P− | P− | [13](Fidel, unpublished data) | ||
| TNF-α | P− | P+ | [34, 114] | ||
| Regulatory | TGF-β | I+ | I+ | [29, 34, 35, 43] | |
| IL-10 | P− | P−, S+ | P+ | [29, 34, 43, 44, 114] | |
| Chemokines | MCP-1 | P− | [29, 30] | ||
| RANTES | P− | [30] | |||
| IL-8/MIP-2 | P−, S+ | P− | [30, 51, 71] | ||
| MIP-1α | P− | [30] | |||
P, protection; S, susceptibility; I, immunoregulation; +, reported role; −, reported lack of a role
3. Paradigm shift for VVC – Role for innate immunity
3.1. Early studies of innate immunity and the development of a human live challenge protocol
Since efforts to gain evidence for adaptive immune mechanisms against VVC continued to show a lack of protection by CMI or antibody responses, studies focused on characterizing innate immune factors that may be associated with host resistance against vaginitis. Vaginal innate immune cell populations were first evaluated in the mouse model in which PMNs were identified to be the predominant cell type among vaginal leukocytes [30]. Despite the strong phagocytic activity against Candida, studies showed that the presence of vaginal PMNs during infection was erratic, and had no effect on clearing vaginal fungal burden [28, 59]. Neutropenia, on the other hand, reduced inflammation associated with vaginitis in mice [59]. Other innate immune cells present in the vaginal cavity such as macrophages and natural killer cells were also evaluated and were found only in small numbers, if any [60, 61]. Subsequent studies evaluating roles of epithelial cells revealed the ability of vaginal epithelial cells isolated from mice, humans and macaques to inhibit the growth of C. albicans in vitro [61-63]. In vaginal and oral epithelial cells, the mechanism of action involved direct cell contact with Candida but no role for soluble factors [61, 64]. In addition, the antifungal activity was fungistatic rather than fungicidal, which could be elicited by intact, not necessarily live, epithelial cells [65]. We recently reported our discovery that a likely candidate for this anti-Candida activity is Annexin A1, a molecule expressed on the surface of epithelial cells and known to be involved in regulating growth pathways in C. albicans [66]. In a study evaluating vaginal epithelial cells from women with RVVC, the anti-Candida activity was significantly reduced in cells from RVVC women compared to those from a control group [67]. Together, these data strongly suggest that vaginal epithelial cells may play a key role as the first line of defense at the vaginal mucosa, and the anti-Candida activity appears to be an innate mechanism to control the growth of Candida in a non-inflammatory fashion. Other molecules that act against Candida in the oral cavity, such as defensins or histatins [68, 69], have not been studied at any depth in VVC. The only study, to date, is a preliminary evaluation of several innate host factors (i.e. lysozyme, myeloperoxidase, C5a) in lavage fluid of women with VVC/RVVC where no one factor was shown in higher or lower levels during symptomatic infection (Fidel, unpublished observation).
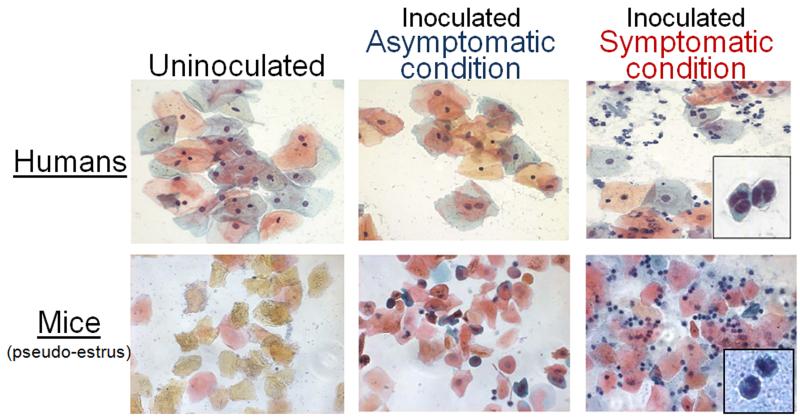
In the subsequent years, we have made dramatic progress in our understanding of host immune responses against VVC with the development of a human live challenge model. In the live challenge study, resistant (no previous VVC episode) or susceptible (with infrequent VVC episodes) women were evaluated for the natural history of infection following intravaginal inoculation with live C. albicans. Results revealed that protection, as evidenced by asymptomatic vaginal colonization with Candida, occurred in the absence of any inflammatory response, while symptomatic infection was accompanied by a heavy vaginal cellular infiltrate consisting of PMNs (Figure 1). In addition, PMN infiltration scores assessed from these women positively correlated with vaginal fungal burden although the hyphal (pathogenic) form of Candida could be present in both asymptomatic and symptomatic conditions [70]. Finally, when tested in an in vitro PMN migration assay, vaginal lavage fluid from women with symptomatic infection showed increased PMN chemotactic activity compared to lavage fluid from those with asymptomatic colonization, suggesting a chemotactic factor(s) (e.g. cytokines, chemokines) had been produced and secreted into the vaginal cavity in response to the Candida challenge [70]. Notably, evaluation of common proinflammatory cytokines and chemokines (G-CSF, TNF-α, IL-1, IL-6, IL-8 and IL-17) in lavage fluids from the study failed to identify candidates for the PMN chemotactic factors (reported in [71]). Moreover, Candida itself fails to directly induce PMN chemotaxis or through any organism-derived factors (Fidel, unpublished observation). Hence, organism virulence or virulence factors alone do not appear to be a major player in the PMN response. These important data from the live challenge study reshaped our knowledge based on the conventional dogma regarding Th1-resistance/Th2-susceptibility to mucosal Candida infections. Furthermore, based on the evidence showing the strong involvement of innate components of host immune responses during VVC, the paradigm has shifted into the current concept that both resistance and susceptibility to VVC are associated with innate immunity.
Figure 1.
Presence of PMNs in vaginal lavage fluid following inoculation with C. albicans. (A) Vaginal smears collected on day 7 post-inoculation from women who acquired a symptomatic infection (symptomatic condition), became asymptomatically colonized (asymptomatic condition) or uninoculated women. (B) Vaginal smears collected on day 7 post-inoculation from estrogen-treated inoculated mice with high PMNs (symptomatic condition), low PMNs (asymptomatic condition) or uninoculated mice. Smear samples were preserved and stained using the Papanicolaou technique. Images are shown at X400 magnification. Inserts show a high-magnified view of PMNs at 120% of X1,000 magnification. Partially reproduced with permission from ASM Press.
To explain the relationship between host susceptibility to VVC and the vaginal presence of Candida̧ the hypothesis was proposed that symptomatic infection is acquired when the number of Candida organisms achieves a threshold which varies between women depending on the sensitivity of vaginal epithelial cells and may ultimately determine the clinical outcome (reviewed in [72]). Based on the hypothesis, we postulate that 1) in women with RVVC, vaginal epithelial cells are extremely sensitive to Candida and respond by secreting a danger signal that promote PMN infiltration after exposure to low numbers of Candida. These women are susceptible to recurrence by small increases in organism numbers (i.e., shortly following disruption or cessation of maintenance antifungal therapy. 2) In women with infrequent history of VVC due to any of the known predisposing factors (i.e., oral contraceptives, hormone replacement therapy, antibiotic usage, etc.), vaginal epithelial cells are less sensitive to Candida and have a higher threshold for Candida. These women remain asymptomatic following exposure to moderate numbers of Candida, but if the numbers rise following the use of antibiotics or estrogen, the threshold will be breached and a similar response will result in a symptomatic condition. 3) In women with no history of VVC, their vaginal epithelial cells are insensitive to even large numbers of Candida and hence the threshold is rarely breached. Therefore, the PMN response rarely, if ever, occurs and these women remain asymptomatic. At present, it is unknown what factors are involved in establishing the level of epithelial cell sensitivity to Candida, but is presumed to be a genetic predisposition. Indeed, some polymorphisms have been identified in women with RVVC [13, 42], On the other hand, no differences in susceptibility to infection have been demonstrated for the various haplotypic strains of mice [71, 73-75].
3.2. Dissecting the vaginal PMN response in animal studies
In parallel with the early clinical studies examining innate immune factors against VVC, the mouse model also showed no role for the erratic presence of vaginal PMNs against Candida [28, 59, 71]. Although PMNs were identified to be the most predominant leukocyte population in vaginal lavage fluid irrespective of time post-inoculation, their erratic presence was presumed to be a product of the pseudo-estrus condition [76, 77].
Consequently, however, a series of studies was conducted to formally classify the PMN response in various conditions. Similar to clinical observations, data showed that a heavy vaginal PMN migration occurred in a subset of inoculated animals irrespective of the haplotype or the duration of infection without affecting fungal burden [71]. Of note, previous studies testing effects of estrogen concentrations, inocula and strains of C. albicans also showed similar PMN infiltration patterns [76-78]. In light of symptomatic/asymptomatic conditions observed in inoculated women from the live challenge study, the high PMN and low PMN responses in mice were classified to be simulating the symptomatic and asymptomatic conditions of VVC, respectively (Figure 1) [71]. Although previous attempts to quantify clinical signs and symptoms of vaginitis in mice (e.g. redness, swelling, irritation, scratching) were unsuccessful and therefore could not be used as correlates to the PMN infiltration, vaginal PMNs levels appear to be rigid criteria based on the association with the clinical symptomatology of VVC. Accordingly, when tested in an in vitro PMN migration assay, results showed vaginal lavage fluids from mice under a high PMN (symptomatic) condition had increased PMN chemotactic activity compared to those from a low PMN (asymptomatic) condition, suggesting the presence of a similar chemotactic factor(s) secreted in response to Candida [71]. Taking into account previous reports showing a lack of strong vaginal cytokine/chemokine responses in humans and mice in VVC, identification of this putative PMN chemotactic factor(s) was crucial to further uncovering immune processes associated with susceptibility to symptomatic disease. The mouse model of VVC was then exploited to further dissect the innate mechanisms associated with the immunopathology of VVC.
4. Immune mediators involved in the immunopathogenesis of VVC
4.1. Identification of PMN chemotactic factors – S100A8 and S100A9 alarmins
Subsequent animal studies by Yano et al. incorporated proteomic approaches in search of a putative factor(s) responsible for the inflammatory response during the symptomatic condition [71]. First, vaginal lavage fluid from mice inoculated with C. albicans was evaluated for protein expression patterns. Based on the PMN-associated symptomatology criteria, two distinct proteins at 10 kDa and 14 kDa were identified by differential expression that positively correlated with the levels of vaginal PMNs. These proteins were further analyzed by mass spectrometry for protein identification and showed a significant match for S100A8 (10 kDa) and S100A9 (14 kDa) calcium-binding proteins. Confirming the initial protein identification, detection by western blots and ELISA showed high abundance of S100A8 and S100A9 in vaginal lavage fluid from inoculated mice with high PMNs, but were extremely low in fluids from those with low PMNs. Consistently, evaluation of vaginal tissues by immunohistochemistry showed elevated S100 protein expression localized at the apical surface of vaginal epithelium of mice with high PMNs, while the expression was dramatically reduced in tissues from those with low PMNs. This observation was further supported by increased S100 expression in vaginal epithelial cells at the mRNA levels, confirming epithelial cells as a primary source of S100 proteins produced under the symptomatic condition. Finally, neutralization of S100A8, but not S100A9, reduced the PMN chemotactic activity of vaginal lavage fluid in an in vitro migration assay, suggesting a role for S100A8 in mediating PMN migration during vaginal infection. Hence, at least in the mouse model, S100A8 appears to be produced by vaginal epithelial cells following interaction with Candida, and may play a key role as a PMN chemotactic factor that initiate the inflammatory response during symptomatic VVC.
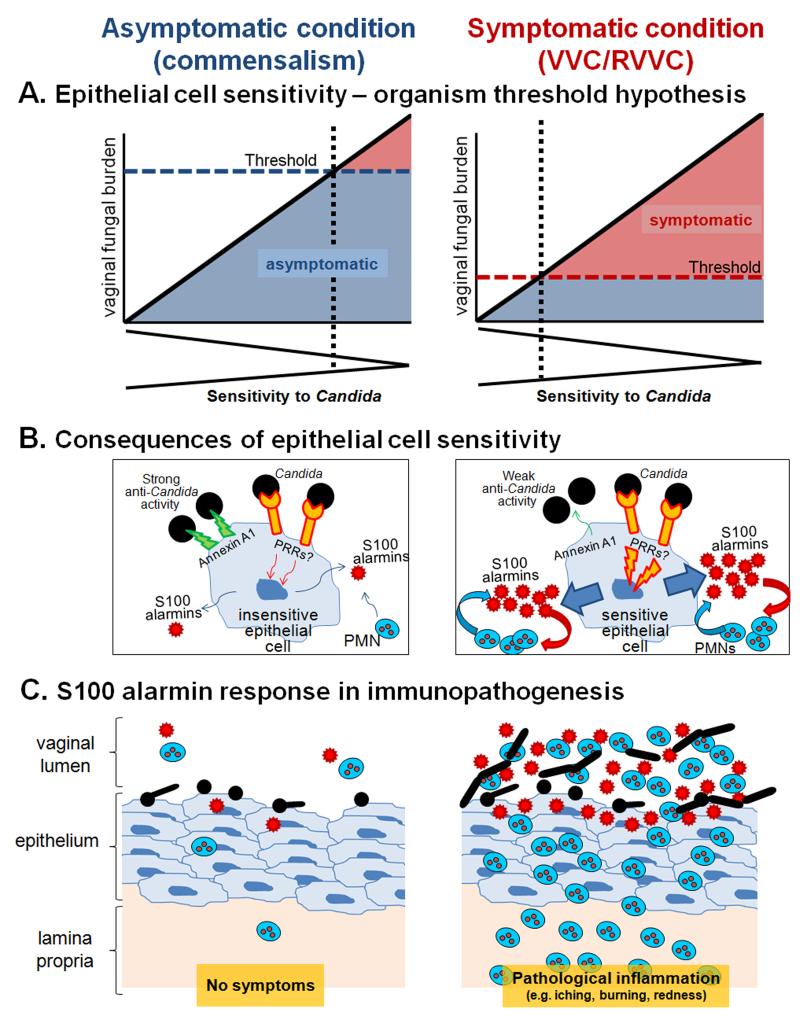
S100A8 and S100A9 are small molecular weight calcium- and zinc-binding proteins of the S100 family and are also known as calgranulin A, myeloid-related protein (MRP) 8, chemotactic protein (CP) 10, and calgranulin B, MRP-14, respectively. S100A8 and S100A9 are found as monomers or heterodimeric complex called calprotectin, which has been shown to elicit antimicrobial properties to various microbial pathogens including C. albicans [55, 79, 80]. These proteins also serve as potent chemoattractants for PMNs and participate in inflammatory processes. Due to their involvement as endogenous danger-signaling chemoattractant molecules, S100A8 and S100A9 have been termed “alarmins”, a subgroup of endogenous damage-associated molecular patterns (DAMPs) eliciting host immune responses in similar mechanisms as exogenous pathogen-associated molecular patterns (PAMPs) [81]. S100 alarmins are produced by phagocytes, monocytes, epithelial cells and endothelial cells and released at sites of inflammation [82-86]. In contrast to other mucosal sites where S100 alarmins are readily detected as calprotectin [55, 79, 80], data from the mouse study showed that S100A8 and S100A9 were mostly present as monomers in the vaginal secretions [71]. In addition, vaginal S100 alarmins exhibited no antimicrobial effect on C. albicans as evidenced by the fact that vaginal fungal burden remained unaffected by the levels of S100 alarmins detected in the vagina. However, the magnitude of PMN infiltration positively correlated with the amount of vaginal S100 alarmins [71]. Although vaginal epithelial cells appear to be a primary source of S100 alarmins during experimental VVC, PMNs also likely contribute to the S100 production once recruited into the vaginal mucosa. A proposed hypothesis is that PMNs recruited in response to the initial epithelial cell-derived S100 alarmins may initiate a second wave of S100 production, amplifying the PMN response as part of positive feedback mechanism. In addition, S100 alarmins have been implicated to stimulate innate immune cells via pattern recognition receptors such as Toll-like receptors (TLRs) and receptor for advanced glycation endproducts (RAGE), further eliciting proinflammatory responses [87]. However, specific receptors for S100A8 and S100A9 remain elusive. The possible mechanism for the effects of vaginal S100 alarmins on PMN migration during VVC is depicted in Figure 2.
Figure 2.
Schematic diagrams representing the mechanism for the effects of vaginal S100 alarmins on PMN migration during VVC. (A) Epithelial cell sensitivity – organism threshold hypothesis. In women with no history of VVC (left panel), their vaginal epithelial cells are insensitive to Candida. These women remain asymptomatic as even in the presence of high numbers for Candida (threshold number to initiate pathological response rarely breached). In women with RVVC (right panel), vaginal epithelial cells are extremely sensitive to Candida. These women are susceptible to symptomatic infection following exposure to even small numbers of Candida (low organism threshold). The thresholds represent an arbitrary organism number of the upper limit for vaginal fungal burden that would initiate symptomatic infection. (B) Consequences of epithelial cell sensitivity. Under asymptomatic condition (left panel), vaginal epithelial cells are insensitive to Candida and remain unstimulated following interaction with Candida. In turn, PMN migration does not occur in the absence of S100 alarmin production. Strong cell-surface Annexin A1-dependent (proposed based on oral epithelial cells) antifungal activity provides non-inflammatory means to maintain Candida at the commensal state. Under symptomatic conditions (right panel), vaginal epithelial cells are extremely sensitive to Candida and exert weak antifungal activity through Annexin A1. Epithelial cells become activated upon recognition of Candida via unidentified PRRs. S100 alarmins are secreted as danger signals toward which vaginal PMNs migrate through vaginal epithelium. Once in the vaginal epithelium, recruited PMNs also produce S100 alarmins as part of positive feedback mechanism to further amplify the PMN response. (C) S100 alarmin response in immunopathogenesis. PMN infiltration remains minimal in the absence of S100 alarmin production by vaginal epithelial cells, therefore, no symptom occurs (left panel). In contrast, high concentrations of S100 alarmins in vaginal epithelium trigger PMN migration to the vaginal cavity, resulting in pathological inflammation associated with the symptoms of infection. The inflammatory process enhances Candida growth and hyphal formation (right panel).
Despite their well-characterized biochemical properties, the discovery of the link between S100A8 and S100A9 alarmins and vaginal infection is relatively new, with only a few previously documented studies. One is a clinical study reported by Hashemi et al. showing cervico-vaginal fluids containing HIV+ monocytes had more immunoreactive S100A8 than those without [88]. These studies also showed that human recombinant S100A8 enhanced HIV production by a human monocytic cell line up to 40-fold compared to untreated cells. Although potential inflammatory properties of vaginal S100A8 were not evaluated in this study, the results provided primary evidence for the vaginal presence of S100A8 and S100A9 and their involvement as HIV-inducing, rather than antimicrobial factors within the vaginal tract. Another study to consider is a histological evaluation of S100A8 and S100A9 expression on epidermal keratinocytes from various types of clinical tissue specimens, including vulva and vaginal mucosa [89]. In this study, Abtin et al. demonstrated constitutive upregulation of both S100 alarmins in the upper layers of the genital epithelia, possibly due to the constant interaction with microbiota. Furthermore, the S100A8 and S100A9 protein production was shown to be induced by bacterial flagellin through a TLR5-dependent mechanism in human foreskin-derived keratinocytes. Although the authors found a lack of S100 induction by these cells when challenged with C. albicans culture supernatant, it would be interesting to test whether the S100 induction occurs by a similar mechanism in the vaginal epithelial cells and to investigate the bioactivity (i.e. antimicrobial and/or chemotactic properties) of S100A8 and S100A9 in the vaginal mucosa in humans.
4.2. Role of S100A8 and S100A9 alarmins in inflammatory diseases
As seen in the mouse model of VVC, there is an increasing body of evidence showing the association of S100A8 and S100A9 alarmins with other inflammatory conditions (Table 2). The S100 alarmins were reported to be elevated in tumor cells, particularly in skin and epidermal carcinomas with localized inflammation [90, 91]. Effects of S100 alarmins on tumor cells also include a tumor-promoting activity involving induction of cell proliferation of colonic tumor cells via NF-κB activation [92]. Additional studies showed that S100A9−/− mice (which lack both functional S100A8 and S100A9 proteins) exhibited reduced growth of lymphomas and sarcoma, while S100A8 and S100A9 were upregulated in splenocytes from tumor-bearing wild-type mice [93]. In cases of infectious diseases, data showed that S100A9−/− mice, having intact TLR4 signaling, had reduced inflammatory responses toward LPS stimulation [94]. Subsequently, addition of extracellular S100A8/S100A9 rescued the impaired phenotype, suggesting a critical role for initiation of S100 alarmin-mediated inflammation following a bacterial challenge. Moreover, a clinical study showed a clear reduction in antimicrobial activity of calprotectin (S100A8/S100A9 heterodimer) against C. albicans in HIV+OPC+ patients [95], implicating a protective role of S100 alarmins against OPC. This antifungal effect of S100A8 appears to be elicited by a Zn2+-chelating property of the molecule whereby the metal ion necessary for the fungal growth becomes depleted from the extracellular microenvironment [96]. The association of S100 alarmins with autoimmune diseases has been long known in various forms of inflammatory conditions. In rheumatoid arthritis, patients exhibit elevated S100A8 and S100A9 levels in both serum and synovial fluid [97, 98] and S100A8/S100A9-producing phagocytes were detected at sites of cartilage destruction [99]. Moreover, S100A9−/− mice displayed reduced leukocyte infiltration and joint inflammation in the experimental antigen-induced arthritis, while wild-type mice showed upregulation of S100 alarmins accompanied by severe signs of the disease [100]. In the gastrointestinal tract, accumulation of S100-positive phagocytes was observed at the site of inflamed colonic tissues from patients with Crohn’s disease and ulcerative colitis, but not in healthy mucosa of the same individuals [101-103]. In agreement with the finding in the experimental VVC study, S100 alarmins were produced and secreted by intestinal epithelial cells at the site of inflammation [104]. All the evidence described above strongly implicates the involvement of S100 alarmins in the induction of disease immunopathology as seen in tissue injury and dysfunction at the various mucosal sites. The regulation of leukocyte dynamics, and microbial growth in some cases, by S100 alarmins within mucosal tissues is quite intriguing and undoubtedly will be the subject of more intense research in several other forms of inflammatory, degenerative and malignant diseases.
Table 2.
Involvement of S100 alarmins in inflammatory diseases
| Disease | Protective | Pathological | Reference | |
|---|---|---|---|---|
| Tumors | Skin/epidermal carcinomas | √ | [90, 91] | |
| Squamous cell carcinomas | √ | √ | [107, 108] | |
| Pterygium (benign conjunctiva growth) |
√ | [116, 117] | ||
| Liver carcinomas | √ | [118] | ||
| Infections | Vaginal candidiasis | √ | [71] | |
| Oral candidiasis | √ | [95] | ||
| HIV | √ | [88, 119] | ||
| Mycobacteriosis | √ | [120] | ||
| Streptococcal pneumonia | √ | [111] | ||
| Urinary tract infection (Ureaplasma) |
√ | [121] | ||
| Autoimmunity | Psoriasis | √ | [122] | |
| Rheumatoid arthritis | √ | [97, 98] | ||
| Cystic fibrosis | √ | [123, 124] | ||
| Chronic inflammatory bowel disease |
√ | [82, 105] | ||
| Sjogren’s syndrome | √ | [125] | ||
| Systemic lupus erythematosus |
√ | [126] | ||
| Others | Atherosclerosis | √ | [127, 128] | |
| Gouty arthritis | √ | [129] | ||
| Meibomian gland disease (an eyelid cyct) |
√ | [130] |
5. Future directions and perspectives - S100A8 and S100A9 alarmins as potential target for diagnostics and therapeutic intervention
In diseases discussed above, S100 alarmins can be detected in serum and mucosal secretions and have been shown to correlate with either the presence or absence of symptomatic disease, including VVC, OPC, inflammatory bowel disease (IBD), rheumatoid arthritis, and several cancers [71, 90, 91, 95, 97, 98, 105-108]. Furthermore, the levels of S100 alarmins in cervical mucus were unaffected during different phases of menstrual cycle and have been proposed for a biomarker of inflammation in the lower female genital tract [109]. Hence, there is a great potential that vaginal S100 alarmins may serve as a diagnostic tool for early detection of an onset of symptomatic VVC or predicting disease relapse.
There is also a wide range of possibilities for S100 alarmins as novel targets for therapeutic interventions. In a clinical study on patients with sepsis, S100 alarmin levels were decreased in sera from surviving individuals during recovery, whereas nonsurvivors exhibited high S100 serum levels [110], suggesting that monitoring and controlling serum S100 alarmins may lead to improved prognosis of the disease. Interestingly, when observed at the tissue levels in a mouse model of streptococcal pneumonia, blockage of S100 alarmins by neutralizing antibodies inhibited phagocyte migration into the alveoli, but not accumulation within the lung tissue, without affecting other chemokine levels [111]. Furthermore, studies using a mouse model of IBD revealed that blockage of carboxylated glycans, putative binding partners of S100 alarmins, by specific antibodies, reduced colonic inflammation and associated tumorigenesis [112]. S100 alarmins were also shown to bind TLR4 on other surrounding cells, including PMNs themselves, and further amplify the inflammatory reaction [94, 113]. Based on the clear implication of S100 alarmins as mediators of pathological inflammation during VVC, neutralizing soluble S100 alarmins or inhibiting their secretion into the vagina could inhibit the acute PMN response and ameliorate the symptoms of infection. Thus, S100 alarmin-targeted immunotherapies may represent a novel treatment option to cure acute VVC and prevent recurrent infection without relying on the conventional antifungal regimens.
6. Conclusions
Current treatment regimens for acute VVC/RVVC are dominated by azole derivatives via topical or oral administration [2]. Although the incidence of antifungal drug resistance is rare in RVVC, the static nature of conventional antifungal therapies often leads to repeated infections caused by relapse of surviving Candida. Thus, eliciting well-balanced host responses between protective and tolerogenic immunity to Candida that works in concert is important to maintain a healthy vaginal microenvironment.
Despite more than two decades of intense research, studies are yet ongoing to answer this fundamental question: what is the underlying immunological factor(s) that allow/create susceptibility to RVVC? Current knowledge on host immune mechanisms against VVC presented herein suggest non-classical immune mediators, S100A8 and S100A9 alarmins, are critical for the immunopathological inflammation and accompanying symptoms of Candida vaginitis. However, as the role of S100 alarmins in human VVC/RVVC remains elusive, additional clinical studies are needed to gain more insight into the mechanisms of local innate immune responses in human VVC/RVVC before exploiting S100 alarmins as a target for clinical intervention.
Highlights.
Cytokines and cells of adaptive immunity do not appear to mediate protection against VVC.
Symptomatic VVC is associated with vaginal PMN influx and ensuing non-protective inflammation.
Epithelial cell-derived S100 alarmins appear to induce the PMN-associated immunopathology.
S100A8 and S100A9 alarmins may serve as novel targets for therapies to treat or prevent VVC.
Acknowledgements
This work was supported by R01 AI32556 (NIAID, National Institute of Health) and partly by Louisiana Vaccine Center and South Louisiana Institute for Infectious Disease Research sponsored by the Louisiana Board of Regents.
Abbreviations
- VVC
vulvovaginal candidiasis
- RVVC
recurrent vulvovaginal candidiasis
- Th
T helper
- PMNs
polymorphonuclear leukocytes
- OPC
oropharyngeal candidiasis
- CMC
chronic mucocutaneous candidiasis
- CMI
cell-mediated immunity
- IFN-γ
interferon-gamma
- DCs
dendritic cells
- MBL
mannose-binding lectin
- MP
mannoprotein
- SAP
secreted aspartyl proteinase
- PBMCs
peripheral blood mononuclear cells
- BD
defensin
- MRP
myeloid-related protein
- CP
chemotactic protein
- DAMP
damage-associated molecular pattern
- PAMP
pathogen-associated molecular pattern
- TLR
toll-like receptor
- RAGE
receptor for advanced glycation endproduct
- LPS
lipopolysaccharide
- IBD
inflammatory bowel disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sobel JD, Faro S, Force R, Foxman B, Ledger WJ, Nyirjesy PR, Reed BD, Summers PR. Vulvovaginal candidiasis: Epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998;178:203–11. doi: 10.1016/s0002-9378(98)80001-x. [DOI] [PubMed] [Google Scholar]
- [2].Sobel JD. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin Infect Dis. 1992;14:S148–S153. doi: 10.1093/clinids/14.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- [3].Fidel PL., Jr. Distinct protective host defenses against oral and vaginal candidiasis. Med Mycol. 2002;40:359–75. [PubMed] [Google Scholar]
- [4].Romani L. Immunity to Candida albicans: Th1, Th2 cells and beyond. Curr Opin Microbiol. 1999;2:363–7. doi: 10.1016/S1369-5274(99)80064-2. [DOI] [PubMed] [Google Scholar]
- [5].Romani L, Mencacci A, Tonnetti L, Spaccapelo R, Cenci E, Puccetti P, Wolf SF, Bistoni F. IL-12 is both required and prognostic in vivo for T-helper type 1 differentiation in murine candidiasis. J immunol. 1994;152:5167–75. [PubMed] [Google Scholar]
- [6].Mathur S, Virella G, Koistinen J, Horger EO, Mahvi TA, Fudenberg HH. Humoral immunity in vaginal candidiasis. Infect Immun. 1977;15:287–94. doi: 10.1128/iai.15.1.287-294.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kirkpatrick CH, Rich RR, Bennett JE. Chronic mucocutaneous candidiasis: Model Building in cellular immunity. Ann Intern Med. 1971;74:955–78. doi: 10.7326/0003-4819-74-6-955. [DOI] [PubMed] [Google Scholar]
- [8].Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–21. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Eyerich K, Foerster S, Rombold S, Seidl HP, Behrendt H, Hofmann H, Ring J, Traidl-Hoffmann C. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J Invest Dermatol. 2008;128:2640–5. doi: 10.1038/jid.2008.139. [DOI] [PubMed] [Google Scholar]
- [11].Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–31. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- [12].Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, Bistoni F, Puccetti P, Kastekein RA, Kopf M, Romani L. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2680–2. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- [13].Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, Jacobs L, Jansen T, Verheijen K, Masthoff L, Morré SA, Vriend G, Williams DL, Perfect JR, Joosten LA, Wijmenga C, van der Meer JW, Adema GJ, Kullberg BJ, Brown GD, Netea MG. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1798–801. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lev-Sagie A, Nyirjesy P, Tarangelo N, Bongiovanni AM, Bayer C, Linhares IM, Giraldo PC, Ledger WJ, Witkin SS. Hyaluronan in vaginal secretions: association with recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2009;201:206.e1–206.e5. doi: 10.1016/j.ajog.2009.05.010. [DOI] [PubMed] [Google Scholar]
- [15].Klein RS, Harris CA, Small CB, Moll B, Lesser M, Friedland GH. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984;311:354–7. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- [16].Clift RA. Candidiasis in the transplant patient. Amer J Med. 1984;77(suppl 4D):34–8. [PubMed] [Google Scholar]
- [17].Samaranayake LP. Oral mycoses in HIV infection. Oral Surg Oral Med Oral Pathol. 1992;73:171–80. doi: 10.1016/0030-4220(92)90191-r. [DOI] [PubMed] [Google Scholar]
- [18].Romani L, Puccetti P, Bistoni F. Biological role of Th cell subsets in candidiasis. In: Romagnani S, editor. Th1 and Th2 cells in health and disease. Karger; Farmington, CT: 1996. pp. 114–37. [Google Scholar]
- [19].Mathews HL, Witek-Janusek L. Host defense against oral, esophageal, and gastrointestinal candidiasis. In: Carderone RA, editor. Candida and Candidiasis. ASM Press; Washington, DC: 2002. pp. 179–92. [Google Scholar]
- [20].Sohnle PG, Bhatti M, Wagner DK. Immunology of cutaneous candidiasis. In: Calderone R, editor. Candida and Candidiasis. ASM Press; Washington, DC: 2002. pp. 211–21. [Google Scholar]
- [21].Fidel PL, Jr., Sobel JD. The role of cell-mediated immunity in candidiasis. Trends Micro. 1994;2:202–6. doi: 10.1016/0966-842x(94)90112-i. [DOI] [PubMed] [Google Scholar]
- [22].Fong IW, McCleary P, Read S. Cellular immunity of patients with recurrent or refractory vulvovaginal moniliasis. Am J Obstet Gynecol. 1992;166:887–90. doi: 10.1016/0002-9378(92)91356-f. [DOI] [PubMed] [Google Scholar]
- [23].Hobbs JR, Briden D, Davidson F, Kahan M, Oates JK. Immunological aspects of candidal vaginitis. Proc R Soc Med. 1977;70:11–4. [PMC free article] [PubMed] [Google Scholar]
- [24].Mendling W, Koldovsky U. Investigations by cell-mediated immunologic tests and therapeutic trials with thymopentin in vaginal mycoses. Infect Dis Obstet Gynecol. 1996;4:225–31. doi: 10.1155/S1064744996000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cu-Uvin S, Hogan JW, Warren D, Klein RS, Peipert J, Schuman P, Holmberg S, Anderson J, Schoenbaum E, Vlahov D, Mayer KH. Prevalence of Lower Genital Tract infections Among Human Immunodeficiency Virus (HIV)-Serpositive and High-Risk HIV-Seronegative Women. Clin Infect Dis. 1999;29:1145–50. doi: 10.1086/313434. [DOI] [PubMed] [Google Scholar]
- [26].White MH. Is vulvovaginal candidiasis an AIDS-related illness. Clin Infect Dis. 1996;22(Suppl 2):S124–S127. doi: 10.1093/clinids/22.supplement_2.s124. [DOI] [PubMed] [Google Scholar]
- [27].Leigh JE, Barousse M, Swoboda RK, Myers T, Hager S, Wolf NA, Cutright JL, Thompson J, Sobel JD, Fidel PL., Jr. Candida-specific systemic cell-mediated immune reactivities in HIV-infected persons with and without mucosal candidiaisis. J Infect Dis. 2001;183:277–85. doi: 10.1086/317944. [DOI] [PubMed] [Google Scholar]
- [28].Fidel PL, Jr., Luo W, Steele C, Chabain J, Baker M, Wormley FL. Analysis of vaginal cell populations during experimental vaginal candidiasis. Infect Immun. 1999;67:3135–40. doi: 10.1128/iai.67.6.3135-3140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Taylor BN, Saavedra M, Fidel PL., Jr. Local Th1/Th2 cytokine production during experimental vaginal candidiasis. Med Mycol. 2000;38:419–31. doi: 10.1080/mmy.38.6.419.431. [DOI] [PubMed] [Google Scholar]
- [30].Saavedra M, Taylor B, Lukacs NW, Fidel PL., Jr. Local production of chemokines during experimental vaginal candidiasis. Infect Immun. 1999;67:5820–9. doi: 10.1128/iai.67.11.5820-5826.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fidel PL, Jr., Wolf NA, KuKuruga MA. T lymphocytes in the murine vaginal mucosa are phenotypically distinct from those in the periphery. Infect Immun. 1996;64:3793–9. doi: 10.1128/iai.64.9.3793-3799.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nandi D, Allison JP. Phenotypic analysis and gamma/delta-T cell receptor repertoire of murine T cells associated with the vaginal epithelium. J Immunol. 1991;147:1773–8. [PubMed] [Google Scholar]
- [33].Ibraghimov AR, Sacco RE, Sandor M, Iakoubov Z, Lynch RG. Resident CD4+ ab T cells of the murine female genital tract: a phenotypically distinct T cell lineage that rapidly proliferates in response to systemic T cell activation stimuli. Int immunol. 1995;7:1763–9. doi: 10.1093/intimm/7.11.1763. [DOI] [PubMed] [Google Scholar]
- [34].Barousse M, Van Der Pol BJ, Fortenberry D, Orr D, Fidel PL., Jr. Vaginal yeast colonization, prevalence of vaginitis, and associated local immunity in adolescents. Sex Trans Infect. 2004;80:48–53. doi: 10.1136/sti.2002.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fidel PL, Jr., Barousse M, Espinosa T, Chesson RR, Dunlap K. Local immune responsiveness following intravaginal challenge with Candida antigen in adult women at different stages of the menstrual cycle. Med Mycol. 2003;41:97–109. doi: 10.1080/mmy.41.2.97.109. [DOI] [PubMed] [Google Scholar]
- [36].Santoni G, Boccanera M, Adriani D, Lucciarini R, Amantini C, Morrone S, Cassone A, De Bernardis F. Immune cell-mediated protection against vaginal candidiasis: Evidence for a major role of vaginal CD4(+) T cells and possible participation of other local lymphocyte effectors. Infect Immun. 2002;70:4791–7. doi: 10.1128/IAI.70.9.4791-4797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].De Bernardis F, Santoni G, Boccanera M, Spreghini E, Adriani D, Morelli L, Cassone A. Local anticandidal immune responses in a rat model of vaginal infection by and protection against Candida albicans. Infect Immun. 2000;68:3297–304. doi: 10.1128/iai.68.6.3297-3304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schaller M, Korting HC, Borelli C, Hamm G, Hube B. Candida albicans-secreted aspartic proteinases modify the epithelial cytokine response in an in vitro model of vaginal candidiasis. Infect Immun. 2005;73:2758–65. doi: 10.1128/IAI.73.5.2758-2765.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wormley FL, Jr, Steele C, Wozniak K, Fujihashi K, McGhee JR, Fidel PL., Jr. Resistance of TCR d-chain knock-out mice to experimental Candida vaginitis. Infect Immun. 2001;69:7162–4. doi: 10.1128/IAI.69.11.7162-7164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wormley FL, Jr., Chaiban J, Fidel PL., Jr. Cell adhesion molecule and lymphocyte activation marker expression during experimental vaginal candidiasis. Infect Immun. 2001;69:5072–9. doi: 10.1128/IAI.69.8.5072-5079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].LeBlanc DM, Barousse MM, Fidel PL., Jr. A role for dendritic cells in immunoregulation during experimental vaginal candidiasis. Infect Immun. 2006;74:3213–21. doi: 10.1128/IAI.01824-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Babula O, Lazdane G, Kroica J, Linhares IM, Ledger WJ, Witkin SS. Frequency of interleukin-4 (IL-4) -589 gene polymorphism and vaginal concentrations of IL-4, nitric oxide, and mannose-binding lectin in women with recurrent vulvovaginal candidiasis. Clin Infect Dis. 2005;40:1258–62. doi: 10.1086/429246. [DOI] [PubMed] [Google Scholar]
- [43].Chen S, Li S, Wu Y, Liu Z, Li J. Local expression of vaginal Th1 and Th2 cytokines in murine vaginal candidiasis under different immunity conditions. J Huazhong Univ Sci Technolog Med Sci. 2008;28:476–9. doi: 10.1007/s11596-008-0423-z. [DOI] [PubMed] [Google Scholar]
- [44].De Bernardis F, Santoni G, Boccanera M, Lucciarini R, Arancia S, Sandini S, Amantini C, Cassone A. Protection against rat vaginal candidiasis by adoptive transfer of vaginal B lymphocytes. FEMS Yeast Res. 2010;10:432–40. doi: 10.1111/j.1567-1364.2010.00620.x. [DOI] [PubMed] [Google Scholar]
- [45].De Bernardis F, Boccanera M, Adriani D, Spreghini E, Santoni G, Cassone A. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect Immun. 1997;65:3399–405. doi: 10.1128/iai.65.8.3399-3405.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Han Y, Morrison RP, Cutler JE. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect Immun. 1998;66:5771–6. doi: 10.1128/iai.66.12.5771-5776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Casadevall A. Antibody immunity and invasive fungal infections. Infect Immun. 1995;63:4211–8. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rigg D, Miller MM, Metzger WJ. Recurrent allergic vulvovaginitis: Treatment with Candida albicans allergen immunotherapy. Am J Obstet Gynecol. 1990;162:332–6. doi: 10.1016/0002-9378(90)90380-p. [DOI] [PubMed] [Google Scholar]
- [49].Witkin SS. Immunologic factors influencing susceptibility to recurrent candidal vaginitis. Clin Obstet Gynecol. 1991;34:662–8. [PubMed] [Google Scholar]
- [50].Witkin SS, Kalo-Klein A, Galland L, Teich M, Ledger WJ. Effect of Candida albicans plus histamine on prostaglandin E2 production by peripheral blood mononuclear cells from healthy women and women with recurrent candidal vaginitis. J Infect Dis. 1991;164:396–9. doi: 10.1093/infdis/164.2.396. [DOI] [PubMed] [Google Scholar]
- [51].Fan SR, Liao QP, Liu XP, Liu ZH, Zhang D. Vaginal allergic response in women with vulvovaginal candidiasis. Int J Gynaecol Obstet. 2008;101:27–30. doi: 10.1016/j.ijgo.2007.08.024. [DOI] [PubMed] [Google Scholar]
- [52].Fidel PL, Jr., Ginsburg KA, Cutright JL, Wolf NA, Leaman D, Dunlap, Sobel JD. Vaginal-associated immunity in women with recurrent vulvovaginal candidiasis: Evidence for vaginal Th1-type responses following intravaginal challenge with Candida antigen. J Infect Dis. 1997;176:728–39. doi: 10.1086/514097. [DOI] [PubMed] [Google Scholar]
- [53].Conti HR, Baker O, Freeman AF, Jang WS, Holland SM, Li RA, Edgerton M, Gaffen SL. New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal Immunol. 2011;4:448–55. doi: 10.1038/mi.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kolls JK, McCray PB, Jr, Chan YR. Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol. 8:829–35. doi: 10.1038/nri2433. 8 A.D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Urban CF, Ermert DSM, Abu-Abed U, Goosman C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Feinen B, Jerse AE, Gaffen SL, Russel MW. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol. 2010;3:312–21. doi: 10.1038/mi.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Scurlock AM, Frazer LC, Andrews CW, Jr, O’Connell CM, Foote IP, Bailey SL, Chandra-Kuntal K, Kolls JK, Darville T. Interleukin-17 contributes to generation of Th1 immunity and neutrophil recruitment during Chlamydia muridarum genital tract infection but is not required for macrophage influx or normal resolution of infection. Infect Immun. 2011;79:1349–62. doi: 10.1128/IAI.00984-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Pietrella D, Rachini A, Pines M, Pandey N, Mosci P, Bistoni F, d’Enfert C, Vecchiarelli A. Th17 cells and IL-17 in protective immunity to vaginal candidiasis. PLoS ONE. 2011;6:e22770. doi: 10.1371/journal.pone.0022770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Black CA, Eyers FM, Russell A, Dunkley ML, Clancy RL, Beagley KW. Acute neutropenia decreases inflammation associated with murine vaginal candidiasis but has no effect on the course of infection. Infect Immun. 1998;66:1273–5. doi: 10.1128/iai.66.3.1273-1275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Parr MB, Parr EL. Langerhans cells and T lymphocyte subsets in the murine vagina and cervix. Biol Reprod. 1991;44:491–8. doi: 10.1095/biolreprod44.3.491. [DOI] [PubMed] [Google Scholar]
- [61].Steele C, Ozenci H, Luo W, Scott M, Fidel PL., Jr. Growth inhibition of Candida albicans by vaginal cells from naive mice. Med Mycol. 1999;37:251–60. [PubMed] [Google Scholar]
- [62].Steele C, Ratterree M, Fidel PL., Jr. Differential susceptibility to experimental vaginal candidiasis in macaques. J Infect Dis. 1999;180:802–10. doi: 10.1086/314964. [DOI] [PubMed] [Google Scholar]
- [63].Barousse MM, Steele C, Dunlap K, Espinosa T, Boikov D, Sobel JD, Fidel PL., Jr. Growth Inhibition of Candida albicans by human vaginal epithelial cells. J Infect Dis. 2001;184:1489–93. doi: 10.1086/324532. [DOI] [PubMed] [Google Scholar]
- [64].Steele C, Leigh JE, Swoboda RK, Fidel PL., Jr. Growth inhibition of Candida by human oral epithelial cells. J Infect Dis. 2000;182:1479–85. doi: 10.1086/315872. [DOI] [PubMed] [Google Scholar]
- [65].Yano J, Lilly E, Steele C, Fortenberry D, Fidel PL., Jr. Oral and vaginal epithelial cell anti-Candida activity is acid-labile and does not require live epithelial cells. Oral Microbiol Immunol. 2005;20:199–205. doi: 10.1111/j.1399-302X.2005.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lilly EA, Yano J, Fidel PL., Jr. Annexin-A1 identified as the oral epithelial cell anti-Candida effector moiety. Mol Oral Microbiol. 2010;25:293–304. doi: 10.1111/j.2041-1014.2010.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Barousse MM, Espinosa T, Dunlap K, Fidel PL., Jr. Vaginal epithelial cell anti-Candida albicans activity is associated with protection against symptomatic vaginal candidiasis. Infect Immun. 2005;73:7765–7. doi: 10.1128/IAI.73.11.7765-7767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Vylkova S, Nayyar N, Li W, Edgerton M. Human beta-defensins kill Candida albicans in an energy-dependent and salt-sensitive manner without causing membrane disruption. Antimicrob Agents Chemother. 2007;51:154–61. doi: 10.1128/AAC.00478-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Peters BM, Zhu J, Fidel PL, Jr, Scheper MA, Hackett W, El Shaye S, Jabra-Rizk MA. Protection of the oral mucosa by salivary histatin-5 against Candida albicans in an ex vivo murine model of oral infection. FEMS Yeast Res. 2010;10:597–604. doi: 10.1111/j.1567-1364.2010.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Fidel PL, Jr., Barousse M, Espinosa T, Ficarra M, Sturtevant J, Martin DH, Quayle AJ, Dunlap K. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun. 2004;72:2939–46. doi: 10.1128/IAI.72.5.2939-2946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yano J, Lilly E, Barousse M, Fidel PL., Jr. Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect Immun. 2010;78:5126–37. doi: 10.1128/IAI.00388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Fidel PL., Jr. History and Update on Host Defense Against Vaginal Candidiasis. Am J Reprod Immunol. 2007;57:2–12. doi: 10.1111/j.1600-0897.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- [73].Black CA, Eyers FM, Dunkley ML, Clancy RL, Beagley KW. Major histocompatibility haplotype does not impact the course of experimentally induced murine vaginal candidiasis. Lab Anim Sci. 1999;49:668–72. [PubMed] [Google Scholar]
- [74].Clemons KV, Spearow JL, Parmar R, Espiritu M, Stevens DA. Genetic susceptibility of mice to Candida albicans vaginitis correlates with host estrogen sensitivity. Infect Immun. 2004;72:4878–80. doi: 10.1128/IAI.72.8.4878-4880.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Calderon L, William R, Martinez M, Clemons KV, Stevens DA. Genetic susceptibility to vaginal candidiasis. Med Mycol. 2003;41:143–7. doi: 10.1080/mmy.41.2.143.147. [DOI] [PubMed] [Google Scholar]
- [76].Fidel PL, Jr., Cutright JL, Steele C. Effects of Reproductive hormones on experimental vaginal candidiasis. Infect Immun. 2000;68:651–7. doi: 10.1128/iai.68.2.651-657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Fidel PL, Jr., Sobel JD. Murine models of Candida vaginal infections. In: Zak O, Sande M, editors. Experimental models in antimicrobial chemotherapy. Academic Press Ltd; London, UK: 1999. pp. 741–8. [Google Scholar]
- [78].Fidel PL, Jr., Cutright JL, Tait L, Sobel JD. A murine model of Candida glabrata vaginitis. J Infect Dis. 1996;173:425–31. doi: 10.1093/infdis/173.2.425. [DOI] [PubMed] [Google Scholar]
- [79].Sohnle PG, Hahn BL, Santhanagopalan V. Inhibition of Candida albicans growth by calprotectin in the absence of direct contact with the organisms. J Infect Dis. 1996;174:1369–72. doi: 10.1093/infdis/174.6.1369. [DOI] [PubMed] [Google Scholar]
- [80].Zimmer DB, Cornwall EH, Landar A, Song W. The S100 protein family: history, function, and expression. Brain Res Bull. 1995;37:417–29. doi: 10.1016/0361-9230(95)00040-2. [DOI] [PubMed] [Google Scholar]
- [81].Ehrchen JM, Sunderkötter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 2009;86:557–66. doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- [82].Foell D, Wittkowski H, Ren Z, Turton J, Pang G, Daebritz J, Ehrchen J, Heidemann J, Borody T, Roth J, Clancy R. Phagocyte-specific S100 proteins are released from affected mucosa and promote immune responses during inflammatory bowel disease. J Pathol. 2008;216:183–92. doi: 10.1002/path.2394. [DOI] [PubMed] [Google Scholar]
- [83].Gebhardt C, Németh J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol. 2006;72:1622–31. doi: 10.1016/j.bcp.2006.05.017. [DOI] [PubMed] [Google Scholar]
- [84].Kumar RK, Yang Z, Bilson S, Thliveris S, Cooke BE, Geczy CL. Dimeric S100A8 in human neutrophils is diminished after phagocytosis. J Leukoc Biol. 2001;70:59–64. [PubMed] [Google Scholar]
- [85].Ross KF, Herzberg MC. Calprotectin expression by gingival epithelial cells. Infect Immun. 2001;69:3248–54. doi: 10.1128/IAI.69.5.3248-3254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 2003;170:3233–42. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- [87].Sims GP, Rowe DC, Rietdijik ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–88. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- [88].Hashemi FB, Mollenhauer J, Madsen LD, Sha BE, Nacken W, Moyer MB, Sorg C, Spear GT. Myeloid-related protein (MRP)-8 from cervico-vaginal secretions activates HIV replication. AIDS. 2001;15:441–9. doi: 10.1097/00002030-200103090-00002. [DOI] [PubMed] [Google Scholar]
- [89].Abtin A, Eckhart L, Gläser R, Gmeiner R, Mildner M, Tschachler E. The antimicrobial heterodimer S100A8/S100A9 (calprotectin) is upregulated by bacterial flagellin in human epidermal keratinocytes. J Invest Dermatol. 2010;130:2423–30. doi: 10.1038/jid.2010.158. [DOI] [PubMed] [Google Scholar]
- [90].Gebhardt C, Breitenbach U, Tuckermann JP, Dittrich BT, Richter KH, Angel P. Calgranulins S100A8 and S100A9 are negatively regulated by glucocorticoids in a c-Fos-dependent manner and overexpressed throughout skin carcinogenesis. Oncogene. 2002;21:4266–76. doi: 10.1038/sj.onc.1205521. [DOI] [PubMed] [Google Scholar]
- [91].Hummerich L, Muller R, Hess J, Kokocinski F, Hahn M, Furstenberger G, Mauch C, Lichter P, Angel P. Identification of novel tumour-associated genes differentially expressed in the process of squamous cell cancer development. Oncogene. 2006;25:111–21. doi: 10.1038/sj.onc.1209016. [DOI] [PubMed] [Google Scholar]
- [92].Turovskaya O, Foell D, Sinha P, Vogl T, Newlin R, Nayak J, Nguyen M, Olsson A, Nawroth PP, Bierhaus A, Varki N, Kronenberg M, Freeze HH, Srikrishna G. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis. 2008;29:2035–43. doi: 10.1093/carcin/bgn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Cheng P, Crozo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–49. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. MRP8 and MRP14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–9. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- [95].Brandtzaeg P, Gabrielsen TO, Dale I, Muller F, Steinbakk M, Fagerhol MK. The leucocyte protein L1 (calprotectin): a putative nonspecific defence factor at epithelial surfaces. Adv Exp Med Bio. 1995;371:201–6. doi: 10.1007/978-1-4615-1941-6_41. [DOI] [PubMed] [Google Scholar]
- [96].Sohnle PG, Hunter MJ, Hahn B, Chazin WJ. Zinc-reversible antimicrobial activity of recombinant calprotectin (migration inhibitory factor-related proteins 8 and 14) J Infect Dis. 2000;182:1272–5. doi: 10.1086/315810. [DOI] [PubMed] [Google Scholar]
- [97].Chen YS, Yan W, Geczy CL, Brown MA, Thomas R. Serum levels of soluble receptor for advanced glycation end products and of S100 proteins are associated with inflammatory, autoantibody, and classical risk markers of joint and vascular damage in rheumatoid arthritis. Arthritis Res Ther. 2009;11:R39. doi: 10.1186/ar2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Hammer HB, Odegard S, Fagerhol MK, Landewé R, van der Heijde D, Uhlig T, Mowinckel P, Kvien TK. Calprotectin (a major leukocyte protein) is strongly and independently correlated with joint inflammation and damage in rheumatoid arthritis. Ann Rheum Dis. 2007;66:1093–7. doi: 10.1136/ard.2006.064741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Youssef P, Roth J, Frosch M, Costello P, Fitzgerald O, Sorg C, Bresnihan B. Expression of myeloid related proteins (MRP) 8 and 14 and the MRP8/14 heterodimer in rheumatoid arthritis synovial membrane. J Rheumatol. 1999;26:2523–8. [PubMed] [Google Scholar]
- [100].van Lent PL, Grevers L, Blom AB, Sloetjes A, Mort JS, Vogl T, Nacken W, van den Berg WB, Roth J. Myeloid-related proteins S100A8/S100A9 regulate joint inflammation and cartilage destruction during antigen-induced arthritis. Ann Rheum Dis. 2008;67:1750–8. doi: 10.1136/ard.2007.077800. [DOI] [PubMed] [Google Scholar]
- [101].Rugtveit J, Nilsen EM, Bakka A, Carlsen H, Brandtzaeg P, Scott H. Cytokine profiles differ in newly recruited and resident subsets of mucosal macrophages from inflammatory bowel disease. Gastroenter. 1997;112:1493–505. doi: 10.1016/s0016-5085(97)70030-1. [DOI] [PubMed] [Google Scholar]
- [102].Sarsfield P, Jones DB, Wright DH. Accessory cells in physiological lymphoid tissue from the intestine: an immunohistochemical study. Histopathology. 1996;28:213–9. doi: 10.1046/j.1365-2559.1996.d01-417.x. [DOI] [PubMed] [Google Scholar]
- [103].Waraich T, Sarsfield P, Wright DH. The accessory cell populations in ulcerative colitis: a comparison between the colon and appendix in colitis and acute appendicitis. Hum Pathol. 1997;28:297–303. doi: 10.1016/s0046-8177(97)90127-1. [DOI] [PubMed] [Google Scholar]
- [104].Roth J, Vogl T, Sorg C, Sunderkötter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. TRENDS Immunol. 2003;24:155–8. doi: 10.1016/s1471-4906(03)00062-0. [DOI] [PubMed] [Google Scholar]
- [105].Leach ST, Yang Z, Messina I, Sorg C, Geczy CL, Cunningham AM, Day AS. Serum and mucosal S100 proteins, calprotectin (S100A8/S100A9) and S100A12, are elevated at diagnosis in children with inflammatory bowel disease. Scand J Gastroenterol. 2007;42:1321–31. doi: 10.1080/00365520701416709. [DOI] [PubMed] [Google Scholar]
- [106].Fagerberg UL, Lööf L, Lindholm J, Hansson LO, Finkel Y. Fecal calprotectin: a quantitative marker of colonic inflammation in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;45:414–20. doi: 10.1097/MPG.0b013e31810e75a9. [DOI] [PubMed] [Google Scholar]
- [107].Qin F, Song Y, Li Z, Zhao Y, Zhang Y, Geng L. S100A8/A9 induces apoptosis and inhibits metastasis of CasKi human cervical cancer cells. Pathol Oncol Res. 2010;16:353–60. doi: 10.1007/s12253-009-9225-2. [DOI] [PubMed] [Google Scholar]
- [108].Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–75. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- [109].Kunimi K, Maegawa M, Kamada M, Yamamoto S, Yasui T, Matsuzaki T, Kuwahara A, Furumoto H, Ohmoto Y, Kido H, Irahara M. Myeloid-related protein-8/14 is associated with proinflammatory cytokines in cervical mucus. J Reprod Immunol. 2006;71:3–11. doi: 10.1016/j.jri.2005.12.010. [DOI] [PubMed] [Google Scholar]
- [110].Payen D, Lukaszewicz AC, Belikova I, Faivre V, Gelin C, Russwurm S, Launay JM, Sevenet N. Gene profiling in human blood leukocytes during recovery from septic shock. Intensive Care Med. 2008;34:1371–6. doi: 10.1007/s00134-008-1048-1. [DOI] [PubMed] [Google Scholar]
- [111].Raquil MA, Anceriz N, Rouleau P, Tessier PA. Blockage of antimicrobial proteins S100A8 and S100A9 inhibits phagocyte migration to the alveoli in streptococcal pneumonia. J Immunol. 2008;180:3366–74. doi: 10.4049/jimmunol.180.5.3366. [DOI] [PubMed] [Google Scholar]
- [112].Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived supressor cells. J Immunol. 2008;181:4666–75. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Frosch M, Ahlmann M, Vogl T, Wittkowski H, Wulffraat N, Foell, Roth J. The myeloid-related proteins 8 and 14 complex, a novel ligand of toll-like receptor 4, and interleukin-1beta form a positive feedback mechanism in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2009;60:883–91. doi: 10.1002/art.24349. [DOI] [PubMed] [Google Scholar]
- [114].De Bernardis F, Lucciarini R, Boccanera M, Amantini C, Arancia S, Morrone S, Mosca M, Cassone A, Santoni G. Phenotypic and functional characterization of vaginal cells in a rat model of Candida albicans vaginitis. Infect Immun. 2006;74:4282–94. doi: 10.1128/IAI.01714-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Weissenbacher TM, Witkin SS, Gingelmaier A, Scholz C, Friese K, Mylonas I. Relationship between recurrent vulvovaginal candidosis and immune mediators in vaginal fluid. Eur J Obstet Gynecol Reprod Biol. 2009;144:59–63. doi: 10.1016/j.ejogrb.2009.01.010. [DOI] [PubMed] [Google Scholar]
- [116].Riau AK, Wong TT, Beuerman RW, Tong L. Calcium-binding S100 protein expression in pterygium. Mol Vis. 2009;15:335–42. [PMC free article] [PubMed] [Google Scholar]
- [117].Zhou L, Beuerman RW, Ang LP, Chan CM, Li SF, Chew FT, Tan DT. Elevation of human alpha-defensins and S100 calcium-binding proteins A8 and A9 in tear fluid of patients with pterygium. Invest Ophthalmol Vis Sci. 2009;50:2077–86. doi: 10.1167/iovs.08-2604. [DOI] [PubMed] [Google Scholar]
- [118].Németh J, Stein I, Haag D, Riehl A, Longerich T, Horwitz E, Breuhahn K, Gebhardt C, Schirmacher P, Hahn M, Ben-Neriah Y, Pikarsky E, Angel P, Hess J. S100A8 and S100A9 are novel nuclear factor kappa B target genes during malignant progression of murine and human liver carcinogenesis. Hepatology. 2009;50:1251–62. doi: 10.1002/hep.23099. [DOI] [PubMed] [Google Scholar]
- [119].Ryckman C, Robichaud GA, Roy J, Cantin R, Tremblay MJ, Tessier PA. HIV-1 transcrition and virus production are both accentuated by the proinflammatory myeloid-related proteins in human CD4+ T lymphocytes. J Immunol. 2002;169:3307–13. doi: 10.4049/jimmunol.169.6.3307. [DOI] [PubMed] [Google Scholar]
- [120].Sunderkötter CH, Tomimori-Yamashita J, Nix V, Maeda SM, Sindrilaru A, Mariano M, Sorg C, Roth J. High expression of myeloid-related proteins 8 and 14 characterizes an inflammatorily active but ineffective response of macrophages during leprosy. Immunology. 2004;111:472–80. doi: 10.1111/j.0019-2805.2004.01836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Reyes L, Alvarez S, Allam A, Reinhard M, Brown MB. Complicated urinary tract infection is associated with uroepithelial expression of proinflammatory protein S100A8. Infect Immun. 2009;77:4265–74. doi: 10.1128/IAI.00458-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Benoit S, Toksoy A, Ahlmann M, Schmidt M, Sunderkötter C, Foell D, Pasparakis M, Roth J, Goebeler M. Elevated serum levels of calcium-binding S100 proteins A8 and A9 reflect disease activity and abnormal differentiation of keratinocytes in psoriasis. Br J Dermatol. 2006;155:62–6. doi: 10.1111/j.1365-2133.2006.07198.x. [DOI] [PubMed] [Google Scholar]
- [123].Lorenz E, Muhlebach MS, Tessier PA, Alexis NE, Hite R Duncan, Seeds MC, Peden DB, Meredith W. Different expression ratio of S100A8/A9 and S100A12 in acute and chronic lung diseases. Respir Med. 2008;102:567–73. doi: 10.1016/j.rmed.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].McMorran BJ, Patat SA, Carlin JB, Grimwood K, Jones A, Armstrong DS, Galati JC, Cooper PJ, Byrnes CA, Francis PW, Robertson CF, Hume DA, Borchers CH, Wainwright CE, Wainwright BJ. Novel neutrophil-derived proteins in bronchoalveolar lavage fluid indicate an exaggerated inflammatory response in pediatric cystic fibrosis. Clin Chem. 2007;53:1782–91. doi: 10.1373/clinchem.2007.087650. [DOI] [PubMed] [Google Scholar]
- [125].Sweet SP, Denbury AN, Challacombe SJ. Salivary calprotectin levels are raised in patients with oral candidiasis or Sjogrens syndrome but decreased by HIV infection. Oral Microbiol Immunol. 2001;16:119–23. doi: 10.1034/j.1399-302x.2001.016002119.x. [DOI] [PubMed] [Google Scholar]
- [126].Lood C, Stenström M, Tydén H, Gullstrand B, Källberg E, Leanderson T, Truedsson L, Sturfelt G, Ivars F, Bengtsson AA. Protein synthesis of the proinflammatory S100A8/A9 complex in plasmacytoid dendritic cells and cell surface S100A8/A9 on leukocyte subpopulations in systemic lupus erythematosus. Arthritis Res Ther. 2011;13:R60. doi: 10.1186/ar3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].McCormick A, Rahimi F, Bobryshev YV, Gaus K, Zreigat H, Cai H, Lord RS, Geczy CL. S100A8 and S100A9 in human arterial wall. Implications for atherogenesis. J Biol Chem. 2005;280:41521–9. doi: 10.1074/jbc.M509442200. [DOI] [PubMed] [Google Scholar]
- [128].Croce K, Gao H, Wang Y, Mooroka T, Sakuma M, Shi C, Sukhova GK, Packard RR, Hogg N, Libby P, Simon DI. Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation. 2009;120:427–36. doi: 10.1161/CIRCULATIONAHA.108.814582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Ryckman C, McColl SR, Vandal K, de Médicis R, Lussier A, Poubelle PE, Tessier PA. Role of S100A8 and S100A9 in neutrophil recruitment in response to monosodium urate monohydrate crystals in the air-pouch model of acute gouty arthritis. Arthritis Rheum. 2003;48:2310–20. doi: 10.1002/art.11079. [DOI] [PubMed] [Google Scholar]
- [130].Tong L, Zhou L, Beuerman RW, Zhao SZ, Li XR. Association of tear proteins with Meibomian gland disease and dry eye symptoms. Br J Ophthalmol. 2011;95:848–52. doi: 10.1136/bjo.2010.185256. [DOI] [PubMed] [Google Scholar]