Abstract
The renin-angiotensin system is an essential regulatory system for blood pressure and fluid homeostasis. Angiotensinogen is the only known precursor of all the peptides generated in this system. While many of the basic understandings of angiotensinogen have come from research efforts to define its role in blood pressure regulation, novel pathophysiological functions of angiotensinogen have been discovered in the last two decades including kidney developmental abnormalities, atherosclerosis, and obesity. Despite the impressive advance in the understanding of angiotensinogen gene structure and protein functions, some fundamental questions remain unanswered. In this short review, we provide contemporary insights into the molecular characteristics of angiotensinogen and its pathophysiological features. In light of the recent progress, we emphasize some newly recognized functional features of angiotensinogen other than its regulation on blood pressure.
Keywords: angiotensinogen, gene, protein, atherosclerosis, obesity
Introduction
Angiotensinogen (AGT) is a glycoprotein that is the unique substrate of the rennin-angiotensin system. Through sequential cleavages by either the classic enzymatic pathway, renin and angiotensin-converting enzyme (ACE), or alternative pathways, AGT gives rise to a spectrum of angiotensin peptides, with angiotensin (Ang) II being the major effector peptide that regulates blood pressure and sodium/water homeostasis. Many components of the renin-angiotensin system including renin, ACE, as well as AngII and its receptors are well characterized and have been comprehensively studied in animal models. By contrast, many attributes of AGT has received less attention. This may be partially due to the traditional view of AGT as a passive substrate and lack of pharmacological inhibitors that directly target the protein. The development of many state-of-the-art techniques including the successful creation of the cell-specific deficient mice is providing new insights into this substrate of the renin-angiotensin system. This review briefly introduces the current knowledge regarding the molecular and pathophysiological features of AGT and explores the similarities between humans and mice since the latter are considered the most convenient animal model to understand the roles of AGT in many human diseases. Specifically, we will highlight atherosclerosis and obesity.
Molecular Characteristics of AGT
Gene Structure of AGT
The AGT gene in humans and rodents was cloned, mapped and characterized throughout the 1980s.[1–7] It is well conserved among vertebrates and has homologs in invertebrates such as fish.[8] Human AGT gene is a single-copy gene, locating within 20 Mb to the end of the long arm (1q42.2) on chromosome 1. It contains 5 exons and 4 introns, which spans 12,063 bp (nucleotide 230,838,274 - 230,850,336) on chromosome 1 and encodes 485 amino acids. The first exon contains 500 bp of the 5’-untranslated region. The second exon codes the 33-amino-acid signal peptide and more than half of the mature protein. Exon 5 encompasses the C-terminus of the protein as well as over 600 bp of the 3’-untranslated region.
The AGT gene in mice is similar to the human gene in terms of genomic size, gene structure, and coding exons (encoding 482 amino acids versus 485 amino acids in humans). It is located on chromosome 8 and close to the chromosome end, within only 4.6 Mb to the telomere which makes genetic manipulations of this gene in mice an arduous task.
Protein Characteristics of AGT
Human AGT is a heterogeneous plasma glycoprotein, mainly synthesized in hepatocytes. After removal of the 33-amino-acid signal peptide, the 452-amino-acid mature protein with the first 10 amino acids corresponding to AngI is secreted into plasma or extracellular compartments. The heterogeneity of plasma AGT is primarily due to variable glycosylation.[9] Human AGT protein contains four putative sites for N-linked glycosylation (Asn-X-Ser/Thr): Asn14, Asn137, Asn271 and Asn295. In vitro site-mutagenesis study demonstrates that all four sites can be glycosylated, with preference at Asn14 and Asn271.[9] Asn14 is close to the renin cleavage site (Leu10-Val11), and its glycosylation has been demonstrated to lower the affinity of AGT for renin.[9] Asn14 glycosylation is also present in mice, but not in rats. These glycosylation sites appear to have no critical roles on folding, intracellular trafficking, or secretion of the AGT protein.[9]
Human AGT protein contains four cysteines, two of them forming Cys18-Cys138 linkage that are conserved in all species.[9,10] The crystal structure of human AGT shows that the formation of Cys18-Cys138 disulphide bridge confers a conformational change that allows access of renin to its cleavage site of AGT. It has been demonstrated that oxidative status of AGT has an impact on the rate of AGT-renin reaction.[11] It is unknown whether this disulphide bridge has similar properties in mouse AGT.
Another noteworthy feature is the species specificity of AGT-renin reaction. For example, human AGT cannot be cleaved by mouse renin.[12,13] While this may be influenced by an amino acid substitution at the renin cleavage site, Leu10-Val11 bond in humans versus Leu10-Leu11 bond in mice,[13] the mechanism of this unique feature has not been completely unraveled.
The cleavage of intact AGT by renin leads to the generation of both AngI and des(AngI)-AGT, the remaining residues after the removal of AngI that accounts for more than 95% of the AGT protein sequence and maintains a typical serpin folding. The relative abundance of intact versus des(AngI) form has not been characterized. There is some evidence that des(AngI)-AGT itself has biological properties that may relate to the serpin characteristics of the protein.[14,15]
Regulation of AGT
AGT gene expression is under developmental and hormonal controls in a cell type-specific manner.[16] It is generally accepted that the predominant regulation of AGT occurs at the transcriptional level, although some post-transcriptional regulation also exists.[17] AngII has been consistently shown to enhance mRNA stability of AGT and exert positive feedback on the AGT protein production.[17–21] AngII upregulates mRNA abundance of AGT in hepatocytes through nuclear factor-kappaB activation,[22] and increases plasma AGT protein via action of signal transducer and activator of transcription 3 upon inducing interleukin-6.[21,23] In our laboratory, we have consistently observed over 2-fold increase of plasma AGT concentrations in mice with exogenous AngII infusion (unpublished data). The positive feedback of AngII on AGT is balanced by its negative feedback on renin, the rate-limiting enzyme in the synthesis of angiotensin peptides.
Multiple putative cis-acting DNA regulatory elements, including glucocorticoids, estrogen, and acute phase responsive elements, are located within a region of 1 kb that is immediate upstream of human AGT gene.[2,6] Dexamethasone administration leads to striking increases of AGT mRNA abundance in liver and modest increases in brain.[24,25] However, increases of the AGT protein are much less than the mRNA increases.[25] Estrogen is another positive regulator of AGT synthesis. Plasma AGT concentrations increase in parallel with estrogen during pregnancy. Synthetic estrogen in oral contraceptive pills also increases plasma AGT concentrations in a dose-dependent manner.[25] In contrast to the direct interactions of glucocorticoids and estrogen on the 5′-region of the AGT gene through their corresponding receptors, thyroid hormones seem to affect AGT mRNA abundance dependent on a secondary gene or protein, since their effects can be blocked by cycloheximide, an inhibitor of protein synthesis.[25] In addition, for all these 3 hormonal regulations, there are many confounding factors, such as a malignantly high dose above the physiological concentrations, a certain approach of administration, or interactions with a secondary gene or protein that complicate the interpretation of the reported findings.[25] Therefore, their roles as primary regulators of AGT require further examination.
Tissue and Cellular Distribution of AGT
AGT is promptly secreted from cells into extracellular compartments.[26] Therefore, the distribution of AGT synthesis is more commonly determined by mRNA rather than protein abundance. AGT mRNA has been consistently detected in many tissues such as liver, adipose, brain, heart, kidneys, and vessels.[27–30] It has also been identified in spinal cord, lungs, adrenal glands, large intestine, stomach, spleen and ovaries with low or variable abundances.[24,30] While most AGT mRNA is found in adult liver, it may be mainly present in adipose tissues, brain, and kidneys at embryonic stage. AGT production rises remarkably after birth and reaches adult level within 24 hours.[31]
At a cellular level, besides hepatocytes, it is widely accepted that adipocytes, proximal tubule epithelial cells, and astrocytes are AGT synthesizing cells.[29,32,33] Among all extra-hepatic tissues synthesizing AGT, only adipose tissue has been show to have an impact on plasma AGT concentrations in adipocyte-specific AGT deficient mice.[34] On the other hand, liver-specific deletion of a floxed human AGT transgene using adenoviral delivery of Cre recombinase largely diminishes plasma human AGT concentrations.[35] It infers that circulating AGT cannot be compensated by extra-hepatic tissues in the absence of hepatocyte-derived AGT. However, a hepatocyte-specific AGT deficient mouse model is needed for a definite conclusion.
Enzymes using AGT as a Substrate
While AGT is the only known substrate of all angiotensin peptides of the rennin-angiotensin system, there are many enzymes that have been identified to use AGT as a substrate.[36–39] Renin, a plasma aspartyl protease, is best known for cleaving AGT into AngI. Indeed, AGT is the only defined substrate for renin.[40] The rate-limiting AGT-renin reaction and AGT being the unique renin substrate make renin the most effective target to inhibit the renin-angiotensin cascade.[41] There is compelling evidence that renal renin is secreted into plasma to cleave AGT released from liver.[37,42–44] Therefore, this is considered a systemic pathway to generate angiotensin peptides. There are controversial findings regarding the local production of renin outside kidneys. While some studies have reported that kidney-derived renin is the only source to catalyze AGT locally,[44,45] there is growing evidence that renin can also be synthesized in tissues other than kidneys or non-juxtaglomerular cells.[46–49] In addition to renin, many other enzymes such as cathepsin D, cathepsin G, kallikrein, pepsin, tissue-plasminogen activator, tonin, and trypsin have been demonstrated to convert AGT into either AngI or AngII.[38,39,50,51] These enzymes are abundant in tissues or some cell types, which has led to speculation that these enzymes may have impacts on the local generation of angiotensin peptides. However, most enzymes require an acidic environment (pH range 4–7) to actively catalyze AGT.[39] Currently, the physiological significance of these enzymes using AGT as a substrate in vivo has not been established.
Plasma Concentrations of AGT
Plasma AGT concentrations in humans are approximately 1 μM or 60 μg/ml (range 28 - 71 μg/ml).[52] This is close to the Michaelis-Menton constant (KM) of renin (1.25 μM).[53] This indicates both AGT and renin concentrations are important for the rate of AngII generation, thus control the tonic activity of the renin-angiotensin system. Mice have much lower plasma AGT concentrations (≈ 20 - 30 nM or 1216 ± 101 ng/ml in C57BL/6 strain).[54] Its implications are not clear that plasma AGT concentrations differ by over one order of magnitude between humans and rodents.
There is no standard method for measuring AGT protein concentration. Two approaches, indirect enzymatic assay and direct radioimmunoassay or ELISA, are currently used by many investigators. Indirect assay only measures intact AGT through equivalent AngI released from the cleavage of AGT by renin in a given amount of time. Direct assay measures total AGT, which consists of both intact AGT and des(AngI)-AGT. Recently, a simple and sensitive sandwich ELISA kit has been developed to measure AGT concentrations in both humans and rodents.[52,54] Unfortunately, this kit does not distinguish the intact AGT from des(AngI)-AGT, which is important when changes in plasma concentrations of intact AGT are not correlated with that of total AGT. For instance, neither sodium depletion nor pharmacological inhibition of the rennin-angiotensin system affects plasma total AGT concentrations, although both intact AGT concentrations and des(AngI)-AGT have significant changes in opposite directions.[55,56]
Plasma and Tissue Catabolism of AGT
The cleavage of AGT by renin is well known. However, there is a relative paucity of information of plasma clearance of either the intact or des(AngI) form of AGT. Using radioiodinated tracer studies of AGT, the half-life of the protein has been estimated to be ~5 hours in rats and rabbits.[57–59] It is unknown whether the intact and the cleaved forms have similar rates of clearance from plasma.
There is also spare evidence of tissues responsible for the catabolism of AGT. A single line of evidence displays that the protein is predominantly accumulated in kidney.[59] However, this study was performed with directly radioiodinated proteins in which the label may not accumulate at the loci of catabolism. More meaningful analysis may be obtained by the conjugation of radioiodinated residualizing labels to track tissue sites of AGT catabolism.[60]
Pathophysiological Features of AGT
Genetic Manipulations of AGT in Mice
It has been reported that mice overexpressing either rat[61,62] or human[63,64] AGT alone do not exhibit any significant phenotype. In contrast, mice carrying both human AGT and renin genes display pronounced phenotypes including increased blood pressure, cardiac hypertrophy, and kidney abnormalities,[63,64] providing evidence for the species specificity of AGT-renin reaction.
AGT deficient mice have been developed by two laboratories.[65,66] Besides expected profound reductions in blood pressure, AGT deficient mice also have renal and cardiac dysfunctions. However, pathologies in these two organs are distinct between transgenic AGT overexpressing mice and AGT deficient mice. While mice with human AGT and renin transgenes develop nephrosclerosis and cardiac hypertrophy,[67] AGT deficient mice exhibit hydronephrosis and dilated cardiomyopathy.[68,69] These distinct pathologies strongly indicate that tight regulation of AGT production is important to maintain normal blood pressure as well as normal renal and cardiac structures and functions.
Characteristics of mice with genetic manipulations of AGT are summarized in Table 1. It is speculated that the phenotypic changes in these mouse models are directly related to the changes of AngII production since the phenotypes in AGT deficient mice are also observed equivalently in renin, ACE, and combined AT1a and AT1b receptor deficient mice.[70–73] However, exogenous AngII or tissue-specific enhancement of AngII cannot fully recover the phenotypes in AGT deficient mice,[74–76] indicating that AGT may have both AngII-dependent and AngII-independent functions. In addition, phenotypes in AGT deficient mice can only be partially rescued by restoring AGT in circulation or one to multiple tissues,[75–80] supporting a theme that this glycoprotein produced in both systemic and cell-specific manners synergistically contribute to its pathophysiological functions. Recently, an AGT floxed mouse model has been reported, which will permit the determination of cell-specific deficiencies of AGT on many pathophysiological processes.[34]
Table 1.
Characteristics of mice with genetic manipulations of AGT
| Manipulation | Strategy | Pathophysiological Features | References | |||
|---|---|---|---|---|---|---|
| BP | kidney | heart | Others | |||
| rat Agt overexpression | rat Agt transgene under the control of the mouse metallothionein I promoter |
↔ | ND | ND | ND | [61] |
| rat Agt transgene under the control of the rat Agt promoter |
↑ | nephro- sclerosis |
hyper- trophy |
ND | [62,76] | |
| human Agt overexpression |
human Agt transgene under the control of the human Agt promoter |
↔ | ↔ | ↔ | ↔ | [63] |
| rat Agt and Ren overexpression |
breeding of rat Agt transgenic mice with rat Ren transgenic mice |
↑ | ND | ND | ND | [61] |
| human Agt and Ren overexpression |
breeding of human Agt transgenic mice with human Ren transgenic mice |
↑ | nephro- sclerosis |
hyper- trophy |
↓ body weight | [64,94] |
| whole body Agt deficiency (Agt −/−) |
insertion of a neo cassette to the exon 2 prior to the start codon of the mouse Agt |
↓ | hydro- nephrosis |
cardio- myopathy |
↓ body weight, fat mass & locomoter activity |
[65,66] |
| rat Agt overexpression in adipocytes |
rat Agt transgene under the control of aP2 promoter in wild type mice |
↑ | ND | ND | ↑ body weight & fat mass, ↓ energy expenditure |
[79] |
| rat Agt adipocyte- specific expression |
rat Agt transgene under the control of aP2 promoter in Agt −/− mice |
↔ | ↔ | ND | ||
|
Agt adipocyte-specific deficiency |
breeding of Agt floxed mice with trangenic mice expressing Cre recombinase under the control of aP2 promoter |
↓ in aged mice |
ND | ND | ↔ body weight & fat mass |
[34] |
Notes: Agt = angiotensinogen gene; Ren = renin gene; BP = blood pressure; ↔ = no change; ↑ = increase; ↓ = decrease; ND = not determined. The pathophysiological changes were determined in comparison with their relative wild type littermates
Genetic Manipulations of AGT in Mouse Atherosclerosis and Obesity
There is compelling evidence that the renin-angiotensin system plays a critical role in the development of atherosclerosis.[81,82] While many mouse atherosclerosis studies have directly targeted AngII type 1 receptors or either of the two critical enzymes (renin and ACE),[49,56,83–87] no study has addressed the role of AGT in atherosclerosis. We have detected AGT protein in mouse atherosclerotic lesions using immunostaining.[85] One study has reported that mice with human AGT and human renin transgenes in the C57BL/6 background had augmented atherosclerosis, compared to the wild type controls, when fed a high-fat diet for 14 weeks.[88] There is no study that has determined the role of AGT deficiency in mouse atherosclerosis. This is possibly due to their low neonatal survival rate and severe phenotypes that hamper the breeding of AGT deficiency mice to hypercholesterolemic apolipoprotein E or low density lipoprotein receptor deficient mice.[68]
Potential associations of AGT with obesity and adipocyte metabolism have long been noticed in animal models and in vitro studies. AGT mRNA abundance is increased in adipose tissues of obese rodents.[89–91] Whole body deficiency of AGT leads to a lean phenotype that is manifested with impaired high fat diet-induced body weight gain, reduced fat mass, and increased locomotor activity in mice.[92,93] However, these mice have severe phenotypes that strongly impact the normal development and growth.[64] The unhealthy condition may confound the interpretation of the observed metabolic phenotypes. In comparison with AGT deficient mice, mice with human AGT and renin transgenes also exhibit reduced body weight gain compared to their wild type controls, although these mice have increased tissue and plasma AGT abundance.[94] The paradoxical observations require systematic research on the effects of this gene in obesity.
Recently AGT floxed mouse model has been developed to probe the effects of cell-specific AGT deficiency in many pathophysiological states.[34] These mice have normal neonatal survival rate and display no gross kidney abnormalities. Using Cre recombinase trangenic approach under the control of a specific promoter, this mouse model will provide an optimal approach to understanding the relationship between AGT and many pathophysiological features as well as the underlying mechanisms in a cell-specific mode.
Genetic Association of AGT with Atherosclerosis and Obesity in Humans
The correlation of AGT gene with human diseases has been investigated primarily through screening its single nucleotide polymorphisms (SNPs). The first compelling genetic evidence of M235T polymorphorism implicating a causal relationship between the AGT gene and essential hypertension in humans was published in 1992.[95] Since this initial report, M235T has become the most frequently studied SNP in AGT for hypertension. It has also been investigated frequently in patients with atherosclerotic diseases. While a few studies failed to define an association of M235T with atherosclerosis,[96] most studies demonstrated that this polymorphism was associated with atherosclerosis in different populations.[97–100] In addition to M235T, a few studies have also reported that T174M, another polymorphism in exon 2 of the AGT gene, is related with risk factors or prevalence of coronary artery disease.[101,102]
Although there is no direct evidence, animal studies and in vitro experiments indicate that AGT may also play an important role in obesity in humans. Investigations of the common AGT polymorphisms have shown a significant association of M235T with obesity in female hypertensive patients in different populations,[103–105] although it failed to display any correlation in males.[106]
Currently, genetic polymorphism analyses of the AGT gene are a cardinal approach to defining an association of this gene with human diseases. Many studies have reported potential links between SNPs in AGT and atherosclerosis or obesity, although their causal relationships remain to be established. For both atherosclerosis and obesity, the reported associations are either modest or only significant in a specific disease state, either of the two genders, or certain age group within one study. Confounding factors including small sample size, population heterogeneity, environmental and culture/ethic differences, multiple disease states, and complex interactions within the gene or with many other genes, may complicate the interpretation of these polymorphism studies. Therefore, it is important to control the confounding factors in order to define a causal link between the AGT gene and atherosclerosis or obesity.
Conclusions and Perspectives
The unique position of AGT in the renin-angiotensin system and its distinct features may make this protein become an attractive target in developing effective therapeutic strategies in many human diseases such as atherosclerosis and obesity. However, despite great efforts on discovering the molecular mechanisms and clinical relevance of AGT gene and protein functions, some fundamental questions remain unanswered. These include: (1) Are the effects of AGT on its pathophysiological phenotypes, in part or in whole, dependent on AngII? (2) What is the correlation of angiotensinogen produced in local tissues with the circulating pool? And what is the relative contribution of local versus circulating angiotensinogen to its pathophysiological functions? (3) What is the fate of des(AngI)-AGT? Is it degraded rapidly after the release of AngI, or is it biologically functional, independent of the classic rennin-angiotensin system? (4) As demonstrated by the crystal structure of AGT, Cys18-Cys138 linkage determines the accessibility of its cleavage site to renin. Is the modulation of this disulphide bond relevant to the development or progress of its pathophysiological functions? These unanswered questions provide both challenges and opportunities to explore the mechanisms and effects of AGT in human diseases. The current advancement of many state-of-the-art techniques, including reconstructing AGT protein and the availability of cell-specific AGT deficient mice, provides excellent tools to answer these questions.
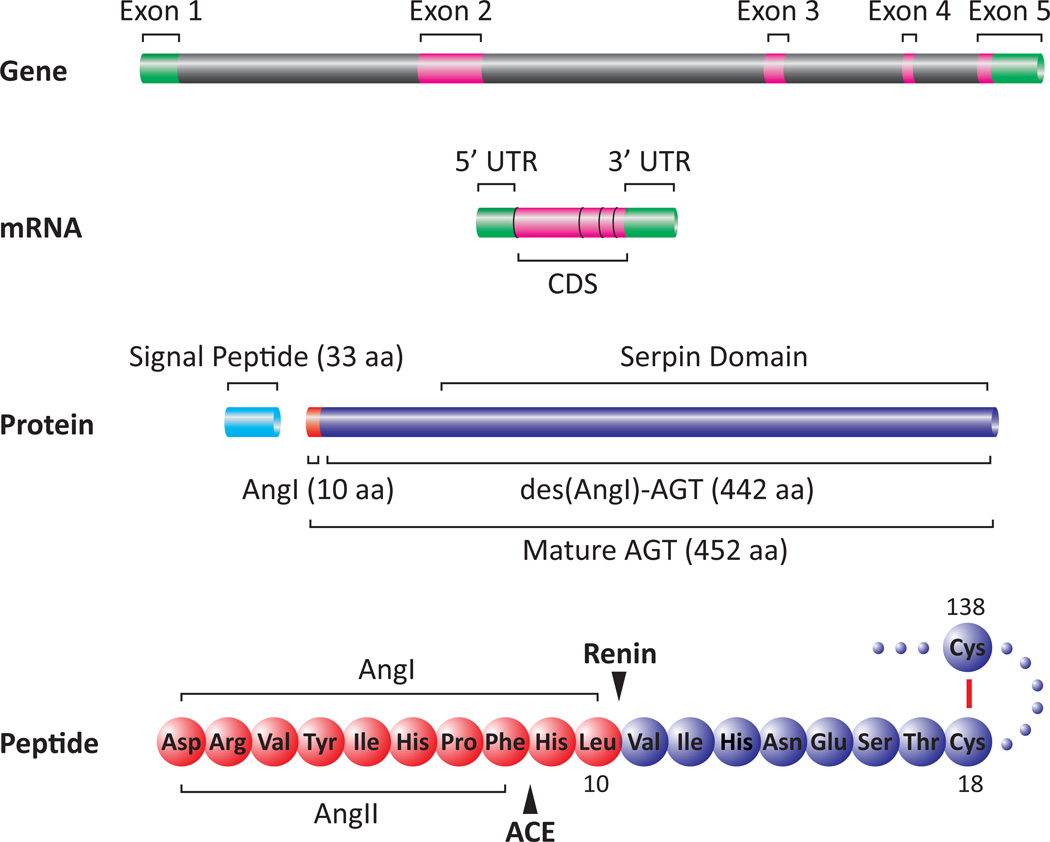
Figure 1. Schematic Representation of Human AGT Gene and Protein.
Human angiotensinogen (AGT) gene contains 5 exons. Exon 2 encodes the majority of the protein. After the removal of a 33 aa signal peptide, the 452 aa mature AGT is secreted. Cleavage of mature AGT by renin gives a decapeptide, AngI, and the 442 aa des(AngI)-AGT. AngI is further cleaved by angiotensin-converting enzyme (ACE) into an octapeptide, AngII. Two cysteines at 18 and 138 form a disulphide bridge (Cys18-Cys138) that confers a conformational change allowing access of renin to the AngI cleavage site of AGT. Diagrams were drawn proportional to actual gene and protein size based on the University of California Santa Cruz Human Genome Browser Feb 2009 Assembly. UTR: untranslated region; CDS: coding sequences; ACE: angiotensin-converting enzyme.
Acknowledgments
The authors’ research work is supported by grants from the National Institutes of Health (HL062846 and HL80100).
References
- 1.Ohkubo H, Kageyama R, Ujihara M, et al. Cloning and sequence analysis of cDNA for rat angiotensinogen. Proc Natl Acad Sci U S A. 1983;80:2196–2200. doi: 10.1073/pnas.80.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaillard I, Clauser E, Corvol P. Structure of human angiotensinogen gene. DNA. 1989;8:87–99. doi: 10.1089/dna.1.1989.8.87. [DOI] [PubMed] [Google Scholar]
- 3.Clouston WM, Evans BA, Haralambidis J, Richards RI. Molecular cloning of the mouse angiotensinogen gene. Genomics. 1988;2:240–248. doi: 10.1016/0888-7543(88)90008-0. [DOI] [PubMed] [Google Scholar]
- 4.Mori M, Ishizaki K, Yamada T, et al. Restriction fragment length polymorphisms of the angiotensinogen gene in inbred rat strains and mapping of the gene on chromosome 19q. Cytogenet Cell Genet. 1989;50:42–45. doi: 10.1159/000132716. [DOI] [PubMed] [Google Scholar]
- 5.Clouston WM, Fournier RE, Richards RI. The angiotensinogen gene is located on mouse chromosome 8. FEBS Lett. 1989;255:419–422. doi: 10.1016/0014-5793(89)81136-6. [DOI] [PubMed] [Google Scholar]
- 6.Fukamizu A, Takahashi S, Seo MS, et al. Structure and expression of the human angiotensinogen gene. Identification of a unique and highly active promoter. J Biol Chem. 1990;265:7576–7582. [PubMed] [Google Scholar]
- 7.Isa MN, Boyd E, Morrison N, et al. Assignment of the human angiotensinogen gene to chromosome 1q42-q43 by nonisotopic in situ hybridization. Genomics. 1990;8:598–600. doi: 10.1016/0888-7543(90)90053-w. [DOI] [PubMed] [Google Scholar]
- 8.Takei Y, Joss JM, Kloas W, Rankin JC. Identification of angiotensin I in several vertebrate species: its structural and functional evolution. Gen Comp Endocrinol. 2004;135:286–292. doi: 10.1016/j.ygcen.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Gimenez-Roqueplo AP, Celerier J, Lucarelli G, Corvol P, Jeunemaitre X. Role of N-glycosylation in human angiotensinogen. J Biol Chem. 1998;273:21232–21238. doi: 10.1074/jbc.273.33.21232. [DOI] [PubMed] [Google Scholar]
- 10.Streatfeild-James RM, Williamson D, Pike RN, et al. Angiotensinogen cleavage by renin: importance of a structurally constrained N-terminus. FEBS Lett. 1998;436:267–270. doi: 10.1016/s0014-5793(98)01145-4. [DOI] [PubMed] [Google Scholar]
- 11.Zhou A, Carrell RW, Murphy MP, et al. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468:108–111. doi: 10.1038/nature09505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Printz MP, Printz JM, Dworschack RT. Human angiotensinogen. Purification partial characterization, and a comparison with animal prohormones. J Biol Chem. 1977;252:1654–1662. [PubMed] [Google Scholar]
- 13.Hatae T, Takimoto E, Murakami K, Fukamizu A. Comparative studies on species-specific reactivity between renin and angiotensinogen. Mol Cell Biochem. 1994;131:43–47. doi: 10.1007/BF01075723. [DOI] [PubMed] [Google Scholar]
- 14.Celerier J, Cruz A, Lamande N, Gasc JM, Corvol P. Angiotensinogen and its cleaved derivatives inhibit angiogenesis. Hypertension. 2002;39:224–228. doi: 10.1161/hy0202.103441. [DOI] [PubMed] [Google Scholar]
- 15.Vincent F, Bonnin P, Clemessy M, et al. Angiotensinogen delays angiogenesis and tumor growth of hepatocarcinoma in transgenic mice. Cancer Res. 2009;69:2853–2860. doi: 10.1158/0008-5472.CAN-08-2484. [DOI] [PubMed] [Google Scholar]
- 16.Nibu Y, Takahashi S, Tanimoto K, Murakami K, Fukamizu A. Identification of cell type-dependent enhancer core element located in the 3'-downstream region of the human angiotensinogen gene. J Biol Chem. 1994;269:28598–28605. [PubMed] [Google Scholar]
- 17.Klett C, Hellmann W, Muller F, et al. Angiotensin II controls angiotensinogen secretion at a pretranslational level. J Hypertens Suppl. 1988;6:S442–S445. doi: 10.1097/00004872-198812040-00139. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura A, Iwao H, Fukui K, et al. Regulation of liver angiotensinogen and kidney renin mRNA levels by angiotensin II. Am J Physiol. 1990;258:E1–E6. doi: 10.1152/ajpendo.1990.258.1.E1. [DOI] [PubMed] [Google Scholar]
- 19.Jamaluddin M, Meng T, Sun J, et al. Angiotensin II induces nuclear factor (NF)-kappaB1 isoforms to bind the angiotensinogen gene acute-phase response element: a stimulus-specific pathway for NF-kappaB activation. Mol Endocrinol. 2000;14:99–113. doi: 10.1210/mend.14.1.0400. [DOI] [PubMed] [Google Scholar]
- 20.Kobori H, Ozawa Y, Satou R, et al. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol. 2007;293:F938–F945. doi: 10.1152/ajprenal.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Villalobos RA, Seth DM, Satou R, et al. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am J Physiol Renal Physiol. 2008;295:F772–F779. doi: 10.1152/ajprenal.00019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brasier AR, Jamaluddin M, Han Y, Patterson C, Runge MS. Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-kappaB (NF-kappaB) transcription factor. Mol Cell Biochem. 2000;212:155–169. [PubMed] [Google Scholar]
- 23.Jain S, Li Y, Patil S, Kumar A. HNF-1alpha plays an important role in IL-6-induced expression of the human angiotensinogen gene. Am J Physiol Cell Physiol. 2007;293:C401–C410. doi: 10.1152/ajpcell.00433.2006. [DOI] [PubMed] [Google Scholar]
- 24.Campbell DJ, Habener JF. Angiotensinogen gene is expressed and differentially regulated in multiple tissues of the rat. J Clin Invest. 1986;78:31–39. doi: 10.1172/JCI112566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deschepper CF. Angiotensinogen: hormonal regulation and relative importance in the generation of angiotensin II. Kidney Int. 1994;46:1561–1563. doi: 10.1038/ki.1994.446. [DOI] [PubMed] [Google Scholar]
- 26.Lynch KR, Peach MJ. Molecular biology of angiotensinogen. Hypertension. 1991;17:263–269. doi: 10.1161/01.hyp.17.3.263. [DOI] [PubMed] [Google Scholar]
- 27.Cassis LA, Lynch KR, Peach MJ. Localization of angiotensinogen messenger RNA in rat aorta. Circ Res. 1988;62:1259–1262. doi: 10.1161/01.res.62.6.1259. [DOI] [PubMed] [Google Scholar]
- 28.Cassis LA, Saye J, Peach MJ. Location and regulation of rat angiotensinogen messenger RNA. Hypertension. 1988;11:591–596. doi: 10.1161/01.hyp.11.6.591. [DOI] [PubMed] [Google Scholar]
- 29.Saye JA, Cassis LA, Sturgill TW, Lynch KR, Peach MJ. Angiotensinogen gene expression in 3T3-L1 cells. Am J Physiol. 1989;256:C448–C451. doi: 10.1152/ajpcell.1989.256.2.C448. [DOI] [PubMed] [Google Scholar]
- 30.Phillips MI, Speakman EA, Kimura B. Levels of angiotensin and molecular biology of the tissue renin angiotensin systems. Regul. Pept. 1993;43:1–20. doi: 10.1016/0167-0115(93)90403-u. [DOI] [PubMed] [Google Scholar]
- 31.Gomez RA, Cassis L, Lynch KR, et al. Fetal expression of the angiotensinogen gene. Endocrinology. 1988;123:2298–2302. doi: 10.1210/endo-123-5-2298. [DOI] [PubMed] [Google Scholar]
- 32.Satou R, Gonzalez-Villalobos RA, Miyata K, et al. Costimulation with angiotensin II and interleukin 6 augments angiotensinogen expression in cultured human renal proximal tubular cells. Am J Physiol Renal Physiol. 2008;295:F283–F289. doi: 10.1152/ajprenal.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stornetta RL, Hawelu-Johnson CL, Guyenet PG, Lynch KR. Astrocytes synthesize angiotensinogen in brain. Science. 1988;242:1444–1446. doi: 10.1126/science.3201232. [DOI] [PubMed] [Google Scholar]
- 34.Batifoulier-Yiannikouris F, Karounos M, Charnigo R, English VL, Rateri DL, Daugherty A, Cassis LA. Adipocyte-specific deficiency of angiotensinogen decreases plasma angiotensinogen concentration and systolic blood pressure in mice. 2011 doi: 10.1152/ajpregu.00323.2011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stec DE, Davisson RL, Haskell RE, Davidson BL, Sigmund CD. Efficient liver-specific deletion of a floxed human angiotensinogen transgene by adenoviral delivery of Cre recombinase in vivo. J Biol Chem. 1999;274:21285–21290. doi: 10.1074/jbc.274.30.21285. [DOI] [PubMed] [Google Scholar]
- 36.Campbell DJ, Bouhnik J, Coezy E, et al. Characterization of precursor and secreted forms of rat angiotensinogen. Endocrinology. 1984;114:776–785. doi: 10.1210/endo-114-3-776. [DOI] [PubMed] [Google Scholar]
- 37.Campbell DJ. Circulating and tissue angiotensin systems. J Clin Invest. 1987;79:1–6. doi: 10.1172/JCI112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dzau VJ. Multiple pathways of angiotensin production in the blood vessel wall: evidence, possibilities and hypotheses. J Hypertens. 1989;7:933–936. doi: 10.1097/00004872-198912000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Belova LA. Angiotensin II-generating enzymes. Biochemistry (Mosc) 2000;65:1337–1345. doi: 10.1023/a:1002848402911. [DOI] [PubMed] [Google Scholar]
- 40.Bouhnik J, Clauser E, Strosberg D, et al. Rat angiotensinogen and des(angiotensin I)angiotensinogen: purification, characterization, and partial sequencing. Biochemistry. 1981;20:7010–7015. doi: 10.1021/bi00527a036. [DOI] [PubMed] [Google Scholar]
- 41.Azizi M, Webb R, Nussberger J, Hollenberg NK. Renin inhibition with aliskiren: where are we now, and where are we going? J Hypertens. 2006;24:243–256. doi: 10.1097/01.hjh.0000202812.72341.99. [DOI] [PubMed] [Google Scholar]
- 42.Beaty O, 3rd, Sloop CH, Schmid HE, Jr, Buckalew VM., Jr Renin response and angiotensinogen control during graded hemorrhage and shock in the dog. Am J Physiol. 1976;231:1300–1307. doi: 10.1152/ajplegacy.1976.231.4.1300. [DOI] [PubMed] [Google Scholar]
- 43.Eggena P, Barrett JD. Renin substrate release in response to perturbations of renin-angiotensin system. Am J Physiol. 1988;254:E389–E393. doi: 10.1152/ajpendo.1988.254.4.E389. [DOI] [PubMed] [Google Scholar]
- 44.Danser AH. Local renin-angiotensin systems: the unanswered questions. Int J Biochem Cell Biol. 2003;35:759–778. doi: 10.1016/s1357-2725(02)00178-4. [DOI] [PubMed] [Google Scholar]
- 45.Katz SA, Opsahl JA, Lunzer MM, Forbis LM, Hirsch AT. Effect of bilateral nephrectomy on active renin, angiotensinogen, and renin glycoforms in plasma and myocardium. 97;30(2 Pt 1):259–266. doi: 10.1161/01.hyp.30.2.259. [DOI] [PubMed] [Google Scholar]
- 46.Karlsson C, Lindell K, Ottosson M, et al. Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II. J Clin Endocrinol Metab. 1998;83:3925–3929. doi: 10.1210/jcem.83.11.5276. [DOI] [PubMed] [Google Scholar]
- 47.Okamura A, Rakugi H, Ohishi M, et al. Upregulation of renin-angiotensin system during differentiation of monocytes to macrophages. J Hyperten. 1999;17:537–545. doi: 10.1097/00004872-199917040-00012. [DOI] [PubMed] [Google Scholar]
- 48.Sun Y, Zhang J, Zhang JQ, Weber KT. Renin expression at sites of repair in the infarcted rat heart. J Mol Cell Cardiol. 2001;33:995–1003. doi: 10.1006/jmcc.2001.1365. [DOI] [PubMed] [Google Scholar]
- 49.Lu H, Rateri DL, Feldman DL, et al. Renin inhibition reduces hypercholesterolemia-induced atherosclerosis in mice. J Clin Invest. 2008;118:984–993. doi: 10.1172/JCI32970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arakawa K, Yuki M, Ikeda M. Chemical identity of tryptensin with angiotensin. Biochem J. 1980;187:647–653. doi: 10.1042/bj1870647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katz SA, Opsahl JA, Forbis LM. Myocardial enzymatic activity of renin and cathepsin D before and after bilateral nephrectomy. Basic Res Cardiol. 2001;96:659–668. doi: 10.1007/s003950170019. [DOI] [PubMed] [Google Scholar]
- 52.Katsurada A, Hagiwara Y, Miyashita K, et al. Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol. 2007;293:F956–F960. doi: 10.1152/ajprenal.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cumin F, Le-Nguyen D, Castro B, Menard J, Corvol P. Comparative enzymatic studies of human renin acting on pure natural or synthetic substrates. Biochim Biophys Acta. 1987;913:10–19. doi: 10.1016/0167-4838(87)90226-3. [DOI] [PubMed] [Google Scholar]
- 54.Kobori H, Katsurada A, Miyata K, et al. Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am J Physiol Renal Physiol. 2008;294:F1257–F1263. doi: 10.1152/ajprenal.00588.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clauser E, Bouhnik J, Gonzalez MF, Corvol P, Menard J. Influence of converting-enzyme inhibition on rat des-angiotensin I-angiotensinogen. Am J Physiol. 1984;246:E129–E133. doi: 10.1152/ajpendo.1984.246.2.E129. [DOI] [PubMed] [Google Scholar]
- 56.Lu H, Balakrishnan A, Howatt DA, et al. Comparative effects of different modes of renin angiotensin system inhibition on hypercholesterolaemia-induced atherosclerosis. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01712.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewicki JA, Printz JM, Printz MP. Clearance of rabbit plasma angiotensinogen and relationship to CSF angiotensinogen. Am J Physiol. 1983;244:H577–H585. doi: 10.1152/ajpheart.1983.244.4.H577. [DOI] [PubMed] [Google Scholar]
- 58.Hilgenfeldt U. Half-life of rat angiotensinogen: influence of nephrectomy and lipopolysaccharide stimulation. Mol Cell Endocrinol. 1988;56:91–98. doi: 10.1016/0303-7207(88)90012-3. [DOI] [PubMed] [Google Scholar]
- 59.Yayama K, Yoshiya M, Takahashi K, et al. Role of the kidney in the plasma clearance of angiotensinogen in the rat: plasma clearance and tissue distribution of 125I-angiotensinogen. Life Sci. 1995;57:1791–1801. doi: 10.1016/0024-3205(95)02157-e. [DOI] [PubMed] [Google Scholar]
- 60.Daugherty A, Thorpe SR, Lange LG, Sobel BE, Schonfeld G. Loci of catabolism of B-very low density lipoproteins in vivo delineated with a residualizing label, 125I-dilactitol tyramine. J. Biol. Chem. 1985;260:14564–14570. [PubMed] [Google Scholar]
- 61.Ohkubo H, Kawakami H, Kakehi Y, et al. Generation of transgenic mice with elevated blood pressure by introduction of the rat renin and angiotensinogen genes. Proc Natl Acad Sci U S A. 1990;87:5153–5157. doi: 10.1073/pnas.87.13.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kimura S, Mullins JJ, Bunnemann B, et al. High blood pressure in transgenic mice carrying the rat angiotensinogen gene. EMBO J. 1992;11:821–827. doi: 10.1002/j.1460-2075.1992.tb05119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takahashi S, Fukamizu A, Hasegawa T, et al. Expression of the human angiotensinogen gene in transgenic mice and transfected cells. Biochem. Biophys. Res. Comm. 1991;180:1103–1109. doi: 10.1016/s0006-291x(05)81180-5. [DOI] [PubMed] [Google Scholar]
- 64.Fukamizu A, Sugimura K, Takimoto E, et al. Chimeric renin-angiotensin system demonstrates sustained increase in blood pressure of transgenic mice carrying both human renin and human angiotensinogen genes. J Biol Chem. 1993;268:11617–11621. [PubMed] [Google Scholar]
- 65.Kim HS, Krege JH, Kluckman KD, et al. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci USA. 1995;92:2735–2729. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanimoto K, Sugiyama F, Goto Y, et al. Angiotensinogen-deficient mice with hypotension. J Biol Chem. 1994:31334–31337. [PubMed] [Google Scholar]
- 67.Kai T, Kino H, Ishikawa K. Role of the renin-angiotensin system in cardiac hypertrophy and renal glomerular sclerosis in transgenic hypertensive mice carrying both human renin and angiotensinogen genes. Hypertens Res. 1998;21:39–46. doi: 10.1291/hypres.21.39. [DOI] [PubMed] [Google Scholar]
- 68.Nagata M, Tanimoto K, Fukamizu A, et al. Nephrogenesis and renovascular development in angiotensinogen-deficient mice. Lab Invest. 1996;75:745–753. [PubMed] [Google Scholar]
- 69.Walther T, Steendijk P, Westermann D, et al. Angiotensin deficiency in mice leads to dilated cardiomyopathy. Eur J Pharmacol. 2004;493:161–165. doi: 10.1016/j.ejphar.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 70.Yanai K, Saito T, Kakinuma Y, et al. Renin-dependent cardiovascular functions and renin-independent blood- brain barrier functions revealed by renin-deficient mice. J Biol Chem. 2000;275:5–8. doi: 10.1074/jbc.275.1.5. [DOI] [PubMed] [Google Scholar]
- 71.Krege JH, John SW, Langenbach LL, et al. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375:146–148. doi: 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- 72.Oliverio MI, Kim HS, Ito M, et al. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci USA. 1998;95:15496–15151. doi: 10.1073/pnas.95.26.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsuchida S, Matsusaka T, Chen X, et al. Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest. 1998;101:755–760. doi: 10.1172/JCI1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lochard N, Silversides DW, van Kats JP, Mercure C, Reudelhuber TL. Brain-specific restoration of angiotensin II corrects renal defects seen in angiotensinogen-deficient mice. J Biol Chem. 2003;278:2184–2189. doi: 10.1074/jbc.M209933200. [DOI] [PubMed] [Google Scholar]
- 75.Ishida J, Sugiyama F, Tanimoto K, et al. Rescue of angiotensinogen-knockout mice. Biochem Biophys Res Commun. 1998;252:610–616. doi: 10.1006/bbrc.1998.9707. [DOI] [PubMed] [Google Scholar]
- 76.Kang N, Walther T, Tian XL, et al. Reduced hypertension-induced end-organ damage in mice lacking cardiac and renal angiotensinogen synthesis. J Mol Med. 2002;80:359–366. doi: 10.1007/s00109-002-0326-6. [DOI] [PubMed] [Google Scholar]
- 77.Davisson RL, Kim HS, Krege JH, et al. Complementation of reduced survival, hypotension, and renal abnormalities in angiotensinogen-deficient mice by the human renin and human angiotensinogen genes. J. Clin. Invest. 1997;99:1258–1264. doi: 10.1172/JCI119283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davisson RL, Ding Y, Stec DE, Catterall JF, Sigmund CD. Novel mechanism of hypertension revealed by cell-specific targeting of human angiotensinogen in transgenic mice. Physiol Genomics. 1999;1:3–9. doi: 10.1152/physiolgenomics.1999.1.1.3. [DOI] [PubMed] [Google Scholar]
- 79.Massiera F, Bloch-Faure M, Ceiler D, et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15:2727–2729. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- 80.Ding Y, Stec DE, Sigmund CD. Genetic evidence that lethality in angiotensinogen-deficient mice is due to loss of systemic but not renal angiotensinogen. J Biol Chem. 2001;276:7431–7436. doi: 10.1074/jbc.M003892200. [DOI] [PubMed] [Google Scholar]
- 81.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 82.Hillaert MA, Lentjes EG, Beygui F, et al. Measuring and targeting aldosterone and renin in atherosclerosis-A review of clinical data. Am Heart J. 2011;162:585–596. doi: 10.1016/j.ahj.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 83.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation. 2001;103:448–454. doi: 10.1161/01.cir.103.3.448. [DOI] [PubMed] [Google Scholar]
- 85.Daugherty A, Rateri DL, Lu H, Inagami T, Cassis LA. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor. Circulation. 2004;110:3849–3857. doi: 10.1161/01.CIR.0000150540.54220.C4. [DOI] [PubMed] [Google Scholar]
- 86.Wassmann S, Czech T, Van Eickels M, et al. Inhibition of diet-induced atherosclerosis and endothelial dysfunction in apolipoprotein E/Angiotensin II type 1A receptor double-knockout mice. Circulation. 2004;110:3062–3067. doi: 10.1161/01.CIR.0000137970.47771.AF. [DOI] [PubMed] [Google Scholar]
- 87.Weiss D, Taylor WR. Deoxycorticosterone acetate salt hypertension in apolipoprotein E−/− mice results in accelerated atherosclerosis: the role of angiotensin II. Hypertension. 2008;51:218–224. doi: 10.1161/HYPERTENSIONAHA.107.095885. [DOI] [PubMed] [Google Scholar]
- 88.Sugiyama F, Haraoka S, Watanabe T, et al. Acceleration of atherosclerotic lesions in transgenic mice with hypertension by the activated renin-angiotensin system. Lab Invest. 1997;76:835–842. [PubMed] [Google Scholar]
- 89.Jones BH, Standridge MK, Taylor JW, Moustaid N. Angiotensinogen gene expression in adipose tissue: analysis of obese models and hormonal and nutritional control. Am J Physiol. 1997;273:236–242. doi: 10.1152/ajpregu.1997.273.1.R236. [DOI] [PubMed] [Google Scholar]
- 90.Rahmouni K, Mark AL, Haynes WG, Sigmund CD. Adipose depot-specific modulation of angiotensinogen gene expression in diet-induced obesity. Am J Physiol Endocrinol Metab. 2004;286:E891–E895. doi: 10.1152/ajpendo.00551.2003. [DOI] [PubMed] [Google Scholar]
- 91.Boustany CM, Bharadwaj K, Daugherty A, et al. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;287:R943–R949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 92.Massiera F, Seydoux J, Geloen A, et al. Angiotensinogen-deficient mice exhibit impairment of diet-induced weight gain with alteration in adipose tissue development and increased locomotor activity. Endocrinology. 2001;142:5220–5225. doi: 10.1210/endo.142.12.8556. [DOI] [PubMed] [Google Scholar]
- 93.Kim S, Urs S, Massiera F, et al. Effects of high-fat diet, angiotensinogen (agt) gene inactivation, and targeted expression to adipose tissue on lipid metabolism and renal gene expression. Horm Metab Res. 2002;34:721–725. doi: 10.1055/s-2002-38263. [DOI] [PubMed] [Google Scholar]
- 94.Kai T, Sugimura K, Shimada S, Kurooka A, Ishikawa K. Renin-angiotensin system stimulates cardiac and renal disorders in Tsukuba hypertensive mice. Clin Exp Pharmacol Physiol. 1999;26:206–211. doi: 10.1046/j.1440-1681.1999.03023.x. [DOI] [PubMed] [Google Scholar]
- 95.Jeunemaitre X, Soubrier F, Kotelevtsev YV, et al. Molecular basis of human hypertension: role of angiotensinogen. Cell. 1992;71:169–180. doi: 10.1016/0092-8674(92)90275-h. [DOI] [PubMed] [Google Scholar]
- 96.Sethi AA, Nordestgaard BG, Gronholdt ML, et al. Angiotensinogen single nucleotide polymorphisms, elevated blood pressure, and risk of cardiovascular disease. Hypertension. 2003;41:1202–1211. doi: 10.1161/01.HYP.0000072334.34433.17. [DOI] [PubMed] [Google Scholar]
- 97.Katsuya T, Koike G, Yee TW, et al. Association of angiotensinogen gene T235 variant with increased risk of coronary heart disease. Lancet. 1995;345:1600–1603. doi: 10.1016/s0140-6736(95)90115-9. [DOI] [PubMed] [Google Scholar]
- 98.Jeunemaitre X, Ledru F, Battaglia S, et al. Genetic polymorphisms of the renin-angiotensin system and angiographic extent and severity of coronary artery disease: the CORGENE study. Hum Genet. 1997;99:66–73. doi: 10.1007/s004390050313. [DOI] [PubMed] [Google Scholar]
- 99.Rodriguez-Perez JC, Rodriguez-Esparragon F, Hernandez-Perera O, et al. Association of angiotensinogen M235T and A(-6)G gene polymorphisms with coronary heart disease with independence of essential hypertension: the PROCAGENE study. Prospective Cardiac Gene. J Am Coll Cardiol. 2001;37:1536–1542. doi: 10.1016/s0735-1097(01)01186-x. [DOI] [PubMed] [Google Scholar]
- 100.Zafarmand MH, van der Schouw YT, Grobbee DE, de Leeuw PW, Bots ML. The M235T polymorphism in the AGT gene and CHD risk: evidence of a Hardy-Weinberg equilibrium violation and publication bias in a meta-analysis. PLoS One. 2008;3:e2533. doi: 10.1371/journal.pone.0002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gardemann A, Stricker J, Humme J, et al. Angiotensinogen T174M and M235T gene polymorphisms are associated with the extent of coronary atherosclerosis. Atherosclerosis. 1999;145:309–314. doi: 10.1016/s0021-9150(99)00082-9. [DOI] [PubMed] [Google Scholar]
- 102.Tsai CT, Hwang JJ, Ritchie MD, et al. Renin-angiotensin system gene polymorphisms and coronary artery disease in a large angiographic cohort: detection of high order gene-gene interaction. Atherosclerosis. 2007;195:172–180. doi: 10.1016/j.atherosclerosis.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 103.Prat-Larquemin L, Oppert JM, Clement K, et al. Adipose angiotensinogen secretion, blood pressure, and AGT M235T polymorphism in obese patients. Obes Res. 2004;12:556–561. doi: 10.1038/oby.2004.63. [DOI] [PubMed] [Google Scholar]
- 104.Takakura Y, Yoshida T, Yoshioka K, Umekawa T, Kogure A, Toda H, Kagawa K, Fukui S, Yoshikawa T. Angiotensinogen gene polymorphism (Met235Thr) influences visceral obesity and insulin resistance in obese Japanese women. 2006;55:6, 819–824. doi: 10.1016/j.metabol.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 105.Procopciuc LM, Sitar-Taut A, Pop D, et al. Renin angiotensin system polymorphisms in patients with metabolic syndrome (MetS) Eur J Intern Med. 2010;21:414–418. doi: 10.1016/j.ejim.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 106.Cooper R, McFarlane-Anderson N, Bennett FI, Wilks R, Puras A, Tewksbury D, Ward R, Forrester T. ACE, angiotensinogen and obesity: a potential pathway leading to hypertension. 97;11:2, 107–111. doi: 10.1038/sj.jhh.1000391. [DOI] [PubMed] [Google Scholar]